Anticorrosion Activity of Low-Zinc Powder Coating Primers Containing Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Powder Coatings Primer
- -
- Epoxy resin: Eponac 825, Epoxy type 3 (Sir Industriale, Macherio, Italy)
- -
- DICY-based curing agent: Vicura MC-2844 (1-o-tolylbiguanide) (Vesta Chemicals, Zwolle, The Netherlands)
- -
- degassing agent: Benzoin (Aldrich, Buchs, Switzerland)
- -
- flow control agent: Byk 360P (Byk-Chemie, Wesel, Germany),
- -
- zinc dust: 4P32 (EverZinc, Liège, Belgium),
- -
- barite (natural, grinded mineral)
- -
- graphene single-walled carbon nanotubes (SWCNTs) in polyethylene wax Tuball: outer diameter: 1.6 ± 0.4 nm, length: >5 µm, G/D ratio: >90, specific surface area of 1 g SWCNT: ≥300 m2, number of SWCNTs in 1 g: 1017 pcs (OCSiAl, Leudelange, Luxembourg).
2.2. Application on the Substrate and Cross-Linking of the Coatings
2.3. Measurements
3. Results and Discussion
3.1. Physical and Mechanical Properties
3.2. Morphology
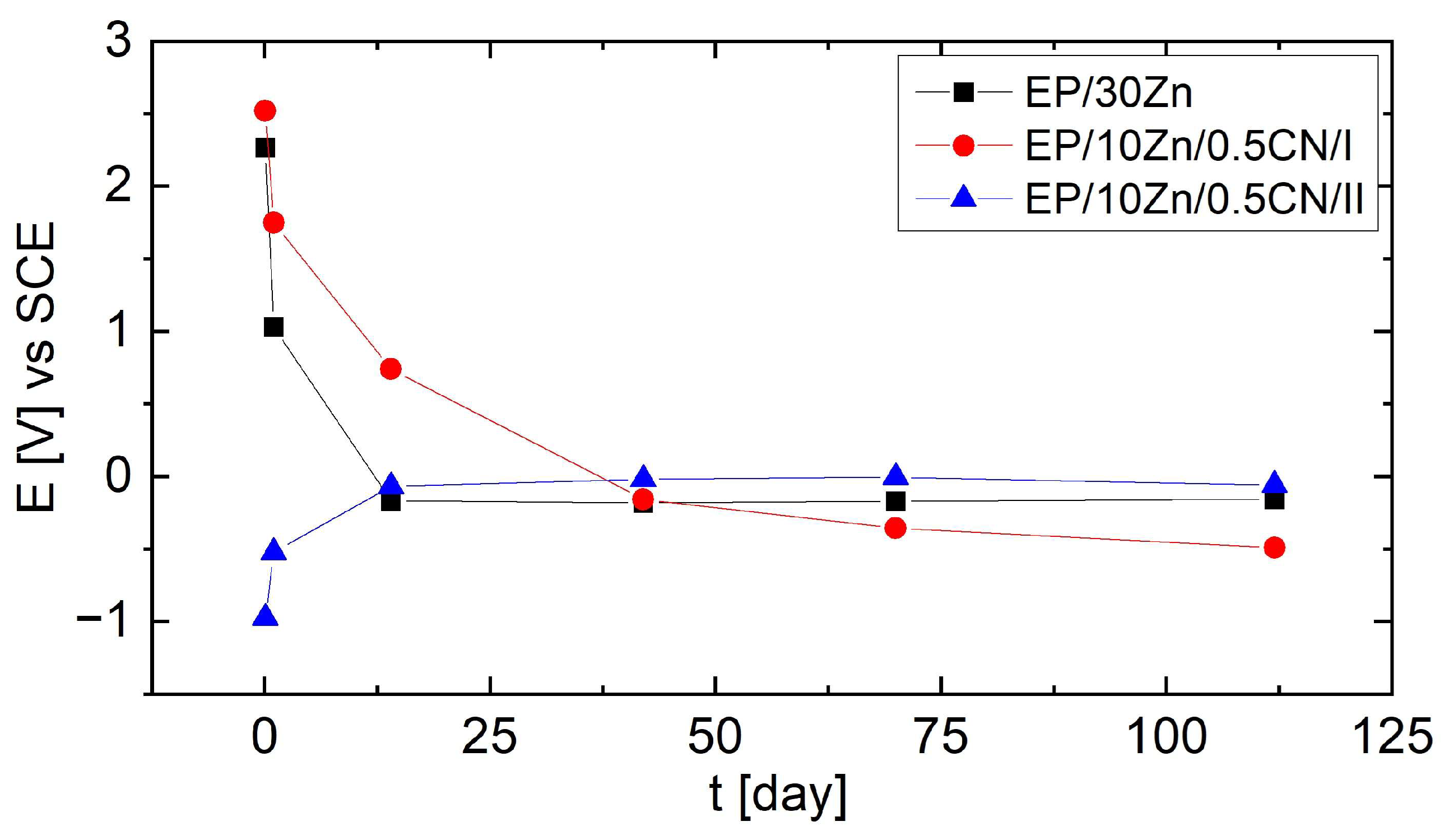
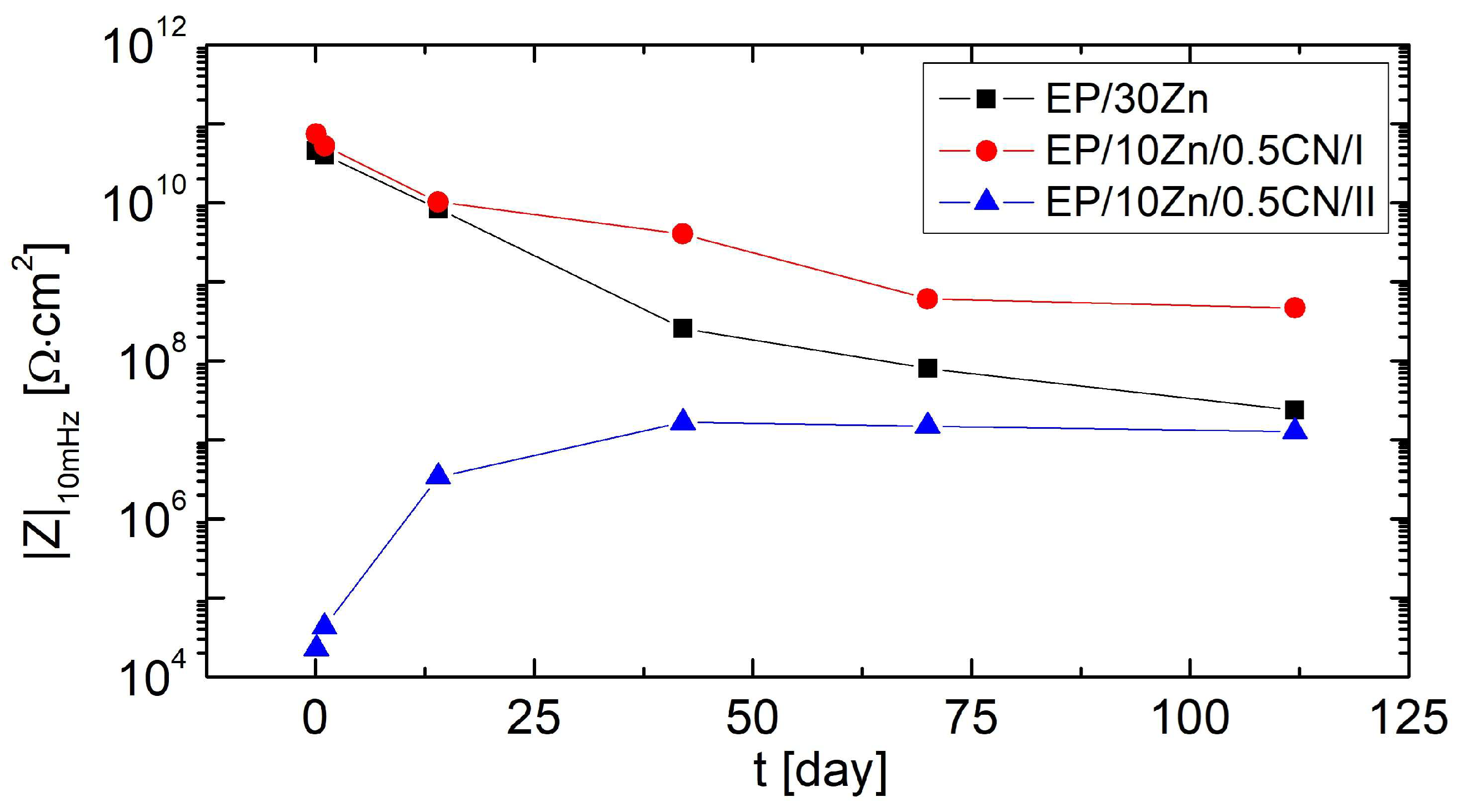
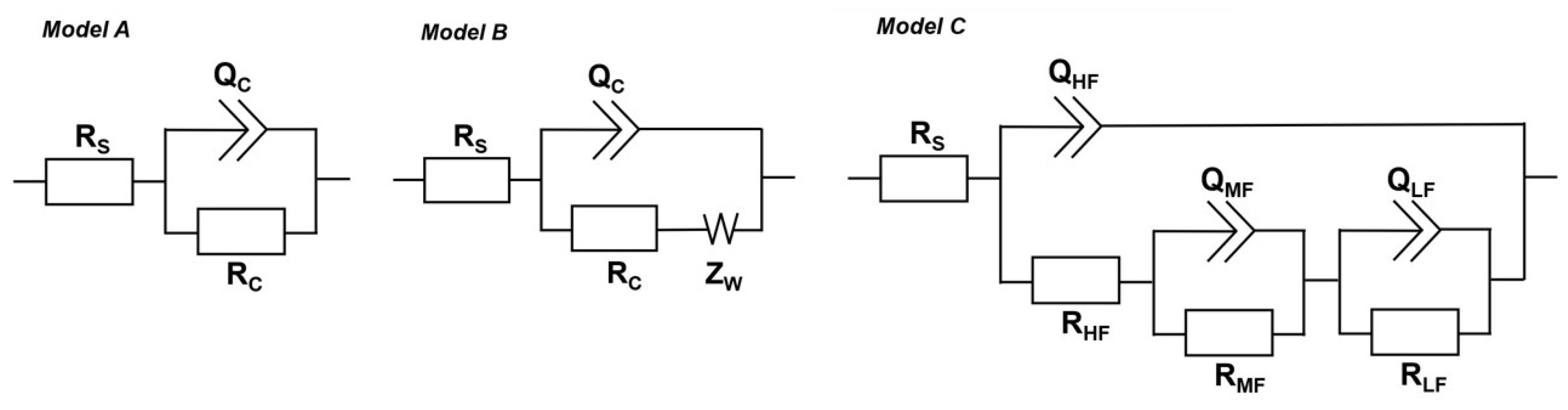
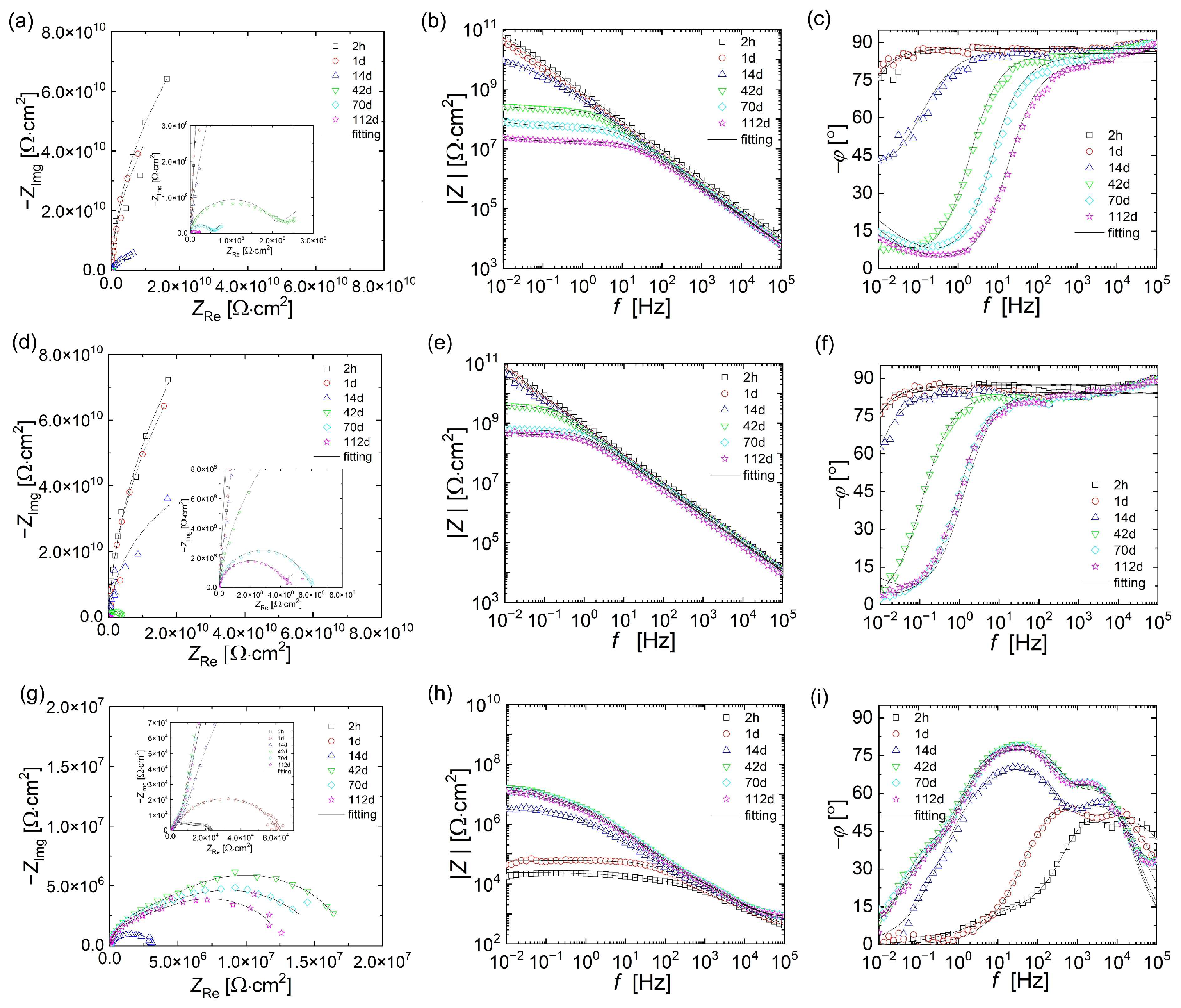
3.3. Electrochemical Measurements
3.4. Corrosion Protection Mechanism
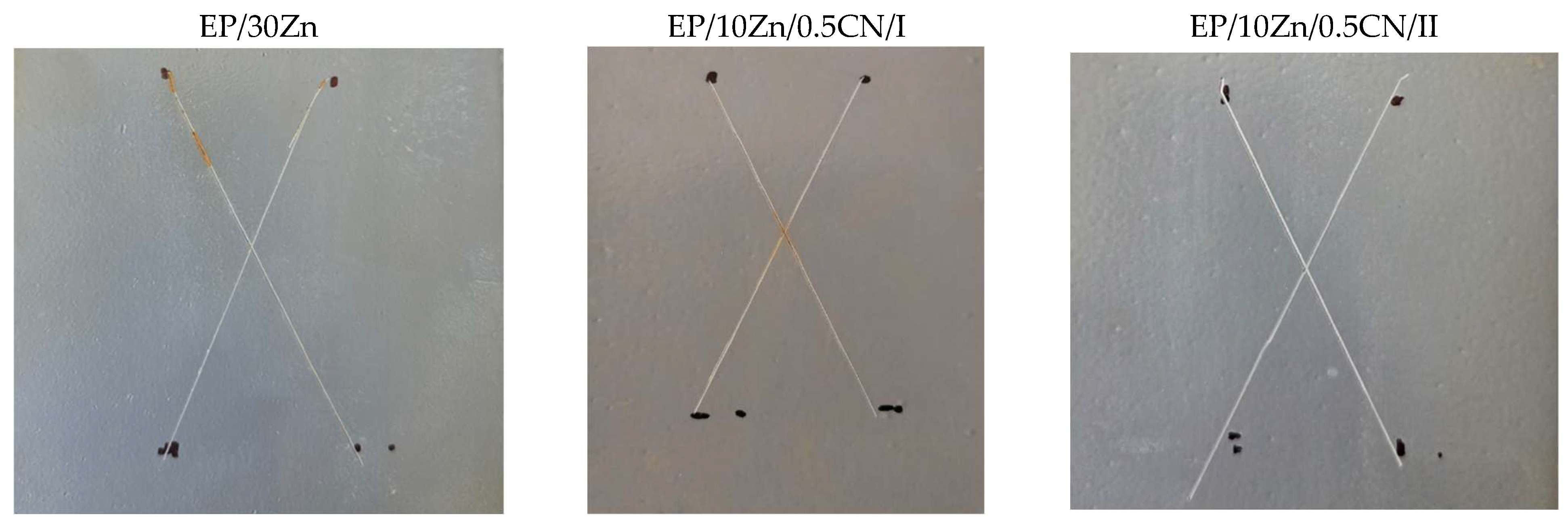
3.4.1. EP/30Zn Coating
3.4.2. EP/10Zn/0.5CN/I Coating
3.4.3. EP/10Zn/0.5CN/II Coating
3.5. Immersion Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Żyłka, W.; Majka, A.; Skała, P.; Szczerba, Z.; Cieniek, B.; Stefaniuk, I. Impact of Degraded Aviation Paints on the Aerodynamic Performance of Aircraft Skin. Materials 2025, 18, 2401. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research Progress in Organic Zinc Rich Primer Coatings for Cathodic Protection of Metals—A Comprehensive