Homometallic 2D Cd2+ and Heterometallic 3D Cd2+/Ca2+, Cd2+/Sr2+ Metal–Organic Frameworks Based on an Angular Tetracarboxylic Ligand
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis
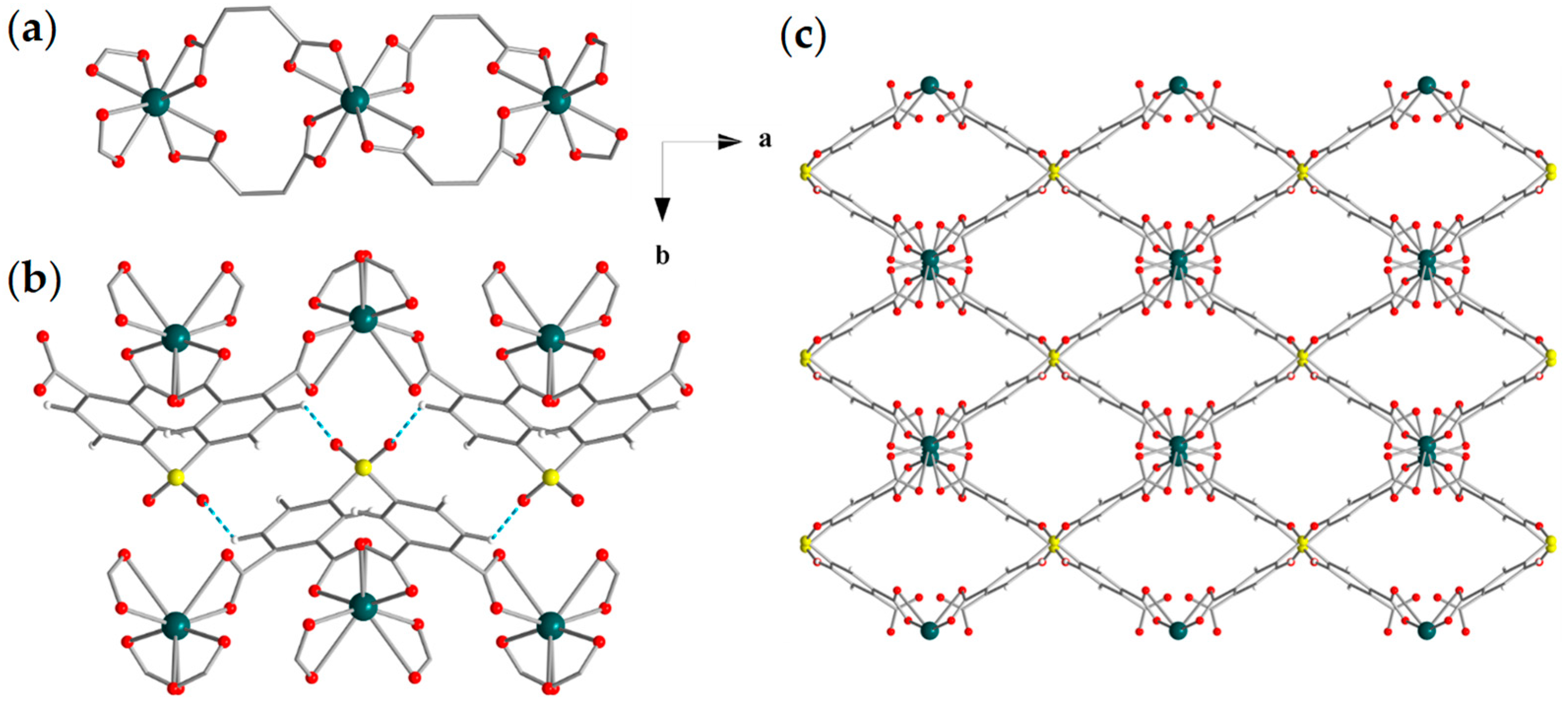
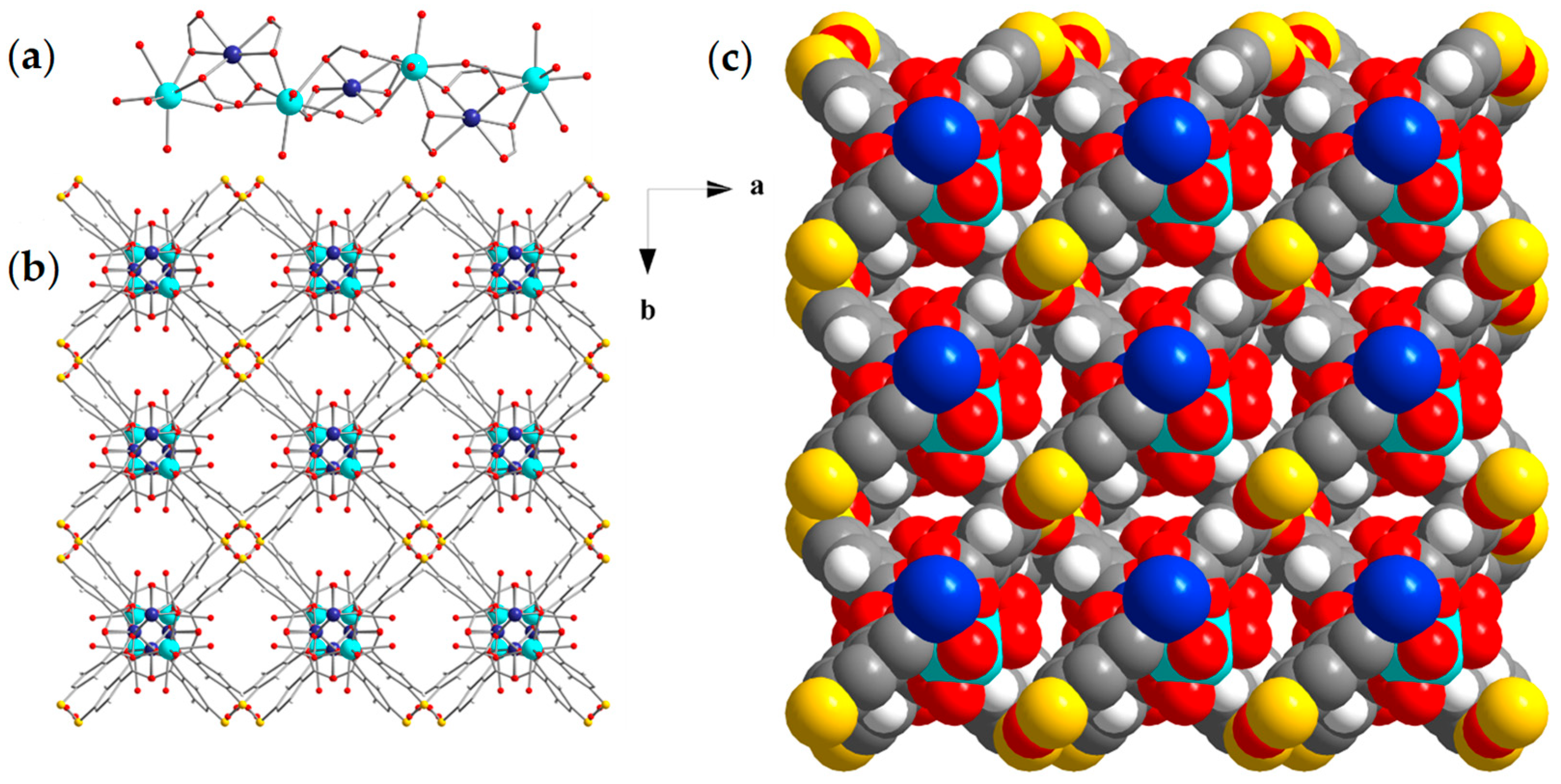
3.2. Structural Characterization
3.3. Physical Characterization
3.4. Gas Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Férey, G. Hybrid Porous Solids: Past, Present, Future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like Metal–Organic Frameworks (ZMOFs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape Engineering of Metal–Organic Frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.; Daniels, E.A.; Curtin, T.; Perry, J.J.; Zaworotko, M.J. Direct Air Capture of CO2 by Physisorbent Materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef] [PubMed]
- Gehre, M.; Guo, Z.; Rothenberg, G.; Tanase, S. Sustainable Separations of C4-Hydrocarbons by Using Microporous Materials. ChemSusChem 2017, 10, 3947–3963. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent Advances in Gas Storage and Separation Using Metal–Organic Frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Liu, J.; Goetjen, T.A.; Wang, Q.; Knapp, J.G.; Wasson, M.C.; Yang, Y.; Syed, Z.H.; Delferro, M.; Notestein, J.M.; Farha, O.K.; et al. MOF-Enabled Confinement and Related Effects for Chemical Catalyst Presentation and Utilization. Chem. Soc. Rev. 2022, 51, 1045–1097. [Google Scholar] [CrossRef] [PubMed]
- Hadjikyprianou, E.; Petrides, S.; Kourtellaris, A.; Tasiopoulos, A.J.; Georgiades, S.N. Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework. Materials 2023, 16, 5298. [Google Scholar] [CrossRef]
- Diamantis, S.A.; Margariti, A.; Pournara, A.D.; Papaefstathiou, G.S.; Manos, M.J.; Lazarides, T. Luminescent Metal–Organic Frameworks as Chemical Sensors: Common Pitfalls and Proposed Best Practices. Inorg. Chem. Front. 2018, 5, 1493–1511. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and Photophysics of MOFs: Steps towards MOF-Based Sensing Enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef] [PubMed]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–Organic Frameworks for Chemical Sensing Devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and Capture of Toxic and Hazardous Gases and Vapors by Metal–Organic Frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–Organic Framework-Based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–Organic Frameworks for Heavy Metal Removal from Water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Kumar, P.; Pournara, A.; Kim, K.-H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-Organic Frameworks: Challenges and Opportunities for Ion-Exchange/Sorption Applications. Prog. Mater. Sci. 2017, 86, 25–74. [Google Scholar] [CrossRef]
- Panagiotou, N.; Liatsou, I.; Pournara, A.; Angeli, G.K.; Giappa, R.M.; Tylianakis, E.; Manos, M.J.; Froudakis, G.E.; Trikalitis, P.N.; Pashalidis, I.; et al. Water-Stable 2-D Zr MOFs with Exceptional UO22+ Sorption Capability. J. Mater. Chem. A 2020, 8, 1849–1857. [Google Scholar] [CrossRef]
- Hanikel, N.; Kurandina, D.; Chheda, S.; Zheng, Z.; Rong, Z.; Neumann, S.E.; Sauer, J.; Siepmann, J.I.; Gagliardi, L.; Yaghi, O.M. MOF Linker Extension Strategy for Enhanced Atmospheric Water Harvesting. ACS Cent. Sci. 2023, 9, 551–557. [Google Scholar] [CrossRef]
