Spectroscopic and Morphological Examination of Co0.9R0.1MoO4 (R = Ho, Yb, Gd) Obtained by Glycine Nitrate Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Preparation
Co0.9Ho0.1MoO4+ CH3COOH + 3.96NO2↑ + 8.69H2O
Co0.9Yb0.1MoO4+ CH3COOH + 3.96NO2↑ + 8.69H2O
Co0.9Gd0.1MoO4+ CH3COOH + 3.96NO2↑ + 8.74H2O
2.2. Sample Characterization
3. Results and Discussion
3.1. Differential Thermal Analyses (DTA)
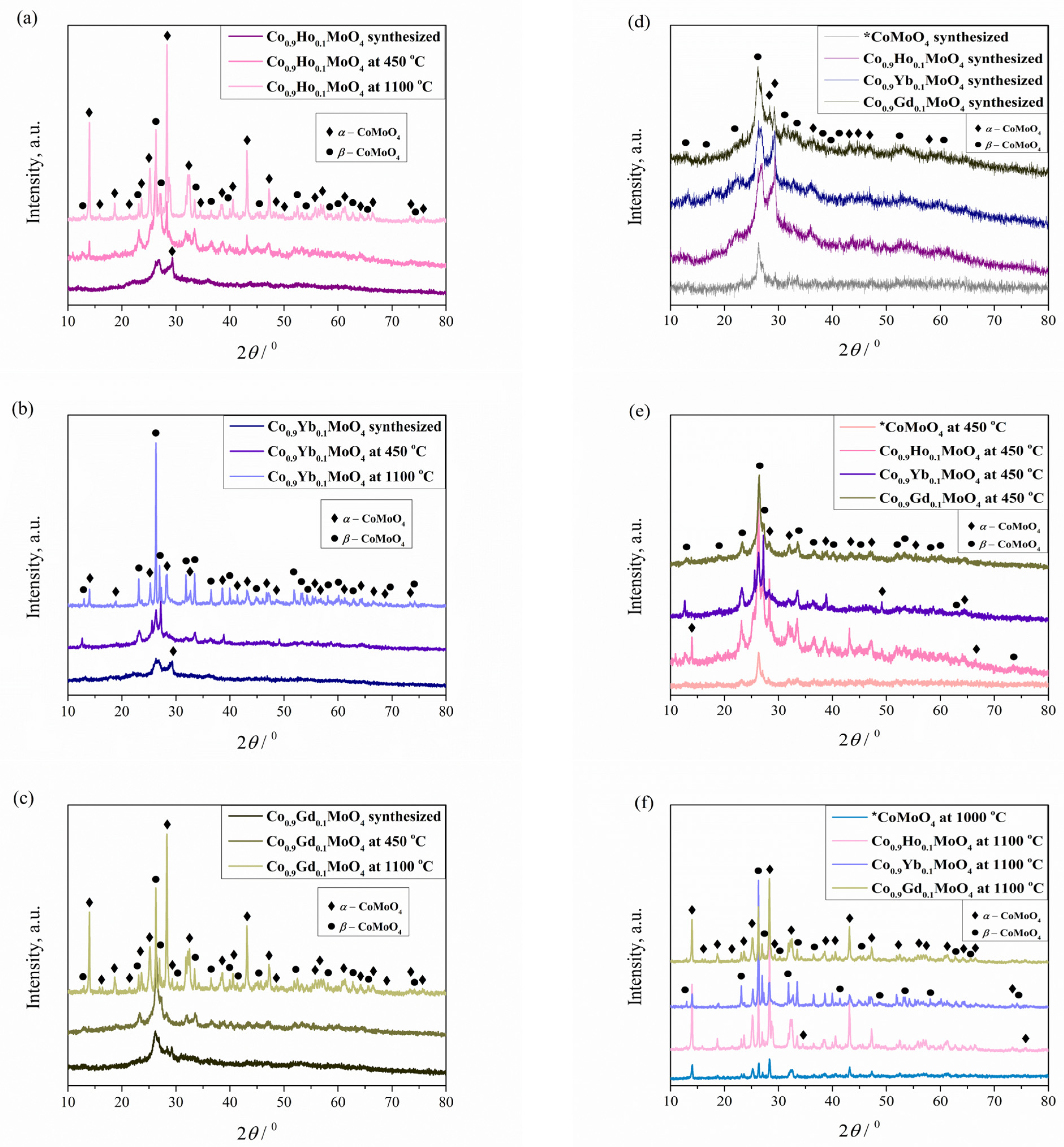
3.2. XRD Analysis
3.3. FTIR
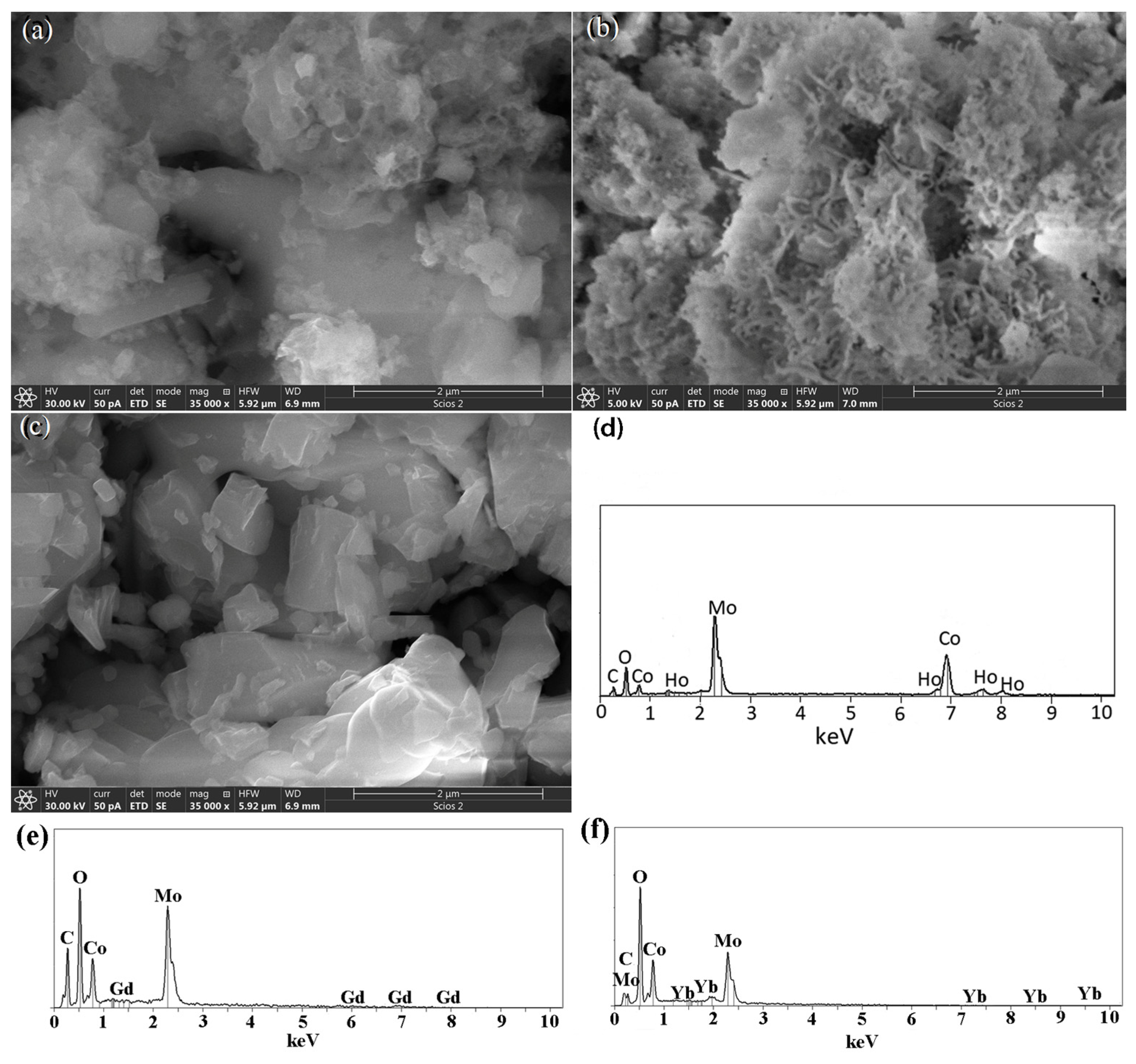
3.4. FESEM Analysis
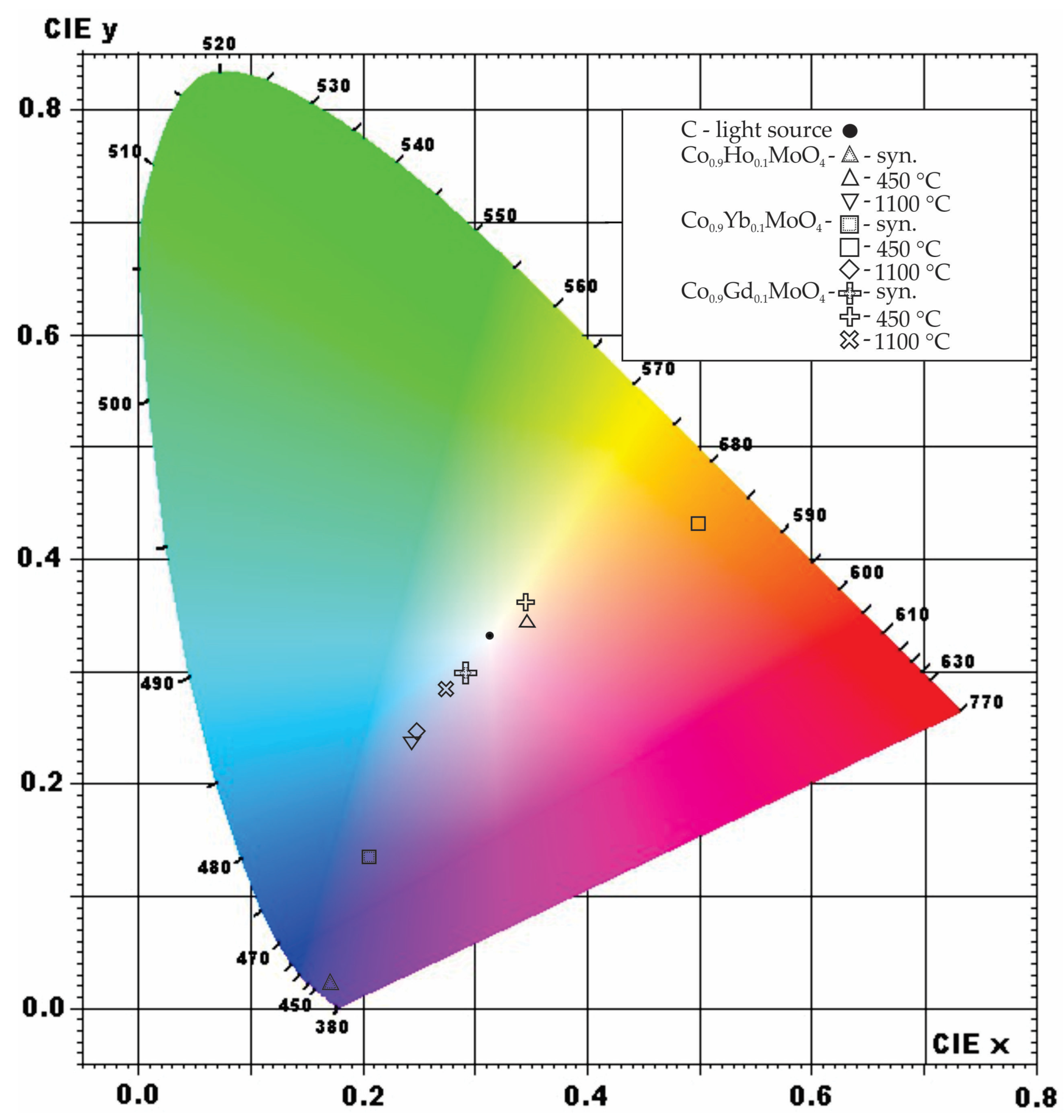
3.5. Spectroscopy
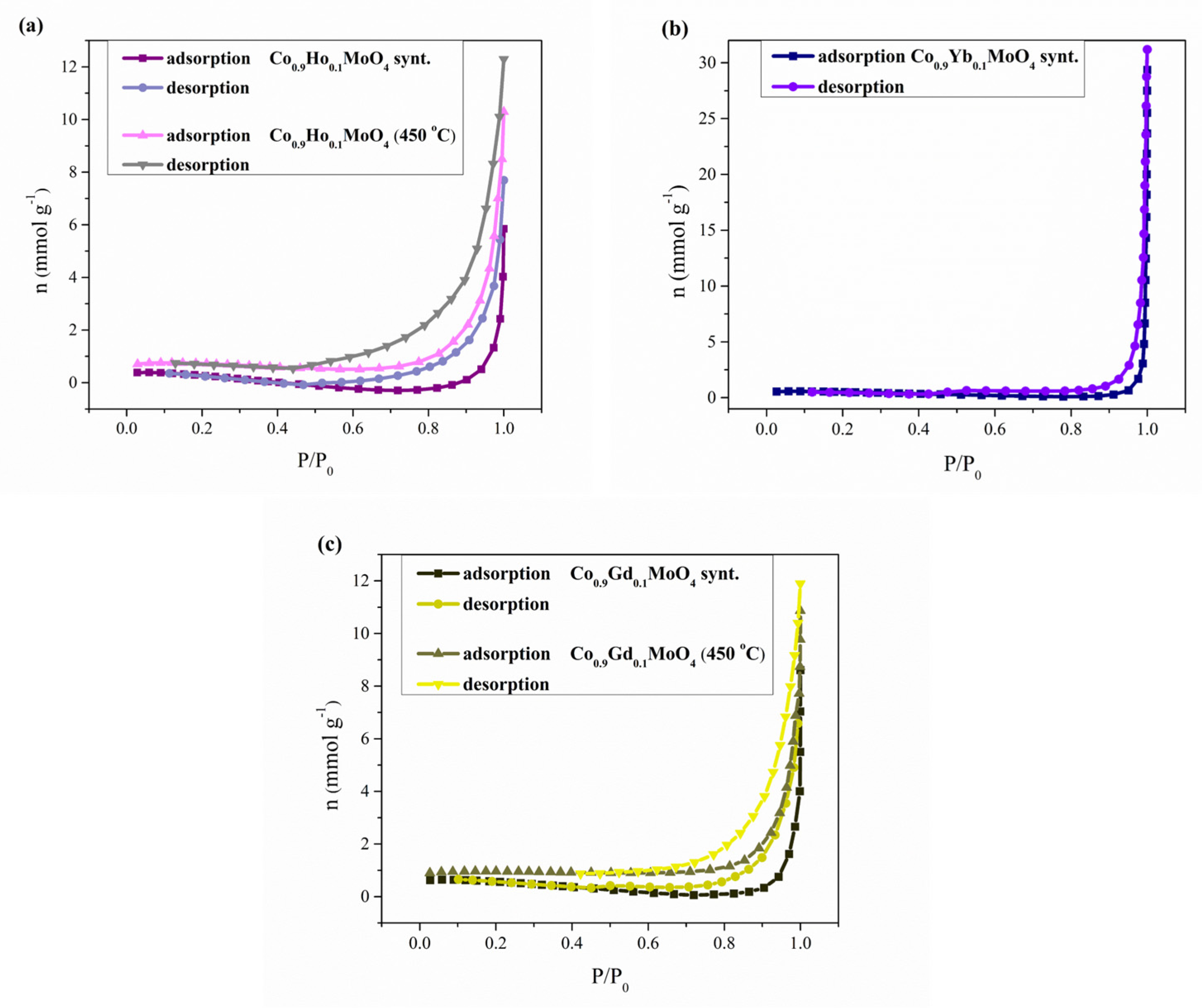
3.6. BET Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, N.; Kraus, H.; Kim, H.J.; Mokina, V.; Tsiumra, V.; Wagner, A.; Zhydachevskyy, Y.; Mykhaylyk, V.B. Characterisation of tungstate and molybdate crystals ABO4 (A = Ca, Sr, Zn, Cd; B = W, Mo) for luminescence lifetime cryothermometry. Materialia 2018, 4, 287–296. [Google Scholar] [CrossRef]
- Seevakan, K.; Manikandan, A.; Devendran, P.; Baykal, A.; Alagesan, T. Electrochemical and magneto-optical properties of cobalt molybdate nano-catalyst as high-performance supercapacitor. Ceram. Int. 2018, 44, 17735–17742. [Google Scholar] [CrossRef]
- Khadka, A.; Samuel, E.; Joshi, B.; Il Kim, Y.; Aldalbahi, A.; El-Newehy, M.; Lee, H.-S.; Yoon, S.S. Bimetallic CoMoO4 Nanosheets on Freestanding Nanofiber as Wearable Supercapacitors with Long-Term Stability. Int. J. Energy Res. 2023, 2023, 2910207. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Wang, G.; Wang, J.; Fan, L.; Hao, J.; Wang, S.; Liu, Y.; Wu, H.; Li, Y.; et al. Preparation of Pie-Shaped CoMoO4 with High Capacitive and Photocatalytic Properties by a Solvothermal Method. Coatings 2022, 12, 1771. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Khiew, P.S.; Siong, C.W.; Kong, I.; Tan, M.T.T. Synthesis of NiMoO4 nanorods on graphene and superior electrochemical performance of the resulting ternary based composites. Ceram. Int. 2017, 43, 13772–13780. [Google Scholar] [CrossRef]
- Yesuraj, J.; Elumalai, V.; Muthuraaman, B.; Suthanthiraraj, S.A.; Elanthamilan, E.; Merlin, J.P. A facile sonochemical assisted synthesis of α-MnMoO4/PANI nanocomposite electrode for supercapacitor applications. J. Electroanal. Chem. 2017, 797, 78–88. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Krishnamoorthy, K.; Radhakrishnan, S.; Kim, N.J.; Sang, J.K. Synthesis, characterization, and electrochemical properties of CoMoO4 nanostructures. Int. J. Hydrogen Energy 2014, 39, 5186–5193. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Roy, S. Exploration of Molybdenum Oxide Compounds—A Review. Catal. Res. 2024, 4, 11. [Google Scholar] [CrossRef]
- Bahari, M.B.; Mamat, C.R.; Jalil, A.A.; Hassan, N.S.; Sawal, M.H.; Rajendran, S.; Alam, M.N.H.Z. Molybdenum as cathode materials: Paving the way for sustainable biohydrogen production in microbial electrolysis cells. Process Saf. Environ. 2024, 191, Part B, 1633–1647. [Google Scholar] [CrossRef]
- Ruan, Q.; Qian, Y.; Xue, M.; Chen, L.; Zhang, Q. Emerging two-dimensional Mo-based materials for rechargeable metal-ion batteries: Advances and perspectives. J. Energy Chem. 2024, 89, 487–518. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, G.; Wang, H.; Yin, J.; Zhang, L.; Xiao, F.; Siddharth, K.; Zhu, S.; Shao, M. Defect Engineering of Molybdenum-Based Materials for Electrocatalysis. Catalysts 2020, 10, 1301. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Meyrick, D.; Lee, Y.-S.; Selvan, R.K. Synthesis and improved electrochemical performances of nano β-NiMoO4-CoMoO4·xH2O composites for asymmetric supercapacitors. RSC Adv. 2013, 3, 16542–16548. [Google Scholar] [CrossRef]
- Rani, B.J.; Swathi, S.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Babu, E.S.; Alfarraj, S.; Alharbi, S.A.; Velauthapillai, D. Solvothermal synthesis of CoMoO4 nanostructures for electrochemical applications. J. Mater. Sci. Mater. Electron. 2021, 32, 5989–6000. [Google Scholar] [CrossRef]
- Li, P.; Ruan, C.; Xu, J.; Xie, Y. Supercapacitive performance of CoMoO4 with oxygen vacancy porous nanosheet. Electrochim. Acta 2020, 330, 135334. [Google Scholar] [CrossRef]
- Rico, J.L.; A’valos-Borja, M.; Barrera, A.; Hargreaves, J.S.J. Template-free synthesis of CoMoO4 rods and their characterization. Mater. Res. Bull. 2013, 48, 4614–4617. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Tabibpour, M.; Kiani, M.A.; Kazemi, H. An advanced asymmetric supercapacitor based on a binder-free electrode fabricated from ultrathin CoMoO4 nano-dandelions. RSC Adv. 2016, 6, 71156–71164. [Google Scholar] [CrossRef]
- Gao, Y.; Tao, J.; Li, J.; Xie, H.; Li, Y.; Wang, T.; Zhang, C. Construction of CoMoO4 nanorods wrapped by Ni-Co-S nanosheets for high-performance supercapacitor. J. Alloys Compd. 2022, 925, 166705. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Parwaz Khan, A.A.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.-H. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Khalid, N.R.; Hamza, S.M.; Ali, F.; Iqbal, T.; Rafique, M.; Imran, M.; Assiri, M.A. Synthesis and photocatalytic performance of CoMoO4/MoO3 composite for wastewater treatment. Mater. Today Commun. 2023, 35, 105816. [Google Scholar] [CrossRef]
