Local Infections Associated with Ventricular Assist Devices: Materials-Related Challenges and Emerging Solutions
Abstract
1. Introduction
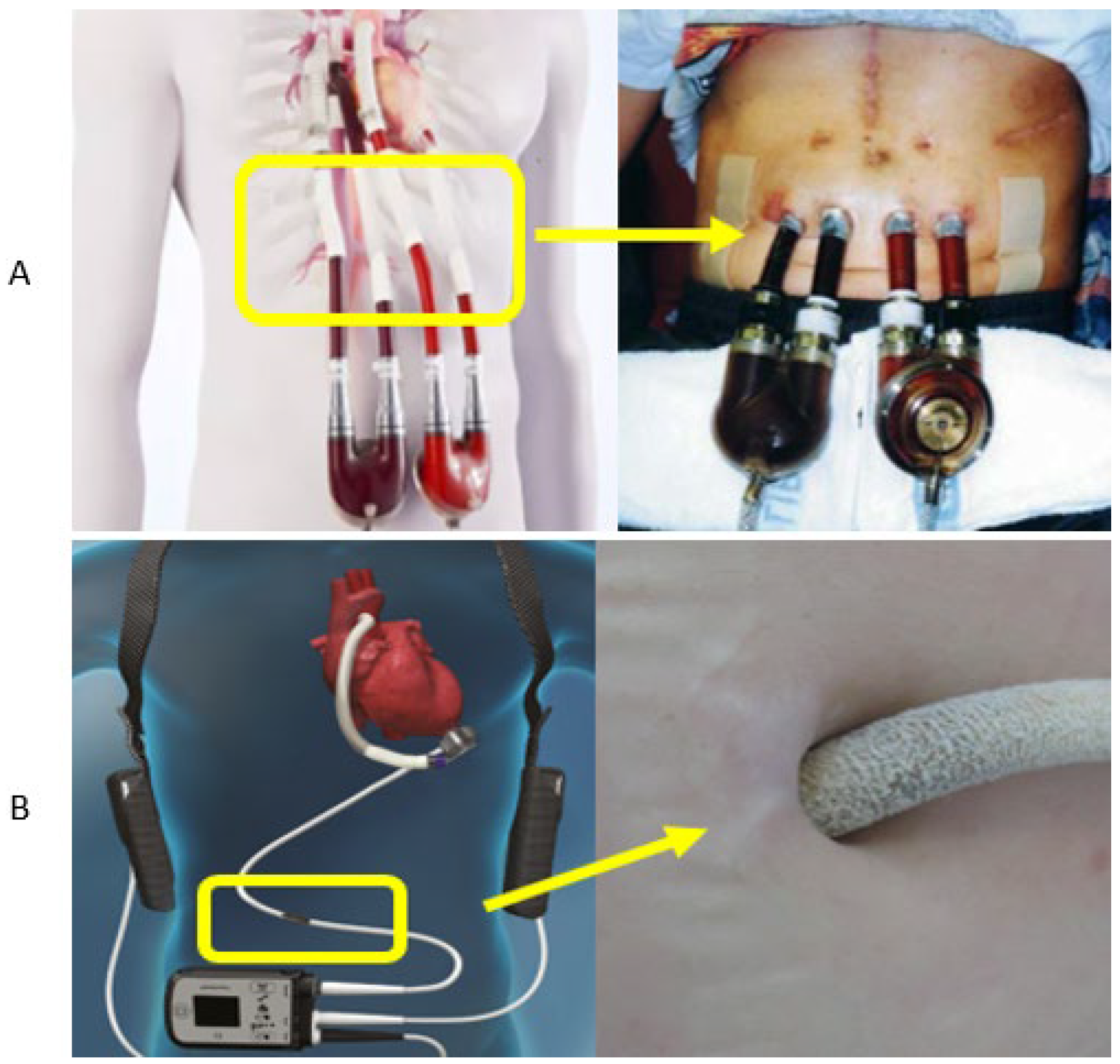
2. Materials Utilized at Percutaneous Exit Sites
3. Etiology and Diagnosis of Local Infections
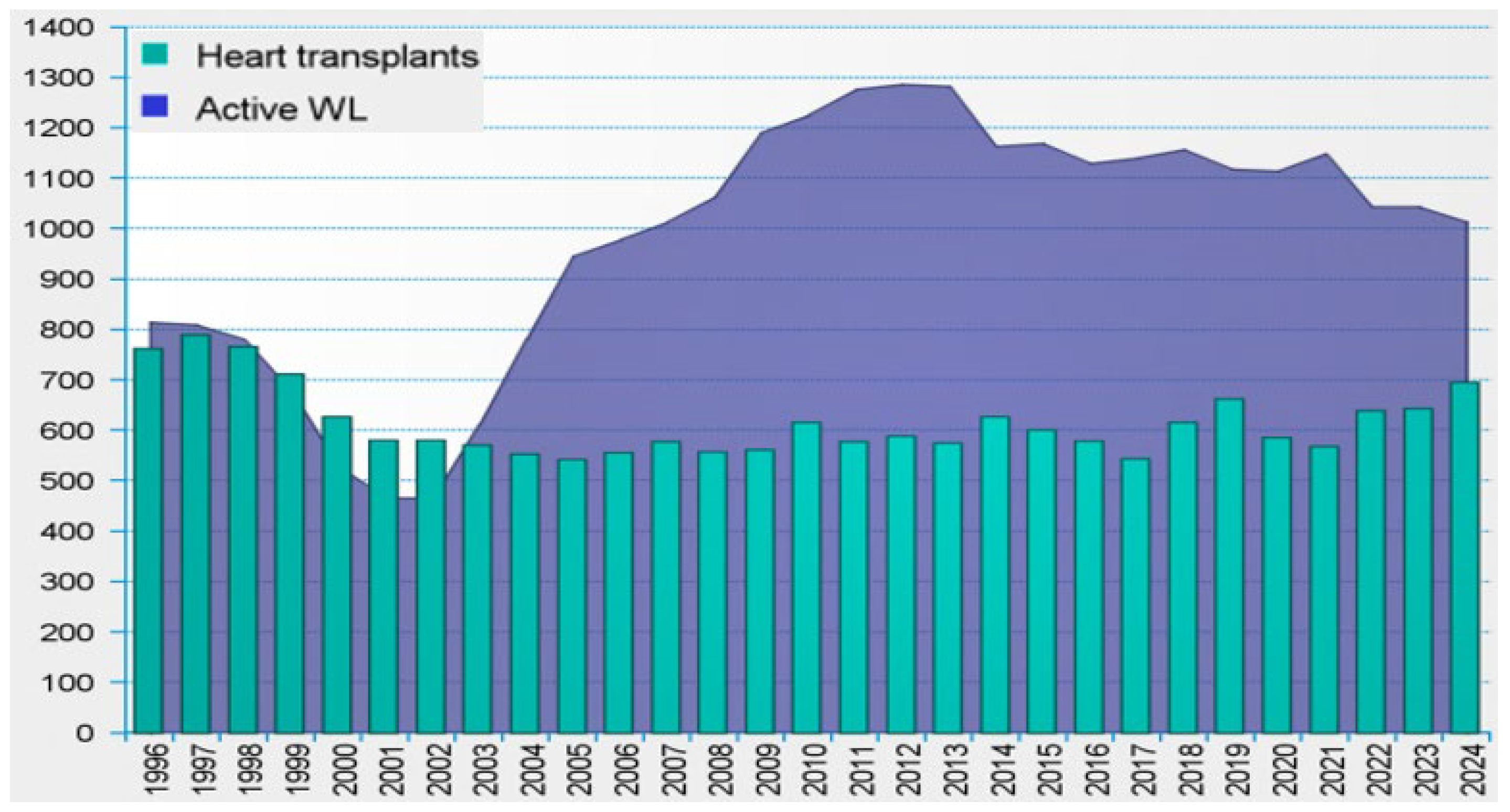
3.1. Epidemiology of Local VAD Infections
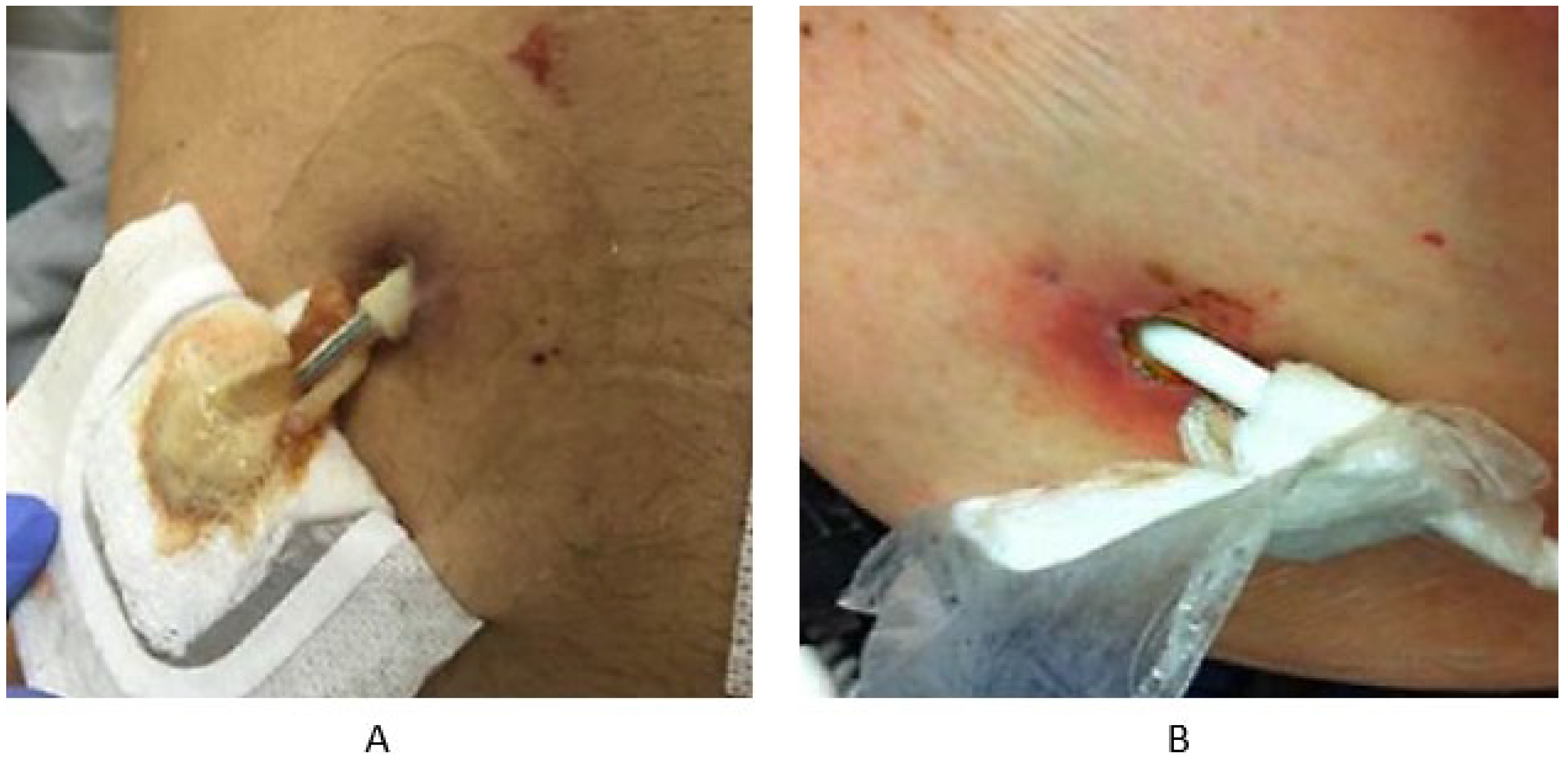
3.2. Etiology of Local Infections
3.3. Biofilm Formation at Transcutaneous Exit Sites
3.4. Diagnostics Approaches
4. Prevention and Management of Local Infections in MCS
5. Economic Considerations
6. Innovative Solutions and Future Directions
6.1. Metal Nanoparticles
6.2. Antibiotics Solutions
6.3. Antibacterial Peptides
7. Shared Challenges in Other Clinical Applications
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurotransplant. Statistics. Available online: https://statistics.eurotransplant.org/ (accessed on 30 January 2025).
