The Influence of Heat Treatment Parameters on the Microstructure and Hardness of High-Chromium Alloy Steel X46Cr13
Abstract
1. Introduction
2. Materials and Methods
- Austenitizing temperature Tγ = 950–1150 °C, in 50 °C increments;
- Holding time in Tγ:tγ = 150–750 s, in 150 s increments;
- Cooling rate from Tγ:Vcooling depended on the use of different cooling media, i.e., still air (A), oil (O), and water (W).
3. Results and Discussion
4. Conclusions
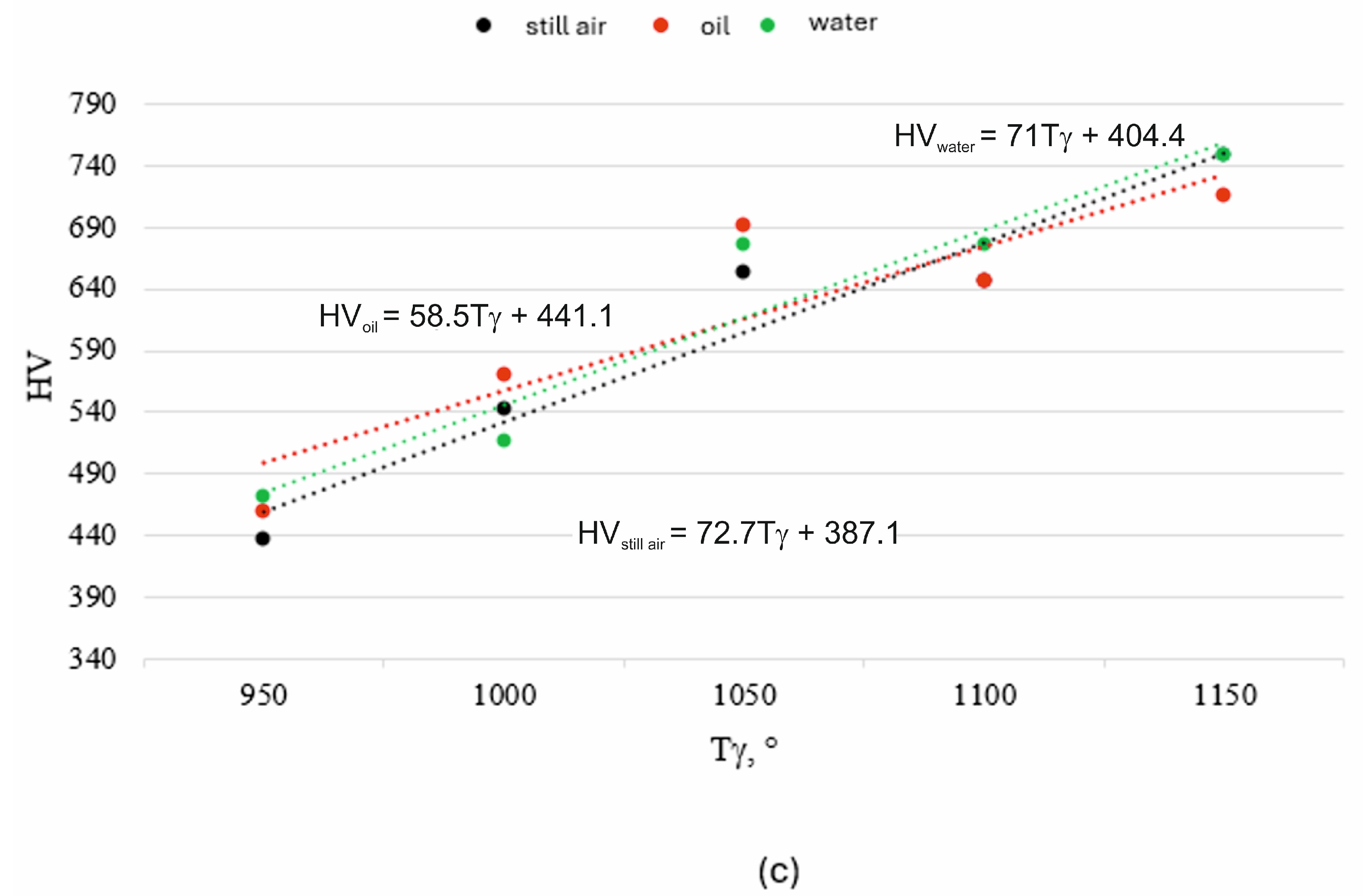
- An increase in austenitizing temperature and time leads to higher hardness of the high-chromium alloy steel X46Cr13, regardless of the quenching medium used in the hardening process.
- The highest hardness of the 5 mm-thick high-chromium alloy steel X46Cr13 sheet—approximately 750 HV (62 HRC)—was achieved through air and water hardening at an austenitizing temperature and time of 1150 °C and 750 s, respectively. Therefore, for further considerations regarding layered casting technology, the value of 62 HRC will be adopted as the maximum achievable hardness.
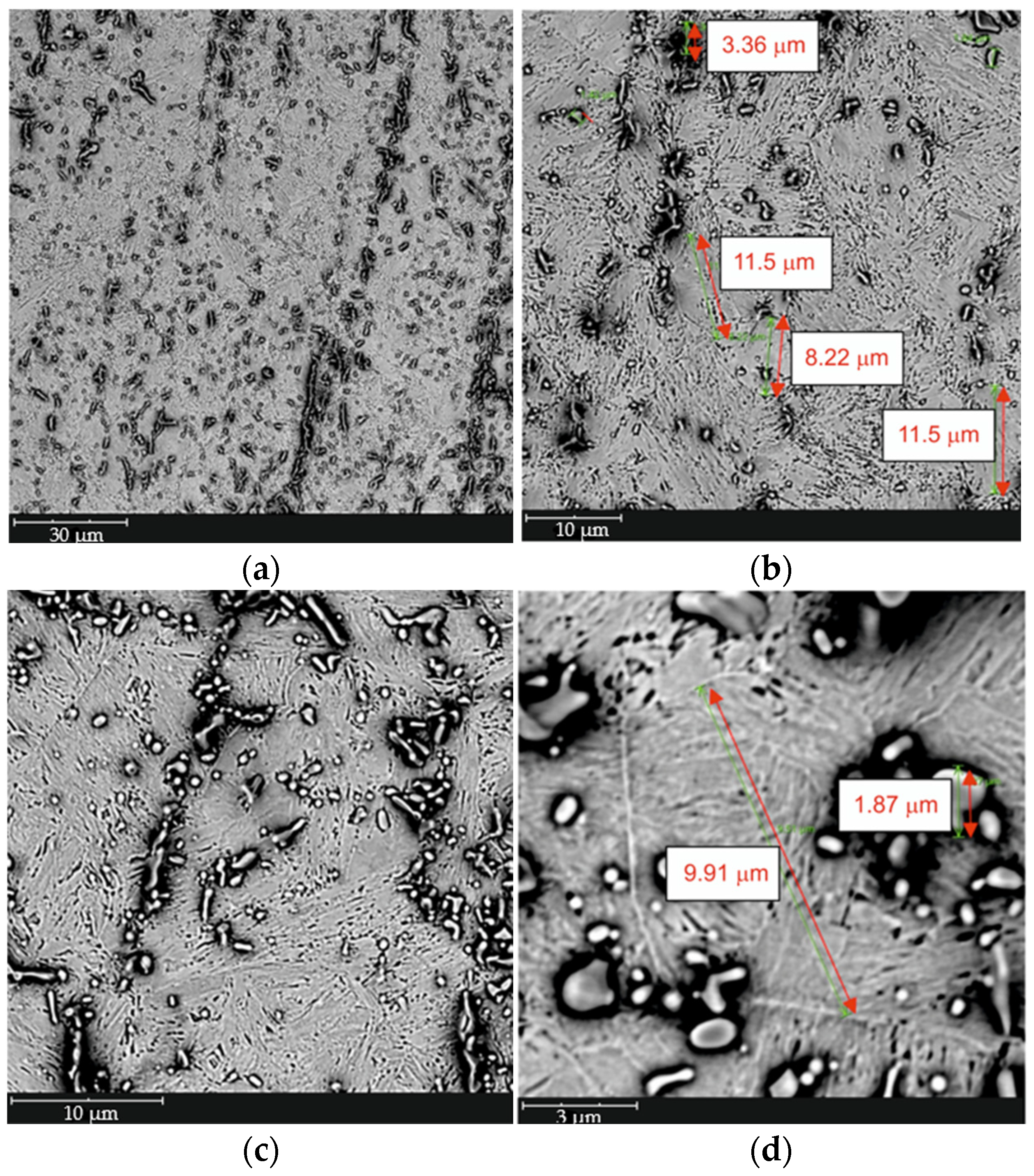
- Proper selection of hardening parameters for the high-chromium alloy steel X46Cr13 allows the formation of a favorable microstructure composed of Cr and Fe carbides in a martensitic matrix, which is desirable in terms of achieving high hardness.
