Metallic Ion Release Behaviors from Cobalt–Chromium Alloys Fabricated by Additive Manufacturing with Mechanical Grinding in an Acidic Saline Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Specimen Characterization
2.3. Static Immersion Test
2.4. Statistical Analysis
3. Results
3.1. Materials Characterization
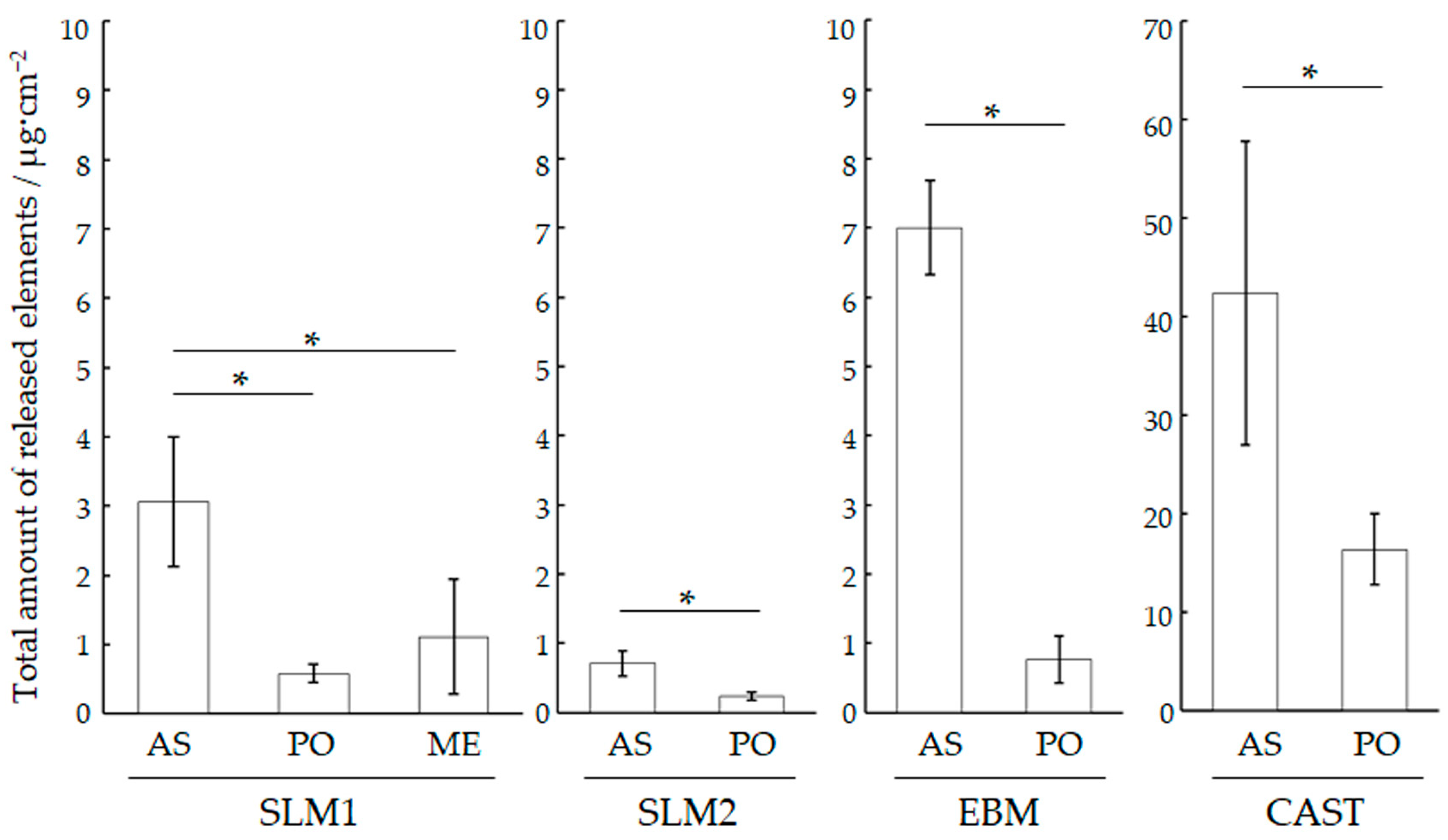
3.2. pH and Amount of Released Metallic Ions
4. Discussion
5. Conclusions
- The segregation of the molten pool on the as-built SLM and EBM specimens fabricated using AM was observed, and the polished and machined specimens had homogenous surfaces.