Review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- EC. Commission EU Directive 2004/73/EC of 29 April 2004 Adapting to Technical Progress for the Twenty-Ninth Time Council Directive 67/548/EEC on the Approximation of the Laws, Regulations and Administrative Provisions Relating to the Classification, Packaging and Labelling of Dangerous Substances. Off. J. Eur. Union 2004, L216, 3–310. [Google Scholar]
- Langer, E.; Zubielewicz, M.; Kuczyńska, H.; Królikowska, A.; Komorowski, L. Anticorrosive Effectiveness of Coatings with Reduced Content of Zn Pigments in Comparison with Zinc-Rich Primers. Corros. Eng. Sci. Technol. 2019, 54, 627–635. [Google Scholar] [CrossRef]
- Qi, C.; Dam-Johansen, K.; Weinell, C.E.; Bi, H.; Wu, H. Enhanced Anticorrosion Performance of Zinc Rich Epoxy Coatings Modified with Stainless Steel Flakes. Prog. Org. Coat. 2022, 163, 106616. [Google Scholar] [CrossRef]
- Arthanareeswari, M.; Kamaraj, P.; Tamilselvi, M.; Devikala, S. A Low Temperature Nano TiO2 Incorporated Nano Zinc Phosphate Coating on Mild Steel with Enhanced Corrosion Resistance. Mater. Today Proc. 2018, 5, 9012–9025. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Naeimi, A.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. A Facile Synthesis Method of an Effective Anti-Corrosion Nanopigment Based on Zinc Polyphosphate through Microwaves Assisted Combustion Method; Comparing the Influence of Nanopigment and Conventional Zinc Phosphate on the Anti-Corrosion Properties of an Epoxy Coating. J. Alloys Compd. 2018, 762, 730–744. [Google Scholar] [CrossRef]
- Zubielewicz, M. Wyroby Lakierowe Do Zabezpieczeń Przeciwkorozyjnych. Available online: https://inzynierbudownictwa.pl/wyroby-lakierowe-do-zabezpieczen-przeciwkorozyjnych/ (accessed on 20 July 2025).
