Microstructure and Mechanical and Corrosion Behavior of Novel High-Entropy CoCrFeNiSiVx (x = 0.25; 0.5; 0.75; 1.0) Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
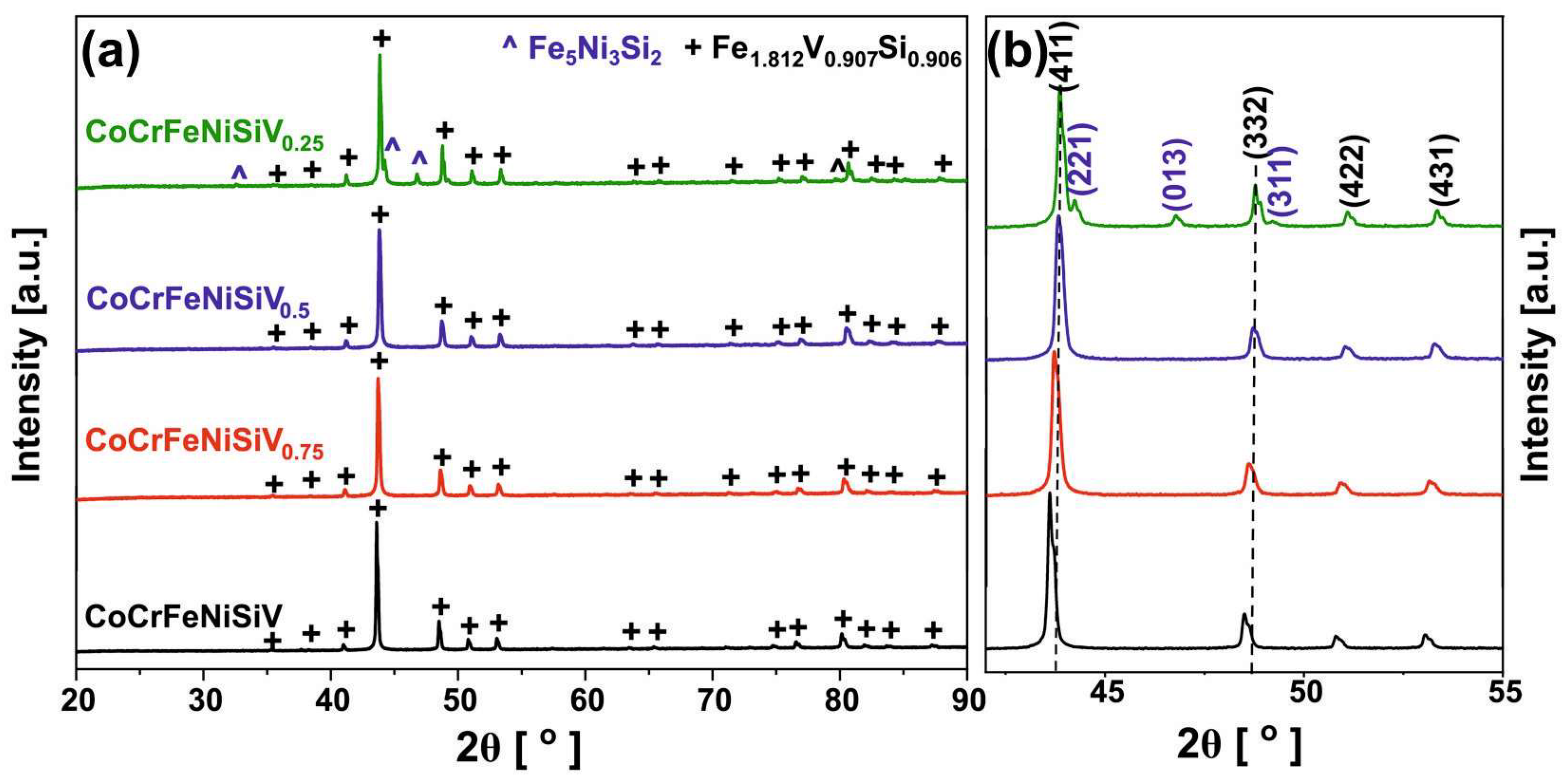
3.1. XRD Analysis
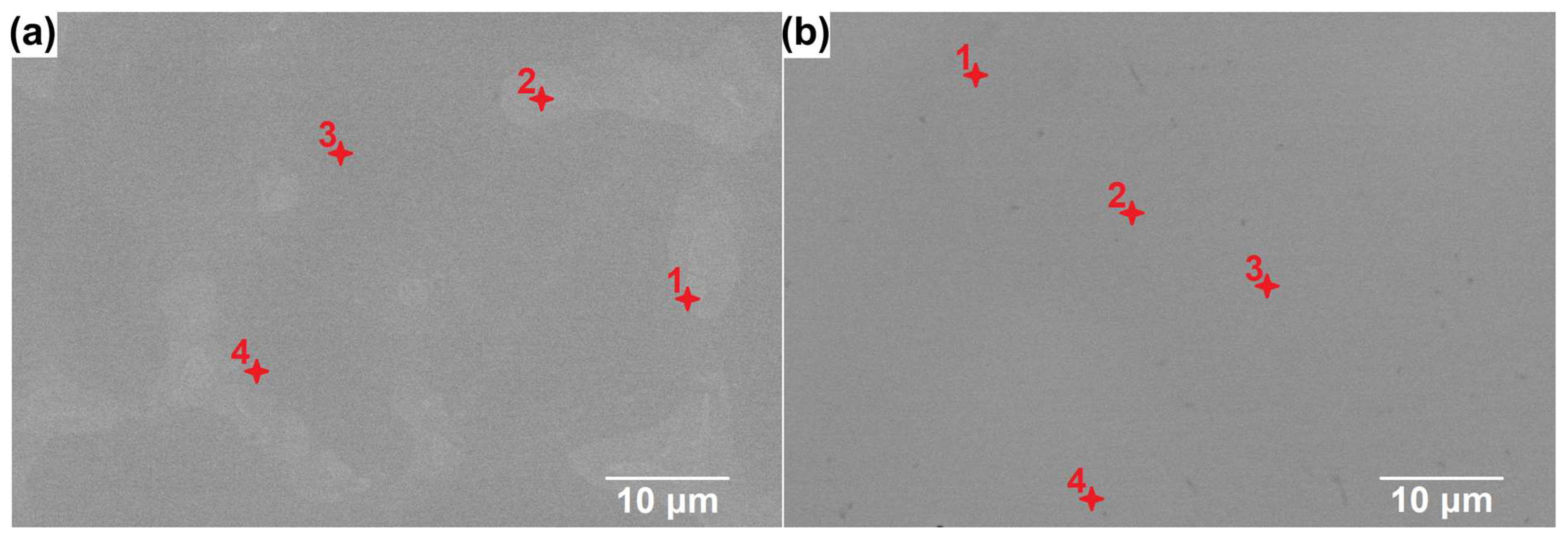
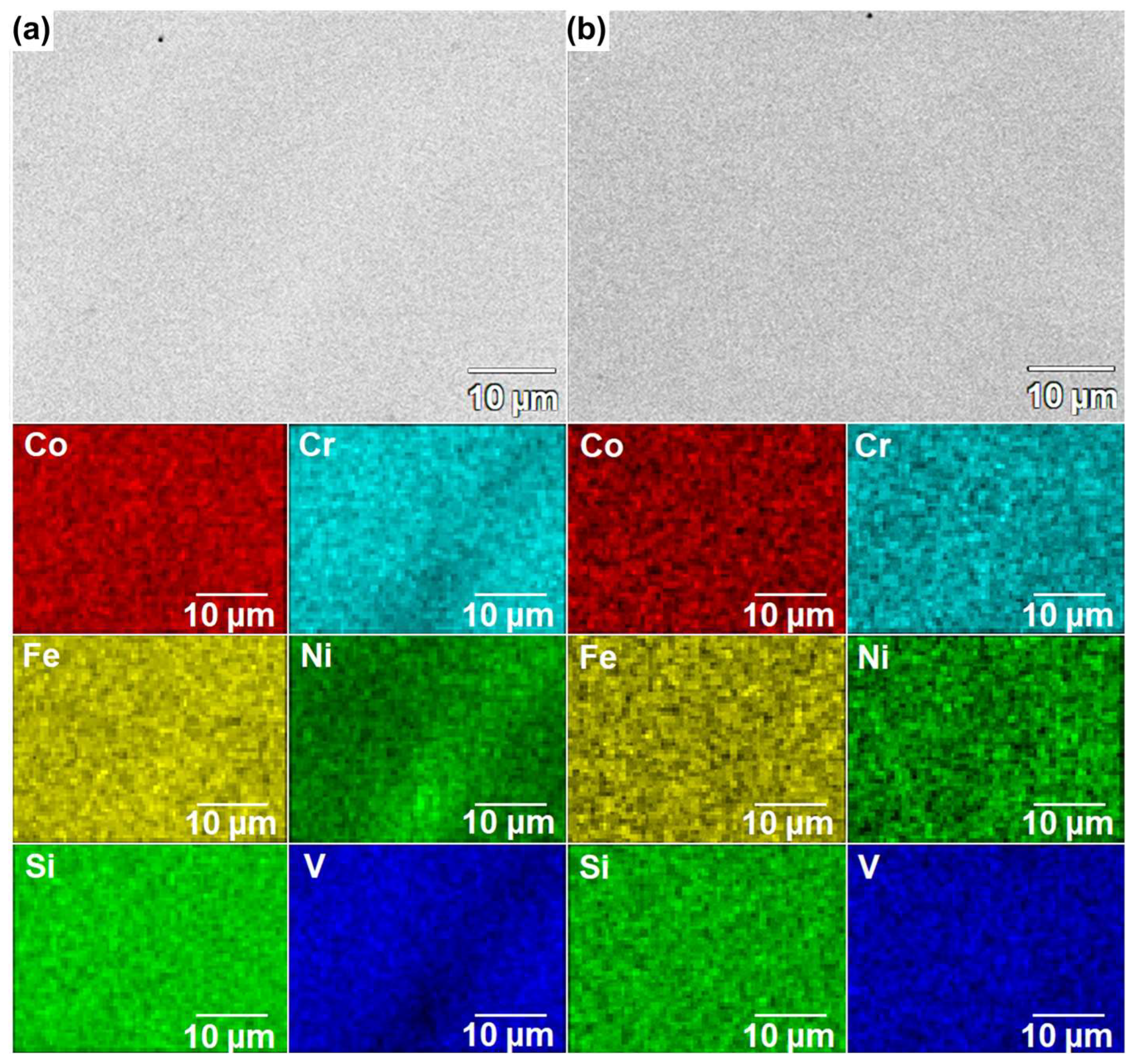
3.2. Microscopic Observations
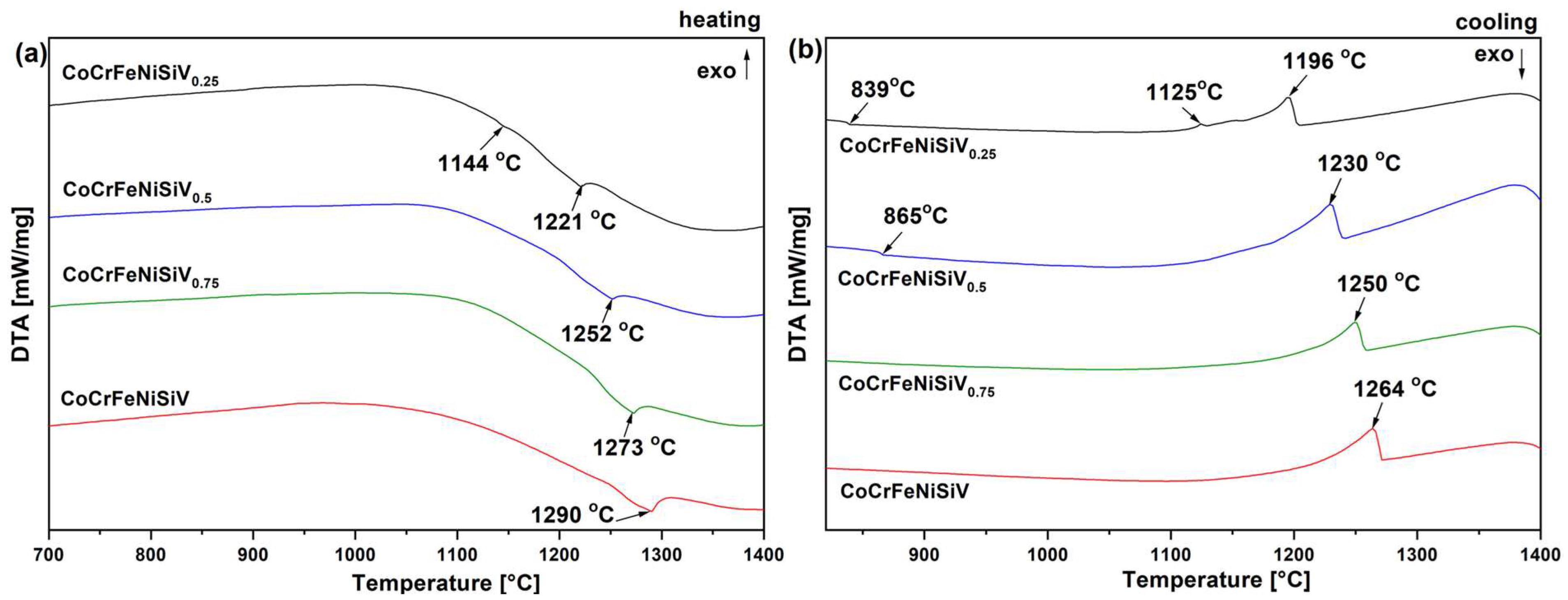
3.3. Differential Thermal Analysis
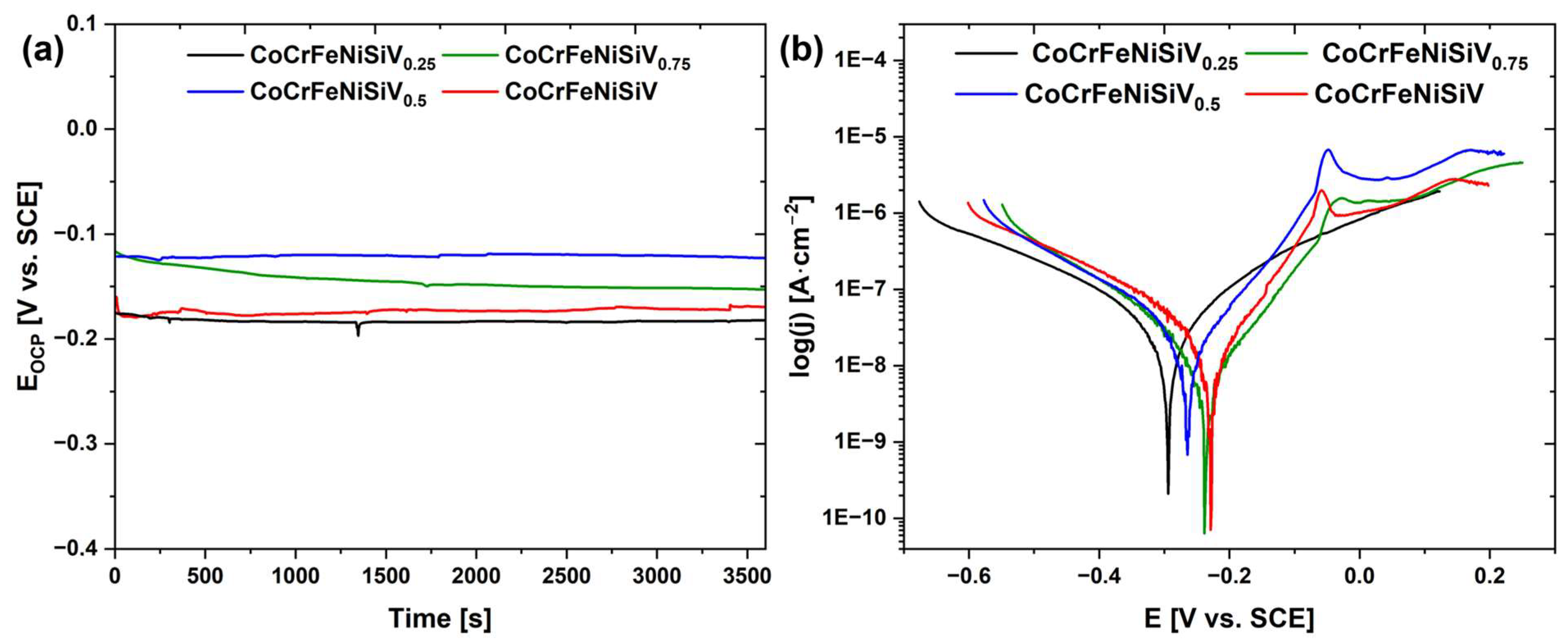
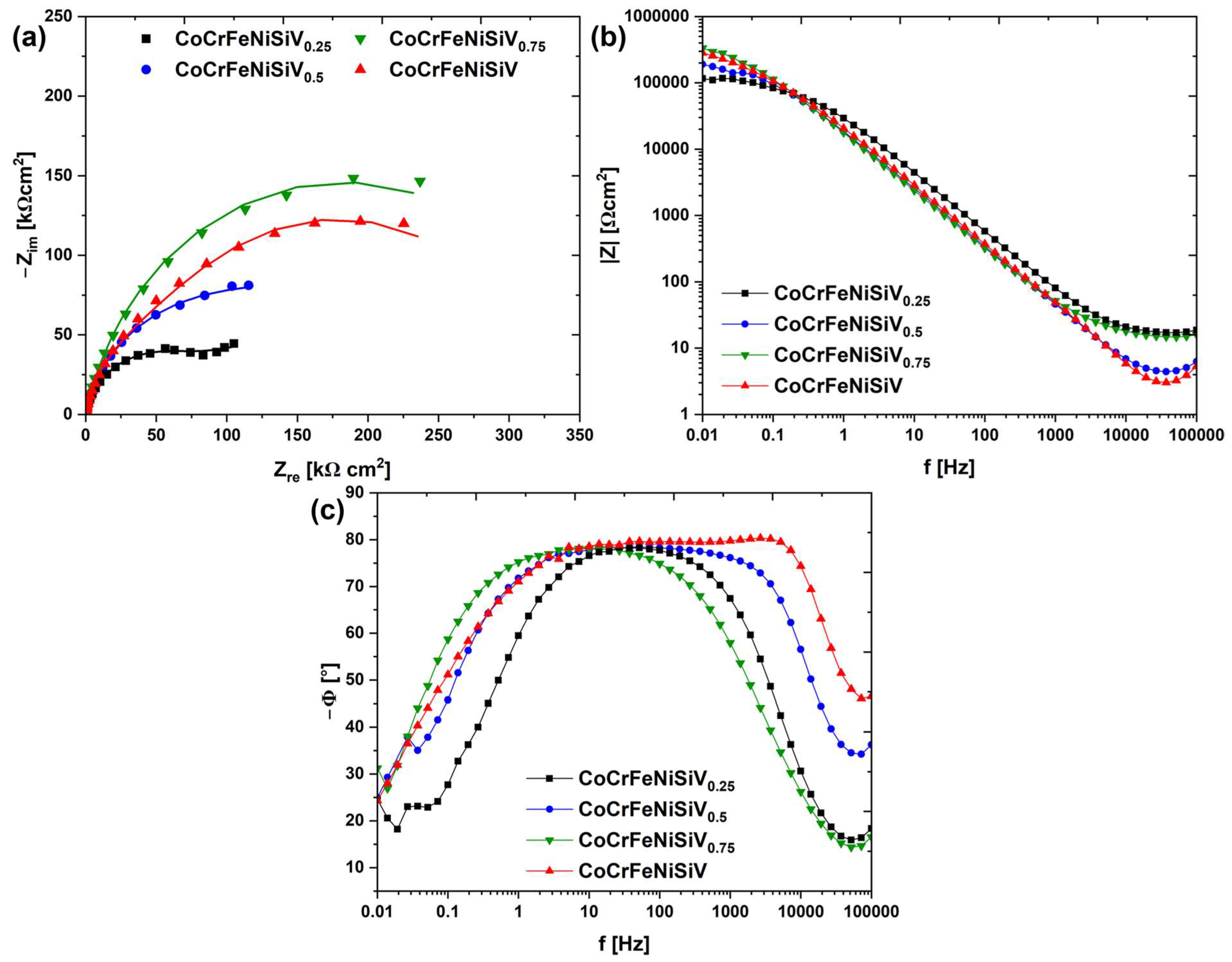
3.4. Electrochemical Investigations
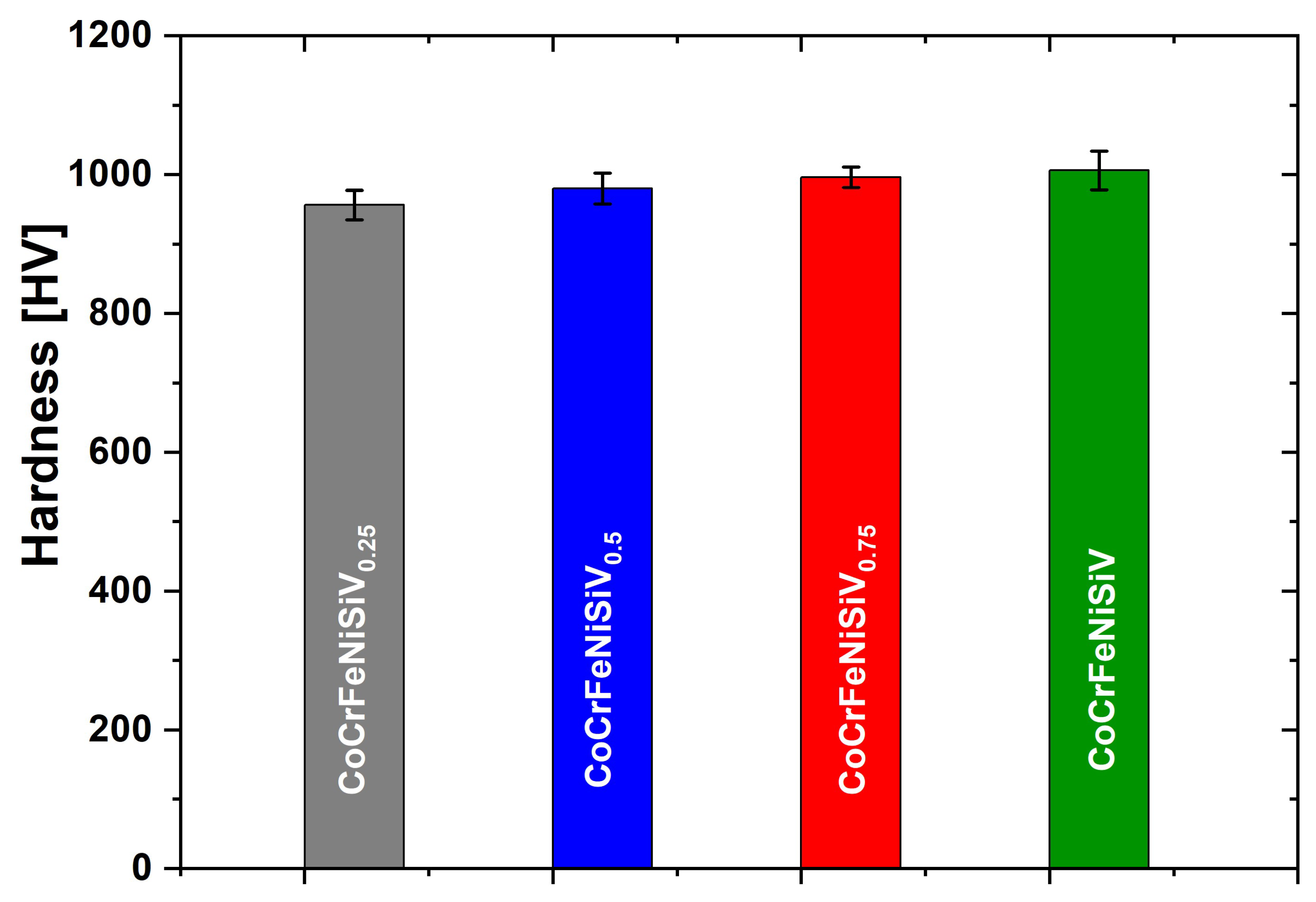
3.5. Hardness
3.6. Wear Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.R.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, J.; Gan, J.Y.; Chin, T.S.; Shun, T.T. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Liu, D.; Yu, Q.; Kabra, S.; Jiang, M.; Forna- Kreutzer, P.; Zhang, R.; Payne, M.; Walsh, F.; Gludovatz, B.; Asta, M.; et al. Exceptional fracture toughness of CrcCoNi-based medium- and high-entropy alloys at 20 kelvin. Science 2022, 378, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ren, Y.; Zeng, Y.; Ma, W.; Morita, K.; Zhan, S.; Lei, Y.; Lv, G.; Li, S.; Wu, J. Recent progress in high-entropy alloys: A focused review of preparation processes and properties. J. Mater. Res. Technol. 2024, 29, 2689–2719. [Google Scholar] [CrossRef]
- Jiao, W.; Miao, J.; Lu, Y.; Chen, X.; Ren, Z.; Yin, G.; Li, T. Designing CoCrFeNi-M (M = Nb, Ta, Zr, and Hf) eutectic high-entropy alloys via a modified simple mixture method. J. Alloys Compd. 2023, 941, 168975. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Soni, V.K.; Sinha, A.K. Effect of alloying elements, phases and heat treatments on properties of high-entropy alloys: A review. Trans. Indian Inst. Met. 2023, 76, 897–914. [Google Scholar] [CrossRef]
- Khulief, Z.T. High entropy alloys, properties, applications, and effect of alloying elements. J. Univ. Babylon Eng. Sci. 2021, 29, 18–30. [Google Scholar]
