Performance Research of Ultra-High Performance Concrete Incorporating Municipal Solid Waste Incineration Fly Ash
Abstract
1. Introduction
2. Materials and Methods
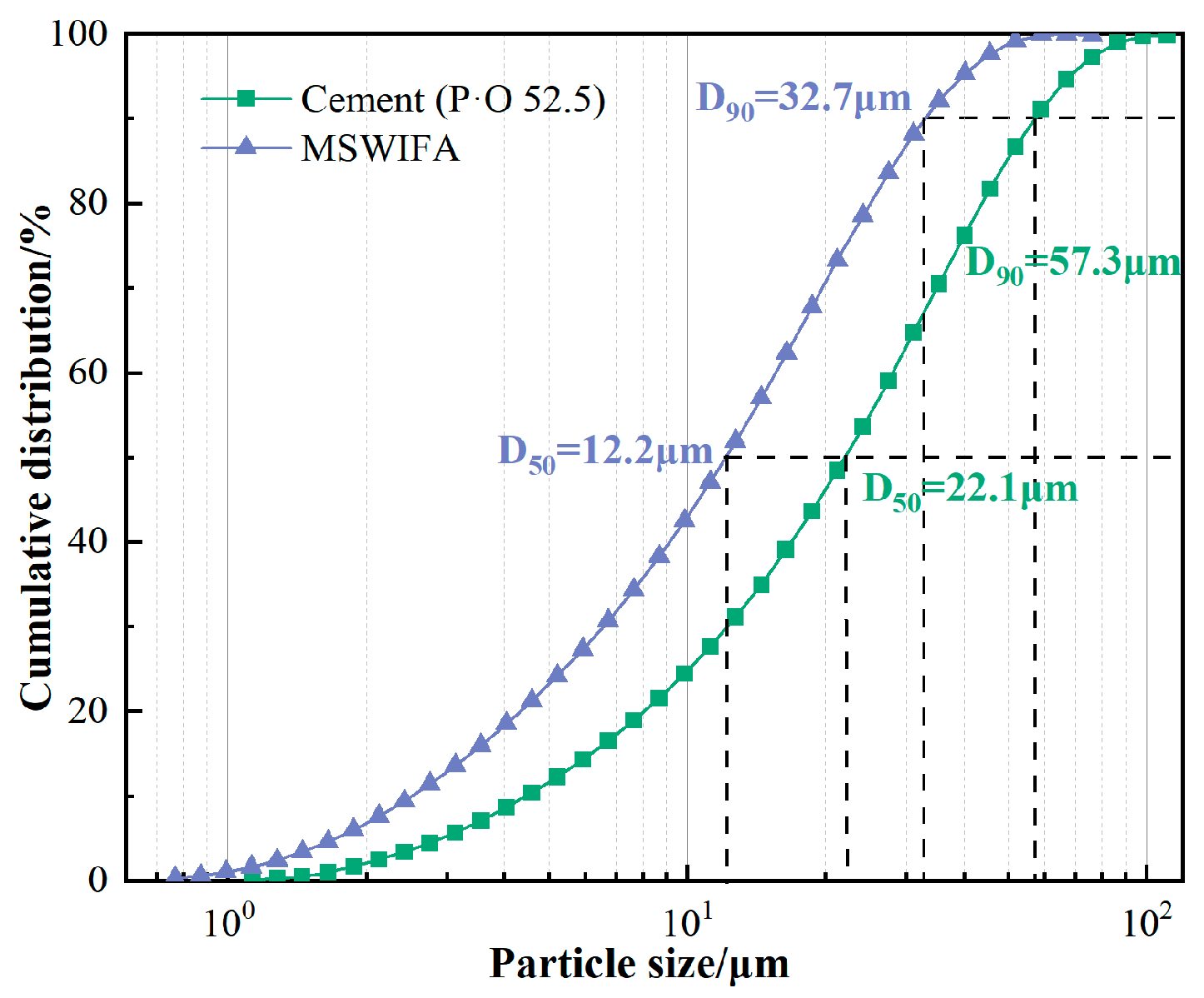
2.1. Raw Materials
2.2. Preparation of UHPC Samples
2.3. Experimental Methods
2.3.1. Physical and Mechanical Properties
2.3.2. Durability
2.3.3. Microscopic Properties
2.3.4. Heavy Metal Leaching Concentration Analysis
3. Results and Discussion
3.1. Effects of MSWIFA Content on the Physical Properties of UHPC
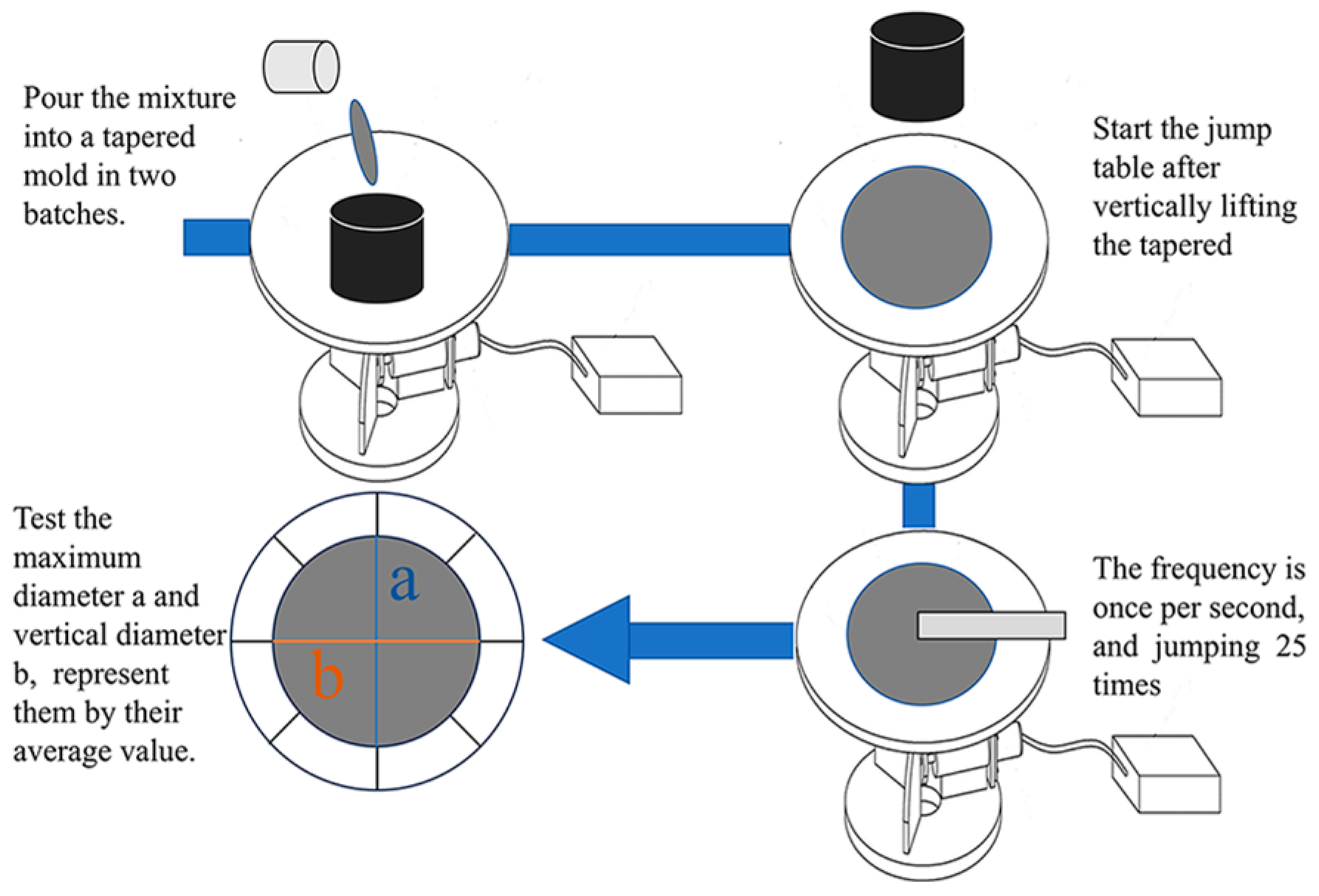
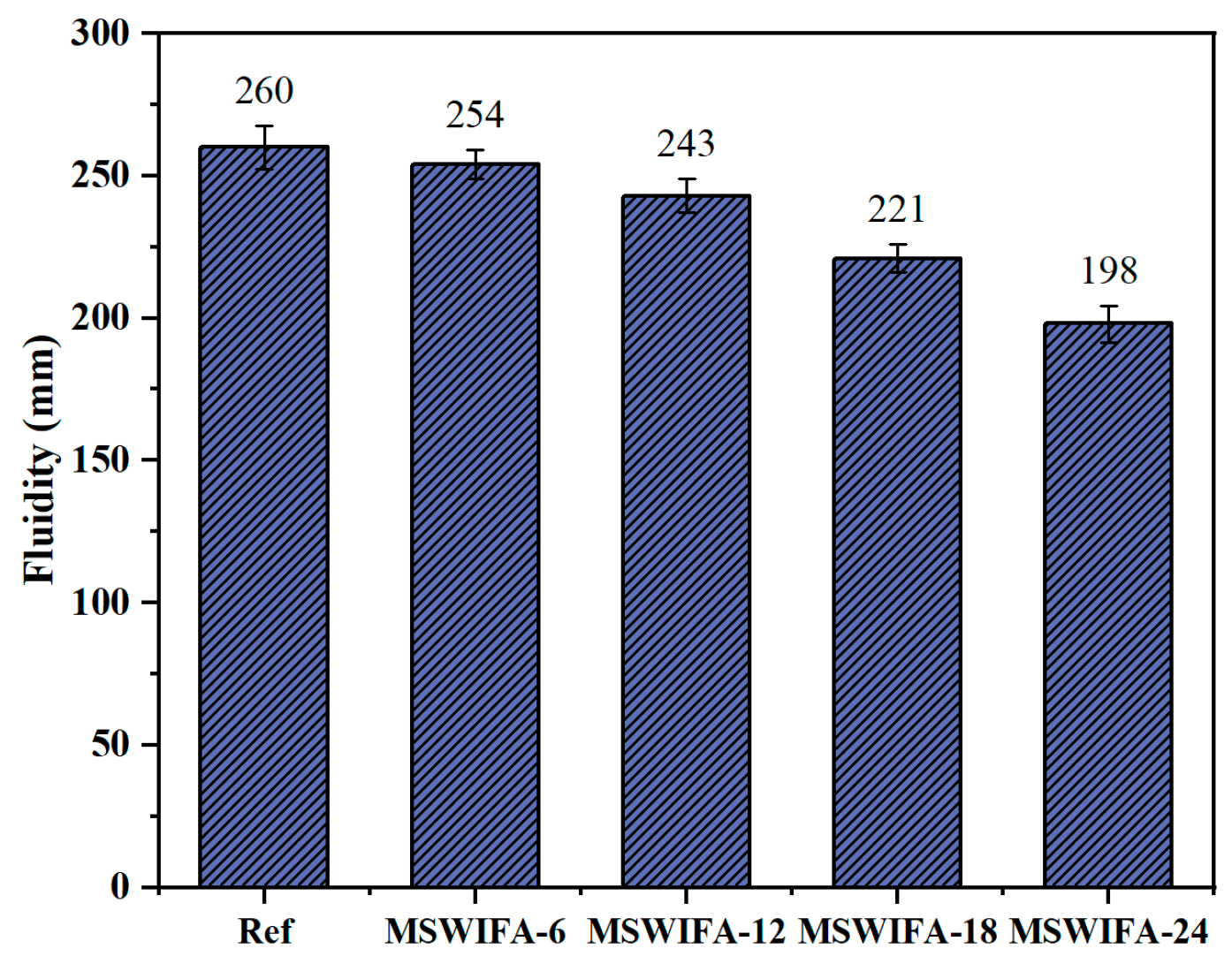
3.1.1. Fluidity
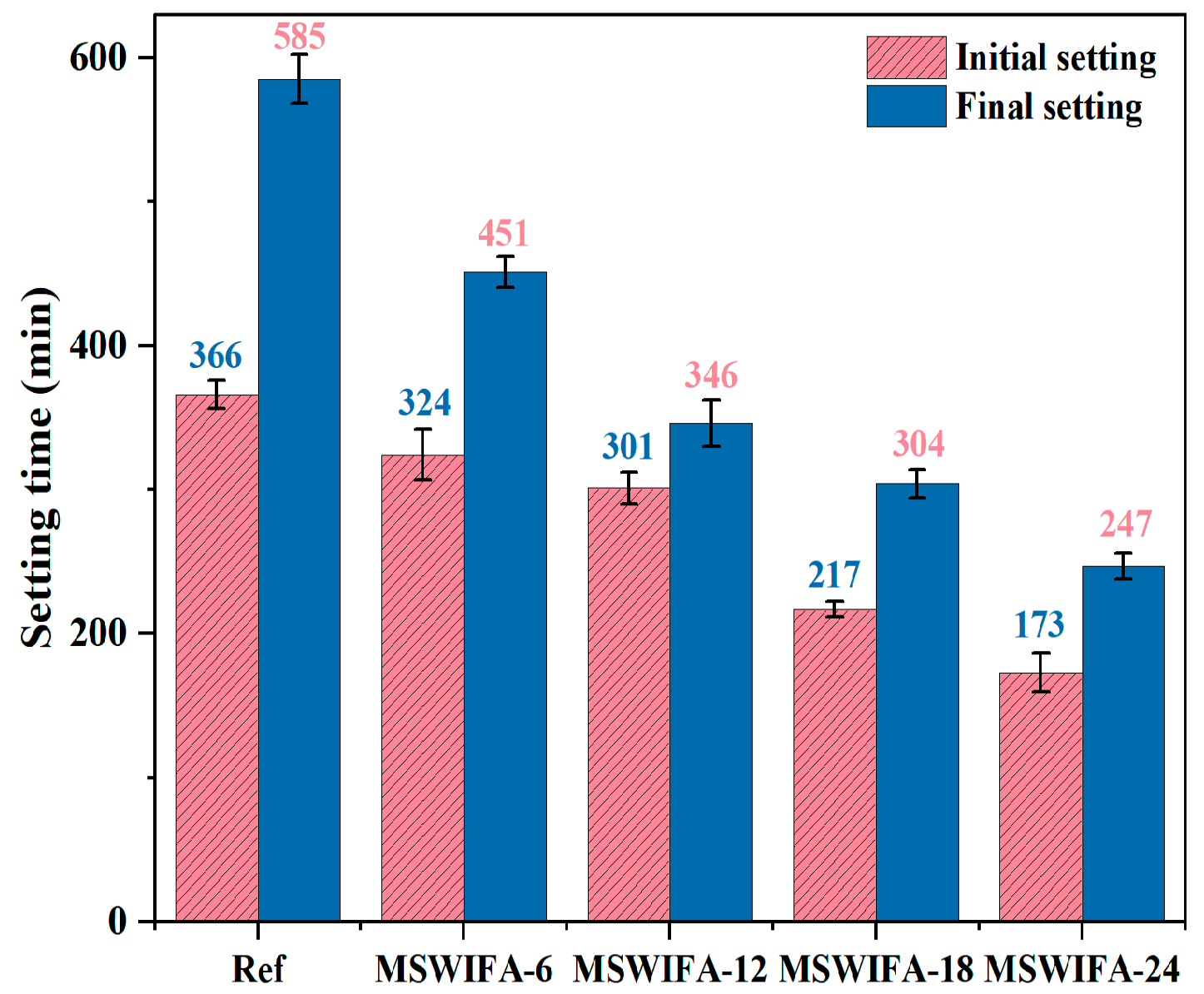
3.1.2. Setting Time
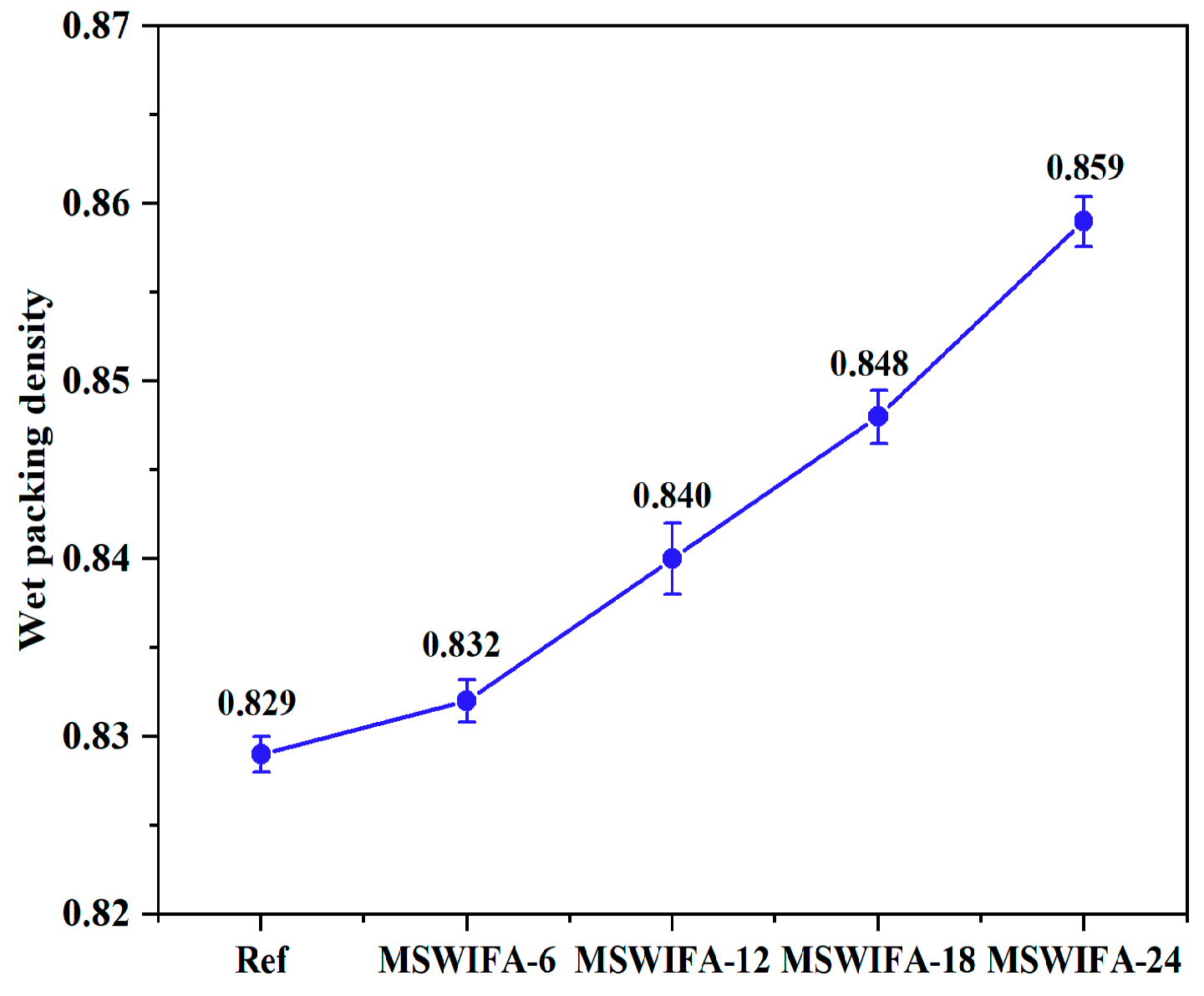
3.1.3. Wet Packing Density
3.2. Effects of MSWIFA Content on the Mechanical Properties of UHPC
3.2.1. Compressive Strength
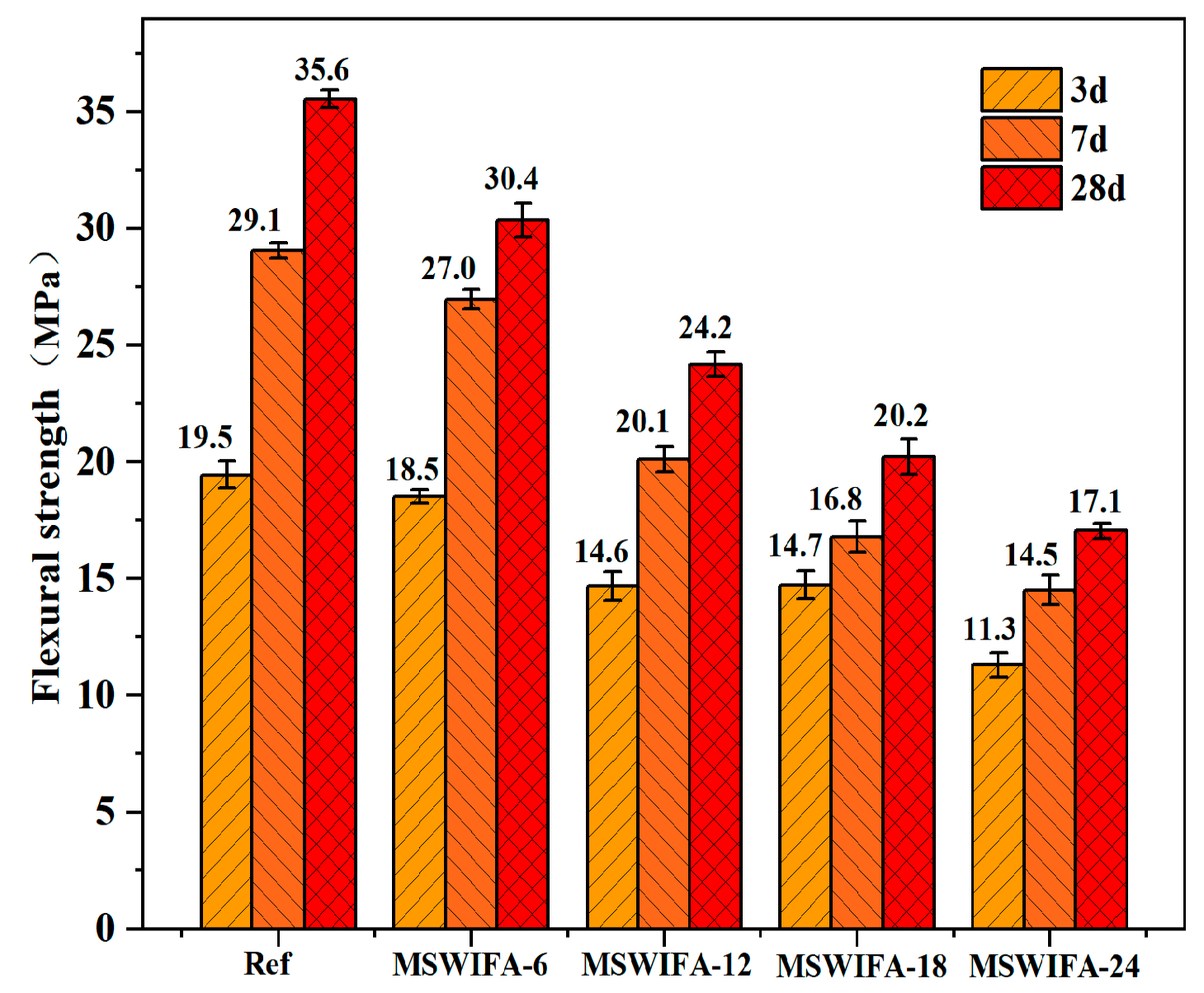
3.2.2. Flexural Strength
3.3. Effects of MSWIFA Content on the Durability of UHPC
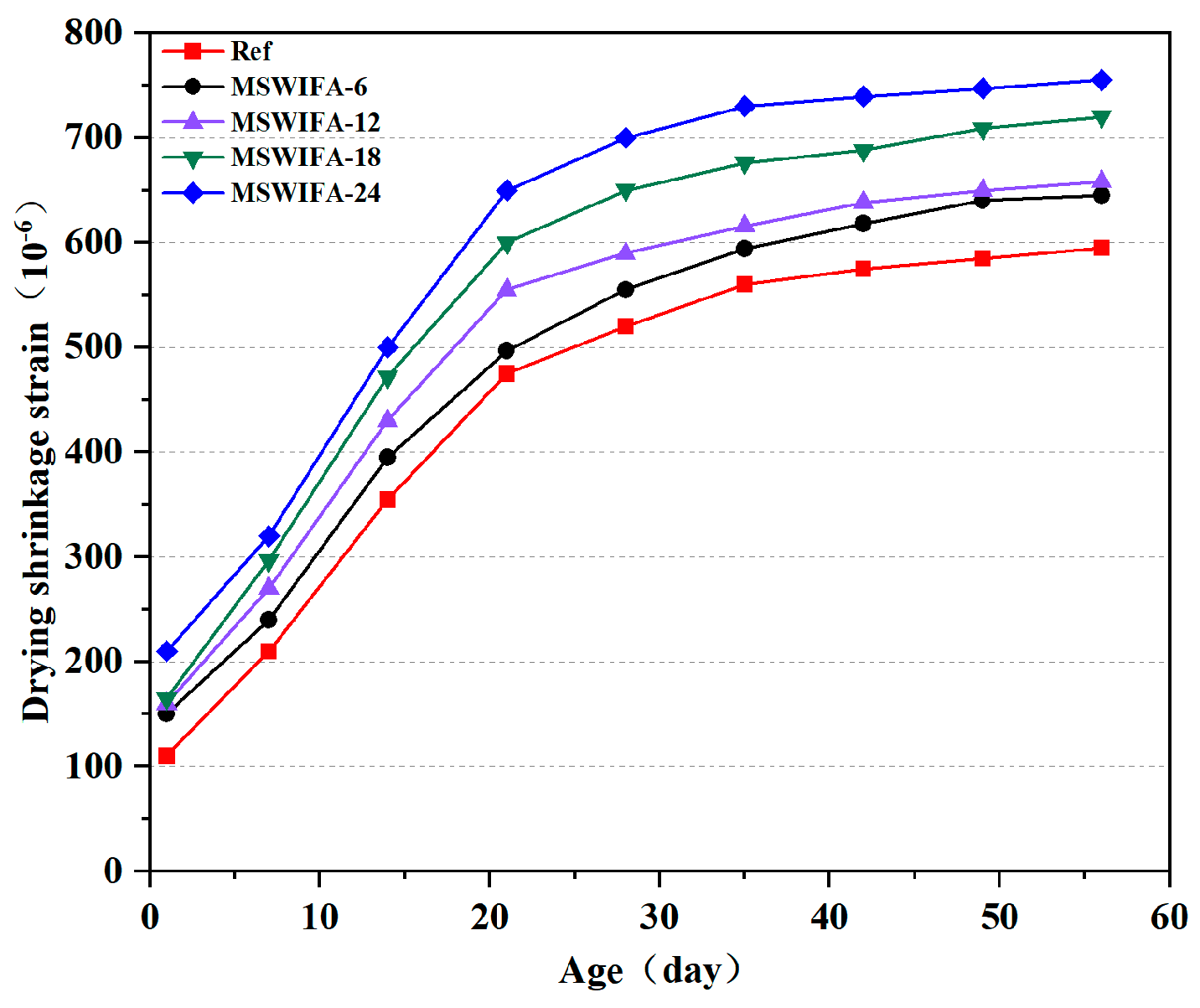
3.3.1. Drying Shrinkage Performance
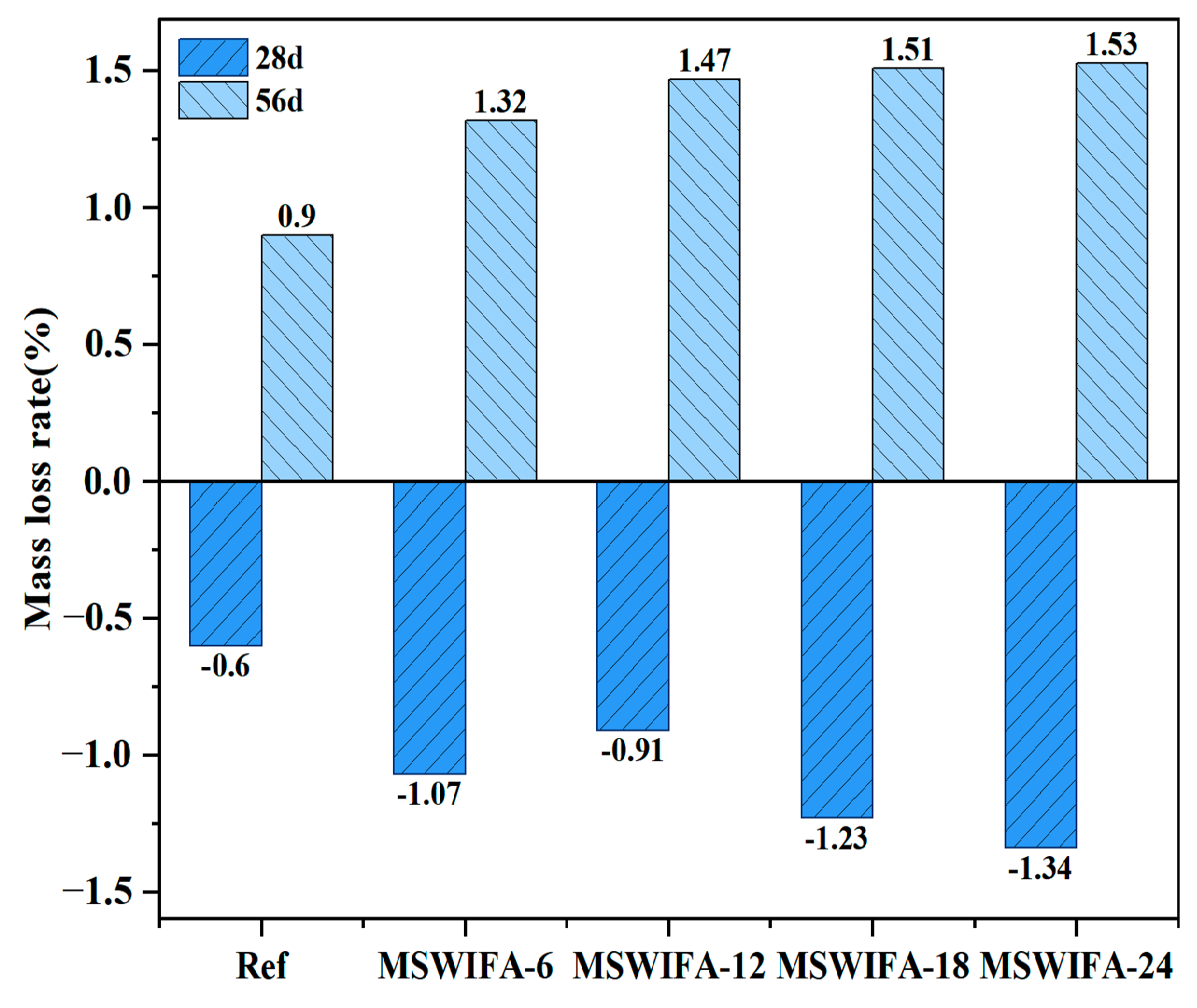
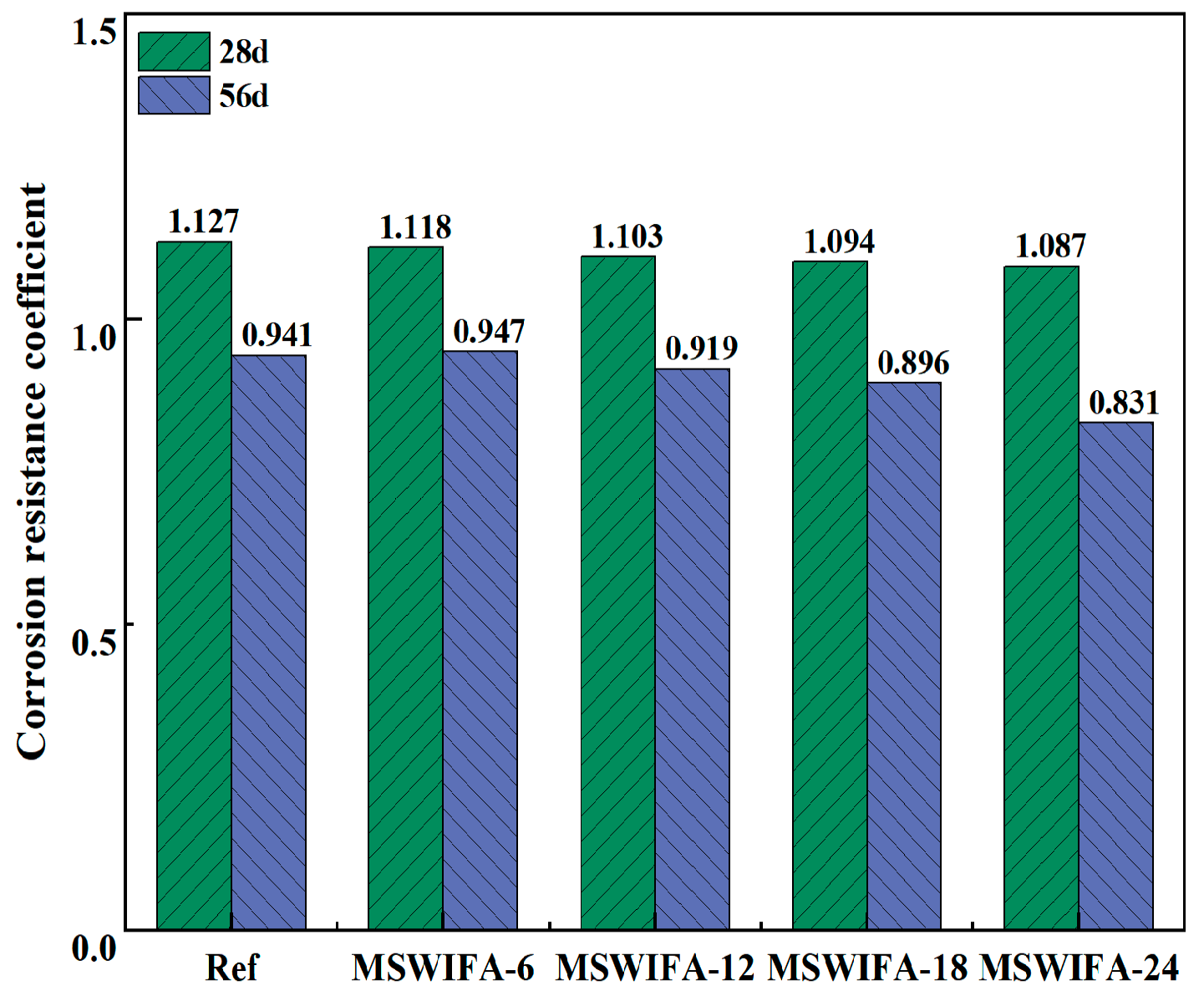
3.3.2. Resistance to Chloride Attack
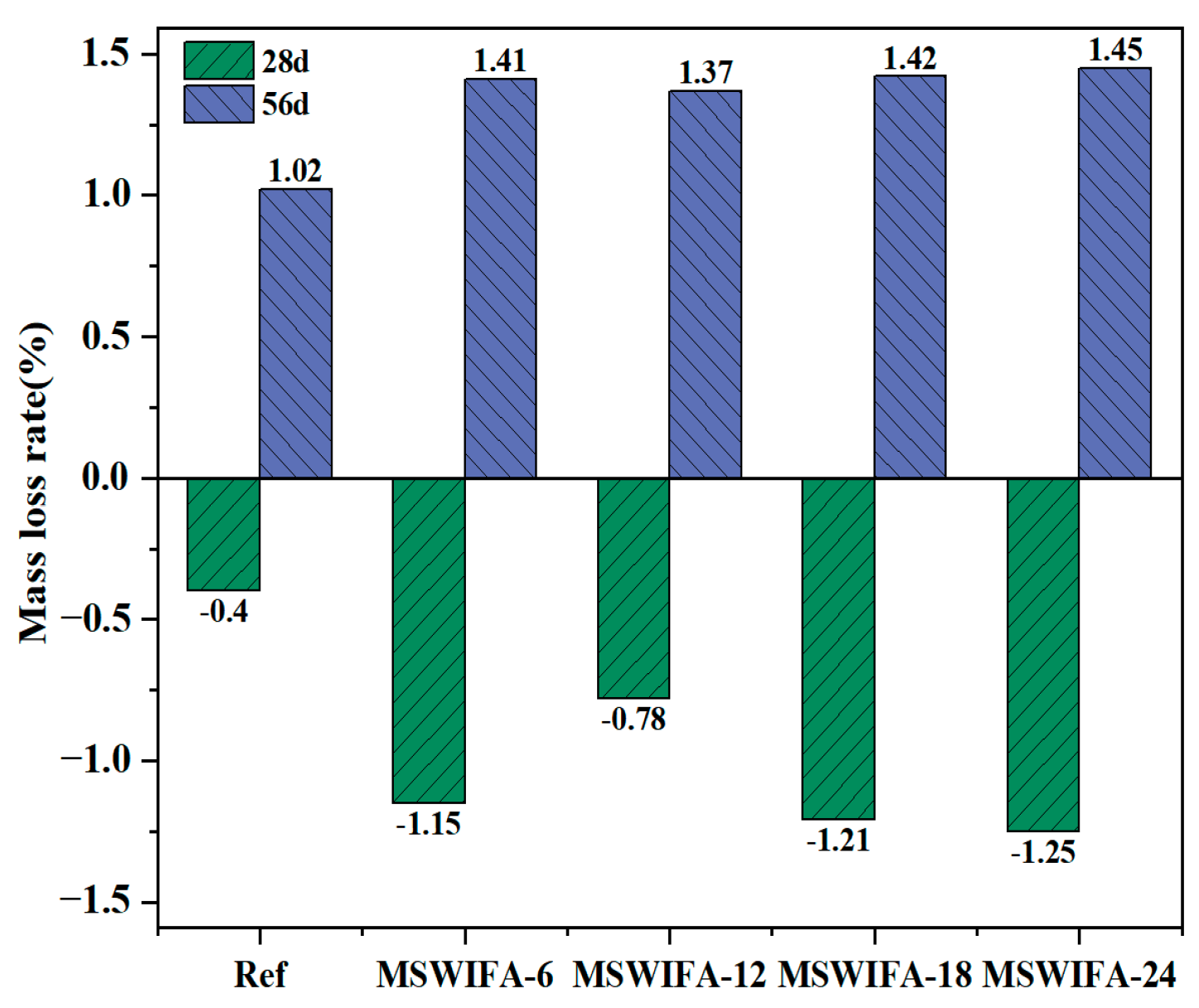
3.3.3. Resistance to Sulfate Attack
3.4. Microscopic Test Analysis of UHPC
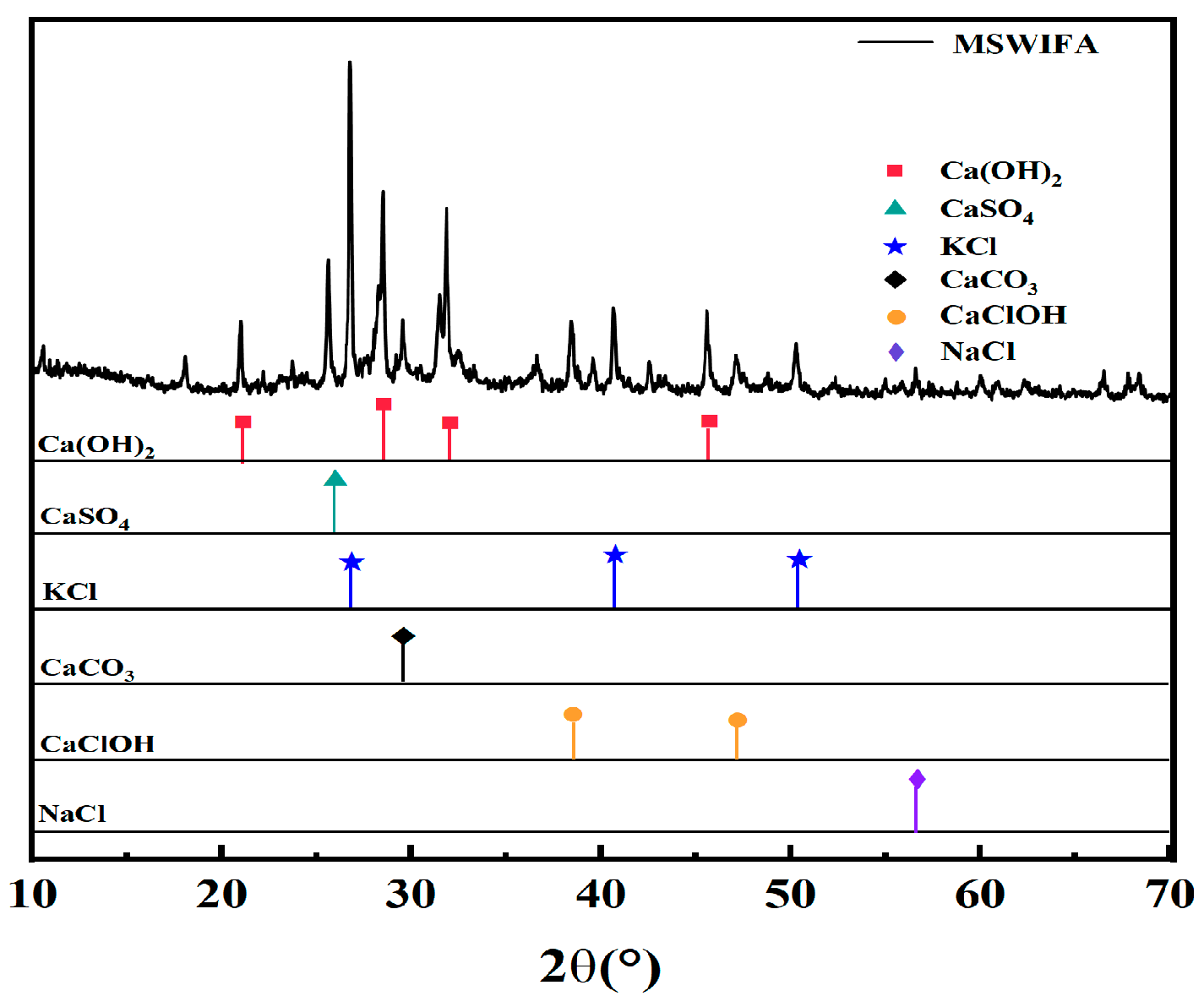
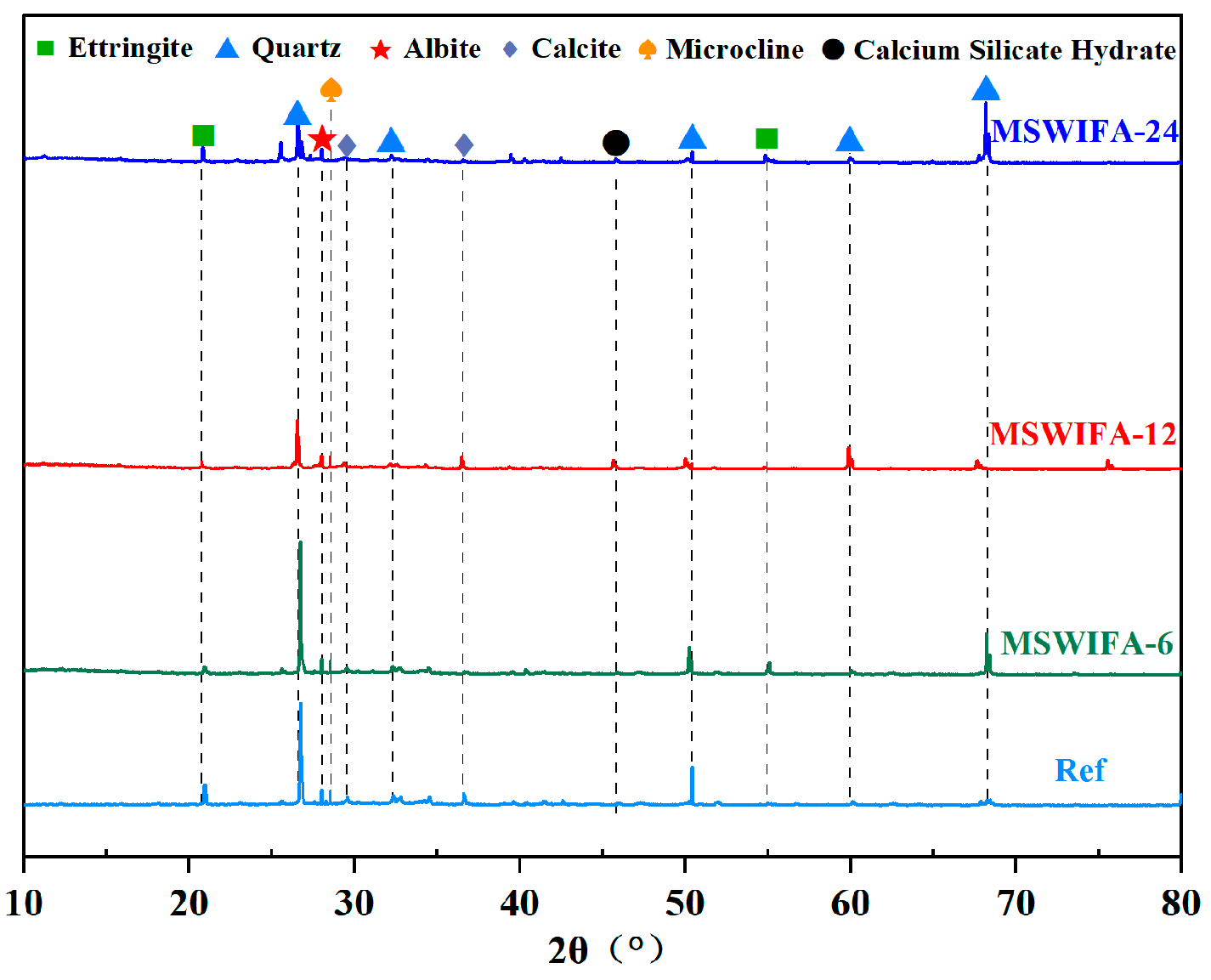
3.4.1. XRD
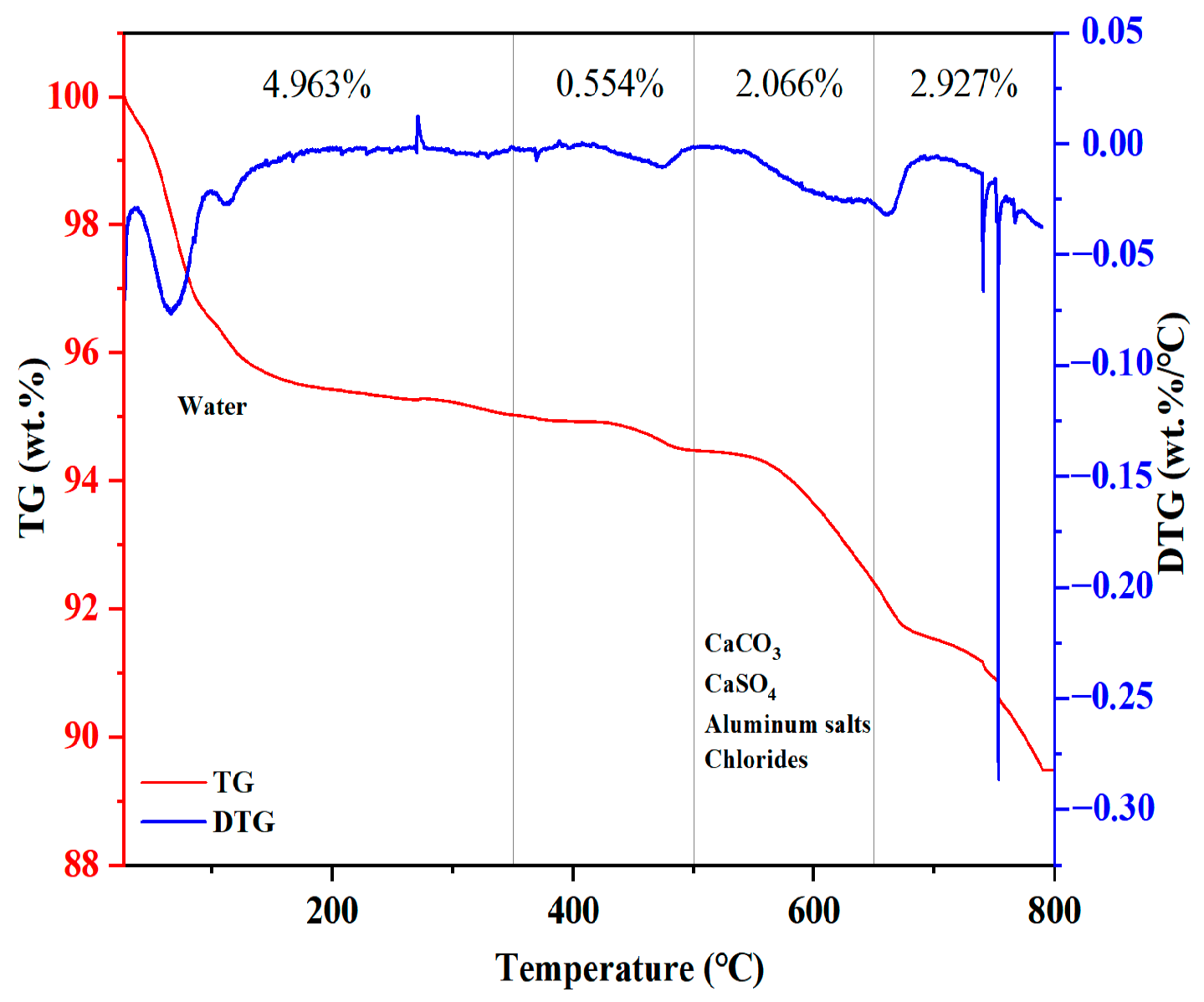
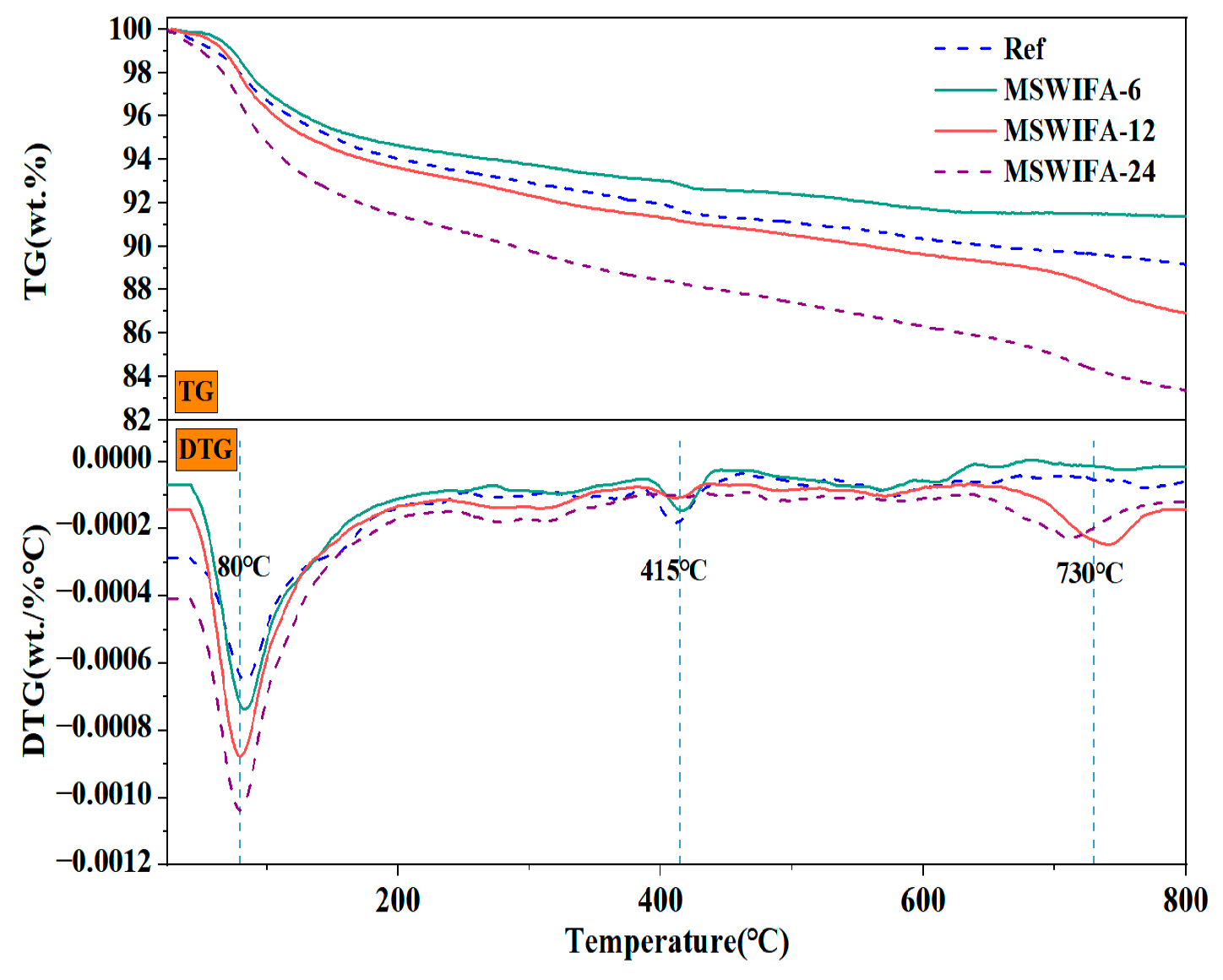
3.4.2. TG/DTA
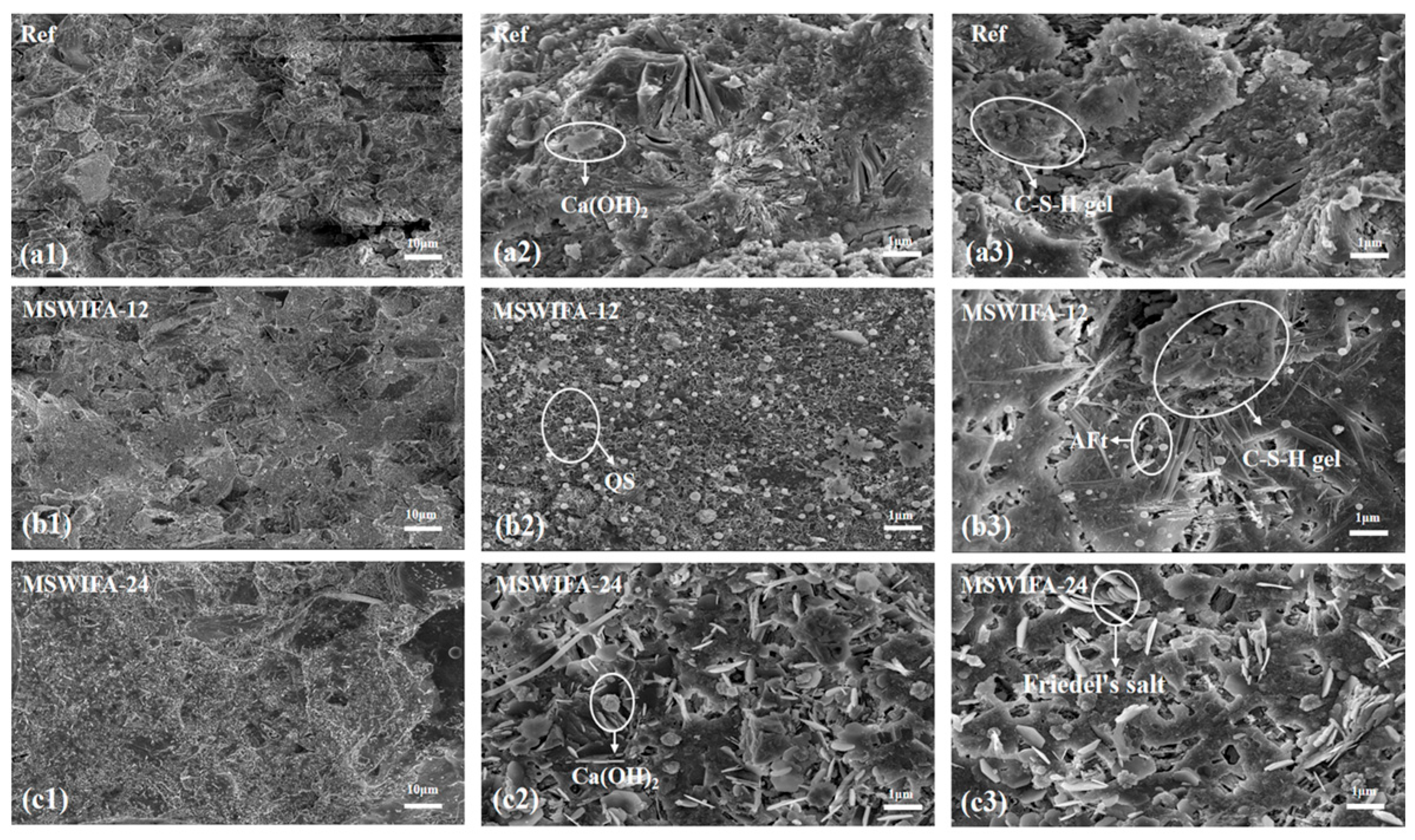
3.4.3. SEM
3.5. Heavy Metal Immobilization Performance Analysis of UHPC
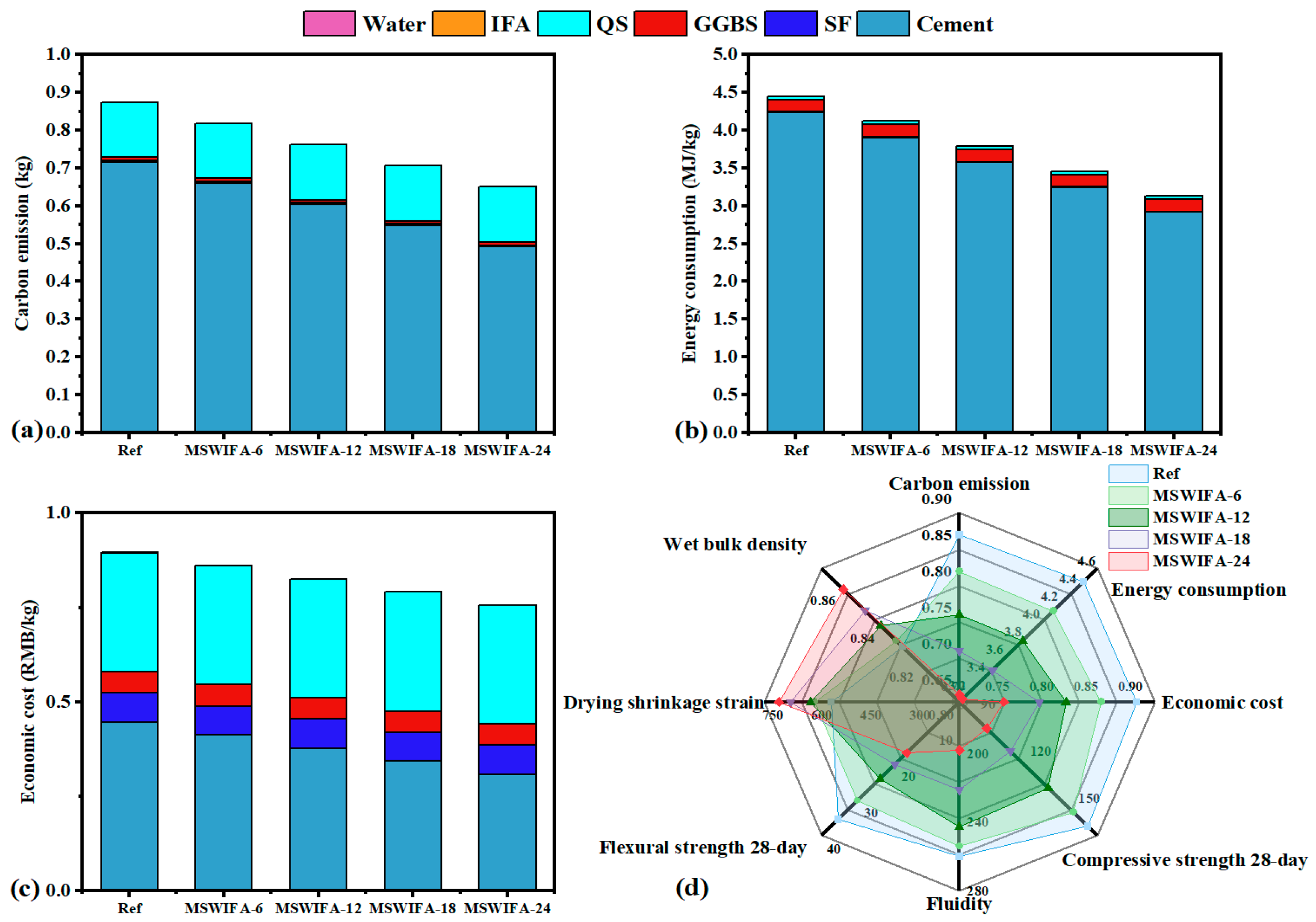
