Effect of B/N Doping on Enhanced Hydrogen Storage in Transition Metal-Modified Graphene: A First-Principles DFT Study
Abstract
1. Introduction
2. Simulation Methods
3. Results and Discussion
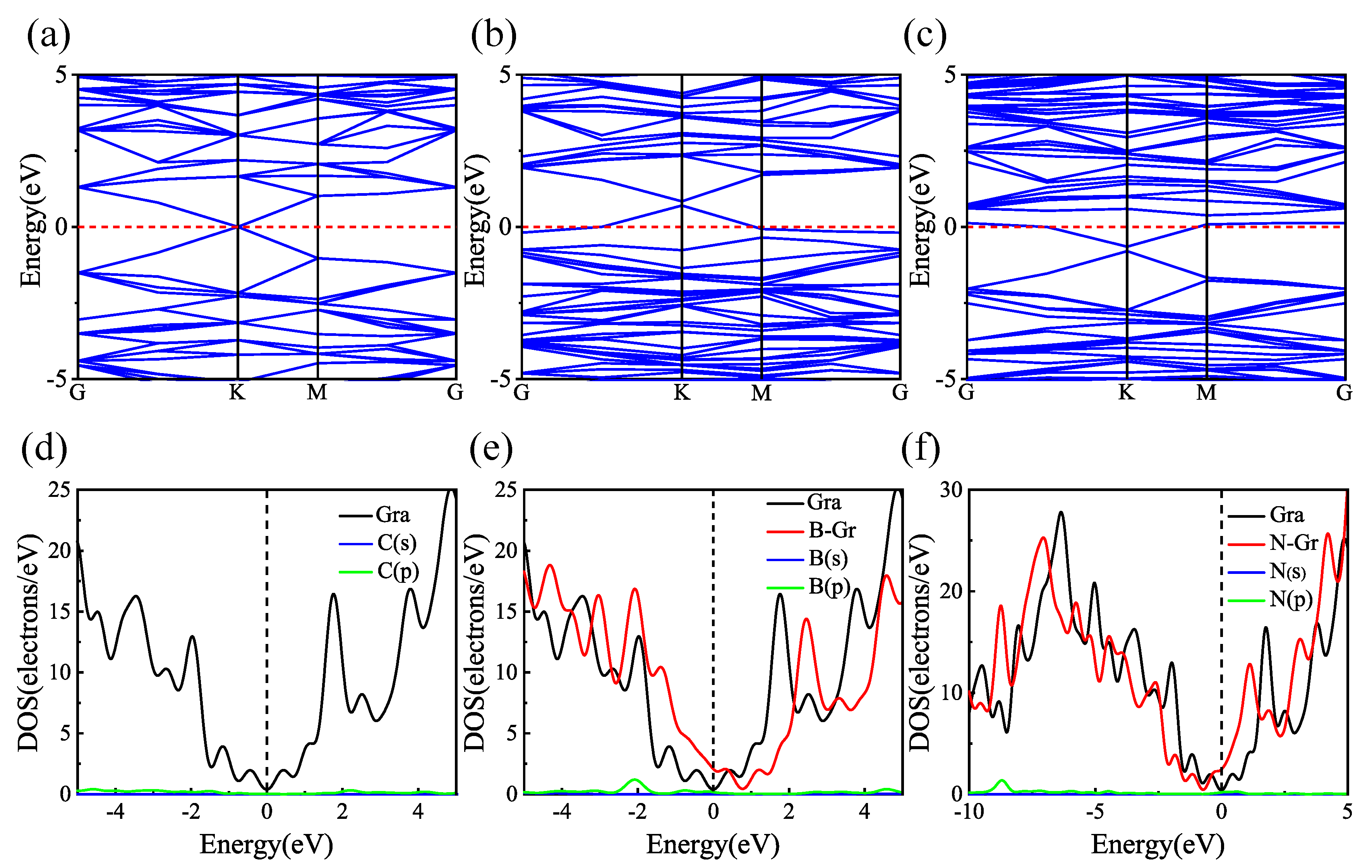
3.1. Sc, Ti, and V Atoms Modify the Structure of Gr, BGr, and NGr
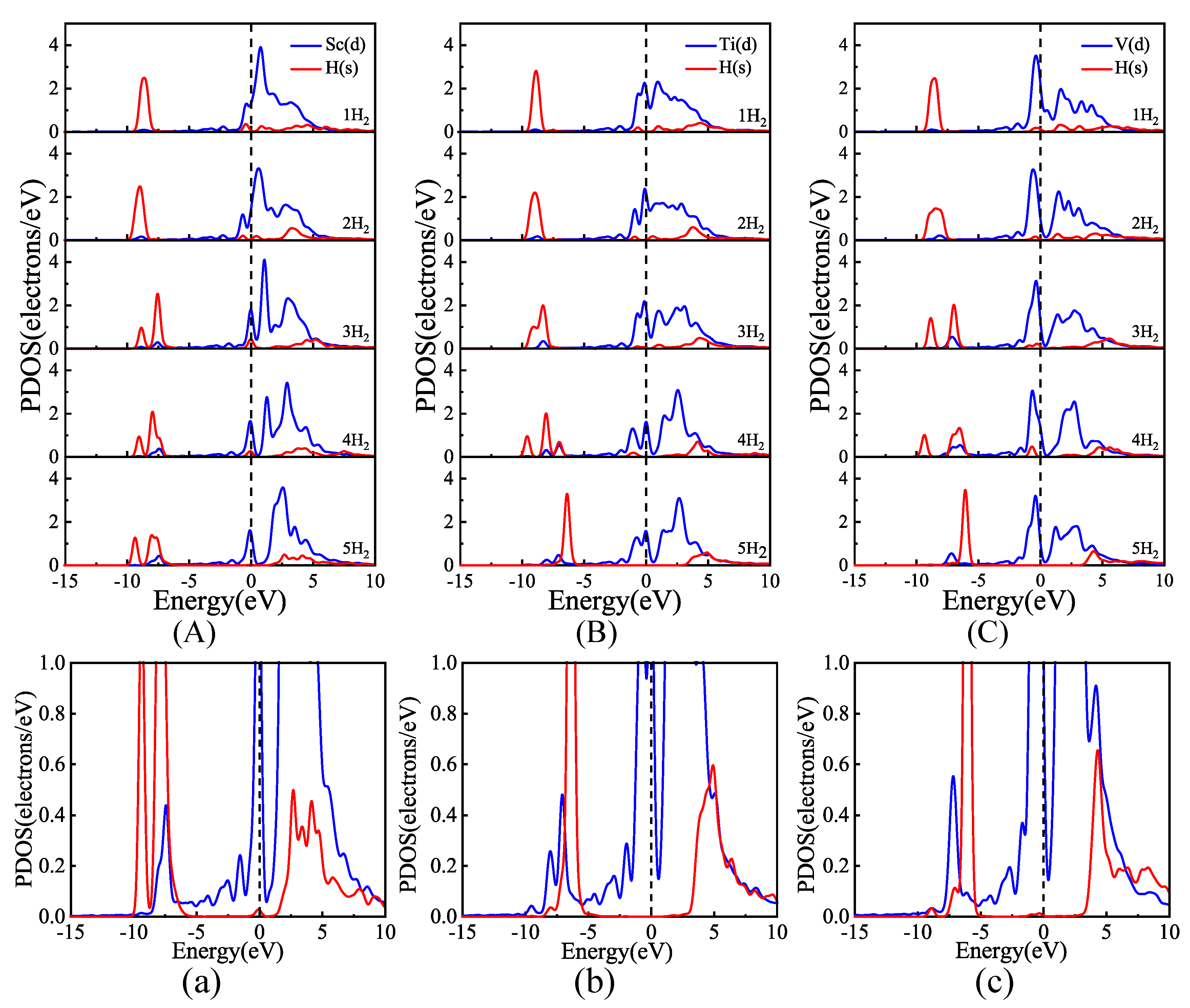
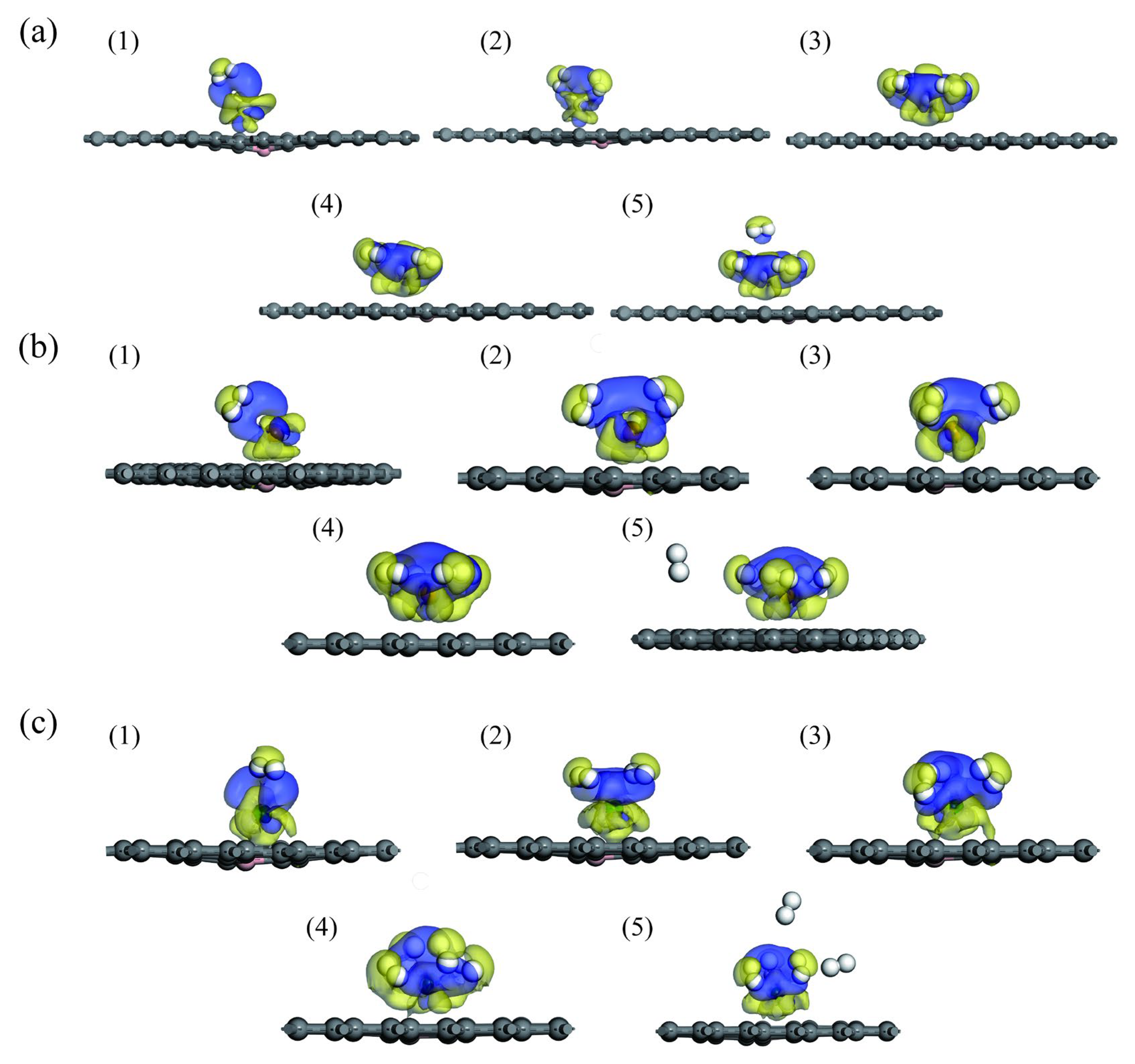
3.2. Hydrogen Adsorption of B-Doped Single Vacancy Defect Graphene Modified by Sc, Ti, and V Atoms
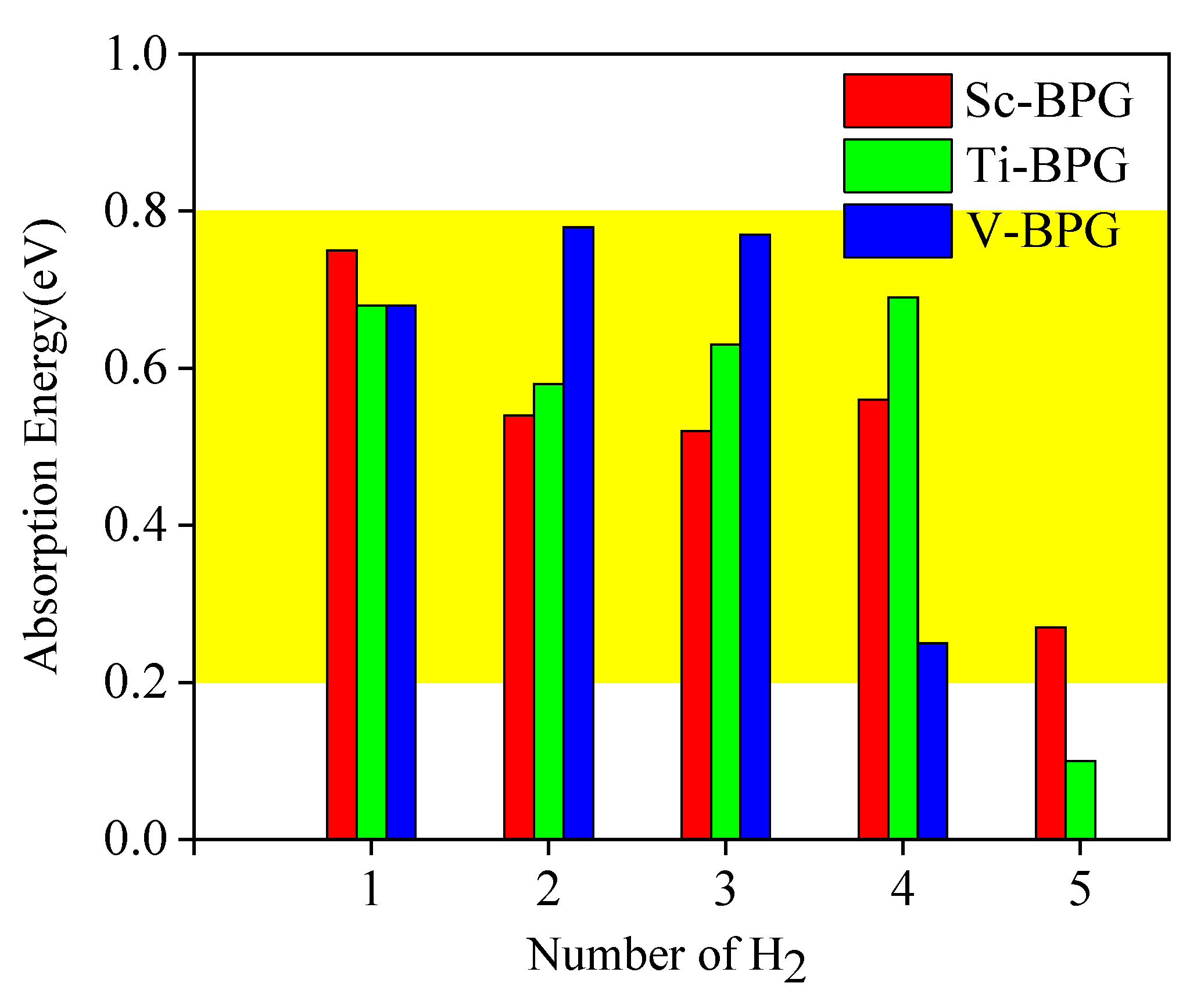
3.3. Comparison of Hydrogen Storage Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Stern, A.G. A new sustainable hydrogen clean energy paradigm. Int. J. Hydrogen Energy 2018, 43, 4244–4255. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- von Colbe, J.B.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Kim, B.-J.; Park, S.-J. Optimization of the pore structure of nickel/graphite hybrid materials for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 648–653. [Google Scholar] [CrossRef]
- Chung, T.-Y.; Tsao, C.-S.; Tseng, H.-P.; Chen, C.-H.; Yu, M.-S. Effects of oxygen functional groups on the enhancement of the hydrogen spillover of Pd-doped activated carbon. J. Colloid Interface Sci. 2015, 441, 98–105. [Google Scholar] [CrossRef]
- Morse, J.R.; Zugell, D.A.; Patterson, E.; Baldwin, J.W.; Willauer, H.D. Hydrogenated graphene: Important material properties regarding its application for hydrogen storage. J. Power Sources 2021, 494, 229734. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, C.-C. Enhancement of hydrogen spillover onto carbon nanotubes with defect feature. Microporous Mesoporous Mater. 2008, 109, 549–559. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, C.-C. Effect of surface characteristics and catalyst loaded amount on hydrogen storage in carbon nanotubes. Microporous Mesoporous Mater. 2008, 112, 553–560. [Google Scholar] [CrossRef]
