Peptide-Guided TiO2/Graphene Oxide–Cellulose Hybrid Aerogels for Visible-Light Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Self-Assembly of Peptides and Biomimetic Mineralization of TiO2
2.3. Preparation of GO/MCC/PNFs-TiO2 Hybrid Aerogels
2.4. Photocatalytic Tests
2.5. Characterization Techniques
3. Results and Discussion
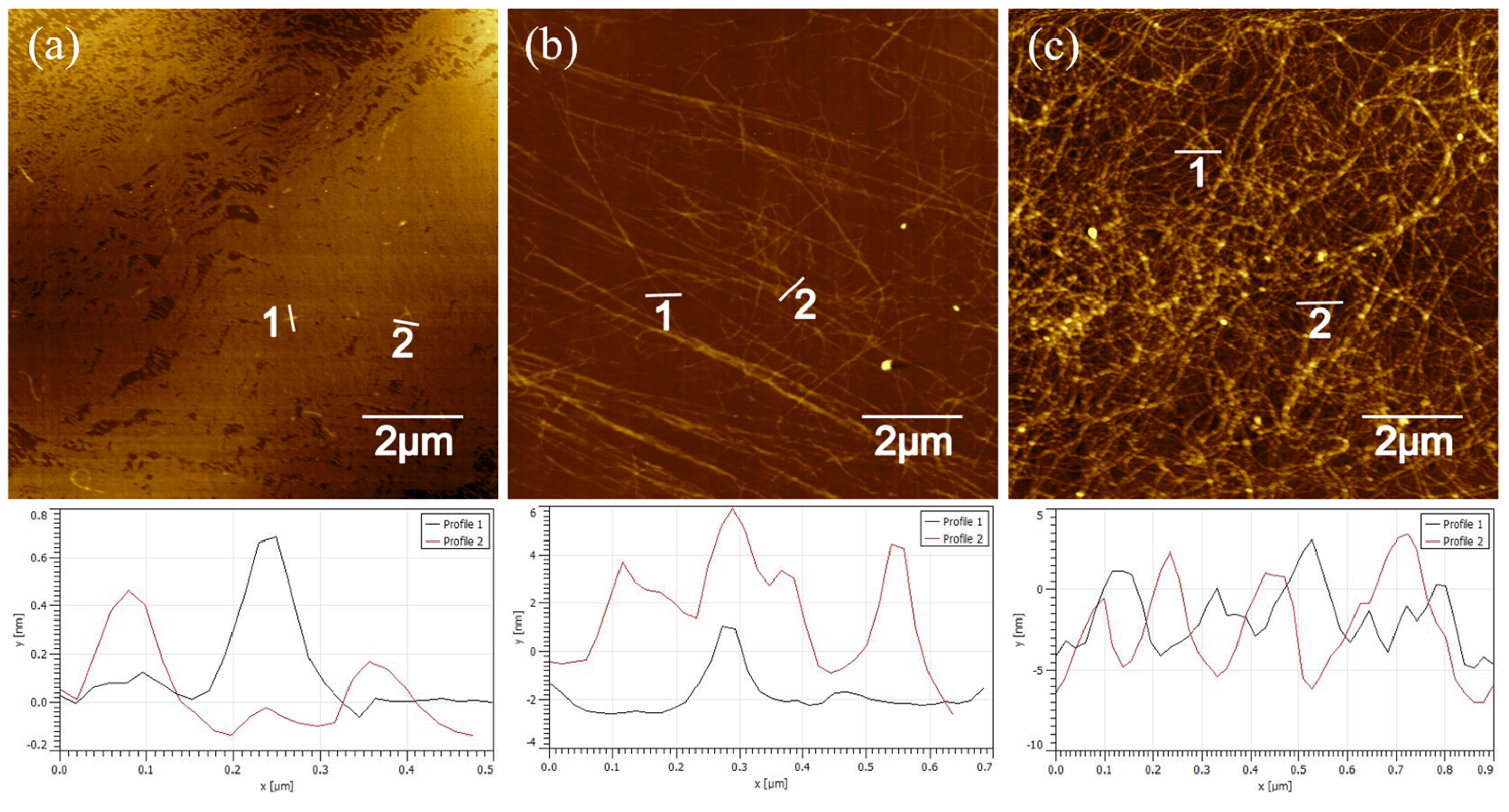
3.1. Self-Assembly and Characterizations of PNFs–TiO2 Composites
3.2. Synthesis and Characterizations of GO/PNFs–TiO2 Suspensions
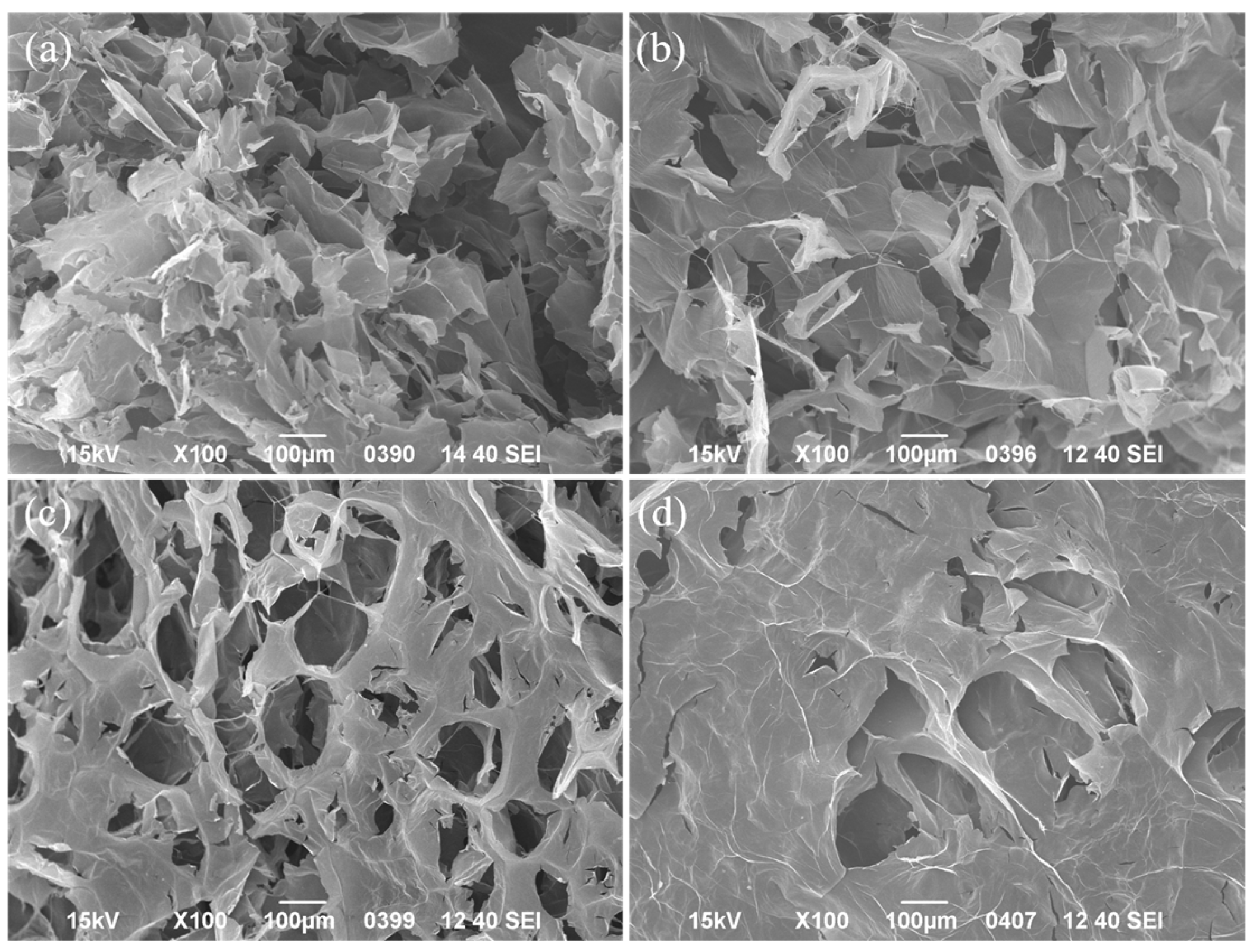
3.3. Preparation and Characterization of GO/MCC/PNFs-TiO2 Hybrid Aerogels
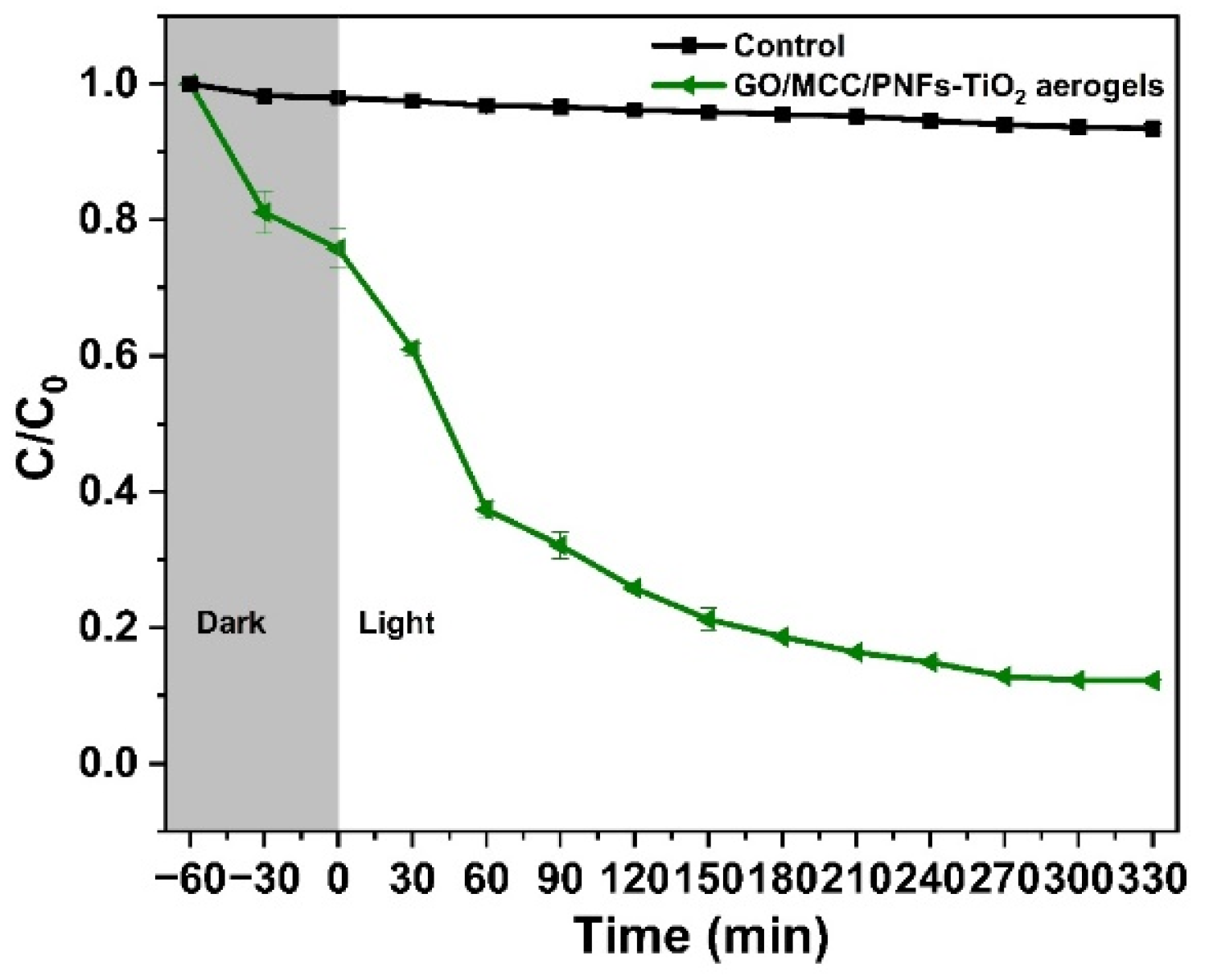
3.4. Photocatalytic Degradation of Organic Pollutants
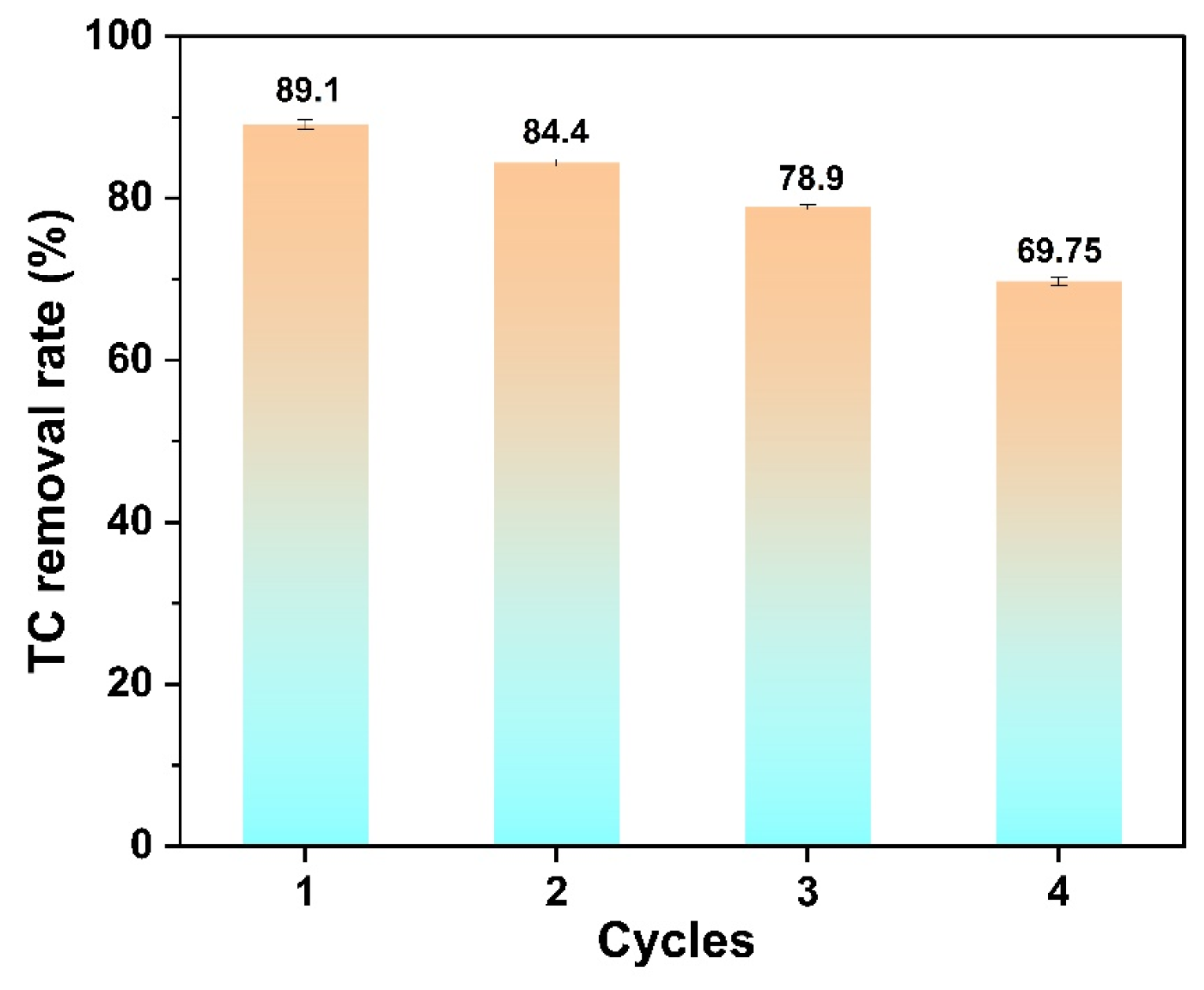
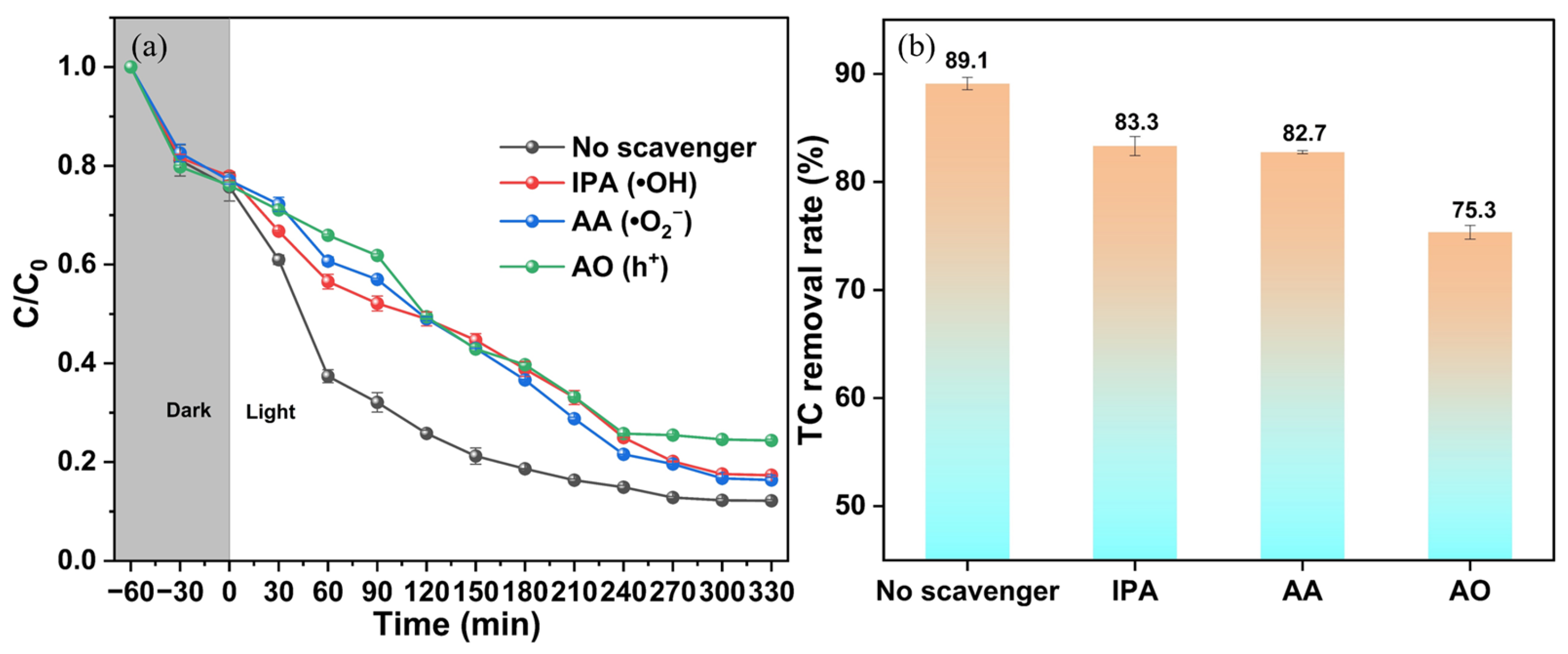
3.5. Cycling Performance and Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Wang, Y.; Fang, T.; Tang, X.; Ding, Z.; Han, Y.; Guo, R.; Qi, P.; Liu, X. Customization of indium-based MOFs for enhanced adsorptive and photocatalytic of organic pollutants: Morphology involvement on performance and mechanism. Chem. Eng. J. 2024, 498, 155449. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wang, Y.; Jiang, S.; Wang, Y.; Liu, X.; Ding, Z. Degradation of methyl parathion in thermally activated peroxymonosulfate processes: Kinetics, reaction mechanism and toxicity evaluation. J. Hazard. Mater. 2025, 491, 137987. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, Y.; He, P.; Wang, Y.; Wei, G. Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation. Materials 2024, 17, 2660. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhu, T.; Sun, J.; Pan, X.; Li, J.; Luo, H. Emerging organic contaminants in sewage sludge: Current status, technological challenges and regulatory perspectives. Sci. Total Environ. 2024, 955, 177234. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhang, Y.; Yuan, X.; Zuo, J. Antibiotic resistance evolution driven synergistically by antibiotics and typical organic pollutants in antibiotic production wastewater. J. Hazard. Mater. 2025, 483, 136543. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Shoronika, A.Z.; Fariha, A.; Hasan, M.; Islam, S.; Marwani, H.M.; Islam, A.; Hasan, M.; Alsukaibi, A.K.; et al. Sustainable toxic dye removal and degradation from wastewater using novel chitosan-modified TiO2 and ZnO nanocomposites. J. Mol. Liq. 2023, 388, 122764. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Geng, J.; Ma, L.; Jiang, J.; Li, B.; Yabo, S.D.; Lu, L.; Fu, D.; Shen, J.; et al. Occurrence, distribution, and risk assessment of quinolone antibiotics in municipal sewage sludges throughout China. J. Hazard. Mater. 2023, 453, 131322. [Google Scholar] [CrossRef]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-light-activated tio2-based photocatalysts for the inactivation of pathogenic bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Navarro, L.K.T.; Jaroslav, C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels 2023, 9, 976. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J. Recent advances in the synthesis of defective TiO2 nanofibers and their applications in energy and catalysis. Chem. Eng. J. 2023, 472, 144831. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Cui, P.; Li, C.; Zhang, B.; Gan, L.; Zhang, S.; Zhang, X.; Zhou, X.; Sun, Z.; et al. Ultrahigh Photocatalytic CO2 Reduction Efficiency and Selectivity Manipulation by Single-Tungsten-Atom Oxide at the Atomic Step of TiO2. Adv. Mater. 2022, 34, 2109074. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Synthesis of N-CNT/TiO2 composites thin films: Surface analysis and optoelectronic properties. E3S Web Conf. 2020, 183, 05002. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Nitrogen doped carbon nanotubes grafted TiO2 rutile nanofilms: Promising material for dye sensitized solar cell application. Optik 2021, 229, 166234. [Google Scholar] [CrossRef]
