Hybrids of Deep HOMO Organic Cyanoacrylic Acid Dyes and Graphene Nanomaterials for Water Splitting Photoanodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Density Functional Theory (DFT) Calculations
2.2. Dye Synthesis and Characterization
2.2.1. (E)-3-(4-((2-(Tert-butyldimethylsilyloxy)ethyl)(methyl)amino)phenyl)-2-cyanoacrylic Acid (ASIL-CNCOOH)
2.2.2. Molecular Optoelectronic Characterization
2.3. Photoanode Preparation and PEC Tests
3. Results and Discussion
3.1. Theoretical Calculations
3.2. Synthesis and Optoelectronic Characterization of Dye Molecules
3.3. PEC Characterization of Dye-Sensitized TiO2 Photoanodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jian, J.; Jiang, G.; van de Krol, R.; Wei, B.; Wang, H. Recent advances in rational engineering of multinary semiconductors for photoelectrochemical hydrogen generation. Nano Energy 2018, 52, 457–480. [Google Scholar] [CrossRef]
- Raub, A.A.M.; Bahru, R.; Nashruddin, S.N.A.M.; Yunas, J. Advances of nanostructured metal oxide as photoanode in photoelectrochemical (PEC) water splitting application. Heliyon 2024, 10, e39079. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lia, F.; Sun, L. Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ. Sci. 2015, 8, 760–775. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, H.; Hua, J.; Tian, H. Recent advances in dye-sensitized photoelectrochemical cells for water splitting. EnergyChem 2019, 1, 100015. [Google Scholar] [CrossRef]