- Jhi, S.-H. Activated boron nitride nanotubes: A potential material for room-temperature hydrogen storage. Phys. Rev. B 2006, 74, 155424. [Google Scholar] [CrossRef]
- Xu, W.C.; Takahashi, K.; Matsuo, Y.; Hattori, Y.; Kumagai, M.; Ishiyama, S.; Kaneko, K.; Iijima, S. Investigation of hydrogen storage capacity of various carbon materials. Int. J. Hydrogen Energy 2007, 32, 2504–2512. [Google Scholar] [CrossRef]
- Jin, H.; Lee, Y.S.; Hong, I. Hydrogen adsorption characteristics of activated carbon. Catal. Today 2007, 120, 399–406. [Google Scholar] [CrossRef]
- Reyhani, A.; Mortazavi, S.Z.; Mirershadi, S.; Moshfegh, A.Z.; Parvin, P.; Golikand, A.N. Hydrogen Storage in Decorated Multiwalled Carbon Nanotubes by Ca, Co, Fe, Ni, and Pd Nanoparticles under Ambient Conditions. J. Phys. Chem. C 2011, 115, 6994–7001. [Google Scholar] [CrossRef]
- Wang, L.F.; Yang, R.T. Hydrogen storage properties of carbons doped with ruthenium, platinum, and nickel nanoparticles. J. Phys. Chem. C 2008, 112, 12486–12494. [Google Scholar] [CrossRef]
- Seenithurai, S.; Pandyan, R.K.; Kumar, S.V.; Saranya, C.; Mahendran, M. Li-decorated double vacancy graphene for hydrogen storage application: A first principles study. Int. J. Hydrogen Energy 2014, 39, 11016–11026. [Google Scholar] [CrossRef]
- Ataca, C.; Aktürk, E.; Ciraci, S. Hydrogen storage of calcium atoms adsorbed on graphene: First-principles plane wave calculations. Phys. Rev. B 2009, 79, 041406. [Google Scholar] [CrossRef]