- Due to the negligible influence of the quenching medium on the hardness of high-chromium alloy steel X46Cr13 in the analyzed heat treatment, air hardening is recommended, as it reduces quenching-induced stresses compared to water hardening.
- The developed heat treatment variant can be applied in the production of cutting tools or casting molds made of X46Cr13 steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, X.; Zheng, Z.; Liu, T.; Long, J.; Wang, S.; Zhang, H.; Zheng, K. Achieving high impact–abrasion–corrosion resistance of high–chromium wear–resistant steel via vanadium additions. J. Mater. Res. Technol. 2024, 29, 2425–2436. [Google Scholar] [CrossRef]
- Shwetha, K.M.; Praveen, B.M.; Devendra, B.K. A review on corrosion inhibitors: Types, mechanisms, electrochemical analysis, corrosion rate and efficiency of corrosion inhibitors on mild steel in an acidic environment. Results Surf. Interfaces 2024, 16, 100258. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control, 5th ed.; John Wiley & Sons: New York, NJ, USA, 2025; pp. 1–8. [Google Scholar]
- Adamczyk, J. Metallic Materials Engineering; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2004; pp. 17–34. (In Polish) [Google Scholar]
- Brytan, Z. Stainless Steel Handbook; Stowarzyszenie Stal Nierdzewna: Warszawa, Poland, 2018; pp. 6–10. (In Polish) [Google Scholar]
- Yuan, J.; Li, P.; Zhang, H.; Yin, S.; Xu, M. Electrochemical Characteristics and Corrosion Mechanisms of High-Strength Corrosion-Resistant Steel Reinforcement under Simulated Service Conditions. Metals 2024, 14, 876. [Google Scholar] [CrossRef]
- Blicharski, M. Material Engineering; WNT: Warszawa, Poland, 2004; pp. 282–306. (In Polish) [Google Scholar]
- Staub, F.; Adamczyk, J.; Cieślakowa, Ł.; Gubała, J.; Maciejny, A. Metallography; Śląskie Wydawnictwo Techniczne: Katowice, Poland, 1994; pp. 433–451. [Google Scholar]
- Raja, V.B.; Palanikumar, K.; Renish, R.R.; Babu, A.G.; Varma, J.; Gopal, P. Corrosion resistance of corten steel–A review. Mater. Today Proc. 2021, 46, 3572–3577. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Wang, D.; Wu, M. Improved corrosion resistance of a new 6% Cr steel in simulated concrete pore solution contaminated by chlorides. Corros. Sci. 2020, 174, 108851. [Google Scholar] [CrossRef]
- Murkute, P.; Pasebani, S.; Isgor, O.B. Production of corrosion-resistant 316L stainless steel clads on carbon steel using powder bed fusion-selective laser melting. J. Mater. Process. Technol. 2019, 273, 116243. [Google Scholar] [CrossRef]
- Bösing, I.; Cramer, L.; Steinbacher, M.; Zoch, H.W.; Thöming, J.; Baune, M. Influence of heat treatment on the microstructure and corrosion resistance of martensitic stainless steel. AIP Adv. 2019, 9, 65317. [Google Scholar] [CrossRef]
- Dalmau, A.; Richard, C.; Igual–Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Shahriari, A.; Ghaffari, M.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Amirkhiz, B.S.; Mohammadi, M. Corrosion resistance of 13wt.% Cr martensitic stainless steels: Additively manufactured CX versus wrought Ni-containing AISI 420. Corros. Sci. 2021, 184, 109362. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Fan, Y.; Zhang, T.; Dong, B.; Chen, L.; Wang, Y. Influence of microstructure on the corrosion behavior of super 13Cr martensitic stainless steel under heat treatment. Mater. Charact. 2021, 175, 111066. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.F.; Man, C.; Hu, Y.B.; Yu, Q.; Li, X.G. Effect of microstructure on corrosion behavior of high strength martensite steel—A literature review. Int. J. Miner. Metall. Mater. 2021, 28, 754–773. [Google Scholar] [CrossRef]
- Ramadan, M. Interface characterization of Bimetallic Casting with 304 Stainless Steel Surface Layer and Gray Cast Iron Base. Adv. Mater. Res. 2015, 1120–1121, 993–998. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Wang, K.; Li, P.; Yang, X.; Tong, W. Diffusion behavior and mechanical properties of high chromium cast iron/low carbon steel bimetal. Mater. Sci. Eng. A 2018, 718, 260–266. [Google Scholar] [CrossRef]
- Wróbel, T. Characterization of bimetallic castings with an austenitic working surface layer and an unalloyed cast steel base. J. Mater. Eng. Perform. 2014, 23, 1711–1717. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Hadad, S.; Mourad, M. Effect of liquid-solid volume ratios on the interfacial microstructure and mechanical properties of high chromium cast iron and low carbon steel bimetal. Mater. Res. Express 2020, 6, 1265c2. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Z.; Wie, D.; Jiao, S.; Chen, D.; Xu, J.; Zhang, X.; Gong, D. Effects of temperature and strain rate on microstructure and mechanical properties of high chromium cast iron/low carbon steel bimetal prepared by hot diffusion-compresion bonding. Mater. Design 2014, 63, 650–657. [Google Scholar] [CrossRef]
- Ramadan, M.; Fathy, N.; Abdel Halim, K.S.; Alghamdi, A.S. New trends and advances in bi-metal casting technologies. Int. J. Adv. Appl. Sci. 2019, 6, 75–80. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, Y.; Liang, Y.; Liu, S.; Xu, R.; Tong, W. Enhanced strength and ductility of high chromium cast iron/low carbon steel bimetal by heterostructure design. Mater. Today Commun. 2023, 37, 107275. [Google Scholar] [CrossRef]
- Rosemann, P.; Kauss, N.; Müller, C.; Halle, T. Influence of solution annealing temperature and cooling medium on microstructure, hardness and corrosion resistance of martensitic stainless steel X46Cr13. Mater Corros. 2015, 66, 1068–1076. [Google Scholar] [CrossRef]
- PN-71/H-86020; Corrosion Resisting Steel (Stainless Steel and Acid Resistant Steel)—Grades. Wydawnictwa Normalizacyjne ALFA: Warszawa, Poland, 1971.
- PN-EN 10088-1:2024-06; Stainless steels—Part 1: List of Stainless Steels. Polish Committee for Standardization: Warszawa, Poland, 2024.
- PN-91/H-04350; Metallic Materials—Brinell Hardness Test. Polish Committee for Standardization: Warszawa, Poland, 1991.
- PN-91/H-04355; Metallic Materials—Rockwell Hardness Test. Scales A, B, C, D, E, F, G, H, K. Polish Committee for Standardization: Warszawa, Poland, 1991.
- Abrams Industries. Available online: https://files.abrams-industries.com/steel/pl_pl/1.4034.pdf (accessed on 8 July 2021).
- Przyszlak, N.; Piwowarski, G. Designing of X46Cr13 steel heat treatment in condition of casting mould. Arch. Foundry Eng. 2023, 23, 119–126. [Google Scholar] [CrossRef]
- PN-EN 10020:2003; Definition and Classification of Steel Grades. Polish Committee for Standardization: Warszawa, Poland, 2003.