- Huang, J.-J.; Yu, J.-H.; Xu, J.-Q. Structural Characterization of Three Semi-Rigid Tetracarboxylate-Containing Transition-Metal Coordination Polymers. Polyhedron 2016, 117, 126–132. [Google Scholar] [CrossRef]
- Bajpai, A.; Chandrasekhar, P.; Govardhan, S.; Banerjee, R.; Moorthy, J.N. Single Crystal-to-Single Crystal Site-Selective Postsynthetic Metal Exchange in a Zn–MOF Based on Semi-Rigid Tricarboxylic Acid and Access to Bimetallic MOFs. Chem. A Eur. J. 2015, 21, 2759–2765. [Google Scholar] [CrossRef]
- Fan, Y.; Si, C.-D.; Hou, C.; Yao, X.-Q.; Hu, D.-C.; Yang, Y.-X.; Liu, J.-C. Three Complexes of Manganese(II) Based on a New Semirigid Tetracarboxylate and N-Containing Ligands: Synthesis, Crystal Structures and Magnetic Properties. Polyhedron 2015, 98, 64–70. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Li, Y.-Z.; Du, H.-B. Synthesis and Properties of Four Coordination Polymers Built from a Semi-Rigid Tripod Carboxylic Acid. CrystEngComm 2013, 15, 8989. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Ju, Z.; Zheng, H. Solvent-Induced Synthesis of Cobalt(II) Coordination Polymers Based on a Rigid Ligand and Flexible Carboxylic Acid Ligands: Syntheses, Structures and Magnetic Properties. Dalton Trans. 2015, 44, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Ahmad, M.; Tripathi, M.R.; Butcher, R.J. Four Novel Coordination Polymers of Transition Metals Built Using a Semi Rigid Oxygen Donor Ligand: Crystal Structures, Novel Topology and Emission Studies. Polyhedron 2013, 50, 169–178. [Google Scholar] [CrossRef]
- Hou, X.; Tang, S.-F. Two New Two-Dimensional Layered Uranyl-Bearing Polycarboxylates from Semi-Rigid Tetracarboxylic Acids. RSC Adv. 2014, 4, 34716. [Google Scholar] [CrossRef]
- Wang, C.; Guo, G.; Wang, P. Two Sodium and Lanthanide(III) MOFs Based on Oxalate and V-Shaped 4,4′-Oxybis(Benzoate) Ligands: Hydrothermal Synthesis, Crystal Structure, and Luminescence Properties. J. Mol. Struct. 2013, 1032, 93–99. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Tseng, F.-S.; Yeh, C.-T.; Chang, T.-G.; Kao, C.-C.; Lin, C.-H.; Liu, W.-R.; Chen, J.-S.; Zima, V. Synthesis, Structures, and Properties of Alkali and Alkaline Earth Coordination Polymers Based on V-Shaped Ligand. CrystEngComm 2012, 14, 6812. [Google Scholar] [CrossRef]
- He, J.-H.; Xiao, D.-R.; Yan, S.-W.; Sun, D.-Z.; Chen, H.-Y.; Wang, X.; Yang, J.; Ye, Z.-L.; Yuan, R.; Wang, E.-B. A Series of Novel 1D Coordination Polymers Constructed from Metal–Quinolone Complex Fragments Linked by Aromatic Dicarboxylate Ligands. Solid State Sci. 2012, 14, 1203–1210. [Google Scholar] [CrossRef]
- He, J.-H.; Sun, D.-Z.; Xiao, D.-R.; Yan, S.-W.; Chen, H.-Y.; Wang, X.; Yang, J.; Wang, E.-B. Syntheses and Structures of Five 1D Coordination Polymers Based on Quinolone Antibacterial Agents and Aromatic Polycarboxylate Ligands. Polyhedron 2012, 42, 24–29. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zheng, X.-J.; Chen, X.-B.; Li, L.-C.; Jin, L.-P. Assembly and Upconversion Luminescence of Lanthanide–Organic Frameworks with Mixed Acid Ligands. Inorganica Chim. Acta 2009, 362, 325–330. [Google Scholar] [CrossRef]
