Tailoring Strength and Corrosion Resistance in Al–Zn–Mg–Cu Alloys by Total (Zn + Mg) Content and Multi-Directional Forging Process
Abstract
1. Introduction
2. Experimental Section
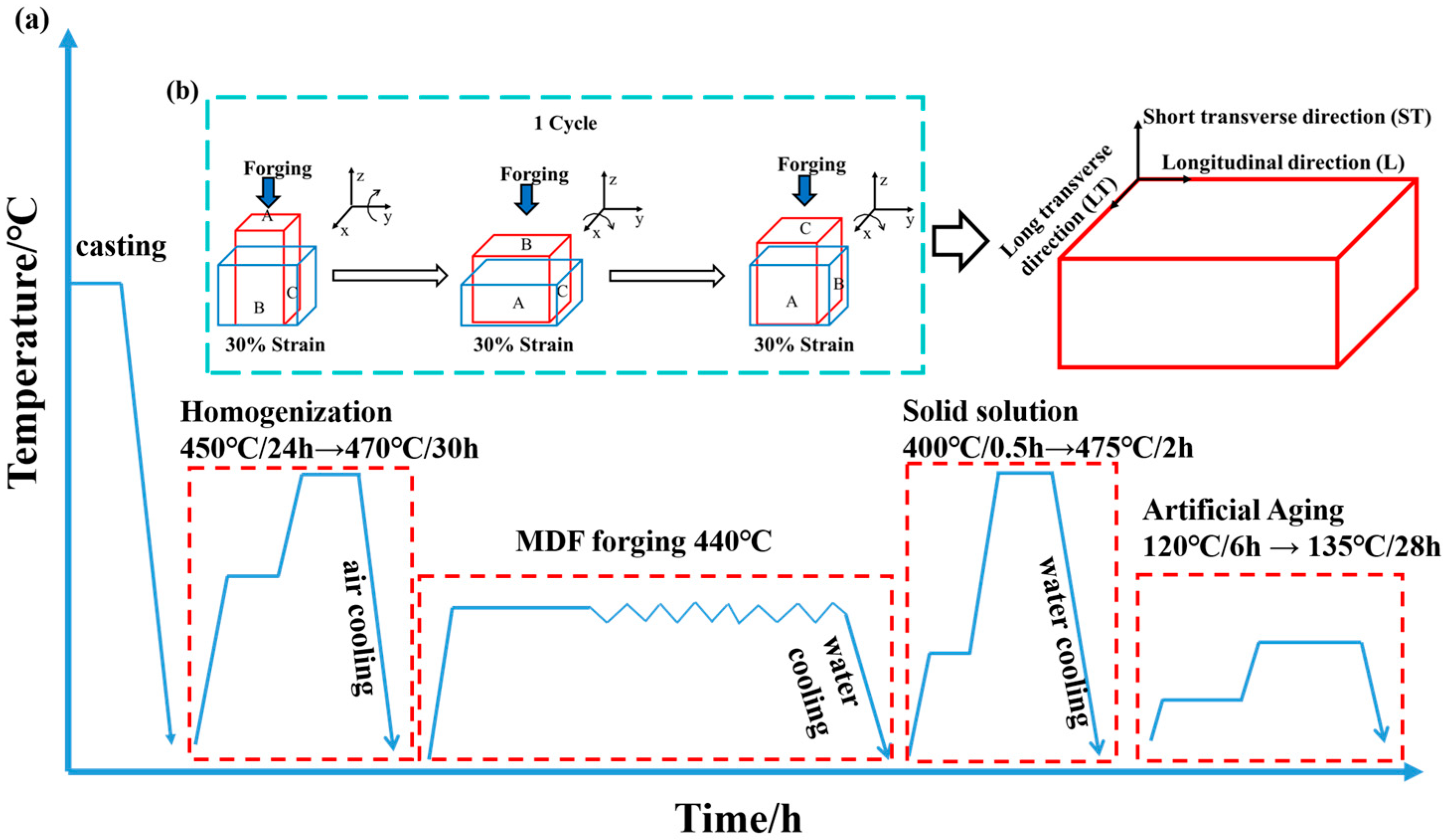
2.1. Materials Preparation
2.2. Mechanical Property Tests