- Collomb, M.-N.; Morales, D.V.; Astudillo, C.N.; Dautreppe, B.; Fortage, J. Hybrid photoanodes for water oxidation combining a molecular photosensitizer with a metal oxide oxygen-evolving catalyst. Sustain. Energy Fuels 2020, 4, 31–49. [Google Scholar] [CrossRef]
- Cecconi, B.; Manfredi, N.; Montini, T.; Fornasiero, P.; Abbotto, A. Dye-sensitized solar hydrogen production: The emerging role of metal-free organic sensitizers. Eur. J. Org. Chem. 2016, 2016, 5194–5215. [Google Scholar] [CrossRef]
- Huang, J.-F.; Lei, Y.; Luo, T.; Liu, J.-M. Photocatalytic H2 production from water by metal-free dye-sensitized TiO2 semiconductors: The role and development process of organic sensitizers. ChemSusChem 2020, 13, 5863–5895. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Huang, J.-F.; Liu, J.-M.; Tsiakaras, P. Recent advances in metal-free photosensitizers for dye-sensitized photoelectrochemical cells. Coord. Chem. Rev. 2025, 522, 216143. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, W.H.; Zhang, Q.; Wu, W.J.; Xu, M.; Ning, Z.J.; Xie, Y.S.; Tian, H. Conveniently synthesized isophoronedyes for high efficiency dye-sensitized solar cells: Tuning photovoltaic performance by structural modification of donor group in donor–π–acceptor system. Chem. Commun. 2009, 13, 1766–1768. [Google Scholar] [CrossRef]
- Duerto, I.; Colom, E.; Andrés, J.M.; Franco, S.; Garín, J.; Orduna, J.; Villacampa, B.; Blesa, M.J. DSSCs based on aniline derivatives functionalized with a tert-butyldimethylsilyl group and the effect of the π–spacer. Dyes Pigment. 