Frictional Characteristics and Tribological Mechanisms of Ionic Liquid Lubricants in Ceramic Tribo-Systems
Abstract
1. Introduction
2. Experiment Details
2.1. Materials
2.2. Preparation of IL
2.3. Characterizations and Instruments
2.4. Tribological Performance
3. Results and Discussion
3.1. Physicochemical Properties
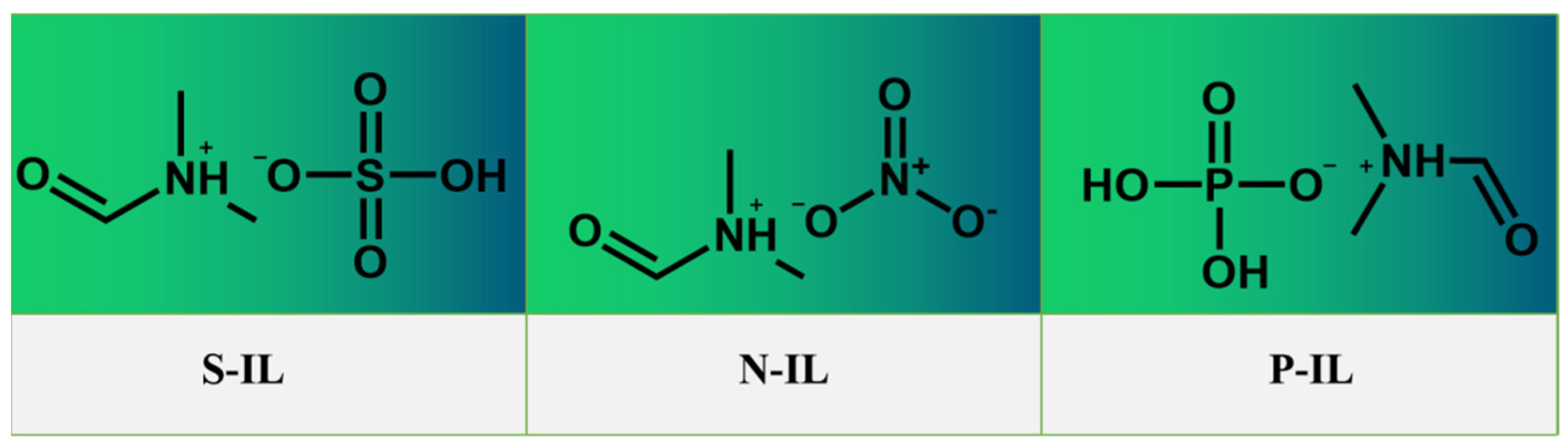
3.1.1. Structure Characterization
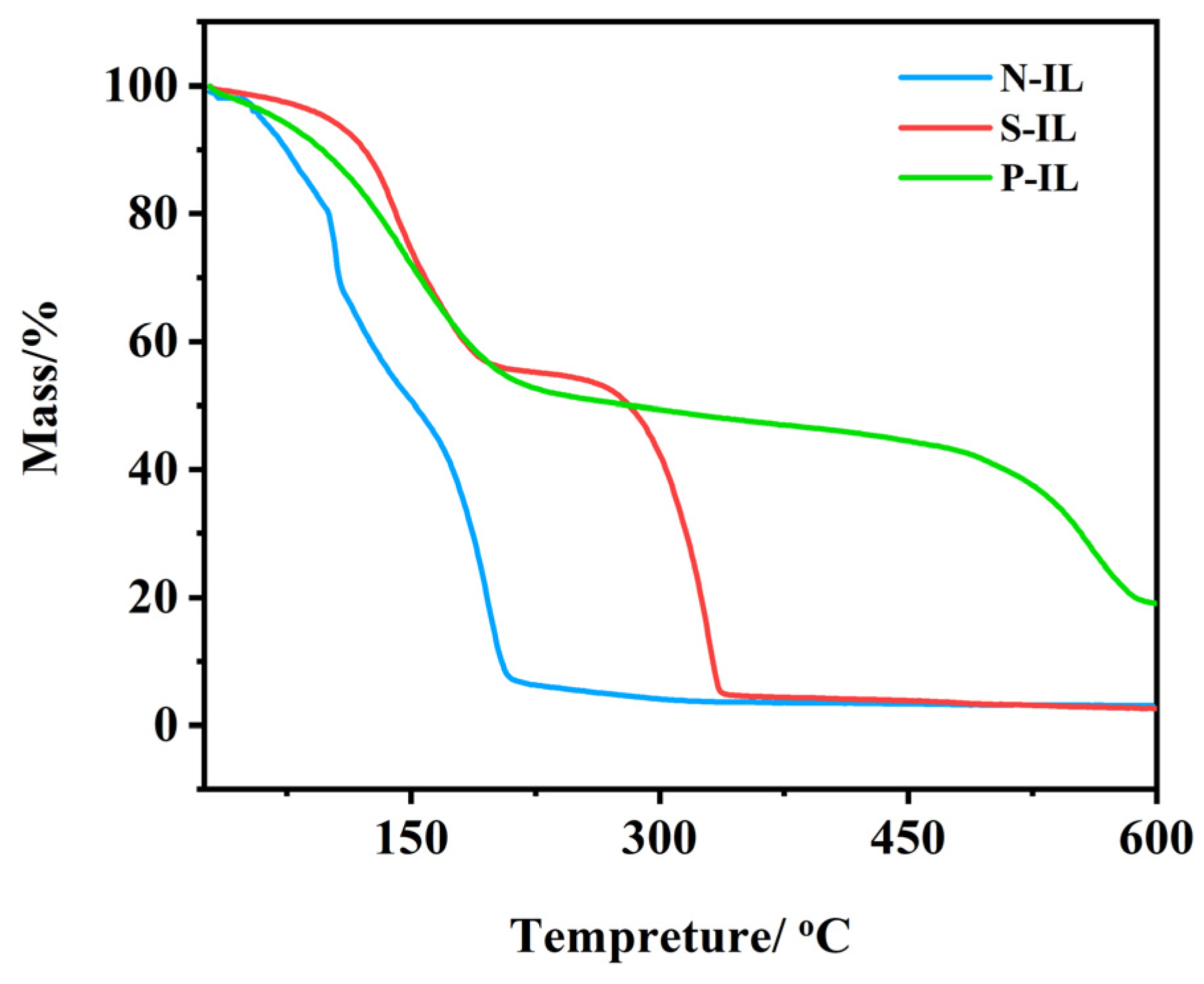
3.1.2. Thermogravimetric Analysis
3.1.3. Viscosity
3.2. Corrosion Analysis
3.3. Tribological Behavior
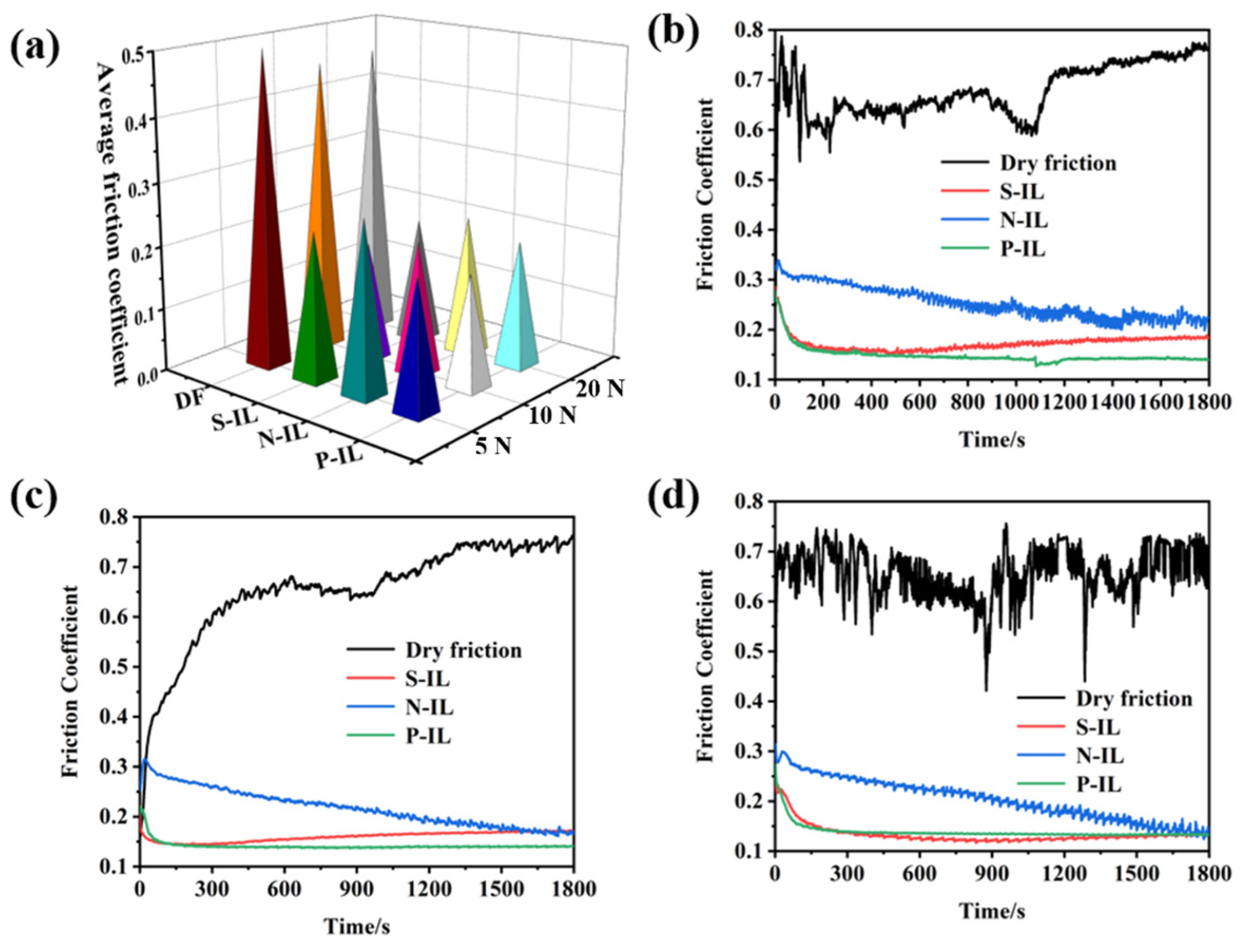
3.3.1. Friction Reduction Under Different Loads
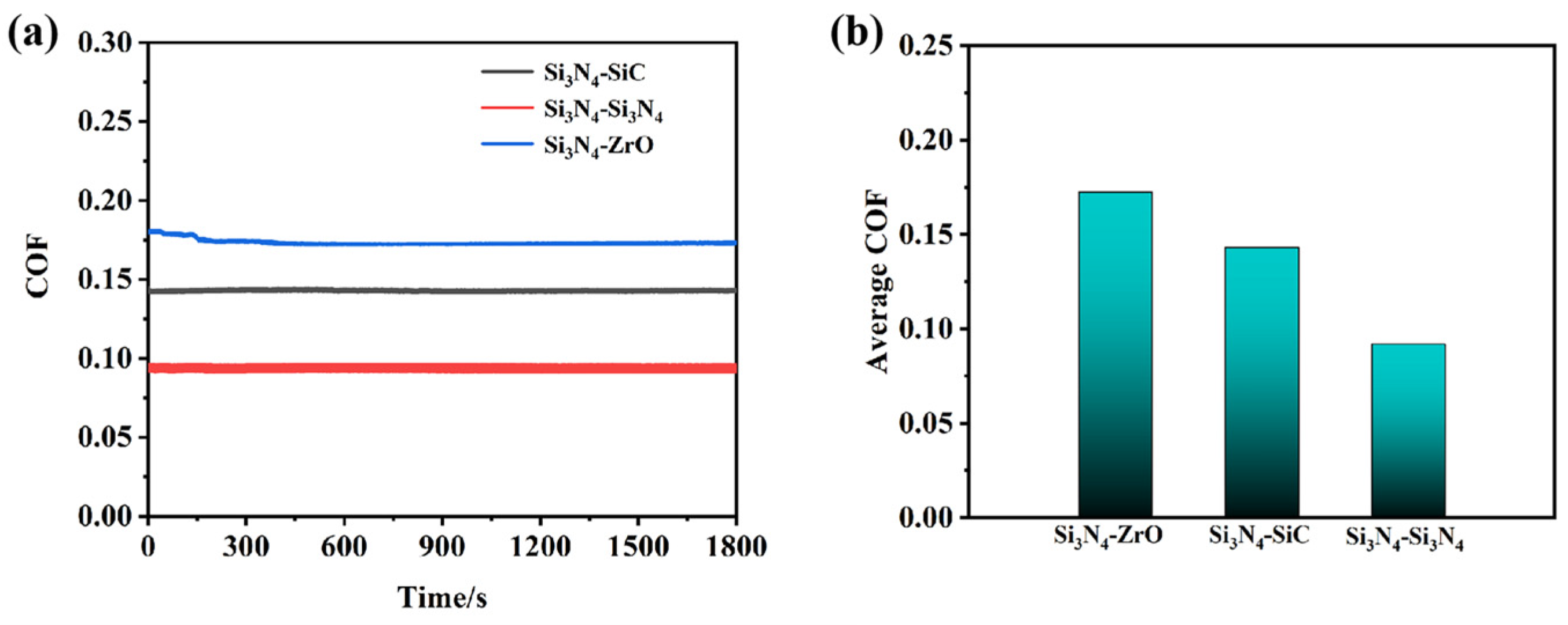
3.3.2. Friction-Reducing and Anti-Wear Properties on Different Friction Pairs
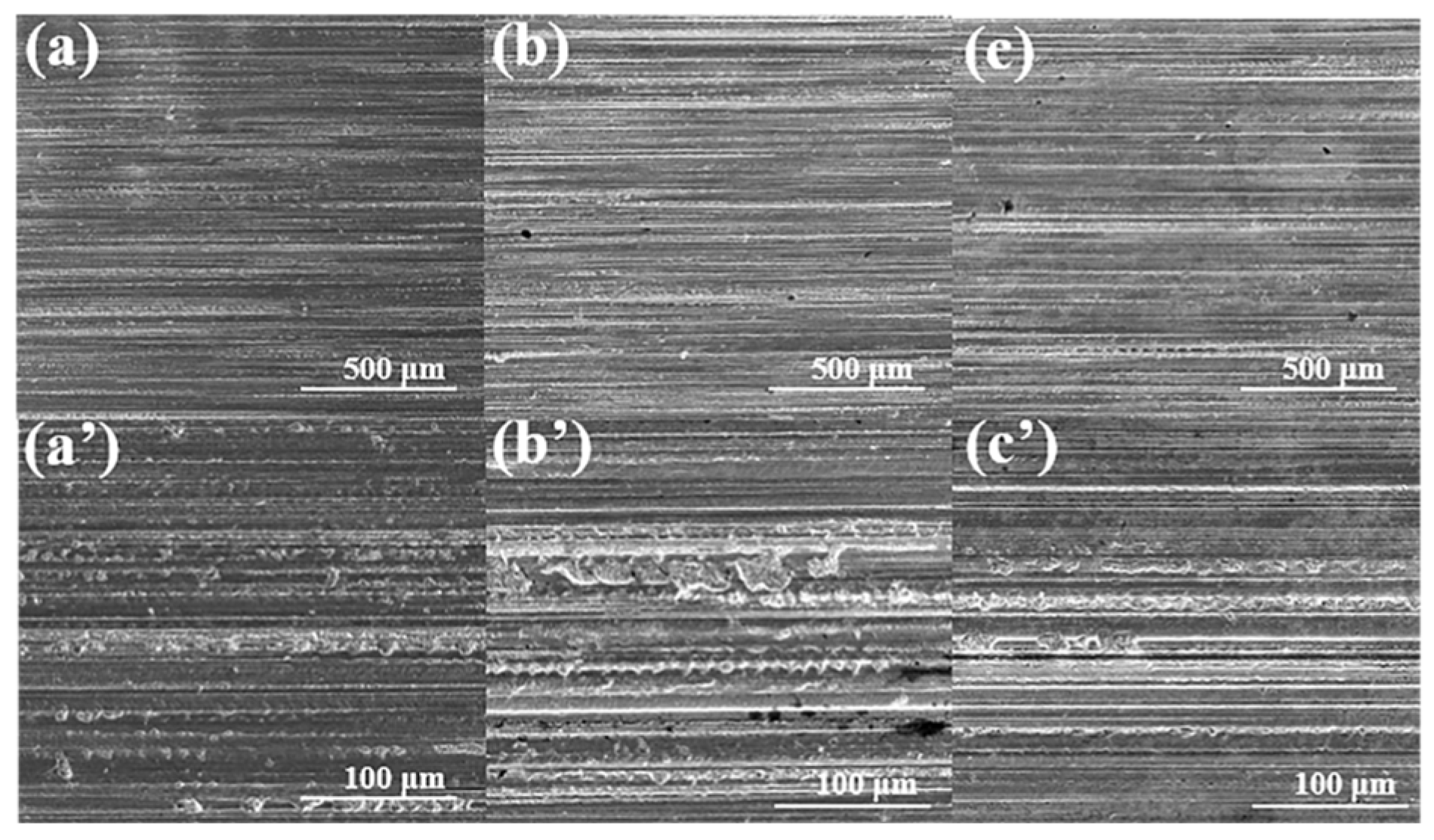
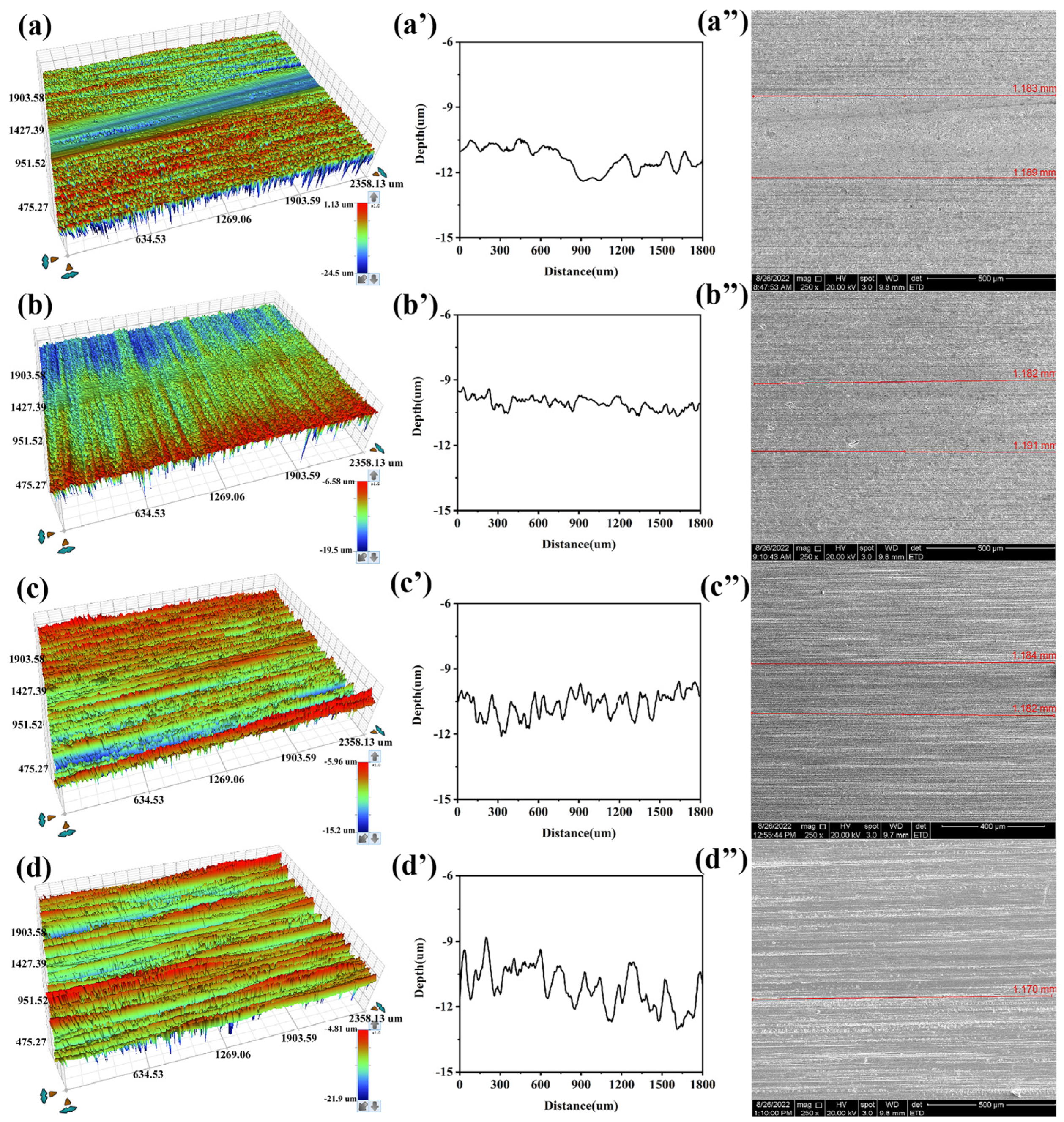
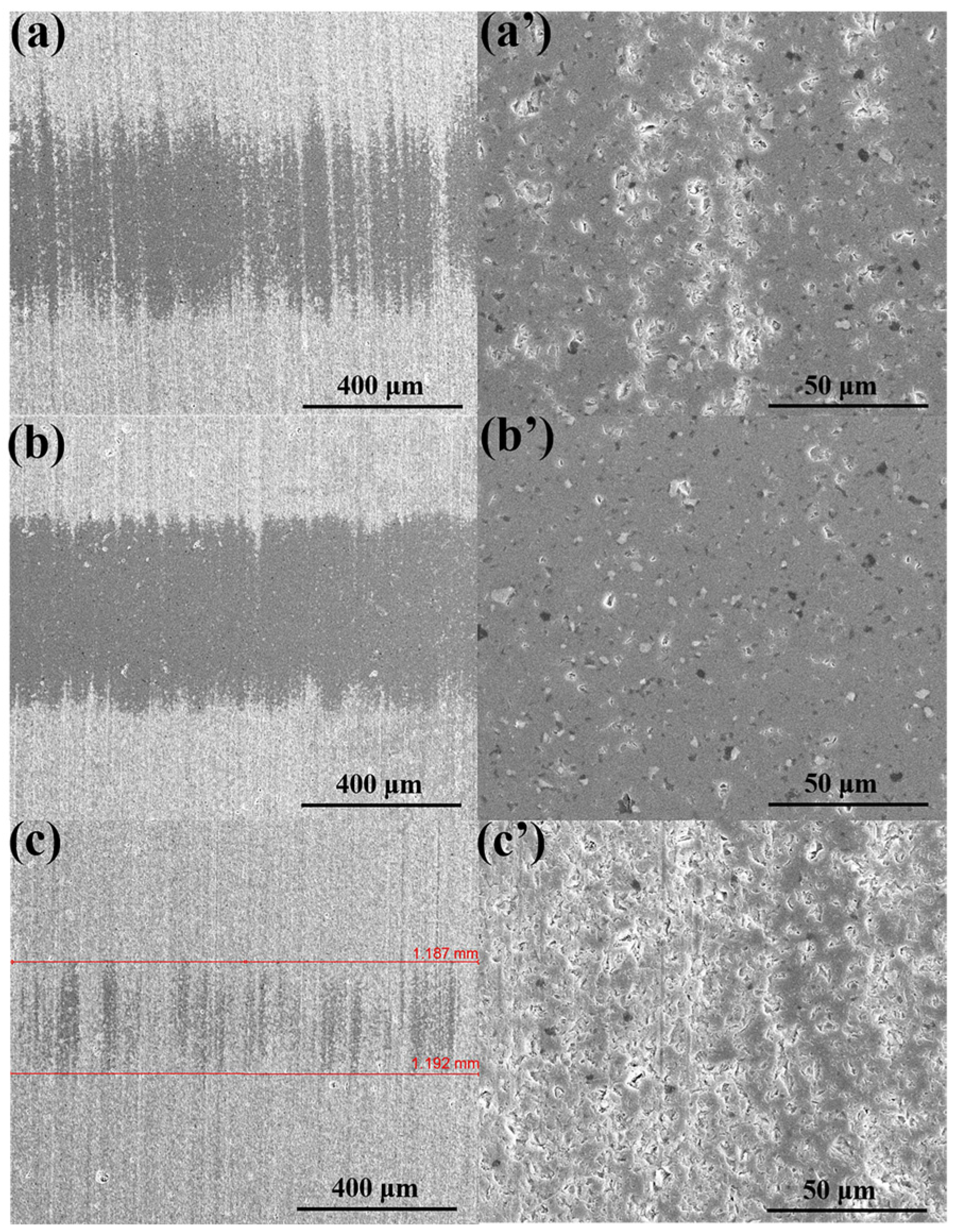
3.4. Surface Analysis (SEM)
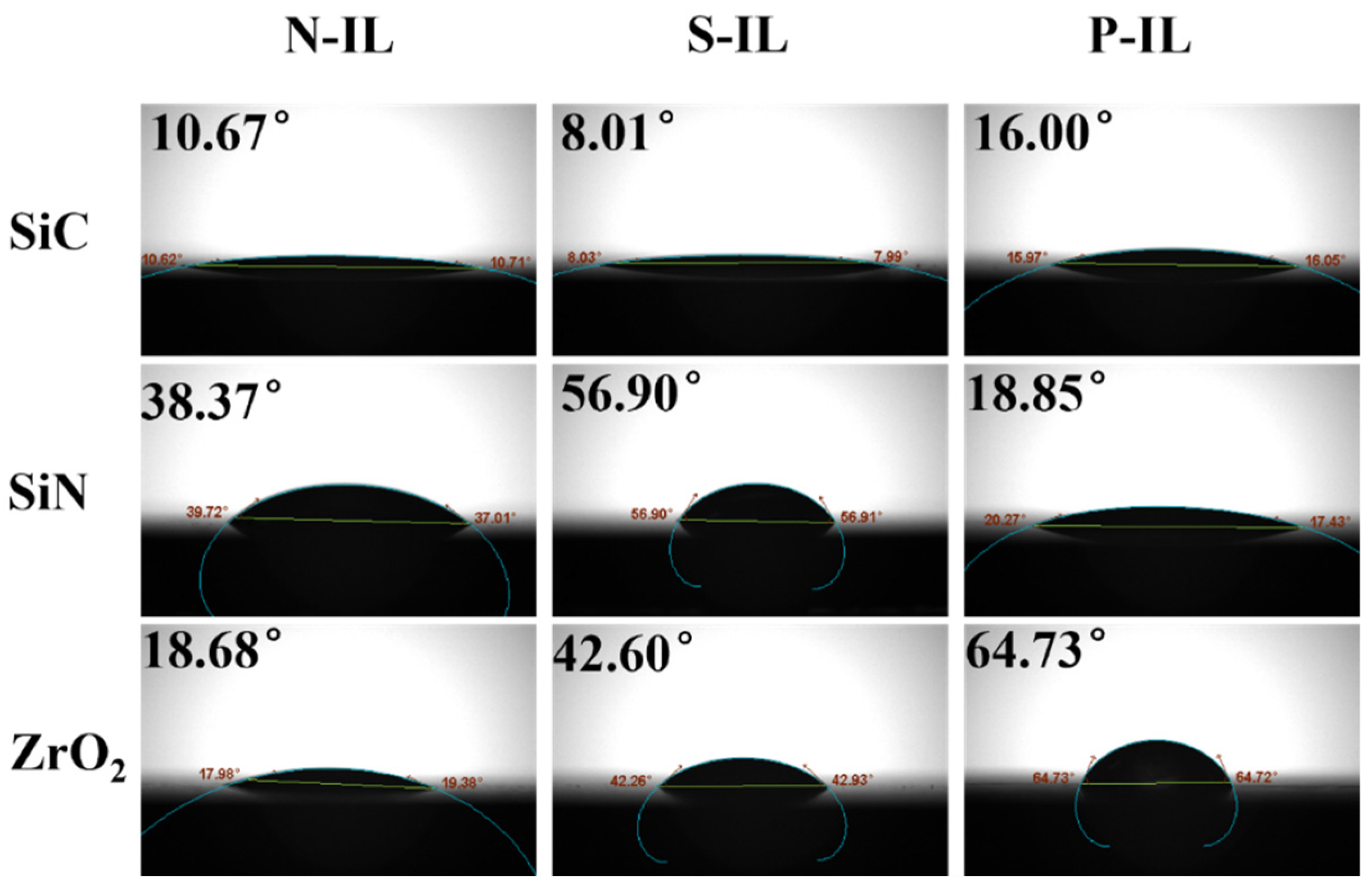
3.5. Wetting Performance
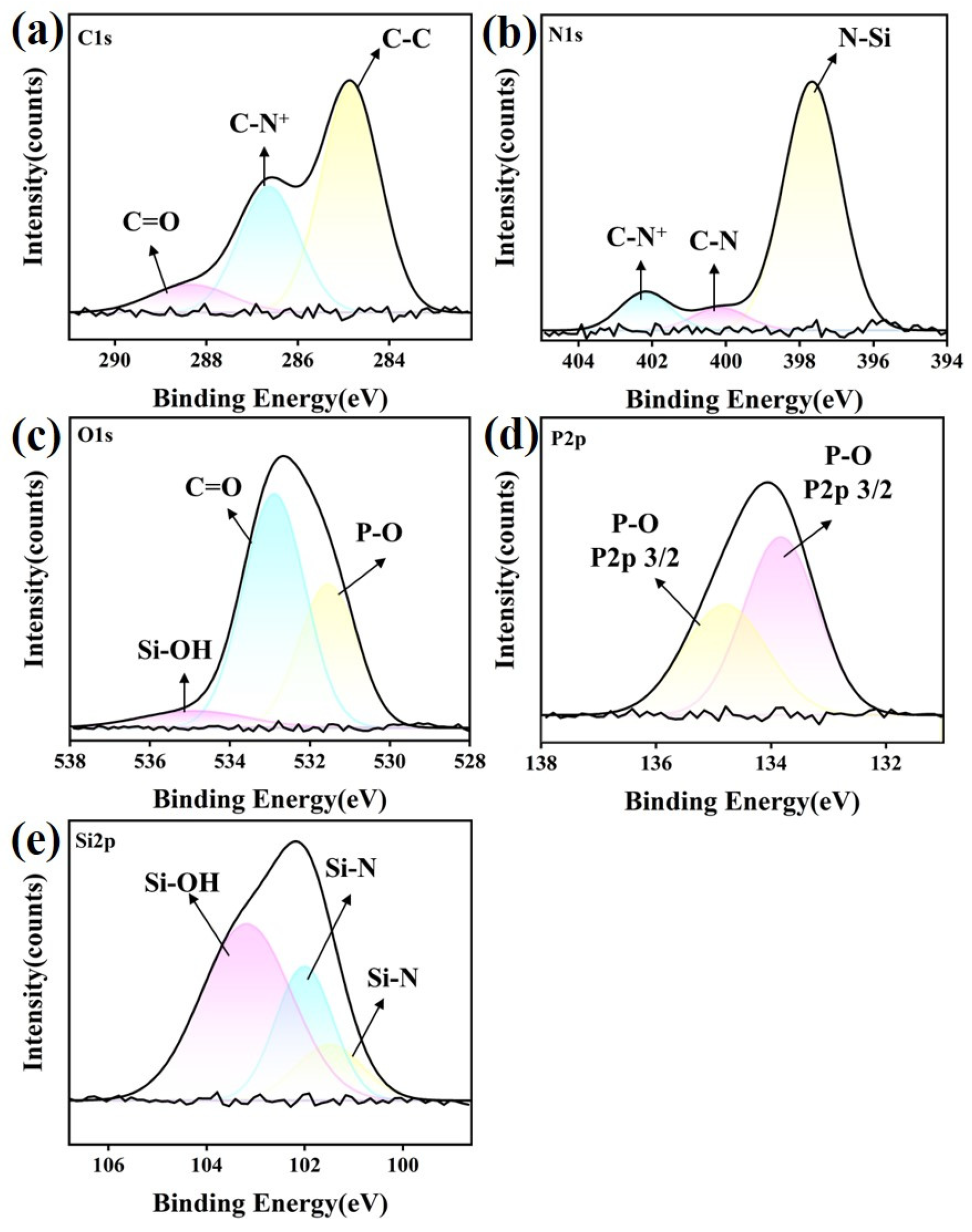
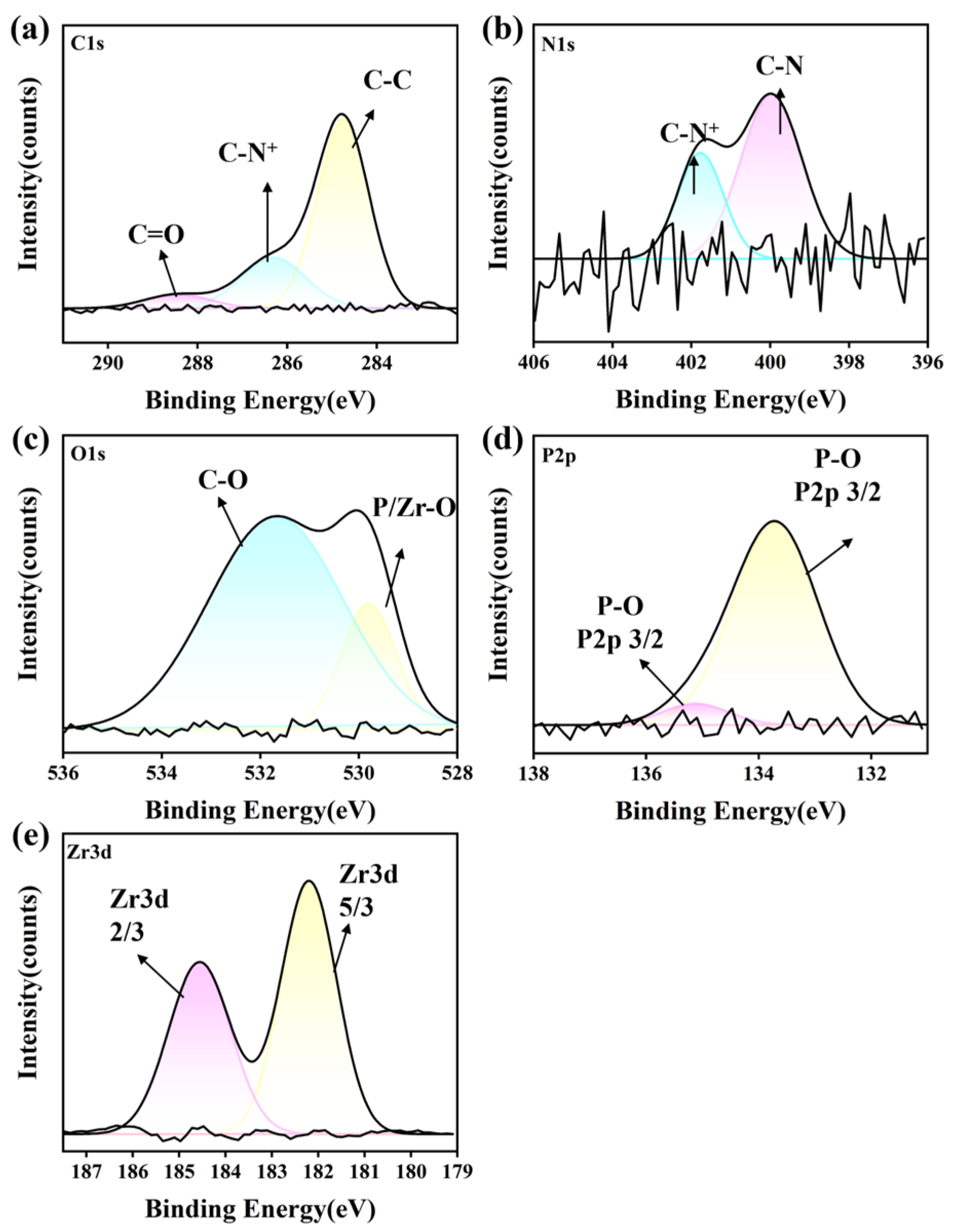
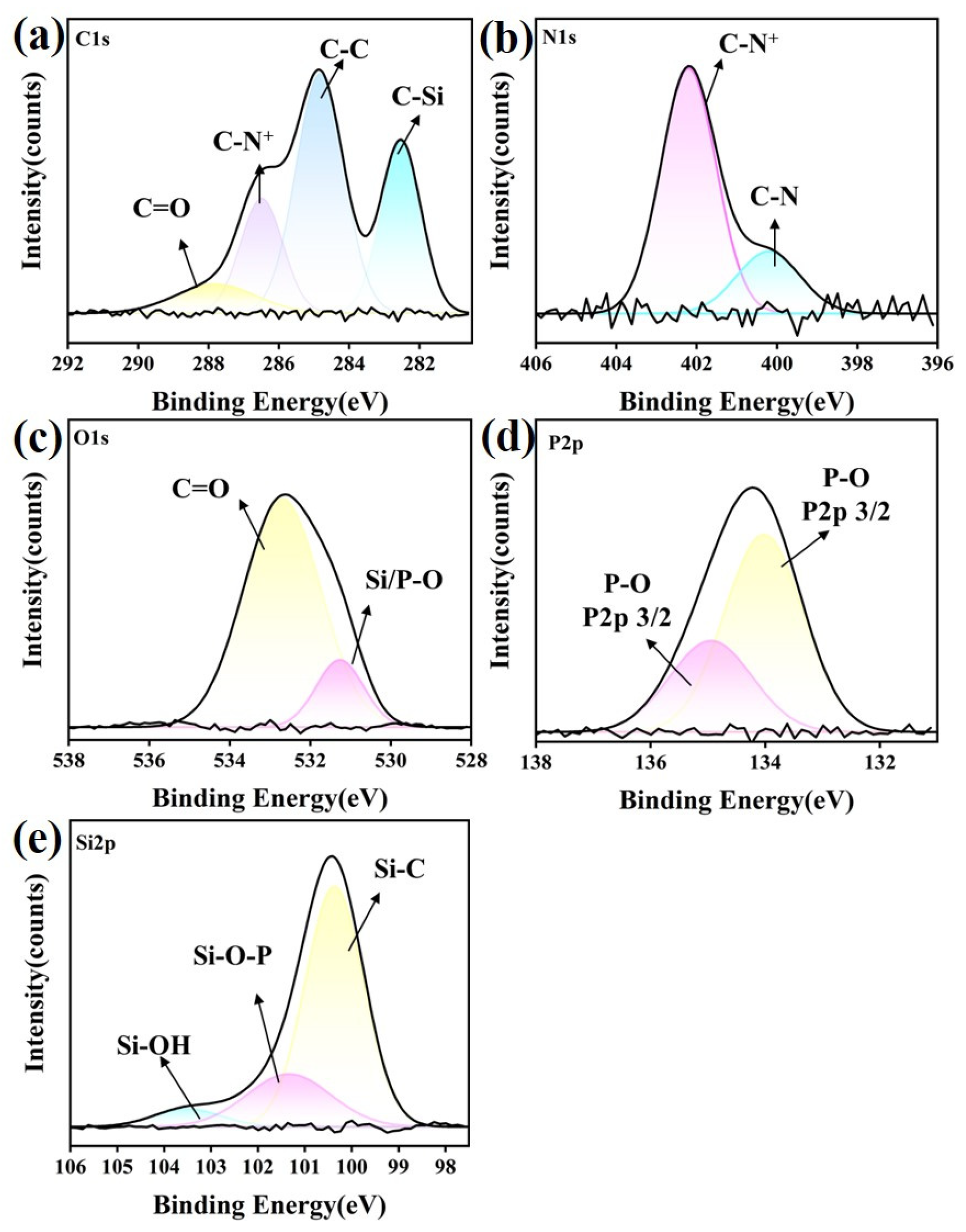
3.6. XPS Analysis
3.6.1. Si3N4 Ceramic
3.6.2. ZrO2 Ceramic
3.6.3. SiC Ceramic
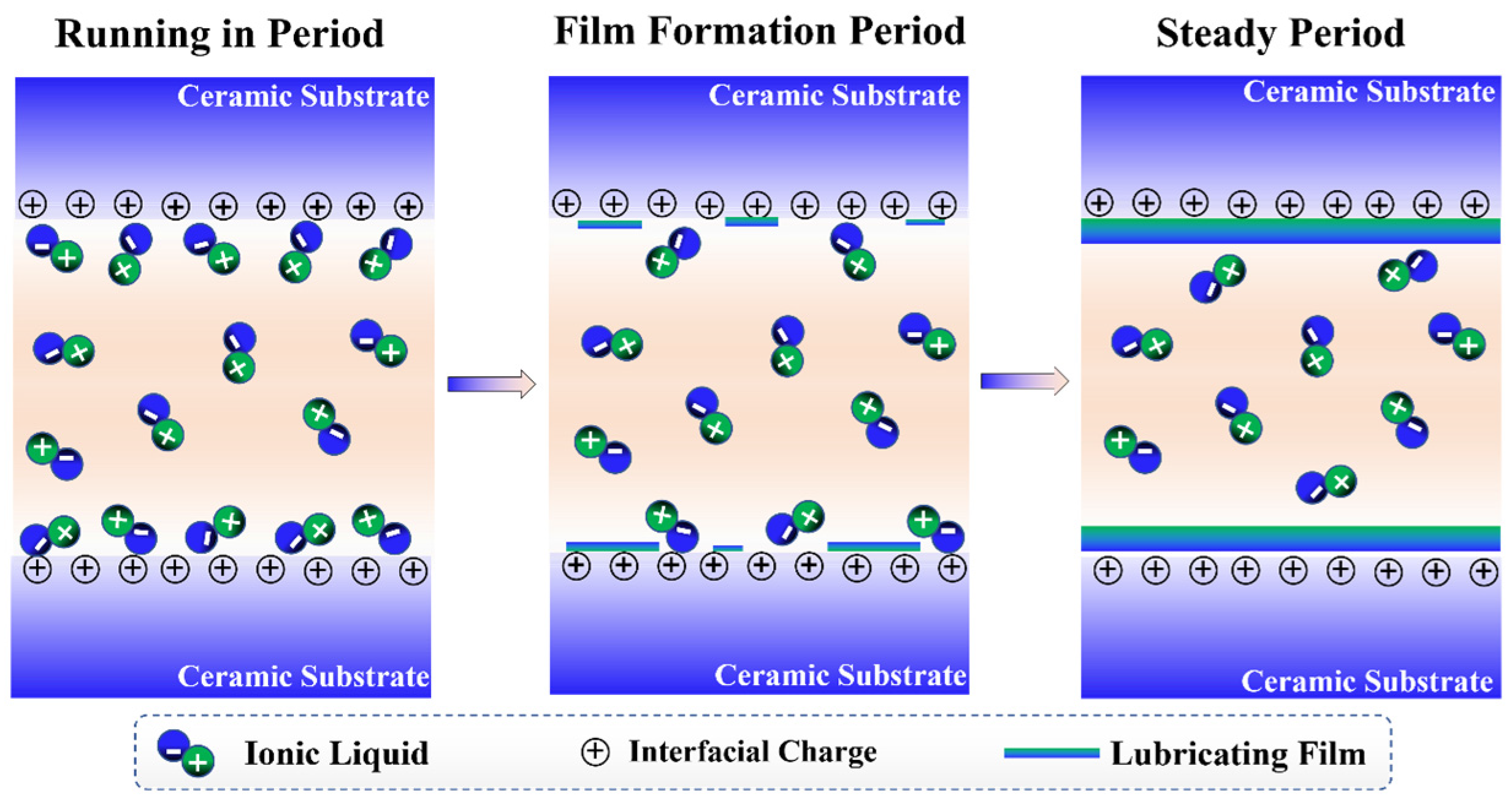
3.7. Lubrication Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.; Zhu, T.; Cheng, Y.; Sun, N.; Wang, H.; Li, Y.; Hu, F.; Xie, Z. Tribological Properties and Wear Mechanisms of WC-ZrO2-Al2O3-GNPs Ceramics against Typical Counterparts. Wear 2025, 564–565, 205705. [Google Scholar] [CrossRef]
- Kasar, A.K.; Rahman, M.H.; D’Souza, B.; Menezes, P.L. Tribological Performance of Ionic Liquid Impregnated Porous Aluminum Borate Ceramic. Tribol. Int. 2023, 180, 108219. [Google Scholar] [CrossRef]
- Zhang, W.; Yamashita, S.; Kita, H. Progress in Tribological Research of SiC Ceramics in Unlubricated Sliding-A Review. Mater. Des. 2020, 190, 108528. [Google Scholar] [CrossRef]
