The Effect of ZrO2 Addition and Thermal Treatment on the Microstructure and Mechanical Properties of Aluminum Metal Matrix Composites (AMMCs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Composite Fabrication
2.2. Thermal Treatment
2.3. Surface Preparation
2.4. X-Ray Diffraction
2.5. Mechanical Testing
3. Results and Discussion
3.1. Microstructural Characterization
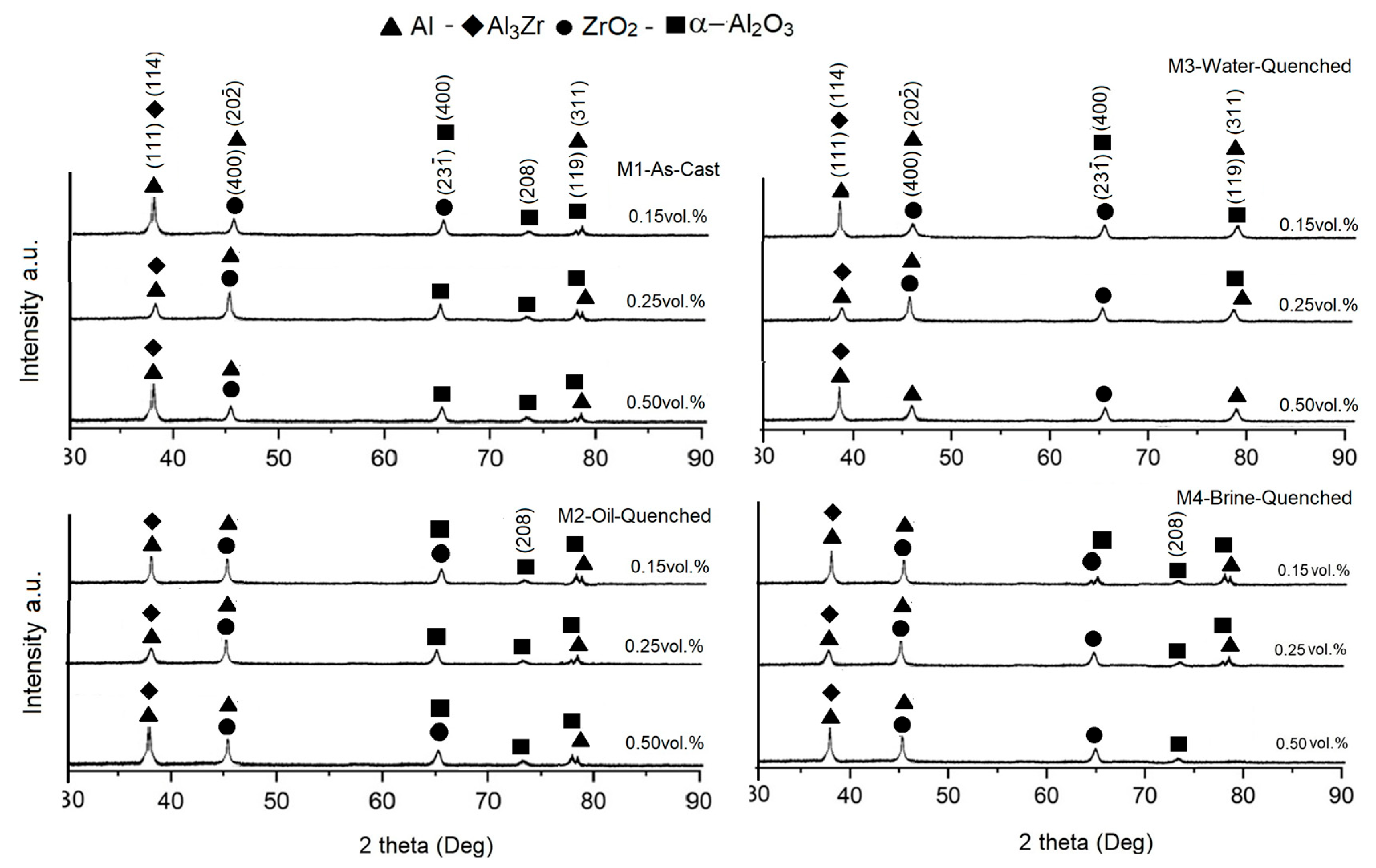
3.2. X-Ray Diffraction Analyses
3.3. Mechanical Behavior
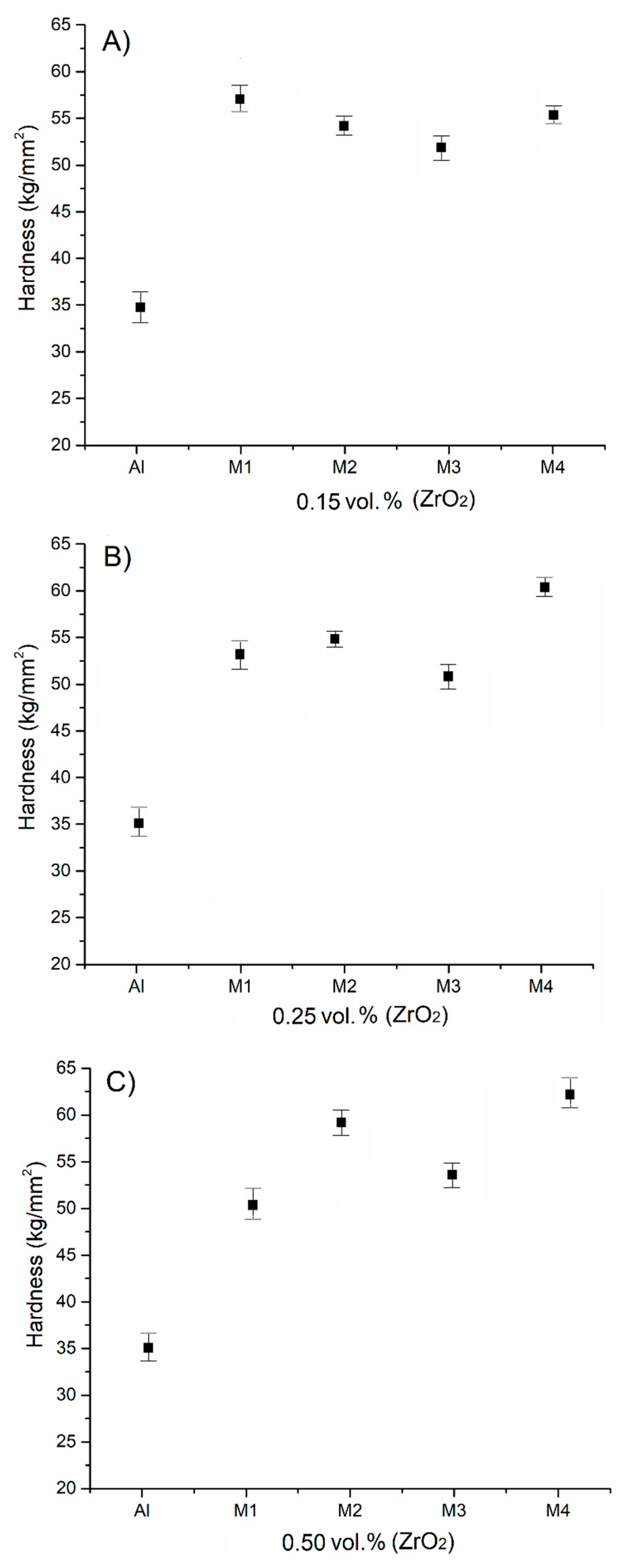
3.3.1. Hardness Evaluation
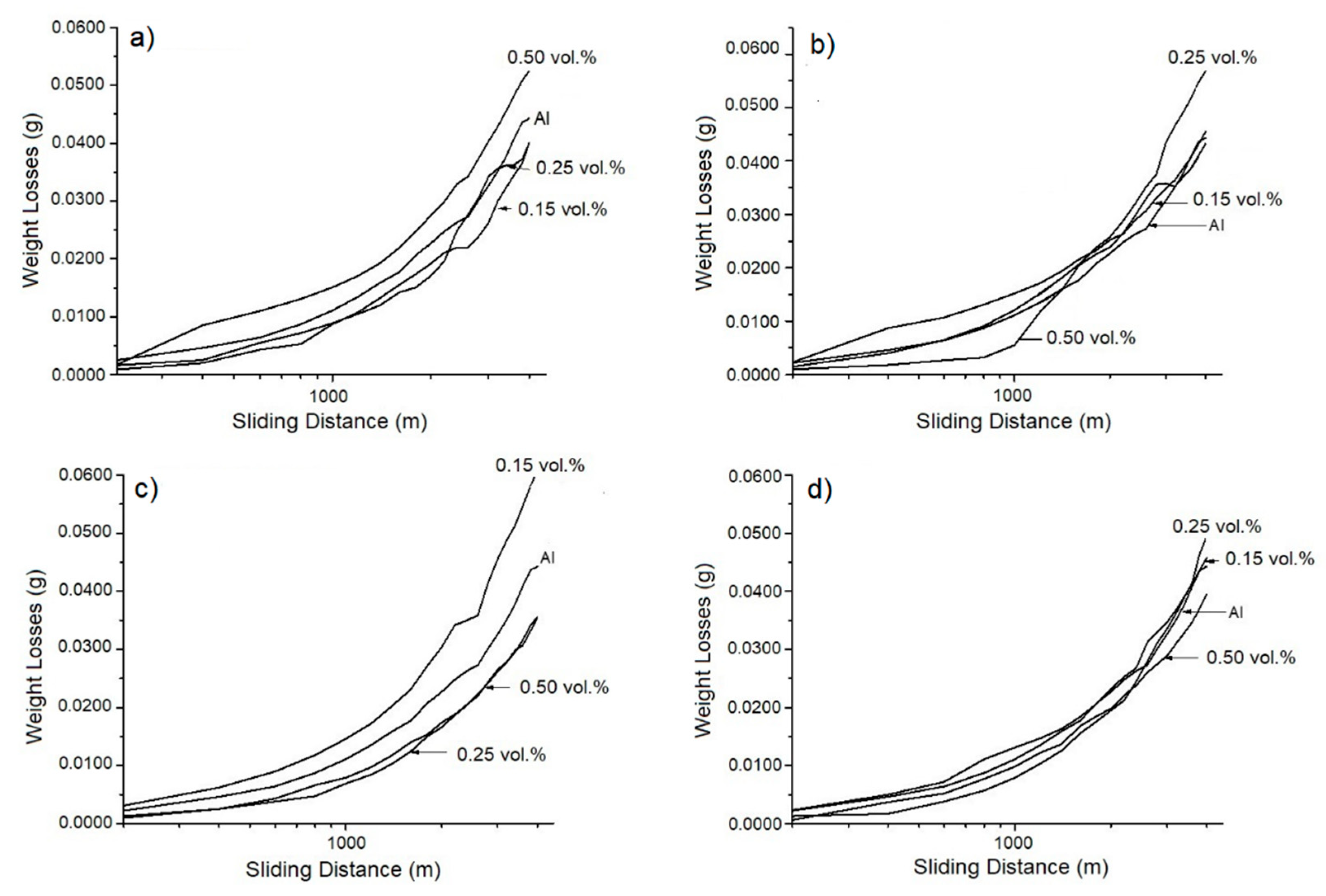
3.3.2. Wear Evaluation
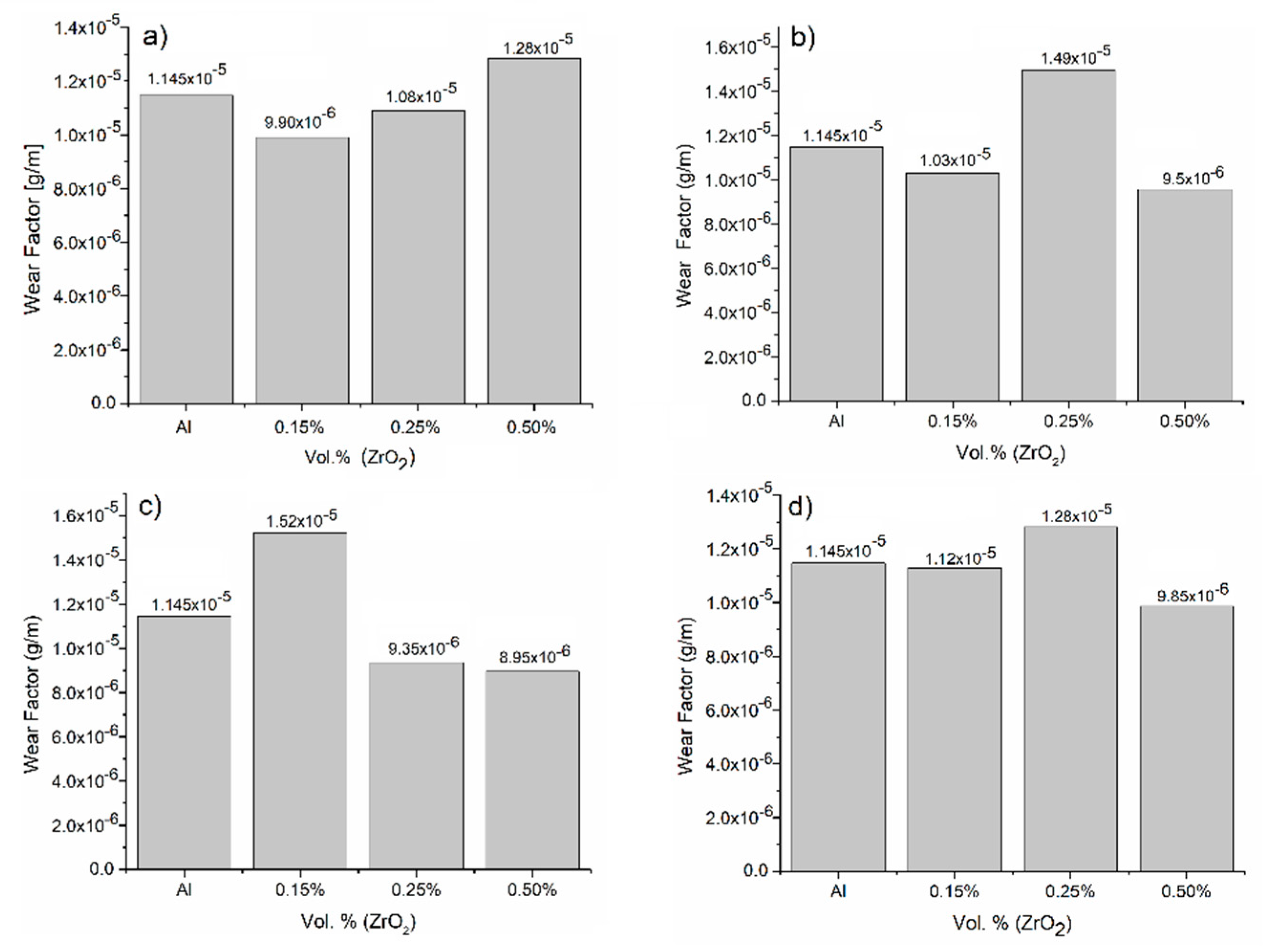
3.3.3. Wear Factor Evaluation
3.3.4. Wear Surface Analyses
4. Conclusions
- -
- The samples with low amounts of ZrO2 added showed a refined dendritic microstructure, while elevated amounts of reinforcement and accelerated cooling rates led to equiaxed grains. Grain size was directly affected by the amount of reinforcement added to the MMCs.
- -
- An evaluation of the hardness of the samples reinforced with 0.50 vol.% of ZrO2 and subjected to thermal treatment that were cooled in synthetic brine showed they exhibited the greatest increase in their values, reaching approximately 30 percent (60 kg/mm2) higher in comparison with the sample without reinforcement.
- -