2018, 148, 61–71. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Hara, K.; Ohtani, B. Robust dye-sensitized overall water splitting system with two-stepphotoexcitation of coumarin dyes and metal oxide semiconductors. Chem. Commun. 2009, 24, 3577–3579. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, T.G.U.; Ghobadi, A.; Buyuktemiz, M.; Yildiz, E.A.; Yildiz, D.B.; Yaglioglu, H.G.; Dede, Y.; Ozbay, E.; Karadas, F. A robust, precious-metal-free dye-sensitized photoanode for water oxidation: A nanosecond-long excited-state lifetime through a Prussian blue analogue. Angew. Chem. Int. Ed. 2020, 59, 4082–4090. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, J.; Ko, K.C.; Park, J.H.; Ko, J.H.; Park, N.; Kim, E.; Ryu, D.H.; Ahn, T.K.; Lee, J.Y.; et al. Phenothiazine-based organic dyes with two anchoring groups on TiO2 for highly efficient visible light-induced water splitting. Chem. Commun. 2012, 48, 11431–11433. [Google Scholar] [CrossRef]
- Li, X.; Cui, S.; Wang, D.; Zhou, Y.; Zhou, H.; Hu, Y.; Liu, J.; Long, Y.; Wu, W.; Hua, J.; et al. New organic donor–acceptor–π–acceptor sensitizers for efficient dye-sensitized solar cells and photocatalytic hydrogen evolution under visible-light irradiation. ChemSusChem 2014, 7, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Wee, K.-R.; Sherman, B.D.; Brennaman, M.K.; Sheridan, M.V.; Nayak, A.; Alibabaei, L.; Meyer, T.J. An aqueous, organic dye derivatized SnO2/TiO2 core/shell photoanode. J. Mater. Chem. A 2016, 4, 2969–2975. [Google Scholar] [CrossRef]
