Tribo-Electrochemical Mechanism of Material Removal Examined for Chemical Mechanical Planarization of Stainless-Steel Using Citrate Buffer as a Complexing Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. CMP Sample and Polishing Slurries
2.2. Instruments and Measurements
2.3. Data Analysis Protocols
3. Results and Discussion
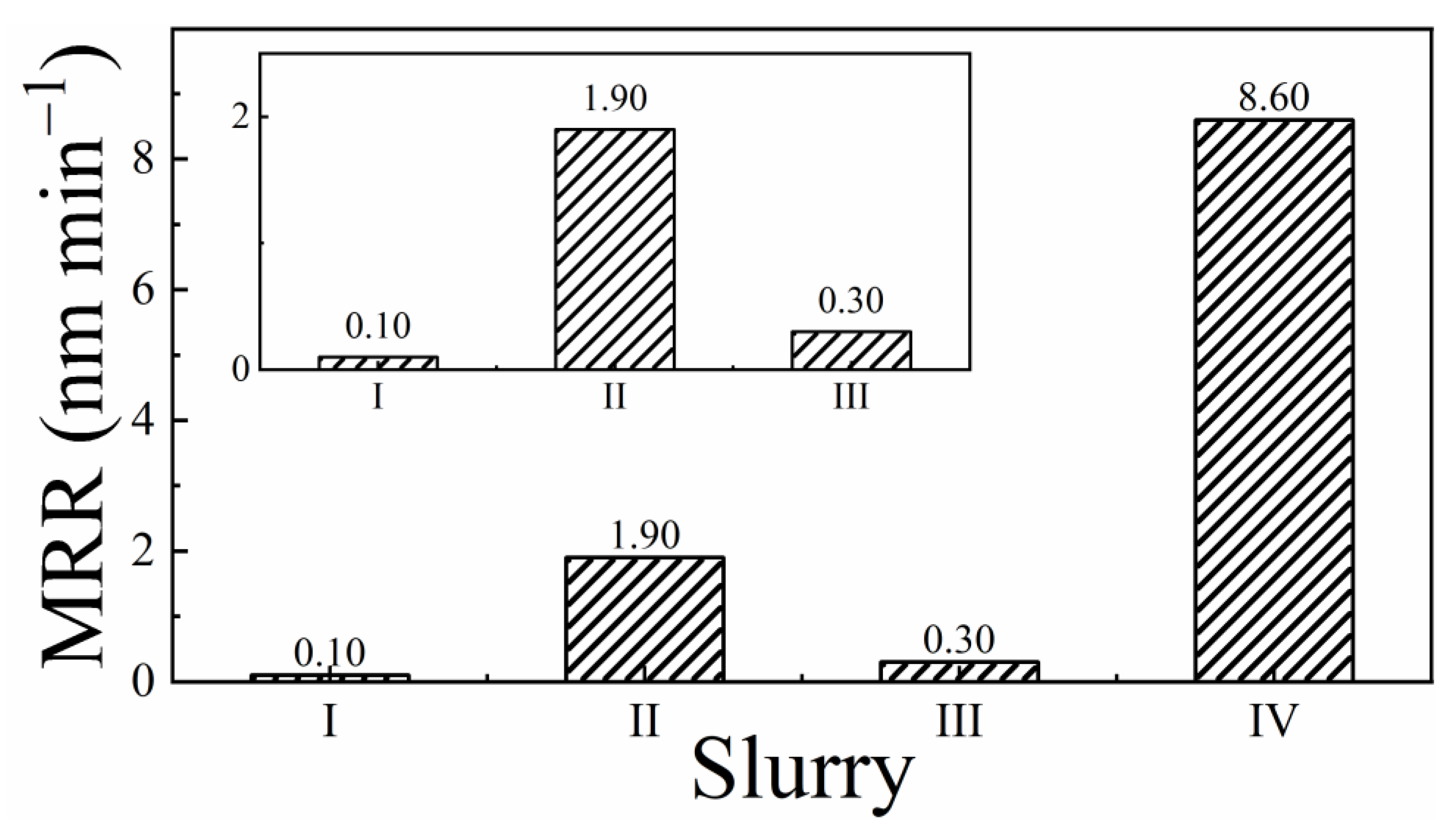
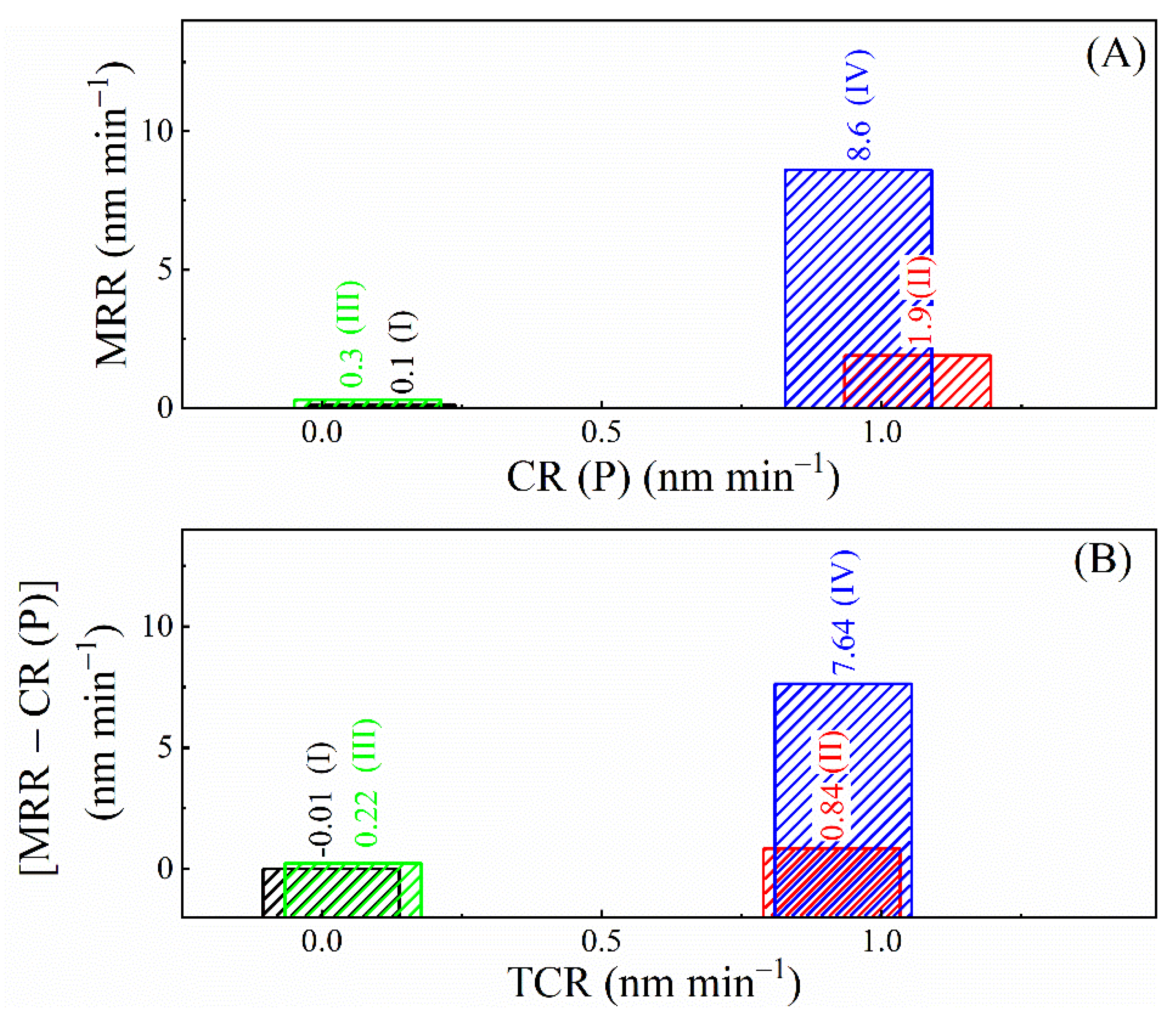
3.1. Results of Material Removal Rate Measurements
3.2. Modes of Material Removal
3.3. Surface Reactions of CMP
3.4. CMP Reactions Probed with Electrochemical Impedance Spectroscopy
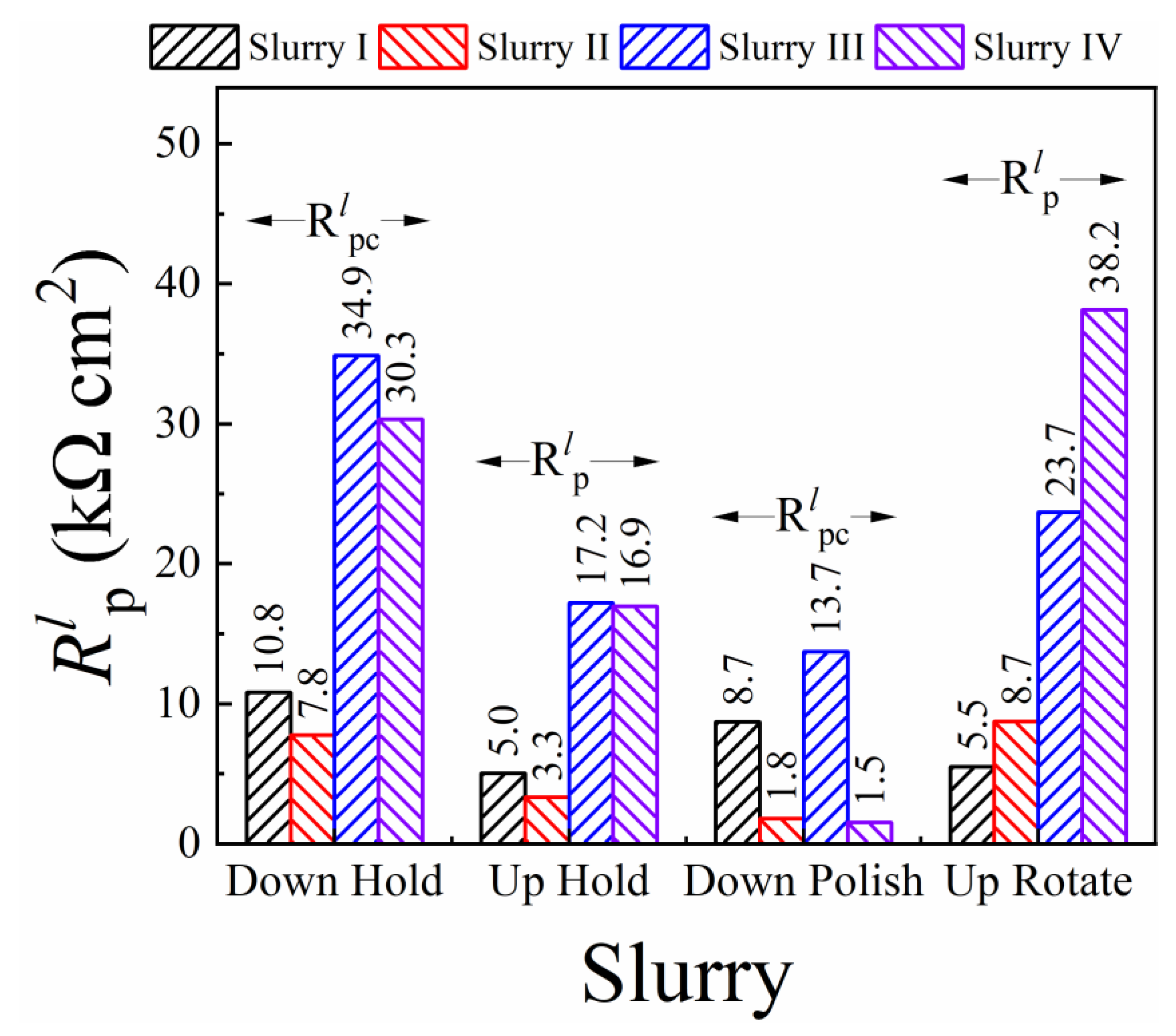
3.5. CMP Mechanism Examined with Linear Polarization Resistance Measurements
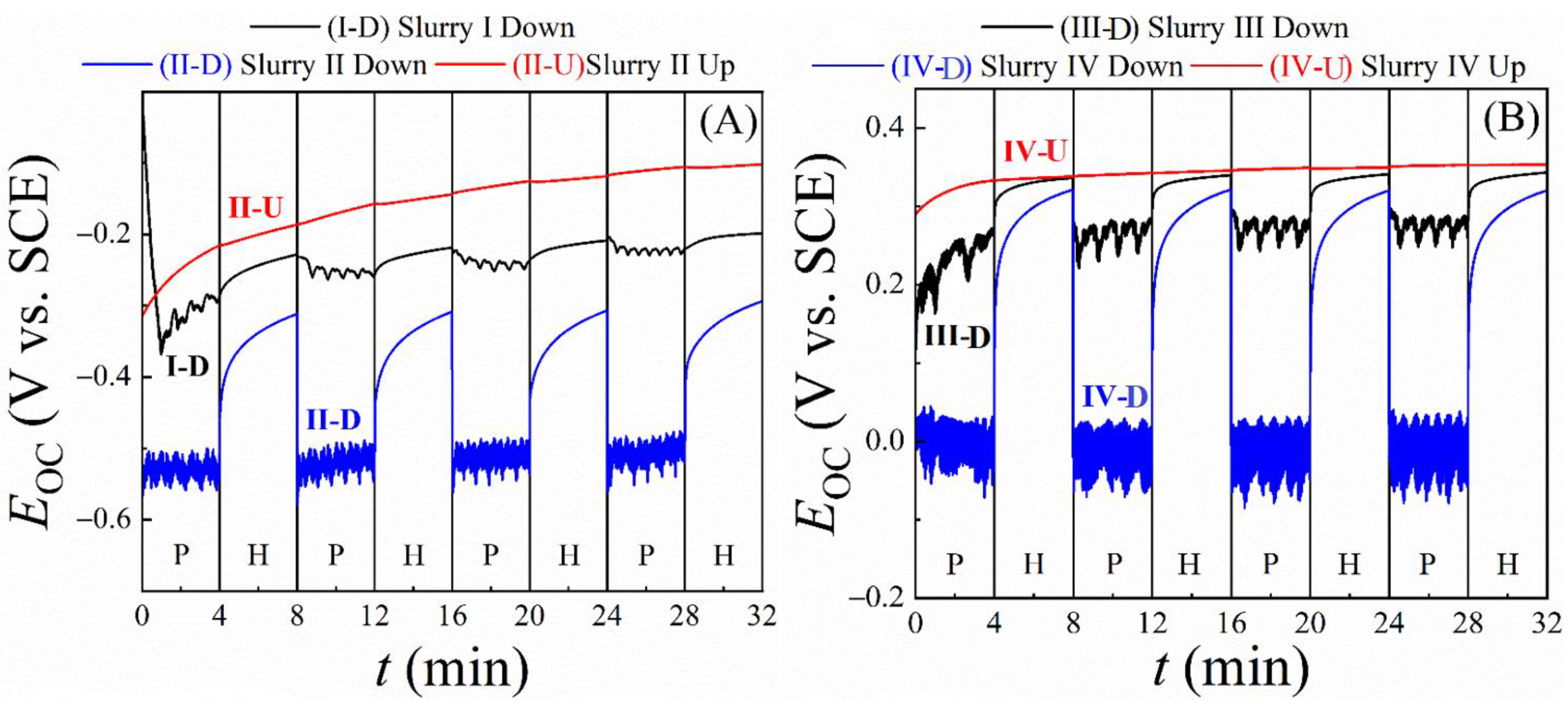
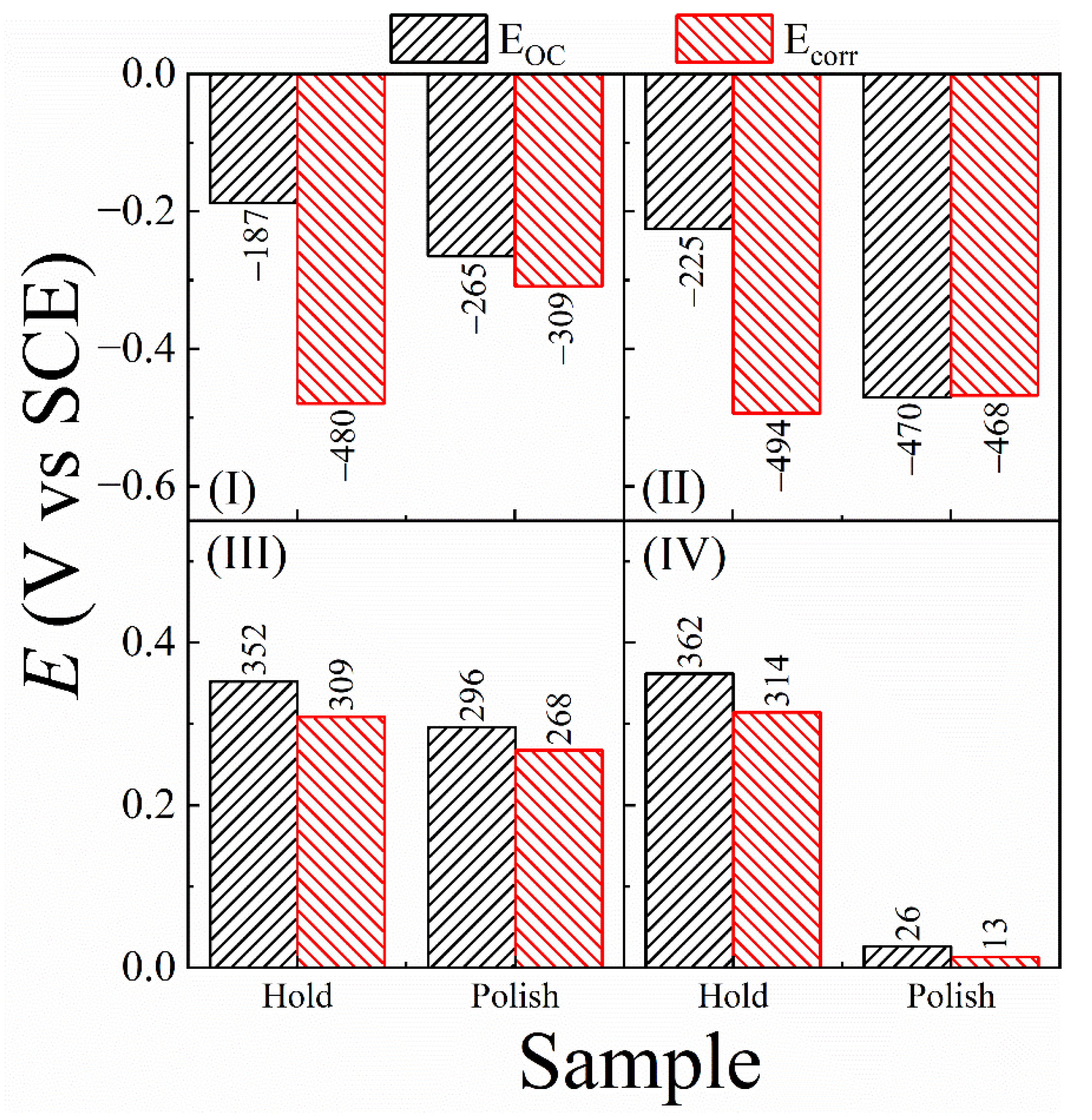
3.6. Probing Tribo-Electrochemistry of CMP Using Intermittent OCP Transient Measurements
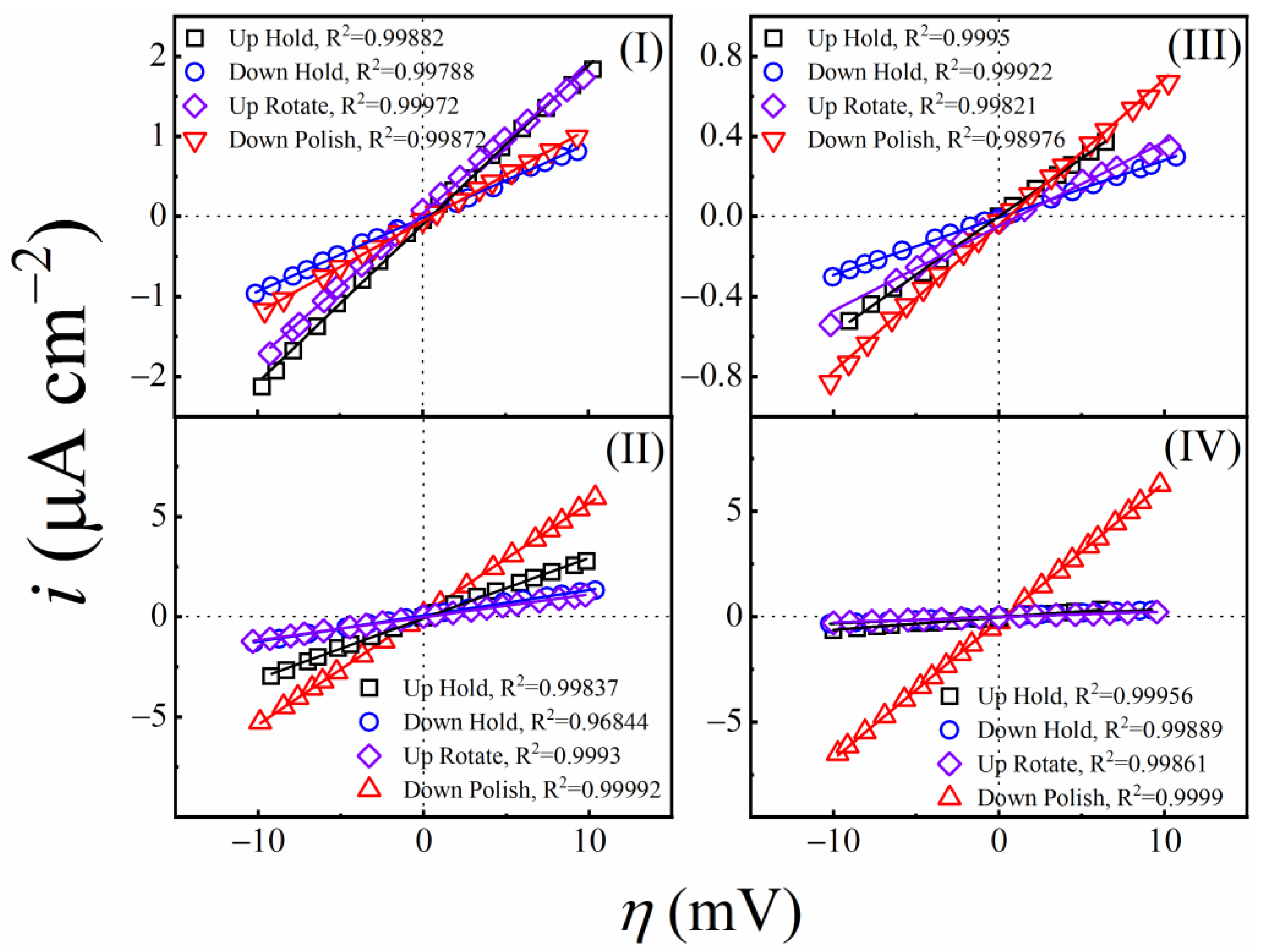
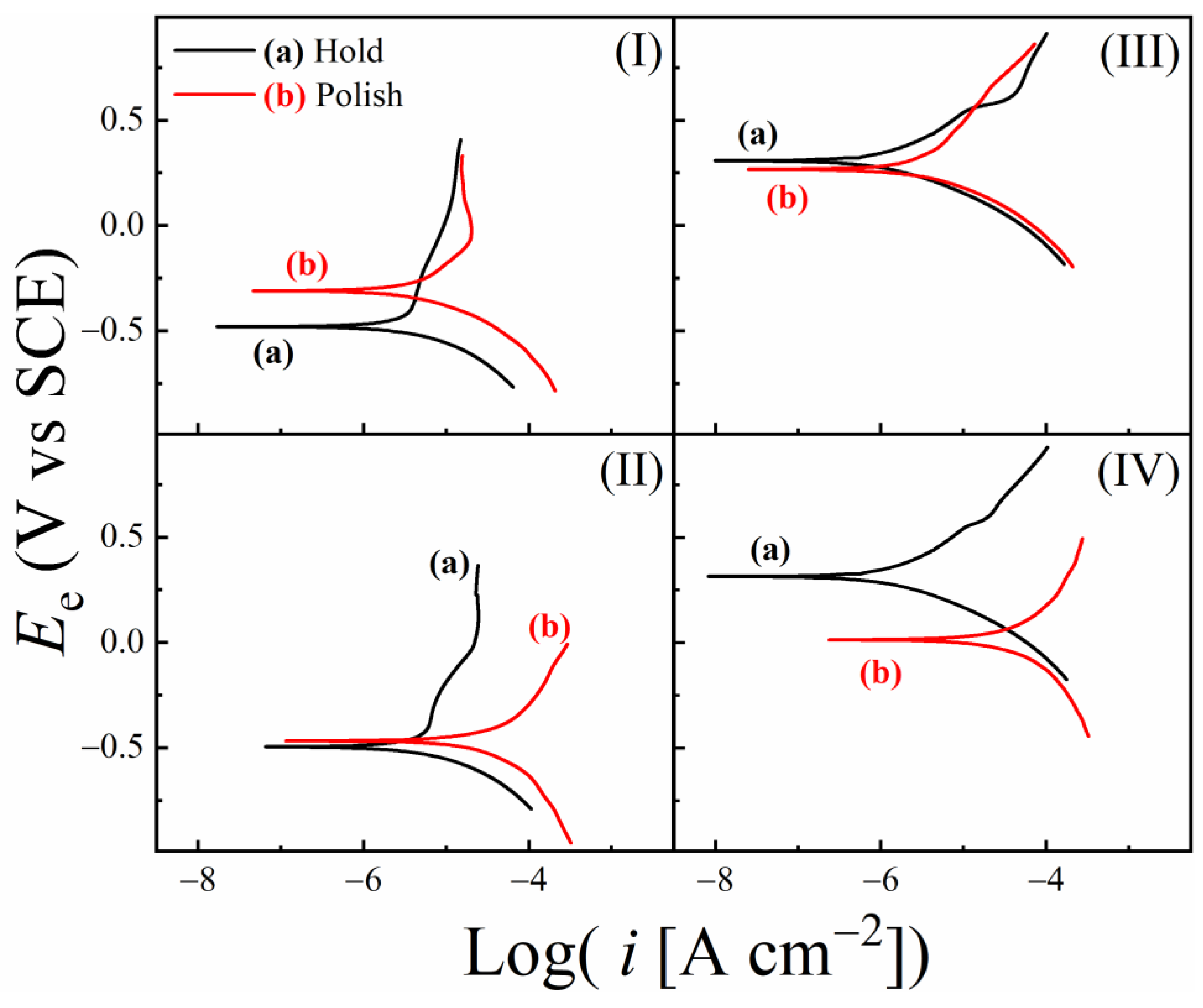
3.7. Results of Tribo-Potentiodynamic Polarization Measurements
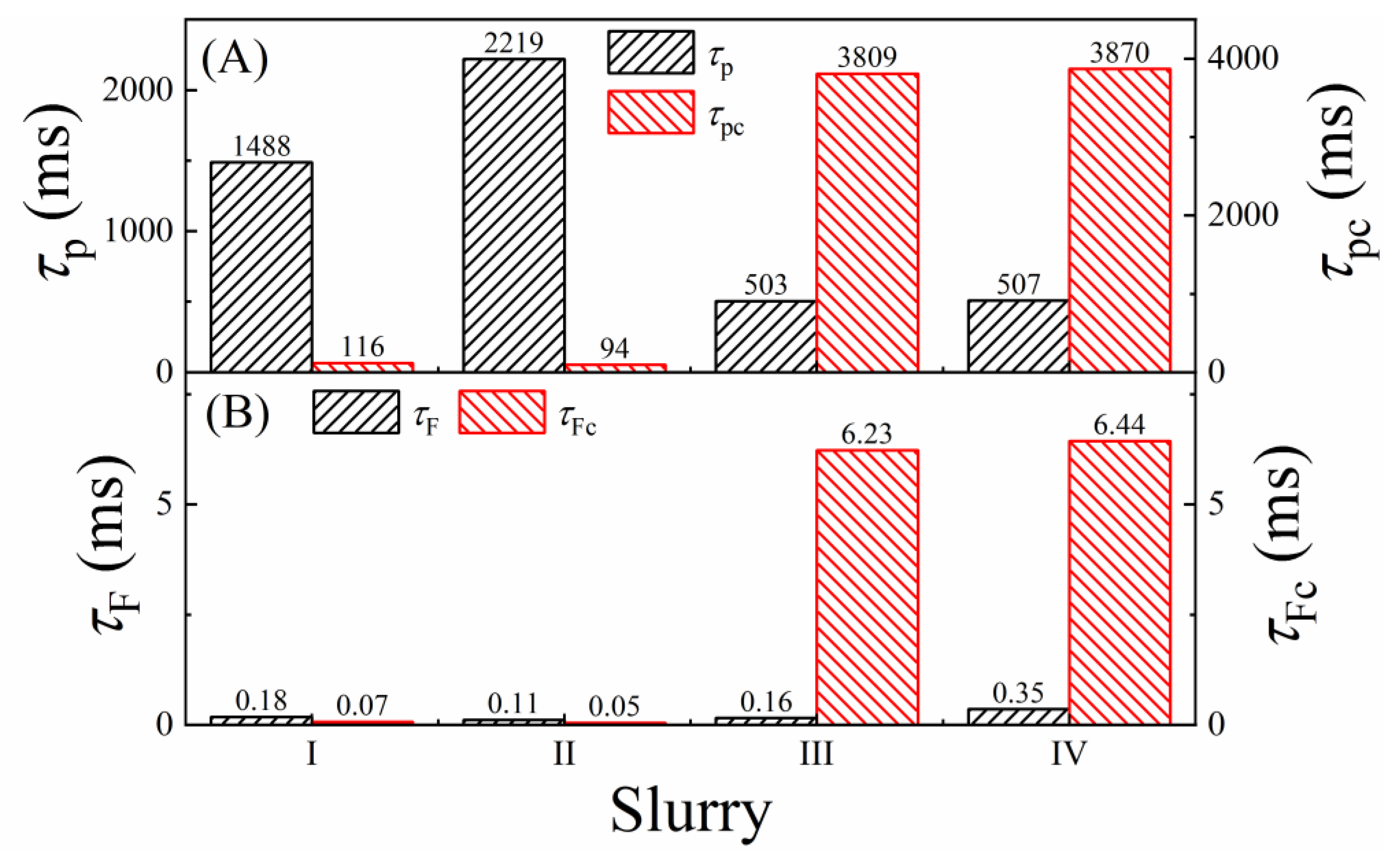
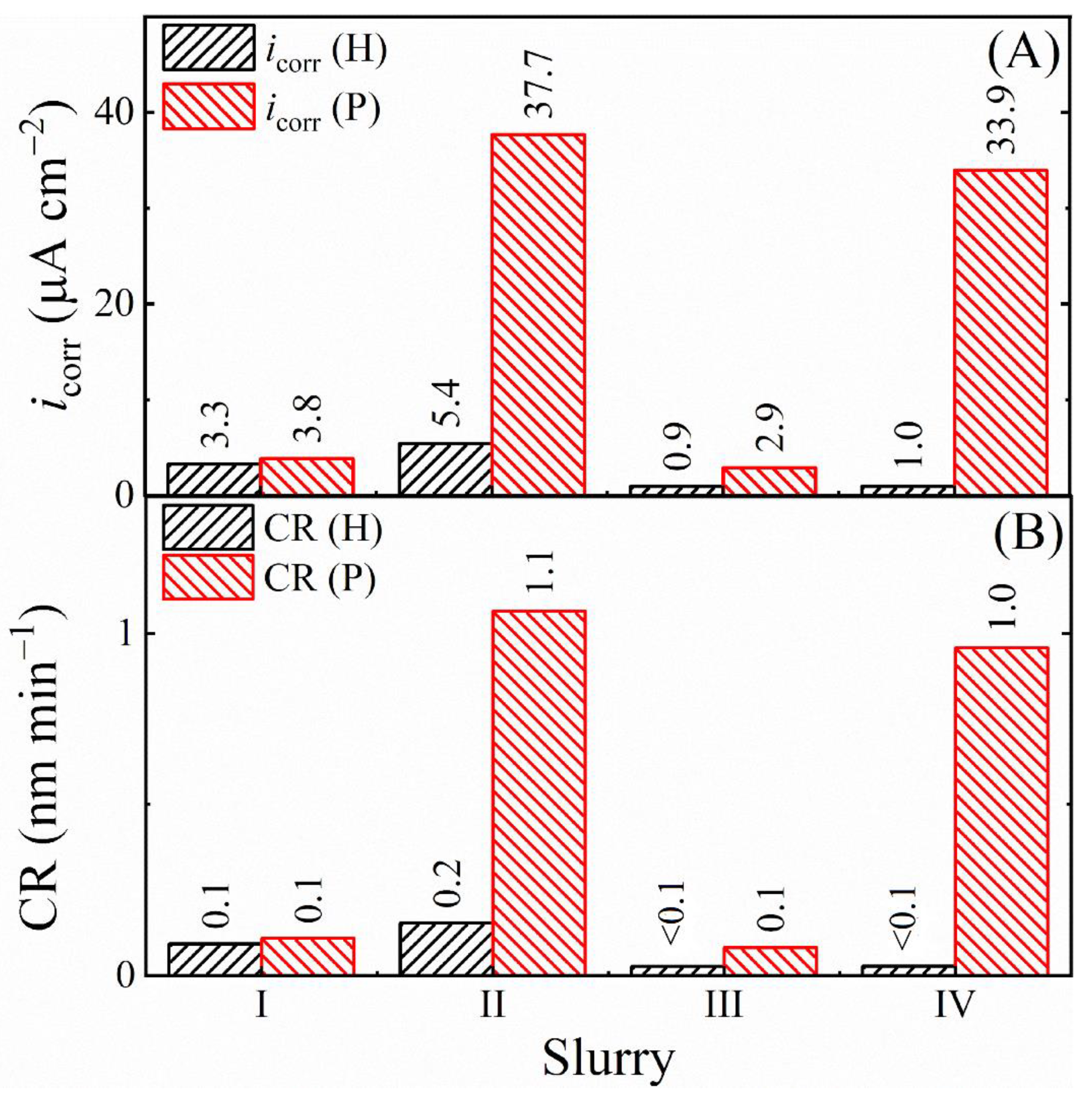
3.8. Comparison of the Rates of Corrosion, Tribo-Corrosion, and Material Removal
3.9. Collective Implications of the Results of Tribo-Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, F.; Herrmann, D.; Powalla, M. Approaches to flexible CIGS thin-film solar cells. Thin Solid Film. 2005, 480, 491–498. [Google Scholar] [CrossRef]
- Hack, M.; Hewitt, R.; Brown, J.J.; Choi, J.W.; Cheon, J.H.; Kim, S.H.; Jang, J. P-11: Analysis of Low Power Consumption AMOLED Displays on Flexible Stainless Steel Substrates. In SID Symposium Digest of Technical Papers; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 210–213. [Google Scholar]
- Mirshojaeian Hosseini, M.J.; Nawrocki, R.A. A review of the progress of thin-film transistors and their technologies for flexible electronics. Micromachines 2021, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.-L.; Qi, J.-F.; He, K.-C.; Bao, N.; Shi, C.-G. Stainless steel sheets as the substrate of disposable electrochemical sensors for analysis of heavy metals or biomolecules. Anal. Chim. Acta 2020, 1124, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, Y.; Yang, Y.; Luo, J. Chemical Mechanical Polishing of Stainless Steel as Solar Cell Substrate. ECS J. Solid State Sci. Technol. 2015, 4, P162–P170. [Google Scholar] [CrossRef]
- Hu, X.; Song, Z.; Liu, W.; Qin, F.; Zhang, Z.; Wang, H. Chemical mechanical polishing of stainless steel foil as flexible substrate. Appl. Surf. Sci. 2012, 258, 5798–5802. [Google Scholar] [CrossRef]
- Qing, L.; Shaokun, C.; Yanan, P.; Hongquan, Q.; Sufang, F.; Jianxiu, S. Chemical mechanical polishing process parameters of 304 stainless steel. Diam. Abras. Eng. 2016, 36, 21–25. [Google Scholar]
- Su, J.X.; Chen, J.P.; Li, Q.; Qin, H.Q. Study on slurry of chemical mechanical polishing 304 stainless steel based on ferric chloride and oxalic acid. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2016; pp. 102–107. [Google Scholar]
- Su, J.X.; Wang, Y.S.; Zhang, X.F.; Liu, H.X.; Wang, Z.K. Selection on Surfactant in Polishing Slurry for Chemical Mechanical Polishing 304 Stainless Steel. Key Eng. Mater. 2020, 866, 125–134. [Google Scholar] [CrossRef]
- Totolin, V.; Göcerler, H.; Rodríguez Ripoll, M.; Jech, M. Tribo-electrochemical study of stainless steel surfaces during chemical–mechanical polishing. Lubr. Sci. 2016, 28, 363–380. [Google Scholar] [CrossRef]