- Tribological evaluations showed that the samples supplemented with 0.50 vol.% of zirconia and oil-quenched presented the lowest weight loss and therefore elevated wear resistance. Two wear mechanisms were identified: oxidative–adhesive for low concentrations and abrasive for higher zirconia concentrations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falcon-Franco, L.; Rosales, I.; García-Villarreal, S.; Curiel, F.F.; Arizmendi-Morquecho, A. Synthesis of magnesium metallic matrix composites and the evaluation of aluminum nitride addition effect. J. Alloys Compd. 2016, 663, 407–412. [Google Scholar] [CrossRef]
- Falcon-Franco, L.; Bedolla-Becerril, E.; Lemus-Ruiz, J.; Gonzalez-Rodríguez, J.G.; Guardian, R.; Rosales, I. Wear performance of TiC as reinforcement of a magnesium alloy matrix composite. Compos. B Eng. 2011, 42, 275–279. [Google Scholar] [CrossRef]
- Macke, A.; Schultz, B.; Rohatgi, P. Metal Matrix Composites Offer the Automotive Industry an Opportunity to Reduce Vehicle Weight, Improve Performance. AM&P Adv. Mater. Proc. 2012, 170, 19–23. [Google Scholar]
- Scudino, S.; Liu, G.; Prashanth, K.G.; Bartusch, B.; Surreddi, K.B.; Murty, B.S.; Eckert, J. Mechanical properties of Al-based metal matrix composites reinforced with Zr-based glassy particles produced by powder metallurgy. Acta Mater. 2009, 57, 2029–2039. [Google Scholar] [CrossRef]
