Large Improvements in the Thermoelectric Properties of SnSe by Fast Cooling
Abstract
1. Introduction
2. Materials and Methods
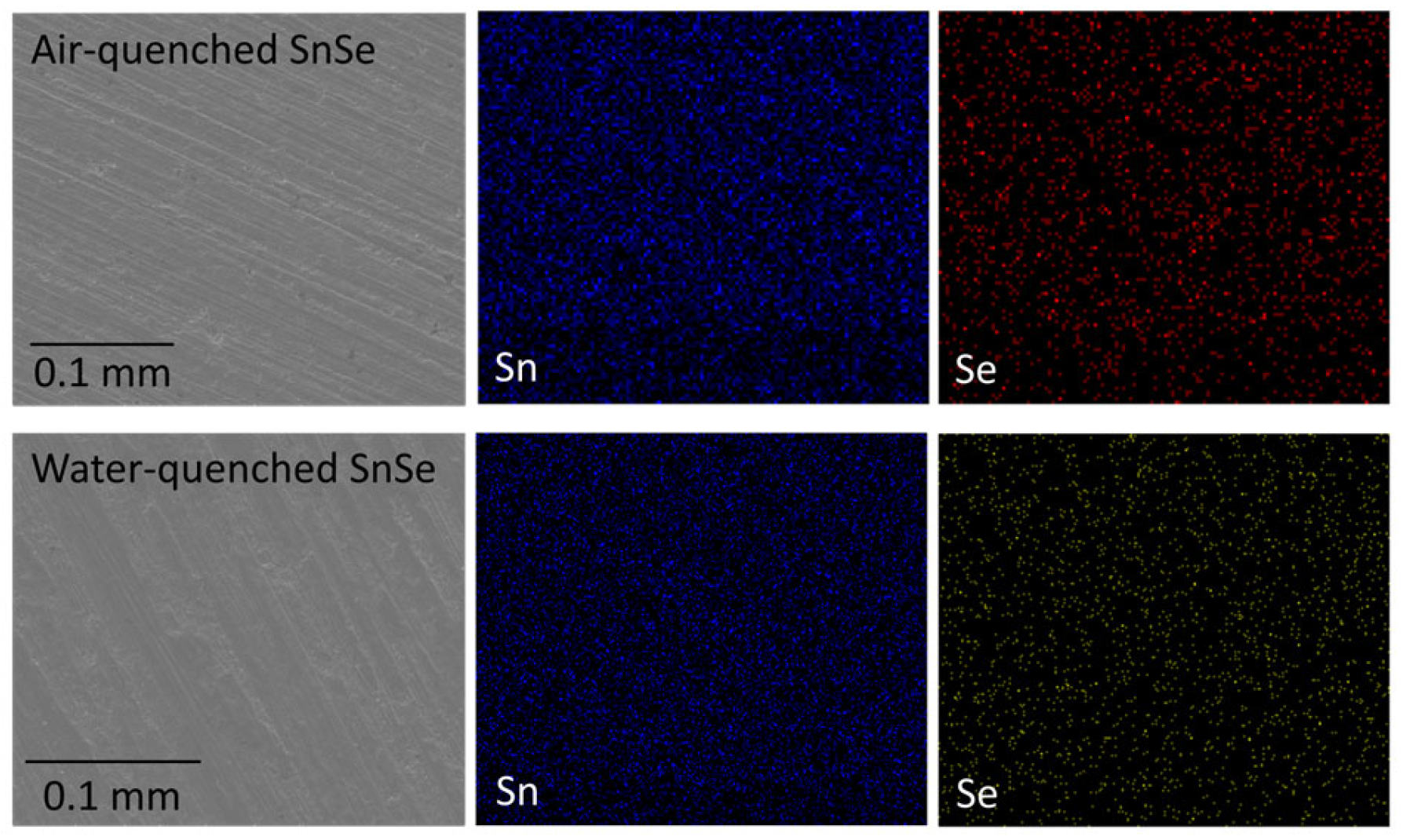
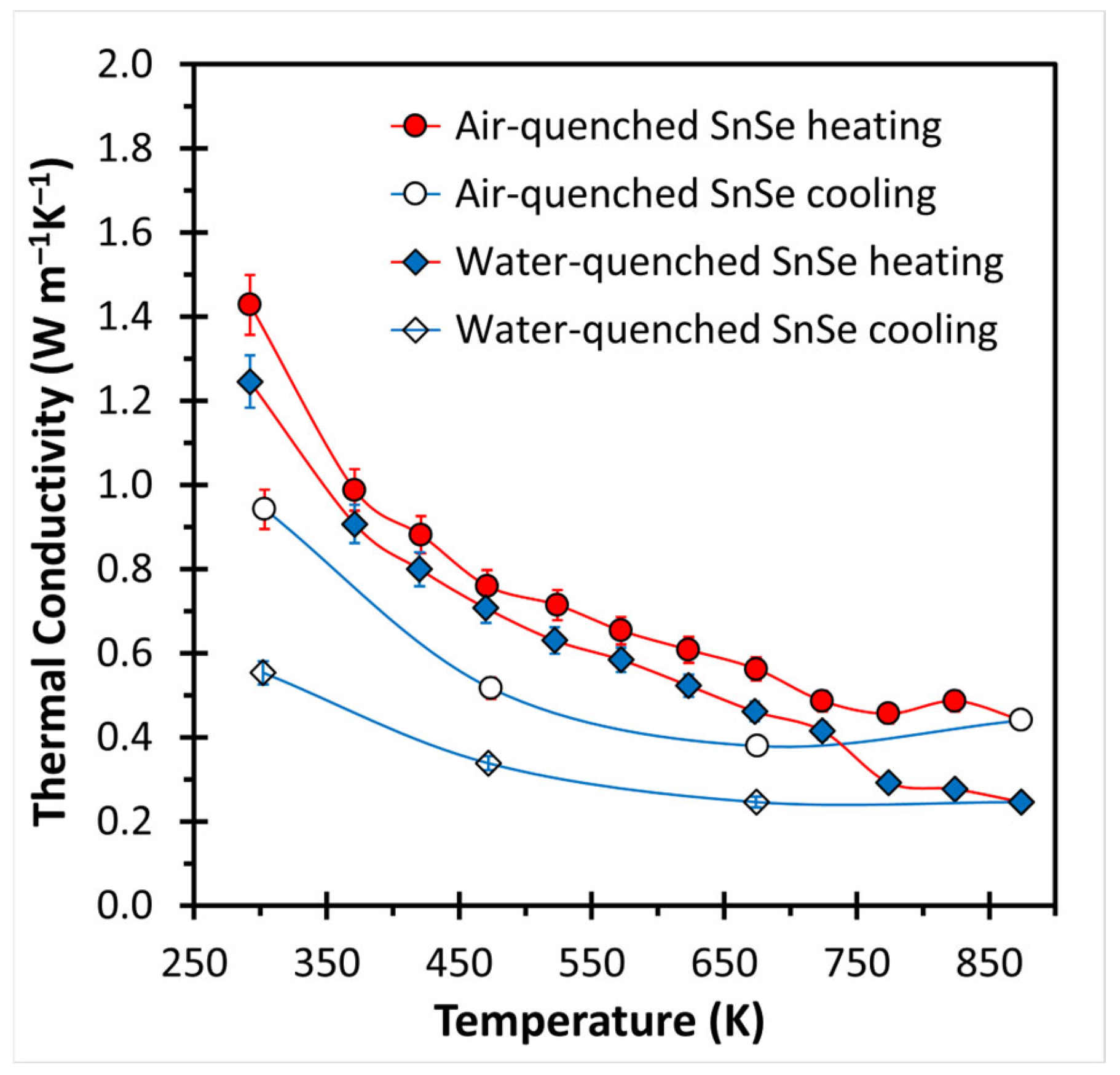
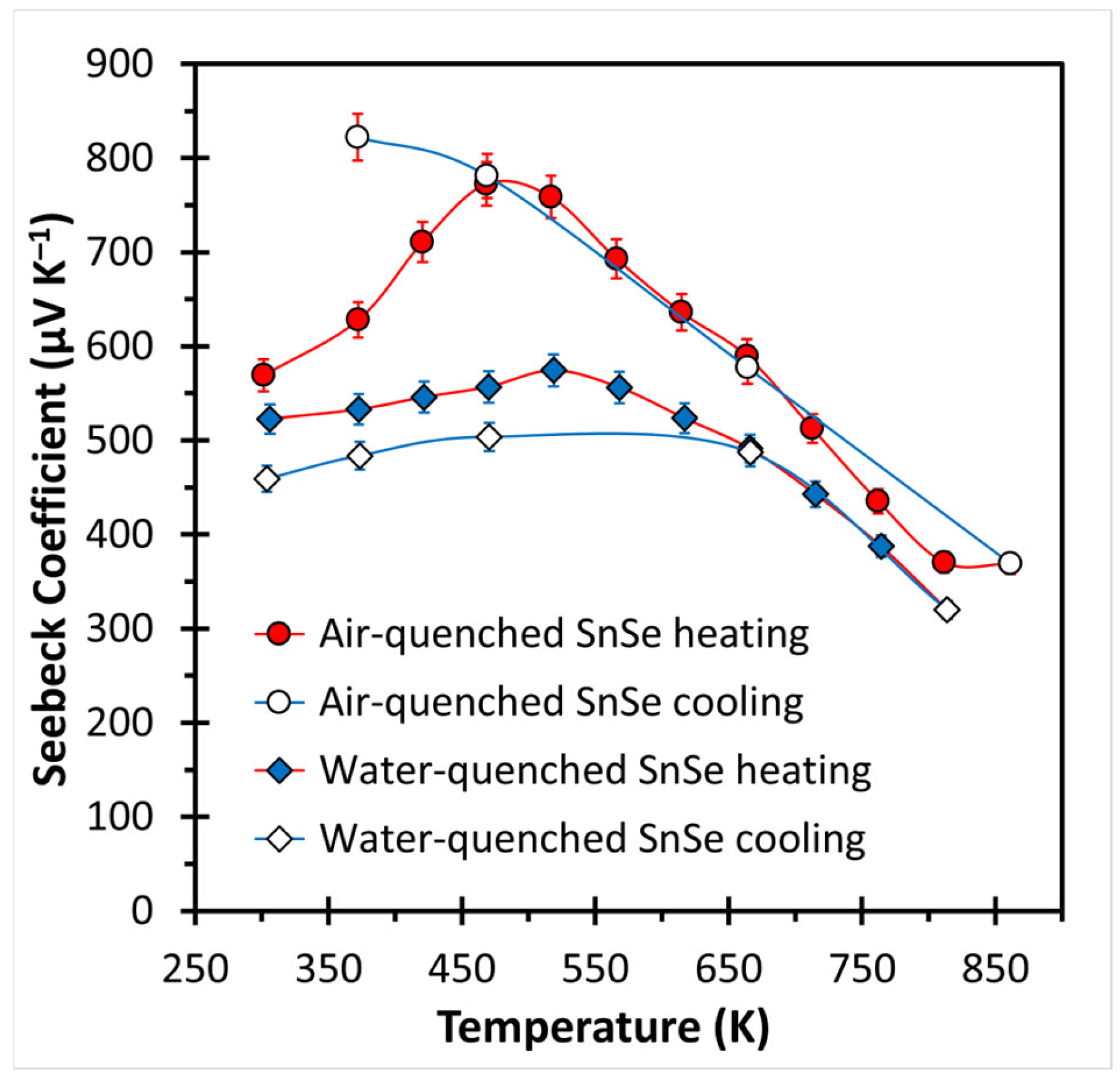
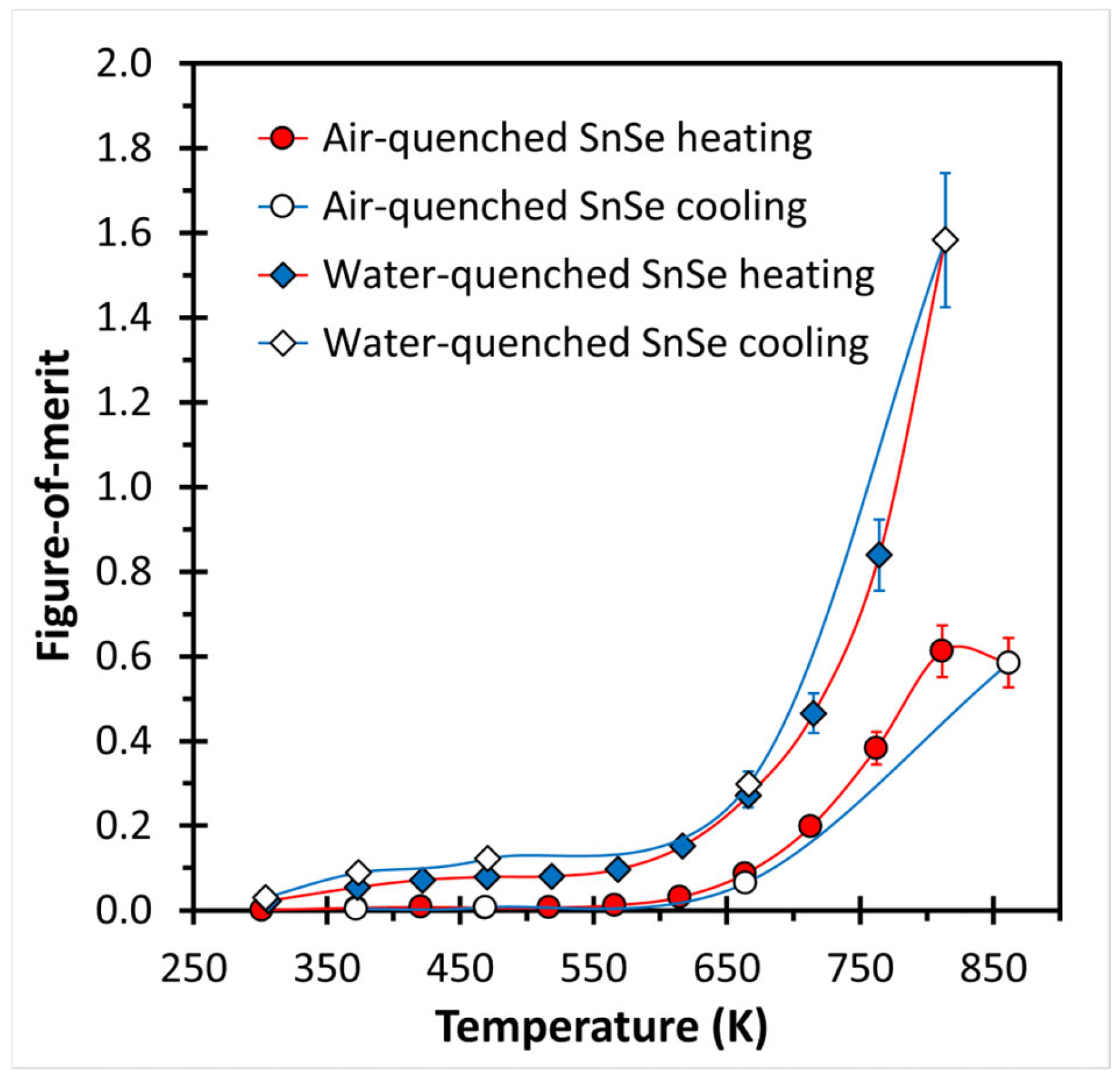
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendricks, T.; Caillat, T.; Mori, T. Keynote Review of Latest Advances in Thermoelectric Generation Materials, Devices, and Technologies 2022. Energies 2022, 15, 7307. [Google Scholar] [CrossRef]
- Furlong, R.R.; Wahlquist, E.J. US Space Missions Using Radioisotope Power Systems. Nucl. News 1999, 42, 26–35. [Google Scholar]
- Yang, J.; Caillat, T. Thermoelectric Materials for Space and Automotive Power Generation. MRS Bull. 2006, 31, 224–229. [Google Scholar] [CrossRef]
- Yang, J.; Stabler, F.R. Automotive Applications of Thermoelectric Materials. J. Electron. Mater. 2009, 38, 1245–1251. [Google Scholar] [CrossRef]
- Matsumoto, M.; Mori, M.; Haraguchi, T.; Ohtani, M.; Kubo, T.; Matsumoto, K.; Matsuda, H. Development of State of the Art Compact and Lightweight Thermoelectric Generator Using Vacuum Space Structure. SAE Int. J. Engines 2015, 8, 1815–1825. [Google Scholar] [CrossRef]
- Orr, B.; Akbarzadeh, A.; Mochizuki, M.; Singh, R. A Review of Car Waste Heat Recovery Systems Utilising Thermoelectric Generators and Heat Pipes. Appl. Therm. Eng. 2016, 101, 490–495. [Google Scholar] [CrossRef]