- Panagiotou, N.; Evangelou, K.; Psalti, A.; Varnava, N.; Angeli, G.K.; Trikalitis, P.N.; Plakatouras, J.C.; Lazarides, T.; Tasiopoulos, A.J. Improving the Cd2+ Detection Capability of a New Anionic Rare Earth Metal–Organic Framework Based on a [RE6(μ3-OH)8]10+ Secondary Building Unit: An Ion-Exchange Approach towards More Efficient Sensors. Mol. Syst. Des. Eng. 2020, 5, 1077–1087. [Google Scholar] [CrossRef]
- Gu, J.; Sun, X.; Kan, L.; Qiao, J.; Li, G.; Liu, Y. Structural Regulation and Light Hydrocarbon Adsorption/Separation of Three Zirconium–Organic Frameworks Based on Different V-Shaped Ligands. ACS Appl. Mater. Interfaces 2021, 13, 41680–41687. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Zhang, B.; Hu, H.-M.; Fu, F.; Wu, X.-L.; Qin, T.; Yang, M.-L.; Xue, G.-L.; Wang, J.-W. Three Novel Heterobimetallic Cd/Zn−Na Coordination Polymers: Syntheses, Crystal Structure, and Luminescence. Cryst. Growth Des. 2008, 8, 3706–3712. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Chen, Y.; Deng, X.-F.; Li, X.; Xu, H.-L.; Yang, Q.; Hu, G.; Qiu, H.-J.; Xiao, D.-R. Two Porous Coordination Polymers Containing Helix-Based Metal-Organic Nanotubes Based on Trigonal N-Donor Ligand. Inorg. Chem. Commun. 2016, 72, 65–68. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Ma, J.-F.; Yang, J.; Liu, Y.-Y.; Wei, G.-H. 1D, 2D, and 3D Metal−Organic Frameworks Based on Bis(Imidazole) Ligands and Polycarboxylates: Syntheses, Structures, and Photoluminescent Properties. Cryst. Growth Des. 2009, 9, 4660–4673. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, J.; Wu, T.; Li, J.; Yang, L.; Cao, R. Synthesis, Structural Characterization and Anion-Sensing Studies of Metal(II) Complexes Based on 3,3′,4,4′-Oxydiphthalate and N-Donor Ligands. Dalton Trans. 2012, 41, 1961–1970. [Google Scholar] [CrossRef]
- Yao, S.; Yi, F.-Y.; Li, G.; Yu, Y.; Wang, J.; Liu, D.; Song, S.-Y. Syntheses, Structures, and Magnetic Properties of Cobalt(II) and Nickel(II) Coordination Polymers Based on a V-Shaped Ligand. J. Solid State Chem. 2017, 250, 6–13. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, J.H.; Moon, D.; Moon, H.R. Luminescent Li-Based Metal–Organic Framework Tailored for the Selective Detection of Explosive Nitroaromatic Compounds: Direct Observation of Interaction Sites. Inorg. Chem. 2013, 52, 589–595. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Wang, Y.-Y.; Zhou, M.-J.; Qin, L.; Zhang, M.-D.; Yang, Q.-X.; Zheng, H.-G. A Three-Dimensional Non-Interpenetrated Porous Metal–Organic Framework Based on Cationic 1-D Chains. Inorg. Chem. Commun. 2014, 40, 148–150. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.; Chen, Z.; Hao, Y.; Li, Y.; Lu, Q.; Zheng, H. Metal–Organic Frameworks Constructed from Flexible V-Shaped Ligands: Adjustment of the Topology, Interpenetration and Porosity via a Solvent System. Chem. Commun. 2012, 48, 10016. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.K.; Suh, M.P.; Moon, H.R. Solvent-Induced Single-Crystal to Single-Crystal Transformation of a Zn 4 O-Containing Doubly Interpenetrated Metal–Organic Framework with a Pcu Net. CrystEngComm 2015, 17, 8807–8811. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Vodak, D.; Sudik, A.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Geometric Requirements and Examples of Important Structures in the Assembly of Square Building Blocks. Proc. Natl. Acad. Sci. USA 2002, 99, 4900–4904. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, M.B.; Kocaarslan, A.; Kaya, K.; Atsay, A.; Svensson Grape, E.; Chen, J.; Yagci, Y. An Unusual Zig-Zag 2D Copper(I) Coordination Polymer as an Outstanding Catalyst for Azide–Alkyne “Click” Chemistry at Room Temperature. Dalton Trans. 2022, 51, 17543–17546. [Google Scholar] [CrossRef]