- Singh, J.; Chauhan, A. Characterization of hybrid aluminum matrix composites for advanced applications A review. J. Mater. Res. Technol. 2016, 5, 159–169. [Google Scholar] [CrossRef]
- Ramnath, B.V.; Elanchezhian, C.; Annamalai, R.M.; Aravind, S.; Atreya, T.S.A.; Vignesh, V.; Subramanian, C. Aluminum Metal Matrix Composites—A Review. Rev. Adv. Mater. Sci. 2013, 38, 55–60. [Google Scholar]
- Lee, D.M.; Suh, B.K.; Kim, B.G.; Lee, J.S.; Lee, C.H. Fabrication, microstructures, and tensile properties of magnesium alloy AZ91/SiCp composites produced by powder metallurgy. Mater. Sci. Technol. 1997, 13, 590–595. [Google Scholar] [CrossRef]
- Subramaniam, B.; Purusothaman, V.R.; Karuppusamy, S.M.; Ganesh, S.H.; Markandan, R.K. Review on properties of aluminium metal matrix composites. J. Mech. Energy Eng. 2020, 4, 57–66. [Google Scholar] [CrossRef]
- Reddy, M.S.; Raju, H. Effect of Nano-ZrO2 Particulates on Wear Characteristics of Al7075 Metal Matrix Composite. Int. J. Veh. Struct. Syst. 2020, 12, 452–455. [Google Scholar]
- Yao, Y.; Chen, L. Processing of B4C Particulate-reinforced Magnesium-matrix Composites by Metal-assisted Melt Infiltration Technique. J. Mater. Sci. Technol. 2014, 30, 661–665. [Google Scholar] [CrossRef]
- Leparoux, M.; Kollo, L.; Kwon, H.; Kallip, K.; Babu, N.K.; AlOgab, K.; Talari, M.K. Solid State Processing of Aluminum Matrix Composites Reinforced with Nanoparticulate Materials. Adv. Eng. Mater. 2018, 20, 1800401. [Google Scholar] [CrossRef]
