Studying How Calcium Silicate and Radiopacifier Proportions Affect the Physicochemical Properties of Endodontic Calcium Silicate-Based Sealers
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting Time
2.2. Flowability
2.3. Radiopacity
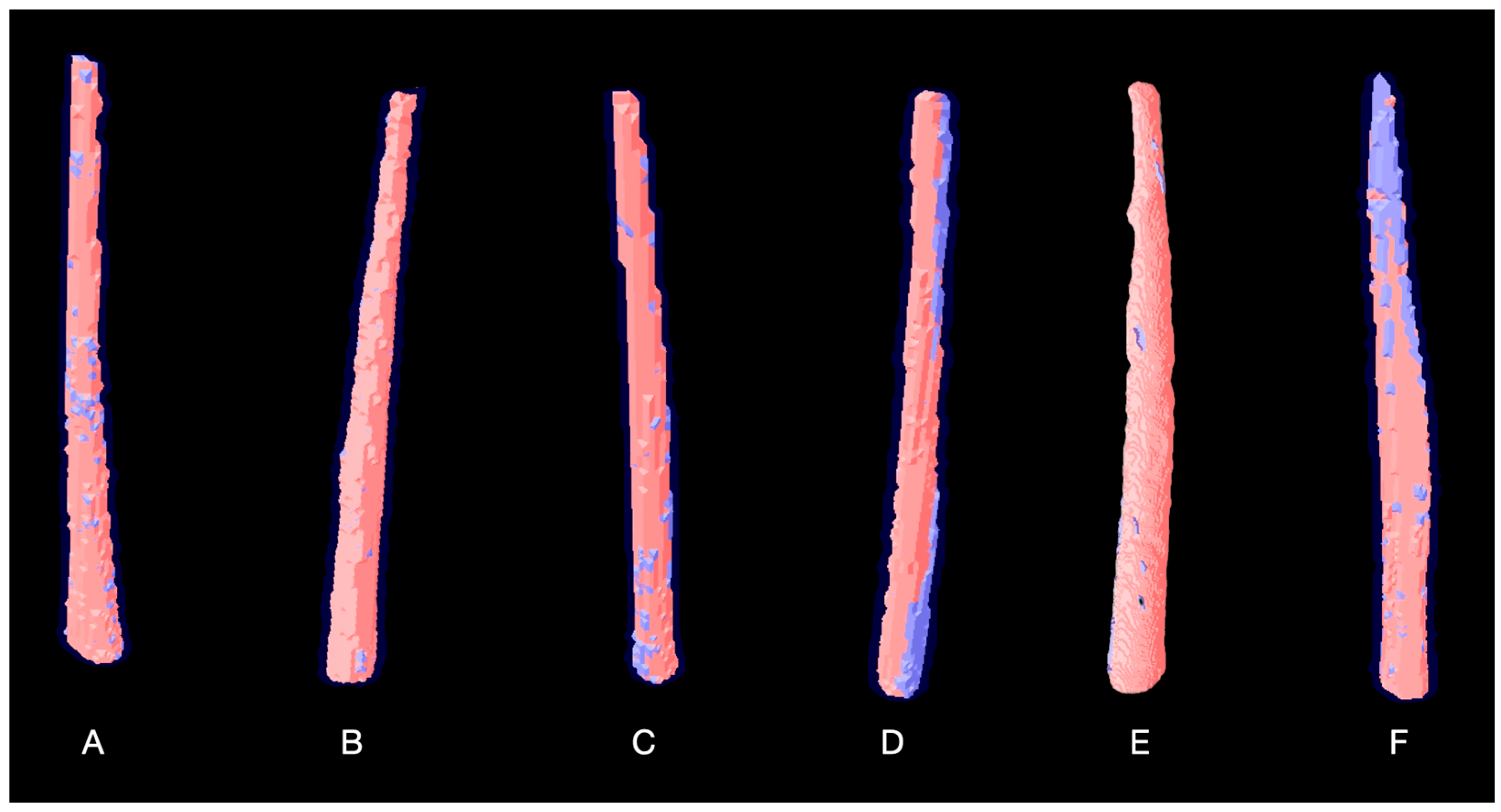
2.4. Volumetric Change Analysis
2.5. Solubility
2.6. pH
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55 (Suppl. S3), 710–777, Erratum in Int. Endod. J. 2023, 56, 402. [Google Scholar] [CrossRef]
- Duarte, M.A.; Demarchi, A.C.; Yamashita, J.C.; Kuga, M.C.; Fraga Sde, C. pH and calcium ion release of 2 root-end filling materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Jiao, K.; Wang, T.D.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.L.; Mao, J.; Chen, J.H.; Pashley, D.H.; et al. A review of the bioactivity of hydraulic calcium silicate cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Candeiro, G.T.; Correia, F.C.; Duarte, M.A.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- López-García, S.; Sánchez-Bautista, S.; García-Bernal, D.; Lozano, A.; Forner, L.; Sanz, J.L.; Murcia, L.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Premixed calcium silicate-based ceramic sealers promote osteogenic/cementogenic differentiation of human periodontal ligament stem cells: A microscopy study. Microsc. Res. Tech. 2024, 87, 1584–1597. [Google Scholar] [CrossRef]
- Mora, A.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Forner, L.; Ghilotti, J.; Lozano, A.; López-García, S. Biocompatibility, bioactivity and immunomodulatory properties of three calcium silicate-based sealers: An in vitro study on hPDLSCs. Clin. Oral Investig. 2024, 28, 416. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Pitt Ford, T.R.; McKendry, D.J.; Abedi, H.R.; Miller, D.A.; Kariyawasam, S.P. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J. Endod. 1997, 23, 225–228. [Google Scholar] [CrossRef]
- Economides, N.; Pantelidou, O.; Kokkas, A.; Tziafas, D. Short-term periradicular tissue response to mineral trioxide aggregate (MTA) as root-end filling material. Int. Endod. J. 2003, 36, 44–48. [Google Scholar] [CrossRef]
- Torabinejad, M.; Watson, T.F.; Pitt Ford, T.R. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef]
- Camilleri, J.; Gandolfi, M.G.; Siboni, F.; Prati, C. Dynamic sealing ability of MTA root canal sealer. Int. Endod. J. 2011, 44, 9–20. [Google Scholar] [CrossRef]
- Khalil, I.; Naaman, A.; Camilleri, J. Properties of Tricalcium Silicate Sealers. J. Endod. 2016, 42, 1529–1535. [Google Scholar] [CrossRef]
