Mechanical and Anti-Icing Properties of Polyurethane/Carbon Fiber-Reinforced Polymer Composites with Carbonized Coffee Grounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CSCG
2.3. Fabrication of PU/CSCG Composite Coating
2.4. Design of Experiments
2.5. Interface-Energy-Driven Deicing Mechanism
2.6. Characteristics
3. Results and Discussion
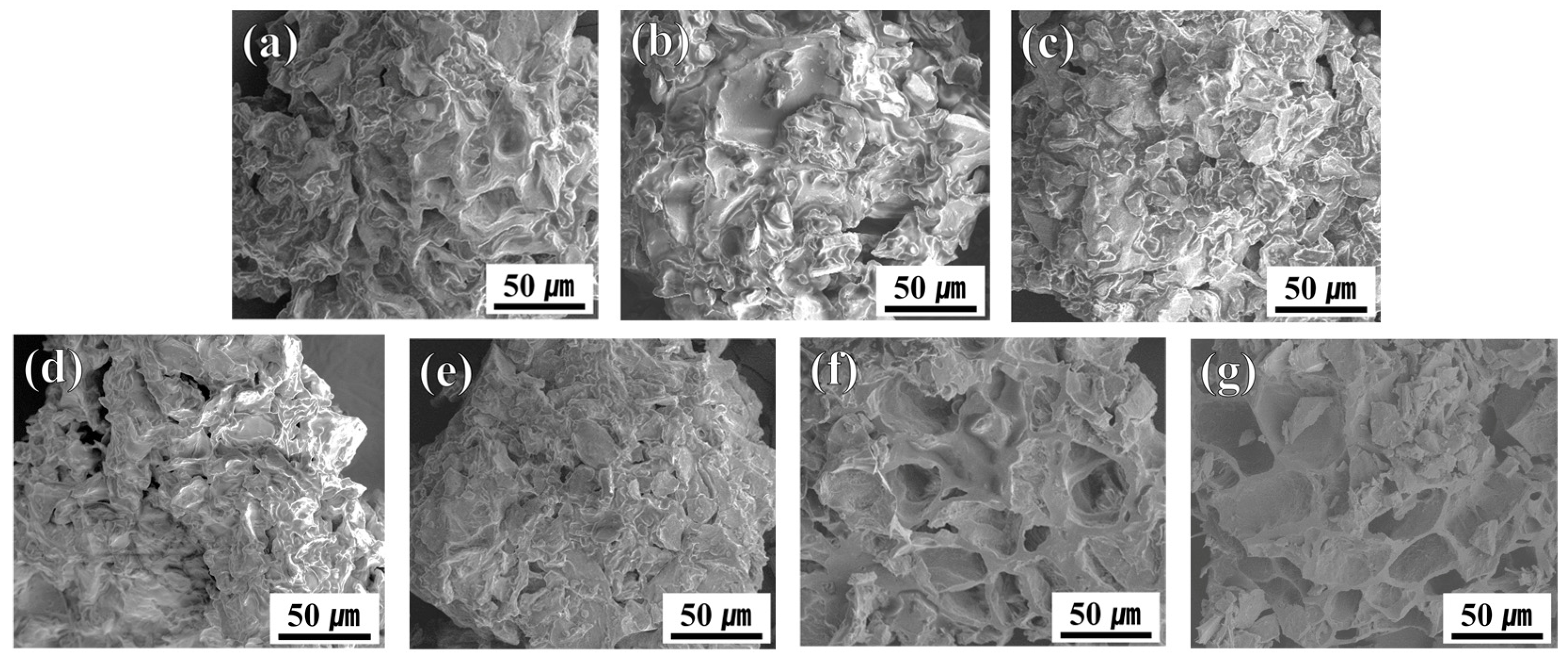
3.1. Morphological Evolution of SCG Under Thermal Treatment
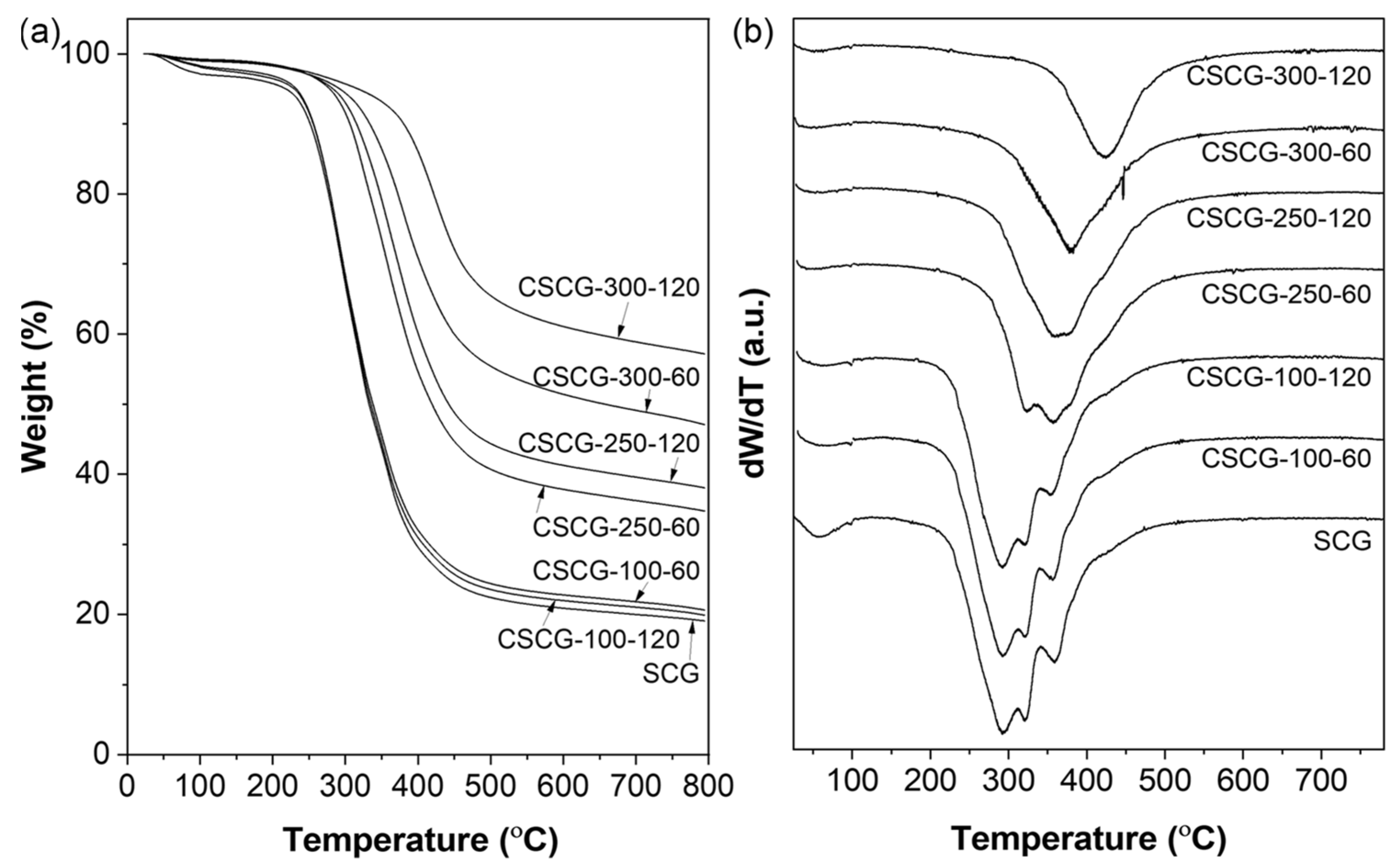
3.2. Thermal Decomposition Behavior (TGA/DTG) of SCG and CSCG
3.3. Chemical and Structural Evolution of SCG During Carbonization
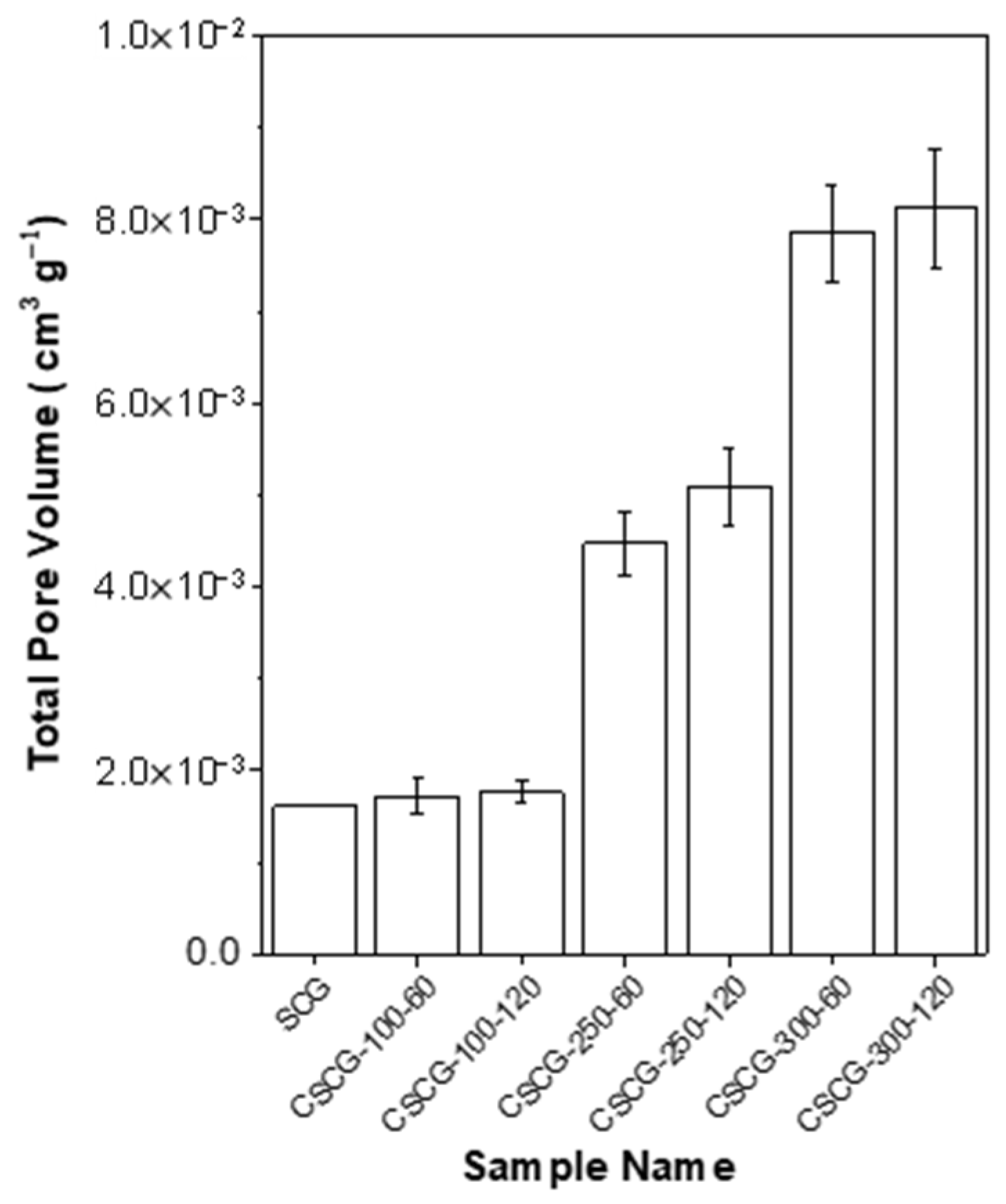
3.4. Pore-Structure Development Assessed by BET Analysis
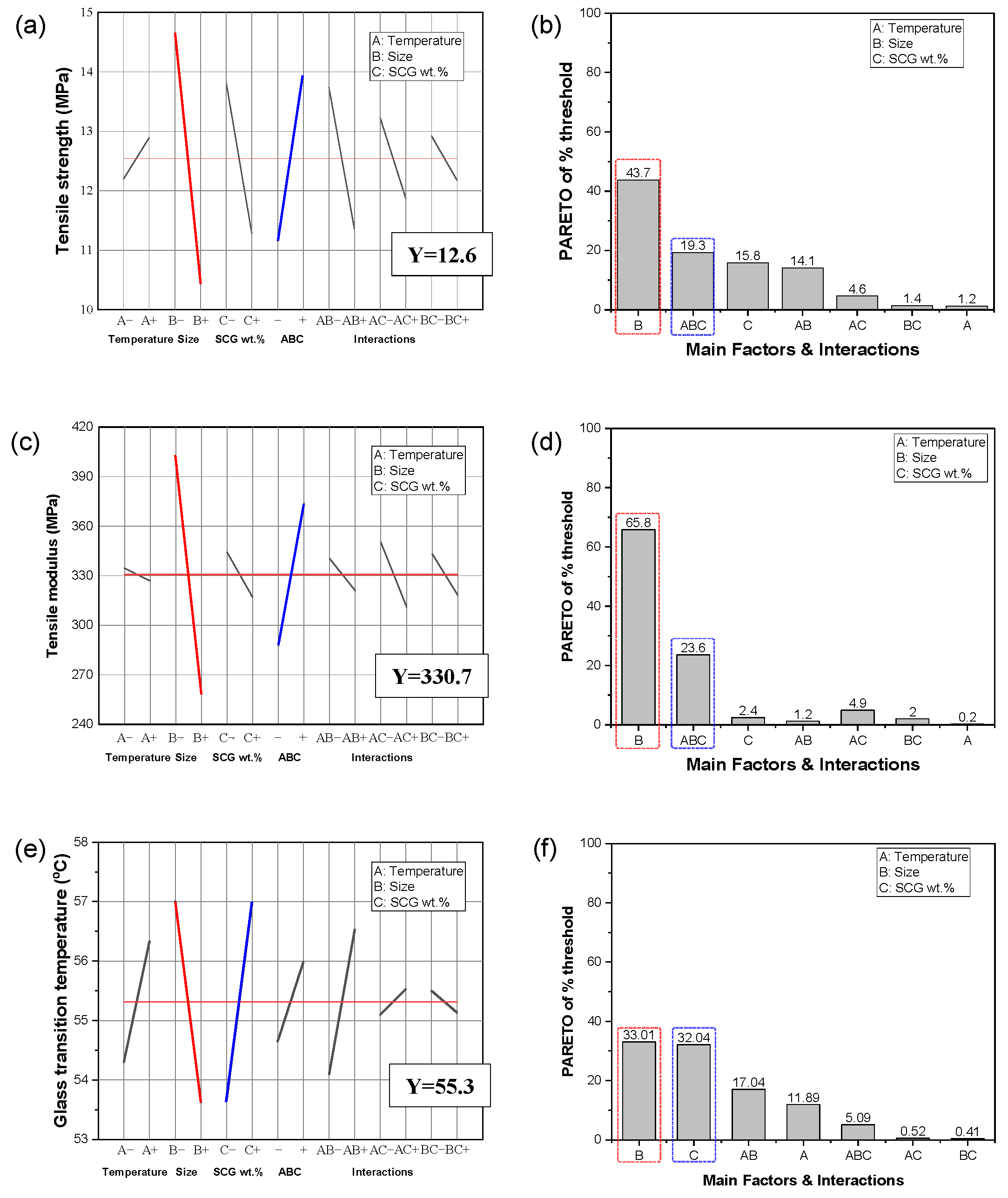
3.5. DOE Analysis of Mechanical and Thermal Properties
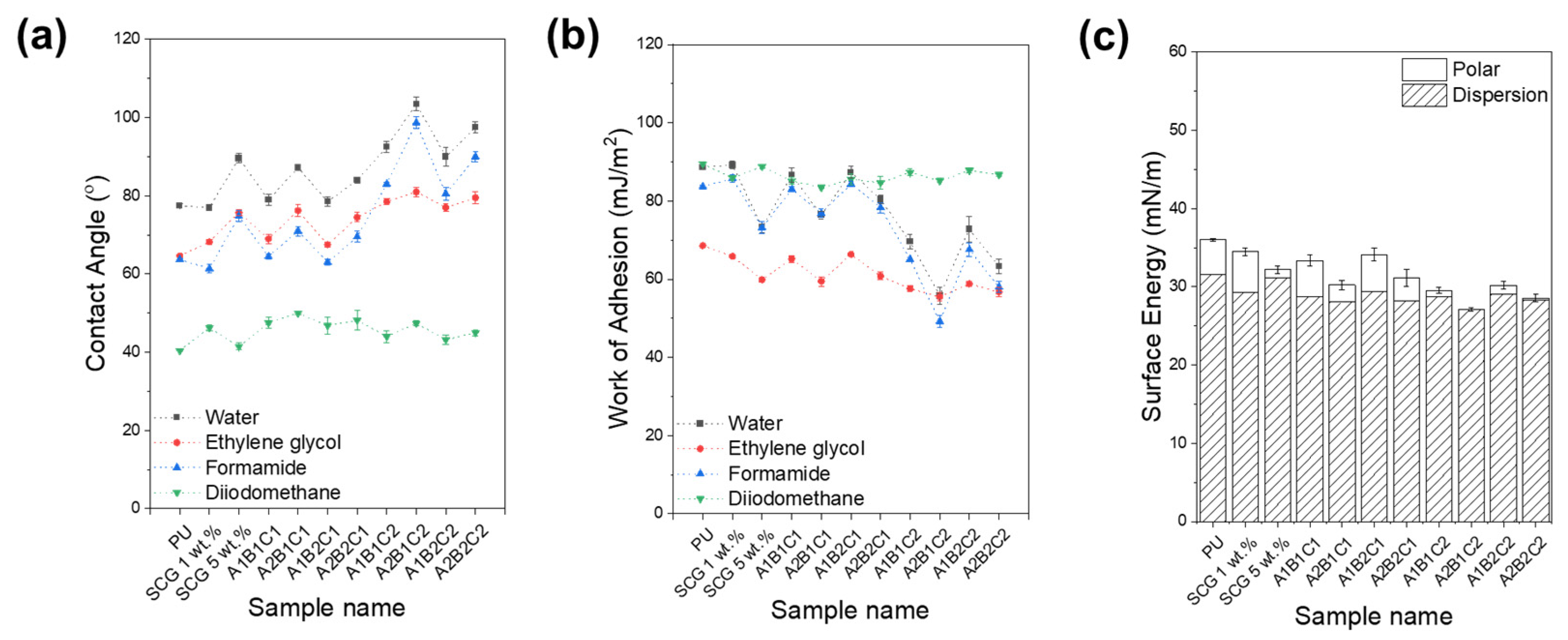
3.6. Surface Wettability and Anti-Icing Performance of PU/SCG-Based Composites
3.7. Microstructural Characteristics of PU/CSC CFRP Composites
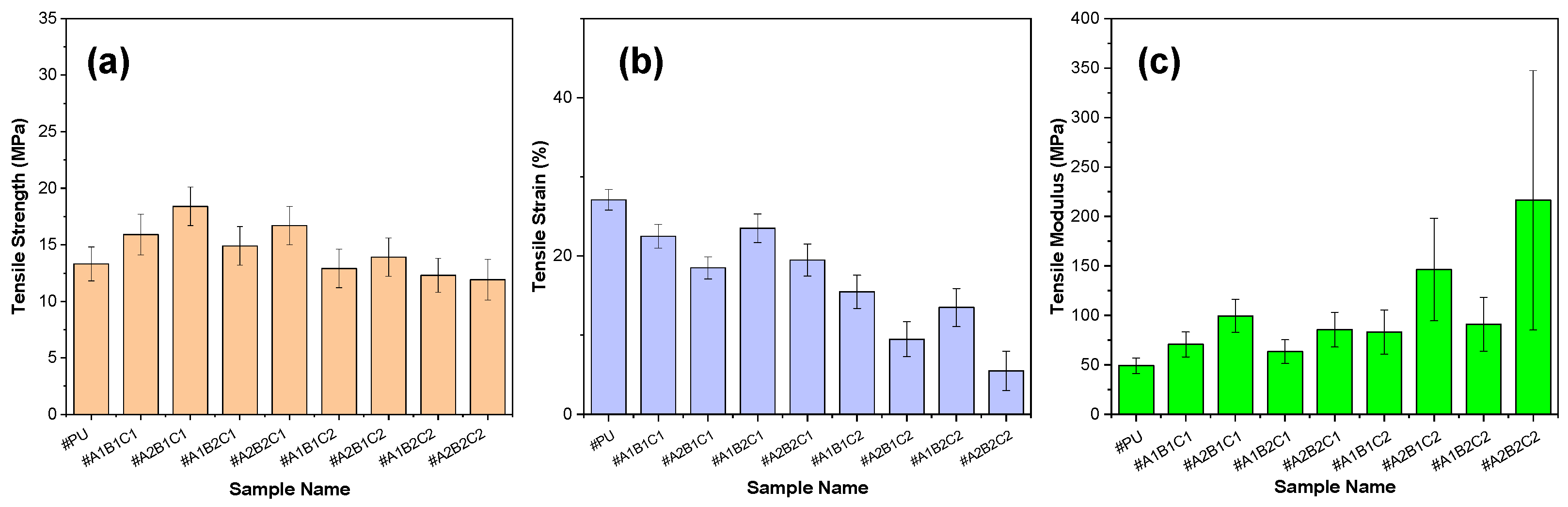
3.8. Mechanical Performance and Anti-Icing Correlation of PU/CSCG Composites
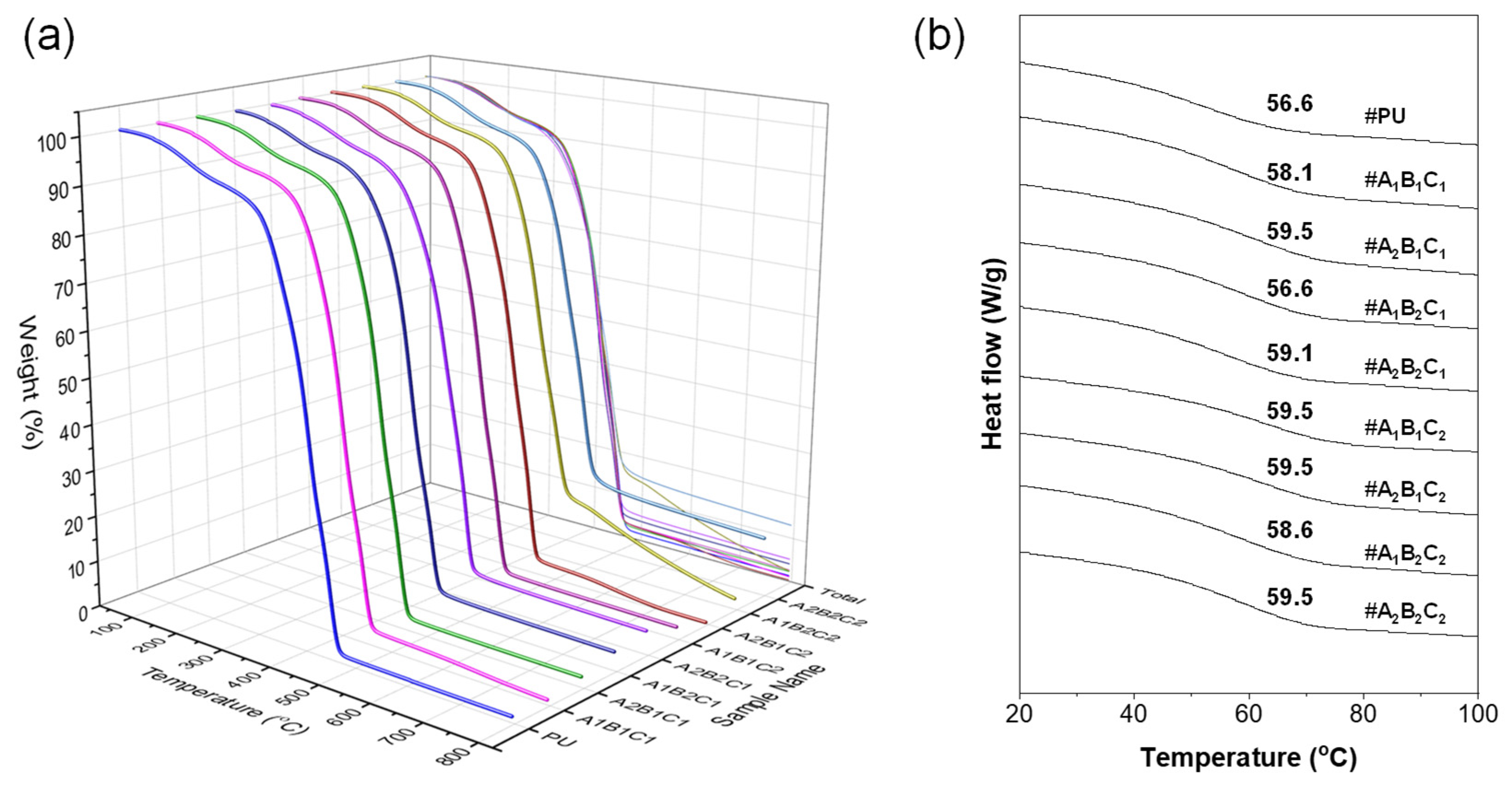
3.9. Thermal Stability and Glass-Transition Behavior of PU/CSCG Composites
3.10. Anti-Icing Performance of PU/SCG- and PU/CSCG-Coated CFRP Composites
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poncet, V.; van Asten, P.; Millet, C.P.; Vaast, P.; Allinne, C. Which Diversification Trajectories Make Coffee Farming More Sustainable? Curr. Opin. Environ. Sustain. 2024, 68, 101432. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef]
- Dimitriou, K.; Bougiatioti, A.; Ramonet, M.; Pierros, F.; Michalopoulos, P.; Liakakou, E.; Solomos, S.; Quehe, P.-Y.; Delmotte, M.; Gerasopoulos, E. Greenhouse Gases (CO2 and CH4) at an Urban Background Site in Athens, Greece: Levels, Sources and Impact of Atmospheric Circulation. Atmos. Environ. 2021, 253, 118372. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse Gases Emissions and Global Climate Change: Examining the Influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Lou, Z.; Zhang, H.; Hussain, A.; Zhan, L.; Yin, K.; Fang, M.; Fei, X. Underestimated Methane Emissions from Solid Waste Disposal Sites Reveal Missed Greenhouse Gas Mitigation Opportunities. Engineering 2024, 36, 12–15. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.; Fang, C.; Li, B.; Nie, M. Differences in the Temperature Dependence of Wetland CO2 and CH4 Emissions Vary with Water Table Depth. Nat. Clim. Change 2021, 11, 766–771. [Google Scholar] [CrossRef]