- Wang, Y.S.; Fei Yuan, P.; Li, M.; Fen Jiang, W.; Sun, Q.; Jia, Y. Calcium-decorated graphyne nanotubes as promising hydrogen storage media: A first-principles study. J. Solid State Chem. 2013, 197, 323–328. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Q.; Jena, P.; Kawazoe, Y. Clustering of Ti on a C60 Surface and Its Effect on Hydrogen Storage. JACS 2005, 127, 14582–14583. [Google Scholar] [CrossRef] [PubMed]
- Psofogiannakis, G.M.; Froudakis, G.E. Fundamental studies and perceptions on the spillover mechanism for hydrogen storage. Chem. Commun. 2011, 47, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Durgun, E.; Ciraci, S.; Yildirim, T. Functionalization of carbon-based nanostructures with light transition-metal atoms for hydrogen storage. Phys. Rev. B 2008, 77, 085405. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Sankaran, M.; Viswanathan, B. The role of heteroatoms in carbon nanotubes for hydrogen storage. Carbon 2006, 44, 2816–2821. [Google Scholar] [CrossRef]
- Cui, H.; Tian, W.; Zhang, Y.; Liu, T.; Wang, Y.; Shan, P.; Chen, Y.; Yuan, H. Study on the hydrogen storage performance of graphene(N)–Sc–graphene(N) structure. Int. J. Hydrogen Energy 2020, 45, 33789–33797. [Google Scholar] [CrossRef]
- Intayot, R.; Rungnim, C.; Namuangruk, S.; Yodsin, N.; Jungsuttiwong, S. Ti4-Decorated B/N-doped graphene as a high-capacity hydrogen storage material: A DFT study. Dalton Trans. 2021, 50, 11398–11411. [Google Scholar] [CrossRef]