- Yang, L.; Ying, J.; Liu, Z.; He, G.; Xu, L.; Liu, M.; Xu, X.; Chen, G.; Guan, M. Synthesis of core/shell cobalt-doped rutile TiO2 nanorods for MB degradation under visible light. RSC Adv. 2025, 15, 10144–10149. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.-C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef]
- Tong, M.-H.; Wang, T.-M.; Lin, S.-W.; Chen, R.; Jiang, X.; Chen, Y.-X.; Lu, C.-Z. Ultra-thin carbon doped TiO2 nanotube arrays for enhanced visible-light photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 623, 156980. [Google Scholar] [CrossRef]
- Yu, L.; Duan, Y.; Wang, D.; Liang, Z.; Liang, C.; Wang, Y. Recent advances in TiO2 modification for improvement in photocatalytic purification of indoor VOCs. RSC Adv. 2025, 15, 28204–28230. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, M.; Gao, K.; Fu, R.; Zhang, S.; Xiao, Y.; Guo, J.; Wang, Z.; Wang, H.; Zhao, Y.; et al. Boosting photocatalytic degradation of levofloxacin over plasmonic TiO2-x/TiN heterostructure. Appl. Surf. Sci. 2024, 655, 159516. [Google Scholar] [CrossRef]
- Che, G.; Yang, W.; Wang, C.; Li, M.; Li, X.; Pan, Q. Efficient photocatalytic oxidative coupling of benzylamine over uranyl–organic frameworks. Inorg. Chem. 2022, 61, 12301–12307. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Zhang, X.; Xu, Y.; An, Y.; Li, C.; Yi, S.; Liu, C.; Wang, K.; Sun, X.; et al. In Situ Construction of Bimetallic Selenides Heterogeneous Interface on Oxidation-Stable Ti3C2Tx MXene Toward Lithium Storage with Ultrafast Charge Transfer Kinetics. Small 2024, 20, 2403078. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Samad, A.A.; Faruk, O.; Reza, S.; Lim, S.; Islam, Z.; Lee, Y.; Kim, D.; Park, J.Y. TiO2 nanoparticles-decorated MXene-PVDF composite carbon nanofibrous mats-based free-standing electrodes for flexible and breathable microsupercapacitors. Carbon 2025, 241, 120381. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Zaidy, M.E. Depollution of industrial dyes by nanocrystalline Ti0.95Bi0.025X0.025O2 (X = Zr, Nb): Visible light harvesting, charge separation and high efficiency. J. Sol-Gel Sci. Technol. 2023, 107, 417–429. [Google Scholar] [CrossRef]
- Yang, G.; Guo, Q.; Kong, H.; Luan, X.; Wei, G. Structural design, biomimetic synthesis, and environmental sustainability of graphene-supported g-C3N4/TiO2 hetero-aerogels. Environ. Sci. Nano 2023, 10, 1257–1267. [Google Scholar] [CrossRef]
- Kim, J.K.; Jang, J.-R.; Salman, M.S.; Tan, L.; Nam, C.-H.; Yoo, P.J.; Choe, W.-S. Harnessing designer biotemplates for biomineralization of TiO2 with tunable photocatalytic activity. Ceram. Int. 2019, 45, 6467–6476. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42. [Google Scholar] [CrossRef]
- Qin, K.; Zheng, Z.; Wang, J.; Pan, H.; Tang, R. Biomineralization strategy: From material manufacturing to biological regulation. Giant 2024, 19, 100317. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, J.; Zhang, X.; Fu, C.; Miao, Y.; Guo, Y.; Tong, Z.; Mao, N. Selective Photocatalytic Activities of Amino Acids/Peptide-Ti3C2Tx-TiO2 Composites Induced by Calcination: Adsorption Enhancement vs Charge Transfer Enhancement. J. Phys. Chem. C 2023, 127, 2231–2245. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Jones, S.E.; Cai, Y.; Ahmad, G.; Naik, R.R.; Kröger, N.; Sandhage, K.H. Identification and Design of Peptides for the Rapid, High-Yield Formation of Nanoparticulate TiO2 from Aqueous Solutions at Room Temperature. Chem. Mater. 