From Construction Industry Waste to High-Performance Insulation: Sustainable Rigid Polyurethane Foams with Recycled Polyol
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Polyurethane Synthesis
2.3. Characterization of PUFs
2.4. Chemical Recycling of Semi-Rigid PU Foams
2.5. Analysis of Recycling Polyol
3. Results and Discussion
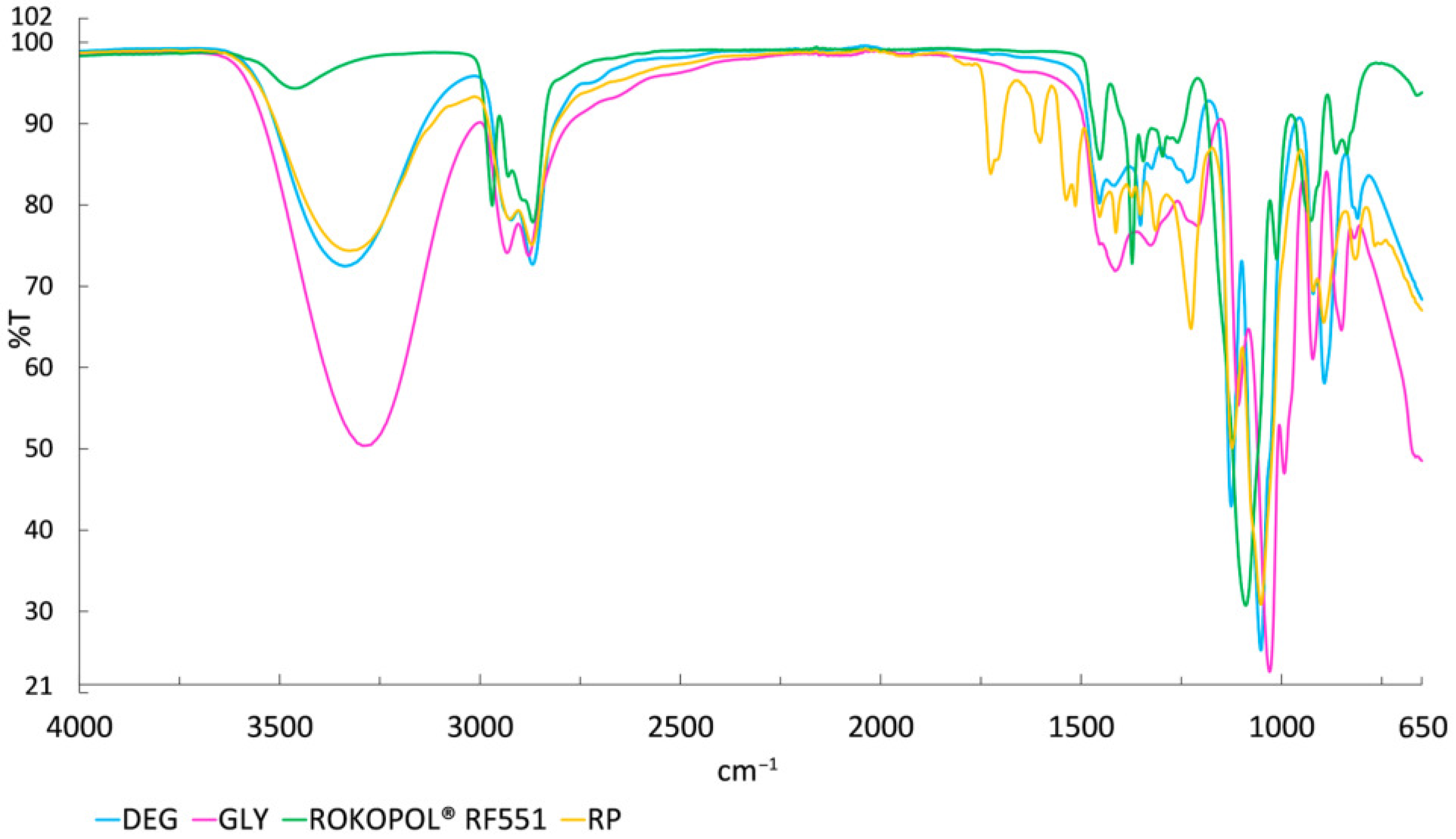
3.1. Characteristics of Recycled Polyol
3.2. Characterization of PU Foams Based on Recycled Polyol
3.2.1. The Effect of RP on the Foaming Process of PUFs
3.2.2. The Influence of Glycolysate on the Closed-Cell Content, Thermal Conductivity Coefficient, Apparent Density, and Cellular Structure of PUFs
3.2.3. The Influence of Glycolysate on the Compressive Strength and Dimensional Stability of PUFs
3.2.4. Evaluation of Flammability of PUFs with RP
3.2.5. The Influence of RP on VOC Release of PUFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polyurethane Foam Market, Industry Size Forecast Report. Available online: https://www.marketsandmarkets.com/Market-Reports/polyurethane-foams-market-1251.html (accessed on 12 June 2025).