- Zhang, W. Tribology of SiC Ceramics under Lubrication: Features, Developments, and Perspectives. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101000. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the Secrets behind Liquid Superlubricity: A State-of-the-Art Review on Phenomena and Mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Strey, N.F.; Ramos, R.; Scandian, C. Superlubricity and Running-in Wear Maps of Water-Lubricated Dissimilar Ceramics. Wear 2022, 498–499, 204328. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Yao, J.; Tian, J.; Xia, Z.; Yan, H.; Gao, L.; Li, S.; Bao, Z. The Effect of Lubricant Viscosity on the Performance of Full Ceramic Ball Bearings. Mater. Res. Express 2022, 9, 015201. [Google Scholar] [CrossRef]
- Si, H.; Yang, Y.; Zhu, T.; Liang, X.; Wang, H.; Li, Y.; Xie, Z.; Ryu, S.-S. Tribological Properties of Oscillatory Pressure Sintered SiC-Based Ceramics: Influence of Load and Sliding Speed. Wear 2025, 574–575, 206074. [Google Scholar] [CrossRef]
- Phillips, B.S.; Zabinski, J.S. Ionic Liquid Lubrication Effects on Ceramics in a Water Environment. Tribol. Lett. 2004, 17, 533–541. [Google Scholar] [CrossRef]
- Liu, C.; Fang, J.; Wen, X.; Tian, Y.; Meng, Y. Active Control of Boundary Lubrication of Ceramic Tribo-Pairs in Sodium Dodecyl Sulfate Aqueous Solutions. Tribol. Lett. 2021, 69, 144. [Google Scholar] [CrossRef]
- Xu, R.; Deng, Y.; Jiang, X.; Liu, Y. Superlubrication and Wear Behavior of SiC/Si3N4 Induced by Nanoparticles in Water-Glycol. Wear 2025, 572–573, 206007. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Song, J.; Hu, L. Tribological Behavior and Lubrication Mechanism of Self-Lubricating Ceramic/Metal Composites: The Effect of Matrix Type on the Friction and Wear Properties. Wear 2017, 372–373, 130–138. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Ma, L.; Jin, Z.; Prakash, B.; Ma, T.; Wang, W. A Review of Advances in Tribology in 2020–2021. Friction 2022, 10, 1443–1595. [Google Scholar] [CrossRef]