- Holland, R.; de Souza, V.; Nery, M.J.; Faraco Júnior, I.M.; Bernabé, P.F.; Otoboni Filho, J.A.; Dezan Júnior, E. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz. Dent. J. 2001, 12, 3–8. [Google Scholar]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Marciano, M.A.; Costa, R.M.; Camilleri, J.; Mondelli, R.F.; Guimarães, B.M.; Duarte, M.A. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J. Endod. 2014, 40, 1235–1240. [Google Scholar] [CrossRef]
- Aguiar, B.A.; Frota, L.M.A.; Taguatinga, D.T.; Vivan, R.R.; Camilleri, J.; Duarte, M.A.H.; de Vasconcelos, B.C. Influence of ultrasonic agitation on bond strength, marginal adaptation, and tooth discoloration provided by three coronary barrier endodontic materials. Clin. Oral Investig. 2019, 23, 4113–4122. [Google Scholar] [CrossRef]
- Lin, H.N.; Wang, L.C.; Chen, M.S.; Chang, P.J.; Lin, P.Y.; Fang, A.; Chen, C.Y.; Lee, P.Y.; Lin, C.K. Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates. Materials 2022, 15, 7934. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Neves, G.S.T.; Kirkpatrick, T.; Letra, A.; Silva, R. Physicochemical and Biological Properties of AH Plus Bioceramic. J. Endod. 2023, 49, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.N.L.; Ferreira, C.M.; Pinto, K.P.; Barbosa, A.F.A.; Colaço, M.V.; Sassone, L.M. Influence of variations in the environmental pH on the solubility and water sorption of a calcium silicate-based root canal sealer. Int. Endod. J. 2021, 54, 1394–1402. [Google Scholar] [CrossRef]
- López-García, S.; Pecci-Lloret, M.R.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodríguez-Lozano, F.J.; Forner, L. Comparative Cytocompatibility and Mineralization Potential of Bio-C Sealer and TotalFill BC Sealer. Materials 2019, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Zordan-Bronzel, C.L.; Esteves Torres, F.F.; Tanomaru-Filho, M.; Chávez-Andrade, G.M.; Bosso-Martelo, R.; Guerreiro-Tanomaru, J.M. Evaluation of Physicochemical Properties of a New Calcium Silicate-based Sealer, Bio-C Sealer. J. Endod. 2019, 45, 1248–1252. [Google Scholar] [CrossRef]
- Marciano, M.A.; Duarte, M.A.; Camilleri, J. Calcium silicate-based sealers: Assessment of physicochemical properties, porosity and hydration. Dent. Mater. 2016, 32, e30–e40. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Modena, E.; Siboni, F.; Prati, C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1107–1123. [Google Scholar] [CrossRef]
- Rosa, S.J.; Duarte, M.A.H.; Silva, E.J.N.L.; Oliveira, M.C.G.; Titato, P.C.G.; Vasconcelos, B.C.; Vivan, R.R.; Alcalde, M.P. Does the Mixing Method of AH Plus Jet Affect its Physicochemical and Mechanical Properties? J. Endod. 2024, 50, 1333–1339. [Google Scholar] [CrossRef]
- ASTM C266-08; Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. ASTM International: West Conshohocken, PA, USA, 2008.
