Few-Layered Black Phosphorene as Hole Transport Layer for Novel All-Inorganic Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
3. Results
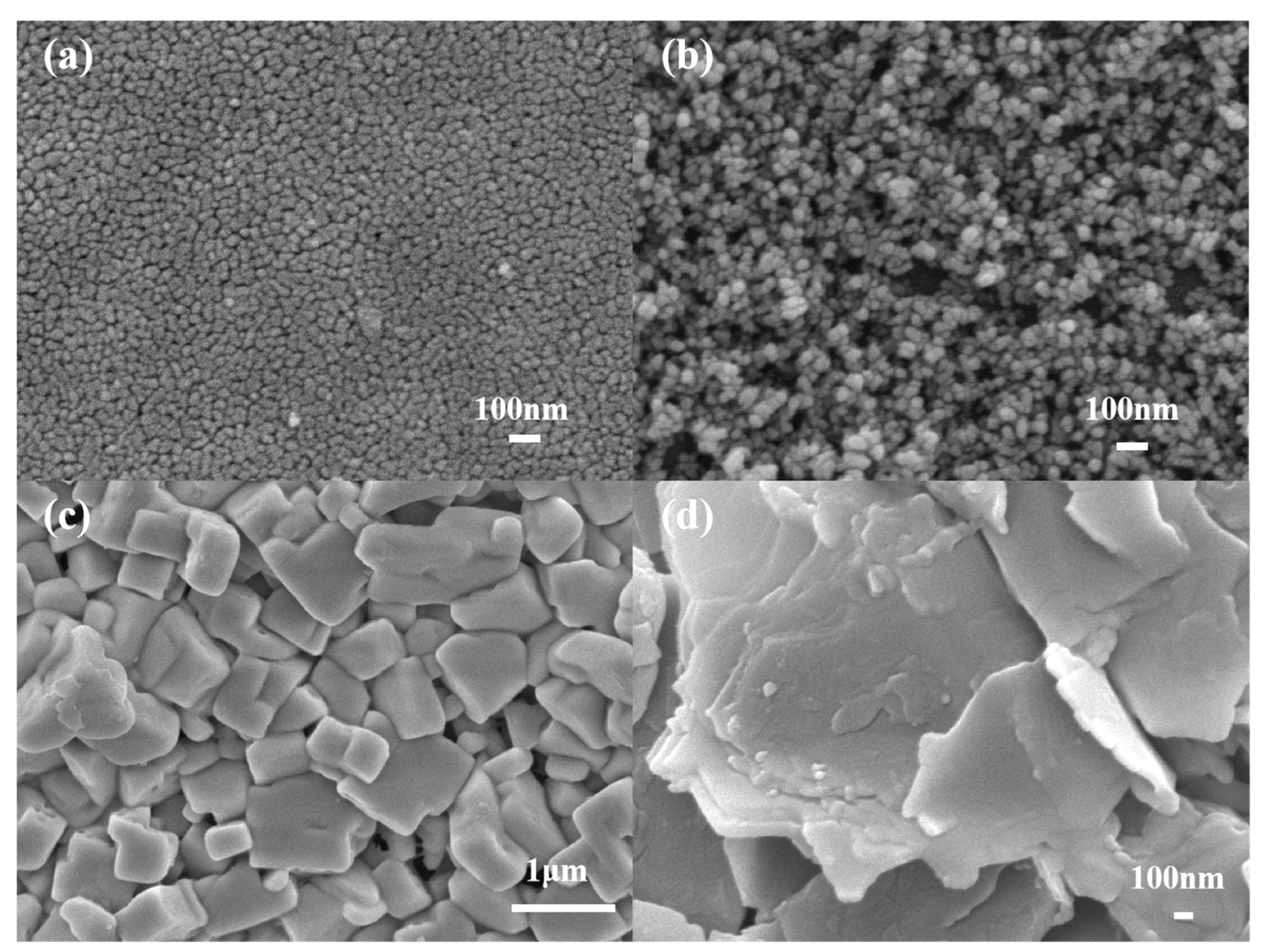
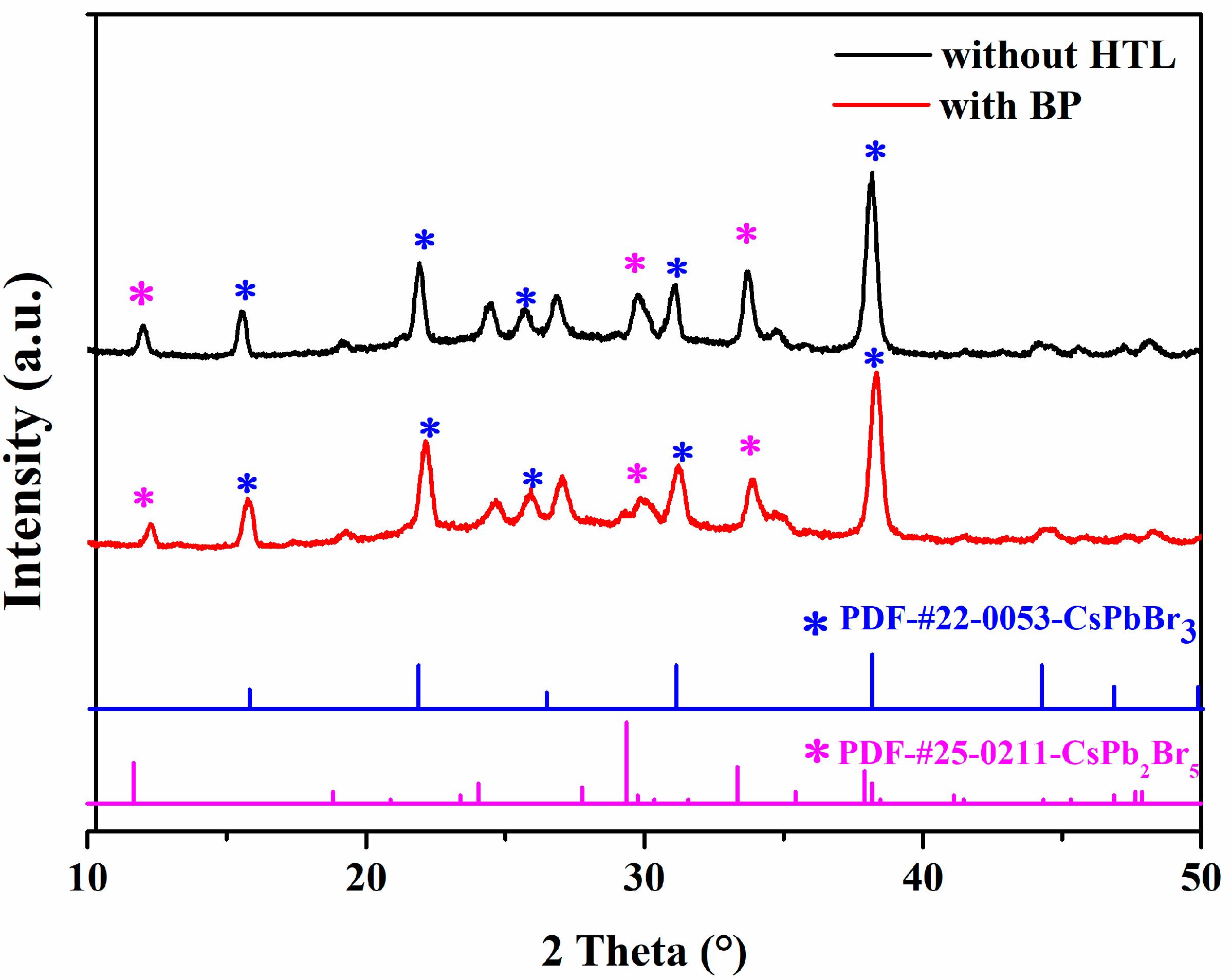
3.1. Characterization of SEM and XRD