- Rokosz, K.; Solecki, G.; Mori, G.; Fluch, R.; Kapp, M.; Lahtinen, J. Effect of polishing on electrochemical behavior and passive layer composition of different stainless steels. Materials 2020, 13, 3402. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Pak, B.; Kim, D.; Jeong, H.; Lee, H. Electrochemical analysis of the slurry composition for chemical mechanical polishing of flexible stainless-steel substrates. J. Frict. Wear 2017, 38, 482–489. [Google Scholar] [CrossRef]
- Xi, M.; Guo, W.; Xu, G.; Luo, W.; Zhu, Q.; Li, Y.; Yu, H.; Chang, T.; Zhang, Y.; Zhang, Z. Two-step chemical mechanical polishing of stainless steel. ECS J. Solid State Sci. Technol. 2022, 11, 044001. [Google Scholar]
- Zhang, D.; Liu, J.; Chen, Y.; Wang, M.; Ge, X. Investigation on S-136 steel surface planarization by chemical mechanical polishing. Microelectron. Eng. 2015, 134, 47–53. [Google Scholar] [CrossRef]
- Jiang, L.; He, Y.; Luo, J. Chemical mechanical polishing of steel substrate using colloidal silica-based slurries. Appl. Surf. Sci. 2015, 330, 487–495. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Liu, J.; Li, Y.; Cui, X.; Liu, L. A novel process of chemical mechanical polishing for FV520B steel. J. Manuf. Process. 2020, 59, 51–57. [Google Scholar] [CrossRef]
- Su, J.X.; Peng, Y.A.; Liu, Z.H.; Wang, Z.K.; Fu, S.F. Analysis on the action of oxidant in chemical mechanical polishing of 304 ultra-thin stainless steel. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2017; pp. 234–239. [Google Scholar]
- Kim, T.; Lee, H. Preliminary study on fluidized bed chemical mechanical polishing (FB-CMP) process for stainless steel 304 (SS304). Micromachines 2020, 11, 705. [Google Scholar] [CrossRef]
- Weng, J.; Lin, R.; Rong, X. Study on polishing slurry of hydrogen peroxide-oxalic acid in CMP 304 stainless steel. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2020; p. 02002. [Google Scholar]
- Zhong, X.; Chen, Y.; Jiang, L.; Li, W.; Qian, L. Synergistic effect of oxidation and complexation on the material removal of pure iron at the nanoscale. Wear 2023, 524, 204771. [Google Scholar] [CrossRef]
- Rock, S.E.; Crain, D.J.; Zheng, J.P.; Pettit, C.M.; Roy, D. Electrochemical investigation of the surface-modifying roles of guanidine carbonate in chemical mechanical planarization of tantalum. Mater. Chem. Phys. 2011, 129, 1159–1170. [Google Scholar] [CrossRef]
- Stojadinović, J.; Bouvet, D.; Mischler, S. Prediction of removal rates in chemical–mechanical polishing (CMP) using tribocorrosion modeling. J. Bio-Tribo-Corros. 2016, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, M.; Gao, F.; Liang, H. Chemical-mechanical polishing (CMP): A controlled tribocorrosion process. In Tribocorrosion of Passive Metals and Coatings; Landolt, D., Mischler, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 498–518e. [Google Scholar]
- Cheng, J.; Wang, T.; Chai, Z.; Lu, X. Tribocorrosion study of copper during chemical mechanical polishing in potassium periodate-based slurry. Tribol. Lett. 2015, 58, 8. [Google Scholar] [CrossRef]
- Kondo, S.; Ichige, Y.; Otsuka, Y. Electrochemical study on metal corrosion in chemical mechanical planarization process. Jpn. J. Appl. Phys. 2017, 56, 07KA01. [Google Scholar] [CrossRef]
- Gamagedara, K.; Roy, D. Experimental Strategies for Studying Tribo-Electrochemical Aspects of Chemical–Mechanical Planarization. Lubricants 2024, 12, 63. [Google Scholar] [CrossRef]
- Peng, W.; Gao, Y.; Jiang, L.; Liu, J.; Qian, L. Attaining ultra-smooth 18CrNiMo7-6 case hardening steel surfaces with chemical mechanical polishing. Lubricants 2022, 10, 199. [Google Scholar] [CrossRef]
