Investigating the Stability of Cu2Se Superionic Thermoelectric Material in Air Atmosphere
Highlights
- Polycrystalline samples of Cu2Se were synthesized and sintered.
- The stability of Cu2Se in an air atmosphere (T = 673–973 K) was investigated.
- A comprehensive study of XRD and SEM-EDS was conducted on oxidized samples.
- TPOx and TGA measurements were carried out.
- Protecting Cu2Se with a protective layer is necessary for its operation in air.
Abstract
1. Introduction
2. Materials and Methods
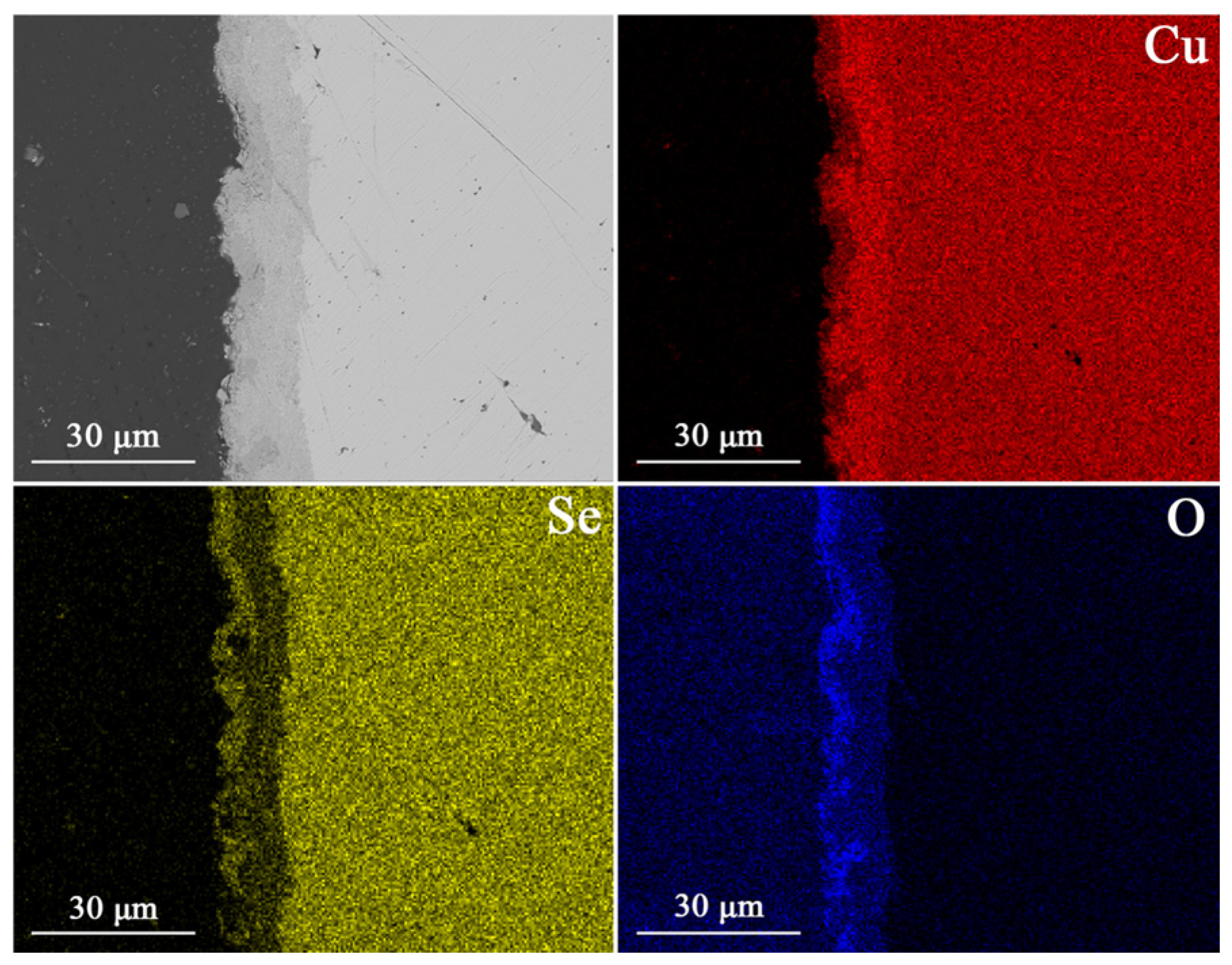
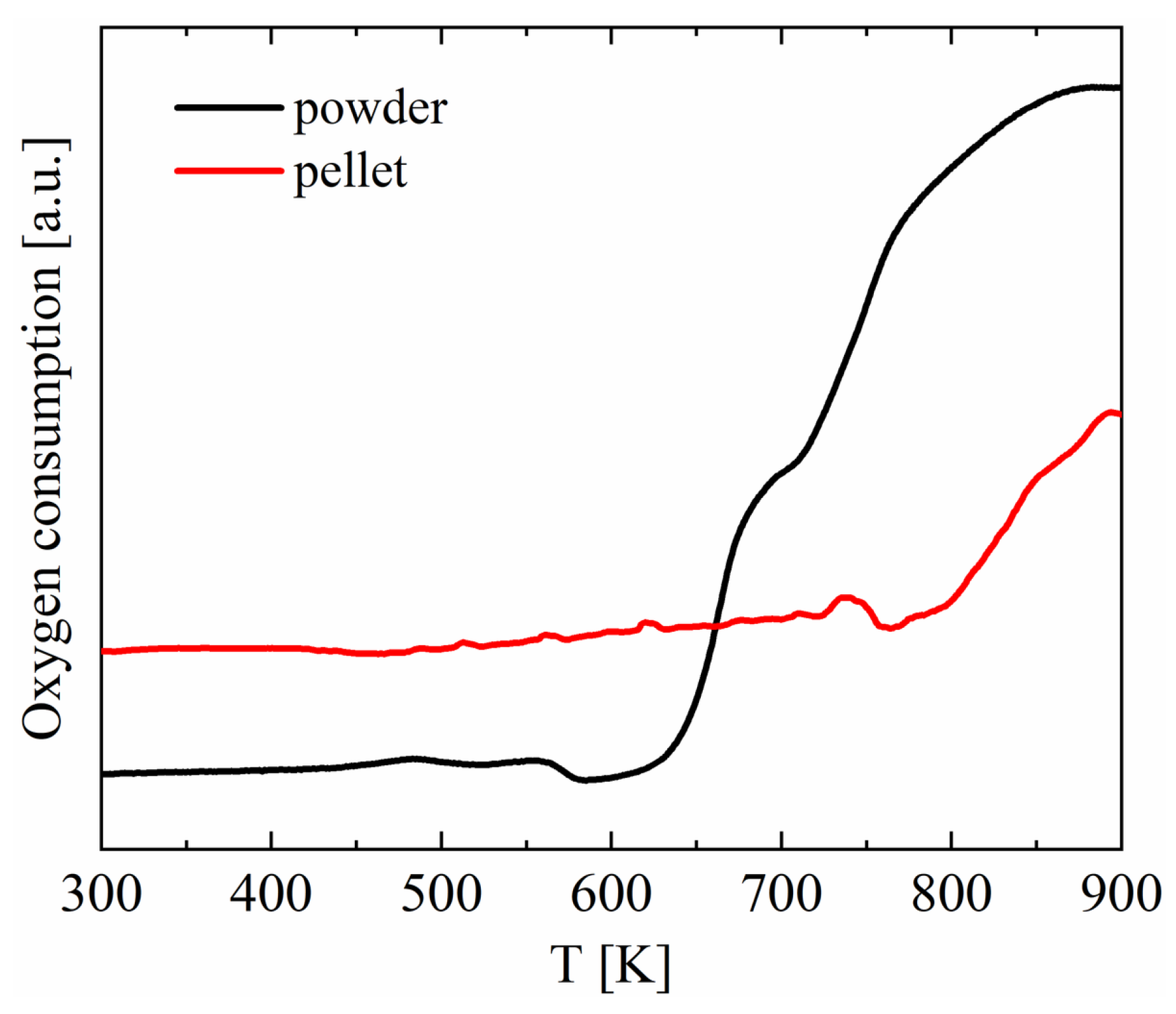
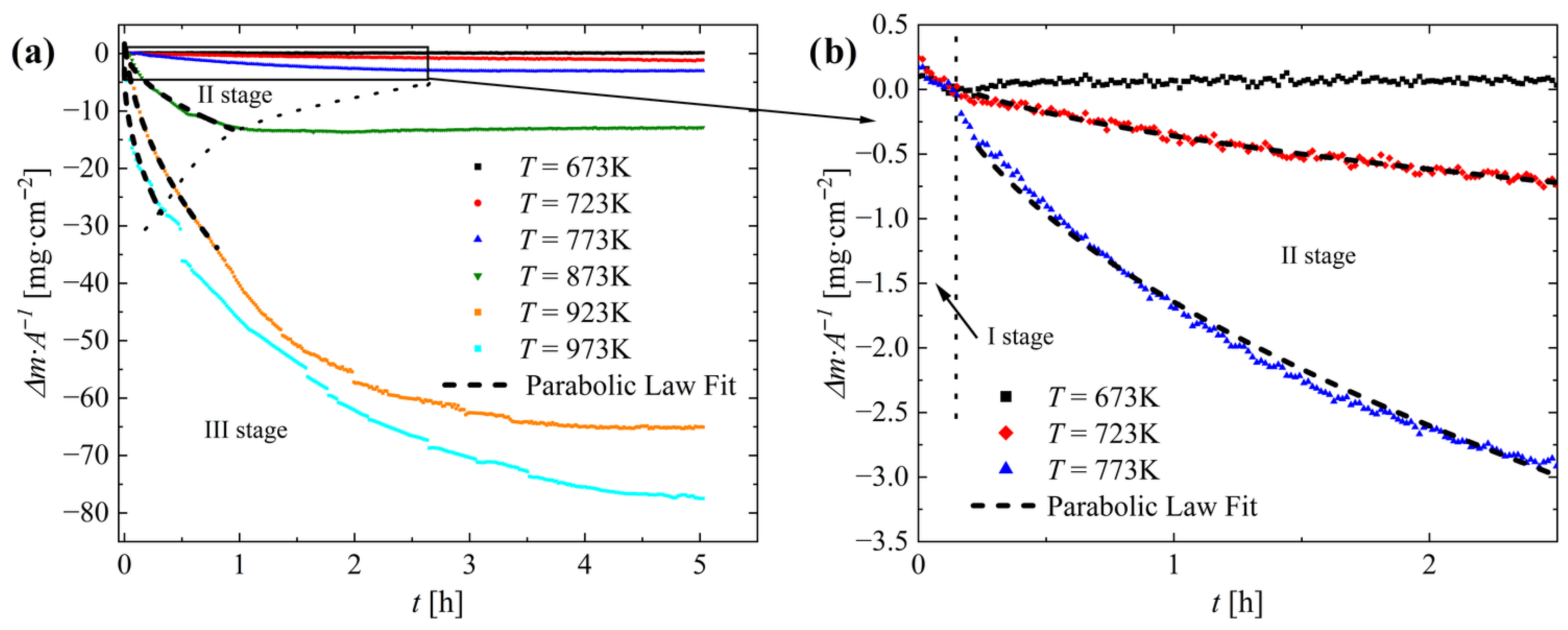
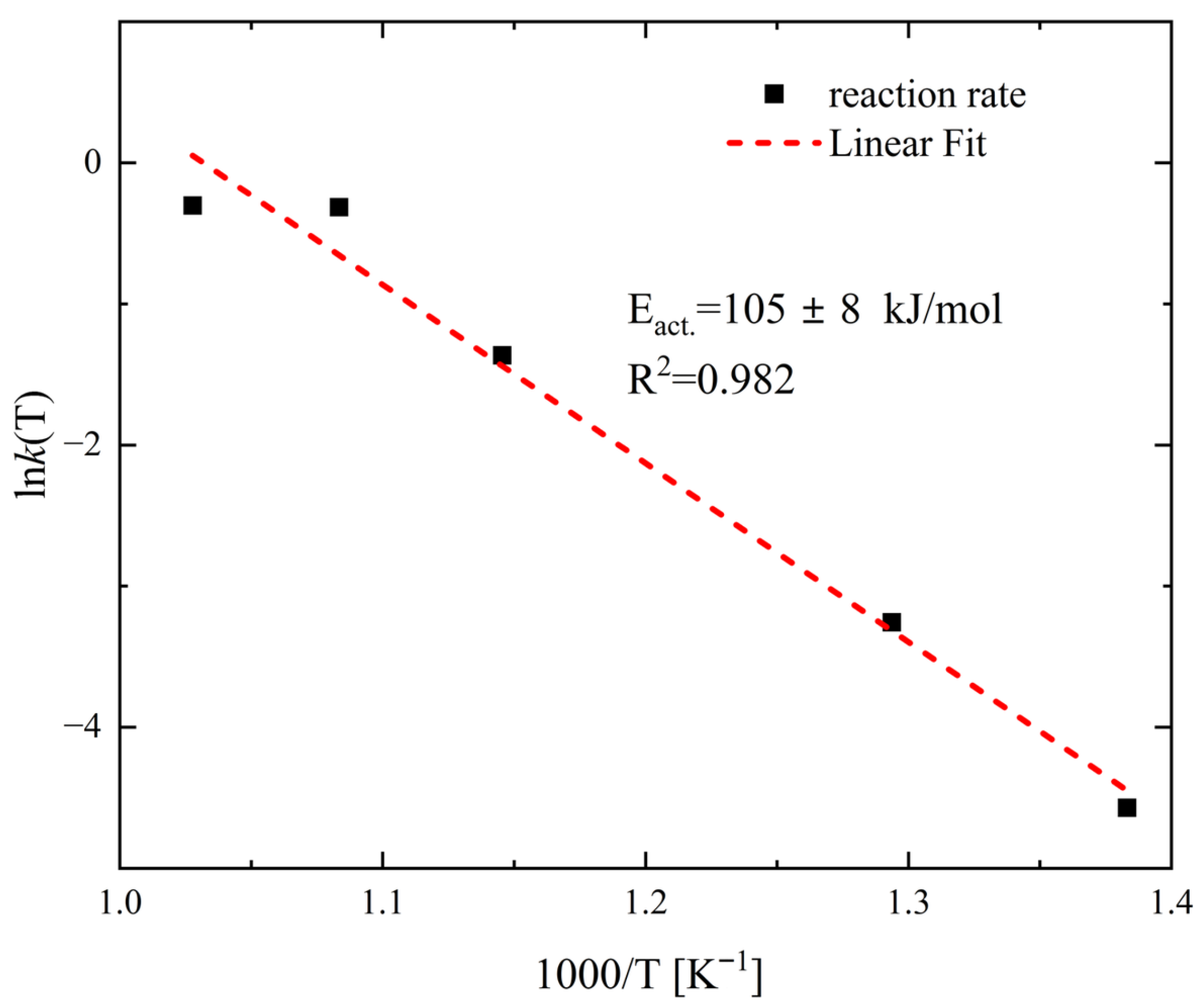
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basit, A.; Xin, J.; Murtaza, G.; Wei, L.; Hameed, A.; Guoyu, W.; Dai, J.Y. Recent advances, challenges, and perspective of copper-based liquid-like thermoelectric chalcogenides: A review. EcoMat 2023, 5, e12391. [Google Scholar] [CrossRef]
- Liu, W.D.; Yang, L.; Chen, Z.G.; Zou, J. Promising and eco-friendly Cu2X-based thermoelectric materials: Progress and applications. Adv. Mater. 2020, 32, 1905703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Qiu, P.; Shi, X.; Chen, L. Recent advances in liquid-like thermoelectric materials. Adv. Funct. Mater. 2019, 30, 1903867. [Google Scholar] [CrossRef]