- The SLM and EBM specimens mainly had an γ-phase, but the polished and machined specimens showed an ε-phase.
- Regardless of if polishing and machining were performed, the SLM and EBM specimens exhibited a lower metallic ion release than the CAST specimens. The total amount of metallic ions released from the SLMs and EBM specimens was less than 7 μg/cm2, but machining and polishing suppressed this amount.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| AS | As-built |
| BSE | Backscattering electron |
| CAD | Computer-aided design |
| CAM | Computer-aided manufacturing |
| Co-Cr | Cobalt–chromium |
| EBM | Electron beam melting |
| EPMA | Electron probe microanalyzer |
| SEM | Scanning electron microscope |
| SLM | Selective laser melting |
| PBF | Powder bed fusion |
| ICP | Inductively coupled plasma optical emission spectrometer |
| XRD | X-ray diffractometer |
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004; pp. 137–153. [Google Scholar]
- Arici, S.; Regan, D. Alternatives to ceramic brackets: The tensile bond strengths of two aesthetic brackets compared ex vivo with stainless steel foil-mesh bracket bases. Br. J. Orthod. 1997, 24, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.A.; Choi, L.K.; Caruso, J.M.; Tomlinson, J.L.; Scott, G.E.; Jeiroudi, M.T. Deformation of metal brackets: A comparative study. Angle Orthod. 1994, 64, 283–290. [Google Scholar] [PubMed]
- Alansari, R.A.; Faydhi, D.A.; Ashour, B.S.; Alsaggaf, D.H.; Shuman, M.T.; Ghoneim, S.H.; Linjawi, A.I.; Marghalani, H.Y.; Dause, R.R. Adult perceptions of different orthodontic appliances. Patient Prefer. Adherence 2019, 13, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Makou, M.; Papadopoulos, T.; Eliades, G. Laboratory evaluation of modern plastic brackets. Eur. J. Orthod. 2012, 34, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef]
- Garhammer, P.; Schmalz, G.; Hiller, K.A.; Reitinger, T. Metal content of biopsies adjacent to dental cast alloys. Clin. Oral Investig. 2003, 7, 92–97. [Google Scholar] [CrossRef]
- Jensen, C.S.; Menné, T.; Lisby, S.; Kristiansen, J.; Veien, N.K. Experimental systemic contact dermatitis from nickel: A dose-response study. Dermatitis 2003, 49, 124–132. [Google Scholar] [CrossRef]
- Hafez, H.S.; Selim, E.M.; Kamel Eid, F.H.; Tawfik, W.A.; Al-Ashkar, E.A.; Mostafa, Y.A. Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: A longitudinal in-vivo study. Am. J. Orthod. Dentofacial Orthop. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal material, properties and design methods of porous biomedical scaffolds for additive manufacturing: A review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2021; Additive Manufacturing—General Principles—Fundamentals and Vocabulary. International Organization for Standardization: Geneva, Switzerland, 2021.