- ISO 6876; Dental Root Canal Sealing Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Húngaro Duarte, M.A.; de Oliveira El Kadre, G.D.; Vivan, R.R.; Guerreiro Tanomaru, J.M.; Tanomaru Filho, M.; de Moraes, I.G. Radiopacity of portland cement associated with different radiopacifying agents. J. Endod. 2009, 35, 737–740. [Google Scholar] [CrossRef]
- Cavenago, B.C.; Pereira, T.C.; Duarte, M.A.; Ordinola-Zapata, R.; Marciano, M.A.; Bramante, C.M.; Bernardineli, N. Influence of powder-to-water ratio on radiopacity, setting time, pH, calcium ion release and a micro-CT volumetric solubility of white mineral trioxide aggregate. Int. Endod. J. 2014, 47, 120–126. [Google Scholar] [CrossRef]
- Cardinali, F.; Camilleri, J. A critical review of the material properties guiding the clinician’s choice of root canal sealers. Clin. Oral Investig. 2023, 27, 4147–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, T.; Xiong, Z.; Sun, Y. Effects of lithium carbonate on performances of sulphoaluminate cement-based dual liquid high water material and its mechanisms. Constr. Build. Mater. 2018, 161, 374–380. [Google Scholar] [CrossRef]
- Janini, A.C.P.; Moraes, B.F.; Pelepenko, L.E.; Dos Santos, V.A.B.; Barros-Costa, M.; Malosá, G.F.; Batista, F.R.S.; Meira, J.A.S.; Matsumoto, M.A.; Antunes, T.B.M.; et al. Physicochemical properties and biological interaction of calcium silicate-based sealers—In vivo model. Clin. Oral Investig. 2025, 29, 86. [Google Scholar] [CrossRef] [PubMed]
- Kandemir Demirci, G.; Çöven, F.O.; Güneri, P.; Karavana, S.Y.; Nalbantsoy, A.; Köse, T.; Kaval, M.E. The solubility, pH value, chemical structure, radiopacity, and cytotoxicity of four different root canal sealers: An in vitro study. Clin. Oral Investig. 2023, 27, 5413–5425. [Google Scholar] [CrossRef]
- Drumond, J.P.S.C.; Maeda, W.; Nascimento, W.M.; Campos, D.L.; Prado, M.C.; de-Jesus-Soares, A.; Frozoni, M. Comparison of Postobturation Pain Experience after Apical Extrusion of Calcium Silicate- and Resin-Based Root Canal Sealers. J. Endod. 2021, 47, 1278–1284. [Google Scholar] [CrossRef]
- Koutroulis, A.; Batchelor, H.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. Investigation of the effect of the water to powder ratio on hydraulic cement properties. Dent. Mater. 2019, 35, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- American National Standards/American Dental Association. Specification No. 57 for Endodontic Sealing Materials; American National Standards/American Dental Association: Chicago, IL, USA, 2000. [Google Scholar]