- Zaia, E.W.; Gordon, M.P.; Yuan, P.; Urban, J.J. Progress and Perspective: Soft Thermoelectric Materials for Wearable and Internet-of-Things Applications. Adv. Electron. Mater. 2019, 5, 1800823. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Park, G.; Park, S.; Khan, S.; Kim, J.; Kim, W. Energy Harvesting Using Thermoelectricity for IoT (Internet of Things) and E-Skin Sensors. J. Phys. Energy 2019, 1, 42001. [Google Scholar] [CrossRef]
- Freer, R.; Powell, A.V. Realising the Potential of Thermoelectric Technology: A Roadmap. J. Mater. Chem. C 2020, 8, 441–463. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as Thermoelectric Materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- González-Barrios, M.; Tabuyo-Martínez, M.; Ávila-Brande, D.; Prado-Gonjal, J. Perspective on Crystal Structures, Synthetic Methods, and New Directions in Thermoelectric Materials. Small Struct. 2024, 5, 2400136. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Salvador, J.R.; Chi, M.; Cho, J.Y.; Wang, H.; Bai, S.; Yang, J.; Zhang, W.; Chen, L. Multiple-Filled Skutterudites: High Thermoelectric Figure of Merit through Separately Optimizing Electrical and Thermal Transports. J. Am. Chem. Soc. 2011, 133, 7837–7846. [Google Scholar] [CrossRef] [PubMed]
