To facilitate sample description, the project team specifies the abbreviation rules for sample categories as follows: the three levels of acetic acid contamination are defined as strong acid (S), weak acid (W), and no acid (N); current and salt spray are denoted by numerical values; and the number of exposure days is also represented by a numerical value. For example, “S-3-10-40” indicates a sample exposed for 40 days under test conditions of salt-spray level 10, contamination level 8, and current level 3.
3.1. Macroscopic Evolution of Corrosion Morphology
Table 2 presents the surface morphology of nine sample groups across different time periods. Visual inspection revealed a progressive dulling of the metallic luster. First, under fixed environmental and test parameters, it is evident that the surface condition undergoes significant changes with increasing exposure time: the metallic luster gradually disappears, the surface becomes increasingly rough, and some samples exhibit white rust or even red rust. Notably, the white rust cannot be washed off by water, ruling out salt deposition. Second, comparing the surface states of samples exposed for 40 days, the condition generally shows a positive correlation with the salt-spray level. When the salt-spray level is 10, the sample surfaces exhibit substantial white rust formation. In comparison, the influence of variations in acidic contamination is relatively minor, whereas the effect of the leakage current is more localized, with corrosion differences occurring only near the graphite electrode contact points and having negligible impact on other areas. Specifically, when both salt spray and electric current are present, raised red corrosion products appear at the contact area between the graphite electrode and the steel foot, indicating local breakthrough of the Zn barrier and cathodic depolarization of underlying Fe. These raised areas are localized near the electrical contact zone—either directly beneath the contact point or on either side of it—and are not observed in other regions. Examples include samples S-10-0-40, N-3-5-40, and N-10-10-40, as well as W-10-5-40 and S-3-10-40. However, optical images of other regions are provided in the table below (