- Man, J.; Wu, B.; Duan, G.; Zhang, L.; Du, X.; Liu, Y.; Esling, C. Super-high strength of a CoCrNiFe based high entropy alloy. J. Mater. Sci. Technol. 2024, 177, 79–84. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Wan, Y.; Chen, H.; Wang, Y.; Yang, J. Strengthening by Ti, Nb, and Zr doping on microstructure, mechanical, tribological, and corrosion properties of CoCrFeNi high-entropy alloys. J. Alloys Compd. 2024, 984, 173819. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, K.; Huang, L.; Xie, B.; Ren, D.; Tang, C.; Feng, W. Effect of doping with different Nb contents on the properties of CoCrFeNi high-entropy alloys. Materials 2023, 16, 6407. [Google Scholar] [CrossRef]
- Lei, H.; Chen, C.; Ye, X.; Kang, H.; Li, Z.; Fu, J.; Zhang, T.; Gao, Z.; Li, B.; Fang, D.; et al. Synergistic effect of Nb and W alloying on the microstructure and mechanical properties of CoCrFeNi high entropy alloys. J. Mater. Res. Technol. 2024, 28, 3765–3774. [Google Scholar] [CrossRef]
- Shi, P.; Yu, J.; Yao, B.; Si, J.; Wu, L.; Wu, X.; Wang, Y. Uncovering the origin of unique elemental distribution behaviors of Vanadium in high entropy alloys. Int. J. Refract. Met. Hard Mater. 2024, 122, 106715. [Google Scholar] [CrossRef]
- Yin, B.; Maresca, F.; Curtin, W.A. Vanadium is an optimal element for strengthening in both fcc and bcc high-entropy alloys. Acta Mater. 2020, 188, 486–491. [Google Scholar] [CrossRef]
- Jain, R.; Rahul, M.R.; Chakraborty, P.; Sabat, R.K.; Samal, S.; Phanikumar, G.; Tewari, R. Design and deformation characteristics of single-phase Co-Cr-Fe-Ni-V high entropy alloy. J. Alloys Compd. 2021, 888, 161579. [Google Scholar] [CrossRef]
- Wang, X.G.; Sun, M.; Liu, J.X.; Liu, X.Q.; Ke, Y.B.; Jiang, W.B.; Wang, H.; Fang, Q.F.; Wang, X.P. Effects of vanadium content on the microstructure and tensile properties of NbTiVxZr high-entropy alloys. J. Alloys Compd. 2024, 987, 174227. [Google Scholar] [CrossRef]
- Qin, G.; Wang, S.; Chen, R.; Zheng, H.; Wang, L.; Su, Y.; Guo, J.; Fu, H. Improvement of microstructure and mechanical properties of CoCrCuFeNi high-entropy alloys by V addition. J. Mater. Eng. Perform. 2019, 28, 1049–1056. [Google Scholar] [CrossRef]