- Roesel, M.J.; Nersesian, G.; Neuber, S.; Thau, H.; Wolff von Gudenberg, R.; Lanmueller, P.; Hennig, F.; Falk, V.; Potapov, E.; Knosalla, C.; et al. LVAD as a Bridge to Transplantation—Current Status and Future Perspectives. Rev. Cardiovasc. Med. 2024, 25, 176. [Google Scholar] [CrossRef]
- Han, J.J.; Acker, M.A.; Atluri, P. Left Ventricular Assist Devices. Circulation 2018, 138, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Stevens, M.C.; Pauls, J.P.; Steinseifer, U. First-generation ventricular assist devices. In Mechanical Circulatory and Respiratory Support; Elsevier: Amsterdam, The Netherlands, 2018; pp. 93–115. [Google Scholar]
- Kuehl, M.; Garbade, J. The evolution of left ventricular assist devices—A moment to reflect. J. Thorac. Dis. 2017, 9, E492–E494. [Google Scholar] [CrossRef]
- Tu, J.; Xu, L.; Li, F.; Dong, N. Developments and Challenges in Durable Ventricular Assist Device Technology: A Comprehensive Review with a Focus on Advancements in China. J. Cardiovasc. Dev. Dis. 2024, 11, 29. [Google Scholar] [CrossRef]
- Kozik, D.; Alsoufi, B. Pediatric Mechanical Circulatory Support—A Review. Indian J. Thorac. Cardiovasc. Surg. 2023, 39 (Suppl. S1), 80–90. [Google Scholar]
- Hernandez, G.A.; Nunez Breton, J.D.; Chaparro, S.V. Driveline Infection in Ventricular Assist Devices and Its Implication in the Present Era of Destination Therapy. Open J. Cardiovasc. Surg. 2017, 9, 1179065217714216. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Peleg, A.Y.; McGiffin, D. Ventricular Assist Device-Specific Infections. J. Clin. Med. 2021, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Juraszek, A.; Smólski, M.; Kołsut, P.; Pięta, A.; Gutmajster, E.; Pająk, K.; Kuśmierczyk, M. Prevalence and Management of Driveline Infections in Mechanical Circulatory Support—A Single Center Analysis. J. Cardiothorac. Surg. 2021, 16, 216. [Google Scholar] [CrossRef]
- Aburjania, N.; Hay, C.; Sohail, M. Continuous-flow left ventricular assist device systems infections: Current outcomes and management strategies. Ann. Cardiothorac. Surg. 2021, 10, 233–239. [Google Scholar] [CrossRef]
- Available online: https://www.openpr.de/news/1206728/Berlin-Heart-gibt-erste-Implantation-einer-Ueberbrueckungsloesung-bei-Patienten-mit-Einkammerherz-bekannt.html (accessed on 10 March 2025).