3.6. Integrated Ecological-Economic Analysis of UHPC
4. Conclusions
- (1)
- Incorporating silica fume alone at ≤6% improves fluidity; at 10% it significantly increases 28-day compressive strength through micro-filler action and C-S-H densification. Ground slag at ≤20% used singly prolongs setting, enhances pumpability and offsets flow loss; at 30% it slightly reduces early strength but surpasses the reference at 56 days. MSWIFA particles exhibit a coarse texture and a large specific surface area. The partial replacement of cement with MSWIFA in UHPC leads to a decrease in fluidity, a reduction in setting time, and an increase in wet packing density.
- (2)
- The substitution of cement with MSWIFA adversely affects the mechanical properties of UHPC. Specimens from the MSWIFA-6 and MSWIFA-12 groups attain 28-day compressive strengths of 150.1 MPa and 134.6 MPa, respectively, alongside flexural strengths of 30.4 MPa and 24.2 MPa at the same curing age. Within the substitution range of 12%, these mechanical properties satisfy the requirements for UHPC. Specimens in the MSWIFA-18 and MSWIFA-24 groups exhibit 28-day compressive strengths of 111.3 MPa and 96.7 MPa, respectively. Within a 24% replacement rate range, their mechanical properties meet the requirements for HPC.
- (3)
- The incorporation of MSWIFA increases the drying shrinkage of UHPC. After immersion in chloride and sulfate solutions, both mass and compressive strength of UHPC exhibit an initial increase followed by a subsequent decrease. The corrosion coefficient demonstrates positive values at 28 days but