- Singh, H.; Brar, G.S.; Kumar, H.; Aggarwal, V. A review on metal matrix composite for automobile applications. Mater. Today Proc. 2021, 43, 320–325. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Chaudhary, R. Recent progress in production of metal matrix composites by stir casting process: An overview. Mater. Today Proc. 2020, 21, 1453–1457. [Google Scholar] [CrossRef]
- Bhandare, R.G.; Sonawane, P.M. Preparation of aluminium matrix composite by using stir casting method. Int. J. Eng. Adv. Technol. 2013, 3, 148–155. [Google Scholar]
- Pandiyarajan, R.; Maran, P.; Marimuthu, S.; Prabakaran, M.P. Investigation on mechanical properties of ZrO2, C and AA6061 metal matrix composites. Adv. Mater. Process. Technol. 2022, 8 (Suppl. S1), 178–186. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Z.; Zhao, J. Thermodynamic assessment of the Al-Zr binary system. J. Phase Equilib. 2001, 22, 544–555. [Google Scholar] [CrossRef]
- Croteau, J.R.; Griffiths, S.; Rossell, M.D.; Leinenbach, C.; Kenel, C.; Jansen, V.; Seidman, D.N.; Dunand, D.C.; Vo, N.Q. Microstructure and mechanical properties of Al-Mg-Zr alloys processed by selective laser melting. Acta Mater. 2018, 153, 35–44. [Google Scholar] [CrossRef]