- Miettunen, K.; Ruan, X.; Saukkonen, T.; Halme, J.; Toivola, M.; Guangsheng, H.; Lund, P. Stability of dye solar cells with photoelectrode on metal substrates. J. Electrochem. Soc. 2010, 157, B814. [Google Scholar] [CrossRef]
- Yagan, R.; Basim, G.B. A fundamental approach to electrochemical analyses on chemically modified thin films for barrier CMP optimization. ECS J. Solid State Sci. Technol. 2019, 8, P3118. [Google Scholar] [CrossRef]
- Zantye, P.B.; Kumar, A.; Sikder, A.K. Chemical mechanical planarization for microelectronics applications. Mater. Sci. Eng. R Rep. 2004, 45, 89–220. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, L.; Zhong, X.; Liu, J.; Qin, N.; Qian, L. Exploring the role of− NH 2 functional groups of ethylenediamine in chemical mechanical polishing of GCr15 bearing steel. Friction 2021, 9, 1673–1687. [Google Scholar] [CrossRef]
- Lee, S.-J.; Chen, Y.-H.; Liu, C.P.; Fan, T.J. Electrochemical mechanical polishing of flexible stainless steel substrate for thin-film solar cells. Int. J. Electrochem. Sci. 2013, 8, 6878–6888. [Google Scholar] [CrossRef]
- Ponthiaux, P.; Wenger, F.; Drees, D.; Celis, J.-P. Electrochemical techniques for studying tribocorrosion processes. Wear 2004, 256, 459–468. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Maghraby, Y.R.; Ibrahim, A.H.; Shouier, K.R.; Alturki, A.M.; El-Shabasy, R.M. Role of green chemistry in sustainable corrosion inhibition: A review on recent developments. Mater. Today Sustain. 2022, 20, 100242. [Google Scholar] [CrossRef]
- Parsons, S.; Poyntz-Wright, O.; Kent, A.; McManus, M. Green chemistry for stainless steel corrosion resistance: Life cycle assessment of citric acid versus nitric acid passivation. Mater. Today Sustain. 2019, 3, 100005. [Google Scholar] [CrossRef]
- Hidber, P.C.; Graule, T.J.; Gauckler, L.J. Citric acid—A dispersant for aqueous alumina suspensions. J. Am. Ceram. Soc. 1996, 79, 1857–1867. [Google Scholar] [CrossRef]
- Burek, B.; Bormann, S.; Hollmann, F.; Bloh, J.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Wei, S.; Johnson, C.; Roy, D. Probing the Mechanisms of Metal CMP Using Tribo-Electroanalytical Measurements: Results for a Copper/Malonate System. ECS J. Solid State Sci. Technol. 2021, 10, 034001. [Google Scholar] [CrossRef]
- Johnson, C.; Wei, S.; Roy, D. An Alkaline Slurry Design for Co-Cu CMP Systems Evaluated in the Tribo-Electrochemical Approach. ECS J. Solid State Sci. Technol. 2018, 7, P38–P49. [Google Scholar] [CrossRef]
- Gamagedara, K.; Roy, D. Mechanisms of Chemically Promoted Material Removal Examined for Molybdenum and Copper CMP in Weakly Alkaline Citrate-Based Slurries. Materials 2024, 17, 4905. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Hong, Y.; Devarapalli, V.K.; Roy, D.; Babu, S.V. Synergistic roles of dodecyl sulfate and benzotriazole in enhancing the efficiency of CMP of copper. J. Electrochem. Soc. 2007, 154, H444–H453. [Google Scholar] [CrossRef]
- Kwon, T.-Y.; Ramachandran, M.; Park, J.-G. Scratch formation and its mechanism in chemical mechanical planarization (CMP). Friction 2013, 1, 279–305. [Google Scholar] [CrossRef]
- Zou, C.; Pan, G.; Gong, H.; Xu, L.; Zhou, Y. Analusis of Scratches Generated on GAN Substrates during polishing. In Proceedings of the 2016 STLE Annual Meeting, Las Vegas, NV, USA, 15–16 May 2016. [Google Scholar]
- Liu, H.; Yang, T.; Zhang, Y.; Lu, Y.; Gao, J.; Li, J.; Luo, J. Effect of grain size on tungsten material removal rate during chemical mechanical planarization process. Mater. Sci. Semicond. Process. 2024, 181, 108618. [Google Scholar] [CrossRef]