Table 2). In the experiment, graphite rods were selected as the electrodes. Since their potential is generally relatively positive, when they come into direct contact with active metals (such as zinc, magnesium, or aluminum) in an electrolyte environment, a galvanic cell is formed. However, by comparing samples W-0-10-40 with N-3-5-40 and W-10-5-40, it can be observed that when no current is applied, there are fewer corrosion products (essentially no protrusions) at the contact area. In contrast, samples subjected to current flow exhibit visible red protrusions, indicating that alternating current promotes electrochemical corrosion. Furthermore, in this experiment, the contact area between the graphite electrodes is extremely small, resulting in a current density at the contact point exceeding 100 A/m
2. This finding is consistent with existing research [
9,
10], which suggests that AC current density must reach a certain threshold to accelerate corrosion.
3.2. SEM/EDS Microanalysis
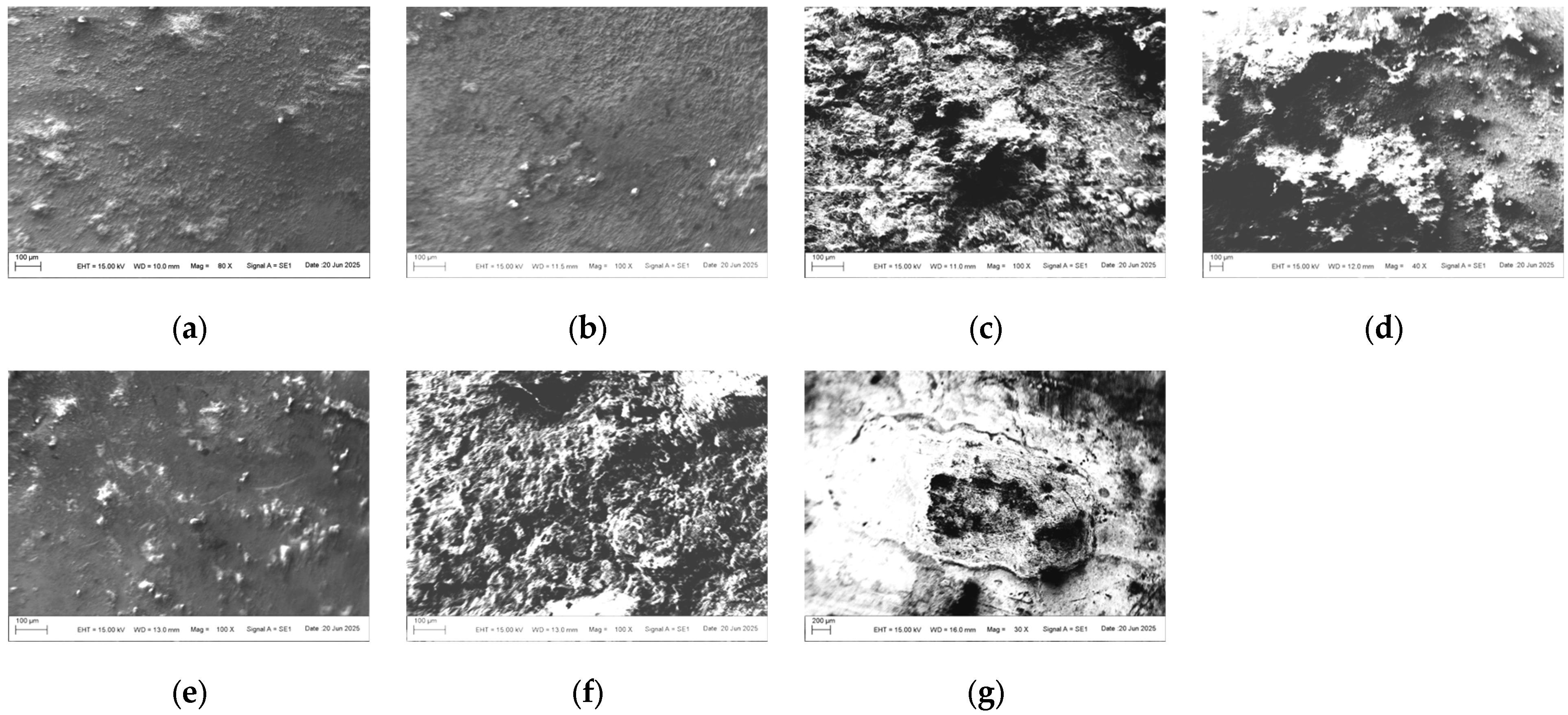
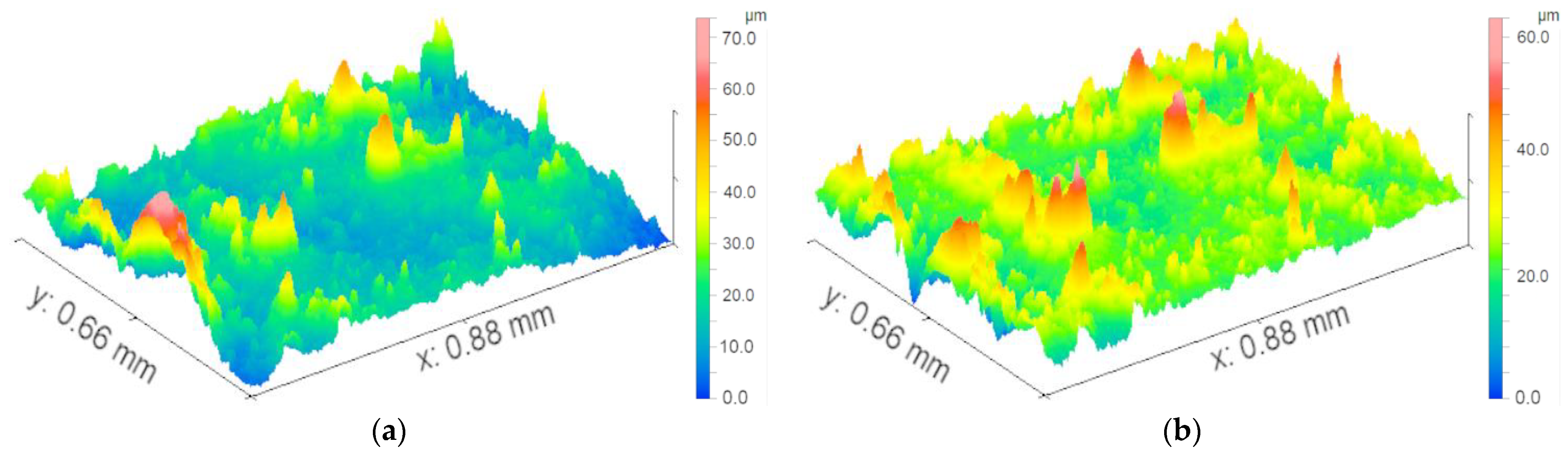
Based on the preliminary results of the optical images, we selected seven representative samples (from the 40-day exposure areas) to test the micro-morphology, as shown in
Figure 2. Regardless of the levels of contamination and electric current, the surfaces of the samples under high-salt-spray corrosion are rough and loose, with even local cavities appearing. When the salt-spray concentration is low, most areas of the sample surfaces remain smooth, indicating that chloride ions (CI
−) cause the greatest damage to the surface galvanized layer. Under high magnification, it can be clearly observed that the corrosion products at the electrical contact areas present mound-like shapes with missing tops (resembling volcanic craters). The missing shapes in the centers of individual corrosion product protrusions are speculated to match the graphite electrode rods. The surfaces of the product protrusions are smooth, but the interior of the missing areas is loose and porous.