- Souto, C.A.; Mirhan, A.L.R.; Yao, J.-Y.; Serrano, L.B.; Travessa, D.N.; Cardoso, K.R. Microstructural characteristics and corrosion behaviour of AlCoCrFeNi high-entropy alloys containing Vanadium. Electrochim. Acta 2024, 503, 144867. [Google Scholar] [CrossRef]
- Ye, Z.; Li, C.; Zhang, X.; Liao, Y.; Gu, J. The influence of vanadium element on the microstructure and mechanical properties of (FeCoNi)100−xVx high-entropy alloys. Mater. Charact. 2022, 192, 112232. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Choi, W.M.; Jo, Y.H.; Kia, D.G.; Sohn, S.S.; Lee, S.; Lee, B.J. A thermodynamic description of the Co-Cr-Fe-Ni-V system for high-entropy alloy design. Calphad 2019, 66, 101624. [Google Scholar] [CrossRef]
- Song, T.J.; Jin, S.B.; Chen, J.A.; Yu, M.Y.; Zhu, J.M. The influences of vanadium addition and temperature on the microstructure, and mechanical properties of Al0.1CoCrFeNiVx multi-principal element alloys. J. Alloys Compd. 2024, 979, 173581. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, X.; Dong, M.; Hu, M.; Yang, Y. Preparation and effect of vanadium addition on the mechanical properties of CoCrFeNiVx high-entropy alloy. J. Mater. Res. Technol. 2023, 27, 7705–7712. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, L.; Shi, H.; Wang, Z.; Yang, Y.; Meng, Y.; Zhang, L.; Cui, Y. Microstructural evolution from dendrites to core-shell equiaxed grain morphology for CoCrFeNiVx high-entropy alloys in metallic casting mold. Metals 2019, 9, 1172. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chuang, M.H. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Tokarewicz, M.; Gradzka-Dahlke, M.; Nowak, W.J.; Gradzik, A.; Szala, M.; Walczak, M. Effect of vanadium addition on the wear resistance of Al0.7CoCrFeNi high-entropy alloy. Materials 2024, 17, 6021. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.L.; Xu, Z.Q.; Cheng, X.W. Effects of vanadium concentration on mechanical properties of VxNbMoTa refractory high-entropy alloys. Mater. Sci. Eng. A 2021, 808, 140848. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Almahmoud, A.; Obeidat, A. Impact of vanadium and zirconium contents on properties of novel lightweight Ti3ZryNbVx refractory high-entropy alloys. Solids 2025, 6, 2. [Google Scholar] [CrossRef]