- Bailey, T.P.; Uher, C. Potential for superionic conductors in thermoelectric applications. Curr. Opin. Green Sustain. Chem. 2017, 4, 58–63. [Google Scholar] [CrossRef]
- Powell, A.V. Recent developments in Earth-abundant copper-sulfide thermoelectric materials. J. Appl. Phys. 2019, 126, 100901. [Google Scholar] [CrossRef]
- Ge, Z.H.; Zhao, L.D.; Wu, D.; Liu, X.; Zhang, B.P.; Li, J.F.; He, J. Low-cost, abundant binary sulfides as promising thermoelectric materials. Mater. Today 2016, 19, 227–239. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as thermoelectric materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Qiu, P.; Shi, X.; Chen, L. Cu-based thermoelectric materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Funahashi, R. Thermoelectric Energy Conversion Theories and Mechanisms, Materials, Devices, and Applications; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Pineda, D.D.; Rezania, A. Thermoelectric Energy Conversion Basic Concepts and Device Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Skipidarov, S.; Nikitin, M. Novel Thermoelectric Materials and Device Design Concepts; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Yazawa, K.; Bahk, J.H.; Shakouri, A. Thermoelectric Energy Conversion Devices and Systems in WSPC Series in Advanced Integration and Packaging; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2021. [Google Scholar]
- Ren, Z.; Lan, Y.; Zhang, Q. Advanced Thermoelectrics Materials, Contacts, Devices, and Systems; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2018. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press LLC: Boca Raton, FL, USA, 1995. [Google Scholar]
- Uher, C. Materials Aspect of Thermoelectricity; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hu, Q.; Zhu, Z.; Zhang, Y.; Li, X.J.; Song, H.; Zhang, Y. Remarkably high thermoelectric performance of Cu2−xLixSe bulks with nanopores. J. Mater. Chem. A 2018, 6, 23417–23424. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Song, H.; Li, X.J. High thermoelectric performance and low thermal conductivity in Cu2−xNaxSe bulk materials with micro-pores. Appl. Phys. A 2019, 125, 572. [Google Scholar] [CrossRef]