2.3. Microstructure Characterization
2.4. Electrochemical Test
3. Results
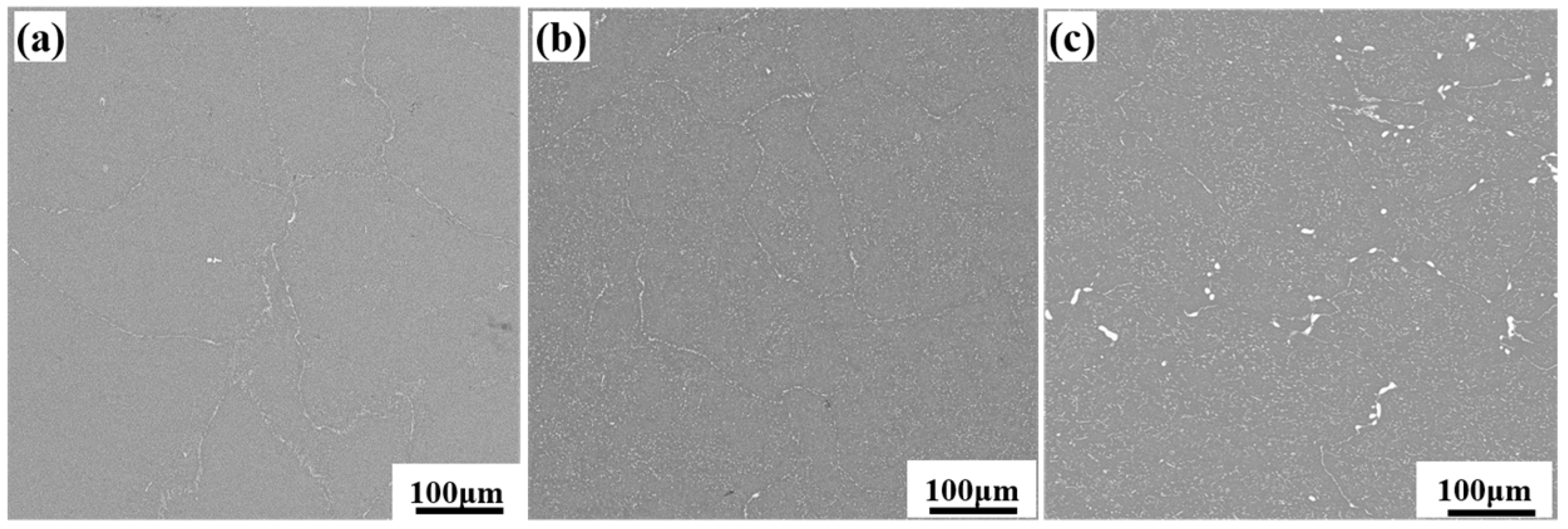
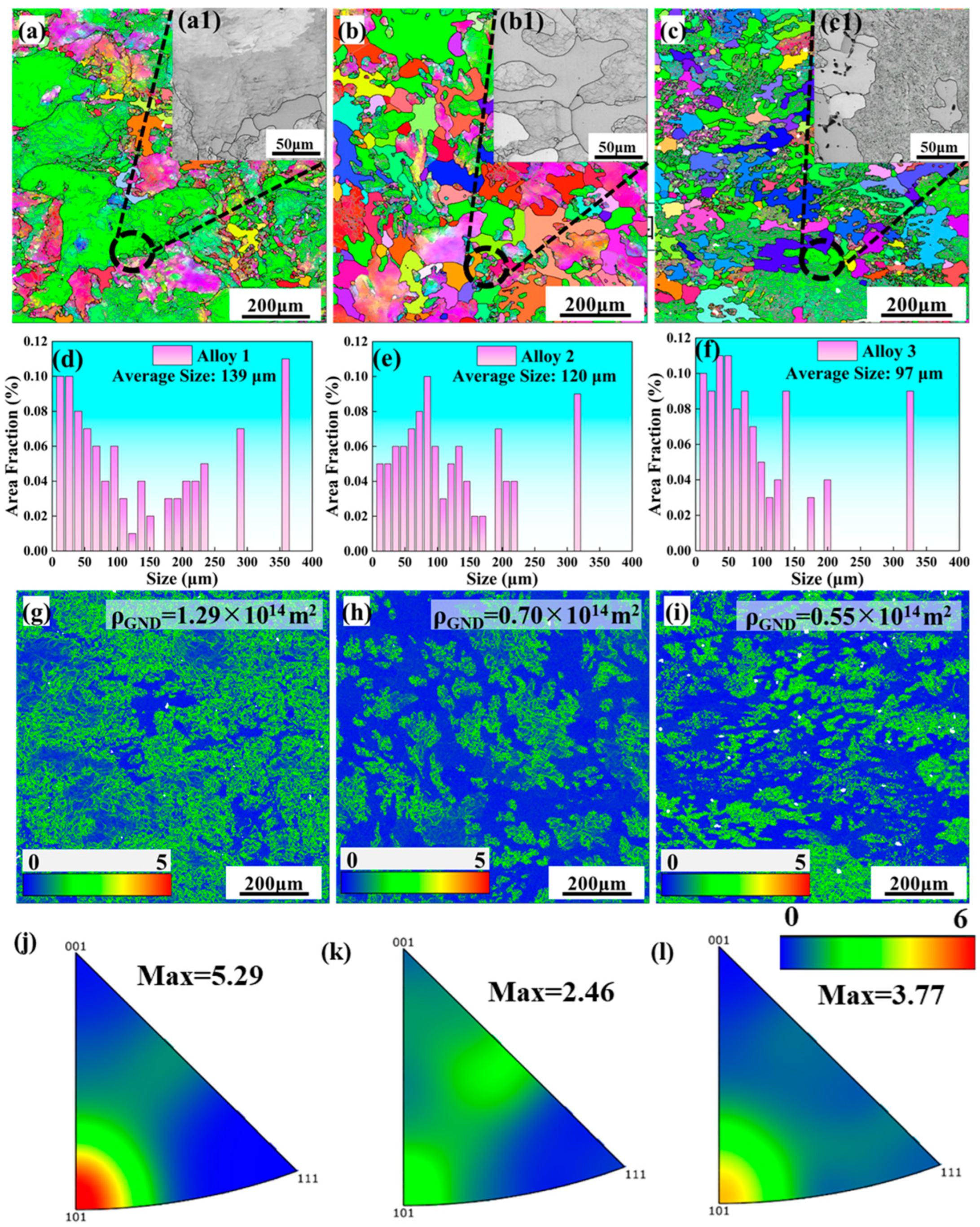
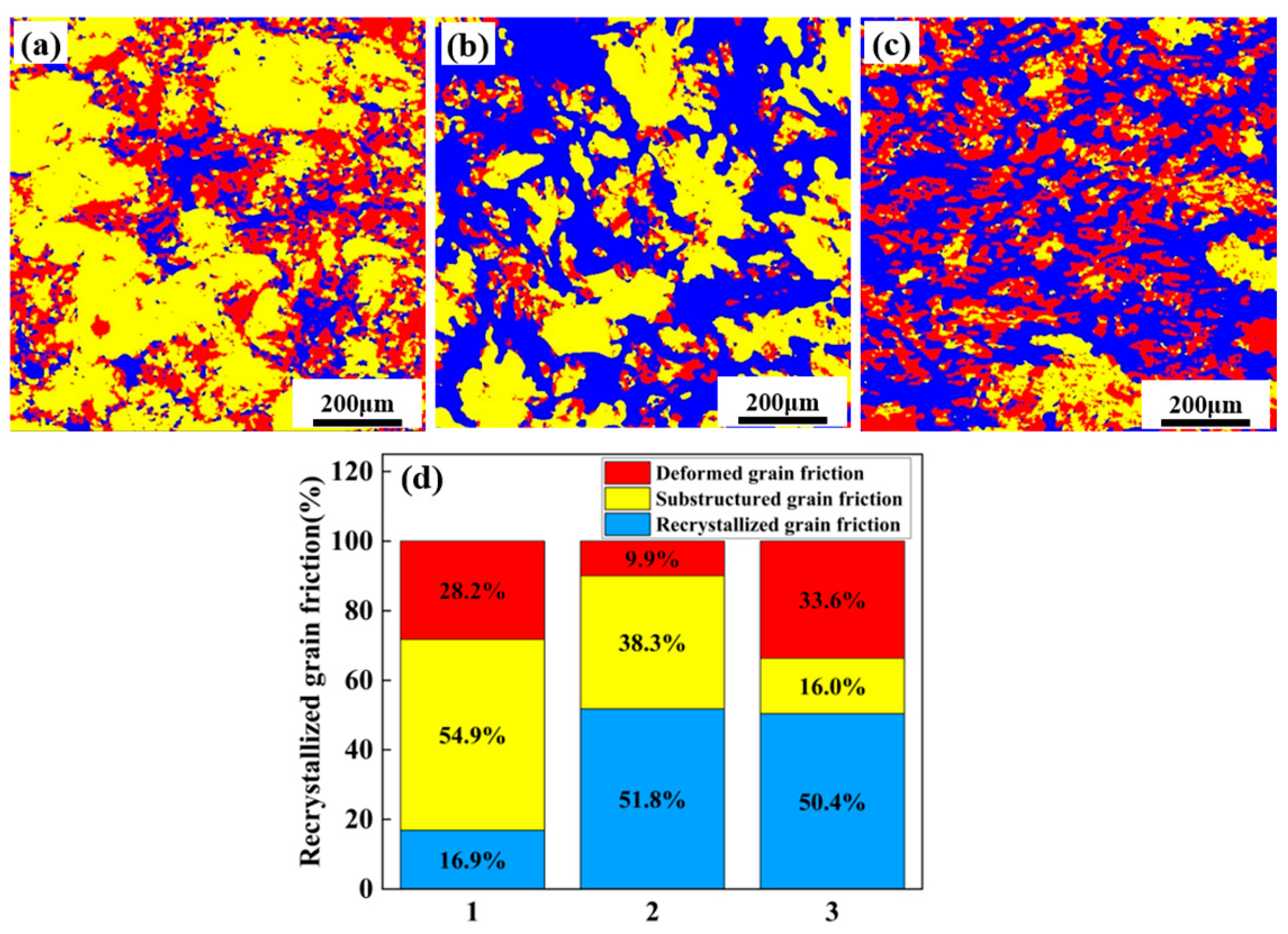
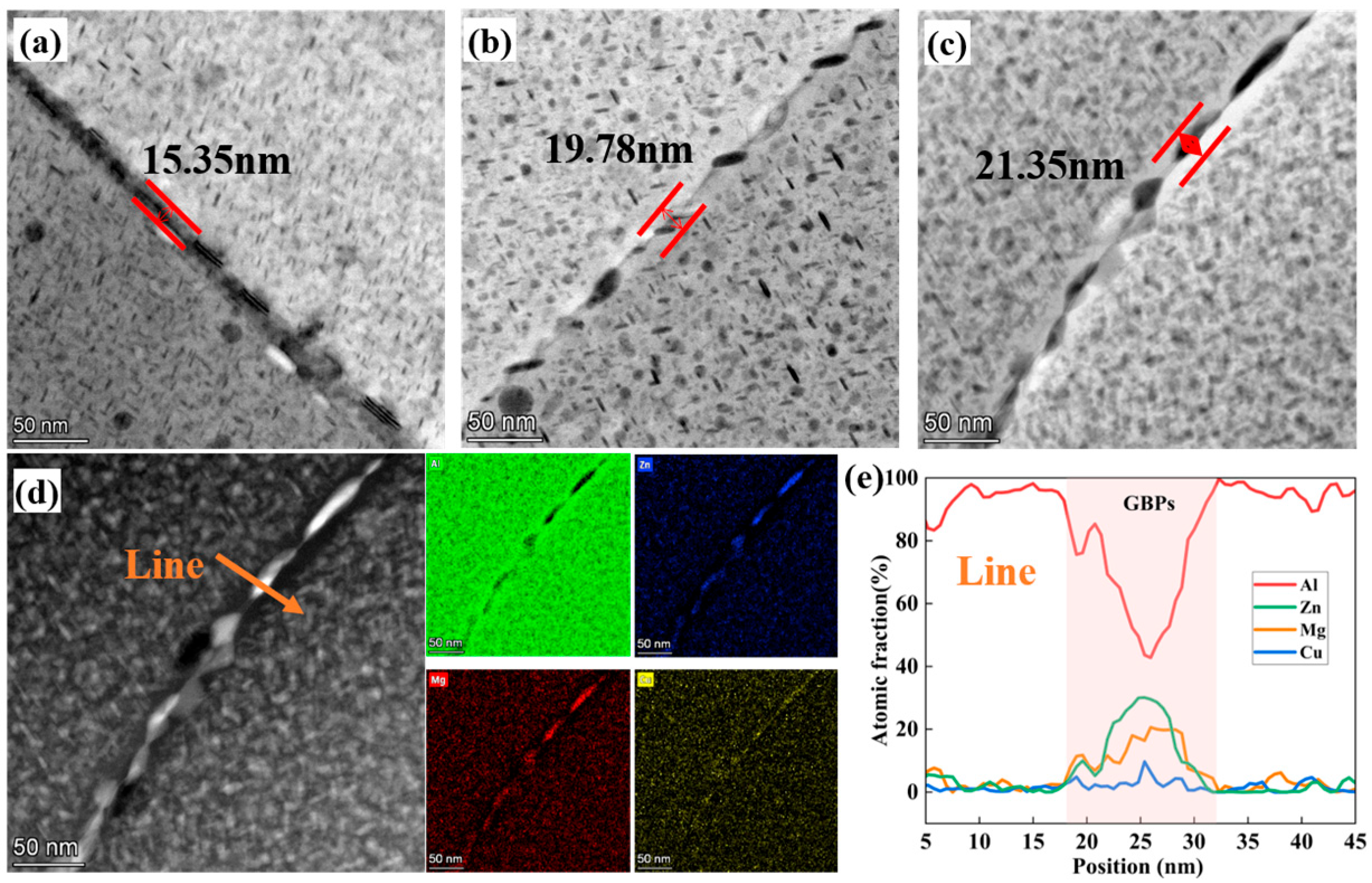
3.1. Microstructure of As-Cast and Forged Alloy