- Sivanantham, A.; Lee, H.; Hwang, S.W.; Lee, H.U.; Cho, S.B.; Ahn, B.; Cho, I.S. Complementary functions of vanadium in boosting electrocatalytic activity of CuCoNiFeMn high-entropy alloy for water splitting. Adv. Funct. Mater. 2023, 33, 2301153. [Google Scholar] [CrossRef]
- Wu, X.; Su, L.; Tieu, A.K.; Cheng, J.; Nguyen, C.; Zhu, H.; Yang, J.; Deng, G. Impact of vanadium (V) content on in-situ oxidation, high temperature mechanical strength and tribological properties of Al0.5CrFeNiVx entropy alloys. Tribol. Int. 2025, 202, 110375. [Google Scholar] [CrossRef]
- Son, S.; Kwak, J.; Haftlang, F.; Kwon, H.; Kim, Y.T.; Lee, S.; Kim, H.S. Corrosion behavior of VCrFeCoNi medium-entropy alloy in chloride solution. J. Mater. Res. Technol. 2023, 22, 2372–2382. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, T.; Du, S.; Wang, Q.; Xiong, R.; Jiao, Z.; Ma, S.; Wang, Z. Tuning the microstructure and strengthening mechanism of the CoCrFeNi high-entropy alloy via doping metalloid and interstitial elements. J. Alloys Compd. 2023, 960, 170622. [Google Scholar] [CrossRef]
- Babilas, R.; Bicz, J.; Radoń, A.; Kądziołka-Gaweł, M.; Łukowiec, D.; Matus, K.; Wyszkowska, E.; Kurpaska, Ł.; Rudomilova, D.; Młynarek-Żak, K. Structure, corrosion resistance and nanomechanical properties of CoCrFeNiX (X = Nb, Mo, B, Si) high entropy alloys. Electrochim. Acta 2025, 525, 145933. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, R.; Liu, T.; Fang, H.; Qin, G.; Su, Y.; Guo, J. Tailoring formation and proportion of strengthening phase in non-equiatomic CoCrFeNi high entropy alloy by alloying Si element. Intermetallics 2022, 147, 107617. [Google Scholar] [CrossRef]
- Guan, Z.; Nan, S.; Feng, C.; Xiao, M.; Song, H.; Qin, G.; Guo, H.; Wu, L.; Gou, H.; Ma, D.; et al. Multicomponent intermetallic compound CoCrFeNiSi: Crystal structure and magnetic properties. J. Magn. Magn. Mater. 2024, 606, 172401. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, R.; Ren, B.; Jiang, A.; Liu, Z.; Liu, J. Influence of Si content on microstructure and mechanical properties of CoCrFeNi high-entropy alloys. Metals 2025, 15, 538. [Google Scholar] [CrossRef]
