Structural, Electronic, and Magnetic Properties of Neutral Borometallic Molecular Wheel Clusters
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
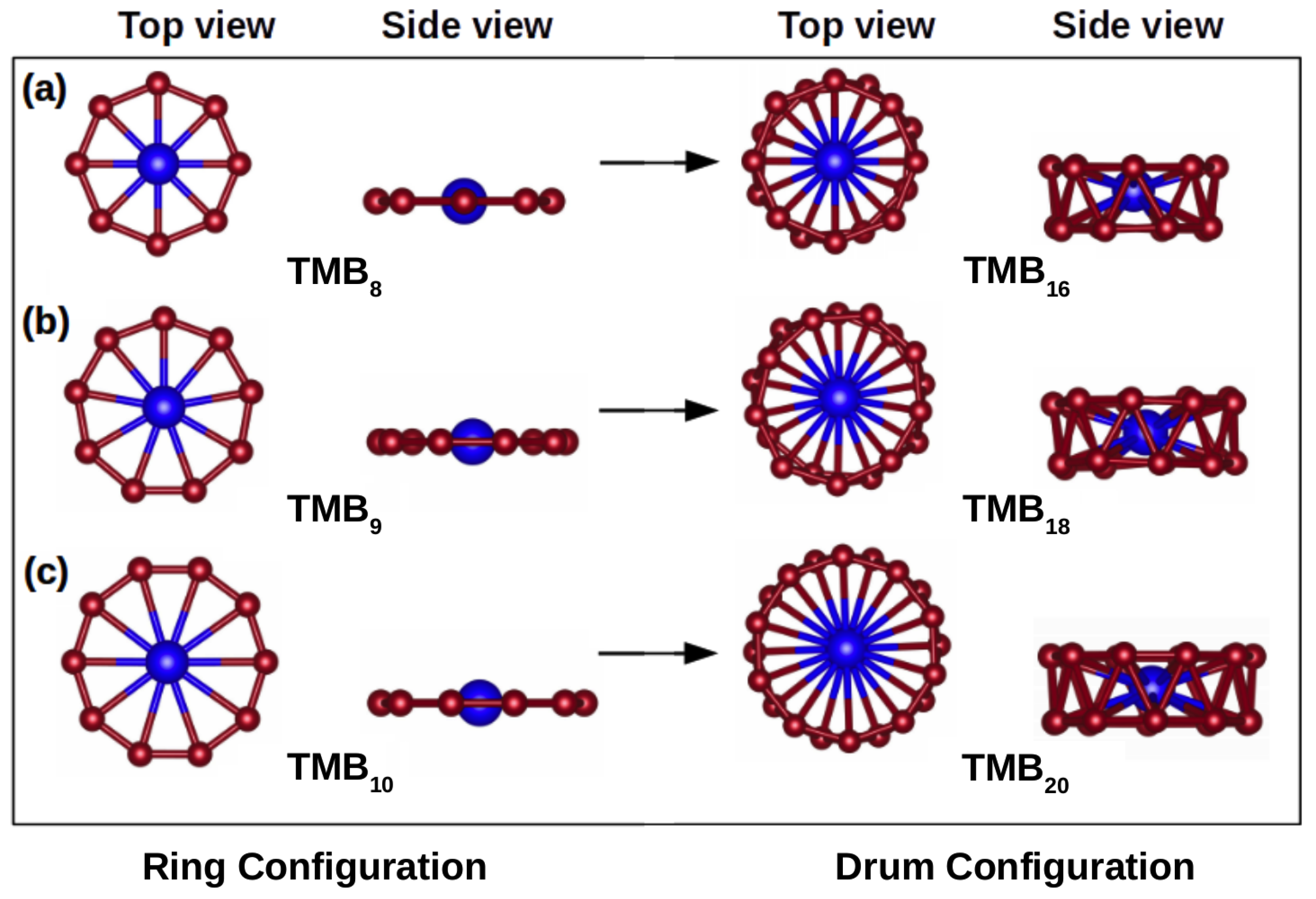
3.1. Structural Properties
3.2. Electronic Properties
3.3. Vibrational Properties
3.4. Dynamic Properties
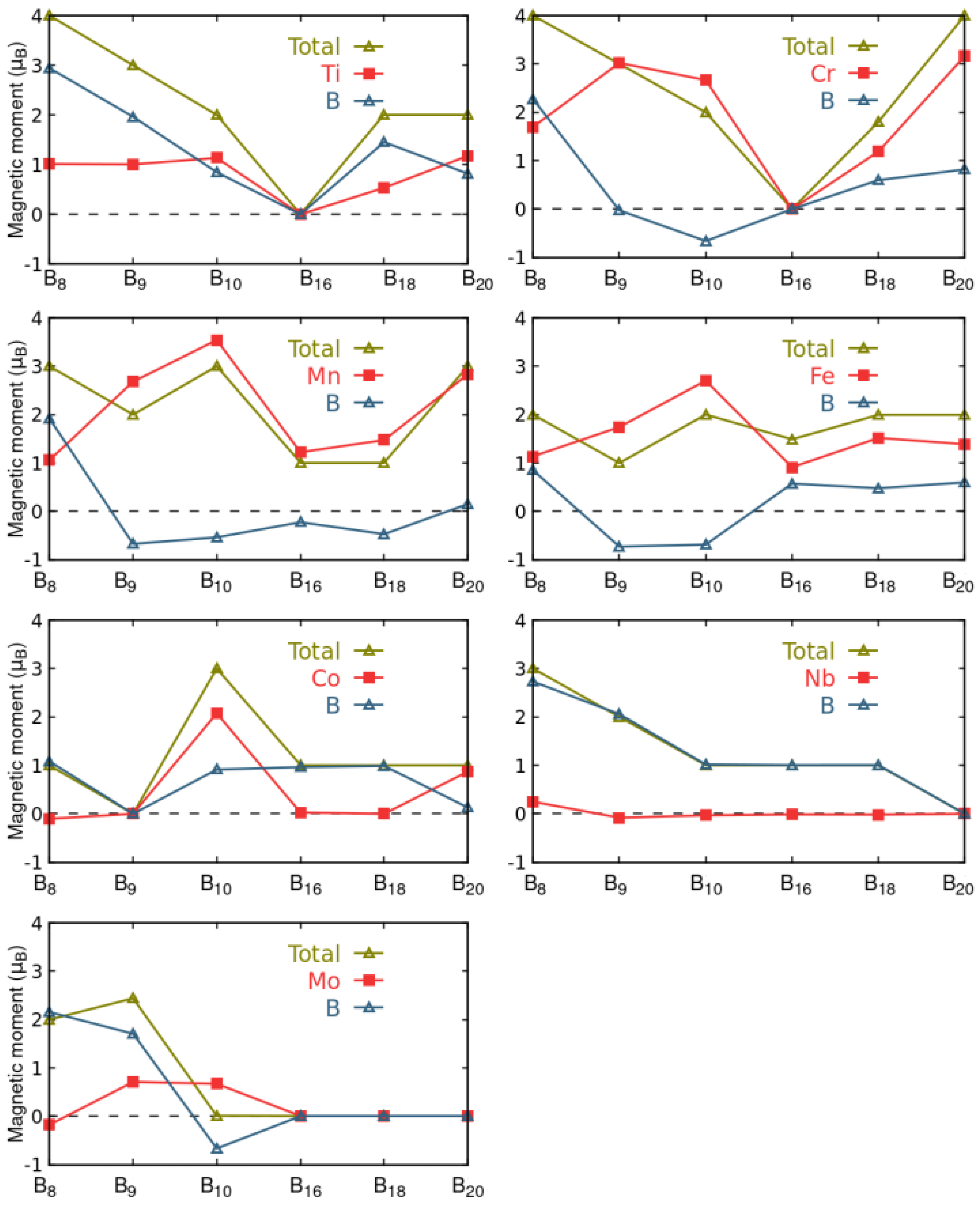
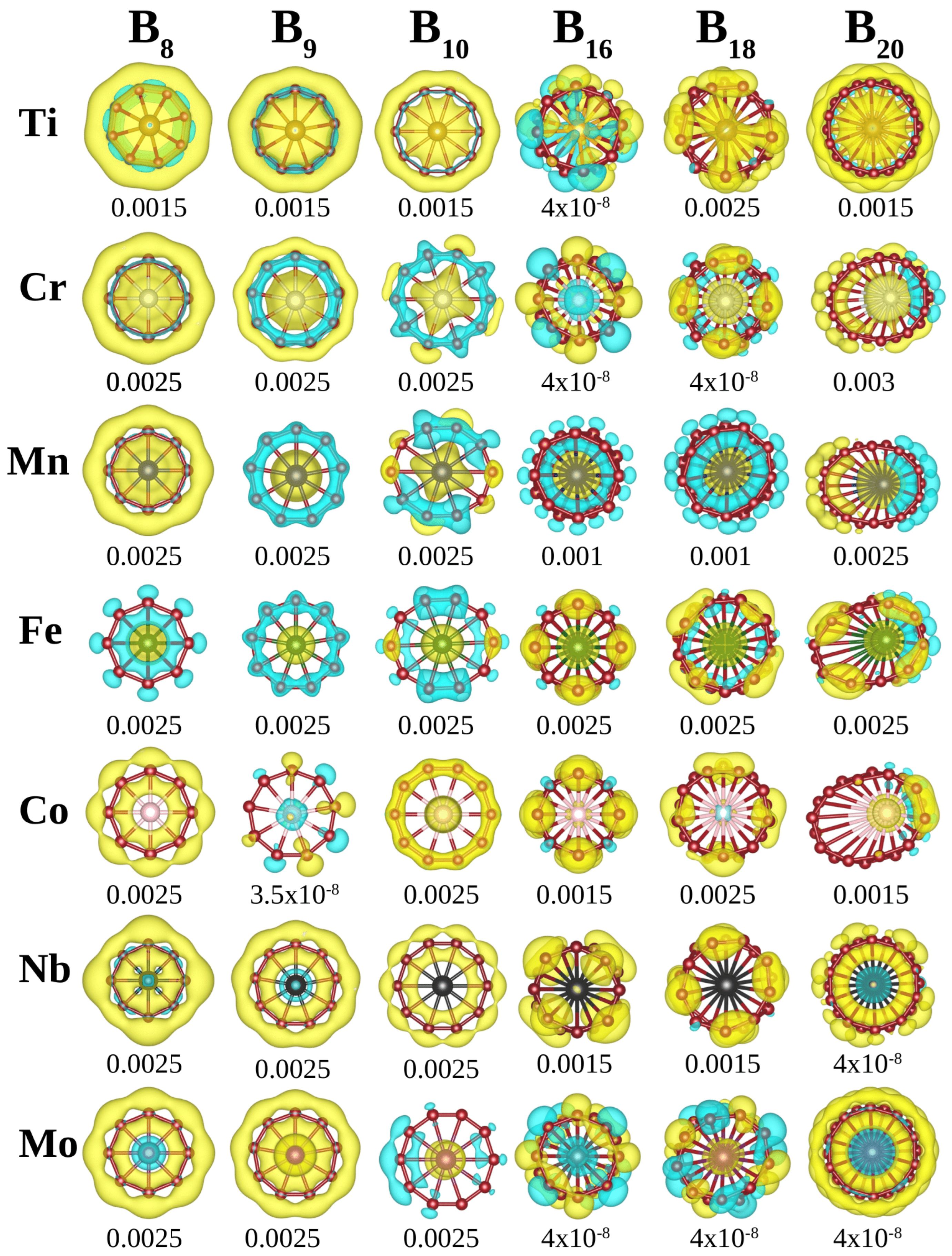
3.5. Magnetic Properties
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stone, F.G.A. Chemical reactivity of the boron hydrides and related compounds. In Advances in Inorganic Chemistry and Radiochemistry; Elsevier: Amsterdam, The Netherlands, 1960; Volume 2, pp. 279–313. [Google Scholar]
- Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, T.; Gonzalez Szwacki, N.; Marchwiany, M. Structure of porous two-dimensional boron crystals. Phys. Rev. B 2021, 104, 195423. [Google Scholar] [CrossRef]
- Tarkowski, T.; Gonzalez Szwacki, N. Boron nanotube structure explored by evolutionary computations. Crystals 2022, 13, 19. [Google Scholar] [CrossRef]
- Tarkowski, T.; Gonzalez Szwacki, N. The structure of thin boron nanowires predicted using evolutionary computations. Solid State Sci. 2023, 142, 107241. [Google Scholar] [CrossRef]
- Li, W.; Wu, K.; Chen, L. Epitaxial growth of borophene on substrates. Prog. Surf. Sci. 2023, 98, 100704. [Google Scholar] [CrossRef]
- Van Duong, L.; Mai, D.T.T.; Pham-Ho, M.P.; Nguyen, M.T. A theoretical approach to the role of different types of electrons in planar elongated boron clusters. Phys. Chem. Chem. Phys. 2019, 21, 13030–13039. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Muya, J.T.; Buendia, F.; Ceulemans, A.; Nguyen, M.T. Formation of the quasiplanar B 50 boron cluster: Topological path from B 10 and disk aromaticity. Phys. Chem. Chem. Phys. 2019, 21, 7039–7044. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jalife, S.; Vásquez-Espinal, A.; Ravell, E.; Pan, S.; Cabellos, J.L.; Liang, W.y.; Cui, Z.h.; Merino, G. Li2B12 and Li3B12: Prediction of the Smallest Tubular and Cage-like Boron Structures. Angew. Chem. Int. Ed. 2018, 57, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Van Duong, L.; Pham, H.T.; Tam, N.M.; Nguyen, M.T. A particle on a hollow cylinder: The triple ring tubular cluster B 27+. Phys. Chem. Chem. Phys. 2014, 16, 19470–19478. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, S.; Bai, H.; Tian, W.; Gao, T.; Li, H.; Miao, C.; Mu, Y.; Lu, H.; Zhai, H.; et al. Cage-Like B41+ and B422+: New Chiral Members of the Borospherene Family. Angew. Chem. Int. Ed. 2015, 54, 8160–8164. [Google Scholar] [CrossRef]
- Tai, T.B.; Tam, N.M.; Nguyen, M.T. Structure of boron clusters revisited, Bn with n = 14–20. Chem. Phys. Lett. 2012, 530, 71–76. [Google Scholar] [CrossRef]
- Li, W.L.; Chen, X.; Jian, T.; Chen, T.T.; Li, J.; Wang, L.S. From planar boron clusters to borophenes and metalloborophenes. Nat. Rev. Chem. 2017, 1, 0071. [Google Scholar] [CrossRef]
- Zhai, H.J.; Alexandrova, A.N.; Birch, K.A.; Boldyrev, A.I.; Wang, L.S. Hepta-and octacoordinate boron in molecular wheels of eight-and nine-atom boron clusters: Observation and confirmation. Angew. Chem. Int. Ed. 2003, 42, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Aromatic metal-centered monocyclic boron rings: Co©B8- and Ru©B9-. Angew. Chem. Int. Ed. 2011, 50, 9334–9337. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Geometric and electronic factors in the rational design of transition-metal-centered boron molecular wheels. J. Chem. Phys. 2013, 138, 134315. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Transition-metal-centered monocyclic boron wheel clusters (M©B n): A new class of aromatic borometallic compounds. Acc. Chem. Res. 2013, 46, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Guo, J.; Li, S. M@ B9 and M@ B10 molecular wheels containing planar nona-and deca-coordinate heavy group 11, 12, and 13 metals (M = Ag, Au, Cd, Hg, In, Tl). Sci. China Ser. B Chem. 2009, 52, 900–904. [Google Scholar] [CrossRef]
- Pu, Z.; Ito, K.; Schleyer, P.v.R.; Li, Q.S. Planar hepta-, octa-, nona-, and decacoordinate first row d-block metals enclosed by boron rings. Inorg. Chem. 2009, 48, 10679–10686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Yuan, Y.; Li, Q.; Jiang, H.; Yang, J.; Lin, W.; Huang, H. Structure and electronic properties of neutral and anionic boron clusters doped with two tantalum atoms. Mol. Phys. 2022, 120, e2029964. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, L.; Yang, J. Double aromaticity in transition metal centered double-ring boron clusters M@B2n (M = Ti, Cr, Fe, Ni, Zn; n = 6, 7, 8). J. Chem. Phys. 2014, 141, 124301. [Google Scholar] [CrossRef]
- Yan, M.; Li, H.; Zhao, X.; Lu, X.; Mu, Y.; Lu, H.; Li, S. Fluxional Bonds in Planar , Tubular Ta@, and Cage-Like . J. Comput. Chem. 2018, 40, 966–970. [Google Scholar] [CrossRef]
- Ren, M.; Jin, S.; Wei, D.; Jin, Y.; Tian, Y.; Lu, C.; Gutsev, G.L. NbB12−: A new member of half-sandwich type doped boron clusters with high stability. Phys. Chem. Chem. Phys. 2019, 21, 21746–21752. [Google Scholar] [CrossRef]
- Chen, B.; He, K.; Dai, W.; Gutsev, G.L.; Lu, C. Geometric and electronic diversity of metal doped boron clusters. J. Phys. Condens. Matter 2023, 35, 183002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.b.; Tiznado, W.; Cui, L.J.; Barroso, J.; Leyva-Parra, L.; Miao, L.h.; Zhang, H.y.; Pan, S.; Merino, G.; Cui, Z.h. Exploring the Use of “Honorary Transition Metals” To Push the Boundaries of Planar Hypercoordinate Alkaline-Earth Metals. J. Am. Chem. Soc. 2024, 146, 16689–16697. [Google Scholar] [CrossRef]