- Ba, D.; Meng, F.; Liu, X. Friction and Wear Behaviors of Surface Nanocrystalline Layer Prepared on Medium Manganese Surfacing Layer under Oil Lubrication. Tribol. Int. 2014, 80, 210–215. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, Y.; Sun, F.; Yue, L.; Zhou, X.; Wei, P.; Zhao, H.; Wen, X.; Bai, P.; Zhao, Q.; et al. The Tribological and Adsorption Performance of Chlorophenyl Silicone Oil Using Different Ceramic Materials under High Temperature. Lubricants 2024, 12, 249. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Meng, X.; Xiao, G.; Chen, Z.; Yi, M.; Zhang, J.; Liu, W.; Xu, C. Influence of Embedding Microcapsules on Tribological Properties of Alumina Ceramics Prepared by Gel Casting. Materials 2025, 18, 2110. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wen, H.; Sun, J.; Wang, W.; Fan, Y.; Jia, J.; Chen, W. Tribological Properties of Si (3)N(4)-hBN Composite Ceramics Bearing on GCr15 under Seawater Lubrication. Materials 2020, 13, 635. [Google Scholar] [CrossRef]
- Qunji, X.; Jianjum, W. Tribochemistry in Ceramic Lubrication. Lubr. Sci. 1996, 8, 369–377. [Google Scholar] [CrossRef]
- Yan, S.; Wei, C.; Zou, H.; Chen, J.; Li, Y.; Shen, T.; Wang, A.; Sui, T.; Lin, B. Fabrication and Tribological Characterization of Laser Textured Engineering Ceramics: Si3N4, SiC and ZrO2. Ceram. Int. 2021, 47, 13789–13805. [Google Scholar] [CrossRef]
- Du, C.; Xie, H.; Liu, J.; Lei, B.; Zhang, R. Improvement of Ti3SiC2/SiC Composite Ceramic Friction and Wear Behavior by Mechanical-Friction Oxidation Synergism in a Wide Temperature Domain of SiC. Wear 2025, 572–573, 205984. [Google Scholar] [CrossRef]