- Oosterom, A.; Jonge, N.; Kirkels, J.H.; Rodermans, B.; Sukkel, E.; Klöpping, C.; Ramjankhan, F.; Lahpor, J.R. End-stage heart failure and mechanical circulatory support: Feasibility of discharge from hospital. Neth. Heart J. 2007, 15, 45–50. [Google Scholar] [CrossRef]
- Zinoviev, R.; Lippincott, C.K.; Keller, S.C.; Gilotra, N.A. In Full Flow: Left Ventricular Assist Device Infections in the Modern Era. Open Forum Infect. Dis. 2020, 7, ofaa124. [Google Scholar] [CrossRef] [PubMed]
- Balestra, N.; Fredericks, S.; Vieira Caetano da Silva, A.; Rodrigues, R.C.M.; Nunes, D.P.; Pedrosa, R.B.S. Driveline dressings used in heartmate patients and local complications: A retrospective cohort. Heart Lung 2023, 62, 271–277. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K., III; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Dettbarn, E.; Prenga, M.; Stein, J.; Müller, M.; Hoermandinger, C.; Schoenrath, F.; Falk, V.; Potapov, E.; Mulzer, J.; Knierim, J. Driveline infections in left ventricular assist devices—Incidence, epidemiology, and staging proposal. Artif. Organs 2024, 48, 83–90. [Google Scholar] [CrossRef]
- Kusne, S.; Mooney, M.; Danziger-Isakov, L.; Kaan, A.; Lund, L.H.; Lyster, H.; Wieselthaler, G.; Aslam, S.; Cagliostro, B.; Chen, J.; et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J. Heart Lung Transplant. 2017, 36, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- McCandless, S.P.; Ledford, I.D.; Mason, N.O.; Alharethi, R.; Rasmusson, B.Y.; Budge, D.; Stoker, S.L.; Clayson, S.E.; Doty, J.R.; Thomsen, G.E.; et al. Comparing velour versus silicone interfaces at the driveline exit site of HeartMate II devices: Infection rates, histopathology, and ultrastructural aspects. Cardiovasc. Pathol. 2015, 24, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zeng, L. Post-operative infection in mechanical circulatory support patients. Ann. Transl. Med. 2020, 8, 831. [Google Scholar] [CrossRef]
- Kremer, J.; El-Dor, A.; Rivinius, R.; Schlegel, P.; Sommer, W.; Warnecke, G.; Karck, M.; Ruhparwar, A.; Meyer, A.L. Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation. Life 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, C.; Sunderkötter, C.; Becker, K.; Grabein, B.; Hagel, S.; Hanses, F.; Wichmann, D.; Thalhammer, F. Left Ventricular Assist Device-Associated Driveline Infections as a Specific Form of Complicated Skin and Soft Tissue Infection/Acute Bacterial Skin and Skin Structure Infection—Issues and Therapeutic Options. Curr. Opin. Infect. Dis. 2024, 37, 95–104. [Google Scholar] [CrossRef]
- Aslam, S.; Cowger, J.; Shah, P.; Stosor, V.; Copeland, H.; Reed, A.; Morales, D.; Giblin, G.; Mathew, J.; Morrissey, O.; et al. The International Society for Heart and Lung Transplantation (ISHLT): 2024 Infection Definitions for Durable and Acute Mechanical Circulatory Support Devices. J. Heart Lung Transplant. 2024, 43, 1039–1050. [Google Scholar] [CrossRef]
- Haglund, N.A.; Davis, M.E.; Tricarico, N.M.; Keebler, M.E.; Maltais, S. Readmissions after Continuous Flow Left Ventricular Assist Device Implantation: Differences Observed Between Two Contemporary Device Types. ASAIO J. 2015, 61, 410–416. [Google Scholar] [CrossRef]
- Zierer, A.; Melby, S.J.; Voeller, R.K.; Guthrie, T.J.; Ewald, G.A.; Shelton, K.; Pasque, M.K.; Moon, M.R.; Damiano, R.J.; Moazami, N. Late Onset Driveline Infections: The Achilles’ Heel of Prolonged Left Ventricular Assist Device Support. Ann. Thorac. Surg. 2007, 84, 515–520. [Google Scholar] [CrossRef]
- Rubinfeld, G.; Levine, J.P.; Reyentovich, A.; DeAnda, A.; Balsam, L.B. Management of Rapidly Ascending Driveline Tunnel Infection. J. Card. Surg. 2015, 30, 853–855. [Google Scholar] [CrossRef]
- Jaquiss, R.D.B.; Imamura, M.; Guleserian, K.J. Berlin Heart Implantation for Congenital Heart Defects. Oper. Tech. Thorac. Cardiovasc. Surg. 2010, 15, 162–171. [Google Scholar] [CrossRef]