- Kong, K.H.; Kim, K.C.; Kim, W.T.; Kim, D.H. Microstructural features of multicomponent FeCoCrNiSix alloys. Appl. Microsc. 2015, 45, 32–36. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.; Su, C.; Geng, Y.; Chen, X. Microstructure and mechanical properties of CoCrFeNiSix (x = 0, 0.25, 0.5, 0.75) high-entropy alloys based on powder plasma arc additive manufacturing. J. Mater. Eng. Perform. 2024, 33, 12413–12423. [Google Scholar] [CrossRef]
- Wei, D.; Gong, W.; Tsuru, T.; Lobzenko, I.; Li, X.; Harjo, S.; Kawasaki, T.; Do, H.S.; Bae, J.W.; Wagner, C.; et al. Si-addition contributes to overcoming the strength-ductility trade-off in high-entropy alloys. Int. J. Plast. 2022, 159, 103443. [Google Scholar] [CrossRef]
- Yaykas, H.; Eskalen, H.; Kavun, Y.; Göğebakan, M.; Kaya, A.H.; Yorulmaz, N. CoCrFeNiSi high entropy alloy: Synthesis, structural and radiation shielding properties. Prog. Nucl. Energy 2023, 165, 104930. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Jia, F.; Zhao, X.; Huang, B.; Ma, J.; Wang, C. Effect of Si element on phase transformation and mechanical properties for FeCoCrNiSix high entropy alloys. Mater. Lett. 2021, 282, 128809. [Google Scholar] [CrossRef]
- Lin, T.; Feng, M.; Lian, G.; Lu, H.; Chen, C.; Huang, X. Effects of Si content on the microstructure and properties of CoCrFeMnNiSix high-entropy alloy coatings by laser cladding. Mater. Charact. 2024, 216, 114246. [Google Scholar] [CrossRef]
- Nagarjuna, C.; Lee, H.; Dewangan, S.K.; Rao, R.K.; Pillai, G.M.; Kumar, V.; Ahn, B. Understanding the role of Si alloying on the structural, mechanical, wear and high temperature oxidation behavior of CrFeNiTiX (X = Si) high entropy alloys. J. Mater. Res. Technol. 2024, 33, 5119–5135. [Google Scholar] [CrossRef]