turns negative at 56 days. The maximum mass loss rate after immersion in both solutions reaches 1.53%, while the maximum corrosion coefficient attains 1.127. At a 24% replacement rate, MSWIFA-UHPC displays excellent resistance to chloride ion penetration and sulfate attack.
- (4)
- This study shows that up to 12 wt% MSWIFA can be incorporated into UHPC. Furthermore, the resulting UHPC maintains a compressive strength above 120 MPa and immobilizes heavy metals to meet both Chinese and EU leaching limits.
- (5)
- The substitution of cement with MSWIFA in UHPC resulted in reductions of 7.42–29.73% in energy consumption, 6.38–25.51% in CO2 emissions, and 2.56–15.38% in cost. This MSWIFA replacement strategy demonstrates significant synergistic environmental and economic benefits, facilitating efficient resource utilization and providing a feasible pathway for the green and low-carbon transformation of UHPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Liu, H.; Yang, N. Comparative study of municipal solid waste incinerator fly ash reutilization in China: Environmental and economic performances. Resour. Conserv. Recycl. 2021, 169, 105541. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, L. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef] [PubMed]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Deng, X.; Xie, C.; Zhang, J. Techno-economic analysis of municipal solid waste treatment for poly-generation system. Sci. Total Environ. 2024, 912, 168869. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Zhu, F. Study on the reduction of chlorine and heavy metals in municipal solid waste incineration fly ash by organic acid and microwave treatment and the variation of environmental risk of heavy metals. Sci. Total Environ. 2023, 870, 161929. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Carcelen-Taboada, V.; Fernández-Jiménez, A. Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Constr. Build. Mater. 2016, 105, 218–226. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Wu, K. Performance and risk assessment of alinite cement-based materials from municipal solid waste incineration fly ash (MSWIFA). Mater. Struct. 2016, 49, 2383–2391. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Xie, G. Resource utilization of municipal solid waste incineration fly ash-cement and alkali-activated cementitious materials: A review. Sci. Total Environ. 2022, 852, 158254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, X.; Liu, R. Manufacture of alkali-activated cementitious materials using municipal solid waste incineration fly ash (MSWIFA): The effect of the Si/Al molar ratio on fresh and hardened properties. Constr. Build. Mater. 2023, 409, 134075. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef]
- Wang, B.; Ding, W.; Fan, C. Immobilization properties and reaction mechanism of municipal solid waste incineration fly ash with alkali activation technology. J. Build. Eng. 2024, 91, 109559. [Google Scholar] [CrossRef]
- Xu, Q.; Shang, N.; Ko, J.H. Utilization of municipal solid waste incineration (MSWIFA) in geopolymer concrete: A study on compressive strength and leaching characteristics. Materials 2024, 17, 4609. [Google Scholar] [CrossRef]