- Rogl, G.; Rogl, P.F. Filled Sb-Based Skutterudites from 1996–2022. Crystals 2022, 12, 1843. [Google Scholar] [CrossRef]
- Shi, Y.; Assoud, A.; Ponou, S.; Lidin, S.; Kleinke, H. A New Material with a Composite Crystal Structure Causing Ultralow Thermal Conductivity and Outstanding Thermoelectric Properties: Tl2Ag12Te7+δ. J. Am. Chem. Soc. 2018, 140, 8578–8585. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance Bulk Thermoelectrics with All-Scale Hierarchical Architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Jia, B.; Wu, D.; Xie, L.; Wang, W.; Yu, T.; Li, S.; Wang, Y.; Xu, Y.; Jiang, B.; Chen, Z.; et al. Pseudo-Nanostructure and Trapped-Hole Release Induce High Thermoelectric Performance in PbTe. Science 2024, 384, 81–86. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Poudel, B.; Li, W.; Kang, H.B.; Zhu, H.; Priya, S. Bismuth Telluride Thermoelectrics with 8% Module Efficiency for Waste Heat Recovery Application. iScience 2020, 23, 101340. [Google Scholar] [CrossRef]
- Gayner, C.; Menezes, L.T.; Natanzon, Y.; Kauffmann, Y.; Kleinke, H.; Amouyal, Y. Development of Nanostructured Bi2Te3 with High Thermoelectric Performance by Scalable Synthesis and Microstructure Manipulations. ACS Appl. Mater. Interfaces 2023, 15, 13012–13024. [Google Scholar] [CrossRef]