2008, 20, 1578–1584. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.; Pastrana-Martínez, L.M.; Han, C.; Morales-Torres, S.; Figueiredo, J.L.; Dionysiou, D.D.; Faria, J.L. Carbon-based TiO2 materials for the degradation of Microcystin-LA. Appl. Catal. B Environ. 2015, 170–171, 74–82. [Google Scholar] [CrossRef]
- Gan, X.; Li, Y.; Li, W.; Li, L.; Lu, L.; Cheng, X. Enhancing the mechanical properties and hydration of cement paste by incorporating GO/nano In(OH)3 composite with high specific surface area. Cem. Concr. Compos. 2024, 149, 105514. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, M.; Van der Bruggen, B.; Lei, L.; Zhu, L. Effect of TiO2 content on the properties of polysulfone nanofiltration membranes modified with a layer of TiO2–graphene oxide. Sep. Purif. Technol. 2020, 242, 116770. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Zhou, K.; Chen, B.-Y.; Chang, C.-T. Graphene/TiO2/ZSM-5 composites synthesized by mixture design were used for photocatalytic degradation of oxytetracycline under visible light: Mechanism and biotoxicity. Appl. Surf. Sci. 2016, 362, 329–334. [Google Scholar] [CrossRef]
- Vasanth, A.; Powar, N.S.; Krishnan, D.; Nair, S.V.; Shanmugam, M. Electrophoretic graphene oxide surface passivation on titanium dioxide for dye sensitized solar cell application. J. Sci. Adv. Mater. Devices 2020, 5, 316–321. [Google Scholar] [CrossRef]
- Jain, R.; Upadhyaya, M.; Singh, A.; Jaiswal, R.P. TiO2 nanoparticles synthesized on graphene nanosheets with varied crystallinity for photocatalytic degradation of MB dye. J. Environ. Chem. Eng. 2024, 12, 113881. [Google Scholar] [CrossRef]
- Wang, T.; Lim, S.; Chiang, C.; Shen, Y.; Raghunath, P.; Li, J.; Lin, Y.; Lin, M. Photocatalytic activity of B-doped nano graphene oxide over hydrogenated NiO-loaded TiO2 nanotubes. Mater. Today Sustain. 2023, 24, 100497. [Google Scholar] [CrossRef]