- Reddy, P.; Raju, H.; Banapurmath, N.R.; Meti, V.K.V. Influence of ZrO2 nano particles on the behavior of mechanical and tribological properties of the AA7075 composite. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2020, 236, 55–62. [Google Scholar] [CrossRef]
- Boppana, S.B.; Dayanand, S.; Kumar, A.; Kumar, V.; Aravinda, T. Synthesis and characterization of nano graphene and ZrO2 reinforced Al 6061 metal matrix composites. J. Mater. Res. Technol. 2020, 9, 7354–7362. [Google Scholar] [CrossRef]
- Umar, M.; Muraliraja, R.; Rathnakumar, P.; Tamilarasan, T. The influence of ZrO2 on tribological and mechanical characteristics of Al composite. Mater. Today Proc. 2023, 115, 17–23. [Google Scholar] [CrossRef]
- Janghorban, A.; Antoni-Zdziobek, A.; Lomello-Tafin, M.; Antion, C.; Mazingue, T.; Pisch, A. Phase equilibria in the aluminium-rich side of the Al–Zr system. J. Therm. Anal. Calorim. 2013, 114, 1015–1020. [Google Scholar] [CrossRef]
- Samanta, B.; Balakrishnan, S.; Pagoti, R.; Ananthasivan, K.; Joseph, K.; Dasgupta, A. Measurement of solidus and liquidus in the system Zr-Al (20–95 at. % Zr) by using the spot-technique. Thermochim. Acta. 2018, 667, 132–139. [Google Scholar] [CrossRef]
- Vander, V.; George, F. Metallography, Principles and Practice; ASM International McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- ASTM E384-22; Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM G99-17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2020.