- Choi, D.J.; Lorente, N.; Wiebe, J.; Von Bergmann, K.; Otte, A.F.; Heinrich, A.J. Colloquium: Atomic spin chains on surfaces. Rev. Mod. Phys. 2019, 91, 041001. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, Z.Y.; Yamashita, M.; Bu, X.H. Recent progress on cyano-bridged transition-metal-based single-molecule magnets and single-chain magnets. Coord. Chem. Rev. 2021, 428, 213617. [Google Scholar] [CrossRef]
- Ferstl, P.; Hammer, L.; Sobel, C.; Gubo, M.; Heinz, K.; Schneider, M.A.; Mittendorfer, F.; Redinger, J. Self-organized growth, structure, and magnetism of monatomic transition-metal oxide chains. Phys. Rev. Lett. 2016, 117, 046101. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Li, W.L.; Popov, I.A.; Lopez, G.V.; Chen, X.; Boldyrev, A.I.; Li, J.; Wang, L.S. Manganese-centered tubular boron cluster–MnB16-: A new class of transition-metal molecules. J. Chem. Phys. 2016, 144, 154310. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Jian, T.; Lopez, G.V.; Boldyrev, A.I.; Wang, L.S. Cobalt-centred boron molecular drums with the highest coordination number in the CoB16- cluster. Nat. Commun. 2015, 6, 8654. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Li, W.L.; Chen, X.; Chen, T.T.; Lopez, G.V.; Li, J.; Wang, L.S. Competition between drum and quasiplanar structures in RhB 18-: Motifs for metallo-boronanotubes and metallo-borophenes. Chem. Sci. 2016, 7, 7020–7027. [Google Scholar] [CrossRef]
- Li, W.L.; Jian, T.; Chen, X.; Li, H.R.; Chen, T.T.; Luo, X.M.; Li, S.D.; Li, J.; Wang, L.S. Observation of a metal-centered B 2-Ta@ B 18- tubular molecular rotor and a perfect Ta@ B 20- boron drum with the record coordination number of twenty. Chem. Commun. 2017, 53, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- MinháTam, N.; TanáPham, H.; VanáDuong, L.; PhuongáPham-Ho, M.; ThoáNguyen, M. Fullerene-like boron clusters stabilized by an endohedrally doped iron atom: B n Fe with n= 14, 16, 18 and 20. Phys. Chem. Chem. Phys. 2015, 17, 3000–3003. [Google Scholar]