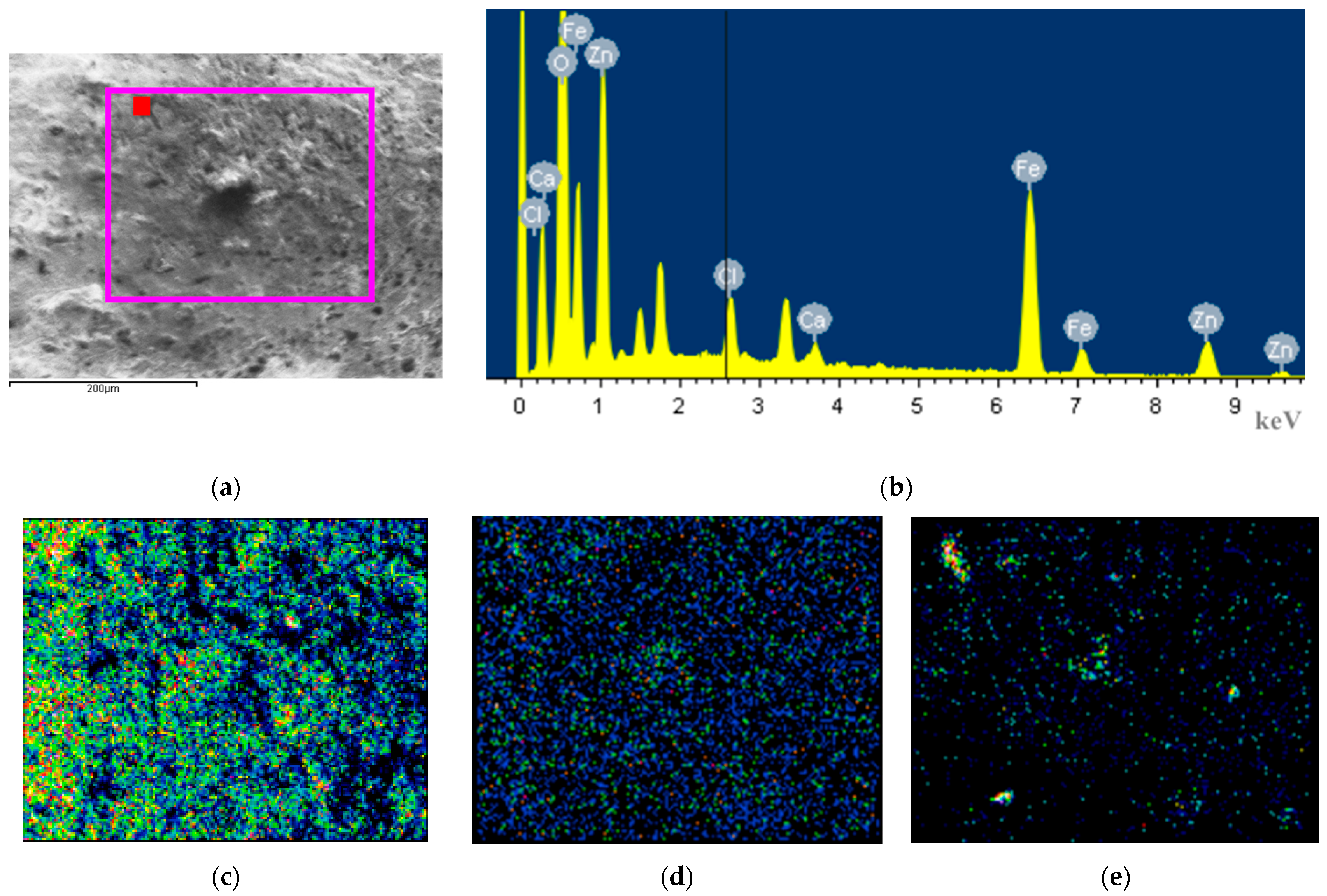
To further determine the elemental composition of the corrosion products, energy dispersive spectroscopy (EDS) point scans and area scans were conducted in the electron microscope field of view, and the results are shown in
Figure 3 and
Table 3.
Judging solely from the atomic percentage of oxygen, the values follow the following order: S-10-0-40 (72.91%) ≈ W-3-0-40 (73.09%) ≈ S-3-10-40 (71.82%) > N-3-5-40 (67.35%) ≈ S-0-5-40 (65.56%) > N-0-10-40 (59.88%) ≈ N-0-0-40 (59.03%). The proportion of oxygen content can generally serve as an indicator of the degree of oxidation reaction. However, due to the non-uniformity of surface corrosion, there will still be fluctuations and errors in oxygen content. When there is no salt spray, contamination, or electric current, and the sample is only exposed to a high-temperature (35 °C) and high-humidity (90% RH) environment, the oxygen content on the surface of hot-dip galvanized coating is around 60%. After exposure to the salt-spray environment, the Cl element content on the sample surface is not high. This may be because the corrosion products dissolve in water, causing the Cl element to be washed away, or it migrates to the loose and porous interior, resulting in a relatively low surface measurement value. The relatively low Si and Ca elements may originate from cement. A small amount of Fe element is detected on the surfaces of S-0-5-40, N-0-10-40, and S-3-10-40, proving that after 40 days of exposure to a high-temperature, high-humidity, and high-salt-spray environment, the hot-dip galvanized coating is damaged. Among them, the atomic percentage of Fe on the surface of S-0-5-40 is 1.77%.
In particular, we conducted an elemental area scan on the central position of the raised corrosion products at the electrical contact area of N-3-5-40. The atomic percentage of iron at this position reaches as high as 19.1%, which is the highest among all the tested positions. At this time, the project team reasonably speculates that the injection point of alternating current will have a synergistic effect with the salt spray, especially the Cl ions, breaking through the hot-dip galvanized protection and causing deep-seated corrosion to the internal carbon steel. Since the leakage current path of alternating-current insulators has randomness and exclusivity, that is, the path depends on the distribution of soluble conductive contaminants, and once one path is conductive, the voltage borne by other paths decreases. Therefore, it is impossible for multiple paths to conduct electricity simultaneously. This situation is highly similar to the current connection method in this test. When the current enters the steel foot at a “point”, it will also have a synergistic effect with corrosive substances, resulting in weak points in the protection.