- Alibabaei, L.; Dillon, R.J.; Reilly, C.E.; Brennaman, M.K.; Wee, K.-R.; Marquard, S.L.; Papanikolas, J.M.; Meyer, T.J. Chromophore-catalyst assembly for water oxidation prepared by atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 39018–39026. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Zhang, S.; Zhang, J.; Yu, Z.; Bai, G.; Du, C. TiO2 nanotube arrays decorated with plasmonic Cu, CuO nanoparticles, and eosin Y dye as efficient photoanode for water splitting. Mater. Chem. Phys. 2019, 231, 27–32. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Huang, D.; Yuan, H.; Wang, M.; Shen, Y. TiO2 nanotubes modified with electrochemically reduced graphene oxide for photoelectrochemical water splitting. Carbon 2014, 80, 591–598. [Google Scholar] [CrossRef]
- Morais, A.; Longo, C.; Araujo, J.R.; Barroso, M.; Durrant, J.R.; Nogueira, A.F. Nanocrystalline anatase TiO2/reduced Graphene oxide composite films as photoanodes for photoelectrochemical water splitting studies: The role of reduced graphene oxide. Phys. Chem. Chem. Phys. 2016, 18, 2608–2616. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, C.; Li, Y.; Han, W.; Fu, W.; He, Y.; Xie, E. Enhanced charge separation and transfer through Fe2O3/ITO nanowire arrays wrapped with reduced graphene oxide for water-splitting. Nano Energy 2016, 30, 892–899. [Google Scholar] [CrossRef]