- Garcia de Andres, C.; Caruana, G.; Alvarez, L. Control of M23C6 carbides in 0.45C–13Cr martensitic stainless steel by means of three representative heat treatment parameters. Mater. Sci. Eng. 1998, 241, 211–215. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, H.; Dong, H. Carbide evolution in high-carbon martensitic stainless cutlery steels during austenitizing. J. Mater. Eng. Perform. 2020, 29, 3868–3875. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Ren, R.; Xue, Z.; Wang, H.; Zhang, Y.; Wan, J.; Wan, J.; Chen, L.; Mu, W. Ferrite formation and decomposition in 316H austenitic stainless steel electro slag remelting ingot for nuclear power applications. Mater. Charact. 2024, 218, 114581. [Google Scholar] [CrossRef]
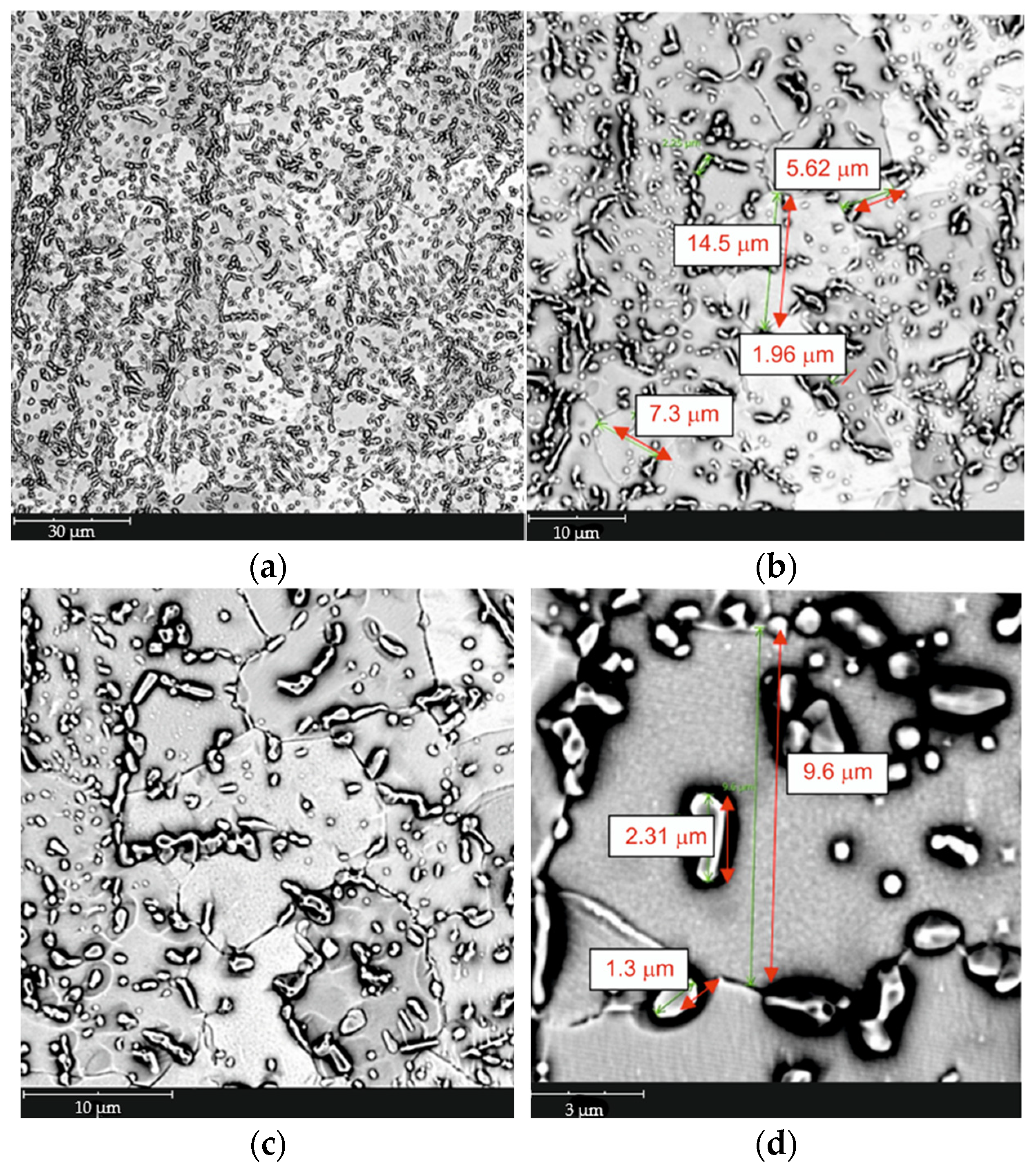
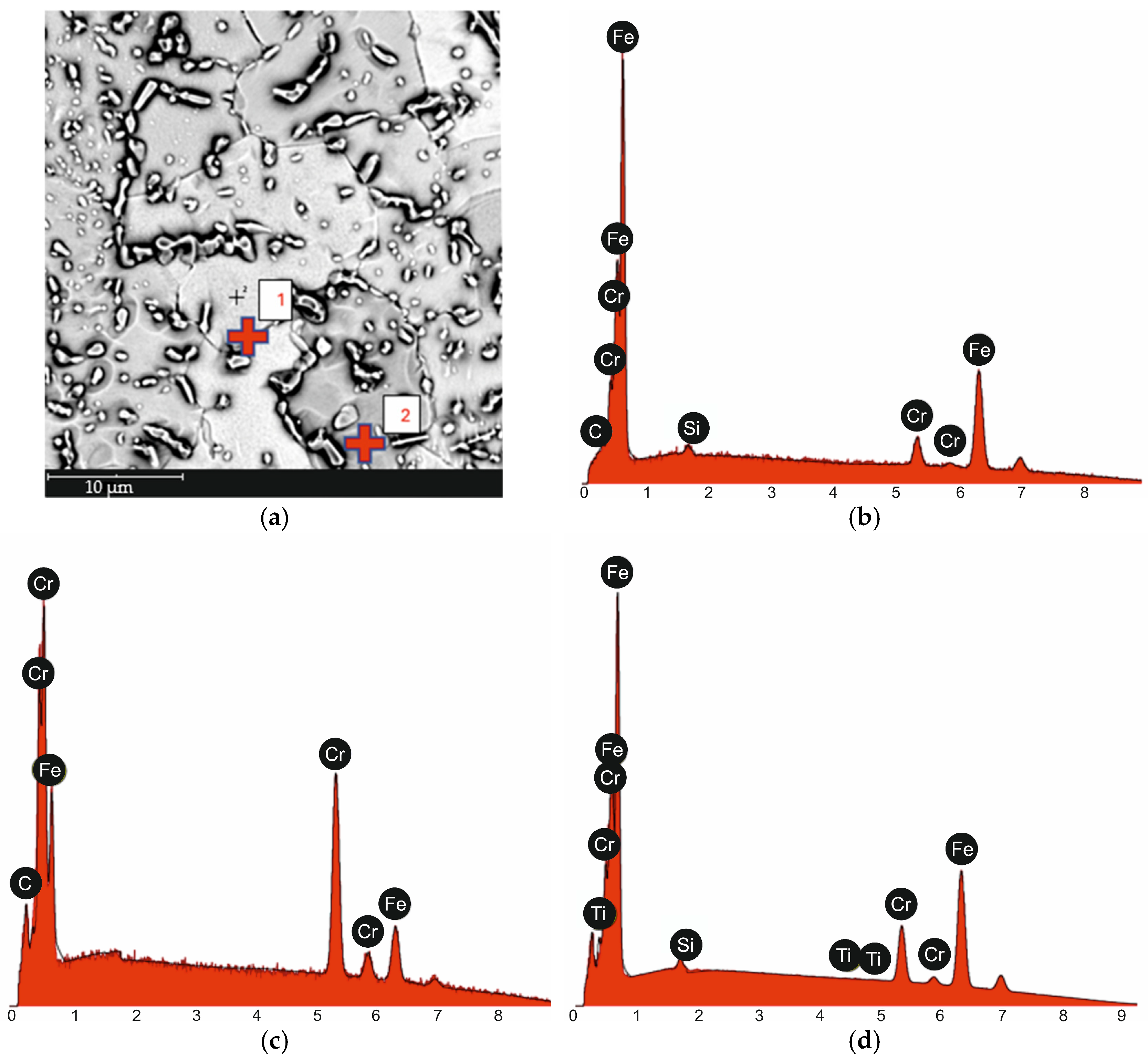
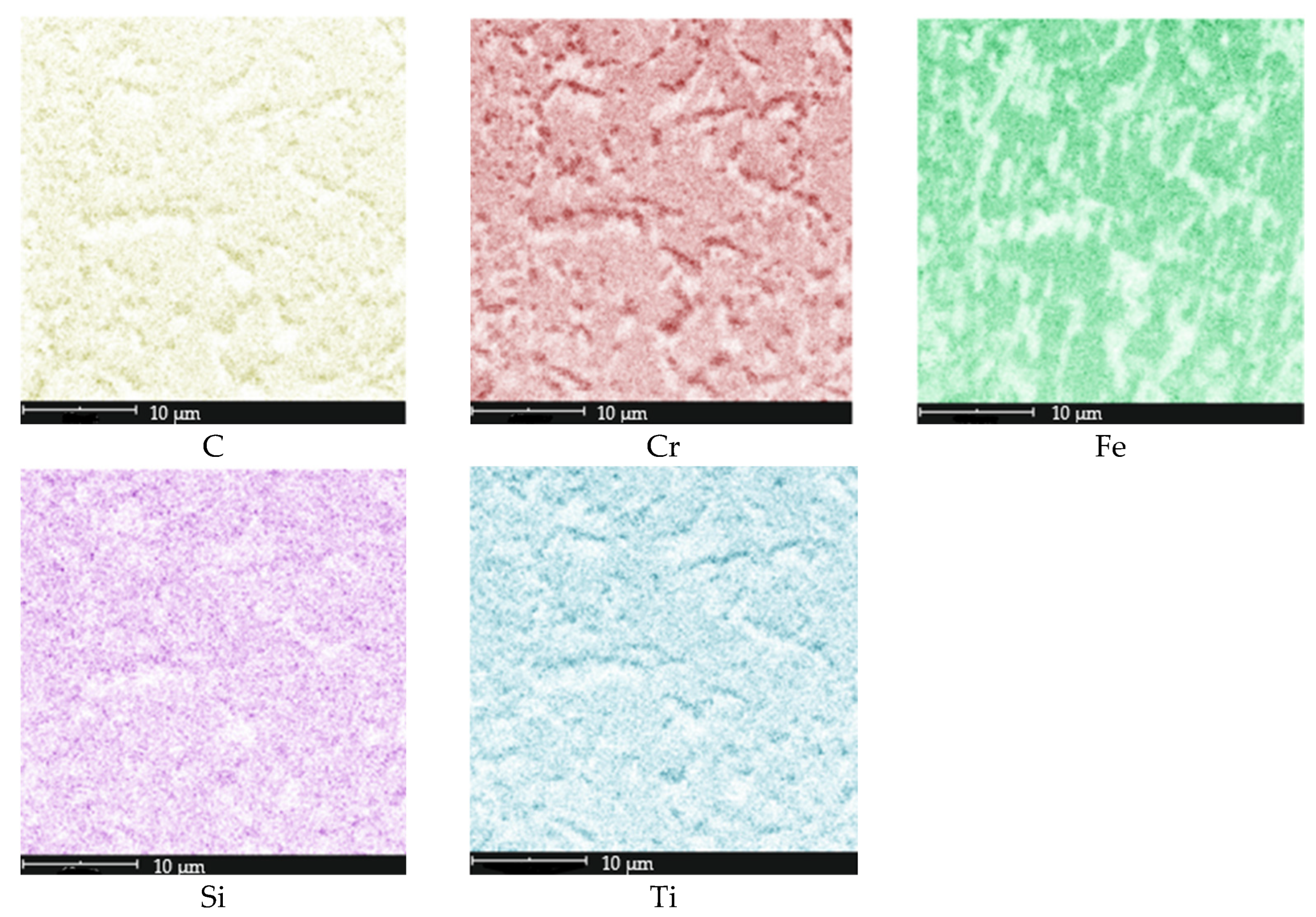
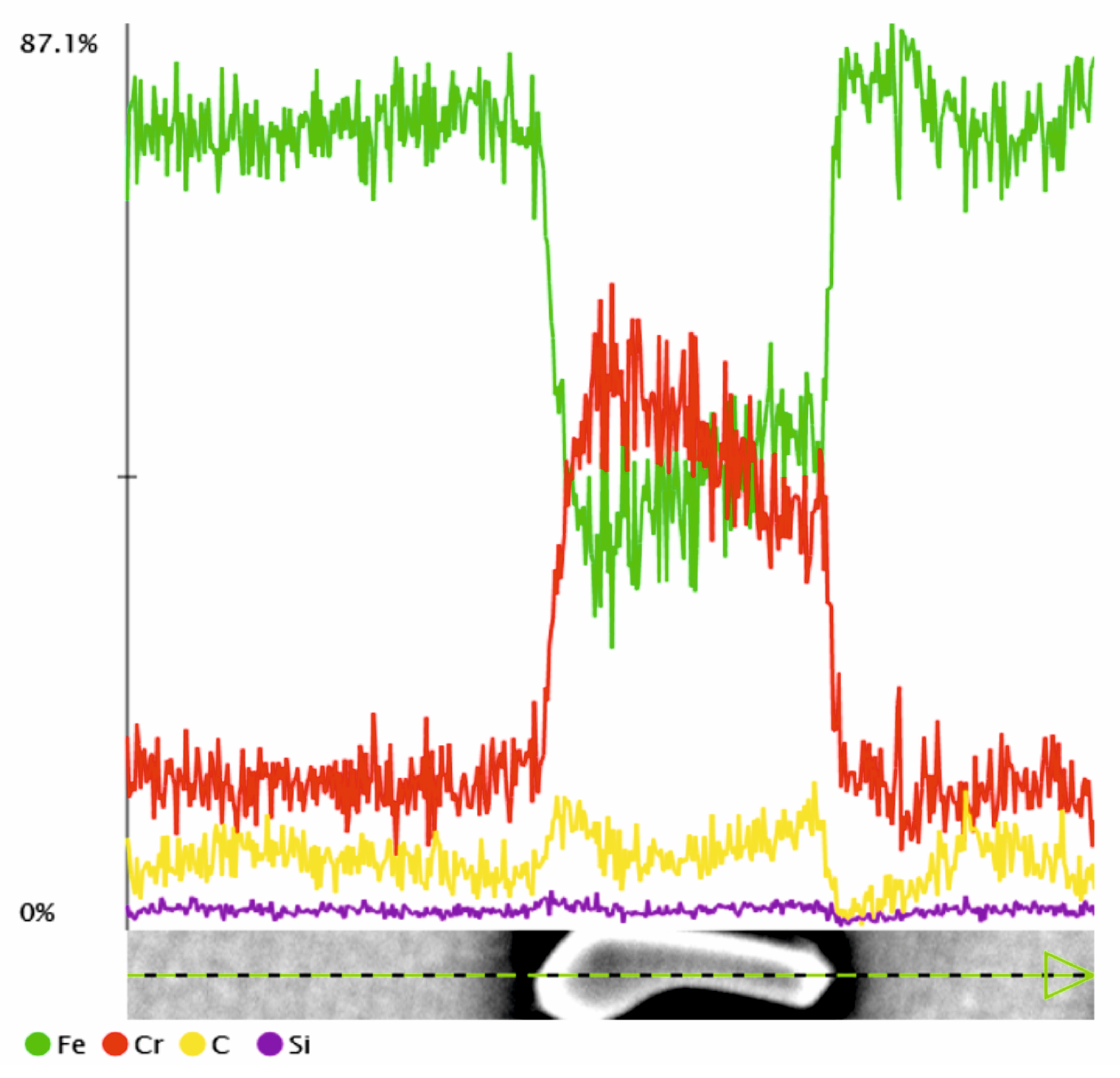

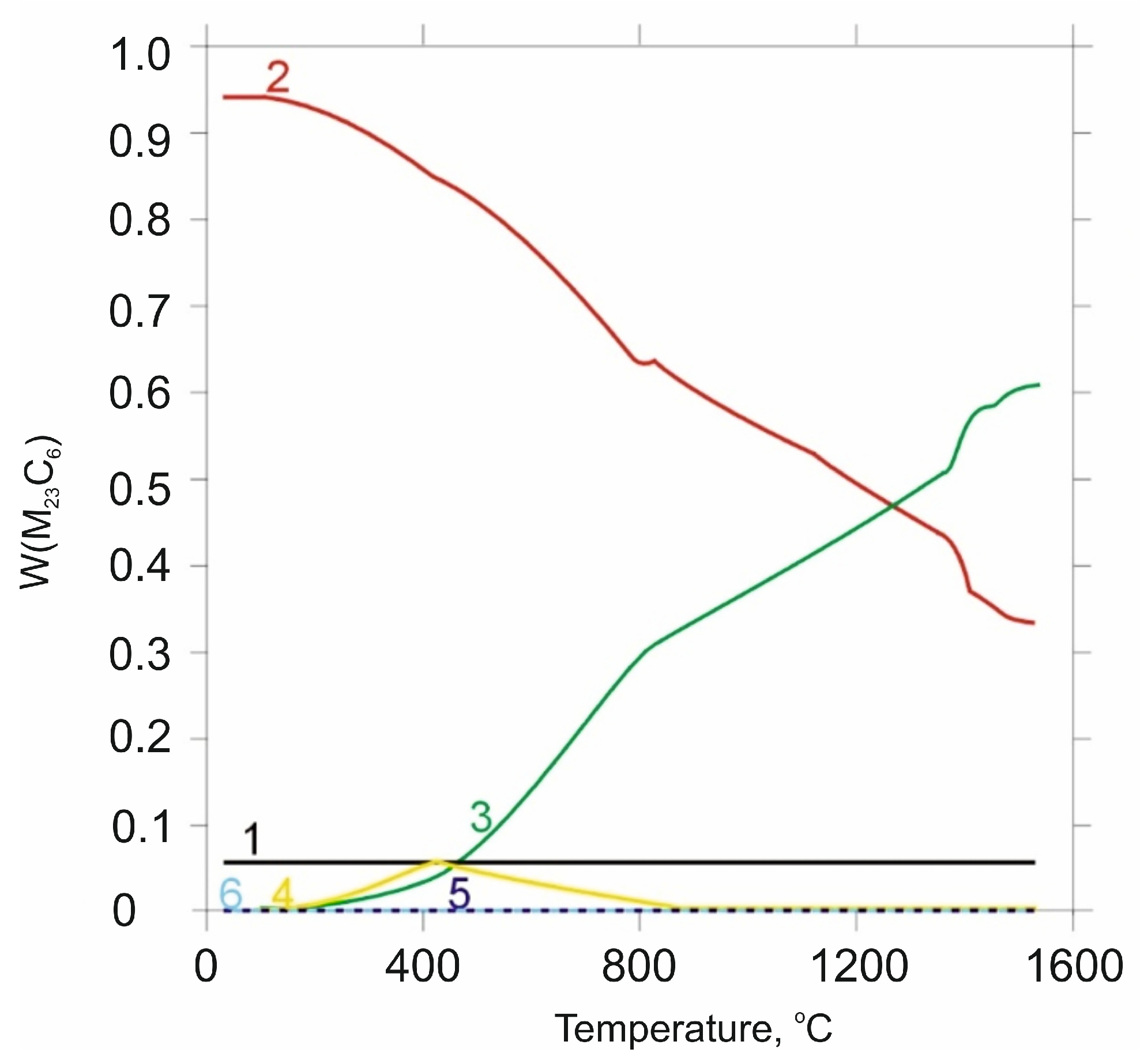
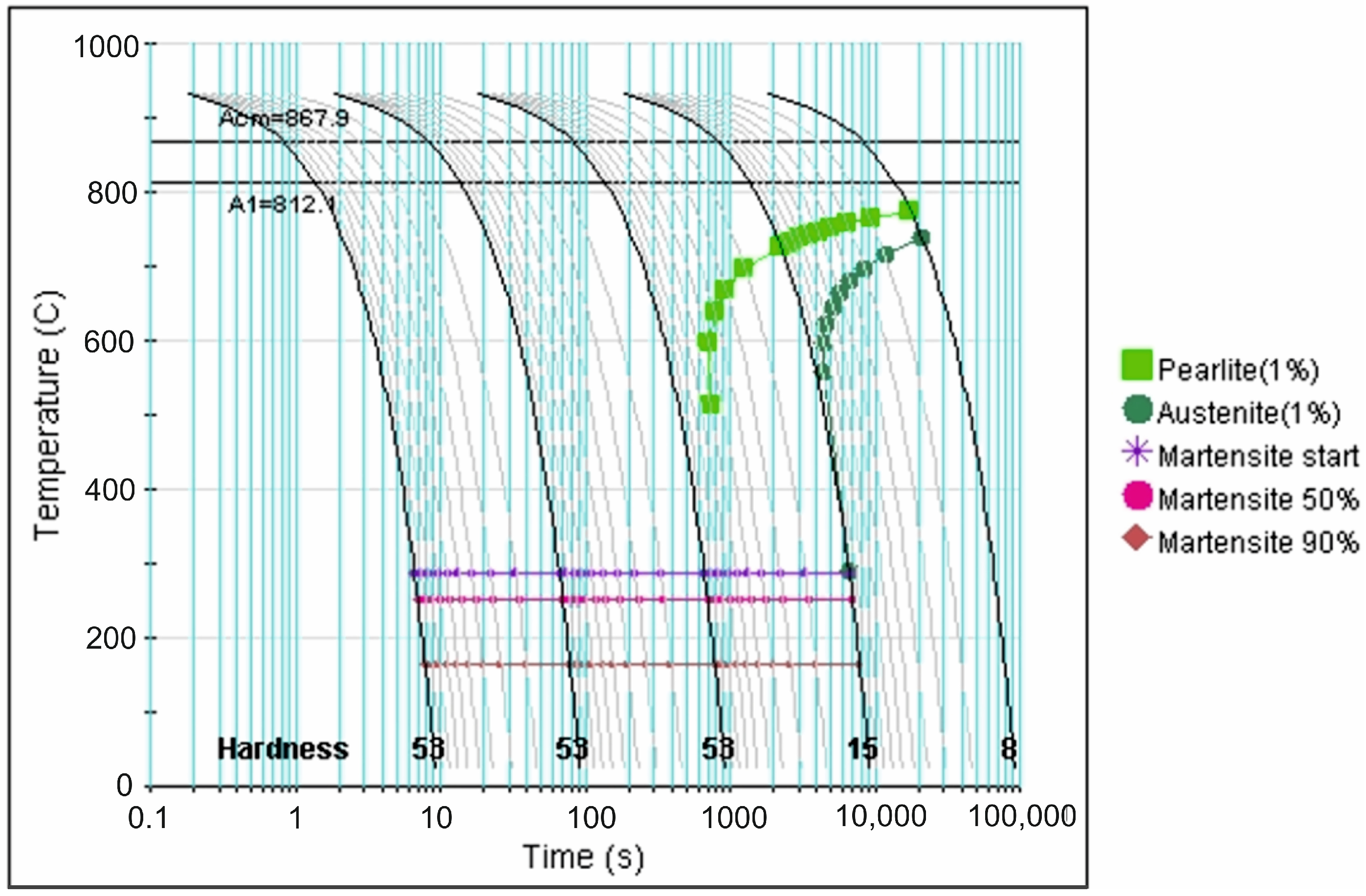


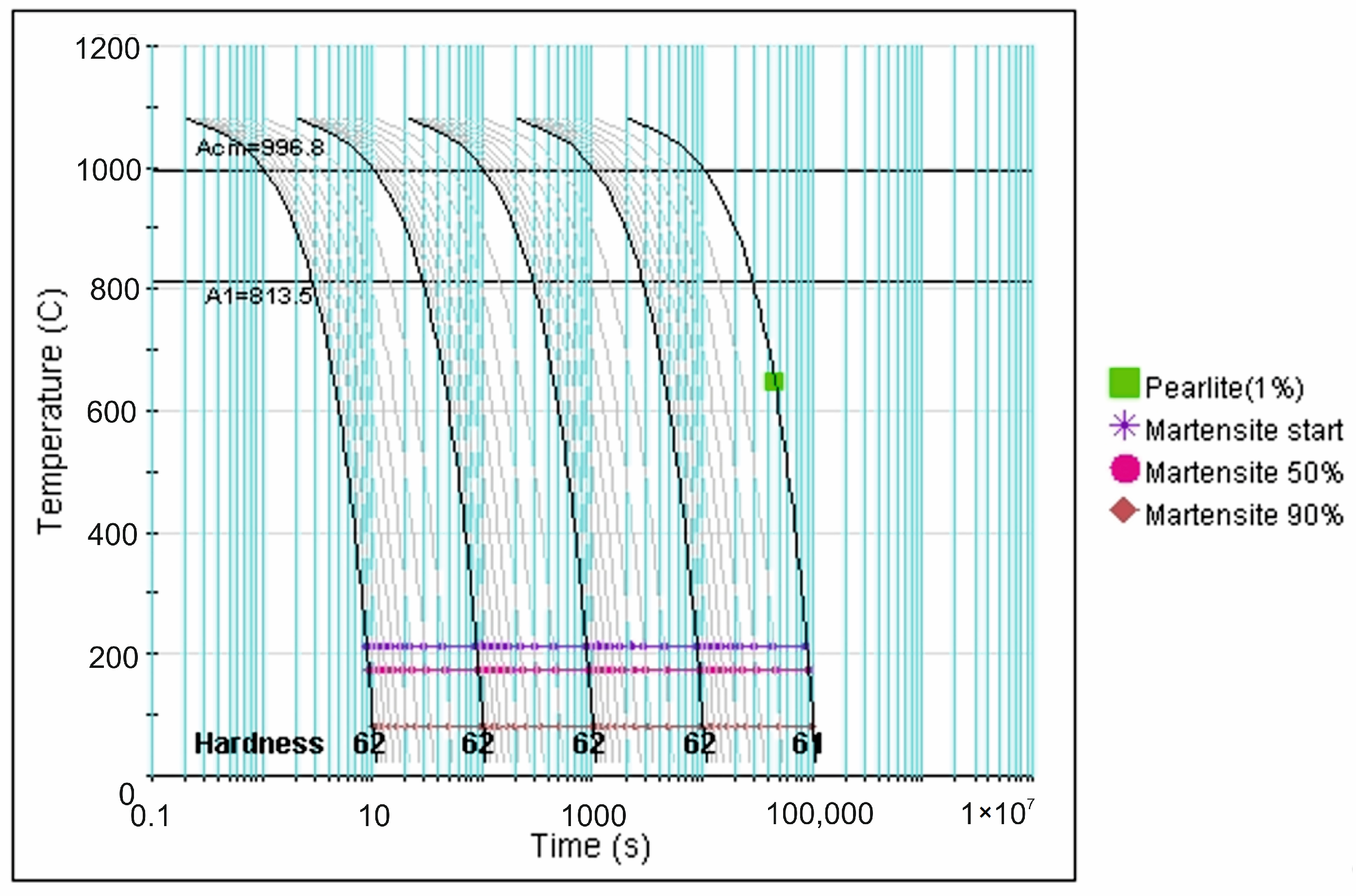
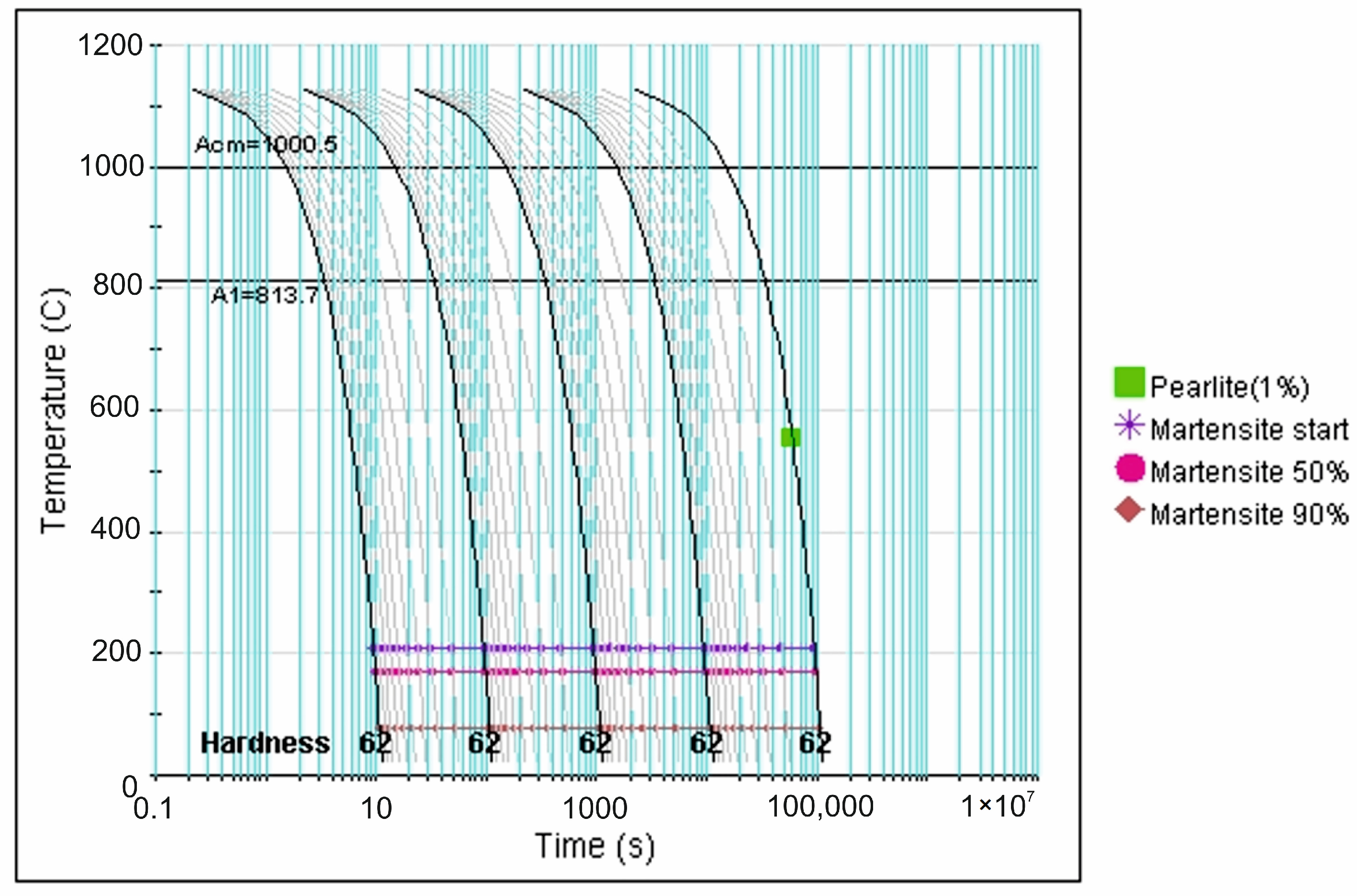
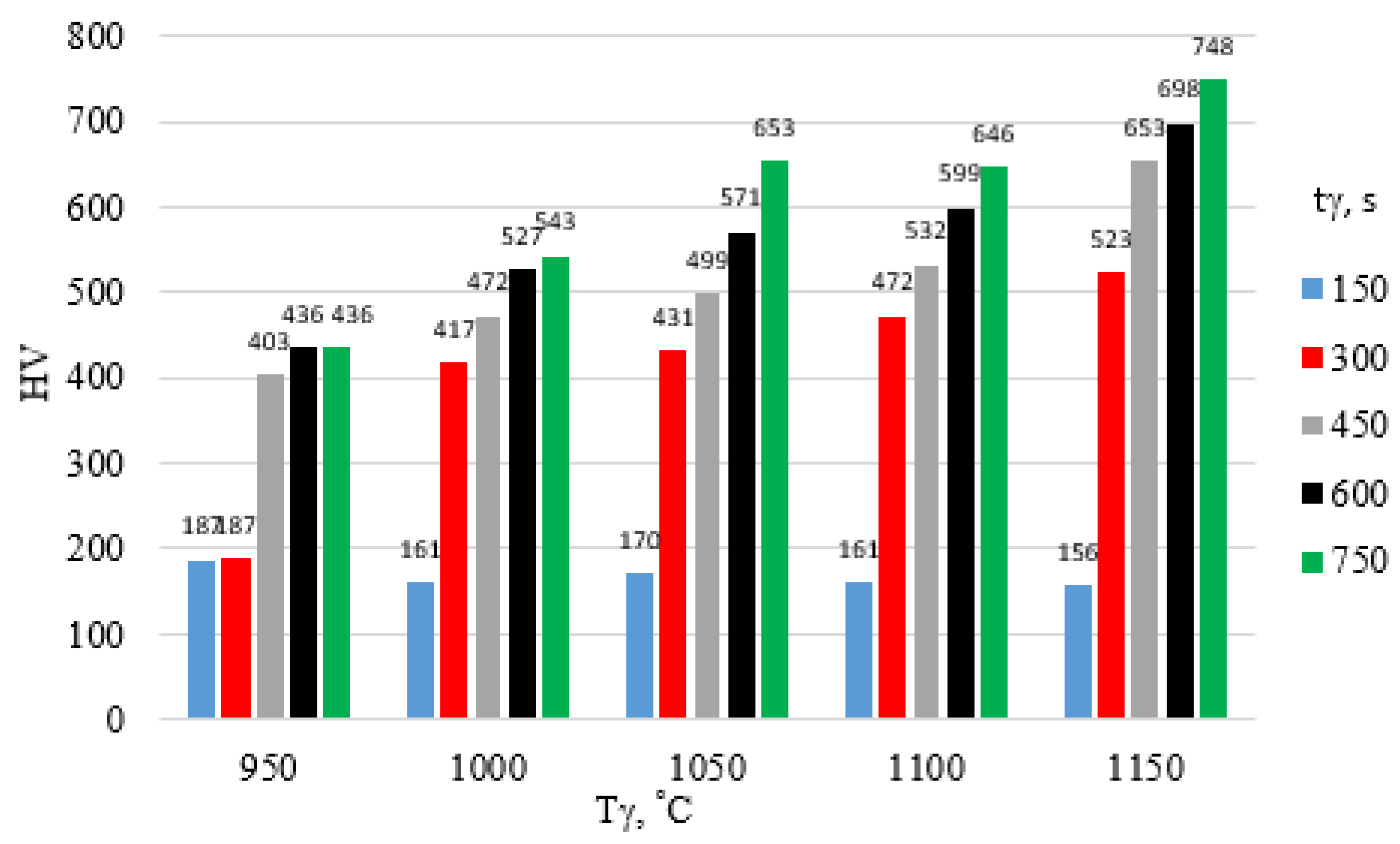
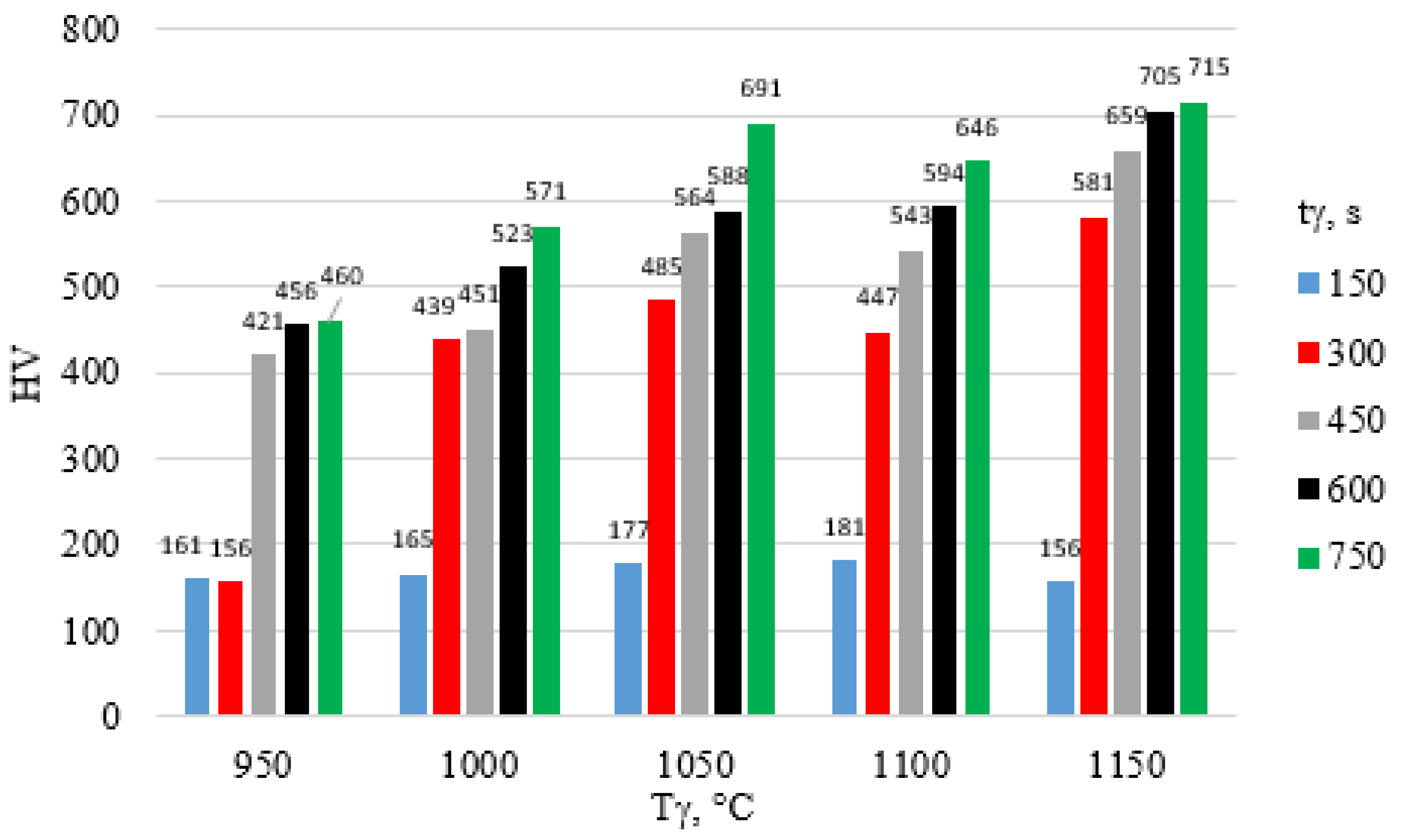
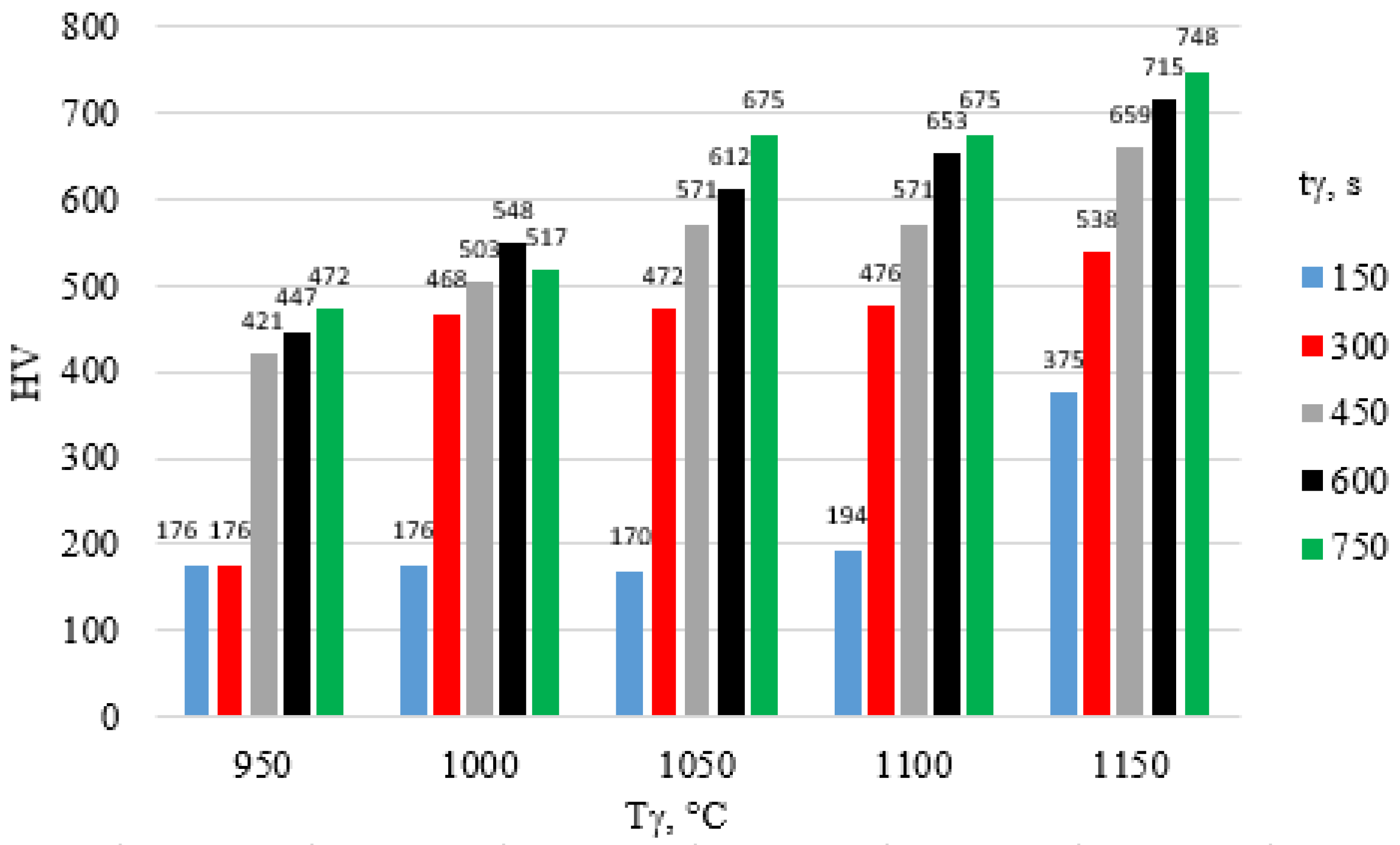
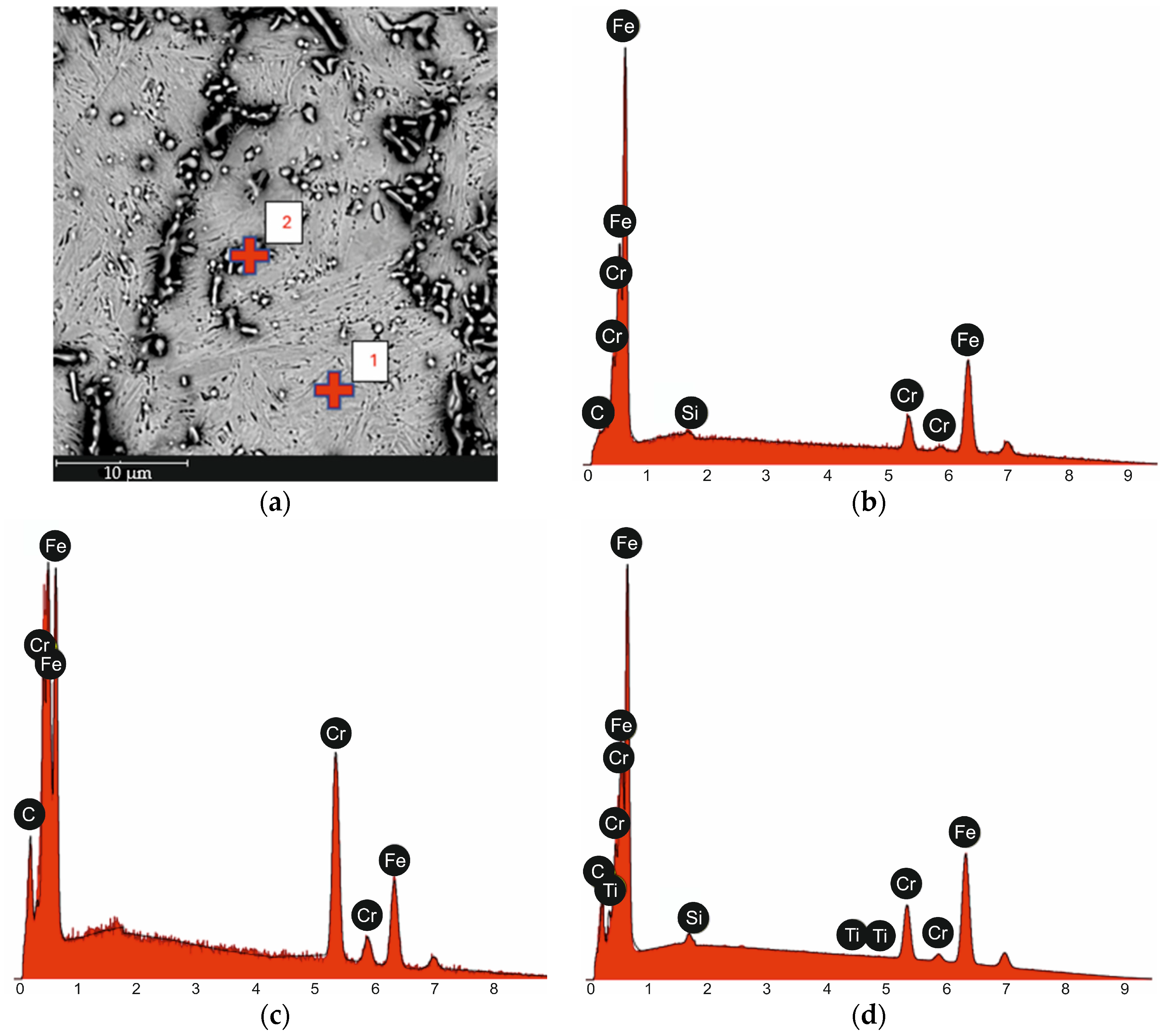



| tγ, s | 150 | 300 | 450 | 600 | 750 | ||||||||||
| Cooling Medium | A | W | O | A | W | O | A | W | O | A | W | O | A | W | O |
| Tγ, °C | 950 | ||||||||||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Tγ, °C | 1000 | ||||||||||||||