- Winterlich, M.; McHugh, D.; O’Toole, E.; Skordi, K.; O’Malley, C.; Sanii, R.; Tasiopoulos, A.; Erxleben, A.; Mayans, J.; Morrison, L.; et al. Expanding the NUIG MOF Family: Synthesis and Characterization of New MOFs for Selective CO2 Adsorption, Metal Ion Removal from Aqueous Systems, and Drug Delivery Applications. Dalton Trans. 2021, 50, 6997–7006. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Kim, J.; Ockwig, N.W.; O’Keeffe, M.; Yaghi, O.M. Control of Vertex Geometry, Structure Dimensionality, Functionality, and Pore Metrics in the Reticular Synthesis of Crystalline Metal−Organic Frameworks and Polyhedra. J. Am. Chem. Soc. 2008, 130, 11650–11661. [Google Scholar] [CrossRef]
- Singh, B.; Mazumder, M.; Balendra; Sundaresan, A.; Pati, S.K.; Ramanan, A. Influence of Noncovalent Interactions on the Magnetic Behavior of Three Isostructural Layered Manganese(II) Dicarboxylate-Based Coordination Polymers. Cryst. Growth Des. 2022, 22, 2534–2546. [Google Scholar] [CrossRef]
- Mohmeyer, A.; Schaate, A.; Brechtken, B.; Rode, J.C.; Warwas, D.P.; Zahn, G.; Haug, R.J.; Behrens, P. Delamination and Photochemical Modification of a Novel Two-Dimensional Zr-Based Metal–Organic Frameworks. Chem. A Eur. J. 2018, 24, 12848–12855. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, E.; An, H.; Li, Y.; Su, Z.; Sun, C. A Bridge between Pillared-Layer and Helical Structures: A Series of Three-Dimensional Pillared Coordination Polymers with Multiform Helical Chains. Chem. A Eur. J. 2006, 12, 6528–6541. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.-J.; Kang, Y.; Cheng, J.-K.; Yao, Y.-G. Hydrothermal Syntheses, Crystal Structures, and Properties of a Novel Class of 3,3‘,4,4‘-Benzophenone-Tetracarboxylate (BPTC) Polymers. Inorg. Chem. 2004, 43, 8085–8091. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.-Y.; Yang, G.-P.; Wang, C.-J.; Wen, G.-L.; Shi, Q.-Z.; Batten, S.R. A Series of Intriguing Metal–Organic Frameworks with 3,3′,4,4′-Benzophenonetetracarboxylic Acid: Structural Adjustment and pH-Dependence. CrystEngComm 2008, 10, 1583. [Google Scholar] [CrossRef]
- Chen, S.-M.; Lian, T.-T. Synthesis, Structure and Properties of a Microporous Cd(II) 3,3′,4,4′-Benzophenone-Tetracarboxylate Framework Material. Inorg. Chem. Commun. 2011, 14, 447–449. [Google Scholar] [CrossRef]
- Balendra; Banday, A.; Tewari, S.; Singh, B.; Murugavel, S.; Ramanan, A. Alkaline-Earth Metal Based Coordination Polymers Assembled from Two Different V-Shaped Ligands: Synthesis, Structure, and Dielectric Properties. Inorg. Chim. Acta 2019, 495, 118940. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Conato, M.; Jacobson, A.J. Amine-Templated Anionic Metal–Organic Frameworks with the 4,4′-(Hexafluoroisopropylidene) Bis(Benzoic Acid) Ligand. Cryst. Growth Des. 2011, 11, 2257–2263. [Google Scholar] [CrossRef]
- Bernini, M.C.; De Paz, J.R.; Snejko, N.; Sáez-Puche, R.; Gutierrez-Puebla, E.; Monge, M.Á. Unusual Magnetic Behaviors and Electronic Configurations Driven by Diverse Co(II) or Mn(II) MOF Architectures. Inorg. Chem. 2014, 53, 12885–12895. [Google Scholar] [CrossRef]