- Swinarew, B. Poliuretany—Nowoczesne wszechstronne materiały. Część II—Pianki poliuretanowe. Przetwórstwo Tworzyw 2015, 21, 428–434. [Google Scholar]
- Wirpsza, Z. Poliuretany: Chemia, Technologia, Zastosowanie; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1991. [Google Scholar]
- Shahee, A.; Abdoos, M.; Aslani, A.; Zahedi, R. Reducing the Energy Consumption of Buildings by Implementing Insulation Scenarios and Using Renewable Energies. Energy Inf. 2024, 7, 18. [Google Scholar] [CrossRef]
- Termomodernizacje Budynków—Ministerstwo Rozwoju i Technologii—Portal Gov.pl. Available online: https://www.gov.pl/web/rozwoj-technologia/potrzebujesz-finansowego-wsparcia-na-termomodernizacje-budynku (accessed on 12 June 2025).
- Wieczorek, K.; Bukowski, P.; Stawiński, K.; Ryłko, I. Recycling of Polyurethane Foams via Glycolysis: A Review. Materials 2024, 17, 4617. [Google Scholar] [CrossRef] [PubMed]
- REDWAVE Part of European Green Deal Sorting Project. Available online: https://www.recyclingproductnews.com/article/38054/redwave-part-of-european-green-deal-project-focused-on-advancing-intelligent-sorting-technologies-for-pu-rigid-foams (accessed on 13 June 2025).
- Stasiak, W. Energooszczędność i Zeroemisyjność w Budownictwie. Budownictwo Trendy i Biznes. 2024. Available online: https://inzynierbudownictwa.pl/energooszczednosc-i-zeroemisyjnosc-w-budownictwie/ (accessed on 13 June 2025).
- Gillham, C.L. Green Paper on a European Strategy on Plastic Waste in the Environment; EU: Brussels, Belgium, 2013. [Google Scholar]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From Polymer Waste to Potential Main Industrial Products: Actual State of Recycling and Recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X.; Guo, X.; Liu, S.; Li, Q.; Liu, Y. Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers 2023, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-Friendly Chemical Recycling of Aliphatic Polyurethanes by Hydrolysis in a CO2-Water System. J. Appl. Polym. Sci. 2018, 135, 45897. [Google Scholar] [CrossRef]
- Asahi, N.; Sakai, K.; Kumagai, N.; Nakanishi, T.; Hata, K.; Katoh, S.; Moriyoshi, T. Methanolysis Investigation of Commercially Available Polyurethane Foam. Polym. Degrad. Stab. 2004, 86, 147–151. [Google Scholar] [CrossRef]
- Recupido, F.; Lama, G.C.; Steffen, S. Efficient Recycling Pathway of Bio-Based Composite Polyurethane Foams via Sustainable Diamine. Ecotoxicol. Environ. Saf. 2024, 269, 115758. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of Polyurethane by Acidolysis: The Effect of Reaction Conditions on the Properties of the Recovered Polyol. Polymer 2021, 219, 123561. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Drinčić, A.; Oreški, A.; Onder, O.C.; Utroša, P.; Pahovnik, D.; Žagar, E. Insight into Chemical Recycling of Flexible Polyurethane Foams by Acidolysis. ACS Sustain. Chem. Eng. 2022, 10, 1323–1332. [Google Scholar] [CrossRef]
- Del Amo, J.; Borreguero, A.M.; Ramos, M.J.; Rodríguez, J.F. Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers 2021, 13, 1418. [Google Scholar] [CrossRef]
- Włoch, M.; Toruńczak, M.; Datta, J. Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates. Materials 2024, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Donadini, R.; Boaretti, C.; Lorenzetti, A.; Roso, M.; Penzo, D.; Dal Lago, E.; Modesti, M. Chemical Recycling of Polyurethane Waste via a Microwave-Assisted Glycolysis Process. ACS Omega 2023, 8, 4655–4666. [Google Scholar] [CrossRef] [PubMed]