- Maji, B.C.; Krishnan, M. The effect of microstructure on the shape recovery of a Fe–Mn–Si–Cr–Ni stainless steel shape memory alloy. Scr. Mater. 2003, 48, 71–77. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Che, Y.; He, J.; Song, J.; Yang, B. A critical review on extraction and refining of vanadium metal. Int. J. Refract. Met. Hard Mater. 2021, 101, 105696. [Google Scholar] [CrossRef]
- Hao, D.; Zhang, N.; Zhang, Y.; Li, D. Effect of vanadium addition on microstructure and properties of Al0.5Cr0.9FeNi2.5 multi-principal alloys. J. Iron Steel Res. Int. 2021, 28, 586–596. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S.; Dong, C.; Li, X. Characterization of electrochemical and passive behaviour of alloy 59 in acid solution. Electrochim. Acta 2014, 135, 412–419. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.; Liu, Y.; Niu, T.; Li, H. Effect of Ti on the corrosion behavior of (FeCrCoNi)100−xTix alloy. Corros. Sci. 2022, 209, 110807. [Google Scholar] [CrossRef]
- Jahani, N.; Reihanian, M.; Gheisari, K. Alloying and corrosion characteristics of FeNiMnCu-based high entropy alloys. Mater. Chem. Phys. 2024, 315, 128990. [Google Scholar] [CrossRef]
- Boissy, C.; Ter-Ovanessian, B.; Mary, N.; Normand, B. Correlation between predictive and descriptive models to characterize the passive film—Study of pure chromium by electrochemical impedance spectroscopy. Electrochim. Acta 2015, 174, 430–437. [Google Scholar] [CrossRef]
- Luo, H.; Zou, S.; Chen, Y.-H.; Li, Z.; Du, C.; Li, X. Influence of carbon on the corrosion behaviour of interstitial equiatomic CoCrFeMnNi high-entropy alloys in a chlorinated concrete solution. Corros. Sci. 2020, 163, 108287. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lu, H.-N.; Tsai, M.-H.; Wu, B.-W.; Lo, Y.-C.; Wang, C.-C.; Chang, S.-Y.; Yen, S.-K. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high-entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Dai, C.; Luo, H.; Li, J.; Du, C.; Liu, Z.; Yao, J. X-ray photoelectron spectroscopy and electrochemical investigation of the passive behavior of high-entropy FeCoCrNiMox alloys in sulfuric acid. Appl. Surf. Sci. 2020, 499, 143903. [Google Scholar] [CrossRef]