- Castillo-Blas, C.; López-Salas, N.; Gutiérrez, M.C.; Puente-Orench, I.; Gutiérrez-Puebla, E.; Ferrer, M.L.; Monge, M.Á.; Gándara, F. Encoding Metal–Cation Arrangements in Metal–Organic Frameworks for Programming the Composition of Electrocatalytically Active Multimetal Oxides. J. Am. Chem. Soc. 2019, 141, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.C.; Platero-Prats, A.E.; Snejko, N.; Gutiérrez-Puebla, E.; Labrador, A.; Sáez-Puche, R.; Romero De Paz, J.; Monge, M.A. Tuning the Magnetic Properties of Transition Metal MOFs by Metal–Oxygen Condensation Control: The Relation between Synthesis Temperature, SBU Nuclearity and Carboxylate Geometry. CrystEngComm 2012, 14, 5493. [Google Scholar] [CrossRef]
- Huang, J.-J.; Xu, W.; Wang, Y.-N.; Yu, J.-H.; Zhang, P.; Xu, J.-Q. New 3-D Coordination Polymers Based on Semi-Rigid V-Shape Tetracarboxylates. J. Solid State Chem. 2015, 226, 206–214. [Google Scholar] [CrossRef]
- Zhong, R.-Q.; Zou, R.-Q.; Du, M.; Yamada, T.; Maruta, G.; Takeda, S.; Xu, Q. Controllable Preparation, Network Structures and Properties of Unusual Metal–Organic Frameworks Constructed from 4,4′-(Hexafluoroisopropylidene)Diphthalic Acid and 4,4′-Bipyridyl. Dalton Trans. 2008, 17, 2346–2354. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Ma, J.-F.; Pang, Y.-Y.; Ma, J.-C.; Yang, J. Four Novel Topological Frameworks Based on 4,4′-(Hexafluoroisopropylidene)Diphthalic Acid and 1,1′-(1,4-Butanediyl)Bis(Imidazole) Ligand. CrystEngComm 2010, 12, 4433. [Google Scholar] [CrossRef]
- Thuéry, P.; Masci, B.; Harrowfield, J. Complexation of Uranyl and Rare-Earth Ions by a Fluorinated Tetracarboxylate. Formation of a Layered Assembly and Three-Dimensional Frameworks. Cryst. Growth Des. 2013, 13, 3216–3224. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, Q.; Akita, T.; Liu, B.; Ohashi, H.; Oji, H.; Honma, T.; Takei, T.; Haruta, M.; Xu, Q. Ultrafine Gold Clusters Incorporated into a Metal–Organic Framework. Chem. A Eur. J. 2011, 17, 78–81. [Google Scholar] [CrossRef]
- Gupta, V.; Mandal, S.K. Design and Construction of a Chiral Cd(II)-MOF from Achiral Precursors: Synthesis, Crystal Structure and Catalytic Activity toward C–C and C–N Bond Forming Reactions. Inorg. Chem. 2019, 58, 3219–3226. [Google Scholar] [CrossRef]
- Gupta, V.; Mandal, S.K. Effect of Unsaturated Metal Site Modulation in Highly Stable Microporous Materials on CO2 Capture and Fixation. Inorg. Chem. 2022, 61, 3086–3096. [Google Scholar] [CrossRef]
- Turcan-Trofin, G.-O.; Avadanei, M.; Shova, S.; Vlad, A.; Cazacu, M.; Zaltariov, M.-F. Metallo-Supramolecular Assemblies of Dinuclear Zn(II) and Mn(II) Secondary Building Units (SBUs) and a Bent Silicon Dicarboxylate Ligand. Inorganica Chim. Acta 2018, 483, 454–463. [Google Scholar] [CrossRef]