- Ziomek-Moroz, M.; Miller, A.; Hawk, J.; Cadien, K.; Li, D. An overview of corrosion–wear interaction for planarizing metallic thin films. Wear 2003, 255, 869–874. [Google Scholar] [CrossRef]
- Nolan, L.M.; Cadien, K.C. Chemically enhanced synergistic wear: A copper chemical mechanical polishing case study. Wear 2013, 307, 155–163. [Google Scholar] [CrossRef]
- Kaufman, F.; Thompson, D.; Broadie, R.; Jaso, M.; Guthrie, W.; Pearson, D.; Small, M. Chemical-mechanical polishing for fabricating patterned W metal features as chip interconnects. J. Electrochem. Soc. 1991, 138, 3460. [Google Scholar] [CrossRef]
- Rock, S.; Crain, D.; Pettit, C.; Roy, D. Surface-complex films of guanidine on tantalum nitride electrochemically characterized for applications in chemical mechanical planarization. Thin Solid Film. 2012, 520, 2892–2900. [Google Scholar] [CrossRef]
- Jiang, L.; He, Y.; Li, J.; Luo, J. Passivation Kinetics of 1, 2, 4-Triazole in Copper Chemical Mechanical Polishing. ECS J. Solid State Sci. Technol. 2016, 5, P272–P279. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, H. A wafer-scale material removal rate profile model for copper chemical mechanical planarization. Int. J. Mach. Tools Manuf. 2011, 51, 395–403. [Google Scholar] [CrossRef]
- Xiao, C.; Deng, C.; Zhang, P.; Qian, L.; Kim, S.H. Interplay between solution chemistry and mechanical activation in friction-induced material removal of silicon surface in aqueous solution. Tribol. Int. 2020, 148, 106319. [Google Scholar] [CrossRef]
- Ilie, F.; Minea, I.-L.; Cotici, C.D.; Hristache, A.-F. The Effects of Friction and Temperature in the Chemical–Mechanical Planarization Process. Materials 2023, 16, 2550. [Google Scholar] [CrossRef]
- Li, L.; Li, C.-Q.; Mahmoodian, M. Effect of applied stress on corrosion and mechanical properties of mild steel. J. Mater. Civ. Eng. 2019, 31, 04018375. [Google Scholar] [CrossRef]
- Virtanen, S. Electrochemical theory|corrosion. In Encyclopedia of Electrochemical Power Sources; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Wei, S.; Roy, D. Galvanodynamic probing of tribologically assisted material removal under chemical control: A cobalt/copper case study for application in chemical mechanical planarization. Tribol. Int. 2023, 179, 108185. [Google Scholar] [CrossRef]
- Ernst, F. Metal-oxide interfaces. Mater. Sci. Eng. R Rep. 1995, 14, 97–156. [Google Scholar]
- Esposito, V.; Castelli, I.E. Metastability at Defective Metal Oxide Interfaces and Nanoconfined Structures. Adv. Mater. Interfaces 2020, 7, 1902090. [Google Scholar] [CrossRef]
- Jie, Z.; Zhang, Z.; Susmel, L.; Zhang, L.; Lu, W. Corrosion fatigue mechanisms and evaluation methods of high-strength steel wires: A state-of-the-art review. Fatigue Fract. Eng. Mater. Struct. 2024, 47, 2287–2318. [Google Scholar] [CrossRef]
- Choi, S.; Doyle, F.M.; Dornfeld, D.A. Material Removal Mechanism during Copper Chemical Mechanical Planarization Based on Nano-Scale Material Behavior. ECS J. Solid State Sci. Technol. 2017, 6, P235–P242. [Google Scholar] [CrossRef]
- Perry, S.C.; Gateman, S.; Stephens, L.; Lacasse, R.; Schulz, R.; Mauzeroll, J. Pourbaix diagrams as a simple route to first principles corrosion simulation. J. Electrochem. Soc. 2019, 166, C3186. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for nickel at 25–300 C. Corros. Sci. 1997, 39, 969–980. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for chromium at 25–300 C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Saji, V.S.; Lee, C.W. Molybdenum, molybdenum oxides, and their electrochemistry. ChemSusChem 2012, 5, 1146–1161. [Google Scholar] [CrossRef]