Interpreting the foregoing results through the lens of diffusion-coupled electro-chemical kinetics reveals a multifaceted corrosion scenario. Chloride ingress not only disrupts the compactness of the passive ZnO film by forming soluble zinc hydroxy-chlorides but also participates in establishing a concentrated brine that elevates the local conductivity of surface electrolytes. When an alternating leakage current is imposed, the resultant potential oscillations exacerbate film rupture, a phenomenon inadequately captured by steady-state DC polarization frameworks. Acetic pollution, often sidelined in the corrosion narrative, acts as a pH buffer that modulates the hydrolysis rates of zincate complexes, thereby exercising a second-order but statistically significant influence on both kinetics and product morphology.
3.3. Electrochemical Impedance Spectroscopy
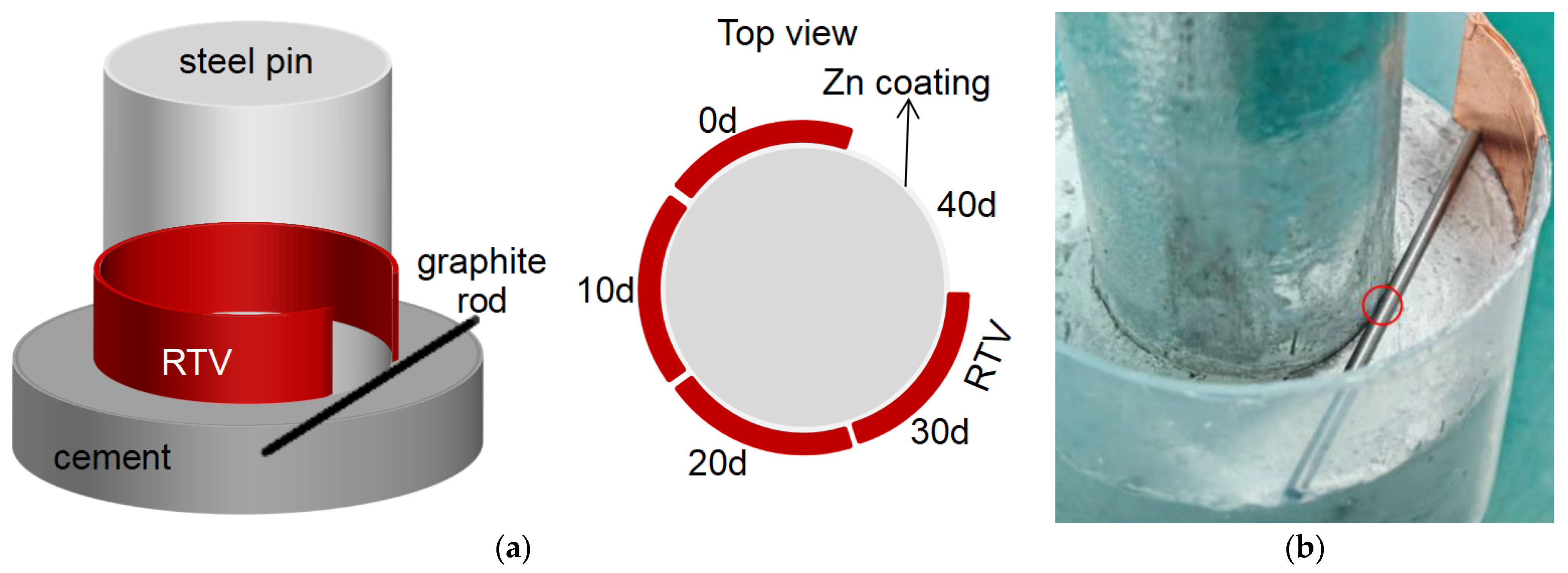
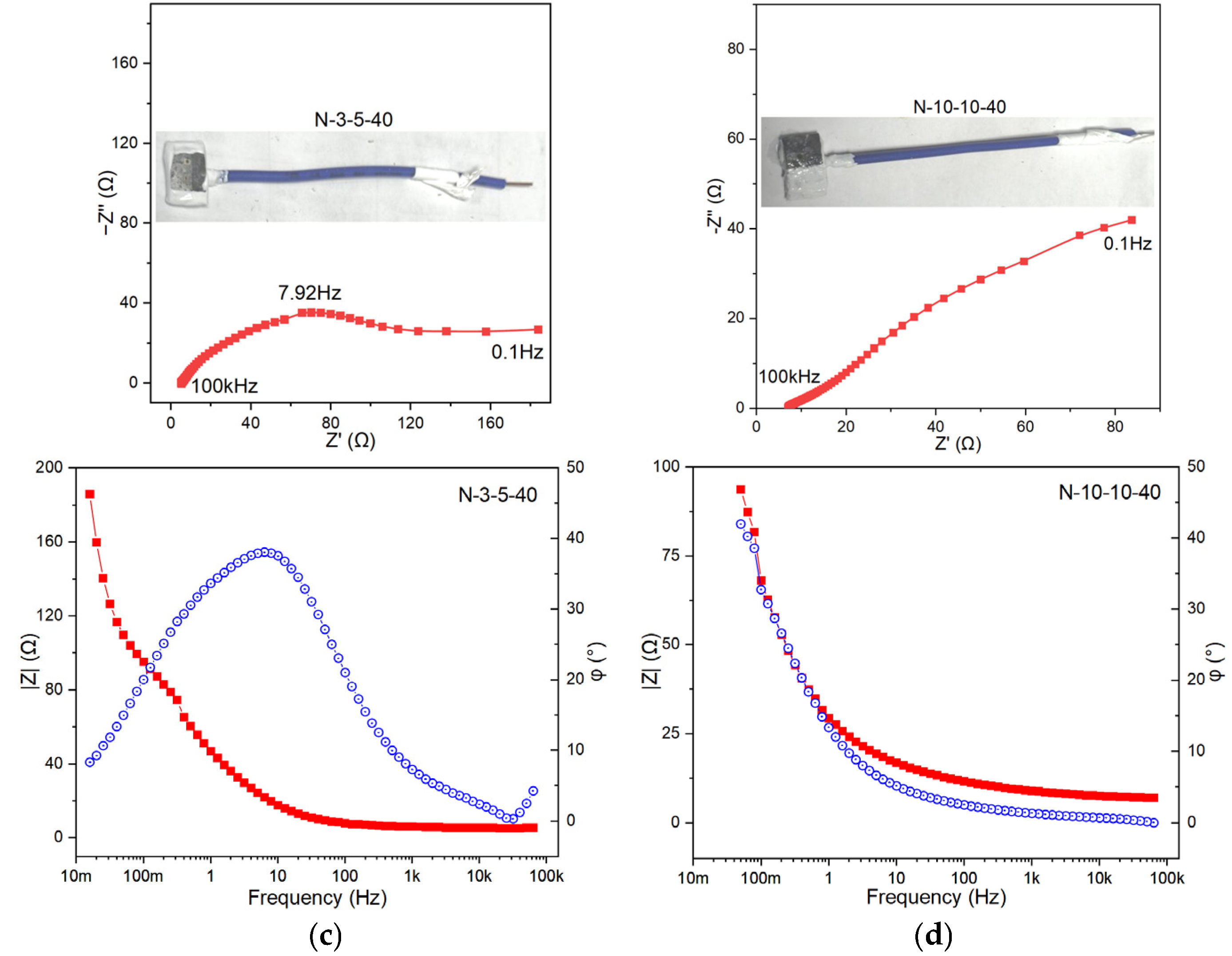
To gain an in-depth understanding of the corrosion behavior and protection performance of metals in different environments from an electrochemical perspective, four samples, namely N-0-10-40, S-10-0-40, N-3-5-40, and N-10-10-40, were selected for electrochemical impedance spectroscopy (EIS) testing. These samples cover the effects of salt spray (high, low, none), acidic contamination (high, none), and leakage current (high, low, none). Wires were welded to the back of the samples, and the redundant parts were sealed with RTV, leaving only a 1 cm × 1 cm corrosion-exposed area. The samples were then immersed in a 0.5 mol/L Na2SO4 solution for testing, and the results are as follows.