- Al Jabbari, Y.S.; Koutsoukis, T.; Barmpagadaki, X.; Zinelis, S. Metallurgical and interfacial characterization of PFM Co-Cr dental alloys fabricated via casting, milling or selective laser melting. Dent. Mater. 2014, 30, e79–e88. [Google Scholar] [CrossRef] [PubMed]
- Al Jabbari, Y.S.; Barmpagadaki, X.; Psarris, I.; Zinelis, S. Microstructural, mechanical, ionic release and tarnish resistance characterization of porcelain fused to metal Co-Cr alloys manufactured via casting and three different CAD/CAM techniques. J. Prosthodont. Res. 2019, 63, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Uriciuc, W.A.; Boșca, A.B.; Băbțan, A.M.; Vermeșan, H.; Cristea, C.; Tertiș, M.; Pășcuță, P.; Borodi, G.; Suciu, M.; Barbu-Tudoran, L.; et al. Study on the surface of cobalt-chromium dental alloys and their behavior in oral cavity as cast materials. Materials 2022, 15, 3052. [Google Scholar] [CrossRef]
- Kajima, Y.; Takaichi, A.; Nakamoto, T.; Kimura, T.; Yogo, Y.; Ashida, M.; Doi, H.; Nomura, N.; Takahashi, H.; Hanawa, T.; et al. Fatigue strength of Co-Cr-Mo alloy clasps prepared by selective laser melting. J. Mech. Behav. Biomed. Mater. 2016, 59, 446–458. [Google Scholar] [CrossRef]
- Qian, B.; Saeidi, K.; Kvetková, L.; Lofaj, F.; Xiao, C.; Shen, Z. Defects-tolerant Co-Cr-Mo dental alloys prepared by selective laser melting. Dent. Mater. 2015, 31, 1435–1444. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C. Preferential dissolution behavior in Ni-Cr dental cast alloy. Bull. Mater. Sci. 2010, 33, 463–468. [Google Scholar] [CrossRef]
- Craig, R.G.; Hanks, C.T. Reaction of fibroblasts to various dental casting alloys. J. Oral. Pathol. 1988, 17, 341–347. [Google Scholar] [CrossRef]
- Conti, M.C.; Karl, A.; Wismayer, P.S.; Buhagiar, J. Biocompatibility and characterization of a Kolsterised(®) medical grade cobalt-chromium-molybdenum alloy. Biomatter. 2014, 4, e27713. [Google Scholar] [CrossRef][Green Version]
- Ganbold, B.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Cho, J. Human Stem Cell Responses and Surface Characteristics of 3D Printing Co-Cr Dental Material. Materials 2019, 12, 3419. [Google Scholar] [CrossRef]
- Grosgogeat, B.; Vaicelyte, A.; Gauthier, R.; Janssen, C.; Le Borgne, M. Toxicological Risks of the Cobalt-Chromium Alloys in Dentistry: A Systematic Review. Materials 2022, 15, 5801. [Google Scholar] [CrossRef]
- Subari, R.; Bellaouchou, A.; Guenbour, A.; Merzouk, N. Influence of temperature and pH on corrosion behavior of Ni-Cr and Co-Cr dental alloys. J. Int. Dent. Med. Res. 2013, 6, 9–14. [Google Scholar]
- ISO 10271: 2020; Dentistry—Corrosion Test Method for Metallic Materials. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 22674: 2022; Dentistry—Metallic Materials for Fixed and Removable Restorations and Appliances. International Organization for Standardization: Geneva, Switzerland, 2022.