- Crista, D.M.A.; El Mragui, A.; Algarra, M.; Esteves da Silva, J.C.G.; Luque, R.; Pinto da Silva, L. Turning Spent Coffee Grounds into Sustainable Precursors for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; ben Mosbah, M.; ben Salem, R.; Moussaoui, Y. Chemical Modification of Lignin Derived from Spent Coffee Grounds for Methylene Blue Adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef]
- Yasemin, S. Isolation and Characterization of Cellulose from Spent Ground Coffee (Coffea arabica L.): A Comparative Study. Waste Manag. 2025, 193, 54–61. [Google Scholar]
- Khoshk, H.R.; Moeenfard, M. Tempo-Oxidized Cellulose Fiber from Spent Coffee Ground: Studying Their Properties as a Function of Particle Size. Heliyon 2025, 11, e41646. [Google Scholar] [CrossRef]
- Maalihan, R.D.; Domalanta, M.R.B.; Corrales, A.C.C.; Caldona, E.B. Advances in Interfacially Engineered Surface-Functionalized Fillers for Multifunctional Polymer Composite Coatings. Polym. Compos. 2025, 46, 5857–5881. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, W.; Liu, M.; Hu, Z.; Jiang, T.; Wang, H.; Zhao, Y. Progress in Mechanism Design of Functional Composites for Anti-Ice/Deicing Materials. Surf. Sci. Technol. 2024, 2, 2. [Google Scholar] [CrossRef]
- Sun, S.; Yu, Q.; Li, M.; Zhao, H.; Wang, Y.; Ji, X. Effect of Carbonization Temperature on Characterization and Water Vapor Adsorption of Coffee-Shell Activated Carbon. Adsorpt. Sci. Technol. 2020, 38, 377–392. [Google Scholar] [CrossRef]
- Strongrich, D.; Lim, F.; Kumar, L.; Alexeenko, A. Fluid Dynamics of Rapid-Depressurization Controlled Ice Nucleation in Pharmaceutical Lyophilization. Phys. Fluids 2025, 37, 036157. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Wang, J. Bioinspired Materials for Controlling Ice Nucleation, Growth, and Recrystallization. Acc. Chem. Res. 2018, 51, 1082–1091. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.D.; Pham, H.T.; Lam, S.S.; Vo, D.-V.N.; Shokouhimehr, M.; Vu, T.H.H.; Nguyen, T.-B.; Kim, S.Y.; Van Le, Q. Anti-Icing Performance on Aluminum Surfaces and Proposed Model for Freezing Time Calculation. Sci. Rep. 2021, 11, 3641. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Tenjimbayashi, M.; Naito, M. Role Variability of Surface Chemistry and Surface Topography in Anti-Icing Performance. iScience 2024, 27, 111039. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Zheng, X.; Tenjimbayashi, M.; Watanabe, I.; Naito, M. De-Icing Performance Evolution with Increasing Hydrophobicity by Regulating Surface Topography. Sci. Technol. Adv. Mater. 2024, 25, 2334199. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Adutwum, L.A.; Oliynyk, A.O.; Luber, E.J.; Olsen, B.C.; Mar, A.; Buriak, J.M. How to Optimize Materials and Devices Via Design of Experiments and Machine Learning: Demonstration Using Organic Photovoltaics. ACS Nano 2018, 12, 7434–7444. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-R.; Teng, D.-Y.; Xue, Y.-F.; Jiang, G.-C. Synthesis of Biomass Polyurethane and Its Properties. J. Polym. Mater. 2025, 42, 359–377. [Google Scholar] [CrossRef]
- Vega-Zuñiga, S.; Rueda-Bayona, J.G.; Ospino-Castro, A. Doe-Anova Analysis to Estimate the Effect of Ambient Temperature, Pressure and Humidity on Surface Wind Speed. Math. Model. Eng. Probl. 2024, 11, 657–663. [Google Scholar]
- Lee, S.; Yoo, K.S. Design of Experiments for Optimizing Silver–Graphene Composite as a Conductive Paste. Korean J. Chem. Eng. 2025, 1–12. [Google Scholar] [CrossRef]
- Waldner, C.; Hirn, U. Modeling Liquid Penetration into Porous Materials Based on Substrate and Liquid Surface Energies. J. Colloid Interface Sci. 2023, 640, 445–455. [Google Scholar] [CrossRef]
- Daniel, D.; Koh, X.Q. Droplet Detachment Force and Its Relation to Young–Dupre Adhesion. Soft Matter 2023, 19, 8434–8439. [Google Scholar] [CrossRef]
- Myoung, S.; Lee, G.; Kim, D. Facile Transition of Surface Wettability in Hierarchical Silica Framework: From Wenzel to Cassie-Baxter Regime. Macromol. Res. 2025, 33, 1117–1123. [Google Scholar] [CrossRef]
- Kumar, S.A.; Narayan, Y.S. Tensile Testing and Evaluation of 3d-Printed Pla Specimens as Per Astm D638 Type Iv Standard. In Innovative Design, Analysis and Development Practices in Aerospace and Automotive Engineering (I-Dad 2018); Springer: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 79–95. [Google Scholar]
- Ahmadi, S.F.; Nath, S.; Iliff, G.J.; Srijanto, B.R.; Collier, C.P.; Yue, P.; Boreyko, J.B. Passive Antifrosting Surfaces Using Microscopic Ice Patterns. ACS Appl. Mater. Interfaces 2018, 10, 32874–32884. [Google Scholar] [CrossRef] [PubMed]