- Gavrish, S.P.; Shova, S.; Gazacu, M.; Lampeka, Y.D. A 2D Coordination Polymerassembled from Nickel(II) Tetraazamacrocyclic Cation and 4,4′-(Dimethylsilanediyl)Diphthalate(3-) Linker. Acta Crystallogr. C Struct. Chem. 2020, 76, 419–426. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Luo, J.-H.; Wu, C.-Y.; Cheng, Y.-J.; Yeh, C.-T.; Chen, Y.-T.; Lo, S.-H.; Lai, Y.-L.; Lin, C.-H. Solvothermal Synthesis, Structural Diversity, and Properties of Alkali Metal–Organic Frameworks Based on V-Shaped Ligand. Cryst. Growth Des. 2013, 13, 3785–3793. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Lin, W.-C.; Lo, S.-H.; Kao, C.-C.; Lin, C.-H.; Yang, C.-C. Microwave Synthesis and Gas Sorption of Calcium and Strontium Metal–Organic Frameworks with High Thermal Stability. CrystEngComm 2012, 14, 1219. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ramanujachary, K.V.; Lofland, S.E.; Magdaleno, T.; Natarajan, S. Stabilization of O–Mn–O Clusters (Mn5) in Three Dimensionally Extended MOF Structures: Synthesis, Structure and Properties. CrystEngComm 2012, 14, 4323. [Google Scholar] [CrossRef]
- Panagiotou, N.; Moscoso, F.G.; Lopes-Costa, T.; Pedrosa, J.M.; Tasiopoulos, A.J. 2-Dimensional Rare Earth Metal–Organic Frameworks Based on a Hexanuclear Secondary Building Unit as Efficient Detectors for Vapours of Nitroaromatics and Volatile Organic Compounds. Inorg. Chem. Front. 2022, 9, 4850–4863. [Google Scholar] [CrossRef]
- Fu, F.; Li, D.-S.; Wu, Y.-P.; Gao, X.-M.; Du, M.; Tang, L.; Zhang, X.-N.; Meng, C.-X. A Versatile V-Shaped Tetracarboxylate Building Block for Constructing Mixed-Ligand Co(Ii) and Mn(Ii) Complexes Incorporating Various N-Donor Co-Ligands. CrystEngComm 2010, 12, 1227–1237. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, X.; Putkham, A.; Hong, K.; Lobkovsky, E.B.; Hurtado, E.J.; Fletcher, A.J.; Thomas, K.M. Surface Interactions and Quantum Kinetic Molecular Sieving for H2 and D2 Adsorption on a Mixed Metal−Organic Framework Material. J. Am. Chem. Soc. 2008, 130, 6411–6423. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Effect of Zn/Co Ratio in MOF-74 Type Materials Containing Exposed Metal Sites on Their Hydrogen Adsorption Behaviour and on Their Band Gap Energy. Int. J. Hydrogen Energy 2011, 36, 10834–10844. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, M.; Li, Y.; Liang, J.; Shi, W.; Chen, J.; Cheng, P. A Porous 3d-4f Heterometallic Metal–Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2010, 35, 8166–8170. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, P.; Chen, J.; Liao, D.-Z.; Yan, S.-P. A Heterometallic Porous Material for Hydrogen Adsorption. Inorg. Chem. 2007, 46, 4530–4534. [Google Scholar] [CrossRef]
- Wei, W.; Xia, Z.; Wei, Q.; Xie, G.; Chen, S.; Qiao, C.; Zhang, G.; Zhou, C. A Heterometallic Microporous MOF Exhibiting High Hydrogen Uptake. Microporous Mesoporous Mater. 2013, 165, 20–26. [Google Scholar] [CrossRef]
- Xu, Y.; Che, Y.; Cheng, F.; Zheng, J. Synthesis, Structures, and Adsorption Properties of Two New La III –Mg II Heterometallic Polymers. Eur. J. Inorg. Chem. 2011, 2011, 5299–5304. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Xu, L.; Li, C.; Zhao, D.; Ge, G.; Wang, Z.; Lin, J. A Heterometallic Metal–Organic Framework Based on Multi-Nuclear Clusters Exhibiting High Stability and Selective Gas Adsorption. Dalton Trans. 2019, 48, 278–284. [Google Scholar] [CrossRef]