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Tuna, S.H.; Özçiçek Pekmez, N.; Kürkçüoğlu, I. Corrosion resistance assessment of Co-Cr alloy frameworks fabricated by CAD/CAM milling, laser sintering, and casting methods. J. Prosthet. Dent. 2015, 114, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, M.C.; Fratto, G.; Valeriani, F.; De Vittori, E.; Giampaoli, S.; Papetti, P.; Romano Spica, V.; Manzon, L. Cobalt-chromium alloys in dentistry: An evaluation of metal ion release. J. Prosthet. Dent. 2015, 114, 602–608. [Google Scholar] [CrossRef]
- Takashima, T.; Koizumi, Y.; Li, Y.; Yamanaka, K.; Saito, T.; Chiba, A. Effect of building position on phase distribution in Co-Cr-Mo alloy additive manufactured by electron-beam melting. Mater. Trans. 2016, 57, 2041–2047. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ceylan, A.; Martinez, E.; Martinez, J.L.; Hernandez, D.H.; Machado, B.I.; Ramirez, D.A.; Medina, F.; Collins, S.; et al. Characterization of titanium aluminide alloy components fabricated by additive manufacturing using electron beam melting. Acta Mater 2010, 58, 1887–1894. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Martinez, E.; Martinez, J.L.; Hernandez, D.H.; Machado, B.I.; Ramirez, D.A.; Wicker, R.B. Characterization of Ti–6Al–4V open cellular foams fabricated by additive manufacturing using electron beam melting. Mat. Sci. Eng. A 2010, 527, 1861–1868. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Murr, L.E.; Li, S.J.; Tian, Y.X.; Martinez, E.; Martinez, J.L.; Machado, B.I.; Gaytan, S.M.; Medina, F.; Wicker, R.B. Open-cellular copper structures fabricated by additive manufacturing using electron beam melting. Mat. Sci. Eng. A. 2011, 528, 5379–5386. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Qian, B.; Shen, Z.; Virtanen, S.; Wallinder, I.O. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, S.; Gan, Y.; Li, J.; Zhao, C.; Zhuo, D.; Lin, J. Investigation on the microstructure, mechanical property and corrosion behavior of the selective laser melted CoCrW alloy for dental application. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 517–525. [Google Scholar] [CrossRef]
- Schuttelaar, M.L.A.; Ofenloch, R.F.; Bruze, M.; Cazzaniga, S.; Elsner, P.; Gonçalo, M.; Naldi, L.; Svensson, Å.; Diepgen, T.L. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: The EDEN Fragrance Study. Contact Dermatitis 2018, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, A.; Suyalatu, N.; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; et al. Microstructures and mechanical properties of Co-29Cr-6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B.; Taylor, R.L.; Colligon, J.S.; Johnson, D. Effect of element concentration on nickel release from dental alloys using a novel ion beam method. Dent. Mater. 2010, 26, 249–256. [Google Scholar] [CrossRef]
- Metikos-Huković, M.; Pilić, Z.; Babić, R.; Omanović, D. Influence of alloying elements on the corrosion stability of CoCrMo implant alloy in Hank’s solution. Acta Biomater. 2006, 2, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Yamanaka, K.; Kuramoto, K.; Ohmura, K.; Ashino, T.; Chiba, A. Effect of carbon on the microstructure, mechanical properties and metal ion release of Ni-free Co-Cr-Mo alloys containing nitrogen. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 145–154. [Google Scholar] [CrossRef]
- Bettini, E.; Eriksson, T.; Boström, M.; Leygraf, C.; Pan, J. Influence of metal carbides on dissolution behavior of biomedical CoCrMo alloy: SEM, TEM and AFM studies. Electrochim. Acta 2011, 56, 9413–9419. [Google Scholar] [CrossRef]