- Wang, H.-H.; Liu, W.-X.; Ma, J.; Liang, Q.; Qin, W.; Lartey, P.O.; Feng, X.-J. Design of (GO/TiO2)N one-dimensional photonic crystal photocatalysts with improved photocatalytic activity for tetracycline degradation. Int. J. Miner. Met Mater. 2020, 27, 830–839. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Nie, J.; Yang, J.-H.; Qi, X.-D.; Zhou, Z.-W.; Wang, Y. Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohydr. Polym. 2018, 198, 546–555. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, T.; Lei, W.; Feng, Y.; Tang, X.; Ding, Z.; Liu, X.; Qi, P.; Wang, Y. A mechanically robust chitosan-based macroporous foam for sustainable Se(IV) elimination from wastewater. Carbohydr. Polym. 2025, 352, 123238. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.-H.; Zhang, N.; Wang, Y.; Zhou, Z.-W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef]
- Amaly, N.; El-Moghazy, A.Y.; Nitin, N.; Sun, G.; Pandey, P.K. Synergistic adsorption-photocatalytic degradation of tetracycline by microcrystalline cellulose composite aerogel dopped with montmorillonite hosted methylene blue. Chem. Eng. J. 2022, 430, 133077. [Google Scholar] [CrossRef]
- Yang, G.; He, P.; Zhu, D.; Wan, K.; Kong, H.; Luan, X.; Fang, L.; Wang, Y.; Wei, G. Functional regulation of polymer aerogels by graphene doping and peptide nanofiber-induced biomineralization as sustainable adsorbents of contaminants. Environ. Sci. Nano 2022, 9, 4497–4507. [Google Scholar] [CrossRef]
- Li, S.; Wen, X.; Miao, X.; Li, R.; Wang, W.; Li, X.; Guo, Z.; Zhao, D.; Lan, K. DFT-guided design of dual dopants in anatase tio2 for boosted sodium storage. Nano Lett. 2024, 24, 14957–14964. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Shang, H.; He, Y.; Gu, S.; Li, S.; Wang, Q.; Wang, S.; Peng, J.; Feng, X.; Li, P.; et al. Engineering the defect distribution via boron doping in amorphous TiO2 for robust photocatalytic NO removal. Appl. Catal. B Environ. 2024, 356, 124239. [Google Scholar] [CrossRef]