- Stulak, J.M.; Davis, M.E.; Haglund, N.; Dunlay, S.; Cowger, J.; Shah, P.; Pagani, F.D.; Aaronson, K.D.; Maltais, S. Adverse events in contemporary continuous-flow left ventricular assist devices: A multi-institutional comparison shows significant differences. J. Thorac. Cardiovasc. Surg. 2016, 151, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Kallel, F.; Ewald, G.A.; Tatooles, A.; Sheridan, B.C.; Brewer, R.J.; Akhter, S.A. Reduction in driveline infection rates: Results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J. Heart Lung Transplant. 2015, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Pelz, G.B.; Hashmi, Z.A.; Moraca, R.J.; Murali, S.; Benza, R.L.; Sokos, G.G.; Magovern, G.J., Jr.; Stutz, S.L.; Bailey, S.H.; Dean, D.A. Battling the Achilles’ Heel of Left Ventricular Assist Devices: A Novel Technique to Reduce Drive Line Infections. J. Heart Lung Transplant. 2010, 29, S7. [Google Scholar] [CrossRef]
- Camboni, D.; Zerdzitzki, M.; Hirt, S.; Tandler, R.; Weyand, M.; Schmid, C. Reduction of INCOR® driveline infection rate with silicone at the driveline exit site. Interact. Cardiovasc. Thorac. Surg. 2016, 24, 222–228. [Google Scholar] [CrossRef]
- Ledford, I.D.; Miller, D.V.; Mason, N.O.; Alharethi, R.A.; Rasmusson, B.Y.; Budge, D.; Stoker, S.L.; Clayson, S.E.; Doty, J.R.; Thomsen, G.E.; et al. Differential infection rates between velour versus silicone interface at the HeartMate II driveline exit site: Structural and ultrastructural insight into possible causes. J. Heart Lung Transplant. 2011, 30 (Suppl. S4), S10–S11. [Google Scholar] [CrossRef]
- Horn, M.V.; Shah, P.; Menteer, J.; Herrington, C.; Guadiz, D.; Dechant, D.; Szmuszkovicz, J. Cannula Site Management Strategies in Pediatric Ventricular Assist Device (VAD) Patients. J. Heart Lung Transplant. 2016, 35 (Suppl. S4), S350. [Google Scholar] [CrossRef]
- Jekiel, K.; Syguła-Cholewińska, J. Assessment of different detection methods in bacteria survival on cotton and polyester textiles. J. Nat. Fibers 2024, 22, 2437533. [Google Scholar] [CrossRef]
- Martin, S.I. Infectious Complications of Mechanical Circulatory Support (MCS) Devices. Curr. Infect. Dis. Rep. 2013, 15, 472–477. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Naftel, D.; Holman, W.; Bellumkonda, L.; Pamboukian, S.V.; Pagani, F.D.; Kirklin, J. Continuous-flow devices and percutaneous site infections: Clinical outcomes. J. Heart Lung Transplant. 2012, 31, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.M.; Xie, R.; Cowger, J.; Schueler, S.; de By, T.; Dipchand, A.I.; Chu, V.H.; Cantor, R.S.; Koval, C.E.; Krabatsch, T.; et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J. Heart Lung Transplant. 2019, 38, 364–373. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, G.; Zhang, M.; Pienta, M.; Chenoweth, C.E.; Pagani, F.D.; Aaronson, K.D.; Fetters, M.D.; Chandanabhumma, P.P.; Cabrera, L.; et al. Michigan Congestive Heart Failure Investigators. Mortality following durable left ventricular assist device implantation by timing and type of first infection. J. Thorac. Cardiovasc. Surg. 2023, 166, 570–579.e4. [Google Scholar] [CrossRef]
- Monkowski, D.H.; Axelrod, P.; Fekete, T.; Hollander, T.; Furukawa, S.; Samuel, R. Infections associated with ventricular assist devices: Epidemiology and effect on prognosis after transplantation. Transpl. Infect. Dis. 2007, 9, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jorde, U.P.; Saeed, O.; Koehl, D.; Morris, A.A.; Wood, K.L.; Meyer, D.M.; Cantor, R.; Jacobs, J.P.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann. Thorac. Surg. 2024, 117, 33–44. [Google Scholar] [CrossRef]
- Cogswell, R.; Smith, E.; Hamel, A.; Bauman, L.; Herr, A.; Duval, S.; John, R.; Roman, D.; Adatya, S.; Colvin-Adams, M.; et al. Substance abuse at the time of left ventricular assist device implantation is associated with increased mortality. J. Heart Lung Transplant. 2014, 33, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Flécher, E.; Auffret, V.; Leclercq, C.; Boulé, S.; Vincentelli, A.; Dambrin, C.; Delmas, C.; Barandon, L.; Veniard, V.; et al. Risk factors and prognostic impact of left ventricular assist device-associated infections. Am. Heart J. 2019, 214, 69–76. [Google Scholar] [CrossRef]