- Murray, J.; Peruzzi, A.; Abriata, J.P. The Al-Zr (aluminum-zirconium) system. J. Phase Equilib. 1992, 3, 277–291. [Google Scholar] [CrossRef]
- Sheedy, P.M.; Caram, H.S.; Chan, H.M.; Harmer, M.P. Effects of Zirconium Oxide on the Reaction Bonding of Aluminum Oxide. J. Am. Ceram. Soc. 2001, 84, 986–990. [Google Scholar] [CrossRef]
- Yar, A.A.; Montazerian, M.; Abdizadeh, H.; Baharvandi, H.R. Microstructure and mechanical properties of aluminum alloy matrix composite reinforced with nano-particle MgO. J. Alloys Compd. 2009, 484, 400–404. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, G.; Zhu, M.; Wang, J.; Zhou, Y. Mechanical properties and fracture mechanisms of aluminum matrix composites reinforced by Al9(Co, Ni)2 intermetallics. Trans. Nonferrous Met. Soc. China 2010, 20, 572–576. [Google Scholar] [CrossRef]
- Baradeswaran, A.; Perumal, A.E. Influence of B4C on the tribological and mechanical properties of Al 7075–B4C composites. Compos. B Eng. 2013, 54, 146–152. [Google Scholar] [CrossRef]
- Dwivedi, D.K. Adhesive wear behaviour of cast aluminium–silicon alloys: Overview. Mater. Des. 2010, 31, 2517–2531. [Google Scholar] [CrossRef]
- El-Azim, A.N.A.; Kassem, M.A.; El-Baradie, Z.M.; Waly, M. Structure and properties of short alumina fibre reinforced AlSi18CuNi produced by stir casting. Mater. Lett. 2002, 56, 963–969. [Google Scholar] [CrossRef]
- Kori, S.; Chandrashekharaiah, T. Studies on the dry sliding wear behaviour of hypoeutectic and eutectic Al–Si alloys. Wear 2007, 263, 745–755. [Google Scholar] [CrossRef]
- Anasyida, A.; Daud, A.; Ghazali, M. Dry sliding wear behaviour of Al–12Si–4Mg alloy with cerium addition. Mater. Des. 2010, 31, 365–374. [Google Scholar] [CrossRef]
- Hassan, A.M.; Alrashdan, A.; Hayajneh, M.T.; Mayyas, A.T. Wear behavior of Al–Mg–Cu–based composites containing SiC particles. Tribol. Int. 2009, 42, 1230–1238. [Google Scholar] [CrossRef]