- Le, T.L.; Le, T.H.; Van, K.N.; Van Bui, H.; Le, T.G.; Vo, V. Controlled growth of TiO2 nanoparticles on graphene by hydrothermal method for visible-light photocatalysis. J. Sci. Adv. Mater. Devices 2021, 6, 516–527. [Google Scholar] [CrossRef]
- Kumari, M.A.; Devi, L.G.; Maia, G.; Chen, T.-W.; Al-Zaqri, N.; Ali, M.A. Mechanochemical synthesis of ternary heterojunctions TiO2(A)/TiO2(R)/ZnO and TiO2(A)/TiO2(R)/SnO2 for effective charge separation in semiconductor photocatalysis: A comparative study. Environ. Res. 2022, 203, 111841. [Google Scholar] [CrossRef]
- Wang, T.; Chang, L.; Wu, H.; Yang, W.; Cao, J.; Fan, H.; Wang, J.; Liu, H.; Hou, Y.; Jiang, Y.; et al. Fabrication of three-dimensional hierarchical porous 2D/0D/2D g-C3N4 modified MXene-derived TiO2@C: Synergy effect of photocatalysis and H2O2 oxidation in NO removal. J. Colloid Interface Sci. 2022, 612, 434–444. [Google Scholar] [CrossRef]
- Feng, X.; Gu, L.; Wang, N.; Pu, Q.; Liu, G. Fe/N co-doped nano-TiO2 wrapped mesoporous carbon spheres for synergetically enhanced adsorption and photocatalysis. J. Mater. Sci. Technol. 2023, 135, 54–64. [Google Scholar] [CrossRef]
- Liccardo, L.; Bordin, M.; Sheverdyaeva, P.M.; Belli, M.; Moras, P.; Vomiero, A.; Moretti, E. Surface defect engineering in colored tio2 hollow spheres toward efficient photocatalysis. Adv. Funct. Mater. 2023, 33, 2212486. [Google Scholar] [CrossRef]
- Dong, Z.; Meng, C.; Li, Z.; Zeng, D.; Wang, Y.; Cheng, Z.; Cao, X.; Ren, Q.; Wang, Y.; Li, X.; et al. Novel Co3O4@TiO2@CdS@Au double-shelled nanocage for high-efficient photocatalysis removal of U(VI): Roles of spatial charges separation and photothermal effect. J. Hazard. Mater. 2023, 452, 131248. [Google Scholar] [CrossRef]