- Vernik Jr, E.D. Simplified procedure for constructing Pourbaix diagrams. Corrosion 1967, 23, 371–373. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for iron at 25–300 C. Corros. Sci. 1996, 38, 2121–2135. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for zinc at 25–300 C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Aksu, S.; Wang, L.; Doyle, F.M. Effect of Hydrogen Peroxide on Oxidation of Copper in CMP Slurries Containing Glycine. J. Electrochem. Soc. 2003, 150, G718–G723. [Google Scholar] [CrossRef]
- Beck, T.R.; Alkire, R.C. Occurrence of salt films during initiation and growth of corrosion pits. J. Electrochem. Soc. 1979, 126, 1662. [Google Scholar] [CrossRef]
- Li, T.; Scully, J.; Frankel, G.S. Localized corrosion: Passive film breakdown vs. pit growth stability: Part IV. The role of salt film in pit growth: A mathematical framework. J. Electrochem. Soc. 2019, 166, C115. [Google Scholar] [CrossRef]
- Russell, P.; Newman, J. Anodic dissolution of iron in acidic sulfate electrolytes: I. Formation and growth of a porous salt film. J. Electrochem. Soc. 1986, 133, 59. [Google Scholar] [CrossRef]
- Li, T.; Frankel, G.S. Repassivation underneath salt film on stainless steel pits. Corros. Sci. 2022, 203, 110353. [Google Scholar] [CrossRef]
- Hunkeler, F.; Królikowski, A.; Böhni, H. A study of the solid salt film on nickel and stainless steel. Electrochim. Acta 1987, 32, 615–620. [Google Scholar] [CrossRef]
- Li, T.; Perea, D.E.; Schreiber, D.K.; Wirth, M.G.; Orren, G.J.; Frankel, G.S. Cryo-based structural characterization and growth model of salt film on metal. Corros. Sci. 2020, 174, 108812. [Google Scholar] [CrossRef]
- Wang, K.; Salasi, M.; Bakhtiari, S.; Iannuzzi, M. On the critical factors for estimating the pit stability product under a salt film. J. Electrochem. Soc. 2021, 168, 061506. [Google Scholar] [CrossRef]
- Isaacs, H. The behavior of resistive layers in the localized corrosion of stainless steel. J. Electrochem. Soc. 1973, 120, 1456. [Google Scholar] [CrossRef]
- Rayment, T.; Davenport, A.J.; Dent, A.J.; Tinnes, J.-P.; Wiltshire, R.J.; Martin, C.; Clark, G.; Quinn, P.; Mosselmans, J.F.W. Characterisation of salt films on dissolving metal surfaces in artificial corrosion pits via in situ synchrotron X-ray diffraction. Electrochem. Commun. 2008, 10, 855–858. [Google Scholar] [CrossRef]
- Lingane, J.J. Polarographic investigation of oxalate, citrate and tartrate complexes of ferric and ferrous iron. J. Am. Chem. Soc. 1946, 68, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Raptopoulou, C.; Terzis, A.; Tangoulis, V.; Mateescu, C.; Salifoglou, A. pH-specific synthesis and spectroscopic, structural, and magnetic studies of a chromium (III)− citrate Species. Aqueous solution speciation of the binary chromium (III)− citrate system. Inorg. Chem. 2007, 46, 2998–3009. [Google Scholar] [CrossRef] [PubMed]
- Hedwig, G.R.; Liddle, J.R.; Reeves, R.D. Complex formation of nickel (II) ions with citric acid in aqueous solution: A potentiometric and spectroscopic study. Aust. J. Chem. 1980, 33, 1685–1693. [Google Scholar] [CrossRef]
- Herting, G.; Wallinder, I.O.; Leygraf, C. A comparison of release rates of Cr, Ni, and Fe from stainless steel alloys and the pure metals exposed to simulated rain events. J. Electrochem. Soc. 2004, 152, B23. [Google Scholar] [CrossRef]
- Sridhar, N.; Dunn, D. In situ study of salt film stability in simulated pits of nickel by Raman and electrochemical impedance spectroscopies. J. Electrochem. Soc. 1997, 144, 4243. [Google Scholar] [CrossRef]