- Mayers, J.; Hofman, B.; Sobiech, I.; Kwesiga, M.P. Insights into the biocompatibility of biodegradable metallic molybdenum for cardiovascular applications-a critical review. Front. Bioeng. Biotechnol. 2024, 12, 1457553. [Google Scholar] [CrossRef]
- Lakhlifi, H.; Jabbar, Y.E.; Guillemet-Fritsch, S.; Durand, B.; Er-Rakho, L.; Ouatib, R.E. Purple nanometrics pigments based on cobalt-doped manganese molybdate: Synthesis, characterization, structural, thermal, optical, colorimetric and chemical properties. J. Mol. Struct. 2022, 1248, 131458. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Murugan, A.; Siva, V.; Asath Bahadur, S.A. Cost-effective synthesis of efficient La doped CoMoO4 nanocomposite electrode for sustainable high-energy symmetric supercapacitors. J. Energy Storage 2023, 73, 108856. [Google Scholar] [CrossRef]
- Ragu, R.; Shanmugam, P.; Sridharan, M.B.; Muniappan, E.; Dheivasigamani, T. Investigation of rare earth metal ions (Sm and Er) doped CoMoO4 polymorphs for photocatalytic dye degradation. Phys. B 2025, 696, 416616. [Google Scholar] [CrossRef]
- Costa, R.K.S.; Teles, S.C.; de Sousa Filho, P.C.; Dias, A.; Siqueira, K.P.F. Influence of europium doping on the structural phase-transition temperature of β- and α- CoMoO4 polymorphs. Mater. Res. Bull. 2019, 118, 110517. [Google Scholar] [CrossRef]
- Hakami, A.; Srinivasan, S.S.; Biswas, P.K.; Krishnegowda, A.; Wallen, S.L.; Stefanakos, E.K. Review on thermochromic materials: Development, characterization, and applications. J. Coat. Technol. Res. 2022, 19, 377–402. [Google Scholar] [CrossRef]