- Kamat, I.; Lamba, H.; Hines-Munson, C.; Hudson, S.; Liao, K.; Muldrew, K.L.; Green, S.; Terwilliger, A.; Kaplan, H.B.; Ramig, R.F.; et al. Identifying causative microorganisms in left ventricular assist device infections as a guide for developing bacteriophage therapy. J. Surg. Res. 2022, 271, 73–81. [Google Scholar] [CrossRef]
- Iyengar, A.; Feinman, J.; Jiang, J.; Song, C.; Kim, S.; Mathew, A.; Golec, S.; Rao, A.; Radakrishnan, A.; Asher, M.; et al. Epidemiology and impact of device-specific infections on patients receiving left ventricular assist devices. JHLT Open 2025, 8, 100208. [Google Scholar] [CrossRef] [PubMed]
- Leuck, A.M. Left ventricular assist device driveline infections: Recent advances and future goals. J. Thorac. Dis. 2015, 7, 2151–2157. [Google Scholar] [PubMed]
- Skalweit, M.J. Left Ventricular Assist Device Infections. In Advanced Concepts in Endocarditis; IntechOpen: London, UK, 2018. [Google Scholar]
- Pieri, M.; Agracheva, N.; Fumagalli, L.; Greco, T.; De Bonis, M.; Calabrese, M.C.; Rossodivita, A.; Zangrillo, A.; Pappalardo, F. I Infections Occurring in Adult Patients Receiving Mechanical Circulatory Support: The Two-Year Experience of an Italian National Referral Tertiary Care Center. Med. Intensiva 2013, 37, 468–475. [Google Scholar] [CrossRef]
- Pitton, M.; Valente, L.G.; Oberhaensli, S.; Casanova, C.; Sendi, P.; Schnegg, B.; Jakob, S.M.; Cameron, D.R.; Que, Y.-A.; Fürholz, M. Dynamics of bacterial pathogens at the driveline exit site in patients with ventricular assist devices: A prospective, observational, single-center cohort study. J. Heart Lung Transplant. 2023, 42, 1445–1454. [Google Scholar] [CrossRef]
- Damyanova, T.; Paunova-Krasteva, T. What we still don’t know about biofilms—Current overview and key research information. Microbiol. Res. 2025, 16, 46. [Google Scholar] [CrossRef]
- Qu, Y.; McGiffin, D.; Kure, C.; Ozcelik, B.; Fraser, J.; Thissen, H.; Peleg, A.Y. Biofilm formation and migration on ventricular assist device drivelines. J. Thorac. Cardiovasc. Surg. 2020, 159, 491–502. [Google Scholar] [CrossRef]
- Qu, Y.; McGiffin, D.; Hayward, C.; McLean, J.; Duncan, C.; Robson, D.; Kure, C.; Shen, R.; Williams, H.; Mayo, S.; et al. Characterization of infected, explanted ventricular assist device drivelines: The role of biofilms and microgaps in the driveline tunnel. J. Heart Lung Transplant. 2020, 39, 1289–1299. [Google Scholar] [CrossRef]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An overview of biofilm formation-combating strategies and mechanisms of action of antibiofilm agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef]
- Hupe, J.; Worthmann, H.; Ravenberg, K.K.; Grosse, G.M.; Ernst, J.; Haverich, A.; Bengel, F.M.; Weissenborn, K.; Schmitto, J.D.; Hanke, J.S.; et al. Interplay between driveline infection, vessel wall inflammation, cerebrovascular events and mortality in patients with left ventricular assist device. Sci. Rep. 2023, 13, 18552. [Google Scholar] [CrossRef]
- Schueler, S.; Silvestry, S.C.; Cotts, W.G.; Slaughter, M.S.; Levy, W.C.; Cheng, R.K.; Beckman, J.A.; Villinger, J.; Ismyrloglou, E.; Tsintzos, S.I.; et al. Cost-effectiveness of left ventricular assist devices as destination therapy in the United Kingdom. ESC Heart Fail. 2021, 8, 3049–3057. [Google Scholar] [CrossRef]
- Giedraitienė, A.; Ružauskas, M.; Šiugždinienė, R.; Tučkutė, S.; Grigonis, K.; Milčius, D. ZnO nanoparticles enhance the antimicrobial properties of two-sided-coated cotton textile. Nanomaterials 2024, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Lacmanova, V.; Nguyenova, H.Y.; Ulbrich, P.; Slepicka, P.; Sajdl, P.; Svorcik, V.; Reznickova, A. Copper layers sputtered on PTFE: Effect of annealing on antibacterial performance. Mater. Today Commun. 2020, 24, 101207. [Google Scholar] [CrossRef]
- Prorokova, N.; Kumeeva, T.; Kholodkov, I. Formation of Coatings Based on Titanium Dioxide Nanosolson Polyester Fibre Materials. Coatings 2020, 10, 82. [Google Scholar] [CrossRef]