- Nachimuthu, S.; He, L.; Cheng, H.-J.; Tiono, R.D.; Jiang, J.-C. A first-principles study on double-sided decorated boron–nitrogen co-doped graphene by vanadium for enhanced low-temperature reversible hydrogen storage. Sustain. Energy Fuels 2021, 5, 2159–2168. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, Y.; Wang, C.; Fang, Y.; Li, K.; Chen, Y. Boron-doping effect on the enhanced hydrogen storage of titanium-decorated porous graphene: A first-principles study. Int. J. Hydrogen Energy 2021, 46, 40301–40311. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.H.; Yuan, L.H.; Zhang, M.L.; Zhang, C.R. Scandium Decoration of Boron Doped Porous Graphene for High-Capacity Hydrogen Storage. Molecules 2019, 24, 2382. [Google Scholar] [CrossRef]
- Chen, I.N.; Wu, S.-Y.; Chen, H.-T. Hydrogen storage in N- and B-doped graphene decorated by small platinum clusters: A computational study. Appl. Surf. Sci. 2018, 441, 607–612. [Google Scholar] [CrossRef]
- Reunchan, P.; Jhi, S.-H. Metal-dispersed porous graphene for hydrogen storage. Appl. Phys. Lett. 2011, 98, 093103. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Fan, X.; Kuo, J.-L.; Deng, W.-Q. DFT Study of Hydrogen Storage by Spillover on Graphene with Boron Substitution. J. Phys. Chem. C 2011, 115, 9241–9249. [Google Scholar] [CrossRef]
- Lueking, A.D.; Psofogiannakis, G.; Froudakis, G.E. Atomic Hydrogen Diffusion on Doped and Chemically Modified Graphene. J. Phys. Chem. C 2013, 117, 6312–6319. [Google Scholar] [CrossRef]
- Weigang, Z. Hydrothermal Doping of Nitrogen in Bamboo-Based Super Activated Carbon for Hydrogen Storage. Bioresources 2017, 12, 6237–6250. [Google Scholar]
- Zhao, W.; Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Wang, H.; Rao, J.; Feo, L.; Fan, M. Synthesis and characterization of Pt-N-doped activated biocarbon composites for hydrogen storage. Compos. B Eng. 2019, 161, 464–472. [Google Scholar] [CrossRef]