- Talvenmaa, P. Introduction to chromic materials. In Woodhead Publishing Series in Textiles, Intelligent Textiles and Clothing; Mattila, H., Ed.; Woodhed Puhlishing Limited: Cambridge, UK, 2006; pp. 193–205. ISBN 9781845690052. [Google Scholar] [CrossRef]
- Hossain, S.; Sadoh, A.; Ravindra, N.M. Principles, properties and preparation of thermochromic materials. Mater. Sci. Eng. Int. J. 2023, 7, 146–156. [Google Scholar] [CrossRef]
- Guzik, M.; Tomaszewicz, E.; Guyot, Y.; Legendziewicz, J.; Boulon, G. Eu3+ luminescence from different sites in a scheelite-type cadmium molybdate red phosphor with vacancies. J. Mater. Chem. C 2015, 3, 8582–8594. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Zou, L.; Gan, S. Formation mechanism and optical properties of CdMoO4 and CdMoO4:Ln3+ (Ln = Pr, Sm, Eu, Dy, Ho and Er) microspheres synthesized via a facile sonochemical route. RSC Adv. 2014, 4, 38455–38465. [Google Scholar] [CrossRef]
- Rosić, M.; Zarubica, A.; Šaponjić, A.; Babić, B.; Zagorac, J.; Jordanov, D.; Matović, B. Structural and photocatalytic examination of CoMoO4 nanopowders synthesized by GNP method. Mater. Res. Bull. 2018, 98, 111–120. [Google Scholar] [CrossRef]
- Patil, K.C.; Hegde, M.S.; Rattan, T.; Aruna, S.T. Chemistry of Nanocrystalline Oxide Materials: Combustion Synthesis, Properties and Applications; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2008; pp. 43–45. Available online: https://ebin.pub/chemistry-of-nanocrystalline-oxide-materials-combustion-synthesis-properties-and-applications-978-981-279-314-0-981-279-314-3.html (accessed on 22 April 2024).