- Majrashi, M.A.A.; Bairwan, R.D.; Mushtaq, R.Y.; Khalil, H.A.; Badr, M.Y.; Alissa, M.; Abdullah, C.; Ali, B.A.; Rizg, W.Y.; Hosny, K.M. Novel Enhancement of Interfacial Interaction and Properties in Biodegradable Polymer Composites Using Green Chemically Treated Spent Coffee Ground Microfiller. Int. J. Biol. Macromol. 2024, 266, 131333. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Bairwan, R.D.; Rizg, W.Y.; Khalil, H.P.S.A.; Murshid, S.S.A.; Sindi, A.M.; Alissa, M.; Saharudin, N.I.; Abdullah, C.K. Enhancement of Spent Coffee Grounds as Biofiller in Biodegradable Polymer Composite for Sustainable Packaging. Polym. Compos. 2024, 45, 9317–9334. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, W.; Li, W.; Li, B.; Shui, Z.; Wang, X.; Jiang, G.; Li, G.; Cheng, J.; Zhang, Z. Adsorption Recovery of Chlorinated Volatile Organic Compounds on Coffee Ground-Based Activated Carbon of Tunable Porosity. Sep. Purif. Technol. 2025, 354, 129271. [Google Scholar] [CrossRef]
- Kwon, G.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A Review of Recent Advancements in Utilization of Biomass and Industrial Wastes into Engineered Biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Luo, S.; Aaqil, M.; Guo, Y.; Zhang, F.; Huang, X.; Gu, Y.; Zhang, Z.; Li, F.; Zhao, C. From Waste to Flavor Enhancer: Differential Pressure Explosion Puffing Modifies Spent Coffee Grounds for Microbial Consortia-Driven Flavor Synthesis in Craft Beer Systems. Food Res. Int. 2025, 218, 116933. [Google Scholar] [CrossRef]
- Djandja, O.S.; Dessie, W.; Huang, Z.; Hou, Q.; Zhuang, S.; Zhao, X.; Kouediatouka, A.N.; Okopi, S.I.; Lu, X.; Kang, S. Activated Nitrogen-Doped Porous Carbon from Organic Solid Waste to Energy Storage Materials: Pore Structure Forming and N-Doping Paths from Recent Methods. J. Energy Storage 2024, 98, 113181. [Google Scholar] [CrossRef]
- Dionisio, M.; Torres, A.; Tinoco, K.; Arrubla-Vélez, J.P. Pyrolysis of Colombian Spent Coffee Grounds (Scgs), Characterization of Bio-Oil, and Study of Its Antioxidant Properties. Int. J. Sustain. Energy 2023, 42, 811–829. [Google Scholar] [CrossRef]
- Fermoso, J.; Mašek, O. Thermochemical Decomposition of Coffee Ground Residues by Tg-Ms: A Kinetic Study. J. Anal. Appl. Pyrolysis 2018, 130, 358–367. [Google Scholar] [CrossRef]