- Antunes, M.; Donato, M.T.; Paz, V.; Caetano, F.; Santos, L.; Colaço, R.; Branco, L.C.; Saramago, B. Improving the Lubrication of Silicon Surfaces Using Ionic Liquids as Oil Additives: The Effect of Sulfur-Based Functional Groups. Tribol. Lett. 2020, 68, 70. [Google Scholar] [CrossRef]
- Zuo, X.; Yu, H.; Zhou, F.; Zhang, X. Synergistic Lubrication Achieved by Proton-Ionic Liquids and MoS2 Ashigh-Performance Water-Based Lubricant Additives. Langmuir 2025, 41, 5534–5545. [Google Scholar] [CrossRef]
- Ye, C.F.; Liu, W.M.; Chen, Y.X.; Yu, L.G. Room-temperature Ionic Liquids: A Novel Versatile Lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Arcifa, A.; Rossi, A.; Ramakrishna, S.N.; Espinosa-Marzal, R.; Sheehan, A.; Spencer, N.D. Lubrication of Si-Based Tribopairs with a Hydrophobic Ionic Liquid: The Multiscale Influence of Water. J. Phys. Chem. C 2018, 122, 7331–7343. [Google Scholar] [CrossRef]
- Cai, Z.-b.; Meyer, H.M.; Ma, C.; Chi, M.; Luo, H.; Qu, J. Comparison of the Tribological Behavior of Steel–Steel and Si3N4–Steel Contacts in Lubricants with ZDDP or Ionic Liquid. Wear 2014, 319, 172–183. [Google Scholar] [CrossRef]
- Khan, A.; Gusain, R.; Khatri, O.P. Organophosphate Anion Based Low Viscosity Ionic Liquids as Oil-Miscible Additives for Lu-Brication Enhancement. J. Mol. Liq. 2018, 272, 430–438. [Google Scholar] [CrossRef]
- Kondo, Y.; Koyama, T.; Tsuboi, R.; Nakano, M.; Miyake, K.; Sasaki, S. Tribological Performance of Halogen-Free Ionic Liquids as Lubricants of Hard Coatings and Ceramics. Tribol. Lett. 2013, 51, 243–249. [Google Scholar] [CrossRef]
- Perez-Martinez, C.S.; Perkin, S. Interfacial Structure and Boundary Lubrication of a Dicationic Ionic Liquid. Langmuir 2019, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qin, J.; Huang, Y.; Liu, X.; Guo, P.; Cai, M.; Zhou, F. Dodecane-Substituted Saturated and Unsaturated Imidazoli-Um-Based Ionic Liquids: Investigated as Potential Lubricants and Lubricating Additives. J. Mol. Liq. 2025, 426, 127341. [Google Scholar] [CrossRef]