- Nair, S.R.; Purohit, R.D.; Tyagi, A.K.; Sinha, P.K.; Sharmaz, B.P. Low-Temperature Sintering of La(Ca)CrO3 Powder Prepared through the Combustion Process. J. Am. Ceram. Soc. 2008, 91, 88–91. [Google Scholar] [CrossRef]
- Rigaku. PDXL Integrated X-Ray Powder Diffraction Software; Rigaku: Tokyo, Japan, 2011. [Google Scholar]
- International Crystallographical Database (ICDD); PDF-2, Release 2023; ICDD: Newtown Square, PA, USA, 2012.
- Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 15 May 2024).
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Available online: http://powdercell-for-windows.software.informer.com/2.4/ (accessed on 15 May 2024).
- Commission Internationale de l’Eclairage. 1931 Commission Internationale de l’Eclairage Proceedings; Huitième Session; Cambridge University Press: Cambridge, UK, 1932; pp. 19–29. [Google Scholar]
- Available online: http://www.radiantimaging.com (accessed on 18 August 2024).
- Barret, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lippens, B.C.; Linsen, B.G.; de Boer, J.H. Studies on Pore Systems in Catalysts I. The Adsorption of Nitrogen; Apparatus and Calculation. J. Catal. 1964, 3, 32–37. [Google Scholar] [CrossRef]
- Eda, K.; Uno, Y.; Nagai, N.; Sotani, N.; Whittingham, M.S. Crystal structure of cobalt molybdate hydrate CoMoO4 * nH2O. J. Solid State Chem. 2005, 178, 2791–2797. [Google Scholar] [CrossRef]
- Wiesmann, M.; Ehrenberg, H.; Wltschek, G.; Zinn, P.; Weitzel, H. Fuess, Crystal structures and magnetic properties of the high-pressure modifications of CoMoO4 and NiMoO4. J. Magn. Magn Mater. 1995, 150, L1–L4. [Google Scholar] [CrossRef]
- Weiss, I.; Muth, C.; Drumm, R.; Kirchner, H. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.Y.; Lu, J.Z.; Yan, F.Y.; Ji, X.Y. The use of oxalates as precursors for the low temperature synthesis of LiNi1-xCoxVO4, Part V New synthetic methods, characterization and theory. In Frontiers of Solid State Chemistry; Feng, S.H., Chen, J.S., Eds.; World Scientific Publishing Co.: Singapore, 2002; p. 515. [Google Scholar]
- Sen, A.; Pramanik, P. A chemical synthetic route for the preparation of fine-grained metal molybdate powders. Mater. Lett. 2002, 52, 140–146. [Google Scholar] [CrossRef]
- Yan, C.H.; Xu, Z.G.; Xheng, F.X.; Wang, Z.M.; Sun, L.D.; Liao, C.S.; Jia, J.T. Nanophased CoFe2O4 prepared by combustion method. Solid State Commun. 1999, 111, 287–291. [Google Scholar] [CrossRef]
- Koul, R.K.; Suri, S.; Singh, V.; Bamzai, K.K. Synthesis, Characterization, and Thermal Kinetics of Mixed Gadolinium: Calcium Heptamolybdate System. Hindawi Publ. Corp. Int. Sch. Res. Not. 2014, 141463. [Google Scholar] [CrossRef][Green Version]
- Shaheen, W.M.; Maksod, I.H.A.E. Thermal solid–solid interactions and physicochemical properties of CO3O4/MoO3 system treated with Al2O3. J. Alloys Compd. 2009, 475, 874–880. [Google Scholar] [CrossRef]
- Costa, R.K.S.; Teles, S.C.; Siqueira, K.P.F. The relationship between crystal structures and thermochromism in CoMoO4. Chem. Pap. 2021, 75, 237–248. [Google Scholar] [CrossRef]
- Zagorac, D.; Schön, J.C.; Rosić, M.; Zagorac, J.; Jordanov, D.; Luković, J.; Matović, B. Theoretical and experimental study of structural phases in CoMoO4. Cryst. Res. Technol. 2017, 52, 1700069. [Google Scholar] [CrossRef]
- Yang, C.; Wöll, C. IR spectroscopy applied to metal oxide surfaces: Adsorbate vibrations and beyond. Adv. Phys. X 2017, 2, 373–408. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Abinaya, M.; Chen, S.-M. Ultrasonic assisted preparation of CoMoO4 nanoparticles modified electrochemical sensor for chloramphenicol determination. J. Solid State Chem. 2021, 302, 122392. [Google Scholar] [CrossRef]