3.2. Microstructure After Aging Treatment
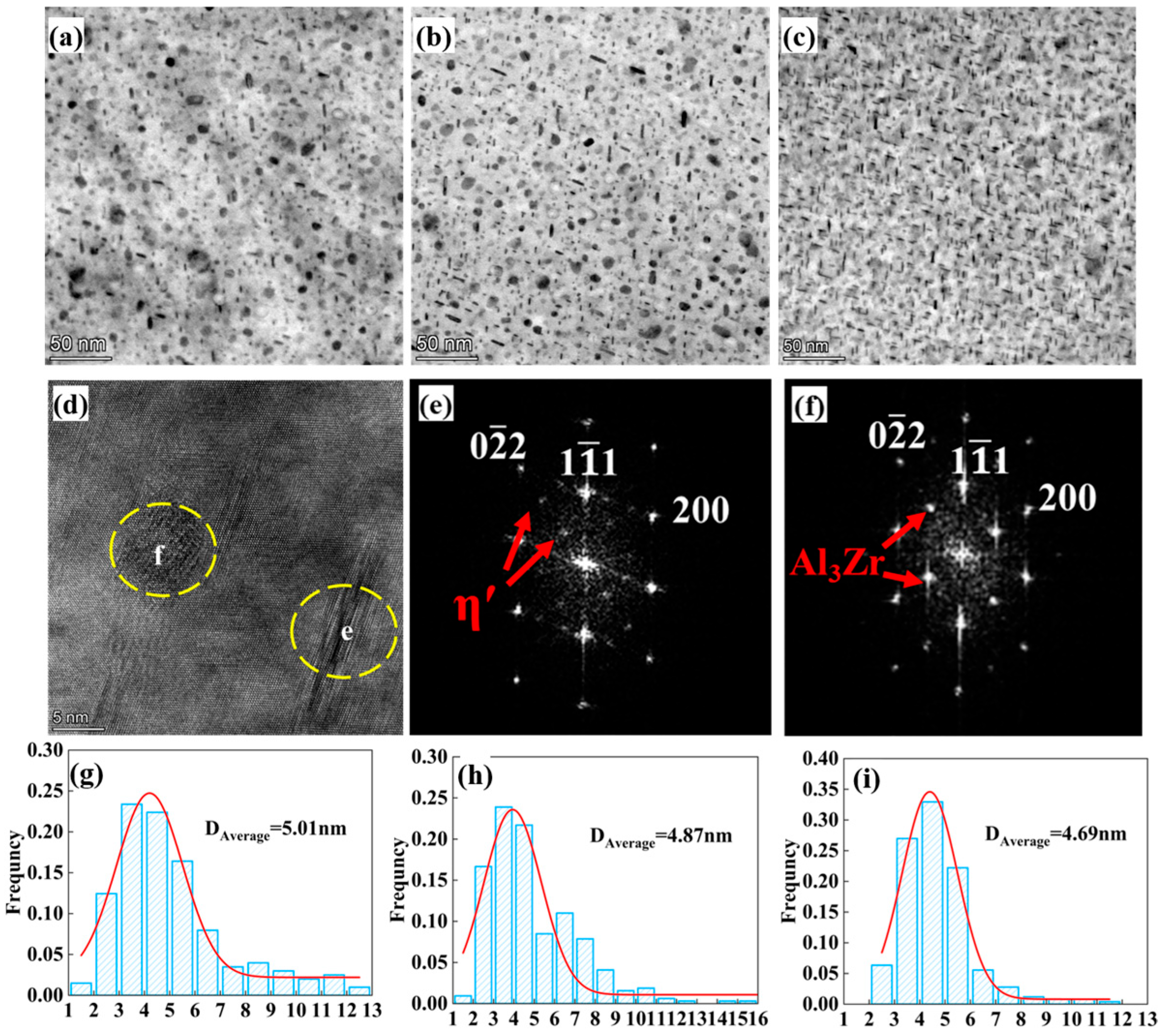
3.3. Precipitation Behavior
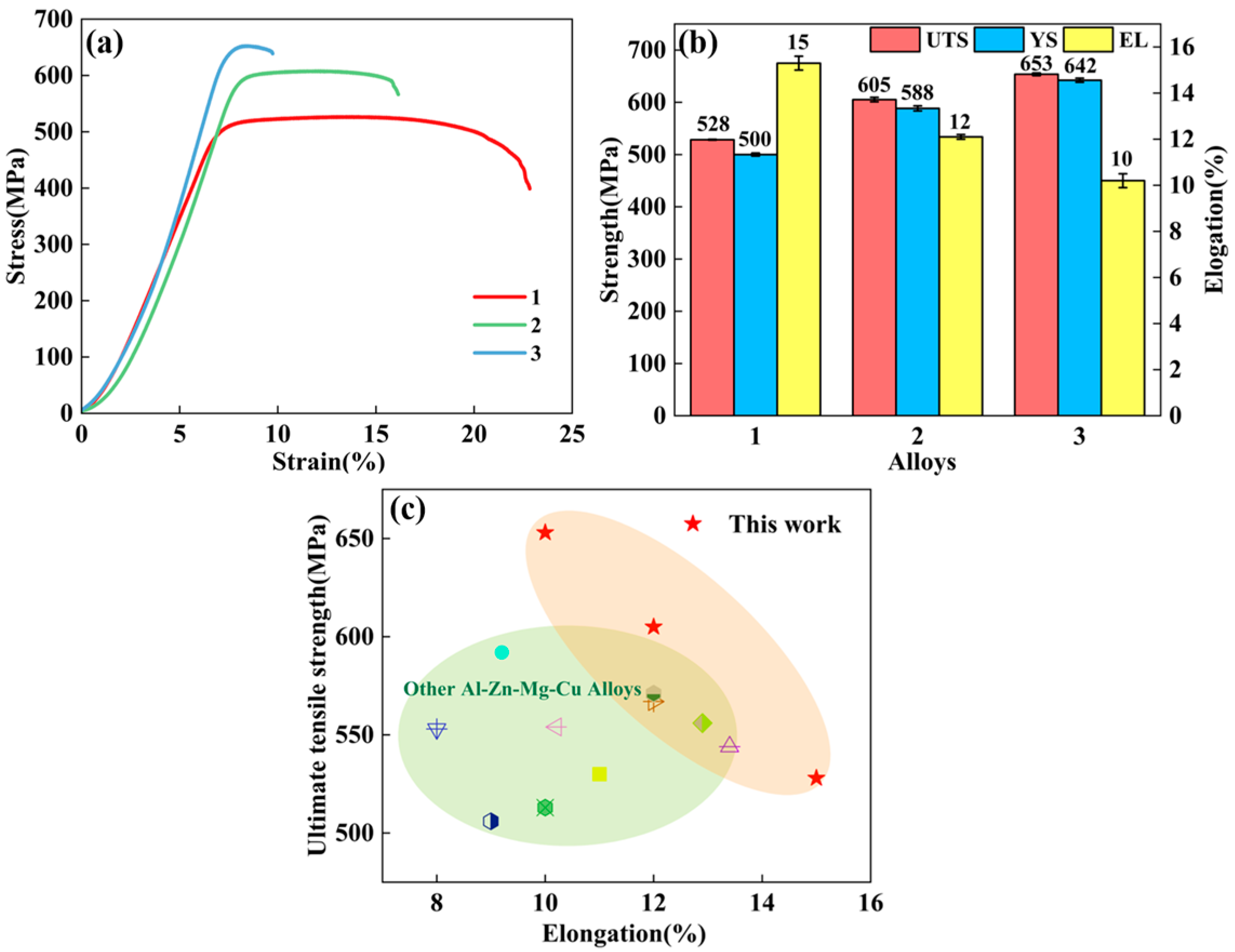
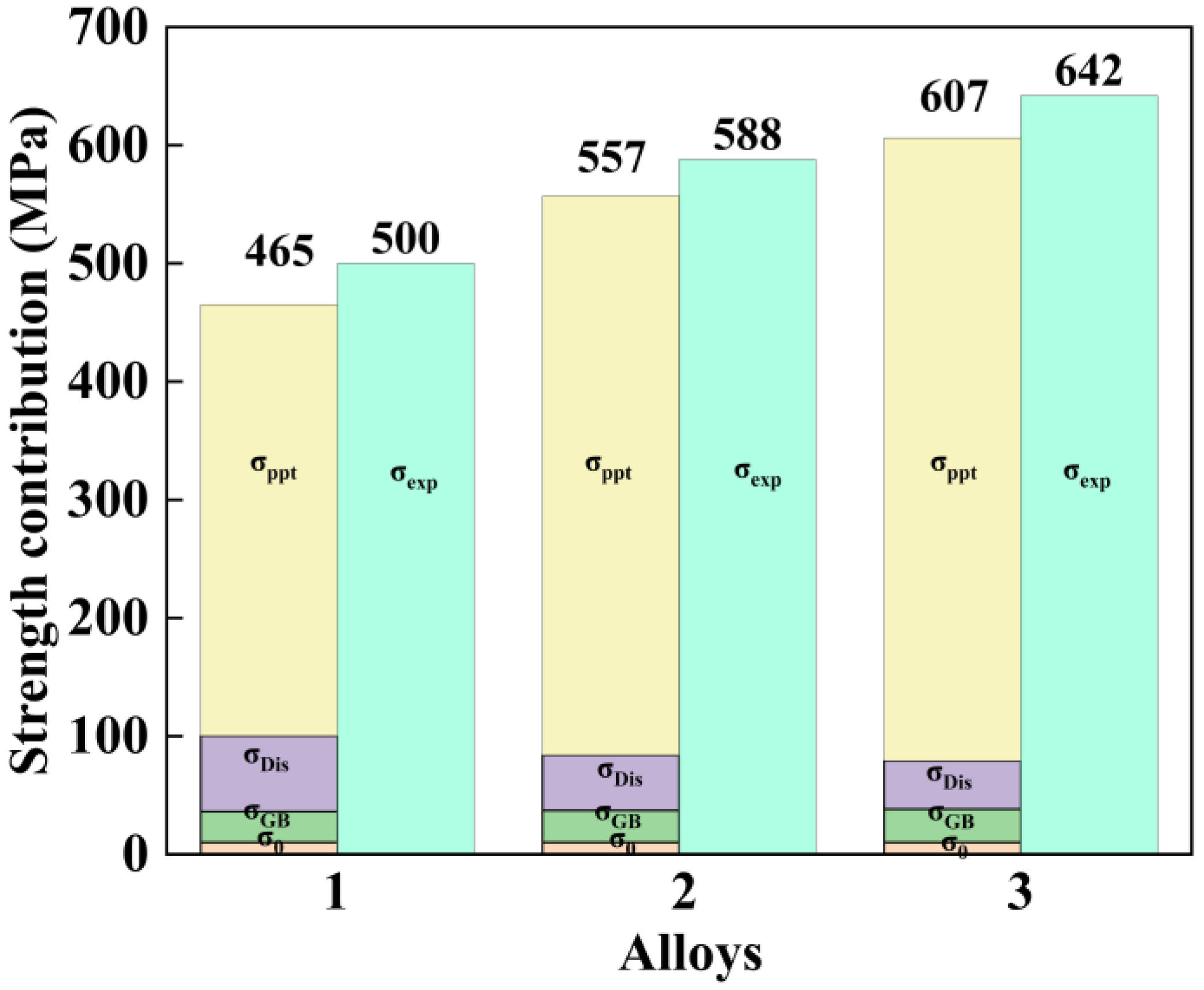
3.4. Mechanical Properties
3.5. Fracture Morphology
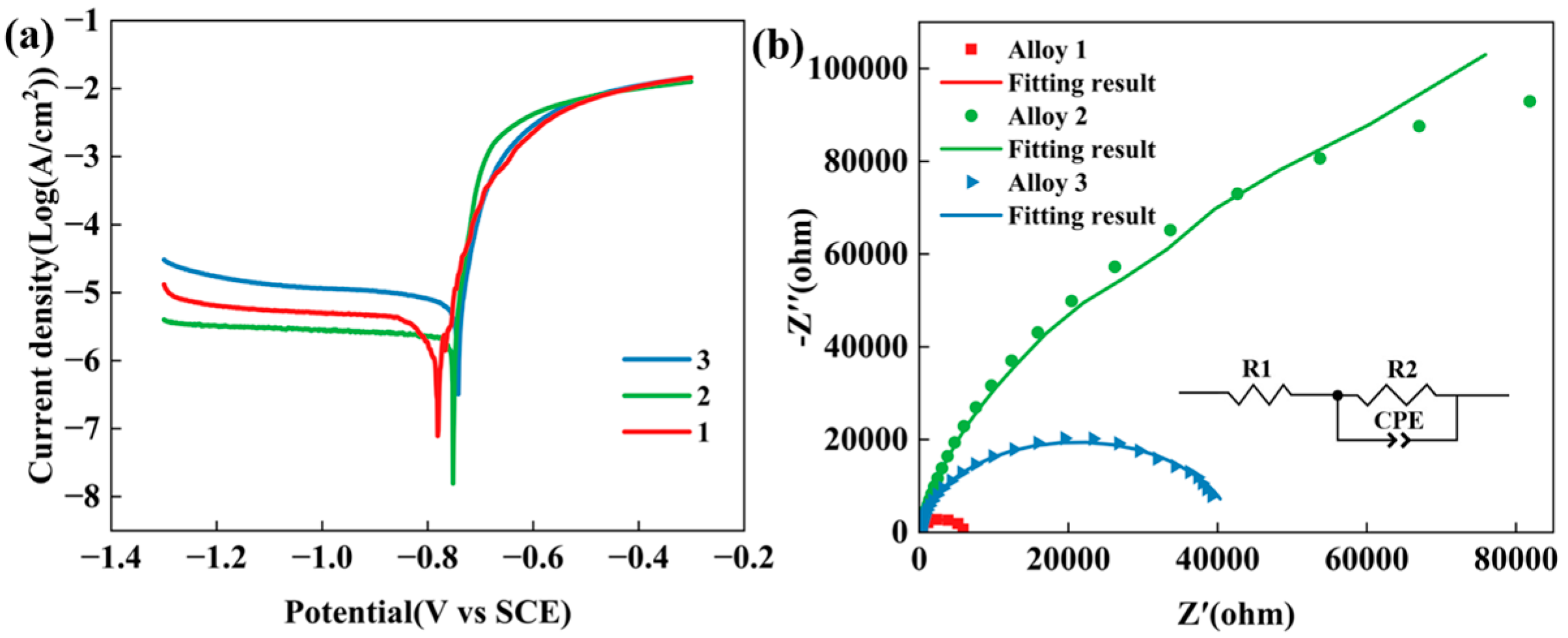