- Galindo-Hernández, F.; Portales, B.; Domínguez, J.M.; Angeles-Beltrán, D. Porosity and fractal study of functionalized carbon nanofibers: Effects of the functionalization degree on hydrogen storage capacity. J. Power Sources 2014, 269, 69–80. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Guan, C.; He, Z.; Lu, Z.; Chen, T.; Liu, J.; Tan, X.; Yang Tan, T.T.; Li, C.M. Surface functionalization-enhanced spillover effect on hydrogen storage of Ni–B nanoalloy-doped activated carbon. Int. J. Hydrogen Energy 2011, 36, 13663–13668. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.T.; He, J.L.; Tian, Y.J. Ab initio investigations of optical properties of the high-pressure phases of ZnO. Phys. Rev. B 2005, 71, 125132. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Johnson, E.; Mackie, I.; DiLabio, G. Dispersion interactions in density-functional theory. J. Phys. Org. Chem. 2009, 22, 1127–1135. [Google Scholar] [CrossRef]
- Parkar, P.; Chaudhari, A. Reversible hydrogen storage on multiple Ti-doped B12C6N6 nanocage. J. Energy Storage 2023, 62, 106910. [Google Scholar] [CrossRef]
- Furlan, S.; Giannozzi, P. The interactions of nitrogen dioxide with graphene-stabilized Rh clusters: A DFT study. Phys. Chem. Chem. Phys. 2013, 15, 15896–15904. [Google Scholar] [CrossRef]
- Hou, M.; Cen, W.; Nan, F.; Li, J.; Chu, Y.; Yin, H. Dissociation of O2 and Its Reactivity on O/S doped Graphene. RSC Adv. 2015, 6, 7015–7021. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zu, X.T.; Gao, F.; Nie, J.L.; Xiao, H.Y. Adsorption of hydrogen on boron-doped graphene: A first-principles prediction. J. Appl. Phys. 2009, 105, 014309. [Google Scholar] [CrossRef]