- Weifan, C.; Fengsheng, L.; Leili, L.; Yang, L. One-step synthesis of nanocrytalline perovskite LaMn03 powders via microwave-Induced solution combustion route. J. Rare Earth 2006, 24, 782–787. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T. Influence of synthesis conditions on physico-chemical and photocatalytic properties of rare earth (Ho, Nd and Sm) oxides. J. Mater. Res. Technol. 2020, 9, 1819–1830. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.; Khajuria, H.; Sheikh, H.N. Facile hydrothermal synthesis of nanocomposites of nitrogen doped graphene with metal molybdates (NG-MMoO4) (M=Mn, Co, and Ni) for enhanced photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 9423–9434. [Google Scholar] [CrossRef]
- Bhiri, N.M.; Dammak, M.; Carvajal, J.J.; Aguiló, M.; Díaz, F.; Pujol, M.C. Stoichiometric dependence and laser heating effect on the luminescence thermometric performance of Er3+, Yb3+: YuGdwVO4 microparticles in the non-saturation regime. Mater. Res. Bull. 2022, 151, 111801. [Google Scholar] [CrossRef]
- Bhemarajam, J.; Varkolu, M.; Prasad, P.S.; Prasad, M. Effect of Yb3+ ions on spectroscopic and optical properties of Bi2O3–B2O3–Li2O–PbO glass system. Results Opt. 2024, 14, 100582. [Google Scholar] [CrossRef]
- Tamrakar, R.K.; Bisen, D.P.; Brahme, N. Comparison of photoluminescence properties of Gd2O3 phosphor synthesized by combustion and solid state reaction method. J. Radiat. Res. Appl. Sci. 2014, 7, 550–559. [Google Scholar] [CrossRef]
- Rosić, M.; Logar, M.; Devečerski, A.; Prekajski, M.; Radosavljević-Mihajlović, A.; Kusigerski, V.; Spasojević, V.; Matović, B. Synthesis, structural and magnetic properties of nanostructured Ca0.9Gd0.1MnO3 obtained by modified glycine nitrate procedure (MGNP). Ceram. Int. 2011, 37, 1313–1319. [Google Scholar] [CrossRef]
- Guineau, B.; Lorblanchet, M.; Gratuze, B.; Dulin, L.; Roger, P.; Akrich, R.; Muller, F. Manganese black pigments in prehistoric paintings: The case of the black frieze of pech merle (France). Archaeometry 2001, 43, 211–225. [Google Scholar] [CrossRef]
- Wilteveen, H.J.; Farnau, E.F. Colors Developed by Cobalt Oxides. J. Ind. Eng. Chem. 1921, 13, 1061–1066. [Google Scholar] [CrossRef]
- International Molybdenum Association. 2008. Available online: https://www.imoa.info/molybdenum/molybdenum-properties.php (accessed on 12 July 2024).
- Huckle, W.G.; Lalor, E. Inorganic Pigments. Ind. Eng. Chem. 1955, 47, 1501–1506. [Google Scholar] [CrossRef]
- Koch, E.-C.; Weiser, V.; Roth, E.; Knapp, S.; Kelzenberg, S. Combustion of Ytterbium Metal, Propellants, Explosives. Pyrotechnics 2012, 37, 9–11. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Charactrization of Porous Solids and Powders: Surface Area, Pore Size and Density; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; p. 44. [Google Scholar]


| Phase | Synthesized | 450 °C | 1100 °C |
|---|---|---|---|
| Co0.9Ho0.1MoO4 ICSD card #78328 | a = 10.43 b = 9.63 c = 7.06 β = 105.33 V = 683.67 85.44% | a = 10.39 b = 9.43 c = 7.06 β = 107.33 V = 660.12 93.49% | a = 10.49 b = 9.53 c = 7.16 β = 106.34 V = 686.69 5.95% |
| Co0.9Ho0.1MoO4 ICSD card #23808 | a = 9.77 b = 8.95 c = 7.86 β = 114.82 V = 623.43 14.56% | a = 9.65 b = 8.78 c = 7.70 β = 112.83 V = 600.88 6.51% | a = 9.61 b = 8.86 c = 7.66 β = 112.82 V = 601.71 94.05% |
| Rp | 6.48 | 7.25 | 10.30 |
| Rwp | 8.23 | 9.95 | 14.74 |
| Rexp | 0.24 | 0.5 | 0.91 |
| Co0.9Yb0.1MoO4 ICSD card #78328 | a = 10.39 b = 9.61 c = 7.06 β = 105.33 V = 679.45 86.26% | a = 10.52 b = 9.56 c = 7.07 β = 106.70 V = 680.81 96.10% | a = 10.39 b = 9.43 c = 7.10 β = 107.33 V = 663.81 89.30% |