- Wang, J.; Zhang, N.X.; Wang, C.Z.; Wu, Q.Y.; Lan, J.H.; Chai, Z.F.; Nie, C.M.; Shi, W.Q. Theoretical probing of twenty-coordinate actinide-centered boron molecular drums. Phys. Chem. Chem. Phys. 2021, 23, 26967–26973. [Google Scholar] [CrossRef]
- Fan, Y.W.; Zhang, W.; Ge, N.N.; Li, Z. Design of Lanthanide Single-Chain Magnets Based on Tubular Segment Clusters. J. Phys. Chem. C 2022, 127, 621–626. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Comprehensive analysis of chemical bonding in boron clusters. J. Comput. Chem. 2006, 28, 251–268. [Google Scholar] [CrossRef]
- Curtarolo, S.; Setyawan, W.; Hart, G.L.; Jahnatek, M.; Chepulskii, R.V.; Taylor, R.H.; Wang, S.; Xue, J.; Yang, K.; Levy, O.; et al. AFLOW: An automatic framework for high-throughput materials discovery. Comput. Mater. Sci. 2012, 58, 218–226. [Google Scholar] [CrossRef]
- Li, W.L.; Ivanov, A.S.; Federič, J.; Romanescu, C.; Černušák, I.; Boldyrev, A.I.; Wang, L.S. On the way to the highest coordination number in the planar metal-centred aromatic Ta©B10- cluster: Evolution of the structures of TaB(n)- (n = 3–8). J. Chem. Phys. 2013, 139, 104312. [Google Scholar] [CrossRef]
- Bystrom, K.; Falletta, S.; Kozinsky, B. Training Machine-Learned Density Functionals on Band Gaps. J. Chem. Theory Comput. 2024, 20, 7516–7532. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Schleyer, P.v.R.; Schaefer, H.F. Predicting Molecules—More Realism, Please! Angew. Chem. Int. Ed. 2008, 47, 7164–7167. [Google Scholar] [CrossRef] [PubMed]

| PG | m | H-L Gap | ||||
|---|---|---|---|---|---|---|
| (Å) | (Å) | () | (eV) | (eV) | ||
| TiB8 | 1.578 | 2.226 | 4 | 0.541 | −9.667 | |