- Kooanantkul, C.; Shelton, R.M.; Camilleri, J. Comparison of obturation quality in natural and replica teeth root-filled using different sealers and techniques. Clin. Oral Investig. 2023, 27, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.F.E.; Zordan-Bronzel, C.L.; Guerreiro-Tanomaru, J.M.; Chávez-Andrade, G.M.; Pinto, J.C.; Tanomaru-Filho, M. Effect of immersion in distilled water or phosphate-buffered saline on the solubility, volumetric change and presence of voids within new calcium silicate-based root canal sealers. Int. Endod. J. 2020, 53, 385–391. [Google Scholar] [CrossRef] [PubMed]

| Sealers and Manufactures | Composition |
|---|---|
| Proportion 1 (P1—Bauru School of Dentistry, University of São Paulo, Bauru, Brazil) | Powder: 30% Calcium silicate, 2% Calcium chloride, 3% Calcium oxide, 40% Tantalum oxide, 25% Zirconium oxide. Liquid: 90% Propylene glycol, 5% Dimethyl sulfoxide, 5% Polycarboxylate |
| Proportion 2 (P2—Bauru School of Dentistry, University of São Paulo, Bauru, Brazil) | Powder: 40% Calcium silicate, 2% Calcium chloride, 3% Calcium oxide, 35% Tantalum oxide, 20% Zirconium oxide. Liquid: 90% Propylene glycol, 5% Dimethyl sulfoxide, 5% Polycarboxylate |
| Proportion 3 (P3—Bauru School of Dentistry, University of São Paulo, Bauru, Brazil) | Powder: 50% Calcium silicate, 2% Calcium chloride, 3% Calcium oxide, 30% Tantalum oxide, 15% Zirconium oxide Liquid: 90% Propylene glycol, 5% Dimethyl sulfoxide, 5% Polycarboxylate |
| AH Plus Bioceramic (Dentsply De Trey, Konstanz, Germany) | Zirconium dioxide (50–75%), Tricalcium silicate (5–15%), Dimethyl sulfoxide (10–30%), Lithium carbonate (<0.5%), Thickening agent (<6%) |
| AH Plus Jet (Dentsply De Trey, Konstanz, Germany) | Paste A: Bisphenol-A epoxy resin, Bisphenol-F epoxy resin, Calcium tungstate, Zirconium oxide, Silica, Iron oxide pigments. Paste B: Dibenzyldiamine, Aminoadamantane, Tricyclodecane-diamine, Calcium tungstate, Zirconium oxide, Silica, Silicone oil. |
| Bio-C Sealer (Angelus, Londrina, Brazil) | Calcium silicate, calcium aluminate, calcium oxide, zirconium oxide, iron oxide, silicon dioxide and dispersing agent |
| Setting Time (min) | ||
|---|---|---|
| Sealers | Initial | Final |
| P1 | 124.0 ± 1.0 a | 407 ± 1.0 a |
| P2 | 155.0 ± 1.0 b | 426 ± 1.0 b |
| P3 | 178.0 ± 1.0 c | 625 ± 1.0 c |
| AH Plus Jet | 847.3 ± 4.93 d | 1725 ± 1.0 d |
| Bio C Sealer | 237.7 ± 6.42 e | 446.7 ± 4.04 e |
| AH Plus Bioc | 115.0 +/− 3.0 a | 265 +/− 1.0 f |
| Scheme | Flowability (mm) | Radiopacity (mm Al) | Volumetric Change (%) | Solubility (%) |
|---|---|---|---|---|
| P1 | 20.69 ± 0.01 a | 9.63 ± 1.39 ac | 2.07 ± 2.14 ªb | 5.82 +/− 0.26 ab |
| P2 | 19.70 ± 0.01 ab | 9.22 ± 1.25 a | 0.18 ± 3.10 ª | 4.68 +/− 2.49 ab |