- Hu, Q.; Ye, C.; Zhang, S.; Wang, X.; Du, C.; Wang, H. Mo content-depended competition between Cr2O3 enrichment and selective dissolution of CoCrFeNiMox high entropy alloys. NPJ Mater. Degrad. 2022, 6, 97. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Zhang, S.; Wang, J. Corrosion Behavior of High Entropy Alloys and Their Application in the Nuclear Industry—An Overview. Metals 2023, 13, 363. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Zirari, T.; Trabadelo, V. A review on wear, corrosion, and wear-corrosion synergy of high entropy alloys. Heliyon 2024, 10, e25867. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Gao, M.C.; Zhang, C.; Zhang, T.; Yang, H.; Qiao, J. Tribological Properties of AlCrCuFeNi2 High-Entropy Alloy in Different Conditions. Metall. Mater. Trans. A 2016, 47, 3312–3321. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Niu, M.; Jiang, Z.; Chen, H.; Sun, Y. High-temperature tribological behavior of CoCrFeNiV high-entropy alloys: A parallel comparison with CoCrFeNiMn high-entropy alloys. Tribol. Int. 2022, 174, 107736. [Google Scholar] [CrossRef]




| Alloy | Identified Phases | Space Group | Lattice Parameters | Statistical Quality Indices | |||
|---|---|---|---|---|---|---|---|
| Rwp (%) | Rpr (%) | Rexp (%) | χ2 | ||||
| CoCrFeNiSiV0.25 | Fe1.812V0.907Si0.906 | a = b = c = 8.7396(4) Å; α = β = γ = 90° | 1.31 | 1.00 | 1.47 | 0.80 | |
| Fe5Ni3Si2 | a = b = c = 6.1289(4) Å; α = β = γ = 90° | ||||||
| CoCrFeNiSiV0.5 | Fe1.812V0.907Si0.906 | a = b = c = 8.7622(3) Å; α = β = γ = 90° | 1.35 | 1.06 | 1.55 | 0.87 | |
| CoCrFeNiSiV0.75 | Fe1.812V0.907Si0.906 | a = b = c = 8.7794(10) Å; α = β = γ = 90° | 1.35 | 1.04 | 1.53 | 0.77 | |
| CoCrFeNiSiV | Fe1.812V0.907Si0.906 | a = b = c = 8.7870(4) Å; α = β = γ = 90° | 1.41 | 1.07 | 1.56 | 0.81 | |
| Alloy | Point | Content [at.%] | |||||
|---|---|---|---|---|---|---|---|
| Co | Cr | Fe | Ni | Si | V | ||
| CoCrFeNiSiV0.25 | 1 | 15.6 | 9.6 | 12.4 | 36.3 | 23.9 | 2.2 |
| 2 | 17.7 | 14.9 | 20.0 | 24.7 | 19.6 | 3.1 | |
| 3 | 19.2 | 21.6 | 17.9 | 16.4 | 18.8 | 6.1 | |
| 4 | 18.6 | 21.0 | 18.3 | 17.1 | 19.1 | 5.9 | |
| CoCrFeNiSiV | 1 | 16.7 | 17.9 | 16.4 | 13.8 | 16.0 | 19.2 |