- Lv, G.-J.; Wu, S.-B.; Lou, R. Kinetic Study of the Thermal Decomposition of Hemicellulose Isolated from Corn Stalk. BioResources 2010, 5, 1281–1291. [Google Scholar] [CrossRef]
- Piyarat, W.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Comparison of Pyrolysis Kinetic Models for Thermogravimetric Analysis of Biomass. Suranree J. Tecnol. 2010, 17, 387–400. [Google Scholar]
- Taiti, C.; Vivaldo, G.; Mancuso, S.; Comparini, D.; Pandolfi, C. Volatile Organic Compounds (Vocs) Fingerprinting Combined with Complex Network Analysis as a Forecasting Tool for Tracing the Origin and Genetic Lineage of Arabica Specialty Coffees. Sci. Rep. 2025, 15, 13709. [Google Scholar] [CrossRef]
- de Paula Protásio, T.; Alves, I.; Melo, N.; Junior, M.; Mendes, R.; Trugilho, P. Thermal Decomposition of Torrefied and Carbonized Briquettes of Residues from Coffee Grain Processing. Ciênc. Agrotec. 2013, 37, 221–228. [Google Scholar] [CrossRef]
- Martinez, C.L.M.; Rocha, E.P.A.; Carneiro, A.d.C.O.; Gomes, F.J.B.; Batalha, L.A.R.; Vakkilainen, E.; Cardoso, M. Characterization of Residual Biomasses from the Coffee Production Chain and Assessment the Potential for Energy Purposes. Biomass Bioenergy 2019, 120, 68–76. [Google Scholar] [CrossRef]
- Lee, C.M.; Kubicki, J.D.; Fan, B.; Zhong, L.; Jarvis, M.C.; Kim, S.H. Hydrogen-Bonding Network and Oh Stretch Vibration of Cellulose: Comparison of Computational Modeling with Polarized Ir and Sfg Spectra. J. Phys. Chem. B 2015, 119, 15138–15149. [Google Scholar] [CrossRef]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative Ft-Ir Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. Tga-Ftir Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Naggi, A.; Torri, G.; Iacomini, M.; Castelli, G.C.; Reggi, M.; Fermani, S.; Dubinsky, Z.; Goffredo, S.; Falini, G. Structure and Function of Stony Coral Intraskeletal Polysaccharides. ACS Omega 2018, 3, 2895–2901. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of Biochar Formation from Various Agricultural by-Products Using Ftir Spectroscopy. Mod. Appl. Sci. 2015, 9, 246. [Google Scholar] [CrossRef]
- Copikova, J.; Cerna, M.; Novotná, M.; Kaasova, J.; Synytsya, A. Application of Ft-Ir Spectroscopy in Detection of Food Hydrocolloids Confectionery Jellies and in Food Supplements. Czech J. Food Sci. 2001, 19, 51–56. [Google Scholar] [CrossRef]