- Gu, J.; Du, Q.; Han, Y.; He, Z.; Li, W.; Zhang, J. Nitrogen-doped carbon supports with terminated hydrogen and their effects on active gold species: A density functional study. Phys. Chem. Chem. Phys. 2014, 16, 25498–25507. [Google Scholar] [CrossRef] [PubMed]
- Nadaraj, S.; Wu, S.-Y.; Chen, H.-T. Boron- and nitrogen-doped penta-graphene as a promising material for hydrogen storage: A computational study. Int. J. Energy Res. 2019, 43, 4867–4878. [Google Scholar]
- Manadé, M.; Viñes, F.; Illas, F. Transition metal adatoms on graphene: A systematic density functional study. Carbon 2015, 95, 525–534. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Pan, R.; An, Y. A first-principles study of Sc-decorated graphene with pyridinic-N defects for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 3106–3113. [Google Scholar] [CrossRef]
- Lebon, A.; Carrete, J.; Longo, R.C.; Vega, A.; Gallego, L.J. Molecular hydrogen uptake by zigzag graphene nanoribbons doped with early 3d transition-metal atoms. Int. J. Hydrogen Energy 2013, 38, 8872–8880. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, X.; Yan, J.; Song, D. Doping effects of B and N on hydrogen adsorption in single-walled carbon nanotubes through density functional calculations. Carbon 2006, 44, 939–947. [Google Scholar] [CrossRef]
- Zhou, Y.; He, X.; Li, M. Roles of doping in enhancing the performance of graphene/graphene-like semiconductors. AIP Adv. 2025, 15, 010701. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Y.; Kang, L.; Zhang, C.; Wang, D.; Wang, C.; Zhang, M.; Wu, X. First-principles investigation of hydrogen storage capacity of Y-decorated porous graphene. Appl. Surf. Sci. 2017, 399, 463–468. [Google Scholar] [CrossRef]