- Qu, G.; Liu, L.; Fang, H.; Li, Y.; Zhang, S.; Hu, L. Exploring the Distinct Lubrication Mechanisms of Functionalized Ionic Liquids in Air and Vacuum: Insights into Molecular-Level Behavior and Performance. Appl. Surf. Sci. 2025, 696, 162955. [Google Scholar] [CrossRef]
- Wu, L.; Gu, L.; Jian, R. Lubrication Mechanism of Graphene Nanoplates as Oil Additives for Ceramics/Steel Sliding Components. Ceram. Int. 2021, 47, 16935–16942. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Huang, G.; Ma, Z.; Shi, Y.; Cai, M.; Zhou, F.; Liu, W. Task-Specific Oil-Miscible Ionic Liquids Lubricate Steel/Light Metal Alloy: A Tribochemistry Study. Adv. Mater. Inter. 2018, 5, 1800791. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Chen, H.; Shi, X.-N.; Liu, X.; Duan, G.-J. Tribological Properties of Ionic Liquids for Steel/Aluminum, Steel/Copper and Steel/Si3N4 Ceramic Contacts under Boundary Lubrication. Ind. Lubr. Tribol. 2018, 70, 1158–1168. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Wang, Y.; Zhao, X.; Chen, D.; Chen, G. Study of Tribological Properties and Lubrication Mechanism of Sur-Factant-Coated Anthracite Sheets Used as Lubricant Additives. Friction 2020, 9, 524–537. [Google Scholar] [CrossRef]
- Chen, G.; Li, P.; Yang, Z.; Ma, Y.; Wang, B. In-Situ Preparation of Ionic Liquid Lubricating Additives for Enhanced Lubrication Performance in Titanium Alloys: Experimental and Molecular Dynamics Simulations. J. Ind. Eng. Chem. 2025, 147, 776–792. [Google Scholar] [CrossRef]
- Kaljusmaa, L.-M.; Tubli, D.; Adamson, J.; Konist, A.; Järvik, O. Thermal Stability of Amine and Carboxylic Acid Based Protic Ionic Liquids from the Perspective of Thermal Energy Storage. J. Mol. Liq. 2025, 427, 127396. [Google Scholar] [CrossRef]