- Wong, G.; Gan, M.; Fan, X. Co-disposal of municipal solid waste incineration fly ash and bottom slag: A novel method of low temperature melting treatment. J. Hazard. Mater. 2021, 408, 124438. [Google Scholar] [CrossRef] [PubMed]
- Bie, R.; Chen, P.; Song, X. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Zhou, Y. Multi-layered cement-hydrogel composite with high toughness, low thermal conductivity, and self-healing capability. Nat. Commun. 2023, 14, 3438. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Wang, F.; Du, Y.; Jiao, D. Wood-inspired cement with high strength and multifunctionality. Adv. Sci. 2021, 8, 2000096. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, Z.; Gu, Z. Impregnate carbonation: CO2–guided in situ growth of robust superhydrophobic structures on concrete surfaces. Adv. Mater. 2024, 36, 2405492. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.; Makul, N.; Fediuk, R. Global carbon recoverability experiences from the cement industry. Case Stud. Constr. Mater. 2022, 17, e01439. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T. Production and properties of ferrite-rich CSAB cement from metallurgical industry residues. Sci. Total Environ. 2020, 712, 136208. [Google Scholar] [CrossRef]
- Gao, P.; Zha, W.; Chu, Y. Calculation model for CO2 emissions of blended cement production. J. Clean. Prod. 2025, 489, 144646. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Qi, Y. Characteristics and leaching behavior of MSWI fly ash in novel solidification/stabilization binders. Waste Manag. 2021, 131, 277–285. [Google Scholar] [CrossRef]