| TiB9 | 1.558 | 2.335 | 3 | 0.090 | −10.692 | |
| TiB10 | 1.536 | 2.485 | 2 | 0.617 | −11.106 | |
| TiB16 | 1.639 | 2.275 | 0 | 0.392 | −8.214 | |
| TiB18 | 1.608 | 2.460 | 2 | 0.246 | −9.245 | |
| TiB20 | 1.579 | 2.593 | 2 | 0.312 | −7.925 | |
| CrB8 | 1.587 | 2.073 | 4 | 0.004 | −11.476 | |
| CrB9 | 1.551 | 2.269 | 3 | 0.367 | −12.495 | |
| CrB10 | 1.524 | 2.466 | 2 | 0.357 | −12.152 | |
| CrB16 | 1.614 | 2.209 | 0 | 0.822 | −11.234 | |
| CrB18 | 1.588 | 2.432 | 1.8 | 0.122 | −10.471 | |
| CrB20 | 1.608 | 2.645 | 4 | 0.425 | −8.272 | |
| MnB8 | 1.572 | 2.054 | 3 | 0.486 | −7.288 | |
| MnB9 | 1.542 | 2.255 | 2 | 0.880 | −7.733 | |
| MnB10 | 1.530 | 2.473 | 3 | 0.264 | −7.128 | |
| MnB16 | 1.602 | 2.235 | 1 | 0.804 | −6.472 | |
| MnB18 | 1.584 | 2.431 | 1 | 0.558 | −5.397 | |
| MnB20 | 1.602 | 2.723 | 3 | 0.439 | −3.518 | |
| FeB8 | 1.564 | 2.065 | 2 | 0.016 | −10.866 | |
| FeB9 | 1.533 | 2.241 | 1 | 0.785 | −12.050 | |
| FeB10 | 1.523 | 2.461 | 2 | 0.274 | −11.099 | |
| FeB16 | 1.594 | 2.231 | 1.49 | 0.063 | −9.955 | |
| FeB18 | 1.581 | 2.445 | 2 | 0.324 | −8.645 | |
| FeB20 | 1.617 | 2.760 | 2 | 0.268 | −7.918 | |
| CoB8 | 1.564 | 2.044 | 1 | 0.001 | −15.709 | |
| CoB9 | 1.539 | 2.234 | 0 | 1.055 | −15.889 | |
| CoB10 | 1.522 | 2.462 | 3 | 0.404 | −14.585 | |
| CoB16 | 1.591 | 2.232 | 1 | 0.297 | −13.398 | |
| CoB18 | 1.579 | 2.443 | 1 | 0.311 | −11.828 | |
| CoB20 | 1.619 | 2.749 | 1 | 0.258 | −11.881 | |
| NbB8 | 1.653 | 2.157 | 3 | 0.504 | −10.702 | |
| NbB9 | 1.573 | 2.300 | 2 | 0.418 | −13.055 | |
| NbB10 | 1.533 | 2.481 | 1 | 0.907 | −13.451 | |
| NbB16 | 1.661 | 2.306 | 1 | 0.322 | −10.318 | |
| NbB18 | 1.561 | 2.477 | 1 | 0.362 | −12.179 | |
| NbB20 | 1.573 | 2.674 | 0 | 0.000 | −10.937 | |
| MoB8 | 1.628 | 2.126 | 2 | 0.419 | −12.395 | |
| MoB9 | 1.560 | 2.281 | 2.44 | 0.080 | −14.021 | |