- Feng, Y.; Ding, Z.; Tang, S.; Liu, X.; Qi, P.; Wang, Y. MXene-confined cobalt-doped lithium ion sieve composites for efficient lithium extraction from seawater. Chem. Eng. J. 2025, 522, 167505. [Google Scholar] [CrossRef]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.-H.; Monteiro, P.J.M.; Yu, L.E. Characterization of photocatalytic TiO2 powder under varied environments using near ambient pressure X-ray photoelectron spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef]
- Guo, L.Q.; Hu, Y.W.; Yu, B.; Davis, E.; Irvin, R.; Yan, X.G.; Li, D.Y. Incorporating TiO2 nanotubes with a peptide of D-amino K122-4 (D) for enhanced mechanical and photocatalytic properties. Sci. Rep. 2016, 6, 22247. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Zhang, W.; Lei, W.; Wang, Y.; Wei, G. Peptide-Guided TiO2/Graphene Oxide–Cellulose Hybrid Aerogels for Visible-Light Photocatalytic Degradation of Organic Pollutants. Materials 2025, 18, 4565. https://doi.org/10.3390/ma18194565
Dai H, Zhang W, Lei W, Wang Y, Wei G. Peptide-Guided TiO2/Graphene Oxide–Cellulose Hybrid Aerogels for Visible-Light Photocatalytic Degradation of Organic Pollutants. Materials. 2025; 18(19):4565. https://doi.org/10.3390/ma18194565
Chicago/Turabian StyleDai, Haonan, Wenliang Zhang, Wensheng Lei, Yan Wang, and Gang Wei. 2025. "Peptide-Guided TiO2/Graphene Oxide–Cellulose Hybrid Aerogels for Visible-Light Photocatalytic Degradation of Organic Pollutants" Materials 18, no. 19: 4565. https://doi.org/10.3390/ma18194565
APA StyleDai, H., Zhang, W., Lei, W., Wang, Y., & Wei, G. (2025). Peptide-Guided TiO2/Graphene Oxide–Cellulose Hybrid Aerogels for Visible-Light Photocatalytic Degradation of Organic Pollutants. Materials, 18(19), 4565. https://doi.org/10.3390/ma18194565