- Luo, F.; Yan, C.; Dang, L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.; Han, Y.; Hu, T.-L.; O’Keeffe, M.; et al. UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Bai, D.; He, M.; Gao, X.; He, Y. Selective Adsorption of C2H2 and CO2 from CH4 in an Isoreticular Series of MOFs Constructed from Unsymmetrical Diisophthalate Linkers and the Effect of Alkoxy Group Functionalization on Gas Adsorption. J. Mater. Chem. A 2018, 6, 3471–3478. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Serra-Crespo, P.; De Lange, M.; Gascon, J.; Kapteijn, F.; Julve, M.; Cano, J.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; et al. Selective Gas and Vapor Sorption and Magnetic Sensing by an Isoreticular Mixed-Metal–Organic Framework. J. Am. Chem. Soc. 2012, 134, 15301–15304. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bu, X.; Zhao, X.; Li, D.-S.; Feng, P. Pore Space Partition in Metal–Organic Frameworks. Acc. Chem. Res. 2017, 50, 407–417. [Google Scholar] [CrossRef]
- Takaishi, K.; Nath, B.D.; Yamada, Y.; Kosugi, H.; Ema, T. Unexpected Macrocyclic Multinuclear Zinc and Nickel Complexes That Function as Multitasking Catalysts for CO2 Fixations. Angew. Chem. 2019, 131, 10089–10093. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, L.; Zhang, X.; Xu, P.; Sun, J. Facile One-Pot Synthesis of Zn/Mg-MOF-74 with Unsaturated Coordination Metal Centers for Efficient CO2 Adsorption and Conversion to Cyclic Carbonates. ACS Appl. Mater. Interfaces 2021, 13, 61334–61345. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, L.; Liu, J.; Huang, Q.; Lu, M.; Ji, W.; Lan, Y. Stable Heterometallic Cluster-Based Organic Framework Catalysts for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-Y.; Zhang, J.-W.; Li, Y.-P.; Li, H.-P.; Wang, Y.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C.; Zhai, Q.-G. Mimic of Ferroalloy To Develop a Bifunctional Fe–Organic Framework Platform for Enhanced Gas Sorption and Efficient Oxygen Evolution Electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 4432–4442. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, D.; Wu, Y.; Zhao, J.; Wu, T.; Zhang, J.; Li, D.; Sun, C.; Feng, P.; Bu, X. Stable Hierarchical Bimetal–Organic Nanostructures as HighPerformance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 4227–4231. [Google Scholar] [CrossRef]
- Iqbal, B.; Saleem, M.; Arshad, S.N.; Rashid, J.; Hussain, N.; Zaheer, M. One-Pot Synthesis of Heterobimetallic Metal–Organic Frameworks (MOFs) for Multifunctional Catalysis. Chem. A Eur. J. 2019, 25, 10490–10498. [Google Scholar] [CrossRef]
- Ouyang, Z.-J.; Mo, X.-Y.; Yang, M.; Zhong, L.; Chen, W.-B.; Gao, S.; Dong, W. High Temperature Fe(III) Spin Crossover Behaviours in Three Unprecedented FeIII–MII–FeIII (M = Fe, Cd) Linear Trinuclear Complexes. Inorg. Chem. Front. 2020, 7, 1526–1531. [Google Scholar] [CrossRef]
- Kawabata, S.; Nakabayashi, K.; Imoto, K.; Klimke, S.; Renz, F.; Ohkoshi, S. Second Harmonic Generation on Chiral Cyanido-Bridged FeII–NbIV Spin-Crossover Complexes. Dalton Trans. 2021, 50, 8524–8532. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Wang, J.; Bai, F.; Ma, Y.; Li, L.; Wang, Q.; Zhao, B.; Cheng, P. The Different Magnetic Relaxation Behaviors in [Fe(CN)6]3− or [Co(CN)6]3− Bridged 3d–4f Heterometallic Compounds. CrystEngComm 2020, 22, 2998–3004. [Google Scholar] [CrossRef]