3.6. Electrochemical Characteristics
4. Discussion
4.1. The Effect of Mortise–Tenon Nested Grain Structure
4.2. The Effect of the Total Amount of (Zn + Mg) on the Mechanical Properties and Precipitated Phase
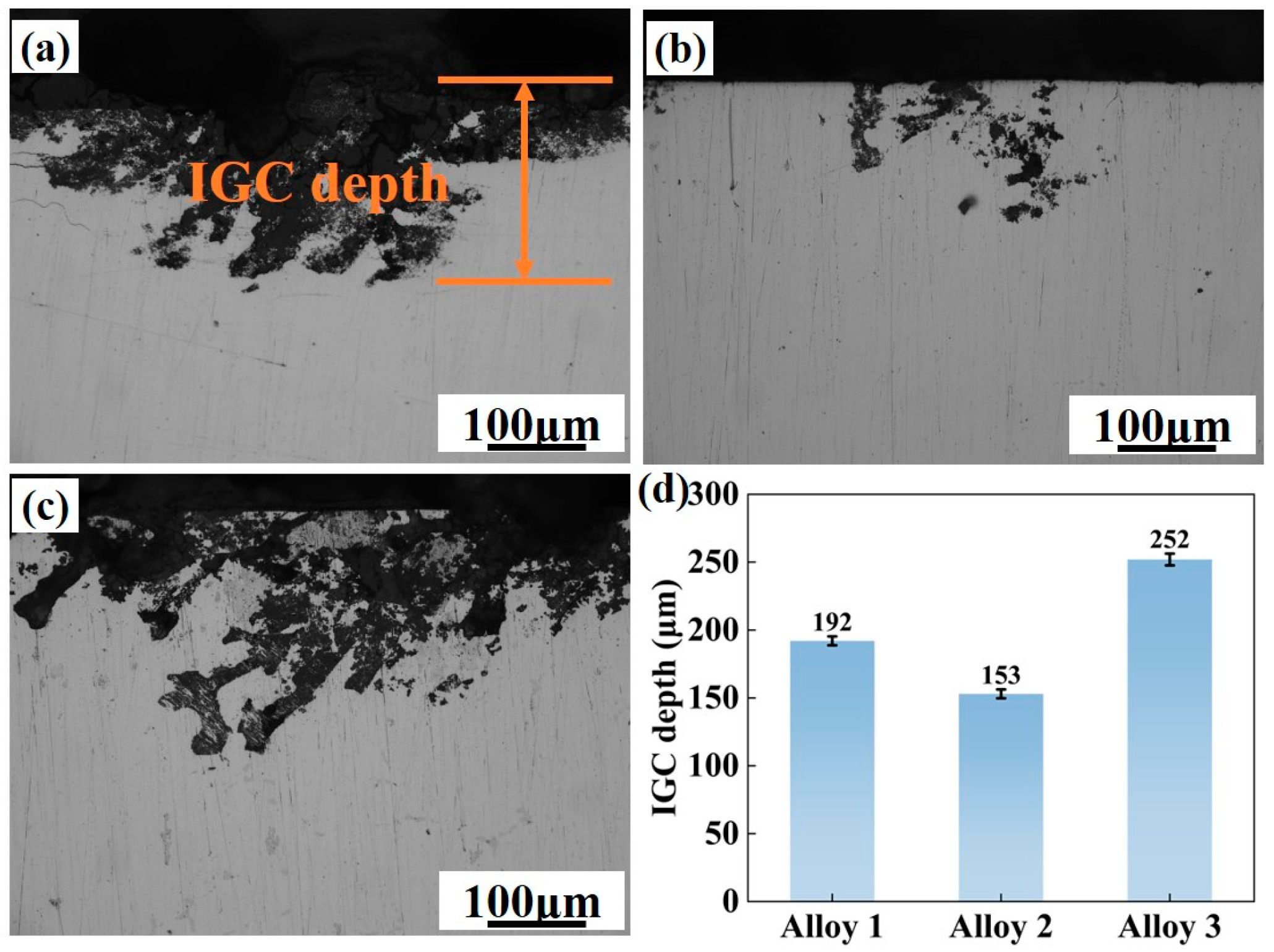
4.3. Corrosion Resistance
5. Conclusions
- (1)
- MDF enables the alloys to obtain a mortise and tenon nested grain structure that suppresses grain boundary slip and strengthens interfaces via mechanical interlocking. Simultaneously, the synergistic deformation between nested submicron and micron grains significantly impedes dislocation motion.