- Liang, H.; Li, H.; Yang, C.; Yang, Q.; Li, J.; Liu, M.; Xia, C.; Nie, Y.; Shen, X.; Peng, Q.; et al. Exploring Viscosity Metrics for Hydrogen-Bond Dominated Liquid Superlubricity Using Ionic Liquid Analogue Models. Colloids Surf. A Phys. Chem. Eng. Asp. 2025, 715, 136614. [Google Scholar] [CrossRef]
- Kronberger, M.; Ripoll, M.R.; Dörr, N.; Linhardt, P. In-Situ Cyclic Voltammetry of an Ionic Liquid as a Lubricant Additive in a Steel-Ceramic Contact. Tribol. Int. 2020, 152, 106264. [Google Scholar] [CrossRef]
- Zhou, Y.; Leonard, D.N.; Guo, W.; Qu, J. Understanding Tribofilm Formation Mechanisms in Ionic Liquid Lubrication. Sci. Rep. 2017, 7, 8426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.J.; Tang, Y.; Hao, X.; Xu, W.; Xiang, D.; Wu, J. Apoptotic Bodies for Advanced Drug Delivery and Therapy. J. Control. Release 2022, 351, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zheng, Z.; Chen, H.; Wang, H.; Liu, X.; Wang, H.; Qiao, D.; Feng, D. Robust Macroscale Superlubricity Enabled by the Protic Ionic Liquid Polyol Aqueous Solution: From Interface Adsorption to Tribofilm Formation. ACS Appl. Mater. Interfaces 2025, 17, 12827–12840. [Google Scholar] [CrossRef]
- Yi, M.; Qin, J.; Wang, Y.; Chen, Q.; Sun, X.; Guo, P.; Liu, X.; Cai, M.; Zhou, F. Construction of Efficiency Protection Film for Harsh Conditions Achieved by Novel Multi-functional Polymeric Ionic Liquids. Chem. Eng. J. 2025, 523, 168021. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Bai, Y.; Wu, Y.; Ma, Z.; Zhang, J.; Zhang, C.; Yu, B.; Zhou, F.; Liu, W.; et al. Towards superior lubricity and anticorrosion performances of proton-type ionic liquids additives for water-based lubricating fluids. Chem. Eng. J. 2020, 383, 123201. [Google Scholar] [CrossRef]
- Cai, M.; Yu, Q.; Zhou, F.; Liu, W. Physicochemistry Aspects on Frictional Interfaces. Friction 2017, 5. [Google Scholar] [CrossRef]