- Hernández-Ferrer, J.; Ansón-Casaos, A.; Víctor-Román, S.; Sanahuja-Parejo, O.; Martínez, M.T.; Villacampa, B.; Benito, A.M.; Maser, W.K. Photoactivity improvement of TiO2 electrodes by thin hole transport layers of reduced graphene oxide. Electrochim. Acta 2019, 298, 279–287. [Google Scholar] [CrossRef]
- Le, C.V.; Nguyen, M.T.T.; Le, N.T.T.; Le, H.K.; Bui, T.M.; Ho, D.H.; Le, V.H.; Ho, T.T.N.; Pham, T.L.C.; Huynh, L.T.N.; et al. Rapidly forming the chemical bond titania–carbon in hybrid composite TiO2/reduced graphene oxide to enhance the efficiency of dye-sensitized solar cells. Arab. J. Sci. Eng. 2022, 47, 387–395. [Google Scholar] [CrossRef]
- Sang, L.; Lei, L.; Lin, J.; Ge, H. Co-sensitization of TiO2 electrode with Eosin Y dye and carbon dots for photoelectrochemical water splitting: The enhanced dye adsorption and the charge transfer route. Int. J. Hydrogen Energy 2017, 42, 29686–29693. [Google Scholar] [CrossRef]
- Kim, D.J.; Choi, Y.S.; Choi, H.-H.; Kwon, S.-J.; Lee, T.-W.; Choi, H.; Kang, I.; Park, J.H.; Hong, B.H. Degradation protection of color dyes encapsulated by graphene barrier films. Chem. Mater. 2019, 31, 7173–7177. [Google Scholar] [CrossRef]
- Esmaili, H.; Kowsari, E.; Ramakrishna, S.; Motamedisade, A.; Andersson, G.G. Sensitization of TiO2 nanoarrays by a novel palladium decorated naphthalene diimide functionalized graphene nanoribbons for enhanced photoelectrochemical water splitting. Mater. Today Chem. 2022, 24, 100900. [Google Scholar] [CrossRef]
- Li, D.; He, X.; Zhao, L.; Li, H.; Zhang, X.; Chen, J.; Jin, Q.; Xu, J. Ultrafast charge transfer dynamics of Rhodamine B with graphene oxide. J. Chem. Phys. 2022, 157, 214701. [Google Scholar] [CrossRef]