- Nabi, M.; Hussain, M.I.U.; Shanaz, S.; Hussain, S.A.; Hussain, I.; Bhat, M.A.; ul Tarfain, N.; Kashoo, Z.A.; Badroo, G.A.; Hassan, M.N.; et al. Antibacterial effectiveness of cotton textiles coated with zinc oxide nanoparticles fabricated using the sonochemical technique. Int. J. Res. Agron. 2024, 7, 175–181. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Kachare, K.; Shendage, S.; Matwal, S.; Walvekar, M.; Vhanbatte, S.; Chang, J.-Y.; Ghule, A. Bio-mediated synthesized zinc oxide coated on cotton fabric for antibacterial and wound healing application. Surf. Coat. Technol. 2024, 491, 131171. [Google Scholar] [CrossRef]
- Dumas, L.; de Souza, M.C.; Bonafe, E.G.; Martins, A.F.; Monteiro, J.P. Optimized incorporation of silver nanoparticles onto cotton fabric using k-carrageenan coatings for enhanced antimicrobial properties. ACS Appl. Bio Mater. 2024, 7, 6908–6918. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Ali, M.; Islam, S.; Iqbal, M.O.; Al-Rawi, M.B.A.; Naseem, M. Enhancing the Antibacterial Properties of Silver Particles Coated Cotton Bandages Followed by Natural Extracted Dye. J. Ind. Text. 2025, 55, 15280837251320571. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, M.H.; Kaczmarek, A.; Mrozińska, Z.; Olczyk, J. Deposition of Copper on Polyester Knitwear Fibers by a Magnetron Sputtering System. Physical Properties and Evaluation of Antimicrobial Response of New Multi-Functional Composite Materials. Appl. Sci. 2020, 10, 6990. [Google Scholar] [CrossRef]
- Gupta, A.; Maruthapandi, M.; Das, P.; Saravanan, A.; Jacobi, G.; Natan, M.; Banin, E.; Luong, J.H.T.; Gedanken, A. Cuprous Oxide Nanoparticles Decorated Fabric Materials with Anti-Biofilm Properties. ACS Appl. Bio Mater. 2022, 5, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of Antimicrobial Properties of Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent advances in TiO2-functionalized textile surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Salama, K.F.; AlJindan, R.; Alfadhel, A.; Akhtar, S.; Al-Suhaimi, E.A. Enhanced Antimicrobial Performance of Textiles Coated with TiO2 Nanoparticles. J. Ind. Text. 2024, 54, 15280837241233743. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y. Lung Toxicity of Inhaled Titanium Dioxide Nanoparticles. J. Appl. Toxicol. 2014, 34, 350–366. [Google Scholar]
- Wolf, S.; Sriram, K.; Camassa, L.M.A.; Pathak, D.; Bing, H.L.; Mohr, B.; Samulin Erdem, J. Systematic Review of Mechanistic Evidence for TiO2 Nanoparticle-Induced Lung Carcinogenicity. Nanotoxicology 2024, 18, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Shadanov, A.A.; Surovtseva, M.A.; Vaver, A.A.; Samoylova, L.M.; Vladimirov, S.V.; Timchenko, T.P.; Kim, I.I.; Poveshchenko, O.V. Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft? Int. J. Mol. Sci. 2024, 25, 965. [Google Scholar] [CrossRef]
- Lazić, I.; Obermeier, A.; Dietmair, B.; Kempf, W.E.; Busch, A.; Tübel, J.; Schneider, J.; von Eisenhart-Rothe, R.; Biberthaler, P.; Burgkart, R.; et al. Treatment of Vascular Graft Infections: Gentamicin-Coated ePTFE Grafts Reveal Strong Antibacterial Properties In Vitro. J. Mater. Sci. Mater. Med. 2022, 33, 30. [Google Scholar] [CrossRef]
- Cristea, A.-G.; Lisă, E.-L.; Iacob, S.; Dragostin, I.; Ștefan, C.S.; Fulga, I.; Anghel, A.M.; Dragan, M.; Morariu, I.D.; Dragostin, O.-M. Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals 2025, 18, 825. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C. Covalent Immobilization of Antimicrobial Peptides (AMPs) onto Biomaterial Surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Youssef, S.H.; Song, Y.; Nayak, U.Y.; Garg, S. Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections. Antibiotics 2025, 14, 379. [Google Scholar] [CrossRef]
- Bach, N.; Chi, T.T.K.; Trung, L.C.; Ngoc, N.H.B.; Hoa, T.P.T.; Huynh, T.N.P.; Khai, T.H.; Dao, T.A.; Tuoc, P.X.; Dung, V.Q. Prevalence, Microbiology, and Outcome of Peritonitis in Peritoneal Dialysis Patients in Vietnam: A Multicenter Study. BMC Nephrol. 2025, 26, 134. [Google Scholar] [CrossRef]
- Hajji, M.; Neji, M.; Agrebi, S.; Ben Nessira, S.; Ben Hamida, F.; Barbouch, S.; Harzallah, A.; Abderrahim, E. Incidence and Challenges in Management of Hemodialysis Catheter-Related Infections. Sci. Rep. 2022, 12, 20536. [Google Scholar] [CrossRef] [PubMed]