- European Union. The Water Framework Directive 2000/60/EC of the European Parliament and of the Council; European Union: Brussels, Belgium, 2000. [Google Scholar]
- Meroufel, A.; Deslouis, C.; Touzain, S. Electrochemical and Anticorrosion Performances of Zinc-Rich and Polyaniline Powder Coatings. Electrochim. Acta 2008, 53, 2331–2338. [Google Scholar] [CrossRef]
- Li, A.; Sun, M.; Ma, Z.; Chen, S.; Zhu, G.; Zhang, Y.; Wang, W. Anticorrosion Performance of Polyvinyl Butyral Composite Coatings Improved by Polyaniline-Multiwalled Carbon Nanotubes/Poly (Methylhydrosiloxane). Thin Solid Film. 2020, 712, 138347. [Google Scholar] [CrossRef]
- Li, W.; Fan, Z.; Li, X.; Jiang, B.; Yan, F.; Zhang, Z.; Wang, X. Improved Anti-Corrosion Performance of Epoxy Zinc Rich Coating on Rusted Steel Surface with Aluminum Triphosphate as Rust Converter. Prog. Org. Coat. 2019, 135, 483–489. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent Advances on Organic Coating System Technologies for Corrosion Protection of Offshore Metallic Structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S.; Hegde, M.B.; Rajitha, K.; Madhusudhana, A.M.; Naik, S.R. Functionalized Multi-Walled Carbon Nanotube/Polyindole Incorporated Epoxy: An Effective Anti-Corrosion Coating Material for Mild Steel. J. Alloys Compd. 2021, 856, 158057. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Rezaei Abadchi, M.; Mirzaee, M.; Ahmadi Tabar, F.; Ramezanzadeh, B. Recent Advances and Future Perspectives for Carbon Nanostructures Reinforced Organic Coating for Anti-Corrosion Application. Surf. Interfaces 2021, 23, 100994. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. A Mechanistic Study of the Enhanced Cathodic Protection Performance of Graphene-Reinforced Zinc Rich Nanocomposite Coating for Corrosion Protection of Carbon Steel Substrate. J. Alloys Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.-J.; Teng, J.-L.; Xia, Y.-Q.; Yu, B.-Q.; Zheng, Y. Preparation and Comparison Anticorrosive Properties of Graphene Anticorrosive Coatings. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012054. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion Protection of Graphene-Modified Zinc-Rich Epoxy Coatings in Dilute NaCl Solution. ACS Appl. Nano Mater. 2019, 2, 180–190. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-Reduced Graphene Oxide for Enhanced Corrosion Protection of Zinc-Rich Epoxy Coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Czachor, D.; Kowalczyk, K.; Pavlova, E.; Wojturski, J.; Florczak, Ł.; Byczyński, Ł. Conductive Polyurethane-Based Powder Clear Coatings Modified with Carbon Nanotubes. Prog. Org. Coat. 2019, 137, 105367. [Google Scholar] [CrossRef]
- Jeon, H.; Park, J.; Shon, M. Corrosion Protection by Epoxy Coating Containing Multi-Walled Carbon Nanotubes. J. Ind. Eng. Chem. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Mohamadzadeh Moghadam, M.H.; Shohani, N.; Mahdavian, M. Effects of Highly Crystalline and Conductive Polyaniline/Graphene Oxide Composites on the Corrosion Protection Performance of a Zinc-Rich Epoxy Coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Deyab, M.A.; Awadallah, A.E. Advanced Anticorrosive Coatings Based on Epoxy/Functionalized Multiwall Carbon Nanotubes Composites. Prog. Org. Coat. 2020, 139, 105423. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic Carbon Nanotubes/Epoxy Nanocomposite Coating by Facile One-Step Spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Park, S.; Shon, M. Effects of Multi-Walled Carbon Nano Tubes on Corrosion Protection of Zinc Rich Epoxy Resin Coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Shen, W.; Feng, L.; Liu, X.; Luo, H.; Liu, Z.; Tong, P.; Zhang, W. Multiwall Carbon Nanotubes-Reinforced Epoxy Hybrid Coatings with High Electrical Conductivity and Corrosion Resistance Prepared via Electrostatic Spraying. Prog. Org. Coat. 2016, 90, 139–146. [Google Scholar] [CrossRef]
- Cai, G.; Xiao, S.; Deng, C.; Jiang, D.; Zhang, X.; Dong, Z. CeO2 Grafted Carbon Nanotube via Polydopamine Wrapping to Enhance Corrosion Barrier of Polyurethane Coating. Corros. Sci. 2021, 178, 109014. [Google Scholar] [CrossRef]