- Colom, E.; Hernández-Ferrer, J.; Galán-González, A.; Ansón-Casaos, A.; Navarro-Rodríguez, M.; Palacios-Lidón, E.; Colchero, J.; Padilla, J.; Urbina, A.; Arenal, R.; et al. Graphene oxide: Key to efficient charge extraction and suppression of polaronic transport in hybrids with poly (3-hexylthiophene) nanoparticles. Chem. Mater. 2023, 35, 3522–3531. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Kula, S.; Szlapa-Kula, A.; Fabiańczyk, A.; Gnida, P.; Libera, M.; Bujak, K.; Siwy, M.; Schab-Balcerzak, E. Effect of thienyl units in cyanoacrylic acid derivatives toward dye-sensitized solar cells. J. Photochem. Photobiol. B 2019, 197, 111555. [Google Scholar] [CrossRef] [PubMed]
- Ansón-Casaos, A.; Martínez-Barón, C.; Angoy-Benabarre, S.; Hernández-Ferrer, J.; Benito, A.M.; Maser, W.K.; Blesa, M.J. Stability of a pyrimidine-based dye-sensitized TiO2 photoanode in sacrificial electrolytes. J. Electroanal. Chem. 2023, 929, 117114. [Google Scholar] [CrossRef]
- Martínez-Barón, C.; Calvo, V.; Hernández-Ferrer, J.; Villacampa, B.; Ansón-Casaos, A.; González-Domínguez, J.M.; Maser, W.K.; Benito, A.M. Towards sustainable TiO2 photoelectrodes based on cellulose nanocrystals as a processing adjuvant. RSC Sustain. 2024, 2, 2015–2025. [Google Scholar] [CrossRef]
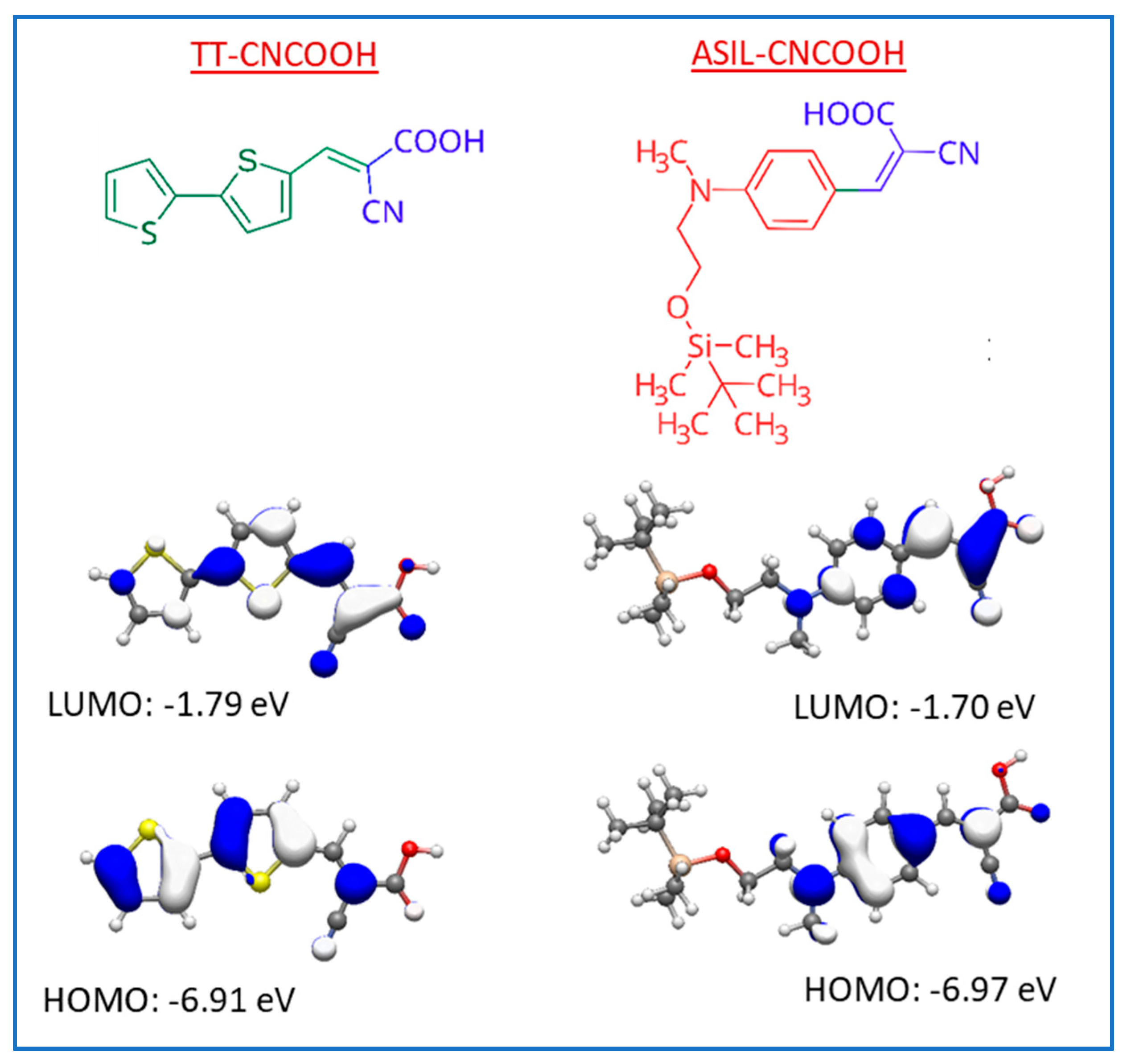
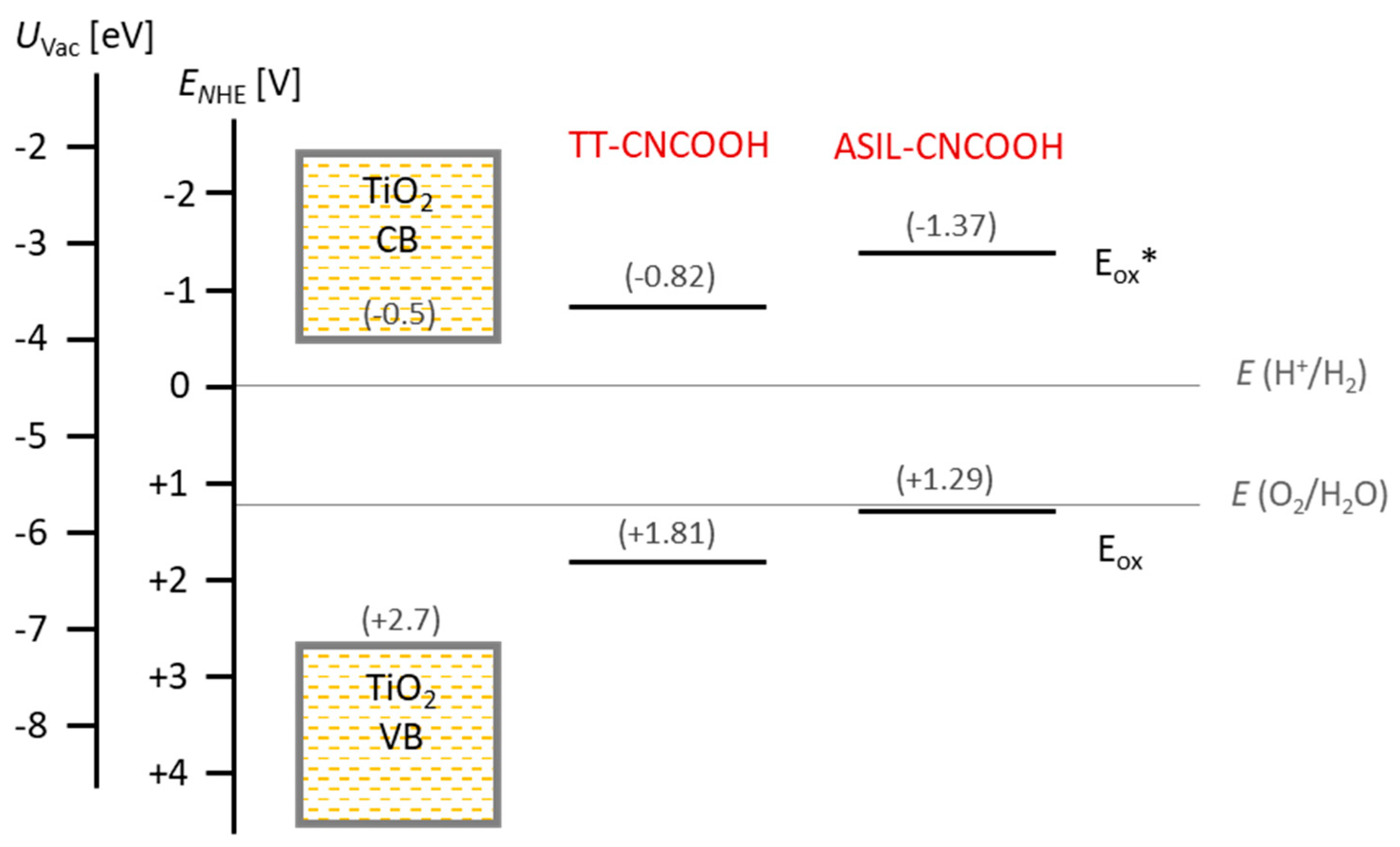
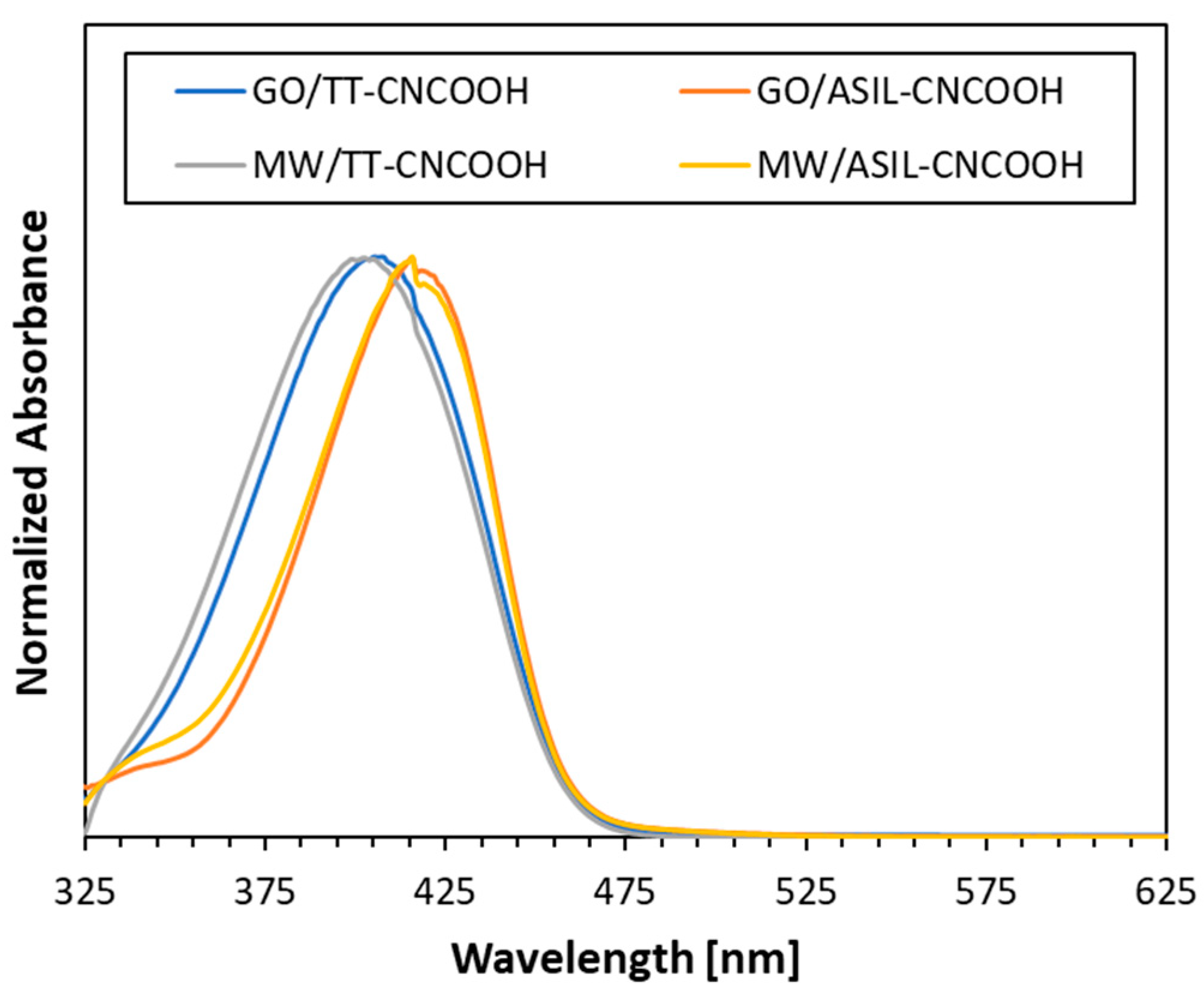
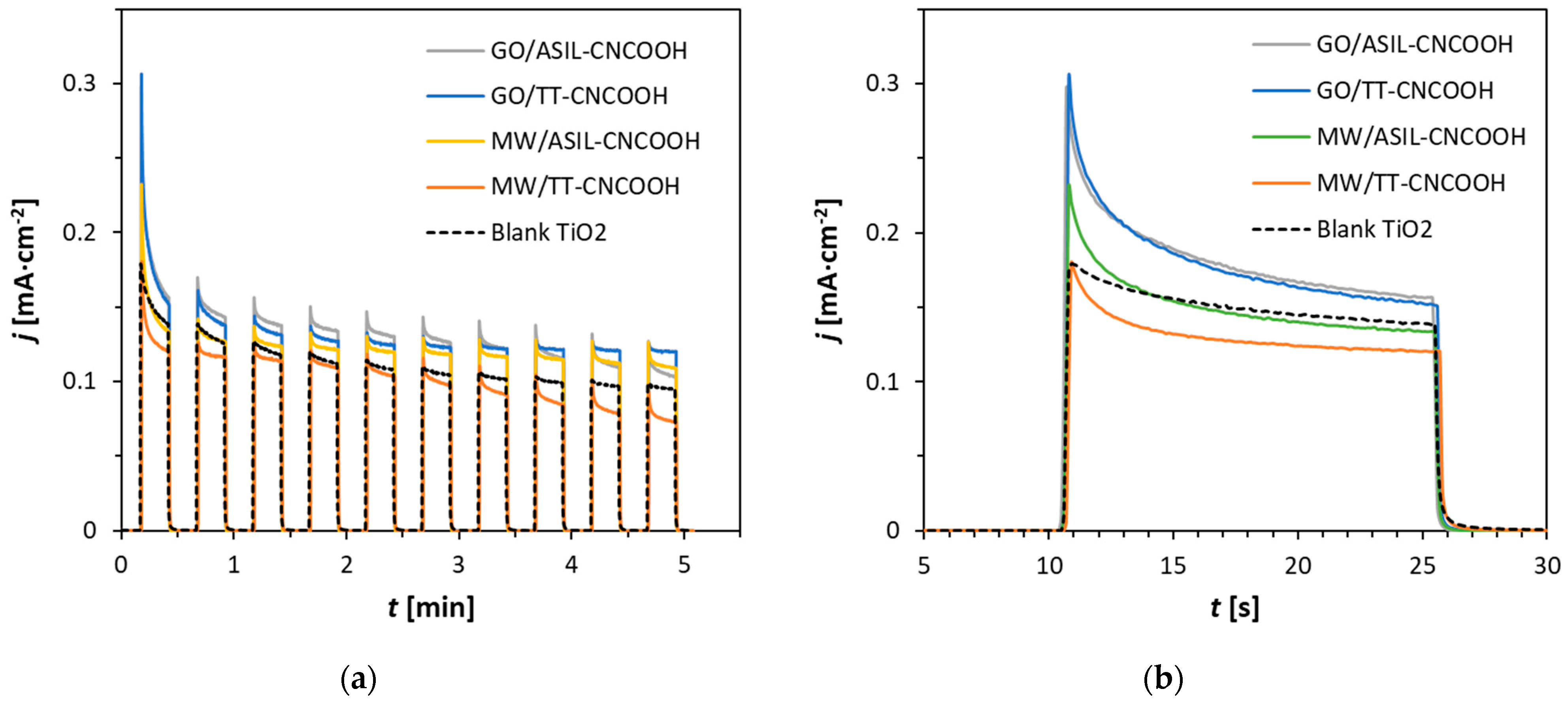