- Kajima, Y.; Takaichi, A.; Kittikundecha, N.; Htat, H.L.; Cho, H.H.W.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N.; Yoneyama, T. Reduction in anisotropic response of corrosion properties of selective laser melted Co-Cr-Mo alloys by post-heat treatment. Dent. Mater. 2021, 37, e98–e108. [Google Scholar] [CrossRef]
- Tasaka, A.; Shimizu, T.; Kato, Y.; Okano, H.; Ida, Y.; Higuchi, S.; Yamashita, S. Accuracy of removable partial denture framework fabricated by casting with a 3D printed pattern and selective laser sintering. J. Prosthodont. Res. 2020, 64, 224–230. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Corrosion behavior and surface characterization of titanium in solution containing fluoride and albumin. Biomaterials 2005, 26, 829–837. [Google Scholar] [CrossRef]







| Specimen | Element (Mass%) | ||||
|---|---|---|---|---|---|
| Co | Cr | Mo | W | Other | |
| SLM1 | Bal. | 28.5 | 6.0 | - | 0.75 (Fe), ≤1.0 (Si, Ni, Mn), 0.75 (C) |
| SLM2 | 60.5 | 28.0 | - | 9.0 | 1.5 (Si), (Mn, N, Nb) |
| EBM | Bal. | 28.0 | 6.0 | - | 0.15(N), ≤1.0(Mn) |
| CAST | 59.5 | 29.2 | 6.6 | 2.6 | 2.1 (Si, Mn, N, C) |
| Specimen | Element (Mass%) | ||||||
|---|---|---|---|---|---|---|---|
| Co | Cr | Mo | W | Si | Other | ||
| SLM1 | AS | 63.4 | 29.7 | 5.4 | 0.7 | Fe, Mn | |
| PO | 63.4 | 30.0 | 5.7 | 0.7 | Fe | ||
| ME | 62.7 | 30.4 | 5.6 | 0.9 | Fe | ||
| SLM2 | AS | 61.2 | 28.5 | - | 8.8 | 1.5 | |
| PO | 60.8 | 29.5 | - | 8.4 | 1.3 | ||
| EBM | AS | 66.2 | 28.4 | 4.9 | 0.6 | Mn, Ni | |
| PO | 65.4 | 28.4 | 4.8 | 0.6 | |||
| CAST | AS | 61.2 | 29.3 | 5.6 | 2.8 | 0.8 | Al |
| PO | 54.5 | 33.1 | 7.4 | 4.0 | 0.9 | ||
| SLM1 | SLM2 | EBM | CAST | |
|---|---|---|---|---|
| AS | 2.26 ± 0.04 | 2.24 ± 0.00 | 2.21 ± 0.01 | 2.25 ± 0.01 |
| PO | 2.26 ± 0.04 | 2.25 ± 0.01 | 2.21 ± 0.01 | 2.22 ± 0.01 |
| ME | 2.26 ± 0.03 |
| Specimen | Element (μg/cm2) | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Co | Cr | Mo | W | Mn | Fe | Ni | (μg/cm2) | |
| SLM1 | 0.22 | 0.05 | - | - | 0.01 | 0.22 | 0.08 | 0.58 ± 0.13 a |
| SLM2 | 0.21 | 0.02 | - | - | - | - | - | 0.24 ± 0.06 a |
| EBM | 0.74 | 0.05 | 0.03 | - | 0.01 | - | - | 0.76 ± 0.34 a |
| CAST | 12.52 | 0.62 | 1.89 | 0.61 | 0.58 | - | - | 16.35 ± 3.60 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, N.; Sawada, T.; Kuwajima, Y.; Yamanaka, K.; Nomura, N.; Kasahara, M.; Chiba, A.; Satoh, K.; Takemoto, S. Metallic Ion Release Behaviors from Cobalt–Chromium Alloys Fabricated by Additive Manufacturing with Mechanical Grinding in an Acidic Saline Solution. Materials 2025, 18, 432. https://doi.org/10.3390/ma18020432
Sakurai N, Sawada T, Kuwajima Y, Yamanaka K, Nomura N, Kasahara M, Chiba A, Satoh K, Takemoto S. Metallic Ion Release Behaviors from Cobalt–Chromium Alloys Fabricated by Additive Manufacturing with Mechanical Grinding in an Acidic Saline Solution. Materials. 2025; 18(2):432. https://doi.org/10.3390/ma18020432
Chicago/Turabian StyleSakurai, Naoto, Tomofumi Sawada, Yukinori Kuwajima, Kenta Yamanaka, Naoyuki Nomura, Masaaki Kasahara, Akihiko Chiba, Kazuro Satoh, and Shinji Takemoto. 2025. "Metallic Ion Release Behaviors from Cobalt–Chromium Alloys Fabricated by Additive Manufacturing with Mechanical Grinding in an Acidic Saline Solution" Materials 18, no. 2: 432. https://doi.org/10.3390/ma18020432
APA StyleSakurai, N., Sawada, T., Kuwajima, Y., Yamanaka, K., Nomura, N., Kasahara, M., Chiba, A., Satoh, K., & Takemoto, S. (2025). Metallic Ion Release Behaviors from Cobalt–Chromium Alloys Fabricated by Additive Manufacturing with Mechanical Grinding in an Acidic Saline Solution. Materials, 18(2), 432. https://doi.org/10.3390/ma18020432