- Trinstancho-Reyes, J.; Sanchez-Carrillo, M.; Sandoval-Jabalera, R.; Orozco-Carmona, V.; Almeraya-Calderon, F.; Chacon-Nava, J.; Gonzalez-Rodriguez, J.; Martinez-Villafane, A. Electrochemical impedance spectroscopy investigation of alloy inconel 718 in molten salts at high temperature. Int. J. Electrochem. Sci. 2011, 6, 419–431. [Google Scholar] [CrossRef]
- Pan, T.; Lin, Y.; Hu, J.; Zeng, C. Electromechanical-Impedance-Spectroscopy Study of Corrosion of Fe and Ni with Molten ZnCl2-KCl Coating in Air at 673K. High Temp. Mater. Process. 2009, 28, 233–244. [Google Scholar] [CrossRef]
- Qiu, J.; Macdonald, D.D.; Schoell, R.; Han, J.; Mastromarino, S.; Scully, J.R.; Kaoumi, D.; Hosemann, P. Electrochemical study of the dissolution of oxide films grown on type 316L stainless steel in molten fluoride salt. Corros. Sci. 2021, 186, 109457. [Google Scholar] [CrossRef]
- Wu, Y.M. Measurements of Double-Layer Capacitance at the Preoxidized Ni/Fused Na2 SO 4 Interface. J. Electrochem. Soc. 1991, 138, 2342. [Google Scholar] [CrossRef]
- Pan, T.; Xue, W.; Chen, D.; Hu, J. Electrochemical-Impedance-Spectroscopy study of corrosion of the alloy NF616 with molten ZnCl2-KCl coating in air at 673 K. High Temp. Mater. Process. 2011, 30, 71–75. [Google Scholar] [CrossRef]
- Thakurta, D.G.; Borst, C.L.; Schwendeman, D.W.; Gutmann, R.J.; Gill, W.N. Pad porosity, compressibility and slurry delivery effects in chemical-mechanical planarization: Modeling and experiments. Thin Solid Film. 2000, 366, 181–190. [Google Scholar] [CrossRef]
- Shi, X.; Simpson, D.; Roy, D. Tribo-electrochemical characterization of Ru, Ta and Cu CMP systems using percarbonate based solutions. ECS J. Solid State Sci. Technol. 2015, 4, P5058. [Google Scholar] [CrossRef]
- McGrath, J.; Davis, C. Polishing pad surface characterisation in chemical mechanical planarisation. J. Mater. Process. Technol. 2004, 153–154, 666–673. [Google Scholar] [CrossRef]
- Turk, M.C.; Walters, M.J.; Roy, D. Experimental considerations for using electrochemical impedance spectroscopy to study chemical mechanical planarization systems. Electrochim. Acta 2017, 224, 355–368. [Google Scholar] [CrossRef]
- Grimm, R.; West, A.; Landolt, D. AC impedance study of anodically formed salt films on iron in chloride solution. J. Electrochem. Soc. 1992, 139, 1622. [Google Scholar] [CrossRef]
- Dell’Oca, C.; Pulfrey, D.; Young, L. Anodic oxide films. In Physics of Thin Films; Elsevier: Amsterdam, The Netherlands, 1971; Volume 6, pp. 1–79. [Google Scholar]
- Ateya, B.; Pickering, H. Effects of ionic migration on the concentrations and mass transfer rate in the diffusion layer of dissolving metals. J. Appl. Electrochem. 1981, 11, 453–461. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Zhang, Z.; Jin, L.; Yang, W.; He, Y.; Ren, L.; Wang, H. Enhanced surface hydroxyl groups by using hydrogen peroxide on hollow tubular alumina for removing fluoride. Microporous Mesoporous Mater. 2020, 297, 110051. [Google Scholar] [CrossRef]
- Orabi, M. Modified description for deposition of particles in small volumes. Phys. Scr. 2023, 98, 095304. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization resistance method for determination of instantaneous corrosion rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Espinoza-Jara, A.; Walczak, M.; Molina, N.; Jahn, W.; Brevis, W. Erosion under turbulent flow: A CFD-based simulation of near-wall turbulent impacts with experimental validation. Eng. Appl. Comput. Fluid Mech. 2022, 16, 1526–1545. [Google Scholar] [CrossRef]