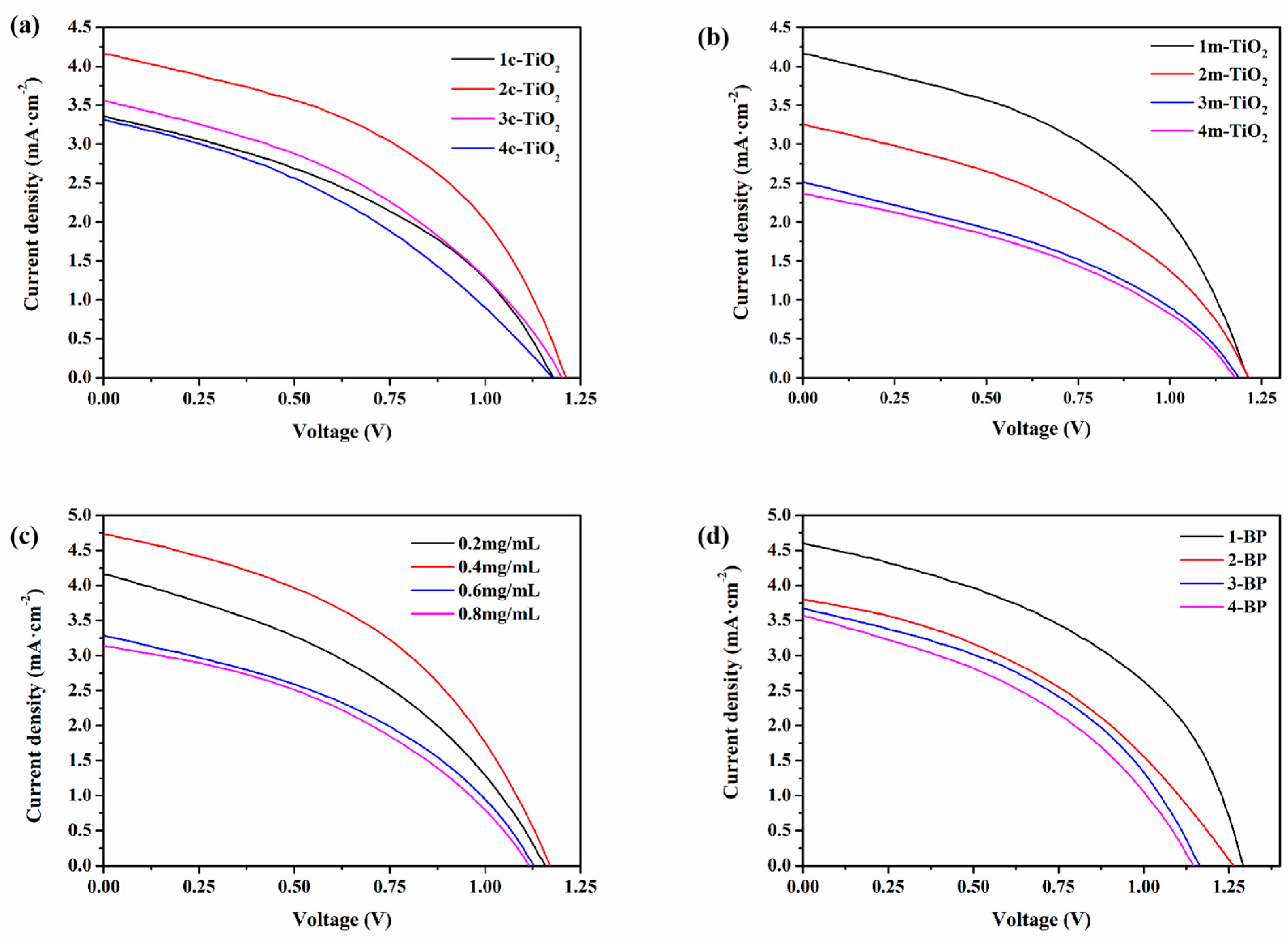
3.2. Preparation and Performance Regulation of CsPbBr3 Solar Cells Based on BP HTL
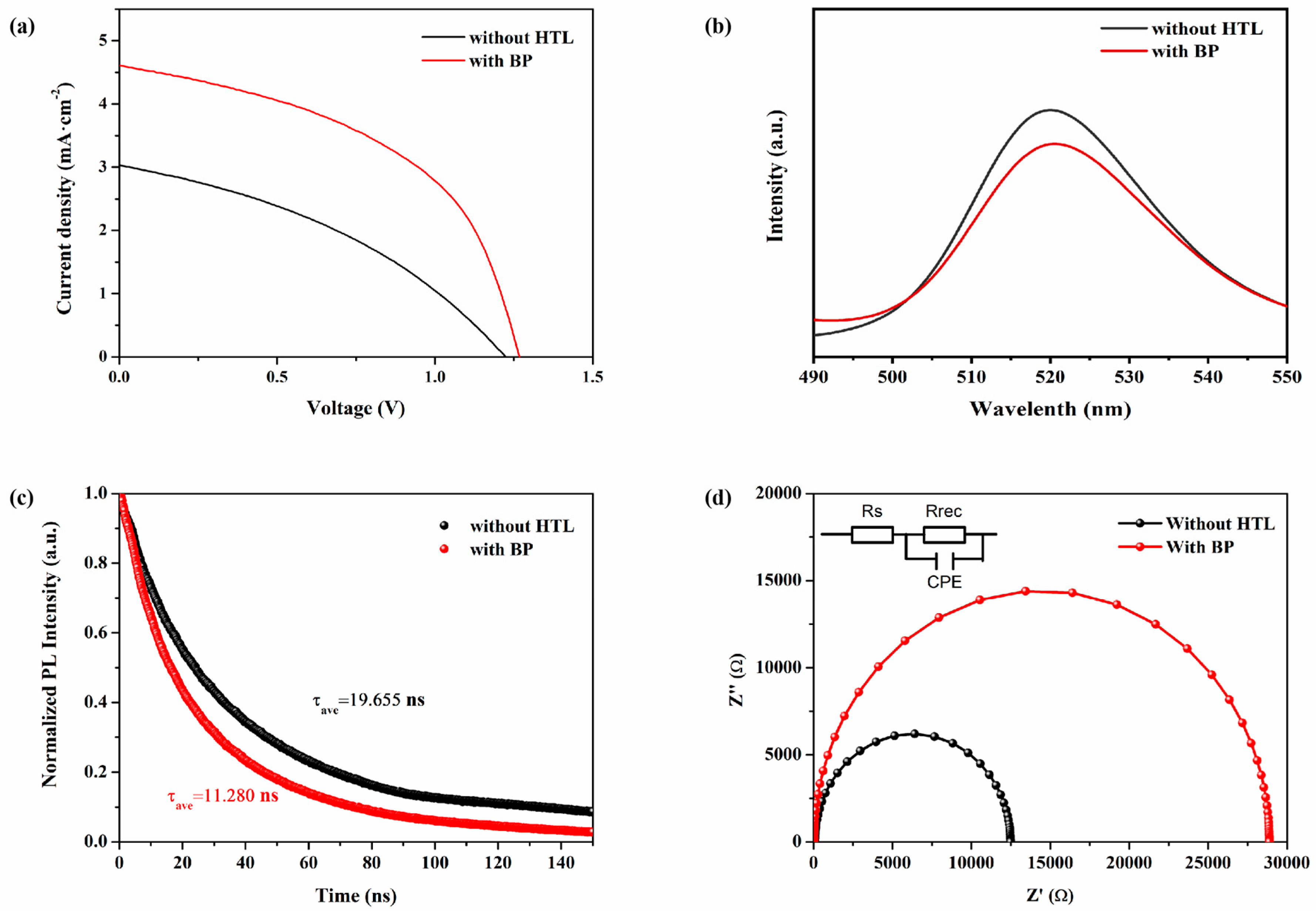
3.3. Photovoltaic Characteristics of CsPbBr3 Solar Cells Based on BP HTL
3.4. Stability Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.M.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G.; et al. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater. 2016, 28, 2253–2258. [Google Scholar] [CrossRef]
- Zhang, P. All Inorganic Perovskite Solar Cells and Absorption Layer Band Gap Regulation. Master’s Thesis, Henan University, Zhengzhou, China, 2019. [Google Scholar]
- National Renewable Energy Laboratory. Best Research-Cell Efficiency Chart; National Renewable Energy Laboratory: Golden, CO, USA, 2024. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 22 July 2024).