| P3 | 19.19 ± 0.01 b | 6.93 ± 2.13 bd | 3.35 ± 5.22 ªb | 2.68 +/− 1.57 a |
| AH Plus Jet | 25.47 ± 0.01 c | 11.73 ± 0.98 c | 1.65 ± 1.47 ªb | 0.05 +/− 0.05 c |
| Bio C Sealer | 26.9 ± 0.99 d | 6.30 ± 0.20 d | 5.49 ± 4.24 b | 8.10 +/−2.15 b |
| AH Plus Bioc | 30.38 +/− 0.01 e | 6.58 +/− 0.59 b | 4.54 ± 2.02 b | 4.49 +/− 1.51 ab |
| pH | ||||
|---|---|---|---|---|
| Sealers | 3 h | 24 h | 72 h | 168 h |
| P1 | 6.86 ± 0.008 ABab | 6.86 ± 0.007 Aa | 6.88 ± 0.019 Bab | 6.87 ± 0.015 ABa |
| P2 | 6.87 ± 0.011 ABabc | 6.87 ± 0.003 ACac | 6.88 ± 0.008 Bab | 6.88 ± 0.010 ABac |
| P3 | 6.86 ± 0.005 Aa | 6.86 ± 0.006 ACac | 6.88 ± 0.015 Aa | 6.88 ± 0.043 Aac |
| AH Plus Jet | 6.88 ± 0.005 Aac | 6.87 ± 0.011 Aac | 6.86 ± 0.007 Ab | 6.87 ± 0.027 Aac |
| Bio C Sealer | 7.15 ± 0.012 Aa | 7.00 ± 0.011 Bbd | 7.25 ± 0.008 Cc | 7.04 ± 0.050 Bb |
| AH Plus Bioceramic | 6.88 ± 0.006 Ac | 6.85 ± 0.013 Ba | 6.88 ± 0.009 Aab | 6.91 ± 0.009 Cc |
| Control | 6.87 ± 0.013 Aac | 6.87 ± 0.013 Ac | 6.87 ± 0.013 Aab | 6.87 ± 0.013 Aac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales de Oliveira Neto, R.; Ferreira da Silva, G.; Carvalho Moreira da Veiga, T.; Jovedi Rosa, S.; de Souza, B.S.W.; Vivan, R.R.; Alcalde, M.P.; Duarte, M.A.H. Studying How Calcium Silicate and Radiopacifier Proportions Affect the Physicochemical Properties of Endodontic Calcium Silicate-Based Sealers. Materials 2025, 18, 4340. https://doi.org/10.3390/ma18184340
Sales de Oliveira Neto R, Ferreira da Silva G, Carvalho Moreira da Veiga T, Jovedi Rosa S, de Souza BSW, Vivan RR, Alcalde MP, Duarte MAH. Studying How Calcium Silicate and Radiopacifier Proportions Affect the Physicochemical Properties of Endodontic Calcium Silicate-Based Sealers. Materials. 2025; 18(18):4340. https://doi.org/10.3390/ma18184340
Chicago/Turabian StyleSales de Oliveira Neto, Raimundo, Guilherme Ferreira da Silva, Tany Carvalho Moreira da Veiga, Stefani Jovedi Rosa, Brenda Stefhany Wilchenski de Souza, Rodrigo Ricci Vivan, Murilo Priori Alcalde, and Marco Antonio Hungaro Duarte. 2025. "Studying How Calcium Silicate and Radiopacifier Proportions Affect the Physicochemical Properties of Endodontic Calcium Silicate-Based Sealers" Materials 18, no. 18: 4340. https://doi.org/10.3390/ma18184340
APA StyleSales de Oliveira Neto, R., Ferreira da Silva, G., Carvalho Moreira da Veiga, T., Jovedi Rosa, S., de Souza, B. S. W., Vivan, R. R., Alcalde, M. P., & Duarte, M. A. H. (2025). Studying How Calcium Silicate and Radiopacifier Proportions Affect the Physicochemical Properties of Endodontic Calcium Silicate-Based Sealers. Materials, 18(18), 4340. https://doi.org/10.3390/ma18184340