- Poulson, B. Advances in understanding hydrodynamic effects on corrosion. Corros. Sci. 1993, 35, 655–665. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Fang, Y.; Raghavan, S. Electrochemical Investigations during the Abrasion of Aluminum/Titanium Thin-Film Stacks in Iodate-Based Slurry. J. Electrochem. Soc. 2004, 151, G878–G881. [Google Scholar] [CrossRef]
- Lee, W.-J.; Park, H.-S. Development of novel process for Ru CMP using ceric ammonium nitrate (CAN)-containing nitric acid. Appl. Surf. Sci. 2004, 228, 410–417. [Google Scholar] [CrossRef]
- Jemmely, P.; Mischler, S.; Landolt, D. Electrochemical modeling of passivation phenomena in tribocorrosion. Wear 2000, 237, 63–76. [Google Scholar] [CrossRef]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Sato, N.; Noda, T. Ion migration in anodic barrier oxide films on iron in acidic phosphate solutions. Electrochim. Acta 1977, 22, 839–843. [Google Scholar] [CrossRef]
- Economy, G.; Speiser, R.; Beck, F.; Fontana, M. Anodic Polarization Behavior of Iron-Nickel Alloys in Sulfuric Acid Solutions. J. Electrochem. Soc. 1961, 108, 337. [Google Scholar] [CrossRef]
- Nakajima, N.; Maekawa, T. Anodic Behaviors of Iron and Its Oxides in Dilute Sulfuric Acid and Akaline Solutions. Trans. Jpn. Inst. Met. 1966, 7, 280–285. [Google Scholar] [CrossRef]
- Vago, E.; Calvo, E.; Stratmann, M. Electrocatalysis of oxygen reduction at well-defined iron oxide electrodes. Electrochim. Acta 1994, 39, 1655–1659. [Google Scholar] [CrossRef]
- Pryor, M.; Cohen, M. The inhibition of the corrosion of iron by some anodic inhibitors. J. Electrochem. Soc. 1953, 100, 203. [Google Scholar] [CrossRef]
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2004. [CrossRef]
- Stein, D.J.; Hetherington, D.; Guilinger, T.; Cecchi, J.L. In Situ Electrochemical Investigation of Tungsten Electrochemical Behavior during Chemical Mechanical Polishing. J. Electrochem. Soc. 1998, 145, 3190–3196. [Google Scholar] [CrossRef]
- Cui, H.; Park, J.-H.; Park, J.-G. Study of Ruthenium Oxides Species on Ruthenium Chemical Mechanical Planarization Using Periodate-Based Slurry. J. Electrochem. Soc. 2012, 159, H335–H341. [Google Scholar] [CrossRef]
- Lee, H. Environmental impact of concentration of slurry components in thick copper CMP. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 13–18. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, C.; Zheng, J.; Sun, P.; Jiang, L.; Qian, L. Preparing an ultra-smooth TaW alloy surface with chemical mechanical polishing via controlling galvanic corrosion. J. Appl. Electrochem. 2024, 54, 839–850. [Google Scholar] [CrossRef]
- Quang, N.M.; Quan, N.N.; Mai, N.T.; Thanh, L.T.P.; Tung, N.T.; Tan, T.N.; Hai, H.T.; Trinh, N.D. A new environmentally friendly chemical mechanical polishing method applied for surface finishing Ti-6Al-4V alloy. J. Mach. Eng. 2023, 23, 64–76. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhang, P.; He, Y.; Yu, D.; Xiao, C. Close-to-Atomic Precision Surface Achieved through a Neutral and Eco-friendly Slurry in Chemical Mechanical Polishing of TC4 Alloy. ACS Sustain. Chem. Eng. 2024, 12, 16082–16090. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Liu, C.; Wang, T.; Dun, J. Material characteristics and chemical–mechanical polishing of aluminum alloy thin films. Thin Solid Film. 1998, 332, 397–403. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhao, Y.; Min, P.; Qi, F.; Liu, X.; Zhao, D. Chemical mechanical planarization of Al alloy in alkaline slurry at low down pressure. J. Mater. Sci. Mater. Electron. 2017, 28, 3364–3372. [Google Scholar] [CrossRef]