- (2)
- Increasing total (Zn + Mg) content enlarges grain boundary PFZs and transitions the η phase from discontinuous to continuous distribution. Electrochemical testing reveals that this elevation diminishes corrosion resistance. However, Al-8.6Zn-1.55Mg-1.9Cu-0.11Zr (Zn + Mg = 10.15 wt.%) exhibits superior electrochemical stability, evidenced by its minimal corrosion current density. IGC testing confirms that this composition provides relatively desirable corrosion resistance.
- (3)
- Elevated total (Zn + Mg) content strengthens alloys through η′ precipitate refinement. Al-9.6Zn-2.1Mg-1.9Cu-0.11Zr (Zn + Mg = 11.7 wt.%) has continuous η–MgZn2 at grain boundaries and widened PFZs induce intergranular corrosion. Al-8.6Zn-1.55Mg-1.9Cu-0.11Zr (Zn + Mg = 10.15 wt.%) optimally balances precipitation strengthening and grain boundary refinement, enhancing both strength and corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, B.; Zhang, S. The Advancement of 7XXX Series Aluminum Alloys for Aircraft Structures: A Review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Dai, Y.; Yan, L.; Sun, S.; Zhang, J.; Li, X.; Liu, J.; Liu, X. In-depth investigation of microstructural evolution induced by Sc, V, and Ni microalloying in Al-Zn-Mg-Cu alloy during hot compression. Mater. Des. 2025, 253, 113857. [Google Scholar] [CrossRef]
- Jo, Y.H.; Jung, H.; Jeon, S.; Choi, H.; Kim, H.-W.; Sung, H. The effect of Zn content on tensile properties and fracture toughness of Al–Zn–Mg–Cu alloy. J. Mater. Res. Technol. 2025, 36, 5696–5706. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Shaban, G.; Ambat, R. Role of aging time and Cu/Zn additions on the microstructure and intergranular corrosion resistance of 6082 Al-Mg-Si alloy. Corros. Sci. 2025, 254, 113027. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Tang, J.; Zhang, J. Effect introduced high density of precipitates on the microstructural evolutions during multi-direction forging of Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2020, 798, 139927. [Google Scholar] [CrossRef]
- Proni, C.T.W.; Torres, L.V.; Haghayeghi, R.; Zoqui, E.J. ECAP: An alternative route for producing AlSiCu for use in SSM processing. Mater. Charact. 2016, 118, 252–262. [Google Scholar] [CrossRef]
- Mazilkin, A.A.; Straumal, B.B.; Borodachenkova, M.V.; Valiev, R.Z.; Kogtenkova, O.A.; Baretzky, B. Gradual softening of Al–Zn alloys during high-pressure torsion. Mater. Lett. 2012, 84, 63–65. [Google Scholar] [CrossRef]
- Sakai, T.; Miura, H.; Goloborodko, A.; Sitdikov, O. Continuous dynamic recrystallization during the transient severe deformation of aluminum alloy 7475. Acta Mater. 2009, 57, 153–162. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Pouraliakbar, H.; Shiran, M.K.G.; Khalaj, G.; Shirazi, M. On the effect of non-isothermal annealing and multi-directional forging on the microstructural evolutions and correlated mechanical and electrical characteristics of hot-deformed Al-Mg alloy. Mater. Sci. Eng. A 2016, 657, 431–440. [Google Scholar] [CrossRef]
- Almeida, N.G.S.; Pereira, P.H.R.; Faria, C.G.D.; Aguilar, M.T.P.; Cetlin, P.R. Mechanical behavior and microstructures of aluminum processed by low strain amplitude multi-directional confined forging. J. Mater. Res. Technol. 2020, 9, 3190–3197. [Google Scholar] [CrossRef]
- Sitdikov, O.; Sakai, T.; Goloborodko, A.; Miura, H.; Kaibyshev, R. Effect of Pass Strain on Grain Refinement in 7475 Al Alloy during Hot Multidirectional Forging. Mater. Trans. 2004, 45, 2232–2238. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Zhang, J.; Ma, Z.; Zhang, Y. Effect of temperature and strain rate on the grain structure during the multi-directional forging of the AlZnMgCu alloy. Mater. Sci. Eng. A 2019, 756, 119–128. [Google Scholar] [CrossRef]
- Sitdikov, O.; Garipova, R.; Avtokratova, E.; Mukhametdinova, O.; Markushev, M. Effect of temperature of isothermal multidirectional forging on microstructure development in the Al-Mg alloy with nano-size aluminides of Sc and Zr. J. Alloys Compd. 2018, 746, 520–531. [Google Scholar] [CrossRef]
- Wu, Z.; Jia, Y.; Mu, Y.; Jia, Y.; Ji, P.; Hu, K.; Wang, Y.; Yang, D.; Ma, P.; Zhao, W.; et al. A mortise-and-tenon structure inspired high strength-ductility 3D printed high-entropy alloys with mechanically interlocked network. Mater. Sci. Eng. A 2024, 899, 146422. [Google Scholar] [CrossRef]
- Wang, D.; Yi, Y.; Li, C.; Huang, S.; He, H.; Zhang, J. Effects of different multidirectional forging processes on the microstructure and three-dimensional mechanical properties of ultra-high strength aluminum alloys. Mater. Sci. Eng. A 2021, 826, 141932. [Google Scholar] [CrossRef]
- Miura, H.; Yu, G.; Yang, X. Multi-directional forging of AZ61Mg alloy under decreasing temperature conditions and improvement of its mechanical properties. Mater. Sci. Eng. A 2011, 528, 6981–6992. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Liu, W.; Ma, Y.; Huang, B. Influence of cumulative strain on microstructure and mechanical properties of multi-directional forged 2A14 aluminum alloy. Mater. Sci. Eng. A 2016, 674, 40–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Liu, Y.; Huang, Y. The microstructures and mechanical properties of a highly alloyed Al-Zn-Mg-Cu alloy: The role of Cu concentration. J. Mater. Res. Technol. 2022, 18, 122–137. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Hu, G.Y.; Chen, K.; Huang, L. Effect of Zn/Mg ratios on SCC, electrochemical corrosion properties and microstructure of Al-Zn-Mg alloy. J. Alloys Compd. 2018, 757, 259–264. [Google Scholar] [CrossRef]
- Tang, J.; Liu, M.; Bo, G.; Jiang, F.; Luo, C.; Teng, J.; Fu, D.; Zhang, H. Unraveling precipitation evolution and strengthening function of the Al-Zn-Mg-Cu alloys with various Zn contents: Multiple experiments and integrated internal-state-variable modeling. J. Mater. Sci. Technol. 2022, 116, 130–150. [Google Scholar] [CrossRef]
- Shu, W.X.; Hou, L.G.; Zhang, C.; Zhang, F.; Liu, J.C.; Liu, J.T.; Zhuang, L.Z.; Zhang, J.S. Tailored Mg and Cu contents affecting the microstructures and mechanical properties of high-strength Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2016, 657, 269–283. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Wang, X.; Ma, Q.; Zhuang, L.; Zhang, J. Effects of Cu addition on the precipitation hardening response and intergranular corrosion of Al-5.2Mg-2.0Zn (wt.%) alloy. Mater. Charact. 2016, 122, 177–182. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between alloy composition, microstructure and exfoliation corrosion in Al–Zn–Mg–Cu alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Guo, M.; Cao, L. Investigation on microstructure and mechanical properties of Al-Zn-Mg-Cu alloys with various Zn/Mg ratios. J. Mater. Sci. Technol. 2021, 85, 106–117. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, Y.; Nie, Z. Effect of Zn Content on the Microstructure and Properties of Super-High Strength Al-Zn-Mg-Cu Alloys. Metall. Mater. Trans. A 2013, 44, 3910–3920. [Google Scholar] [CrossRef]
- GB/T 7998-2023; Method for Evaluating the Susceptibility to Intergranular Corrosion of Aluminum Alloys. State Administration for Market Regulation (SAMR)/Standardization Administration of China (SAC): Beijing, China, 2023.