- Spychaj, T.; Kowalczyk, K.; Kugler, S. Kompozycja Polimerowa Do Podłoży Metalowych, Drewnianych i z Tworzyw Sztucznych. PL232158, 31 May 2019. [Google Scholar]
- Cubides, Y.; Castaneda, H. Corrosion Protection Mechanisms of Carbon Nanotube and Zinc-Rich Epoxy Primers on Carbon Steel in Simulated Concrete Pore Solutions in the Presence of Chloride Ions. Corros. Sci. 2016, 109, 145–161. [Google Scholar] [CrossRef]
- Camps, M.; Paulsen, A.; Bargallo, J.; Vostracka, T. Anti-Corrosive Zinc Primer Coating Compositions. WO2015132366A1, 5 March 2015. [Google Scholar]
- Mcmullin, R.; Coppola, M.; Hanitzsch, N. Process for Providing Metallic Substrates with Corrosion Resistance. EP2931818A1, 19 October 2016. [Google Scholar]
- Zhu, X.; Wei, X.; Cong, W.; Zhang, Y. Epoxy Anticorrosive Paint Added with Carbon Nanotubes and Flaky Zinc Powder. CN112143340A, 13 October 2020. [Google Scholar]
- Ma, X.; Wang, X. Carbon Nanotube Graphene Zinc-Rich Oxygen-Containing Anti-Corrosive Coating. CN109971313A, 27 November 2020. [Google Scholar]
- Ma, X.; Wang, X. A Kind of Oxygen-Containing Anticorrosive Paint of CNT Graphene Zinc-Rich. CN107573818A, 12 January 2018. [Google Scholar]
- Baozhu, W.; Ximei, W.; Wei, W.; Cangang, L.; Chunyan, S. Nano-Modified Polyaspartate Polyurea Heavy Anti-Corrosion Coating and Preparation Method Thereof. CN111662623A, 17 September 2021. [Google Scholar]
- Li, Y.; Wu, M.; Wang, Y.; Wang, J.; Huang, J.; Wang, C.; Wei, W.; Chen, Y.; Miao, X. Influence of TiO2-MWCNTs Nanohybrid Material on the Corrosion Resistance of Epoxy Low-Zinc Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135326. [Google Scholar] [CrossRef]
- Verma, C.; Srivastava, V.; Quadri, T.W.; Ebenso, E.E.; Hussain, C.M. Smart Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780323951586. [Google Scholar]
- Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Knowles, K.M.; Hammer, P. A Comparative Study on Graphene Oxide and Carbon Nanotube Reinforcement of PMMA-Siloxane-Silica Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2016, 8, 16339–16350. [Google Scholar] [CrossRef]
- Ashfaque, P.M.; Basha, K.A.; Safiullah, S.M. Corrosion Protection Performance of Poly(PyM-Co-GMA)/SWCNT Nanocomposites Coating on Mild Steel. Electrochim. Acta 2024, 508, 145272. [Google Scholar] [CrossRef]
- PN-EN ISO 12085; Profile Method—Motifs Parameters. Polish Committee for Standardization (PKN): Warsaw, Poland, 1999.
- PN-EN ISO 2813; Paints and Varnishes—Determination of Gloss Value at 20°, 60° and 85°. Polish Committee for Standardization (PKN): Warsaw, Poland, 2014.
- PN-EN ISO 2808; Paints and Varnishes—Determination of Film Thickness. Polish Committee for Standardization (PKN): Warsaw, Poland, 2019.
- PN-EN ISO 1522; Paints and Varnishes—Pendulum Damping Test. Polish Committee for Standardization (PKN): Warsaw, Poland, 2022.
- PN-EN ISO 2409; Paints and Varnishes—Cross-Cut Test. Polish Committee for Standardization (PKN): Warsaw, Poland, 2020.
- EN 828; Adhesives—Wettability—Determination by Measurement of Contact Angle and Surface Free Energy of Solid Surface. iTeh Suite: Newark, NJ, USA, 2000.
- EN ISO 3915; Plastics—Measurement of Resistivity of Conductive Plastics. iTeh Suite: Newark, NJ, USA, 1999.
- Atkins, P.; Jones, L. Chemia Ogólna. Cząsteczki, Materia, Reakcje; PWN: Warsaw, Poland, 2014. [Google Scholar]
- PN-ISO 7724; Paints and Varnishes—Colorimetry Part 1: Principles, Part 2: Colour Measurement. Polish Committee for Standardization (PKN): Warsaw, Poland, 2003.
- PN-EN ISO 2812-1; Paints and Varnishes—Determination of Resistance to Liquids —Part 1: Immersion in Liquids Other than Water. Polish Committee for Standardization (PKN): Warsaw, Poland, 2018.
- PN-EN ISO 17872; Paints and Varnishes—Guidelines for the Introduction of Scribe Marks Through Coatings on Metallic Panels for Corrosion Testing. Polish Committee for Standardization (PKN): Warsaw, Poland, 2020.
- PN-EN ISO 4628-8; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance. Polish Committee for Standardization (PKN): Warsaw, Poland, 2013.