- Kim, H.-J.; Oh, S.-C. Hydrothermal Carbonization of Spent Coffee Grounds. Appl. Sci. 2021, 11, 6542. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, A.; Dubey, A. Characterization of Biochars from Various Agricultural by- Products Using Ftir Spectroscopy, Sem Focused with Image Processing. Int. J. Agric. Environ. Biotechnol. 2020, 13, 423–430. [Google Scholar] [CrossRef]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef]
- Gabisa, E.W.; Ratanatamskul, C. Recycling of Waste Coffee Grounds as a Photothermal Material Modified with Zncl2 for Water Purification. Sci. Rep. 2024, 14, 10811. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N. Activated Carbon from Biomass: Preparation, Factors Improving Basicity and Surface Properties for Enhanced CO2 Capture Capacity—A Review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Suhas; Chaudhary, M.; Chaudhary, S.; Chaubey, S.; da Paixão Cansado, I.P.; Dehghani, M.H.; Tyagi, I.; Gaur, R. Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study. Resources 2025, 14, 34. [Google Scholar] [CrossRef]
- Várhegyi, G.; Szabó, P.; Antal, M.J. Reaction Kinetics of the Thermal Decomposition of Cellulose and Hemicellulose in Biomass Materials. In Advances in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 760–770. [Google Scholar][Green Version]
- Chen, W.-H.; Wang, C.-W.; Ong, H.C.; Show, P.L.; Hsieh, T.-H. Torrefaction, Pyrolysis and Two-Stage Thermodegradation of Hemicellulose, Cellulose and Lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Cuadros-Lugo, E.; Piñon-Espitia, M.; Martinez-Rodríguez, H.A.; Lardizabal-Gutierrez, D.; Estrada-Guel, I.; Herrera-Ramirez, J.M.; Carreño-Gallardo, C. Turbostratic Carbon/Graphene Prepared Via the Dry Ice in Flames Method and Its Purification Using Different Routes: A Comparative Study. Materials 2022, 15, 2501. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, M.; de Jong, W.; Kortlever, R. Tuning the Properties of N-Doped Biochar for Selective CO2 Electroreduction to CO. ACS Catal. 2023, 13, 10309–10323. [Google Scholar] [CrossRef]
- Shokrani Havigh, R.; Chenari, H.M. A Comprehensive Study on the Effect of Carbonization Temperature on the Physical and Chemical Properties of Carbon Fibers. Sci. Rep. 2022, 12, 10704. [Google Scholar] [CrossRef]