- Ii, Y.; Li, P.; Zhao, G.; Liu, X.; Cui, J. The constituents in Al–10Zn–2.5Mg–2.5Cu aluminum alloy. Mater. Sci. Eng. A 2005, 397, 204–208. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, G.; Zhao, X.; Li, Y.; Lv, Z. Effect of cumulative plastic deformation on microstructure and strengthening mechanism of extruded Al-Zn-Mg-Cu alloy. Vacuum 2025, 232, 113894. [Google Scholar] [CrossRef]
- Takayama, Y.; Szpunar, J.A. Stored Energy and Taylor Factor Relation in an Al-Mg-Mn Alloy Sheet Worked by Continuous Cyclic Bending. Mater. Trans. 2004, 45, 2316–2325. [Google Scholar] [CrossRef]
- Ramesh, S.; Anne, G.; Naik, G.M.; Jagadeesh, C.; Nayaka, H.S. Microstructural and mechanical characterisation of Al-Zn-Mg-Cu alloy processed by multi-directional cryo-forging. Mater. Today Proc. 2021, 46, 5752–5756. [Google Scholar] [CrossRef]
- Aoba, T.; Kobayashi, M.; Miura, H. Effects of aging on mechanical properties and microstructure of multi-directionally forged 7075 aluminum alloy. Mater. Sci. Eng. A 2017, 700, 220–225. [Google Scholar] [CrossRef]
- Wang, D.; Lin, C.; Wang, Y. Influence of a multi-pass process combining three-dimensional cryogenic deformation and solution treatment on microstructure and comprehensive properties of high-strength aluminum alloy. J. Alloys Compd. 2025, 1039, 183178. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, J.H.; Lee, S.-J.; Jung, J.-G. Precipitation behavior and mechanical property anisotropy of Al–Zn–Mg–Cu alloy forgings. Mater. Sci. Eng. A 2025, 926, 147957. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Tan, X.; Wu, M.; Yuan, S.; Xiao, D.; Huang, L. Eliminating Anisotropy of 7085 Alloy Forgings via Temperature Combination Control During Two-Stage Multi-Directional Forging. Materials 2025, 18, 391. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, M.; Wang, Z.; Wang, S.; Liu, C.; Qian, L.; Li, L.; Zhao, H. Effects of cold temperatures, strain rates and anisotropy on the mechanical behavior and fracture morphology of an Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2021, 806, 140691. [Google Scholar] [CrossRef]
- Wei, C.; Lei, Z.; Du, S.; Chen, R.; Yin, Y.; Niu, C.; Xu, Z. Microstructures and Mechanical Properties of Al-Zn-Mg-Cu Alloys under Multi-Directional Severe Strain and Aging. Materials 2023, 16, 4441. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Zhang, J.; Tang, J. Effect of forging speed on the formability, microstructure and mechanical properties of isothermal precision forged of Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2019, 767, 138366. [Google Scholar] [CrossRef]
- Rhee, Y.; Thronsen, E.; Ryggetangen, O.; Marioara, C.D.; Holmestad, R.; Kobayashi, E. Effect of Pre-Deformation on Precipitation in Al–Zn–Mg–Cu Alloy. Met. Mater. Int. 2024, 30, 3294–3310. [Google Scholar] [CrossRef]
- Gao, J.; Fan, H.; Wang, E.; Song, Y.; Sun, G. Exploring the effect of magnesium content on the electrochemical performance of aluminum anodes in alkaline batteries. Electrochim. Acta 2020, 353, 136497. [Google Scholar] [CrossRef]
- Zhang, W.; Su, R.; Li, G.; Qu, Y. Effect of pre-aging process on microstructure and properties of 7075-T8 aluminium alloy. J. Alloys Compd. 2023, 960, 170953. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Fu, Y.; Hu, X.; Cao, X.; Pan, Z.; Wei, Y.; Luo, H.; Li, X. Effect of cold deformation on corrosion behavior of selective laser melted 316L stainless steel bipolar plates in a simulated environment for proton exchange membrane fuel cells. Corros. Sci. 2022, 201, 110257. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Wang, X.; Ye, J.; Huang, Z.; Xiang, S.; Liu, B. Effect of laser shock peening on corrosion behaviors of ultra-high strength Al-Zn-Mg-Cu alloys prepared by spray forming and ingot metallurgy. Corros. Sci. 2022, 205, 110458. [Google Scholar] [CrossRef]
- Ma, L.; Xue, J.; Dai, W.; Zhang, X.; Zhao, X. Moment-rotation relationship of mortise-through-tenon connections in historic timber structures. Constr. Build. Mater. 2020, 232, 117285. [Google Scholar] [CrossRef]
- Chung, T.F.; Yang, Y.L.; Huang, B.M.; Shi, Z.; Lin, J.; Ohmura, T.; Yang, J.R. Transmission electron microscopy investigation of separated nucleation and in-situ nucleation in AA7050 aluminium alloy. Acta Mater. 2018, 149, 377–387. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Evolution of precipitate microstructures during the retrogression and re-ageing heat treatment of an Al-Zn-Mg-Cu alloy. Acta Mater. 2010, 58, 4814–4826. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jung, J.-G.; Baik, S.-I.; Seidman, D.N.; Kim, M.-S.; Lee, Y.-K.; Euh, K. Precipitation strengthening in naturally aged Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2021, 803, 140719. [Google Scholar] [CrossRef]
- Yin, H.; Wen, K.; Li, Z.; Li, X.; Li, Y.; Yan, L.; Yan, H.; Yu, M.; Zhang, Y.; Xiong, B. Investigation on precipitate behavior and mechanical properties of Al-Zn-Mg-Cu alloys with various Zn/Mg ratios. J. Mater. Res. Technol. 2024, 33, 5769–5783. [Google Scholar] [CrossRef]
- Cabibbo, M. Microstructure strengthening mechanisms in different equal channel angular pressed aluminum alloys. Mater. Sci. Eng. A 2013, 560, 413–432. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, D.; Yuan, S.; Tang, S.; Li, Z.; Yin, X.; Huang, L.; Liu, W. Development of bimodal grain-structured Al-Zn-Mg-Cu-Zr alloys for strength-ductility synergy via microalloying with Hf and Sc. Mater. Charact. 2023, 205, 113349. [Google Scholar] [CrossRef]