- Li, Z.; Dolocan, A.; Morales-Collazo, O.; Sadowski, J.T.; Celio, H.; Chrostowski, R.; Brennecke, J.F.; Mangolini, F. Lubrication Mechanism of Phosphonium Phosphate Ionic Liquid in Nanoscale Single-Asperity Sliding Contacts. Adv. Mater. Inter. 2020, 7, 2000426. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gajda, I.; Salar-García, M.J.; Greenman, J.; Hernández-Fernández, F.J.; Ieropoulos, I. Study of the Effects of Ionic Liquid-Modified Cathodes and Ceramic Separators on MFC Performance. Chem. Eng. J. 2016, 291. [Google Scholar] [CrossRef]
- Hwang, D.-H.; Zum Gahr, K.-H. Transition from Static to Kinetic Friction of Unlubricated or Oil Lubricated Steel/Steel, Steel/Ceramic and Ceramic/Ceramic Pairs. Wear 2003, 255, 365–375. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Mu, L.; Shi, Y.; Dong, Y.; Feng, X.; Lu, X. High Load Capacity with Ionic Liquid-Lubricated Tribological System. Tribol. Int. 2016, 94, 315–322. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Han, Y.; Zhang, L.; Feng, D.; Shi, L. Application of Alkylphosphate Ionic Liquids as Lubricants for Ceramic Material. Ind. Eng. Chem. Res. 2015, 54, 12813–12825. [Google Scholar] [CrossRef]
- Ciudad, E.; Borrero-López, O.; Ortiz, A.L.; Guiberteau, F. Microstructural Effects on the Sliding-Wear Resistance of Pressureless Liquid-Phase-Sintered SiC under Diesel Fuel. J. Eur. Ceram. Soc. 2013, 33, 879–885. [Google Scholar] [CrossRef]
- Ge, D.; Deng, J.; Duan, R.; Li, X.; Liu, Y.; Yue, H. Effect of Surface Wettability on Tribological Properties of Al2O3/TiC Ceramic under Wet Lubrication. Ceram. Int. 2019, 45, 24554–24563. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Zhang, C.; Liu, Y.; Luo, J. Superlubricity and Antiwear Properties of In Situ-Formed Ionic Liquids at Ceramic Interfaces Induced by Tribochemical Reactions. ACS Appl. Mater. Interfaces 2019, 11, 6568–6574. [Google Scholar] [CrossRef] [PubMed]