- Kubas, G.J. Fundamentals of H2 Binding and Reactivity on Transition Metals Underlying Hydrogenase Function and H2 Production and Storage. Chem. Rev. 2007, 107, 4152–4205. [Google Scholar] [CrossRef]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
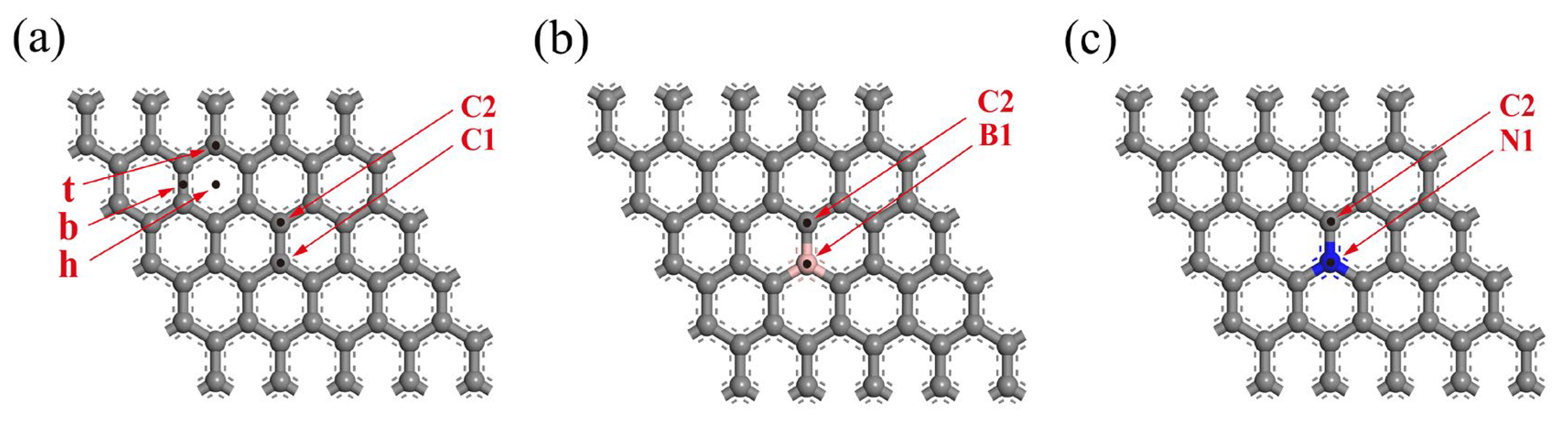
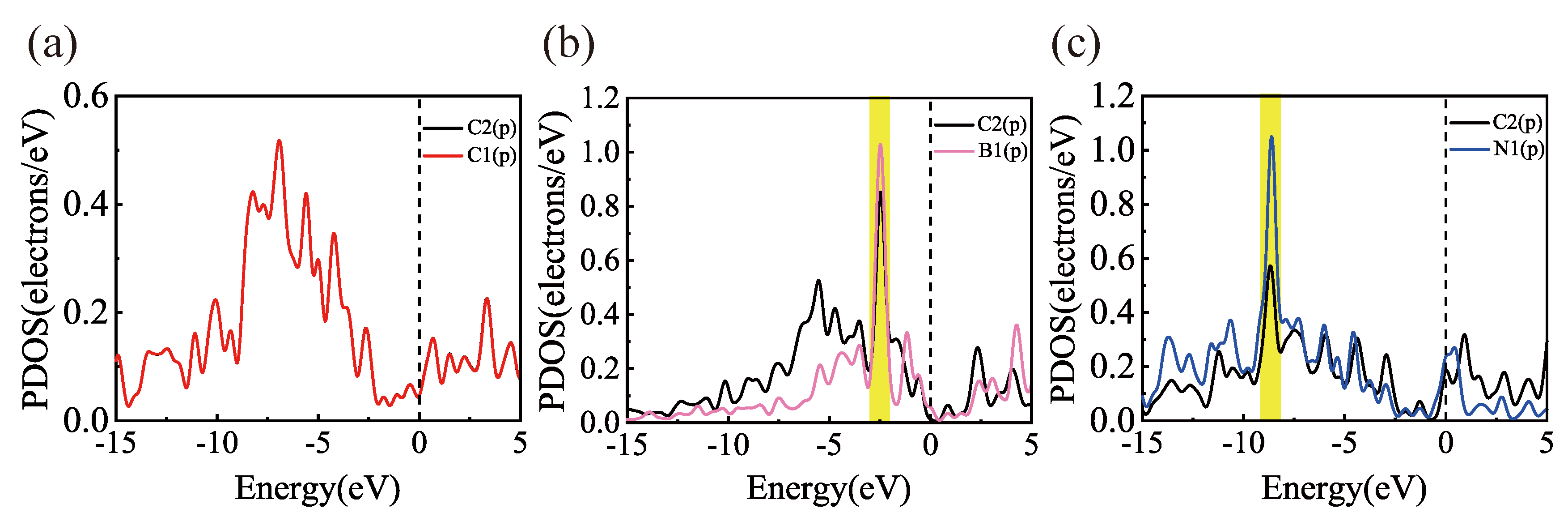
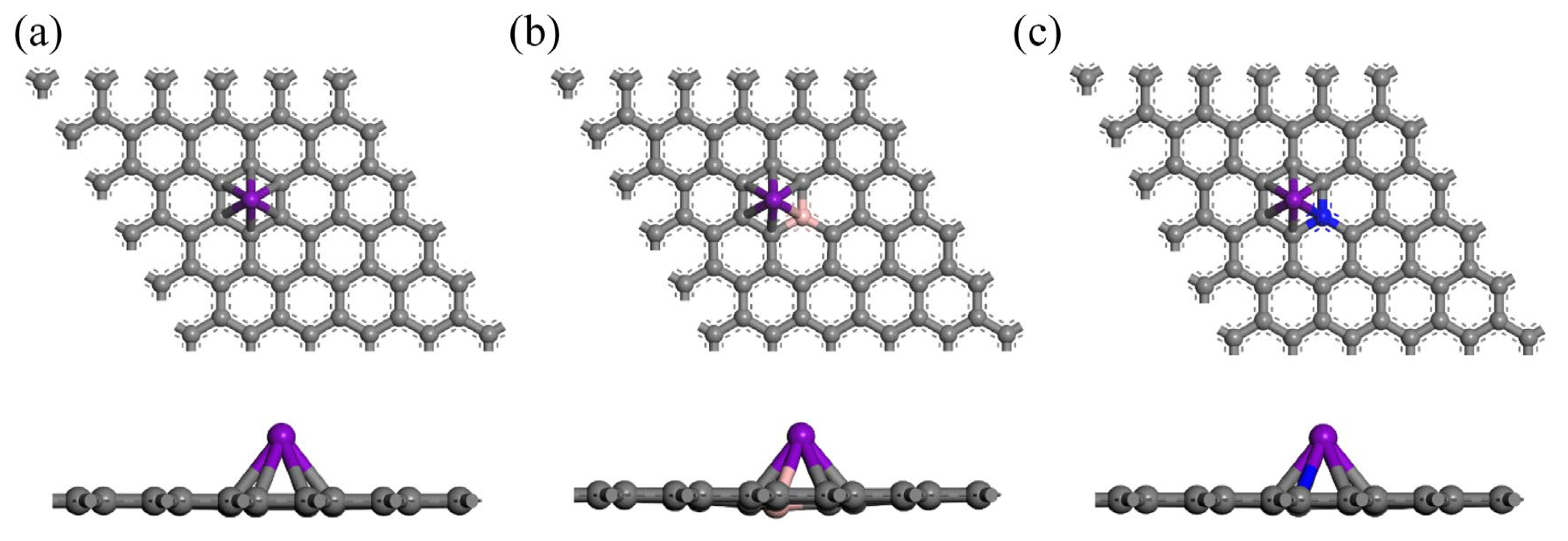
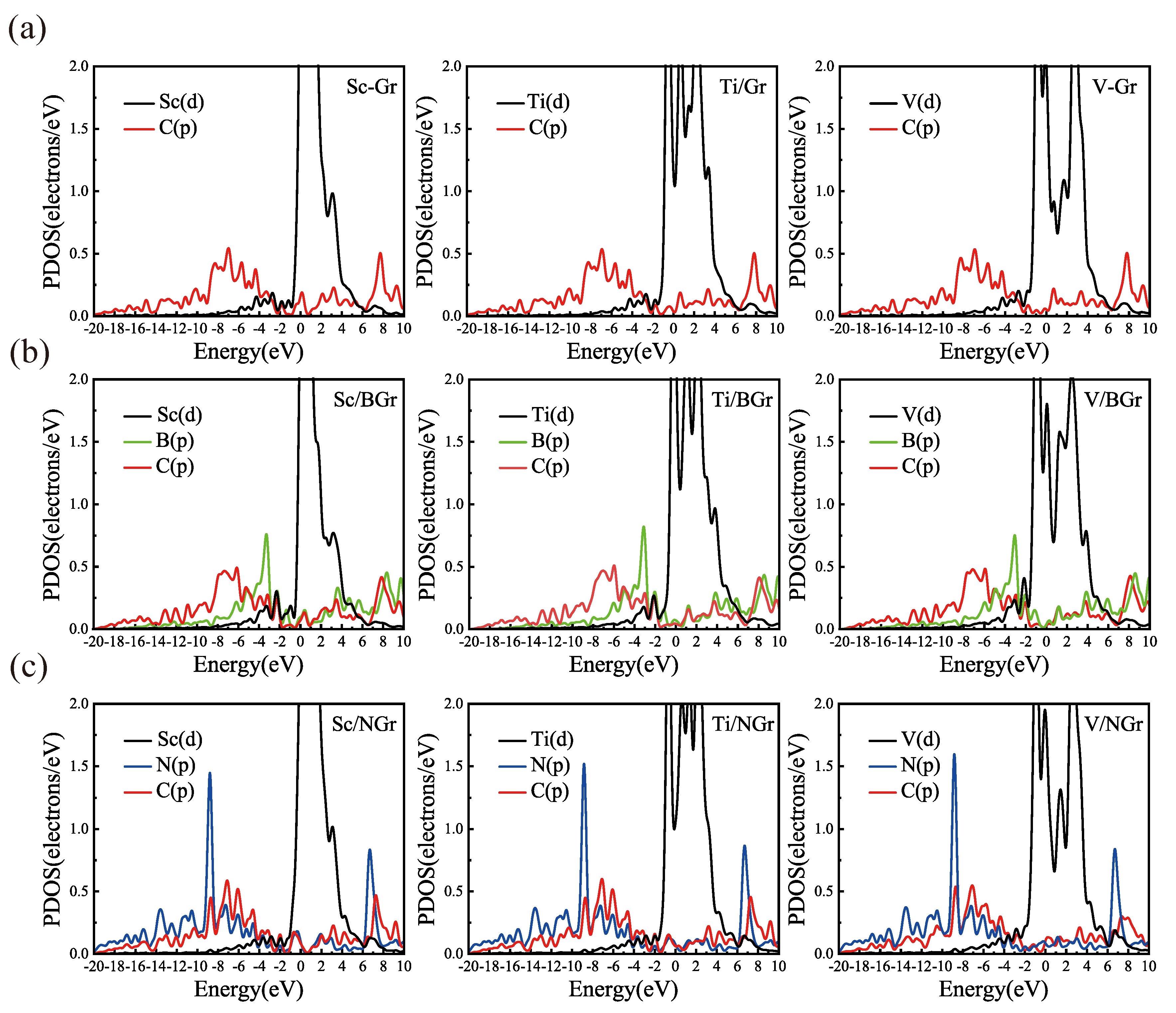
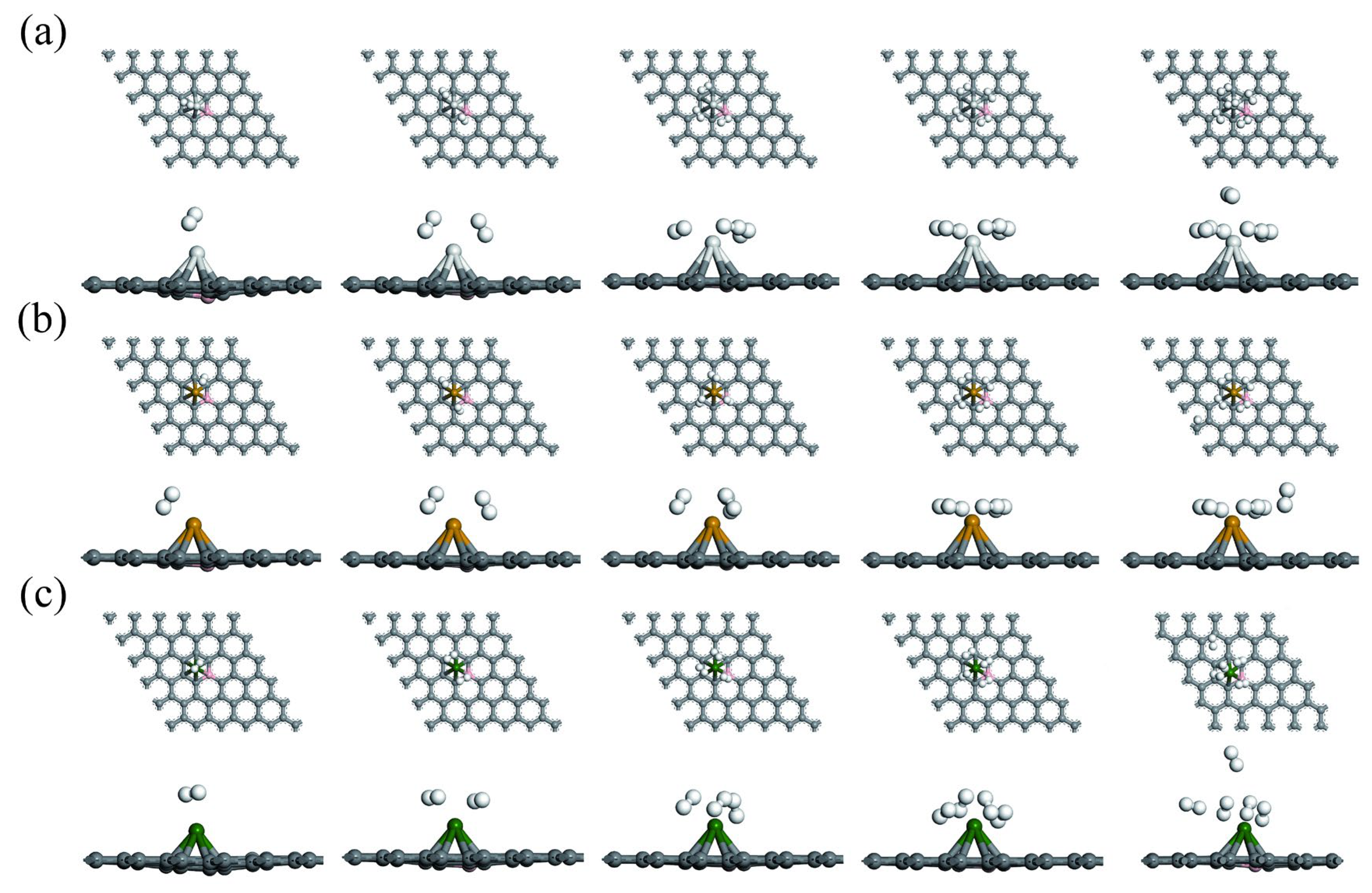
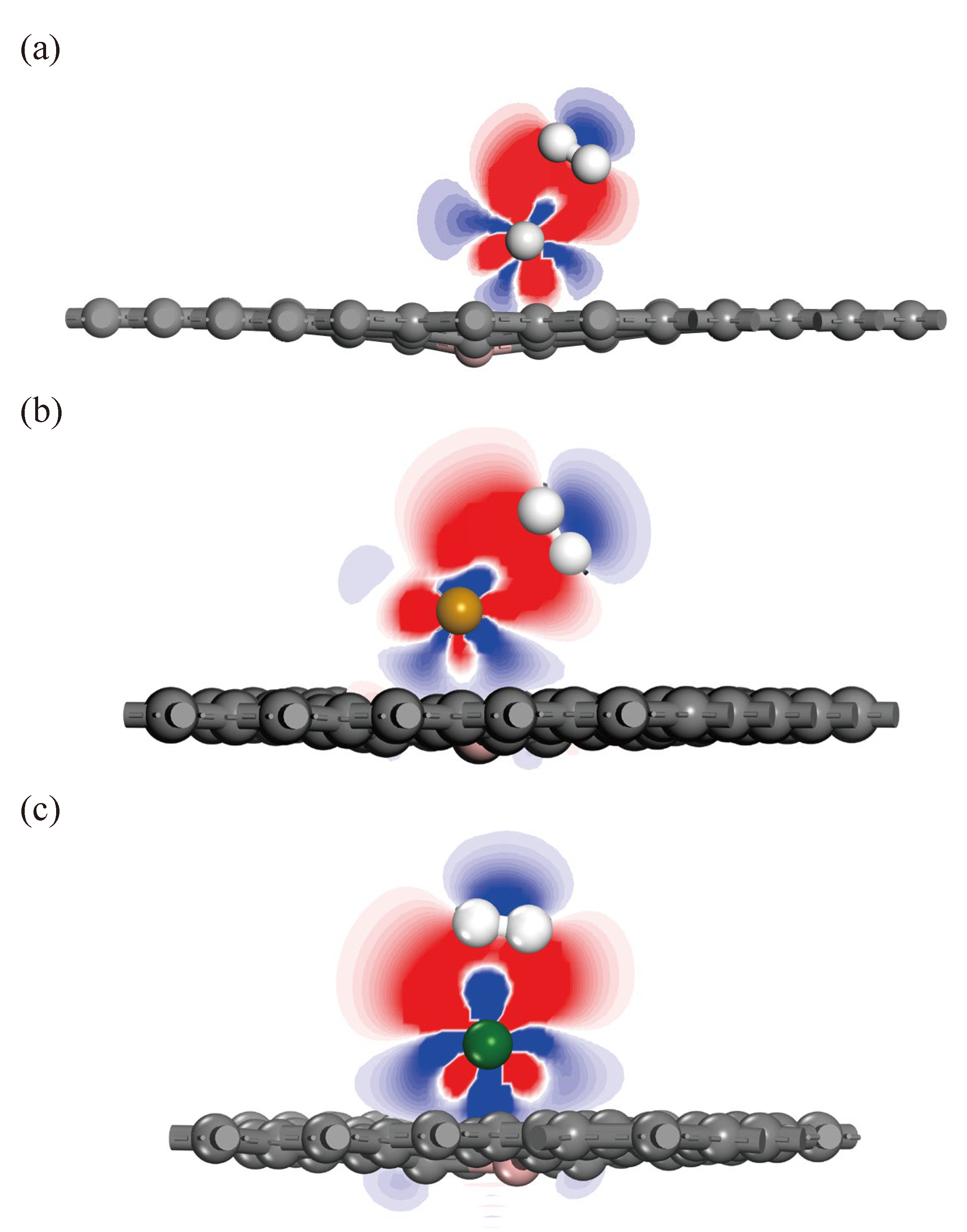
| Substrate | Adsorption Site | TM-C (Å) | TM-B (Å) | TM-N (Å) | H (Å) | ||
|---|---|---|---|---|---|---|---|
| Sc-Gr | H | −1.34 | 2.374 | - | - | 1.893 | +0.43 |
| Sc-BGr | −3.21 | - | 2.412 | - | 1.892 | +0.52 | |
| Sc-NGr | −1.45 | - | - | 2.270 | 1.822 | +0.44 | |
| Ti-Gr | −1.80 | 2.333 | - | - | 1.842 | +0.32 | |
| Ti-BGr | −3.54 | - | 2.349 | - | 1.806 | +0.48 | |
| Ti-NGr | −1.86 | - | - | 2.208 | 1.769 | +0.30 | |
| V-Gr | −1.30 | 2.315 | - | - | 1.820 | +0.25 | |
| V-BGr | −2.99 | - | 2.314 | - | 1.757 | +0.43 | |
| V-NGr | −1.06 | - | - | 2.106 | 1.684 | +0.24 |
| Substrate | ||||||
|---|---|---|---|---|---|---|
| Sc-BGr | −0.75 | −0.750 | 2.008 | 2.353 | 0.837 | |
| −0.54 | −0.645 | 2.062 | 2.396 | 0.808 | ||
| −0.52 | −0.603 | 1.927 | 2.450 | 0.860 | ||
| −0.56 | −0.593 | 2.012 | 2.474 | 0.814 | ||
| −0.27 | −0.528 | 2.420 | 2.477 | 0.765 | ||
| Ti-BGr | −0.68 | −0.680 | 1.946 | 2.309 | 0.809 | |
| −0.58 | −0.630 | 1.975 | 2.304 | 0.799 | ||
| −0.63 | −0.630 | 1.949 | 2.344 | 0.799 | ||