- Hornbogen, E.; Starke, E.A. Overview no. 102 Theory assisted design of high strength low alloy aluminum. Acta Metall. Mater. 1993, 41, 1–16. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, S.; Chen, X.; Pan, S.; Yang, C.; Pan, H.; Zhou, D.; Zhang, D.; Qin, G. Deformation faulting in ultrafine-grained aluminum alloys: Nucleation mechanisms and critical assessment of strengthening-ductilization contributions. Acta Mater. 2025, 286, 120750. [Google Scholar] [CrossRef]
- Yang, C.; Chen, X.; Xia, W.; Hu, J.; Zhang, J.; Zhang, S.; Zhou, D.; Lu, W.; Shu, D.; Zhang, D. Strong and stable ultrafine-grained Al-Mg alloys via polymer-derived in situ nanoparticles. Mater. Sci. Eng. A 2024, 915, 147298. [Google Scholar] [CrossRef]
- Dai, S.; Khan, M.A.; Liao, L.; Zhang, X.; Zhao, D.; Wang, H.; Afifi, M.A.; Li, J. Effect of hot extrusion, novel stepwise-rolling, and heat treatment on microstructure, mechanical properties, and precipitate chemistry of ultra-high strength Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2025, 1010, 177910. [Google Scholar] [CrossRef]
- Sahu, B.P.; Xi, Z.; Kayitmazbatir, M.; Song, E.; Qi, L.; Misra, A. Comparative Analysis of Precipitation Hardening Mechanisms in Al-7075 Alloy under different quenching media. J. Mater. Res. Technol. 2025, 37, 4973–4984. [Google Scholar] [CrossRef]
- Dumont, M.; Lefebvre, W.; Doisneau-Cottignies, B.; Deschamps, A. Characterisation of the composition and volume fraction of η′ and η precipitates in an Al–Zn–Mg alloy by a combination of atom probe, small-angle X-ray scattering and transmission electron microscopy. Acta Mater. 2005, 53, 2881–2892. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, J.; Xin, Z.; Qin, L.; Jia, Y.; Jiang, Y. Microstructure and properties of Cu-Zn-Cr-Zr alloy treated by multistage thermo-mechanical treatment. Mater. Sci. Eng. A 2023, 870, 144679. [Google Scholar] [CrossRef]
- Sun, Z.; Hao, S.; Duan, H.; Zhu, S.; Wang, D.; Li, L.; Du, K. Microstructure, strength and corrosion resistance in an Al-Zn-Mg-Cu alloy after artificial aging and subsequent long-term natural aging. J. Alloys Compd. 2025, 1036, 181915. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals: A Review of the Critical Factors. J. Electrochem. Soc. 1998, 145, 2186. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Su, Y.; Mou, X.; Xiao, Y.; Liu, J. Investigation on the corrosion behavior of aluminum alloys 3A21 and 7A09 in chloride aqueous solution. Mater. Des. 2013, 50, 15–21. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of recrystallization on intergranular fracture and corrosion of Al–Zn–Mg–Cu–Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.; Chen, K.; Jiao, H.; Huang, L.; Zhang, Z.; Yang, Z. Enhancing the stress corrosion cracking resistance of a low-Cu containing Al-Zn-Mg-Cu aluminum alloy by step-quench and aging heat treatment. Corros. Sci. 2019, 161, 108184. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Corrosion behaviour of different tempers of AA7075 aluminium alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Song, F.; Zhang, X.; Liu, S.; Tan, Q.; Li, D. The effect of quench rate and overageing temper on the corrosion behaviour of AA7050. Corros. Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Zhang, Y.; Zhang, X.; Deng, Y. Effect of microstructure on the quench sensitivity of AlZnMgCu alloys. J. Alloys Compd. 2010, 507, 53–61. [Google Scholar] [CrossRef]
- Ramgopal, T.; Gouma, P.I.; Frankel, G.S. Role of grain-boundary precipitates and solute-depleted zone on the intergranular corrosion of aluminum alloy 7150. Corrosion 2002, 58, 687–697. [Google Scholar] [CrossRef]



| Alloys | Zn | Mg | Cu | Zr | Al | (Zn + Mg) |
|---|---|---|---|---|---|---|
| Alloy 1 | 8.6 | 1 | 1.9 | 0.11 | Bal. | 9.6 |
| Alloy 2 | 8.6 | 1.55 | 1.9 | 0.11 | Bal. | 10.15 |
| Alloy 3 | 9.6 | 2.1 | 1.9 | 0.11 | Bal. | 11.7 |
| Alloys | Ecorr (V) | icorr (A·cm−2) | R1 (Ω·cm2) | R2 (Ω·cm2) | CPE (SSn cm−2) |
|---|---|---|---|---|---|
| Alloy 1 | −0.78 | 2.93 × 10−6 | 30.11 ± 0.36 | 6087 ± 97 | 5.16 × 10−6 ± 0.14 × 10−6 |
| Alloy 2 | −0.75 | 2.71 × 10−6 | 67.43 ± 1.01 | 226,140 ± 14,986 | 9.34 × 10−6 ± 0.18 × 10−6 |
| Alloy 3 | −0.74 | 8.52 × 10−6 | 29.38 ± 0.22 | 42,225 ± 500 | 6.71 × 10−6 ± 0.081 × 10−6 |
| Sample | /MPa |
|---|---|
| Alloy 1 | 26.2 |
| Alloy 2 | 27.0 |
| Alloy 3 | 28.2 |
| Sample | /1014 m−2 | /MPa |
|---|---|---|
| Alloy 1 | 1.29 | 64 |
| Alloy 2 | 0.70 | 47 |
| Alloy 3 | 0.55 | 41 |
| Sample | /nm | f/% | |
|---|---|---|---|
| Alloy 1 | 2.04 | 1.4 | 365 |
| Alloy 2 | 1.99 | 2.1 | 473 |
| Alloy 3 | 1.91 | 2.4 | 527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Liu, T.; Wu, M.; Yuan, S.; Li, Z.; Huang, Y.; Yin, X.; Huang, L.; Liu, W.; Xiao, D. Tailoring Strength and Corrosion Resistance in Al–Zn–Mg–Cu Alloys by Total (Zn + Mg) Content and Multi-Directional Forging Process. Materials 2025, 18, 4476. https://doi.org/10.3390/ma18194476
Lin J, Liu T, Wu M, Yuan S, Li Z, Huang Y, Yin X, Huang L, Liu W, Xiao D. Tailoring Strength and Corrosion Resistance in Al–Zn–Mg–Cu Alloys by Total (Zn + Mg) Content and Multi-Directional Forging Process. Materials. 2025; 18(19):4476. https://doi.org/10.3390/ma18194476
Chicago/Turabian StyleLin, Junfu, Tangjian Liu, Mingdong Wu, Shuo Yuan, Zeyu Li, Yang Huang, Xiao Yin, Lanping Huang, Wensheng Liu, and Daihong Xiao. 2025. "Tailoring Strength and Corrosion Resistance in Al–Zn–Mg–Cu Alloys by Total (Zn + Mg) Content and Multi-Directional Forging Process" Materials 18, no. 19: 4476. https://doi.org/10.3390/ma18194476
APA StyleLin, J., Liu, T., Wu, M., Yuan, S., Li, Z., Huang, Y., Yin, X., Huang, L., Liu, W., & Xiao, D. (2025). Tailoring Strength and Corrosion Resistance in Al–Zn–Mg–Cu Alloys by Total (Zn + Mg) Content and Multi-Directional Forging Process. Materials, 18(19), 4476. https://doi.org/10.3390/ma18194476