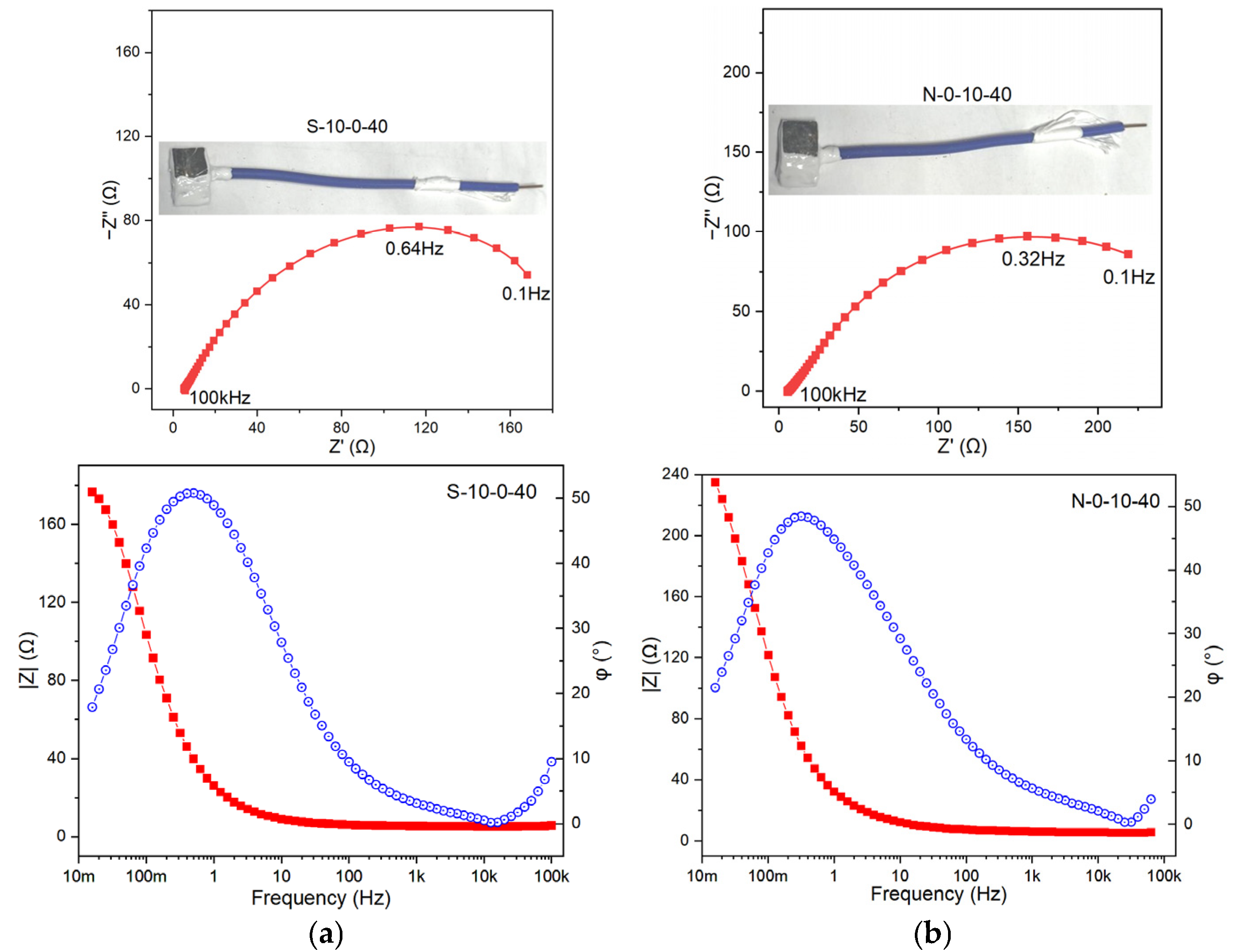
Based on the characteristics of the Bode plots in the test results (
Figure 4a–d), the |Z| (impedance modulus) of N-0-10-40 is the largest, and its phase angle reaches approximately 40°, which is a typical double-time-constant system. N-3-5-40 shows a slight inflection in the low-frequency region. The phase angle of N-10-10-40 fluctuates significantly, and the curve is unstable, suggesting a strong surface non-uniformity. The Randles equivalent circuit, the simplest and most commonly used equivalent circuit, was employed for fitting. This circuit describes the basic characteristics of the co-existence of Faraday current (charge-transfer resistance) and non-Faraday current (double-layer capacitance) at the electrode/electrolyte interface. The charge-transfer resistance (R
ct) and double-layer capacitance (C
dl) parameters were extracted to evaluate the corrosion behavior, interface state, and protective film performance. R
ct reflects the resistance of the corrosion reaction; a higher R
ct value indicates better corrosion resistance. C
dl represents the interfacial reaction activity and surface state, which are affected by factors such as surface roughness and adsorption layers. A large Cdl implies a rough surface, numerous active sites, or loose corrosion products, while a small C
dl indicates a dense surface or well-formed protective film. The R
ct values follow the order of N-0-10-40 > S-10-0-40 > N-3-5-40 > N-10-10-40. This suggests that the corrosion induced by either the electric current or the salt spray alone is limited; however, their combined effect exhibits a synergistic interaction, resulting in substantial deterioration of the protective layer. N-3-5-40 has the smallest C
dl, suggesting a relatively dense surface or weak charge-storage capacity. N-10-0-40 and N-10-10-40 have relatively high C
dl values, indicating that their interfaces may be rough or have thick adsorption layers. This implies that the influence of salt spray on the metal surface is widespread, while the effect of the electric current is more localized. Notably, localized corrosion exerts a relatively small influence on C
dl, which is consistent with the results of the surface morphology analysis.
3.4. 3D Profilometry and Corrosion Volume
To quantitatively investigate the volume of corrosion products per unit area, white-light interferometry mode combined with a 20× objective lens was employed. By manipulating the control handle and fine-tuning the Z-axis, continuous imaging was performed within a specific height range of targeted areas to acquire localized elevation data. Leveraging the optical system’s zoom functionality, the focal range was dynamically adjusted to ensure clear imaging of surface details (e.g., micron-scale corrosion pits) across regions with varying depths or curvatures, thereby meeting the measurement demands of complex morphologies. For larger regions of interest (e.g., those spanning welds or interfaces), the area was scanned in sections, and multiple localized 3D images were automatically stitched to generate comprehensive large-scale three-dimensional topographic data, eliminating data loss caused by field-of-view limitations during single-scan acquisitions.