- Delistefani, F.; Wallbach, M.; Müller, G.A.; Koziolek, M.J.; Grupp, C. Risk factors for catheter-related infections in patients receiving permanent dialysis catheter. BMC Nephrol. 2019, 20, 199. [Google Scholar] [CrossRef] [PubMed]


| Material/ Strategy | Mechanism | Advantages | Limitations |
|---|---|---|---|
| Zinc oxide (ZnO) | Zn2+ ions and reactive oxygen species damage bacterial membranes and cellular components | Effective against Gram-positive, Gram-negative bacteria and fungi, cost-effective | Efficacy varies with coating thickness, particle size, deposition method, long-term durability unclear |
| Silver (Ag) | Ag+ ions disrupt bacterial membranes, proteins and DNA, leading to membrane permeability disruption | Broad-spectrum activity, long-used in biomaterials | Cytotoxicity concerns, diminishing ion release over time, coating stability challenges |
| Copper (Cu) | Cu2+ ions rapidly inactivates bacteria and damage bacterial membranes and enzymes | Strong activity including against resistant strains, cost-effective | Coating stability issues, potential cytotoxicity depending on formulation |
| Titanium dioxide (TiO2) | TiO2 produces reactive oxygen species under light exposure that damage bacterial membranes and biomolecules, inhibiting growth and biofilm formation | Biocompatible, stable, provide durable coatings on textiles and polymers | Safety concerns with nanoparticle inhalation |
| Antibiotic-eluting coatings | Controlled diffusion of antibiotic from surface into surrounding tissue, creating a local concentration that inhibits bacterial adhesion and early biofilm formation | Provides targeted antimicrobial effect at the biomaterial surface, reduces systemic exposure, allows adaptation to different antibiotics depending on expected pathogens | Short drug release duration, potential resistance, uneven coating, coating stability under mechanical stress remains uncertain |
| Smart dressings | Antibiotic or peptide release from dressing matrix upon skin-material contact, sometimes triggered by local environmental changes such as pH, enzymes, or moisture, enabling on-demand antimicrobial activity | Localized, responsive antimicrobial delivery, versatile antibiotic options, reduced need for systemic therapy, possibility of integrating monitoring or sensing functions | Limited evidence from long-term clinical use, mechanical stability and drug release reproducibility under real-life conditions remain challenging |
| Antibacterial peptides | Disrupt bacterial membranes, causing leakage of cellular contents and cell death, or penetrate intact membranes and interfere with intracellular processes by binding to nucleic acids or proteins. | Dual strategy (surface-immobilized contact-active and hydrogel carriers), low resistance risk | Immobilization limits effect to contact zones, release systems may lose activity, mechanical stability needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewa, K.; Kurtyka, P.; Szuber-Dynia, A.; Kapis, A.; Gawlikowski, M. Local Infections Associated with Ventricular Assist Devices: Materials-Related Challenges and Emerging Solutions. Materials 2025, 18, 4541. https://doi.org/10.3390/ma18194541
Cholewa K, Kurtyka P, Szuber-Dynia A, Kapis A, Gawlikowski M. Local Infections Associated with Ventricular Assist Devices: Materials-Related Challenges and Emerging Solutions. Materials. 2025; 18(19):4541. https://doi.org/10.3390/ma18194541
Chicago/Turabian StyleCholewa, Klaudia, Przemysław Kurtyka, Agnieszka Szuber-Dynia, Artur Kapis, and Maciej Gawlikowski. 2025. "Local Infections Associated with Ventricular Assist Devices: Materials-Related Challenges and Emerging Solutions" Materials 18, no. 19: 4541. https://doi.org/10.3390/ma18194541
APA StyleCholewa, K., Kurtyka, P., Szuber-Dynia, A., Kapis, A., & Gawlikowski, M. (2025). Local Infections Associated with Ventricular Assist Devices: Materials-Related Challenges and Emerging Solutions. Materials, 18(19), 4541. https://doi.org/10.3390/ma18194541