| 2 | 16.4 | 17.6 | 15.6 | 15.1 | 16.4 | 18.9 | |
| 3 | 16.8 | 17.4 | 16.4 | 14.2 | 16.4 | 18.8 | |
| 4 | 16.8 | 17.5 | 16.0 | 15.8 | 16.0 | 17.9 | |
| Alloy | EOCP [V] (±0.01) | Ecorr [V] (±0.01) | Rp [kΩcm2] (±0.1) | jcorr [μA/cm2] (±0.01) |
|---|---|---|---|---|
| CoCrFeNiSiV0.25 | −0.182 | −0.294 | 173.5 | 2.01 |
| CoCrFeNiSiV0.5 | −0.123 | −0.264 | 367.6 | 0.37 |
| CoCrFeNiSiV0.75 | −0.153 | −0.237 | 465.2 | 0.32 |
| CoCrFeNiSiV | −0.169 | −0.228 | 514.3 | 0.17 |
| Alloy | Rs [Ωcm2] | R1 [kΩcm2] | CPE1 [μΩ−1 cm−2 sn] | n1 | R2 [kΩcm2] | CPE2 [μΩ−1 cm−2 sn] | n2 |
|---|---|---|---|---|---|---|---|
| CoCrFeNiSiV0.25 | 16.2 | 81.5 | 5.9 | 0.88 | 152.0 | 46.8 | 0.61 |
| CoCrFeNiSiV0.5 | 3.2 | 177.9 | 10.8 | 0.87 | - | - | - |
| CoCrFeNiSiV0.75 | 14.6 | 367.5 | 11.9 | 0.86 | - | - | - |
| CoCrFeNiSiV | 1.3 | 51.4 | 15.4 | 0.89 | 274.8 | 20.9 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babilas, R.; Spilka, M.; Młynarek-Żak, K.; Radoń, A.; Łoński, W.; Matus, K.; Bicz, J. Microstructure and Mechanical and Corrosion Behavior of Novel High-Entropy CoCrFeNiSiVx (x = 0.25; 0.5; 0.75; 1.0) Alloys. Materials 2025, 18, 4616. https://doi.org/10.3390/ma18194616
Babilas R, Spilka M, Młynarek-Żak K, Radoń A, Łoński W, Matus K, Bicz J. Microstructure and Mechanical and Corrosion Behavior of Novel High-Entropy CoCrFeNiSiVx (x = 0.25; 0.5; 0.75; 1.0) Alloys. Materials. 2025; 18(19):4616. https://doi.org/10.3390/ma18194616
Chicago/Turabian StyleBabilas, Rafał, Monika Spilka, Katarzyna Młynarek-Żak, Adrian Radoń, Wojciech Łoński, Krzysztof Matus, and Jakub Bicz. 2025. "Microstructure and Mechanical and Corrosion Behavior of Novel High-Entropy CoCrFeNiSiVx (x = 0.25; 0.5; 0.75; 1.0) Alloys" Materials 18, no. 19: 4616. https://doi.org/10.3390/ma18194616
APA StyleBabilas, R., Spilka, M., Młynarek-Żak, K., Radoń, A., Łoński, W., Matus, K., & Bicz, J. (2025). Microstructure and Mechanical and Corrosion Behavior of Novel High-Entropy CoCrFeNiSiVx (x = 0.25; 0.5; 0.75; 1.0) Alloys. Materials, 18(19), 4616. https://doi.org/10.3390/ma18194616