- Lee, Y.K.; Luo, Z.; Cho, S.P.; Kanatzidis, M.G.; Chung, I. Surface Oxide Removal for Polycrystalline SnSe Reveals Near-Single-Crystal Thermoelectric Performance. Joule 2019, 3, 719–731. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, Y.K.; Yu, Y.; Byun, S.; Luo, Z.Z.; Lee, H.; Ge, B.; Lee, Y.L.; Chen, X.; Lee, J.Y.; et al. Polycrystalline SnSe with a Thermoelectric Figure of Merit Greater than the Single Crystal. Nat. Mater. 2021, 20, 1378–1384. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Chang, C.; Tan, G.; Kanatzidis, M.G. SnSe: A Remarkable New Thermoelectric Material. Energy Environ. Sci. Energy Environ. Sci 2016, 9, 3044–3060. [Google Scholar] [CrossRef]
- Lo, C.-W.T.; Song, S.; Tseng, Y.-C.; Tritt, T.M.; Bogdan, J.; Mozharivskyj, Y. Microstructural Instability and Its Effects on Thermoelectric Properties of SnSe and Na-Doped SnSe. ACS Appl. Mater. Interfaces 2024, 16, 49442–49453. [Google Scholar] [CrossRef] [PubMed]
- Golabek, A.; Barua, N.K.; Menezes, L.T.; Niknam, E.; Yang, Z.; Kleinke, H. Influence of the Densification Process on the Thermoelectric Properties of P-Type SnSe Co-Doped with Na and Cu as Well as Na and Ag. ACS Appl. Energy Mater. 2024, 7, 11852–11858. [Google Scholar] [CrossRef]
- Tang, Y.; Li, D.; Chen, Z.; Deng, S.; Sun, L.; Liu, W.; Shen, L.; Deng, S. Excellent Thermal Stability and Thermoelectric Properties of Pnma-Phase SnSe in Middle Temperature Aerobic Environment. Chinese Phys. B 2018, 27, 118105. [Google Scholar] [CrossRef]