- Molero, C.; de Lucas, A.; Rodríguez, J.F. Recovery of Polyols from Flexible Polyurethane Foam by “Split-Phase” Glycolysis: Study on the Influence of Reaction Parameters. Polym. Degrad. Stab. 2008, 93, 353–361. [Google Scholar] [CrossRef]
- Kiss, G.; Rusu, G.; Peter, F.; Tănase, I.; Bandur, G. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Ivashchuk, O.S.; Suprun, W.Y.; Atamanyuk, V.M.; Kurhanskyi, V.S.; Nahurskyi, A.O.; Chyzhovych, R.A. Depolymerization of Rigid Polyurethane Waste by Catalytic Glycolysis with Diethylene Glycol. S. Afr. J. Chem. Eng. 2025, 52, 61–67. [Google Scholar] [CrossRef]
- Aguado, A.; Martínez, L.; Moral, A.; Fermoso, J.; Irusta, R. Chemical Recycling of Polyurethane Foam Waste Via Glycolysis. Chem. Eng. Trans. 2011, 24, 1069–1074. [Google Scholar] [CrossRef]
- Shin, S.; Kim, H.; Liang, J.; Lee, S.; Lee, D. Sustainable Rigid Polyurethane Foams Based on Recycled Polyols from Chemical Recycling of Waste Polyurethane Foams. J. Appl. Polym. Sci. 2019, 136, 47916. [Google Scholar] [CrossRef]
- PN-EN 14315-1-2013; Thermal Insulating Products for Buildings. In-Situ Formed Spayed Rigid Polyurethane (PUR) and Polyisocyanurate (PIR) Foam Products. Polish Committee for Standardization: Warszawa, Poland, 2013.
- ISO 845:2009; Cellular Plastics and Rubbers—Determination of Apparent Density. International Organization for Standardization: Geneva, Switzerland, 2009.
- ASTM D7487-24; Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test. ASTM International: West Conshohocken, PA, USA, 2024.
- ISO 4590:2016; Rigid Cellular Plastics—Determination of the Volume Percentage of Open Cells and of Closed Cells. International Organization for Standardization: Geneva, Switzerland, 2016.
- EN 12667:2002; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods—Products of High and Medium Thermal Resistance. European Committee for Standardization: Brussels, Belgium, 2002.
- EN 1604:2013; Thermal Insulating Products for Building Applications—Determination of Dimensional Stability Under Specified Temperature and Humidity Conditions. European Committee for Standardization: Brussels, Belgium, 2013.
- ISO 844; Rigid Cellular Plastics—Determination of Compression Properties. International Organization for Standardization: Geneva, Switzerland, 2014.
- DIN 4102-1:1998-05; Fire Behaviour of Building Materials and Components—Part 1: Building Materials; Concepts, Requirements and Tests. Deutsches Institut für Normung (DIN): Berlin, Germany, 1998.
- ISO 1716:2023; Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). International Organization for Standardization: Geneva, Switzerland, 2023.
- Hanbat National University Industry-Academic Cooperation Foundation. Manufacturing Method Thereof of Recycled Polyol Increased Functionality and Polyurethane Using the Same—Patent View—Eureka. KR101061839B1, 5 September 2011.
- ASTM E1899-23; Standard Test Method for Hydroxyl Groups Using Reaction with p-Toluenesulfonyl Isocyanate (TSI) and Potentiometric Titration with Tetrabutylammonium Hydroxide. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D4662-20; Standard Test Methods for Polyurethane Raw Materials: Determination of Acid and Alkalinity Numbers of Polyols. ASTM International: West Conshohocken, PA, USA, 2020.
- ISO 3219-2:2021; Rheology Part 2: General Principles of Rotational and Oscillatory Rheometry. International Organization for Standardization: Geneva, Switzerland, 2021.
- PN-81/C-04959; Determining the Water Content by the Karl Fischer Method in Organic and Inorganic Products. Polish Committee for Standardization: Warsaw, Poland, 1981.