- Tome, S.; Etoh, M.A.; Etame, J. Characterization and leachability behaviour of geopolymer cement synthesised from municipal solid waste incinerator fly ash and volcanic ash blends. Recycling 2018, 3, 50. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, D.; Zhao, Z. High-performance geopolymers with municipal solid waste incineration fly ash: Influence on the mechanical and environmental properties. Buildings 2024, 14, 3518. [Google Scholar] [CrossRef]
- Tan, J.; Dan, H.; Li, J. Use of municipal waste incineration fly ashes (MSWI FA) in metakaolin-based geopolymer. Environ. Sci. Pollut. Res. 2022, 29, 80727–80738. [Google Scholar] [CrossRef]
- Shao, Y.; Hou, H.; Wang, G. Characteristics of the stabilized/solidified municipal solid wastes incineration fly ash and the leaching behavior of Cr and Pb. Front. Environ. Sci. Eng. 2016, 10, 192–200. [Google Scholar] [CrossRef]
- Holmes, R.R.; Hart, M.L.; Kevern, J.T. Heavy metal removal capacity of individual components of permeable reactive concrete. J. Contam. Hydrol. 2017, 196, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.; Huang, S.S.; Onaizi, A.M. Recent trends in ultra-high performance concrete (UHPC): Current status, challenges, and future prospects. Constr. Build. Mater. 2022, 352, 129029. [Google Scholar] [CrossRef]
- Lande, I.; Thorstensen, R.T. Comprehensive sustainability strategy for the emerging ultra-high-performance concrete (UHPC) industry. Clean. Mater. 2023, 8, 100183. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Phase development in normal and ultra high performance cementitious systems by quantitative X-ray analysis and thermoanalytical methods. Cem. Concr. Res. 2009, 39, 69–76. [Google Scholar] [CrossRef]