- Li, T.; Sun, H.; Wang, D.; Huang, J.; Li, D.; Lei, F.; Sun, D. High-performance chemical mechanical polishing slurry for aluminum alloy using hybrid abrasives of zirconium phosphate and alumina. Appl. Surf. Sci. 2021, 537, 147859. [Google Scholar] [CrossRef]

| Element | Fe | Cr | Ni | Mo | Mn | Si | Cu | Ti | S | N | C | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median % | 65.9 | 17.5 | 12.5 | 1.50 | 1.00 | 0.50 | 0.50 | 0.35 | 0.18 | 0.05 | 0.04 | 0.02 |
| Slurry | Composition | RS (Ω) |
|---|---|---|
| I | 0.1 M KNO3 + 0.1 M CB (Ref) | 59.1 |
| II | Ref + 3 wt% 0.3 μm Alumina | 65.9 |
| III | Ref + 1 wt% H2O2 | 75.8 |
| IV | Ref + 1 wt% H2O2 + 3 wt% 0.3 μm Alumina | 66.6 |
| Configuration of Sample Setup | System Designation |
|---|---|
| (a) SS sample surface lifted by 1 mm above the polishing pad maintaining sample surface parallel to the pad surface. Both the sample and the pad are held stationary. | Up-Hold |
| (b) SS sample surface lifted by 1 mm above the polishing pad maintaining sample surface parallel to the pad surface. Both the sample and the pad are rotated at a common angular speed of 95 rpm. | Up-Rotate |
| (c) SS sample surface pressed down onto the polishing pad at a pressure of 0.014 MPa, while both the sample and the pad are held stationary. | Down-Hold |
| (d) SS sample surface pressed down onto the polishing pad at a pressure of 0.014 MPa, while both the sample and the pad are rotated at a common angular speed of 95 rpm. | Down-Polish |
| EEC Parameter * | CMP Systems (Up, Down) | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Rs (Ω), Rsc (Ω cm2) | 59.1, 1704 | 65.9, 1254 | 75.8, 1210 | 66.6, 1091 |
| CoFc (μF cm−2) | 82.1 | 79.1 | 1.9 | 2.3 |
| RoFc (kΩ cm2) | 60.7 | 19.1 | 0.21 | 0.17 |
| τoFc (ms) | 4983 | 1511 | 0.40 | 0.39 |
| CF, CFc (μF cm−2) | 8.94, 0.80 | 5.28, 0.81 | 3.74, 5.41 | 5.19, 5.87 |
| RF, RFc (Ω cm2) | 20.5, 87.1 | 21.7, 59.1 | 42.1, 1152 | 68.4, 1097 |
| Yd, Ydc (μS sd cm−2) | 24.0, 115.4 | 15.2, 139.5 | 6.2, 21.2 | 6.4, 21.8 |
| d, dc | 1, 0.54 | 1, 0.54 | 1, 0.85 | 1, 0.85 |
| Ra (Ω cm2) | 66.5 | 63.9 | 87.0 | 88.9 |
| Ya (μF cm−2) | 83.3 | 48.3 | 14.1 | 13.4 |
| Ca (μF cm−2) | 26.63 | 16.05 | 3.93 | 2.01 |
| a | 0.82 | 0.84 | 0.82 | 0.78 |
| τa (ms) | 1.77 | 1.02 | 0.34 | 0.18 |
| Rp, Rpc (kΩ cm2) | 62.0, 2.71 | 146, 2.00 | 81.2, 147 | 79.2, 144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santefort, D.R.; Gamagedara, K.U.; Roy, D. Tribo-Electrochemical Mechanism of Material Removal Examined for Chemical Mechanical Planarization of Stainless-Steel Using Citrate Buffer as a Complexing Agent. Materials 2025, 18, 317. https://doi.org/10.3390/ma18020317
Santefort DR, Gamagedara KU, Roy D. Tribo-Electrochemical Mechanism of Material Removal Examined for Chemical Mechanical Planarization of Stainless-Steel Using Citrate Buffer as a Complexing Agent. Materials. 2025; 18(2):317. https://doi.org/10.3390/ma18020317
Chicago/Turabian StyleSantefort, David R., Kassapa U. Gamagedara, and Dipankar Roy. 2025. "Tribo-Electrochemical Mechanism of Material Removal Examined for Chemical Mechanical Planarization of Stainless-Steel Using Citrate Buffer as a Complexing Agent" Materials 18, no. 2: 317. https://doi.org/10.3390/ma18020317
APA StyleSantefort, D. R., Gamagedara, K. U., & Roy, D. (2025). Tribo-Electrochemical Mechanism of Material Removal Examined for Chemical Mechanical Planarization of Stainless-Steel Using Citrate Buffer as a Complexing Agent. Materials, 18(2), 317. https://doi.org/10.3390/ma18020317