- Chen, Z.G.; Shi, X.; Zhao, L.D.; Zou, J. High-Performance SnSe Thermoelectric Materials: Progress and Future Challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Barua, N.K.; Golabek, A.; Oliynyk, A.O.; Kleinke, H. Experimentally Validated Machine Learning Predictions of Ultralow Thermal Conductivity for SnSe Materials. J. Mater. Chem. C 2023, 11, 11643–11652. [Google Scholar] [CrossRef]
- Wang, H.; Porter, W.D.; Böttner, H.; König, J.; Chen, L.; Bai, S.; Tritt, T.M.; Mayolett, A.; Senawiratne, J.; Smith, C.; et al. Transport Properties of Bulk Thermoelectrics: An International Round-Robin Study, Part II: Thermal Diffusivity, Specific Heat, and Thermal Conductivity. J. Electron. Mater. 2013, 42, 1073–1084. [Google Scholar] [CrossRef]
- Wang, H.; Porter, W.D.; Böttner, H.; König, J.; Chen, L.; Bai, S.; Tritt, T.M.; Mayolet, A.; Senawiratne, J.; Smith, C.; et al. Transport Properties of Bulk Thermoelectrics—An International Round-Robin Study, Part I: Seebeck Coefficient and Electrical Resistivity. J. Electron. Mater. 2013, 42, 654–664. [Google Scholar] [CrossRef]
- Sist, M.; Zhang, J.; Iversen, B.B. Crystal Structure and Phase Transition of Thermoelectric SnSe. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 310–316. [Google Scholar] [CrossRef]