- Fan, X.-T.; Yang, H.; Li, D.-C.; Tian, H.-Q.; Cao, F.; Dou, J.-M. Three New Heterometallic ZnII–LnIII Complexes with a Windmill-like Framework and Field-Induced SMM Behavior. New J. Chem. 2020, 44, 2555–2560. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Dong, J.; Ni, W.-Y.; Zhang, B.-W.; Cui, J.-Z.; Zhao, B. Unique Chiral Interpenetrating d–f Heterometallic MOFs as Luminescent Sensors. Inorg. Chem. 2015, 54, 5266–5272. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.; Yang, Y.-Y.; Song, X.-Q. A Multifunctional CaII–EuIII Heterometallic Organic Framework with Sensing and Selective Adsorption in Water. Inorg. Chem. 2024, 63, 6871–6882. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Silver, M.A.; Gui, D.; Bai, Z.; Wang, Y.; Liu, W.; Chen, L.; Diwu, J.; Chai, Z.; et al. Superprotonic Conduction through One-Dimensional Ordered Alkali Metal Ion Chains in a Lanthanide-Organic Framework. Chem. Commun. 2018, 54, 4429–4432. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Zhang, Z.; Plonka, A.M.; Li, J.; Parise, J.B. A Calcium Coordination Framework Having Permanent Porosity and High CO2/N2 Selectivity. Cryst. Growth Des. 2012, 12, 2162–2165. [Google Scholar] [CrossRef]
- Machattos, R.P.; Panagiotou, N.; Tasiopoulos, A.J. Highlighting the Structure—Directing Capability of the Functional Groups of Angular Dicarboxylic Ligands: New 2-Dimensional Cu2+ MOFs from Analogous Synthetic Routes. Polyhedron 2021, 205, 115299. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Sarkisov, L.; Bueno-Perez, R.; Sutharson, M.; Fairen-Jimenez, D. Materials Informatics with PoreBlazer v4.0 and the CSD MOF Database. Chem. Mater. 2020, 32, 9849–9867. [Google Scholar] [CrossRef]
- Lu, Y.-K.; Wang, H.-H.; Hu, Q.-X.; Ma, Y.-Y.; Hou, L.; Wang, Y.-Y. A Stable Cd(II)-Based MOF with Efficient CO2 Capture and Conversion, and Fluorescence Sensing for Ronidazole and Dimetridazole. J. Solid State Chem. 2021, 295, 121890. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Oxford Diffraction. CrysAlis CCD and CrysAlis RED; Version P171.38.46; Oxford Diffraction Ltd.: Abingdon, UK, 2017. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND; Version 2003.2001d; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Czepirski, L.; Jagiello, J. Virial-Type Thermal Equation of Gas Solid Adsorption. Chem. Eng. Sci. 1988, 44, 787–801. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machattos, R.P.; Panagiotou, N.; Karagianni, V.I.; Manos, M.J.; Moushi, E.E.; Tasiopoulos, A.J. Homometallic 2D Cd2+ and Heterometallic 3D Cd2+/Ca2+, Cd2+/Sr2+ Metal–Organic Frameworks Based on an Angular Tetracarboxylic Ligand. Materials 2025, 18, 4647. https://doi.org/10.3390/ma18204647
Machattos RP, Panagiotou N, Karagianni VI, Manos MJ, Moushi EE, Tasiopoulos AJ. Homometallic 2D Cd2+ and Heterometallic 3D Cd2+/Ca2+, Cd2+/Sr2+ Metal–Organic Frameworks Based on an Angular Tetracarboxylic Ligand. Materials. 2025; 18(20):4647. https://doi.org/10.3390/ma18204647
Chicago/Turabian StyleMachattos, Rafail P., Nikos Panagiotou, Vasiliki I. Karagianni, Manolis J. Manos, Eleni E. Moushi, and Anastasios J. Tasiopoulos. 2025. "Homometallic 2D Cd2+ and Heterometallic 3D Cd2+/Ca2+, Cd2+/Sr2+ Metal–Organic Frameworks Based on an Angular Tetracarboxylic Ligand" Materials 18, no. 20: 4647. https://doi.org/10.3390/ma18204647
APA StyleMachattos, R. P., Panagiotou, N., Karagianni, V. I., Manos, M. J., Moushi, E. E., & Tasiopoulos, A. J. (2025). Homometallic 2D Cd2+ and Heterometallic 3D Cd2+/Ca2+, Cd2+/Sr2+ Metal–Organic Frameworks Based on an Angular Tetracarboxylic Ligand. Materials, 18(20), 4647. https://doi.org/10.3390/ma18204647