| Dye | λabs 2 [nm] | fosc 3 | Eox 4 [V] | E0-0 [eV] | Eox* 5 [V] |
|---|---|---|---|---|---|
| ASIL-CNCOOH | 387 | 1.26 | 1.41 | 3.02 | −1.61 |
| TT-CNCOOH | 416 | 1.08 | 1.90 | 2.71 | −0.81 |
| Dye | λabs [nm] | Ɛ [×104 M−1 cm−1] | λcut [nm] | Eopt 1 [eV] |
|---|---|---|---|---|
| ASIL-CNCOOH | 429 | 2.92 ± 0.11 | 465 | 2.66 |
| TT-CNCOOH | 421 | 2.41 ± 0.29 | 472 | 2.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansón-Casaos, A.; Benito, A.M.; Maser, W.K.; Orduna, J.; Villacampa, B.; Blesa, M.-J. Hybrids of Deep HOMO Organic Cyanoacrylic Acid Dyes and Graphene Nanomaterials for Water Splitting Photoanodes. Materials 2025, 18, 463. https://doi.org/10.3390/ma18020463
Ansón-Casaos A, Benito AM, Maser WK, Orduna J, Villacampa B, Blesa M-J. Hybrids of Deep HOMO Organic Cyanoacrylic Acid Dyes and Graphene Nanomaterials for Water Splitting Photoanodes. Materials. 2025; 18(2):463. https://doi.org/10.3390/ma18020463
Chicago/Turabian StyleAnsón-Casaos, Alejandro, Ana M. Benito, Wolfgang K. Maser, Jesús Orduna, Belén Villacampa, and María-Jesús Blesa. 2025. "Hybrids of Deep HOMO Organic Cyanoacrylic Acid Dyes and Graphene Nanomaterials for Water Splitting Photoanodes" Materials 18, no. 2: 463. https://doi.org/10.3390/ma18020463
APA StyleAnsón-Casaos, A., Benito, A. M., Maser, W. K., Orduna, J., Villacampa, B., & Blesa, M.-J. (2025). Hybrids of Deep HOMO Organic Cyanoacrylic Acid Dyes and Graphene Nanomaterials for Water Splitting Photoanodes. Materials, 18(2), 463. https://doi.org/10.3390/ma18020463