- Szycher, M. Structure–Property Relations in Polyurethanes. In Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 37–86. ISBN 978-1-4398-3958-4. [Google Scholar]
- Modesti, M.; Simioni, F.; Munari, R.; Baldoin, N. Recycling of Flexible Polyurethane Foams with a Low Aromatic Amine Content. React. Funct. Polym. 1995, 26, 157–165. [Google Scholar] [CrossRef]
- Del Amo, J.; Simón, D.; Ramos, M.J.; Rodríguez, J.F.; De Lucas, A.; Borreguero, A.M. Scaled-up and Economic Assessment Approach of the Split-Phase Glycolysis Process for the Recycling of Flexible Polyurethane Foam Wastes. J. Mater. Cycles Waste Manag. 2022, 24, 1059–1071. [Google Scholar] [CrossRef]
- Gu, X.; Luo, H.; Lv, S.; Chen, P. Glycolysis Recycling of Waste Polyurethane Rigid Foam Using Different Catalysts. JRM 2021, 9, 1253–1266. [Google Scholar] [CrossRef]
- Miguel-Fernández, R.; Amundarain, I.; Asueta, A.; García-Fernández, S.; Arnaiz, S.; Miazza, N.L.; Montón, E.; Rodríguez-García, B.; Bianca-Benchea, E. Recovery of Green Polyols from Rigid Polyurethane Waste by Catalytic Depolymerization. Polymers 2022, 14, 2936. [Google Scholar] [CrossRef] [PubMed]
- Molero, C.; de Lucas, A.; Rodríguez, J.F. Purification by Liquid Extraction of Recovered Polyols. Solvent Extr. Ion Exch. 2006, 24, 719–730. [Google Scholar] [CrossRef]
- Amundarain, I.; Miguel-Fernández, R.; Asueta, A.; García-Fernández, S.; Arnaiz, S. Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers 2022, 14, 1157. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Cheng, C.-M.; Huang, H.-C. Glycolysis of Waste Flexible Polyurethane Foam. Polym. Degrad. Stab. 2003, 80, 103–111. [Google Scholar] [CrossRef]
- Datta, J.; Rohn, M. Glycolysis of polyurethane wastes. Part II. Purification and use of glycolysis products. Polimery 2007, 52, 627–633. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shrewsbury, UK, 2005; ISBN 978-1-84735-035-0. [Google Scholar]
- Fierascu, R.C.; Lungulescu, E.-M.; Fierascu, I.; Stan, M.S.; Voinea, I.C.; Dumitrescu, S.I. Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials? Polymers 2023, 15, 4570. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December. Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Eur. Union 2006, L396, 1–849. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006R1907 (accessed on 3 August 2025).
- Ocepek, M.; Zabret, J.; Kecelj, J. Monitoring of Polyurethane Dispersions after the Synthesis. Mater. Tehnol. 2015, 49, 495–501. [Google Scholar] [CrossRef]
- Gotkiewicz, O.; Kirpluks, M.; Hodan, J.; Parcheta-Szwindowska, P.; Cabulis, U.; Beneš, H. Fast Recycling of Polyurethane Foams Containing Bio-Based Ester-Cleavable Segments. J. Polym. Environ. 2025, 33, 3716–3732. [Google Scholar] [CrossRef]
- Choi, S.W.; Jung, J.M.; Yoo, H.M.; Kim, S.H.; Lee, W.I. Analysis of Thermal Properties and Heat Transfer Mechanisms for Polyurethane Foams Blown with Water. J. Therm. Anal. Calorim. 2018, 132, 1253–1262. [Google Scholar] [CrossRef]
- Moradpour, N.; Yang, J.; Tsai, P.A. Liquid Foam: Fundamentals, Rheology, and Applications of Foam Displacement in Porous Structures. Curr. Opin. Colloid Interface Sci. 2024, 74, 101845. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A Systematic Study Substituting Polyether Polyol with Palm Kernel Oil Based Polyester Polyol in Rigid Polyurethane Foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Ganguly, S. Role of Porous MXenes: Foams and Aerogels in EMI Shielding. In MXene Nanocomposites; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-328151-1. [Google Scholar]
- Paruzel, A.; Michałowski, S.; Hodan, J.; Horák, P.; Prociak, A.; Beneš, H. Rigid Polyurethane Foam Fabrication Using Medium Chain Glycerides of Coconut Oil and Plastics from End-of-Life Vehicles. ACS Sustain. Chem. Eng. 2017, 5, 6237–6246. [Google Scholar] [CrossRef]
- Dusek, K.; Spirkova, M.; Havlicek, I. Network Formation of Polyurethanes Due to Side Reactions. Macromolecules 1990, 23, 1774–1781. [Google Scholar] [CrossRef]
- The Dimensional Stability of Spray Polyurethane Foam|SOPREMA. Available online: https://www.soprema.ca/en/learning-centre/blog/understand-the-dimensional-stability-of-spray-foam (accessed on 11 August 2025).