- Shen, J.-J.; Zhu, T.-J.; Zhao, X.-B.; Zhang, S.-N.; Yang, S.-H.; Yin, Z.-Z. Recrystallization Induced in Situ Nanostructures in Bulk Bismuth Antimony Tellurides: A Simple Top down Route and Improved Thermoelectric Properties. Energy Environ. Sci. 2010, 3, 1519–1523. [Google Scholar] [CrossRef]
- Liu, W.D.; Yin, L.C.; Li, L.; Yang, Q.; Wang, D.Z.; Li, M.; Shi, X.L.; Liu, Q.; Bai, Y.; Gentle, I.; et al. Grain Boundary Re-Crystallization and Sub-Nano Regions Leading to High Plateau Figure of Merit for Bi2Te3 Nanoflakes. Energy Environ. Sci. 2023, 16, 5123–5135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golabek, A.; Barua, N.K.; Niknam, E.; Menezes, L.T.; Kleinke, H. Large Improvements in the Thermoelectric Properties of SnSe by Fast Cooling. Materials 2025, 18, 358. https://doi.org/10.3390/ma18020358
Golabek A, Barua NK, Niknam E, Menezes LT, Kleinke H. Large Improvements in the Thermoelectric Properties of SnSe by Fast Cooling. Materials. 2025; 18(2):358. https://doi.org/10.3390/ma18020358
Chicago/Turabian StyleGolabek, Andrew, Nikhil K. Barua, Ehsan Niknam, Luke T. Menezes, and Holger Kleinke. 2025. "Large Improvements in the Thermoelectric Properties of SnSe by Fast Cooling" Materials 18, no. 2: 358. https://doi.org/10.3390/ma18020358
APA StyleGolabek, A., Barua, N. K., Niknam, E., Menezes, L. T., & Kleinke, H. (2025). Large Improvements in the Thermoelectric Properties of SnSe by Fast Cooling. Materials, 18(2), 358. https://doi.org/10.3390/ma18020358