- Bentz, D.P.; Conway, J.T. Computer modeling of the replacement of “coarse” cement particles by inert fillers in low w/c ratio concretes: Hydration and strength. Cem. Concr. Res. 2001, 31, 503–506. [Google Scholar] [CrossRef]
- Abbas, S.; Nehdi, M.L.; Saleem, M.A. Ultra-high performance concrete: Mechanical performance, durability, sustainability and implementation challenges. Int. J. Concr. Struct. Mater. 2016, 10, 271–295. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, L.; Wang, J. Performance of ultra-high-performance concrete incorporating municipal solid waste incineration fly ash. Case Stud. Constr. Mater. 2022, 17, e01155. [Google Scholar] [CrossRef]
- Mao, J.; Zhou, A.; Liang, Y. Innovative dual-benefit recycling and sustainable management of municipal solid waste incineration fly ash via ultra-high performance concrete. Sci. Total Environ. 2024, 957, 177852. [Google Scholar] [CrossRef]
- Xing, F.; Zheng, X.; Wu, D. Study on synergistic solidification method for municipal solid waste incineration fly ash: Core chemical stabilization and shell physical encapsulation. Case Stud. Constr. Mater. 2025, 22, e04651. [Google Scholar] [CrossRef]
- Partanen, J.; Backman, P.; Backman, R. Absorption of HCl by limestone in hot flue gases. Part II: Importance of calcium hydroxychloride. Fuel 2005, 84, 1674–1684. [Google Scholar] [CrossRef]
- GB/T2419-2005; Test Method for Fluidity of Cement Mortar. Standardization Administration of China: Beijing, China, 2021.