- Xayrullo O‘g, P.U.; Shermatovich, K.B. Comparative Analysis of Thermal and Thermochemical Activation of Bio-Waste for Carbon Adsorbent Production. Conf. Mod. Sci. Pedagog. 2025, 1, 646–652. [Google Scholar]
- Kikuchi, K.; Yamashita, R.; Sakuragawa, S.; Saeki, T.; Oikawa, K.; Kume, T. Pore Structure and Chemical Composition of Activated Carbon Derived from Composted Spent Coffee Grounds. Carbon 2021, 175, 604. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, J.-T.; Lee, K.-T.; Ong, H.C.; Park, Y.-K.; Huang, C.-C. Pore Volume Upgrade of Biochar from Spent Coffee Grounds by Sodium Bicarbonate during Torrefaction. Chemosphere 2021, 275, 129999. [Google Scholar] [CrossRef]
- Phothong, K.; Tangsathitkulchai, C.; Lawtae, P. The Analysis of Pore Development and Formation of Surface Functional Groups in Bamboo-Based Activated Carbon during CO2 Activation. Molecules 2021, 26, 5641. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Naghizadeh, R.; Zolriasatein, A.; Bagheri, S.; Mele, C.; Corcione, C.E. Hydrophobic, Mechanical, and Physical Properties of Polyurethane Nanocomposite: Synergistic Impact of Mg(OH)2 and SiO2. Polymers 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Hirvi, J.T.; Pakkanen, T.A. Enhanced Hydrophobicity of Rough Polymer Surfaces. J. Phys. Chem. B 2007, 111, 3336–3341. [Google Scholar] [CrossRef] [PubMed]
- Hakim, I.A.; Donaldson, S.L.; Meyendorf, N.G.; Browning, C.E. Porosity Effects on Interlaminar Fracture Behavior in Carbon Fiber-Reinforced Polymer Composites. Mater. Sci. Appl. 2017, 8, 170–187. [Google Scholar] [CrossRef]
- Hörrmann, S.; Adumitroaie, A.; Kralovec, C.; Schagerl, M. The Influence of Resin Starved Area Manufacturing Imperfections on the Mechanical Performance of Non-Crimp Fabric Cfrp Laminate. In Proceedings of the 21st International Conference on Composite Materials (ICCM21), Xi’an, China, 20–25 August 2017. [Google Scholar]
- Pavlyuchkova, E.A.; Malkin, A.Y.; Kornev, Y.V.; Simonov-Emel’yAnov, I.D. Distribution of Filler in Polymer Composites. Role of Particle Size and Concentration. Polym. Sci. Ser. A 2024, 66, 113–120. [Google Scholar] [CrossRef]
- Samal, S. Effect of Shape and Size of Filler Particle on the Aggregation and Sedimentation Behavior of the Polymer Composite. Powder Technol. 2020, 366, 43–51. [Google Scholar] [CrossRef]