| −0.69 | −0.645 | 1.836 | 2.391 | 0.840 | ||
| −0.0998 | −0.536 | 4.701 | 2.394 | 0.752 | ||
| V-BGr | −0.68 | −0.680 | 1.886 | 2.237 | 0.823 | |
| −0.78 | −0.730 | 1.873 | 2.276 | 0.811 | ||
| −0.77 | −0.743 | 1.740 | 2.262 | 0.864 | ||
| −0.25 | −0.620 | 1.672 | 2.284 | 0.891 | ||
| 0.14 | - | 3.968 | 2.307 | 0.755 |
| Before Adsorption (e) | Charge (e) | After Adsorption (e) | Charge (e) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sc | Ti | V | Sc | Ti | V | H | ||||
| Sc-BGr | 1.39 | - | - | −1.39 | 1.86 | - | - | −0.19 | −0.16 | −1.51 |
| Ti-BGr | - | 1.34 | - | −1.34 | - | 1.63 | - | −0.16 | −0.10 | −1.37 |
| V-BGr | - | - | 1.33 | −1.33 | - | - | 1.57 | −0.18 | −0.16 | −1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Q.; Wang, L.; Chen, Y.; Nie, Z. Effect of B/N Doping on Enhanced Hydrogen Storage in Transition Metal-Modified Graphene: A First-Principles DFT Study. Materials 2025, 18, 4635. https://doi.org/10.3390/ma18194635
Nie Q, Wang L, Chen Y, Nie Z. Effect of B/N Doping on Enhanced Hydrogen Storage in Transition Metal-Modified Graphene: A First-Principles DFT Study. Materials. 2025; 18(19):4635. https://doi.org/10.3390/ma18194635
Chicago/Turabian StyleNie, Qian, Lei Wang, Ye Chen, and Zhengwei Nie. 2025. "Effect of B/N Doping on Enhanced Hydrogen Storage in Transition Metal-Modified Graphene: A First-Principles DFT Study" Materials 18, no. 19: 4635. https://doi.org/10.3390/ma18194635
APA StyleNie, Q., Wang, L., Chen, Y., & Nie, Z. (2025). Effect of B/N Doping on Enhanced Hydrogen Storage in Transition Metal-Modified Graphene: A First-Principles DFT Study. Materials, 18(19), 4635. https://doi.org/10.3390/ma18194635