- Static Check STC Mika. Available online: https://www.intec-do.de/en/products/static-check-stc-mika/ (accessed on 29 July 2025).
- Dante, R.C. Abrasives, Ceramic, and Inorganic Materials. In Handbook of Friction Materials and Their Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–121. [Google Scholar]
- Zinc Metal Properties. Available online: https://galvanizeit.org/design-and-fabrication/design-considerations/zinc-metal-properties (accessed on 28 July 2025).
- Das, R. (Ed.) Introduction. In Nanohybrid Catalyst Based on Carbon Nanotube. Carbon Nanostructures; Springer: Cham, Switzerland, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically Conductive Polymers and Composites for Biomedical Applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Wiechuła, B.; Latocha, C. Bezpieczeństwo przeciwwybuchowe wielowarstwowych kompozytów powłokowych na odizolowanym podłożu metalowym. Lakiernictwo Przemysłowe 2010, 5. Available online: https://www.lakiernictwo.net/dzial/143-artykuly-branzowe/artykuly/specjalne-wlasciwosci,861#:~:text=Bezpiecze%C5%84stwo%20przeciwwybuchowe%20wielowarstwowych%20kompozyt%C3%B3w%20pow%C5%82okowych%20na%20odizolowanym,sk%C5%82ada%20si%C4%99%20najcz%C4%99%C5%9Bciej%20z%203%2D4%20warstw:%20warstwy (accessed on 29 September 2025).



| Component/ Symbol of Coating | Epoxy Resin, wt% | Curing Agent, wt% | Barite wt% | Benzoin wt% | Byk 360 P wt% | Zinc Dust wt% | SWCNT wt% |
|---|---|---|---|---|---|---|---|
| EP/30Zn | 65.07 | 3.43 | - | 0.5 | 1.0 | 30.0 | - |
| EP/10Zn/0,5CN/I | 65.07 | 3.43 | 19.5 | 0.5 | 1.0 | 10.0 | 0.5 |
| EP/10Zn/0,5CN/II | 65.07 | 3.43 | 19.5 | 0.5 | 1.0 | 10.0 | 0.5 |
| Symbol of Coating | EP/30Zn | EP/10Zn/0.5CN/I | EP/10Zn/0.5CN/II | |
|---|---|---|---|---|
| Current intensity in the powder cloud | µA | 8.4 ± 0.3 | 5.4 ± 0.2 | 6.3 ± 0.2 |
| Roughness PN-EN ISO 12085 [41] | Ra, µm Rz, µm | 0.76 ± 0.02 3.92 ± 0.45 | 0.66 ± 0.02 3.50 ± 0.12 | 0.49 ± 0.03 2.66 ± 0.17 |
| Gloss for the angle of 60 deg PN-EN ISO 2813 [42] | GU | 36.2 ± 0.9 | 34.1 ± 0.8 | 49.0 ± 1.1 |
| Thickness | µm | 98.7 ± 0.1 | 100.1 ± 0.1 | 103.0 ± 0.1 |
| Relative hardness PN-EN ISO 1522 [44] | - | 0.59 ± 0.02 | 0.64 ± 0.02 | 0.69 ± 0.02 |
| Adhesion to the steel PN-EN ISO 2409 [45] | 0-best 5-worst | 0 | 0 | 0 |
| Water contact angle PN-EN 828 [46] | deg |  100.70 ± 1.10 |  97.33 ± 1.05 |  98.42 ± 1.21 |
| Diiodomethan contact angle PN-EN 828 [46] | deg |  34.62 ± 0.71 |  36.48 ± 0.96 |  39.68 ± 0.92 |
| Surface free energy | mN/m | 46.63 ± 1.25 | 43.90 ± 1.20 | 42.29 ± 1.17 |
| Surface resistivity EN ISO 3915 [47] | Ω | 1.2 × 1012 ± 0.20 × 1012 | 1.3 × 109 ± 0.20 × 109 | 12.68 × 103 ± 0.08 × 103 |
| Amount of Zn released in 100 cm3 of seawater solution | g/cm2 | 2.03 × 10−4 ± 0.03 × 10−4 | 1.33 × 10−4 ± 0.02 × 10−4 | 1.38 × 10−4 ± 0.03 × 10−4 |
| Color PN-ISO 7724 [49] | - | L* = 47.57 ± 0.33 a* = −1.27 ± 0.04 b* = −3.10 ± 0.16 - | L* = 46.38 ± 0.36 a* = −1.16 ± 0.07 b* = −3.05 ± 0.15 ΔE* = 1.20 ± 0.09 | L* = 44.64 ± 0.32 a* = −1.27 ± 0.04 b* = −3.43 ± 0.13 ΔE* = 3.48 ± 0.19 |
| Coating | Time, day | QC, S·sn/cm2 | nC | RC, Ω·cm2 | ZW, S·s0.5/cm2 | Chi2 |