- Yuliusman; Nasruddin; Afdhol, M.K.; Haris, F.; Amiliana, R.A.; Hanafi, A.; Ramadhan, I.T. Production of Activated Carbon from Coffee Grounds Using Chemical and Physical Activation Method. Adv. Sci. Lett. 2017, 23, 5751–5755. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Niu, C.; Dalai, A.K. Experimental and Modeling Studies of Torrefaction of Spent Coffee Grounds and Coffee Husk: Effects on Surface Chemistry and Carbon Dioxide Capture Performance. ACS Omega 2021, 7, 638–653. [Google Scholar] [CrossRef]
- Padilla-Martínez, E.; Pérez-Buendía, S.; López-Sandoval, R.; Sánchez-Rodríguez, C. Electrochemical Energy Storage from Spent Coffee Grounds-Derived Carbon by Koh Activation. J. Energy Storage 2023, 71, 108115. [Google Scholar] [CrossRef]
- Rabbani, S.; Bakhshandeh, E.; Jafari, R.; Momen, G. Superhydrophobic and Icephobic Polyurethane Coatings: Fundamentals, Progress, Challenges and Opportunities. Prog. Org. Coat. 2022, 165, 106715. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate–Polymer Composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Yu, S.; Yek, W.M.; Ho, S.Y.; Rannou, S.A.D.; Lim, S.H. Microstructure and Impact Strength of Polyamide 6 Composites. Mater. Today Commun. 2015, 4, 199–203. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.B.; Woo, M.J.; Lee, D.; Kim, J.-H.; Nam, S.Y.; Kwon, D.-J. Mechanical and Anti-Icing Properties of Polyurethane/Carbon Fiber-Reinforced Polymer Composites with Carbonized Coffee Grounds. Materials 2025, 18, 4533. https://doi.org/10.3390/ma18194533
Yang SB, Woo MJ, Lee D, Kim J-H, Nam SY, Kwon D-J. Mechanical and Anti-Icing Properties of Polyurethane/Carbon Fiber-Reinforced Polymer Composites with Carbonized Coffee Grounds. Materials. 2025; 18(19):4533. https://doi.org/10.3390/ma18194533
Chicago/Turabian StyleYang, Seong Baek, Min Ji Woo, Donghyeon Lee, Jong-Hyun Kim, Sang Yong Nam, and Dong-Jun Kwon. 2025. "Mechanical and Anti-Icing Properties of Polyurethane/Carbon Fiber-Reinforced Polymer Composites with Carbonized Coffee Grounds" Materials 18, no. 19: 4533. https://doi.org/10.3390/ma18194533
APA StyleYang, S. B., Woo, M. J., Lee, D., Kim, J.-H., Nam, S. Y., & Kwon, D.-J. (2025). Mechanical and Anti-Icing Properties of Polyurethane/Carbon Fiber-Reinforced Polymer Composites with Carbonized Coffee Grounds. Materials, 18(19), 4533. https://doi.org/10.3390/ma18194533