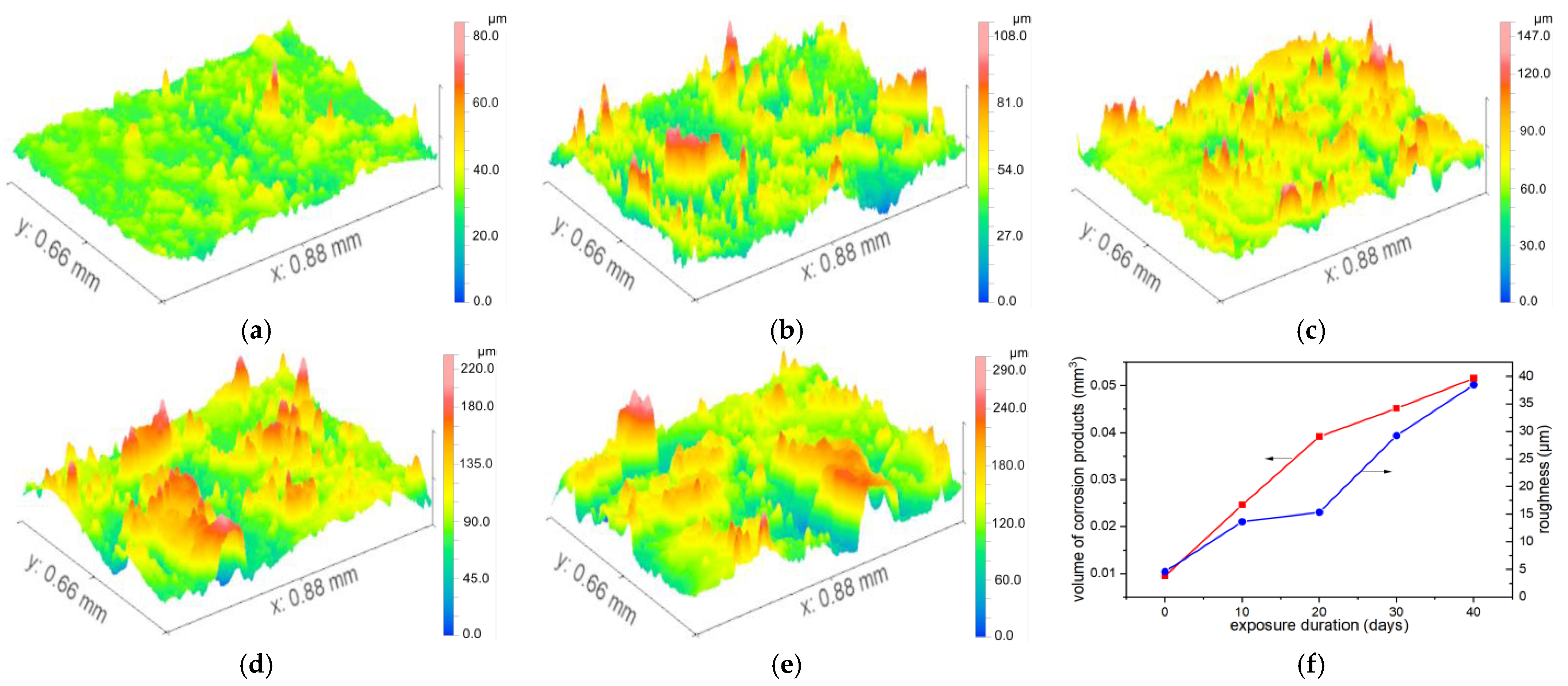
During the post-processing phase, background subtraction was performed for curved surface features (e.g., spherical or cylindrical surfaces) on the metal samples using software. A fitting model was selected based on the actual geometric shape of the sample. Given that the sample surface was cylindrical, the least-squares method was applied to fit the cylindrical surface equation (with its axis parallel to the x-axis). The fitting parameters included the coordinates of the cylinder’s central axis and its radius. The fitting algorithm was required to achieve a residual (the deviation between raw data and the fitted surface) of <5 μm to ensure the accuracy of background subtraction. By performing point-by-point subtraction between the original 3D data and the fitted surface model, a residual surface was obtained—this surface exclusively represented the corrosion morphology (i.e., the deviation of actual corrosion pits from the ideal cylindrical surface). Typical results comparing the original and background-subtracted surfaces are presented in
Figure 5. The processed images can be used to calculate morphological features such as volume, roughness and slope, as shown in
Table 4.
Taking the condition of salt spray = 10, pollution = 0, and current = 10 as an example, we analyzed the growth of corrosion volume and roughness within an area of 0.88 mm × 0.66 mm over exposure time, as shown in
Figure 6. The results revealed that the rate of corrosion product volume accumulation began to decelerate after 20 days, indicating that excessive product buildup can inhibit further corrosion progression. The growth trends of roughness and volume are basically the same. Salt spray exhibited the largest range, identifying it as the primary factor influencing corrosion volume. Salt spray elevates electrolyte concentration, facilitating the electron-loss process of the anode metal and thereby accelerating corrosion reactions. Current ranked second, manifesting as a linear increase in the anode dissolution rate. Contamination showed the smallest range, suggesting it acts primarily as an auxiliary factor with dual effects: physical shielding and catalyzing the formation of localized micro-cells. The
p-values for salt spray, alternating current, and contamination were all significantly <0.001, statistically confirming their strong positive impacts on corrosion volume.