| MoB10 | 1.524 | 2.467 | 0 | 0.092 | −13.998 | |
| MoB16 | 1.658 | 2.249 | 0 | 0.616 | −11.811 | |
| MoB18 | 1.602 | 2.431 | 0 | 0.415 | −12.663 | |
| MoB20 | 1.568 | 2.760 | 0 | 1.088 | −10.871 |
| Ring | (cm−1) | Drum | (cm−1) |
|---|---|---|---|
| TiB8 | 206.22 | TiB16 | 48.41 |
| TiB9 | 123.91 | TiB18 | 65.62 |
| TiB10 | 85.00 | TiB20 | 78.17 |
| CrB8 | 87.93 | CrB16 | 160.53 |
| CrB9 | 196.47 | CrB18 | 59.27 |
| CrB10 | 44.03 | CrB20 | 89.9 |
| MnB8 | 140.21 | MnB16 | 122.13 |
| MnB9 | 135.68 | MnB18 | 130.70 |
| MnB10 | 69.60 | MnB20 | 27.00 |
| FeB8 | 133.40 | FeB16 | 75.14 |
| FeB9 | 128.48 | FeB18 | 79.27 |
| FeB10 | −22.1 | FeB20 | 64.30 |
| CoB8 | 132.22 | CoB16 | 55.24 |
| CoB9 | 102.07 | CoB18 | 70.30 |
| CoB10 | 78.0 | CoB20 | 90.34 |
| NbB8 | 45.69 | NbB16 | 153.38 |
| NbB9 | 93.35 | NbB18 | 38.60 |
| NbB10 | 87.85 | NbB20 | 102.61 |
| MoB8 | 29.26 | MoB16 | 86.96 |
| MoB9 | 95.29 | MoB18 | 41.17 |
| MoB10 | 68.08 | MoB20 | 106.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, S.; Gonzalez Szwacki, N. Structural, Electronic, and Magnetic Properties of Neutral Borometallic Molecular Wheel Clusters. Materials 2025, 18, 459. https://doi.org/10.3390/ma18020459
Perveen S, Gonzalez Szwacki N. Structural, Electronic, and Magnetic Properties of Neutral Borometallic Molecular Wheel Clusters. Materials. 2025; 18(2):459. https://doi.org/10.3390/ma18020459
Chicago/Turabian StylePerveen, Saira, and Nevill Gonzalez Szwacki. 2025. "Structural, Electronic, and Magnetic Properties of Neutral Borometallic Molecular Wheel Clusters" Materials 18, no. 2: 459. https://doi.org/10.3390/ma18020459
APA StylePerveen, S., & Gonzalez Szwacki, N. (2025). Structural, Electronic, and Magnetic Properties of Neutral Borometallic Molecular Wheel Clusters. Materials, 18(2), 459. https://doi.org/10.3390/ma18020459