- Hong, Y.T.; Jeon, J.K.; Jeon, J.H.; Sung, K.S. A Method for Preparation of Recycled Polyols and a Method for Manufacturing Polyurethane Foams with Improved Thermal Insulation Property. AU Patent AU704932B2, 6 May 1999. [Google Scholar]
- Głowacz-Czerwonka, D.; Zakrzewska, P.; Zygmunt-Kowalska, B.; Zarzyka, I. Thermal and Flammability Analysis of Polyurethane Foams with Solid and Liquid Flame Retardants: Comparative Study. Polymers 2025, 17, 1977. [Google Scholar] [CrossRef]
- Zemła, M.; Prociak, A.; Michałowski, S. Bio-Based Rigid Polyurethane Foams Modified with Phosphorus Flame Retardants. Polymers 2021, 14, 102. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Mojaver, P.; Khalilarya, S.; Azdast, T.; Chitsaz, A.; Mojaver, M. Polyurethane Foam Waste Upcycling into an Efficient and Low Pollutant Gasification Syngas. Polymers 2022, 14, 4938. [Google Scholar] [CrossRef]
- DIN ISO 16000-3:2013-01; Indoor Air—Part 3: Determination of Formaldehyde and Other Carbonyl Compounds in Indoor Air and Test Chamber Air—Active Sampling Method (ISO 16000-3:2011). German Institute for Standardisation: Berlin, Germany, 2023.
- Parsons, N.S.; Lam, M.H.W.; Hamilton, S.E. Chemical Characterization of Automotive Polyurethane Foam Using Solid-Phase Microextraction and Gas Chromatography—Mass Spectrometry. J. Forensic Sci. 2013, 58, S186–S191. [Google Scholar] [CrossRef]





| PUF_REF | PUF_5% | PUF_10% | PUF_20% | PUF_30% | PUF_40% | PUF_50% | |
|---|---|---|---|---|---|---|---|
| Rokopol® RF551, php | 100 | 95 | 90 | 80 | 70 | 60 | 50 |
| Recycled polyol, php | 0 | 5 | 10 | 20 | 30 | 40 | 50 |
| Dabco T, php | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 |
| TCPP, php | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| Tegostab B84730, php | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 |
| Rokafenol N8P7, php | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 |
| Water, php | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 |
| Index NCO | 110 | 110 | 110 | 110 | 110 | 110 | 110 |
| Recycled Polyol Code | Glycolysis Agent | Weight Ratio Glycolysis Agent: PU | Catalyst | Amount of Catalyst (wt%/PU) | Temperature of Reaction (°C) |
|---|---|---|---|---|---|
| RP | DEG:GLY (4:1) | 2:1 | DBTDL | 0.2 | 185 |
| Polyol Name | Ohv, mg KOH/g | Av, mg KOH/g | η (25 °C), mPa·s | %H2O, wt% |
|---|---|---|---|---|
| RP | 590 ± 20 | 17.2 ± 0.2 | 1500 ± 100 | 0.07 ± 0.02 |
| Rokopol® RF551 | 420 ± 20 | 0.08 ± 0.02 | 4000 ± 200 | 0.08 ± 0.02 |
| Foam Symbol | Cream Time (s) | Gel Time (s) | Tack-Free Time (s) | Rise Time (s) |
|---|---|---|---|---|
| PUF_REF | 12 ± 1 | 44 ± 2 | 58 ± 2 | 50 ± 2 |
| PUF_5% | 10 ± 1 | 38 ± 2 | 50 ± 2 | 39 ± 2 |
| PUF_10% | 9 ± 1 | 37 ± 2 | 48 ± 2 | 39 ± 2 |
| PUF_20% | 9 ± 1 | 30 ± 2 | 38 ± 1 | 31 ± 2 |
| PUF_30% | 9 ± 1 | 23 ± 1 | 31 ± 1 | 24 ± 1 |
| PUF_40% | 9 ± 1 | 16 ± 1 | 22 ± 1 | 21 ± 1 |
| PUF_50% | 8 ± 1 | 20 ± 1 | 32 ± 1 | 18 ± 1 |