- Selvam, J.D.R.; Smart, D.R.; Dinaharan, I. Synthesis and Characterization of Al6061-Fly Ashp-SiCp Composites by Stir Casting and Compocasting Methods. Energy Procedia 2013, 34, 637–646. [Google Scholar] [CrossRef]
- Kalaiselvan, K.; Murugan, N.; Parameswaran, S. Production and characterization of AA6061–B4C stir cast composite. Mater. Des. 2011, 32, 4004–4009. [Google Scholar] [CrossRef]
- Kumar, A.; Lal, S.; Kumar, S. Fabrication and characterization of A359/Al2O3 metal matrix composite using electromagnetic stir casting method. J. Mater. Res. Technol. 2013, 2, 250–254. [Google Scholar] [CrossRef]
- Kök, M. Abrasive wear of Al2O3 particle reinforced 2024 aluminium alloy composites fabricated by vortex method. Compos. Part A Appl. Sci. Manuf. 2006, 37, 457–464. [Google Scholar] [CrossRef]
- Sajjadi, S.; Ezatpour, H.; Beygi, H. Microstructure and mechanical properties of Al–Al2O3 micro and nano composites fabricated by stir casting. Mater. Sci. Eng. A 2011, 528, 8765–8877. [Google Scholar] [CrossRef]
- Mazahery, A.; Abdizadeh, H.; Baharvandi, H. Development of high-performance A356/nano-Al2O3 composites. Mater. Sci. Eng. A 2009, 518, 61–64. [Google Scholar] [CrossRef]
- Sawla, S.; Das, S. Combined effect of reinforcement and heat treatment on the two body abrasive wear of aluminum alloy and aluminum particle composites. Wear 2004, 257, 555–561. [Google Scholar] [CrossRef]
- Hassan, S.B.; Aponbiede, O.; Aigbodion, V.S. Precipitation hardening characteristics of Al–Si–Fe/SiC particulate composites. J. Alloys Compd. 2008, 466, 268–272. [Google Scholar] [CrossRef]

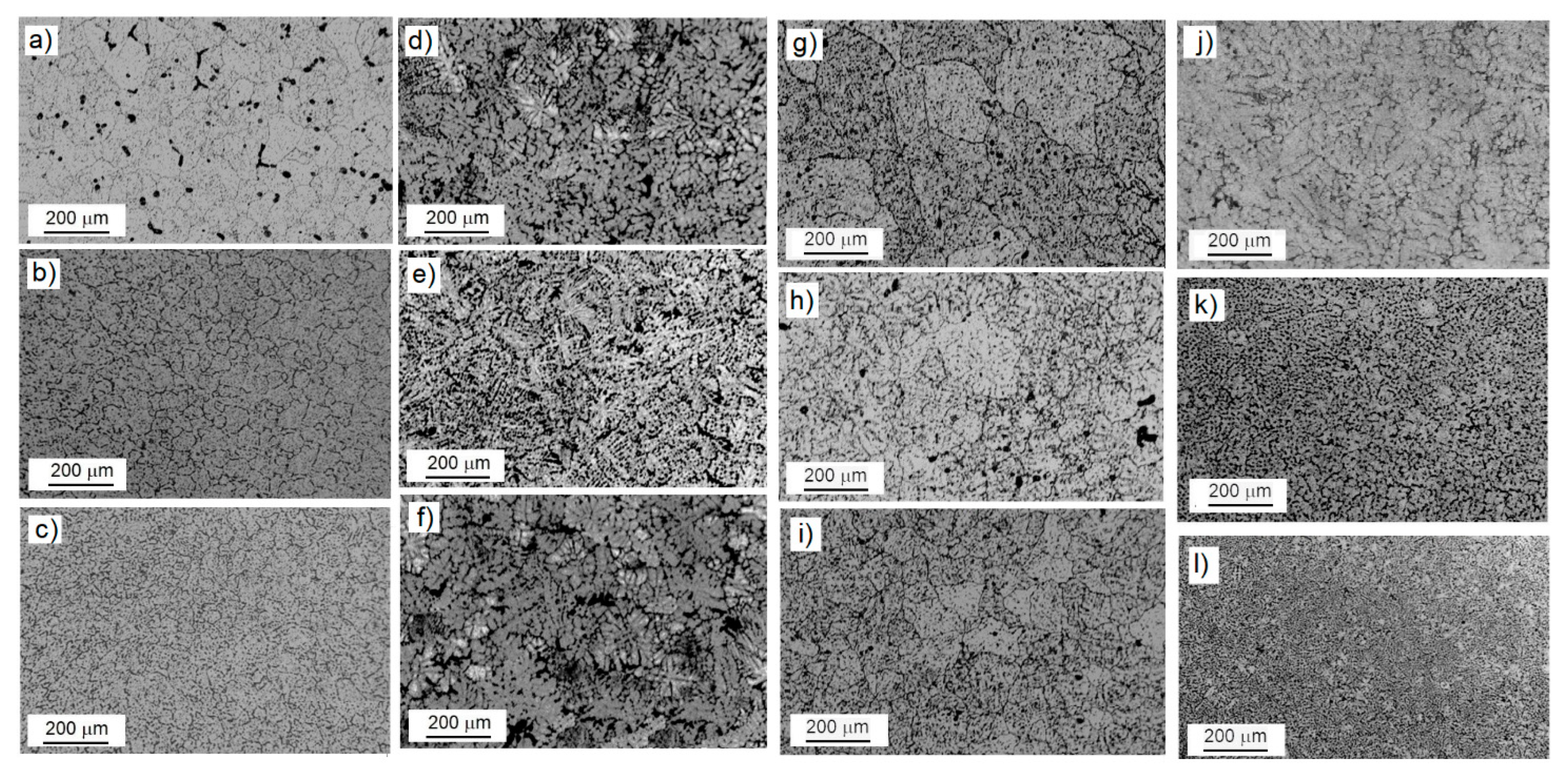

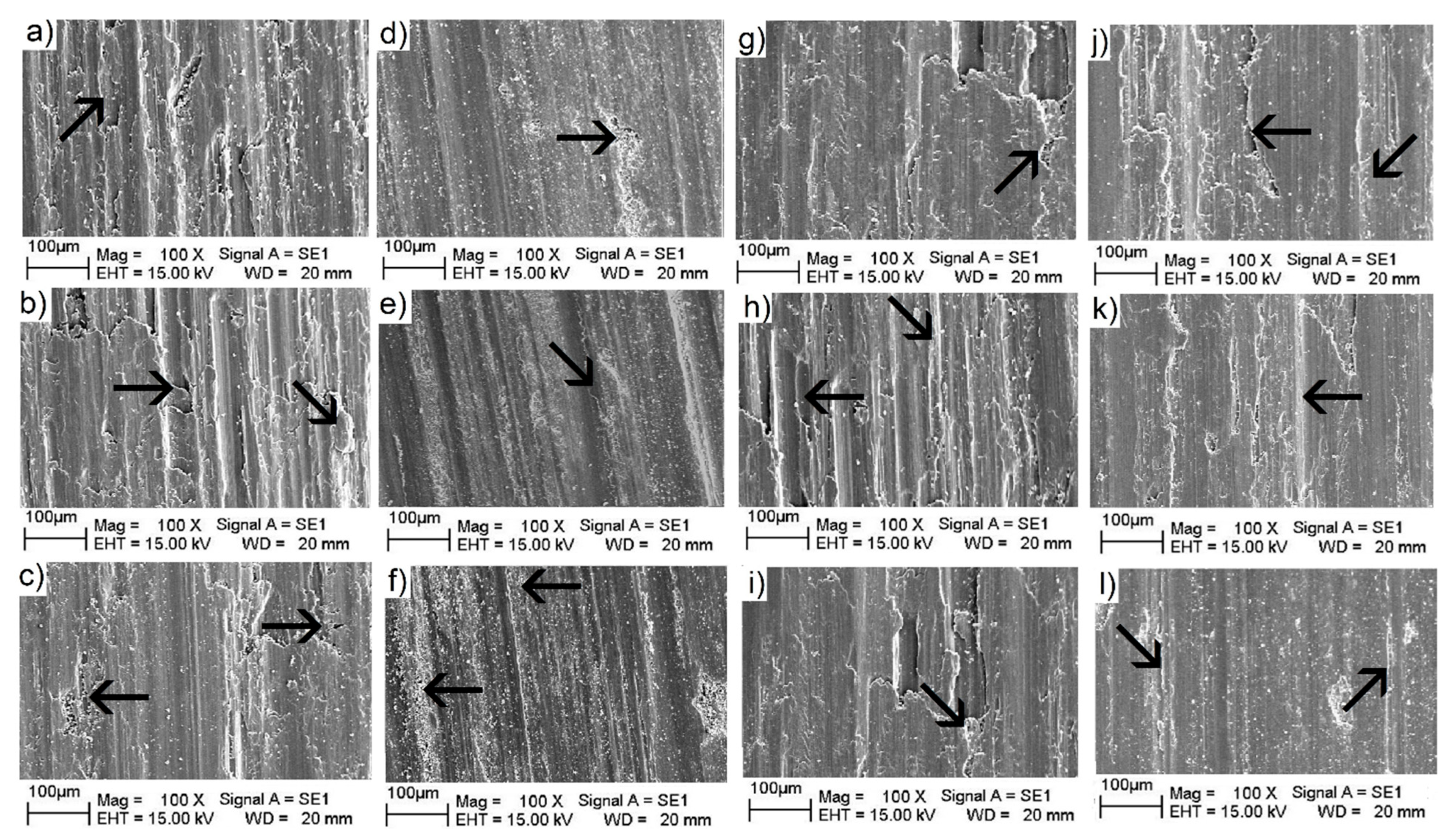
| Sample Designation | Heat Treatment | Designation | ZrO2 Additions in [vol.%] | Porosity (%) |
|---|---|---|---|---|
| M1 | As-cast | (a) | 0.15 | 1.872 |
| (b) | 0.25 | 1.484 | ||
| (c) | 0.50 | 1.732 | ||
| M2 | Oil quenched + thermal treatment | (d) | 0.15 | 3.323 |