| Co0.9Yb0.1MoO4 ICSD card #23808 | a = 9.77 b = 8.95 c = 7.78 β = 114.82 V = 617.08 13.74% | a = 9.63 b = 8.90 c = 7.69 β = 113.88 V = 602.48 3.90% | a = 9.57 b = 8.90 c = 7.66 β = 112.82 V = 601.64 10.70% |
| Rp | 4.92 | 8.86 | 14.08 |
| Rwp | 6.30 | 11.84 | 22.91 |
| Rexp | 0.22 | 0.35 | 1.05 |
| Co0.9Gd0.1MoO4 ICSD card #78328 | a = 10.39 b = 9.43 c = 7.07 β = 106.74 V = 663.28 87.20% | a = 10.46 b = 9.43 c = 7.06 β = 107.33 V = 664.33 94.97% | a = 10.39 b = 9.43 c = 7.08 β = 107.31 V = 662.45 38.27% |
| Co0.9Gd0.1MoO4 ICSD card #23808 | a = 9.76 b = 8.76 c = 7.86 β = 114.82 V = 609.69 12.80% | a = 9.76 b = 8.94 c = 7.69 β = 112.82 V = 618.59 5.03% | a = 9.71 b = 8.85 c = 7.66 β = 114.08 V = 601.35 61.73% |
| Rp | 5.14 | 9.15 | 9.33 |
| Rwp | 6.69 | 12.79 | 13.53 |
| Rexp | 0.23 | 0.38 | 0.70 |
| CoMoO4 * ICSD card #78328 | a = 10.57 b = 9.63 c = 7.21 β = 106.59 V = 703.08 100% | a = 10.57 b = 9.62 c = 7.22 β = 107.33 V = 700.84 100% | / |
| CoMoO4 * ICSD card #23808 | / | / | a = 9.71 b = 8.85 c = 7.66 β = 114.10 V = 600.70 100% |
| Rp | 3.53 | 4.21 | 4.12 |
| Rwp | 4.49 | 5.60 | 6.05 |
| Rexp | 0.17 | 0.21 | 0.32 |
| Oxides (wt.%) | Oxides (wt.%) | Oxides (wt.%) | |||
| CoO | 26.49 | CoO | 26.54 | CoO | 26.64 |
| MoO2 | 66.58 | MoO2 | 66.48 | MoO2 | 66.82 |
| Ho2O3 | 6.93 | Yb2O3 | 6.99 | Gd2O3 | 6.53 |
| Elements (at.%) | Elements (at.%) | Elements (at.%) | |||
| Co | 38.82 | Co | 38.95 | Co | 38.91 |
| Mo | 57.15 | Mo | 57.15 | Mo | 57.15 |
| Ho | 4.03 | Yb | 3.90 | Gd | 3.94 |
| Ho/(Ho + Co) | 9.4 | Yb/(Yb + Co) | 9.1 | Gd/(Gd + Co) | 9.2 |
| Sample | Dc (nm) | Pc (%) | |
|---|---|---|---|
| Co0.9Ho0.1MoO4 | synt. | 427 | 99.8 |
| 450 °C | 589 | 4.4 | |
| 1100 °C | 473 | 38.6 | |
| Co0.9Yb0.1MoO4 | synt. | 456 | 65.3 |
| 450 °C | 585 | 78.7 | |
| 1100 °C | 472 | 32.8 | |
| Co0.9Gd0.1MoO4 | synt. | 469 | 13.1 |
| 450 °C | 577 | 7.4 | |
| 1100 °C | 475 | 20.3 |
| Sample | Sbet, (m2/g−1) | Smeso, (m2/g−1) | Vmic (cm3/g−1) | Vtotal (cm3/g−1) | Rmed, (nm) | Rmax, (nm) |
|---|---|---|---|---|---|---|
| Co0.9Ho0.1MoO4 synt. | 14.879 | 8.589 | 0.0119 | 0.0433 | 9.0389 | 7.1224 |
| Co0.9Yb0.1MoO4 synt. | 10.424 | 11.94 | 0.0113 | 0.0077 | / | 0.3195 |
| Co0.9Gd0.1MoO4 synt. | 11.627 | 15.88 | 0.0139 | 0.012 | 11.737 | 4.396 |
| Co0.9Ho0.1MoO4 (450 °C) | 3.614 | 19.78 | 0.0116 | 0.0072 | 10 | 7.4783 |
| Co0.9Gd0.1MoO4 (450 °C) | 16.215 | 1.54 | 0.0089 | 0.0443 | 11.002 | 8.6617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosić, M.; Milošević, M.; Čebela, M.; Dodevski, V.; Lojpur, V.; Čakar, U.; Stopic, S. Spectroscopic and Morphological Examination of Co0.9R0.1MoO4 (R = Ho, Yb, Gd) Obtained by Glycine Nitrate Procedure. Materials 2025, 18, 397. https://doi.org/10.3390/ma18020397
Rosić M, Milošević M, Čebela M, Dodevski V, Lojpur V, Čakar U, Stopic S. Spectroscopic and Morphological Examination of Co0.9R0.1MoO4 (R = Ho, Yb, Gd) Obtained by Glycine Nitrate Procedure. Materials. 2025; 18(2):397. https://doi.org/10.3390/ma18020397
Chicago/Turabian StyleRosić, Milena, Maja Milošević, Maria Čebela, Vladimir Dodevski, Vesna Lojpur, Uroš Čakar, and Srecko Stopic. 2025. "Spectroscopic and Morphological Examination of Co0.9R0.1MoO4 (R = Ho, Yb, Gd) Obtained by Glycine Nitrate Procedure" Materials 18, no. 2: 397. https://doi.org/10.3390/ma18020397
APA StyleRosić, M., Milošević, M., Čebela, M., Dodevski, V., Lojpur, V., Čakar, U., & Stopic, S. (2025). Spectroscopic and Morphological Examination of Co0.9R0.1MoO4 (R = Ho, Yb, Gd) Obtained by Glycine Nitrate Procedure. Materials, 18(2), 397. https://doi.org/10.3390/ma18020397