| Foam Symbol | Content of Closed Cell, % | Thermal Conductivity Coefficient at 10 °C, mWm·K | Thermal Conductivity Coefficient at 0 °C, mWm·K | Apparent Density, kg/m3 |
|---|---|---|---|---|
| PUF_REF | 88.3 ± 0.73 | 25.62 ± 0.16 | 24.39 ± 0.30 | 44.0 ± 0.83 |
| PUF_5% | 90.6 ± 0.72 | 22.84 ± 0.19 | 21.72 ± 0.35 | 42.0 ± 0.75 |
| PUF_10% | 90.4 ± 0.91 | 23.8 ± 0.16 | 22.86 ± 0.23 | 41.1 ± 0.54 |
| PUF_20% | 91.4 ± 1.15 | 23.6 ± 0.23 | 22.54 ± 0.25 | 40.0 ± 0.97 |
| PUF_30% | 89.0 ± 0.60 | 25.4 ± 0.26 | 24.37 ± 0.28 | 41.2 ± 1.18 |
| PUF_40% | 87.7 ± 2.30 | 26.3 ± 0.29 | 25.15 ± 0.27 | 44.9 ± 0.78 |
| PUF_50% | 80.1 ± 4.16 | 27.0 ± 0.50 | 25.89 ± 0.43 | 48.7 ± 1.21 |
| Foam Symbol | Compressive Strength, Parallel, kPa | Compressive Strength, Perpendicular, kPa |
|---|---|---|
| PUF_REF | 208 ± 12 | 156 ± 10 |
| PUF_5% | 176 ± 14 | 144 ± 8 |
| PUF_10% | 170 ± 12 | 140 ± 11 |
| PUF_20% | 175 ± 16 | 141 ± 9 |
| PUF_30% | 193 ± 15 | 154 ± 13 |
| PUF_40% | 294 ± 14 | 203 ± 11 |
| PUF_50% | 262 ± 13 | 195 ± 8 |
| Foam Symbol | A1, % | A2, % | A3, % |
|---|---|---|---|
| PUF_REF | 0.05 ± 0.15 | 0.07 ± 0.10 | 0.50 ± 0.36 |
| PUF_5% | 0.12 ± 0.08 | −0.09 ± 0.06 | 0.39 ± 0.28 |
| PUF_10% | 0.07 ± 0.16 | −0.05 ± 0.08 | 0.24 ± 0.21 |
| PUF_20% | 0.08 ± 0.11 | 0.07 ± 0.11 | 0.15 ± 0.25 |
| PUF_30% | 0.11 ± 0.12 | 0.06 ± 0.08 | 0.26 ± 0.19 |
| PUF_40% | 0.02 ± 0.06 | 0.00 ± 0.06 | 0.12 ± 0.17 |
| PUF_50% | 0.04 ± 0.05 | 0.02 ± 0.05 | 0.18 ± 0.19 |
| Foam Symbol | A1, % | A2, % | A3, % |
|---|---|---|---|
| PUF_REF | 0.58 ± 0.11 | 0.15 ± 0.11 | 0.69 ± 0.45 |
| PUF_5% | 0.67 ± 0.12 | 0.12 ± 0.09 | 0.59 ± 0.36 |
| PUF_10% | 0.48 ± 0.16 | 0.09 ± 0.04 | 0.63 ± 0.33 |
| PUF_20% | 0.37 ± 0.13 | 0.11 ± 0.12 | 0.48 ± 0.19 |
| PUF_30% | 0.23 ± 0.17 | 0.13 ± 0.12 | 0.50 ± 0.25 |
| PUF_40% | 0.19 ± 0.10 | 0.10 ± 0.09 | 0.38 ± 0.34 |
| PUF_50% | 0.18 ± 0.08 | 0.08 ± 0.07 | 0.28 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, K.; Bobak, Ł.; Bukowski, P. From Construction Industry Waste to High-Performance Insulation: Sustainable Rigid Polyurethane Foams with Recycled Polyol. Materials 2025, 18, 4179. https://doi.org/10.3390/ma18174179
Wieczorek K, Bobak Ł, Bukowski P. From Construction Industry Waste to High-Performance Insulation: Sustainable Rigid Polyurethane Foams with Recycled Polyol. Materials. 2025; 18(17):4179. https://doi.org/10.3390/ma18174179
Chicago/Turabian StyleWieczorek, Kinga, Łukasz Bobak, and Przemysław Bukowski. 2025. "From Construction Industry Waste to High-Performance Insulation: Sustainable Rigid Polyurethane Foams with Recycled Polyol" Materials 18, no. 17: 4179. https://doi.org/10.3390/ma18174179
APA StyleWieczorek, K., Bobak, Ł., & Bukowski, P. (2025). From Construction Industry Waste to High-Performance Insulation: Sustainable Rigid Polyurethane Foams with Recycled Polyol. Materials, 18(17), 4179. https://doi.org/10.3390/ma18174179