- Zhong, B.; Zhang, Y.; Li, W.; Chen, Z.; Cui, J.; Li, W.; Xie, Y.; Hao, Q.; He, Q. High superionic conduction arising from aligned large lamellae and large figure of merit in bulk Cu1.94Al0.02Se. Appl. Phys. Lett. 2014, 105, 123902. [Google Scholar] [CrossRef]
- Nunna, R.; Qiu, P.; Yin, M.; Chen, H.; Hanus, R.; Song, Q.; Zhang, T.; Chou, M.Y.; Agne, M.T.; He, J.; et al. Ultrahigh thermoelectric performance in Cu2Se-based hybrid materials with highly dispersed molecular CNTs. Energy Environ. Sci. 2017, 10, 1928–1935. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Jiang, Y.; Zhuang, H.L.; Thong, H.C.; Su, B.; Li, J.W.; Han, Z.; Li, H.; Pei, J.; et al. Interface-Enhanced High-Temperature Thermoelectricity in Cu1.99Se/B4C Composites with Synergistically Improved Mechanical Strength. Adv. Energy Mater. 2024, 14, 2303942. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Hu, H.; Jiang, Y.; Zhuang, H.L.; Li, H.; Su, B.; Li, J.W.; Han, Z.; Wang, Z.; et al. Ultralow thermal conductivity and high ZT of Cu2Se-based thermoelectric materials mediated by TiO2−n nanoclusters. Joule 2024, 8, 2652–2666. [Google Scholar] [CrossRef]