- Bournel, F.; Gallet, J.-J.; Köhler, U.; Ellakhmissi, B.B.; Kubsky, S.; Carniato, S.; Rochet, F. Propanoate Grafting on (H,OH)-Si(0 0 1)-2 × 1. J. Phys. Condens. Matter 2015, 27, 054005. [Google Scholar] [CrossRef] [PubMed]
- Gates, R.S.; Hsu, S.M. Tribochemistry between Water and Si3N4 and SiC: Induction Time Analysis. Tribol. Lett. 2004, 17, 399–407. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Zhang, C.; Wang, Z.; Luo, J. Superlubricity of 1-Ethyl-3-Methylimidazolium Trifluoromethanesulfonate Ionic Liquid Induced by Tribochemical Reactions. Langmuir 2018, 34, 5245–5252. [Google Scholar] [CrossRef]
- Muhammad, S.; Hussain, S.T.; Waseem, M.; Naeem, A.; Hussain, J.; Jan, M.T. Surface Charge Properties of Zirconium Dioxide. Iran. J. Sci. Technol. 2012, A4, 481–486. [Google Scholar] [CrossRef]



| Samples | Viscosity (mm2/s), 25 °C |
|---|---|
| N-IL | 2.43 |
| S-IL | 7.98 |
| P-IL | 95.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, S.; Cui, L.; Cao, C. Frictional Characteristics and Tribological Mechanisms of Ionic Liquid Lubricants in Ceramic Tribo-Systems. Materials 2025, 18, 4504. https://doi.org/10.3390/ma18194504
Yang Z, Li S, Cui L, Cao C. Frictional Characteristics and Tribological Mechanisms of Ionic Liquid Lubricants in Ceramic Tribo-Systems. Materials. 2025; 18(19):4504. https://doi.org/10.3390/ma18194504
Chicago/Turabian StyleYang, Zehui, Shujuan Li, Limu Cui, and Congjun Cao. 2025. "Frictional Characteristics and Tribological Mechanisms of Ionic Liquid Lubricants in Ceramic Tribo-Systems" Materials 18, no. 19: 4504. https://doi.org/10.3390/ma18194504
APA StyleYang, Z., Li, S., Cui, L., & Cao, C. (2025). Frictional Characteristics and Tribological Mechanisms of Ionic Liquid Lubricants in Ceramic Tribo-Systems. Materials, 18(19), 4504. https://doi.org/10.3390/ma18194504