| No. | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Tγ, °C | 1050 | ||||||||||||||
| No. | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 |
| Tγ, °C | 1100 | ||||||||||||||
| No. | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Tγ, °C | 1150 | ||||||||||||||
| No. | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 |
| Element Concentrations, wt.% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Ni | Mn | Mo | Co | Si | Al | Cu | V | W | P |
| 0.43 | 13.6 | 0.125 | 0.375 | 0.015 | 0.011 | 0.383 | 0.003 | 0.069 | 0.099 | 0.021 | 0.025 |
| Measurement Point | Element | %at. | %wt. |
|---|---|---|---|
| 1 from Figure 2a | Fe | 89.0 | 90.0 |
| Cr | 10.3 | 9.7 | |
| Si | 0.7 | 0.4 | |
| C | 0.0 | 0.0 | |
| 2 from Figure 2a | Cr | 52.1 | 57.2 |
| Fe | 33.1 | 39.0 | |
| C | 14.9 | 3.8 | |
| EDS Surface Analysis Result from Figure 2a | Fe | 71.1 | 81.1 |
| Cr | 14.2 | 15.1 | |
| C | 14.0 | 3.4 | |
| Si | 0.7 | 0.4 | |
| Ti | 0.0 | 0.0 |
| Still Air | Oil | Water | |
|---|---|---|---|
| HV = f(T, t) | HV = 1.02 T + 0.71 t − 943.2 | HV = 1.1 T + 0.71 t − 1009.74 | HV = 1.22 T + 0.69 t – 1087.96 |
| R | 0.91 | 0.89 | 0.92 |
| R2 | 0.83 | 0.79 | 0.84 |
| s | 79.64 | 91.25 | 75.93 |
| F | 54.79 | 42.03 | 56.64 |
| F0.5 | 3.12 | ||
| Measurement Point | Element | %at. | %wt. |
|---|---|---|---|
| 1 from Figure 16a | Fe | 86.0 | 87.5 |
| Cr | 12.9 | 12.2 | |
| C | 0.8 | 0.2 | |