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, W.-J.; Chiu, C.-C.; Juwita, R.; Tsai, H.-H.G. First-Principles Investigation of the Thermal Degradation Mechanisms of Methylammonium Lead Triiodide Perovskite. J. Phys. Chem. C 2020, 124, 14521–14530. [Google Scholar] [CrossRef]
- Imran, M.; Saleem, A.; Khan, N.A.; Kamboh, A.H. Enhanced efficiency and stability of perovskite solar cells by partial replacement of CH3NH3+ with inorganic Cs+ in CH3NH3PbI3 perovskite absorber layer. Phys. B Condens. Matter 2019, 572, 1–11. [Google Scholar] [CrossRef]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium Enhances Long-Term Stability of Lead Bromide Perovskite-Based Solar Cells. J. Phys. Chem. Lett. 2015, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72 % Efficiency. Angew. Chem. Int. Ed. Engl. 2018, 57, 3787–3791. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Abhishek, S.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xu, F.; Li, Y.; Zhao, Y. A Facile Low Temperature Fabrication of High Performance CsPbI2Br All-Inorganic Perovskite Solar Cells. Sol. RRL 2018, 2, 1700180. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xu, G.; Xue, R.; Wang, S.; Li, Y.; Li, Y. Precise Control of Crystal Growth for Highly Efficient CsPbI2Br Perovskite Solar Cells. Joule 2019, 3, 191–204. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho-Baillie, A.W.Y. Hole Transport Layer Free Inorganic CsPbIBr2 Perovskite Solar Cell by Dual Source Thermal Evaporation. Adv. Energy Mater. 2016, 6, 1502202. [Google Scholar] [CrossRef]
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M.J.; et al. Thermal unequilibrium of strained black CsPbI3 thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef]
- Yuan, S.; Deng, J.; Xiong, H.; Wu, W.; Ma, Z.; Wang, M.; Li, W.; Fan, J. In-depth understanding the temperature-dependent reversible phase transition in CsPbI3-XBrX perovskites and its associated photophysical properties. J. Mater. Chem. A 2023, 11, 19685–19695. [Google Scholar] [CrossRef]
- Hou, S.; Wu, S.; Li, X.; Yan, J.; Xing, J.; Liu, H.; Hao, H.; Dong, J.; Huang, H. Efficient CsPbBr3 Perovskite Solar Cells with Storage Stability > 340 Days. Energies 2022, 15, 7740. [Google Scholar] [CrossRef]
- Zou, L.; Li, X.; Yang, M.; Yan, J.; Wang, J.; Cheng, J.; Xing, J.; Liu, H.; Hao, H.; Dong, J. ZnPc/CsPbBr3 QDs collaborative interface modification to improve the performance of CsPbBr3 perovskite solar cells. Sol. Energy Mater. Sol. Cells 2023, 251, 112157. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Lin, Z. A multifunctional 2D black phosphorene-based platform for improved photovoltaics. Chem. Soc. Rev. 2021, 50, 13346–13371. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Long, L.; Tang, X.; Qiu, M.; Liang, W.; Cao, R.; Zhang, Q.; Wang, D.; Zhang, H. Advancing Applications of Black Phosphorus and BP-Analog Materials in Photo/Electrocatalysis through Structure Engineering and Surface Modulation. Adv. Sci. 2020, 7, 2001431. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Guan, L.; Li, Q.; Li, Y.; Zhang, T.; Pan, H.; Sun, Q.; Shen, Y.; Grätzel, C.; Zakeeruddin, S.M.; et al. Black phosphorus quantum dots in inorganic perovskite thin films for efficient photovoltaic application. Sci. Adv. 2020, 6, eaay5661. [Google Scholar] [CrossRef] [PubMed]
- Aidarkhanov, D.; Yelzhanova, Z.; Ren, Z.; Nigmetova, G.; Lau, S.P.; Balanay, M.P.; Hu, H.; Surya, C.; Djurišić, A.B.; Ng, A. Synergic effects of incorporating black phosphorus for interfacial engineering in perovskite solar cells. Surf. Interfaces 2023, 43, 103531. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, M.; Wang, W.; Ma, C.; Wang, S.; Liu, Q.; Lian, T.; Huang, J.; Lin, Z. Synergistic Cascade Carrier Extraction via Dual Interfacial Positioning of Ambipolar Black Phosphorene for High-Efficiency Perovskite Solar Cells. Adv. Mater. 2020, 32, 2000999. [Google Scholar] [CrossRef]
- Lin, S.; Liu, S.; Yang, Z.; Li, Y.; Ng, T.W.; Xu, Z.; Bao, Q.; Hao, J.; Lee, C.-S.; Surya, C.; et al. Solution-Processable Ultrathin Black Phosphorus as an Effective Electron Transport Layer in Organic Photovoltaics. Adv. Funct. Mater. 2016, 26, 864–871. [Google Scholar] [CrossRef]
- Gu, B.; Du, Y.; Chen, B.; Zhao, R.; Lu, H.; Xu, Q.; Guo, C. Black Phosphorus Quantum Dot-Engineered Tin Oxide Electron Transport Layer for Highly Stable Perovskite Solar Cells with Negligible Hysteresis. ACS Appl. Mater. Interfaces 2022, 14, 11264–11272. [Google Scholar] [CrossRef] [PubMed]
- Muduli, S.K.; Varrla, E.; Kulkarni, S.A.; Han, G.; Thirumal, K.; Lev, O.; Mhaisalkar, S.; Mathews, N. 2D black phosphorous nanosheets as a hole transporting material in perovskite solar cells. J. Power Sources 2017, 371, 156–161. [Google Scholar] [CrossRef]
- Dong, W.; Xiong, S.; Yang, J.; Qiao, W.; Zeng, Q.; Wang, X.; Yao, Y.; Bao, Q. Black phosphorus doped Poly(triarylamine) as hole transport layer for highly efficient perovskite solar cells. Org. Electron. 2021, 89, 106052. [Google Scholar] [CrossRef]
- Liu, S.; Lyu, J.; Zhou, D.; Zhuang, X.; Shi, Z.; Sun, R.; Liu, L.; Wu, Y.; Liu, B.; Liu, D.; et al. Dual Modification Engineering via Lanthanide-Based Halide Quantum Dots and Black Phosphorus Enabled Efficient Perovskite Solar Cells with High Open-Voltage of 1.235 V. Adv. Funct. Mater. 2022, 32, 2112647. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Yi, Y.; Wang, J.; Kang, Y.; Chu, P.K.; Ong, H.C.; Yu, X.F. In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets. Chem. Commun. 