| (e) | 0.25 | 2.567 | ||
| (f) | 0.50 | 3.425 | ||
| M3 | Water quenched + thermal treatment | (g) | 0.15 | 2.220 |
| (h) | 0.25 | 1.914 | ||
| (i) | 0.50 | 2.362 | ||
| M4 | Brine quenched + thermal treatment | (j) | 0.15 | 3.451 |
| (k) | 0.25 | 2.321 | ||
| (l) | 0.50 | 1.284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-Cadena, I.; Falcon-Castrejon, R.A.; Guardian-Tapia, R.; Roman-Zubillaga, J.L.; Gonzaga-Segura, S.R.; Falcon-Franco, L.A.; Martinez-Landeros, V.H.; Servin, R. The Effect of ZrO2 Addition and Thermal Treatment on the Microstructure and Mechanical Properties of Aluminum Metal Matrix Composites (AMMCs). Materials 2025, 18, 4507. https://doi.org/10.3390/ma18194507
Rosales-Cadena I, Falcon-Castrejon RA, Guardian-Tapia R, Roman-Zubillaga JL, Gonzaga-Segura SR, Falcon-Franco LA, Martinez-Landeros VH, Servin R. The Effect of ZrO2 Addition and Thermal Treatment on the Microstructure and Mechanical Properties of Aluminum Metal Matrix Composites (AMMCs). Materials. 2025; 18(19):4507. https://doi.org/10.3390/ma18194507
Chicago/Turabian StyleRosales-Cadena, Isai, Reyna Anahi Falcon-Castrejon, Rene Guardian-Tapia, Jose Luis Roman-Zubillaga, Sergio Ruben Gonzaga-Segura, Lazaro Abdiel Falcon-Franco, Victor Hugo Martinez-Landeros, and Rumualdo Servin. 2025. "The Effect of ZrO2 Addition and Thermal Treatment on the Microstructure and Mechanical Properties of Aluminum Metal Matrix Composites (AMMCs)" Materials 18, no. 19: 4507. https://doi.org/10.3390/ma18194507
APA StyleRosales-Cadena, I., Falcon-Castrejon, R. A., Guardian-Tapia, R., Roman-Zubillaga, J. L., Gonzaga-Segura, S. R., Falcon-Franco, L. A., Martinez-Landeros, V. H., & Servin, R. (2025). The Effect of ZrO2 Addition and Thermal Treatment on the Microstructure and Mechanical Properties of Aluminum Metal Matrix Composites (AMMCs). Materials, 18(19), 4507. https://doi.org/10.3390/ma18194507