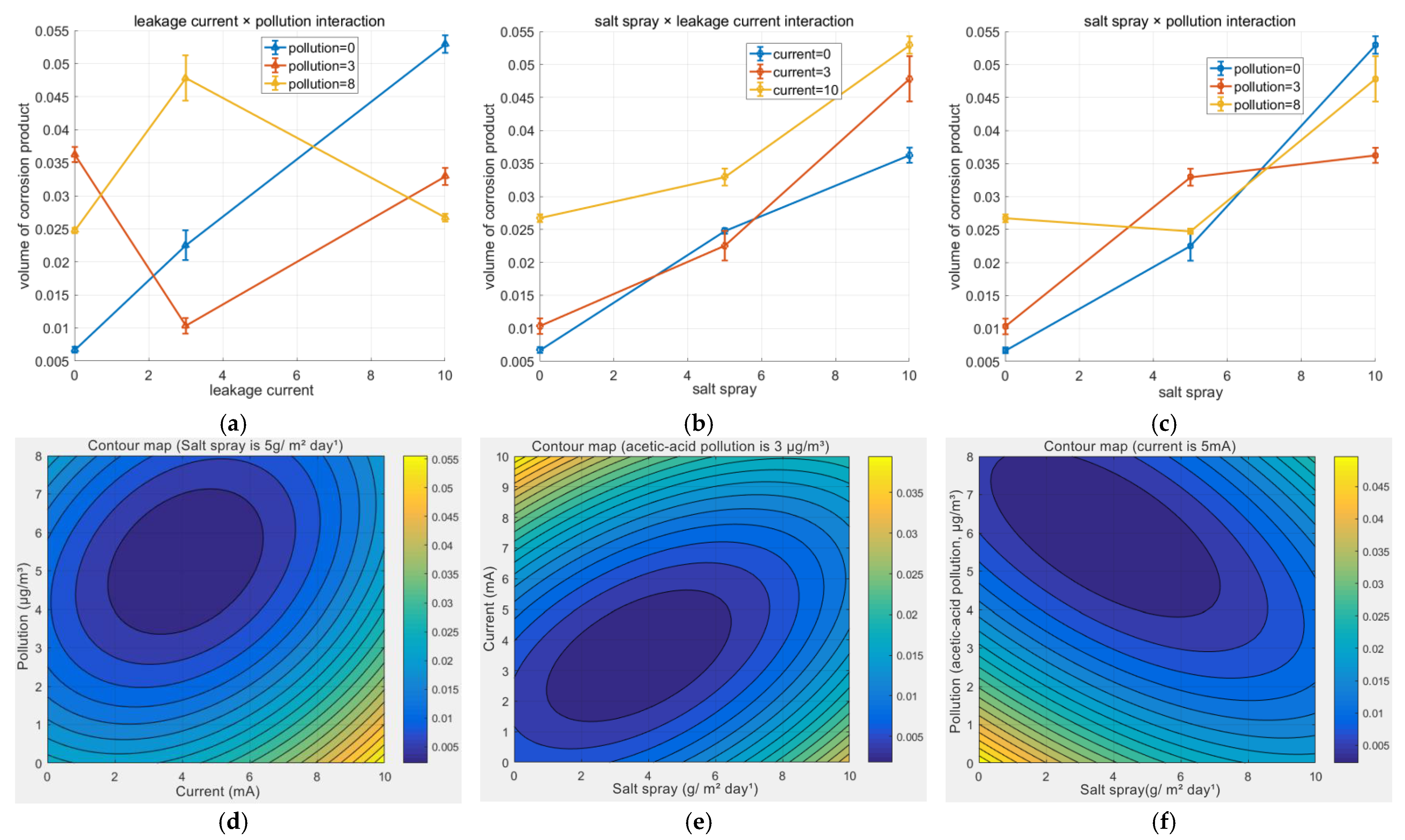
As shown in
Figure 7a–c, the interaction between salt spray and current was the most pronounced; their combined increase led to a nonlinear synergistic amplification of corrosion volume. The interaction between salt spray and pollution indicated that under high-salt-spray conditions, the barrier effect of contamination weakened. The interaction between current and pollution demonstrated that even under dominant current influence, contamination retained its regulatory role. These interaction effects collectively prove that the corrosion process is far from a simple linear superposition of individual factors. Surface roughness increased quasi-linearly with exposure time (R
2 = 0.91) until 20 days, then plateaued. Additionally,
Figure 7d–f display the corresponding contour plots, which depict the link between couple factors more clearly.
Finally, based on the result data, we used a full quadratic regression model that included main effects, quadratic terms, and interaction terms to predict the corrosion volume.
Here, Y represents the corrosion volume, X1 stands for salt spray, X2 represents current, and X3 is pollution. Salt spray has the most significant and strongly positive impact on corrosion volume. Current is the second-most influential factor, also positively promoting corrosion. Contamination also shows a positive effect, but with a relatively small coefficient, indicating its weak independent influence. The quadratic terms of salt spray and current are negative, demonstrating the law of diminishing marginal returns (i.e., when their levels are excessively increased, corrosion no longer grows linearly). The quadratic term of contamination is nearly zero, presenting a linear trend. The positive interaction term between salt spray and current is the largest, suggesting that the combination of high salt spray and high current will synergistically amplify corrosion. As mentioned earlier, the damage caused by electric current to the metal surface primarily stems from locally high current density. Such localized high current density significantly accelerates the oxidation reaction of the metal, leading to the breakdown of the passive film and the formation of an initiation site for pitting corrosion. Subsequently, Cl− ions from the environment preferentially accumulate and penetrate into this damaged area, further intensifying metal dissolution and promoting the nucleation and propagation of pitting corrosion. The interaction effects between salt spray and contamination, as well as between current and contamination, are relatively small but cannot be completely ignored.