2018, 54, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yan, J.; Wang, J.; Jiang, Y.; Xing, J.; Liu, H.; Hao, H.; Dong, J. Preparation of CsPbBr3 Perovskite Solar Cells Using a Green Solvent. Energies 2023, 16, 6426. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, H.; Jin, X.; Wang, H.; Wang, P.; Ijaz, M.; Xu, Q. Highly Stable All-inorganic CsPbBr3 Perovskite Solar Cells Based on Pulsed Laser Deposition. Appl. Phys. Lett. 2023, 123, 092103. [Google Scholar] [CrossRef]



| Sample | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 1c-TiO2 | 1.18 | 3.36 | 40.55 | 1.61 |
| 2c-TiO2 | 1.21 | 4.15 | 45.98 | 2.31 |
| 3c-TiO2 | 1.20 | 3.56 | 39.73 | 1.70 |
| 4c-TiO2 | 1.18 | 3.31 | 36.68 | 1.43 |
| 1m-TiO2 | 1.21 | 4.15 | 45.98 | 2.31 |
| 2m-TiO2 | 1.21 | 3.25 | 40.96 | 1.61 |
| 3m-TiO2 | 1.19 | 2.47 | 38.02 | 1.12 |
| 4m-TiO2 | 1.21 | 2.22 | 39.86 | 1.07 |
| 0.2 mg/mL BP | 1.16 | 4.15 | 39.45 | 1.90 |
| 0.4 mg/mL BP | 1.17 | 4.73 | 43.70 | 2.42 |
| 0.6 mg/mL BP | 1.13 | 3.28 | 40.25 | 1.49 |
| 0.8 mg/mL BP | 1.12 | 3.13 | 40.02 | 1.40 |
| 1-BP | 1.29 | 4.60 | 45.52 | 2.70 |
| 2-BP | 1.26 | 3.80 | 40.02 | 1.91 |
| 3-BP | 1.16 | 3.67 | 42.56 | 1.81 |
| 4-BP | 1.15 | 3.57 | 39.71 | 1.63 |
| Sample | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Without HTL | 1.22 | 2.65 | 39.51 | 1.27 |
| With BP | 1.15 | 3.57 | 39.71 | 1.63 |
| Structure | τ1 | τ2 |
|---|---|---|
| Without HTL | 4.931 | 23.961 |
| with BP | 4.055 | 14.337 |
| Sample | Rs (Ω) | Rrec (Ω) | CPE |
|---|---|---|---|
| Without HTL | 91.37 | 12,397 | 9.554 × 10−9 |
| With BP | 22.75 | 28,862 | 6.798 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Yang, L.; Wang, Z.; Li, F.; Zhang, X.; Zhou, J.; Lv, D.; Ding, Y.; Sun, W. Few-Layered Black Phosphorene as Hole Transport Layer for Novel All-Inorganic Perovskite Solar Cells. Materials 2025, 18, 415. https://doi.org/10.3390/ma18020415
Xu S, Yang L, Wang Z, Li F, Zhang X, Zhou J, Lv D, Ding Y, Sun W. Few-Layered Black Phosphorene as Hole Transport Layer for Novel All-Inorganic Perovskite Solar Cells. Materials. 2025; 18(2):415. https://doi.org/10.3390/ma18020415
Chicago/Turabian StyleXu, Shihui, Lin Yang, Zhe Wang, Fuyun Li, Xiaoping Zhang, Juan Zhou, Dongdong Lv, Yunfeng Ding, and Wei Sun. 2025. "Few-Layered Black Phosphorene as Hole Transport Layer for Novel All-Inorganic Perovskite Solar Cells" Materials 18, no. 2: 415. https://doi.org/10.3390/ma18020415
APA StyleXu, S., Yang, L., Wang, Z., Li, F., Zhang, X., Zhou, J., Lv, D., Ding, Y., & Sun, W. (2025). Few-Layered Black Phosphorene as Hole Transport Layer for Novel All-Inorganic Perovskite Solar Cells. Materials, 18(2), 415. https://doi.org/10.3390/ma18020415