- JGJ/T70-2009; Standard for Test method of Performance on Building Mortar. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- Wong, H.H.C.; Kwan, A.K.H. Packing density of cementitious materials: Part 1—Measurement using a wet packing method. Mater. Struct. 2008, 41, 689–701. [Google Scholar] [CrossRef]
- GB/T17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- GB/T50082-2024; Standard for test methods of long-term Performance and Durability of Ordinary Concrete. Standardization Administration of China: Beijing, China, 2024.
- HJ/T300-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2007.
- HJ 557-2010; Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2010.
- Li, Z.; Zhang, W.; Jin, H. Research on the durability and sustainability of an artificial lightweight aggregate concrete made from municipal solid waste incinerator bottom ash (MSWIBA). Constr. Build. Mater. 2023, 365, 129993. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Zhang, W. Effects of different supplementary cementitious materials on the performance and environment of eco-friendly mortar prepared from waste incineration bottom ash. Constr. Build. Mater. 2022, 356, 129277. [Google Scholar] [CrossRef]
- Guo, P.; Meng, W.; Du, J. Lightweight ultra-high-performance concrete (UHPC) with expanded glass aggregate: Development, characterization, and life-cycle assessment. Constr. Build. Mater. 2023, 371, 130441. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, H.; Shi, W. Feasibility of preparing self-compacting mortar via municipal solid waste incineration bottom ash: An experimental study. Arch. Civ. Mech. Eng. 2023, 23, 251. [Google Scholar] [CrossRef]
- He, W.; Yang, Y.; Zhu, X. Experimental research on mechanical and impact properties of ceramsite prepared from secondary aluminum dross and municipal solid waste incineration ash. Sustain. Environ. Res. 2025, 35, 2. [Google Scholar] [CrossRef]



| Specific Surface Area (m2·kg−1) | Soundness | Setting Time (min) | Compressive Strength (MPa) | Flexural Strength (MPa) | |||
|---|---|---|---|---|---|---|---|
| Initial | Final | 7 Days | 28 Days | 7 Days | 28 Days | ||
| 348 | Qualified | 195 | 255 | 36.6 | 60.1 | 5.9 | 8.4 |
| Materials | CaO | Cl | SO3 | SiO2 | K2O | Na2O | MgO | Al2O3 | Fe2O3 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 56.90 | - | 2.36 | 24.30 | 0.87 | 0.13 | 3.01 | 7.07 | 2.51 | 2.85 |
| Silica fume | 0.65 | - | 0.47 | 95.06 | 0.27 | 0.09 | 0.59 | 0.25 | 0.08 | 2.54 |
| Slag | 34.00 | - | 1.62 | 34.20 | - | - | 6.21 | 17.60 | 1.01 | 5.36 |
| MSWIFA | 43.06 | 22.15 | 7.85 | 6.08 | 5.74 | 5.70 | 3.50 | 2.02 | 1.68 | 2.22 |
| QS | 0.21 | 0.31 | 0.35 | 98.20 | 0.08 | 0.05 | 0.12 | 0.61 | 0.14 | 0.11 |
| Heavy Metal Ions | Pb | Zn | Cu | Cr | Cd |
|---|---|---|---|---|---|
| Leaching concentration (mg/L) | 5.18 | 13.97 | 3.42 | 0.72 | 1.83 |
| Standard limits for leaching toxicity of hazardous wastes in China (mg/L) | 5 | 100 | 100 | 5 | 1 |
| EU standard limits for leaching toxicity of hazardous wastes (mg/L) | 0.25 | 50 | 10 | 0.5 (Cr6+) | 0.04 |
| MSW landfill control standards (mg/L) | 0.25 | 100 | 40 | 0.5 | 0.15 |
| UHPC Sample | Cement (g) | Silica Fume(g) | Slag (g) | MSWIFA (g) | QS (g) | Water (g) | Superplasticizer * (%) |
|---|---|---|---|---|---|---|---|
| Ref | 615.92 | 84.96 | 99.12 | 0 | 464 | 144 | 2 |
| MSWIFA-6 | 567.92 | 84.96 | 99.12 | 48 | 464 | 144 | 2 |
| MSWIFA-12 | 519.92 | 84.96 | 99.12 | 96 | 464 | 144 | 2 |
| MSWIFA-18 | 471.92 | 84.96 | 99.12 | 144 | 464 | 144 | 2 |
| MSWIFA-24 | 423.92 | 84.96 | 99.12 | 192 | 464 | 144 | 2 |
| Heavy Metal | Pb | Zn | Cu | Cr | Cd | |
|---|---|---|---|---|---|---|
| Leaching concentration (mg/L) | MSWIFA-6 | 0.11 | 0.12 | 0.006 | 0.012 | 0.002 |
| MSWIFA-12 | 0.10 | 0.98 | 0.019 | 0.034 | 0.007 | |
| MSWIFA-18 | 0.17 | 4.34 | 2.58 | 0.15 | 0.70 | |
| MSWIFA-24 | 0.21 | 8.55 | 2.81 | 0.50 | 0.80 | |
| Standard limits for leaching toxicity of hazardous wastes (mg/L) | 5 | 100 | 100 | 5 | 1 | |
| EU standard limits for leaching toxicity of hazardous wastes (mg/L) | 0.25 | 50 | 10 | 0.5 (Cr6+) | 0.04 | |
| Control standard for municipal solid waste landfills (mg/L) | 0.25 | 100 | 40 | 0.5 | 0.15 | |
| Materials | Energy Consumption (MJ/kg) | Carbon Emission (kg) | Economic Cost (RMB/kg) |
|---|---|---|---|
| Cement (52.5) | 5.5 | 0.93 | 0.48 |
| Silica fume | 0.06 | 0.03 | 0.73 |
| Slag | 1.33 | 0.07 | 0.18 |
| Quartz sand | 0.067 | 0.233 | 0.50 |
| MSWIFA | 0 | 0 | 0 |
| Water | 0.01 | 0.001 | 0.0028 |
| UHPC Sample | Energy Consumption (MJ/kg) | Reduction Rates (%) | Carbon Emission (kg) | Reduction Rates (%) | Economic Cost (RMB/kg) | Reduction Rates (%) |
|---|---|---|---|---|---|---|
| Ref | 4.45 | 0.00 | 0.87 | 0.00 | 0.78 | 0.00 |
| MSWIFA-5 | 4.12 | 7.42 | 0.82 | 6.38 | 0.76 | 2.56 |
| MSWIFA-10 | 3.79 | 14.86 | 0.76 | 12.76 | 0.73 | 6.41 |
| MSWIFA-20 | 3.46 | 22.30 | 0.71 | 19.14 | 0.69 | 11.54 |
| MSWIFA-30 | 3.13 | 29.73 | 0.65 | 25.51 | 0.66 | 15.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; He, Y.; Liu, J.; Zhang, F.; Hao, X.; Liu, C. Performance Research of Ultra-High Performance Concrete Incorporating Municipal Solid Waste Incineration Fly Ash. Materials 2025, 18, 4623. https://doi.org/10.3390/ma18194623
Liu F, He Y, Liu J, Zhang F, Hao X, Liu C. Performance Research of Ultra-High Performance Concrete Incorporating Municipal Solid Waste Incineration Fly Ash. Materials. 2025; 18(19):4623. https://doi.org/10.3390/ma18194623
Chicago/Turabian StyleLiu, Fengli, Yize He, Junhua Liu, Feiyang Zhang, Xiaofei Hao, and Chang Liu. 2025. "Performance Research of Ultra-High Performance Concrete Incorporating Municipal Solid Waste Incineration Fly Ash" Materials 18, no. 19: 4623. https://doi.org/10.3390/ma18194623
APA StyleLiu, F., He, Y., Liu, J., Zhang, F., Hao, X., & Liu, C. (2025). Performance Research of Ultra-High Performance Concrete Incorporating Municipal Solid Waste Incineration Fly Ash. Materials, 18(19), 4623. https://doi.org/10.3390/ma18194623