|---|---|---|---|---|---|---|
| EP/30Zn | 0.08 | 2.16 × 10−10 | 0.972 | 3.25 × 1011 | - | 2.87 × 10−3 |
| 1 | 3.39 × 10−10 | 0.974 | 2.39 × 1011 | - | 1.12 × 10−3 | |
| 14 | 4.07 × 10−10 | 0.961 | 3.62 × 109 | 5.30 × 10−10 | 6.54 × 10−3 | |
| 42 | 4.73 × 10−10 | 0.951 | 1.99 × 108 | 4.77 × 10−8 | 2.54 × 10−3 | |
| 70 | 5.75 × 10−10 | 0.937 | 5.02 × 107 | 1.05 × 10−7 | 2.44 × 10−3 | |
| 112 | 7.62 × 10−10 | 0.918 | 1.66 × 107 | 4.37 × 10−7 | 3.04 × 10−3 | |
| EP/10Zn/0.5CN/I | 0.08 | 1.92 × 10−10 | 0.966 | 3.91 × 1011 | - | 8.22 × 10−4 |
| 1 | 2.16 × 10−10 | 0.952 | 3.25 × 1011 | - | 2.87 × 10−3 | |
| 14 | 3.07 × 10−10 | 0.936 | 1.03 × 1011 | - | 6.03 × 10−3 | |
| 42 | 3.44 × 10−10 | 0.932 | 3.89 × 109 | - | 2.02 × 10−3 | |
| 70 | 3.62 × 10−10 | 0.932 | 5.61 × 108 | 6.57 × 10−8 | 1.70 × 10−3 | |
| 112 | 3.69 × 10−10 | 0.934 | 3.97 × 108 | 3.36 × 10−8 | 1.81 × 10−3 |
| Coating | Time, Day | QHF, S·sn/cm2 | nHF | RHF, Ω·cm2 | QMF, S·sn/cm2 | nMF | RMF, Ω·cm2 | QLF, S·sn/cm2 | nLF | RLF, Ω·cm2 | Chi2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EP/10Zn/0.5CN/II | 0.08 | 1.87 × 107 | 0.728 | 4.45 × 103 | 8.10 × 10−6 | 0.571 | 1.21 × 104 | 1.52 × 10−8 | 1.000 | 7.79 × 103 | 1.70 × 10−4 |
| 1 | 1.85 × 107 | 0.735 | 1.17 × 104 | 4.31 × 10−8 | 0.876 | 4.90 × 104 | - | - | - | 1.61 × 10−3 | |
| 14 | 1.57 × 107 | 0.743 | 3.49 × 104 | 1.42 × 10−8 | 1.000 | 3.11 × 106 | - | - | - | 5.27 × 10−3 | |
| 42 | 3.67 × 108 | 0.874 | 3.22 × 104 | 1.02 × 10−8 | 1.000 | 5.71 × 106 | 1.53 × 10−7 | 0.837 | 1.19 × 107 | 6.73 × 10−4 | |
| 70 | 3.98 × 108 | 0.862 | 2.51 × 104 | 1.24 × 10−8 | 1.000 | 4.72 × 106 | 1.74 × 10−7 | 0.737 | 1.09 × 107 | 5.30 × 10−3 | |
| 112 | 4.67 × 108 | 0.861 | 3.93 × 104 | 1.10 × 10−8 | 1.000 | 4.40 × 106 | 2.20 × 10−7 | 0.785 | 8.38 × 106 | 1.35 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilch-Pitera, B.; Florczak, Ł.; Czachor-Jadacka, D.; Bellucco, F.; Węgrzyniak-Kściuczyk, E.; Daszykowska, K.; Żychowicz, M. Anticorrosion Activity of Low-Zinc Powder Coating Primers Containing Single-Walled Carbon Nanotubes. Materials 2025, 18, 4587. https://doi.org/10.3390/ma18194587
Pilch-Pitera B, Florczak Ł, Czachor-Jadacka D, Bellucco F, Węgrzyniak-Kściuczyk E, Daszykowska K, Żychowicz M. Anticorrosion Activity of Low-Zinc Powder Coating Primers Containing Single-Walled Carbon Nanotubes. Materials. 2025; 18(19):4587. https://doi.org/10.3390/ma18194587
Chicago/Turabian StylePilch-Pitera, Barbara, Łukasz Florczak, Dominika Czachor-Jadacka, Francesco Bellucco, Elwira Węgrzyniak-Kściuczyk, Katarzyna Daszykowska, and Małgorzata Żychowicz. 2025. "Anticorrosion Activity of Low-Zinc Powder Coating Primers Containing Single-Walled Carbon Nanotubes" Materials 18, no. 19: 4587. https://doi.org/10.3390/ma18194587
APA StylePilch-Pitera, B., Florczak, Ł., Czachor-Jadacka, D., Bellucco, F., Węgrzyniak-Kściuczyk, E., Daszykowska, K., & Żychowicz, M. (2025). Anticorrosion Activity of Low-Zinc Powder Coating Primers Containing Single-Walled Carbon Nanotubes. Materials, 18(19), 4587. https://doi.org/10.3390/ma18194587