| Si | 0.3 | 0.2 | |
| 2 from Figure 16a | Cr | 39.6 | 45.7 |
| Fe | 39.2 | 48.6 | |
| C | 21.3 | 5.7 | |
| EDS Surface Analysis Result from Figure 16a | Fe | 67.8 | 80.0 |
| Cr | 13.8 | 15.2 | |
| C | 17.8 | 4.5 | |
| Si | 0.5 | 0.3 | |
| Ti | 0.1 | 0.1 |
| Austenitizing Time tγ, s | Cooling Medium | β, ° |
|---|---|---|
| 450 | Still air | 48.24 |
| Oil | 48.64 | |
| Water | 47.41 | |
| 600 | Still air | 50.00 |
| Oil | 48.69 | |
| Water | 52.04 | |
| 750 | Still air | 55.48 |
| Oil | 49.48 | |
| Water | 54.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przyszlak, N.; Wróbel, T. The Influence of Heat Treatment Parameters on the Microstructure and Hardness of High-Chromium Alloy Steel X46Cr13. Materials 2025, 18, 4183. https://doi.org/10.3390/ma18174183
Przyszlak N, Wróbel T. The Influence of Heat Treatment Parameters on the Microstructure and Hardness of High-Chromium Alloy Steel X46Cr13. Materials. 2025; 18(17):4183. https://doi.org/10.3390/ma18174183
Chicago/Turabian StylePrzyszlak, Natalia, and Tomasz Wróbel. 2025. "The Influence of Heat Treatment Parameters on the Microstructure and Hardness of High-Chromium Alloy Steel X46Cr13" Materials 18, no. 17: 4183. https://doi.org/10.3390/ma18174183
APA StylePrzyszlak, N., & Wróbel, T. (2025). The Influence of Heat Treatment Parameters on the Microstructure and Hardness of High-Chromium Alloy Steel X46Cr13. Materials, 18(17), 4183. https://doi.org/10.3390/ma18174183