- Jian, Q.; Gong, Y.; Chen, C.; Sun, R.; Zhao, S.; Shen, T.; Zhang, Q.; Geng, Y.; Li, Y.; Dou, W.; et al. Composite Engineering Facilitates High-Performance Cu2Se-GeTe Thermoelectrics. ACS Appl. Mater. Interfaces 2025, 17, 15527–15534. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Li, J.; Bai, H.; Wang, S.; Li, Z.; Tang, H.; Tang, K.; Luo, T.; Yan, Y.; et al. Blocking Ion Migration Stabilizes the High Thermoelectric Performance in Cu2Se Composites. Adv. Mater. 2020, 32, 2003730. [Google Scholar] [CrossRef]
- Lu, P.; Liu, H.; Yuan, X.; Xu, F.; Shi, X.; Zhao, K.; Qiu, W.; Zhang, W.; Chen, L. Multiformity and fluctuation of Cu ordering in Cu2Se thermoelectric materials. J. Mater. Chem. A 2015, 3, 6901–6908. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef]
- Nieroda, P.; Kusior, A.; Leszczyński, J.; Rutkowski, P.; Koleżyński, A. Thermoelectric Properties of Cu2Se Synthesized by Hydrothermal Method and Densified by SPS Technique. Materials 2021, 14, 3650. [Google Scholar] [CrossRef]
- Nieroda, P.; Kruszewski, M.J.; Leszczyński, J.; Mars, K.; Koleżyński, A. Influence of DC and AC current in the SPS sintering process on homogeneity of thermoelectric properties of Cu2S and Cu2Se. Ceram. Int. 2023, 49, 9681–9690. [Google Scholar] [CrossRef]
- Palaporn, D.; Tanusilp, S.; Sun, Y.; Pinitsoontorn, S.; Kurosaki, K. Thermoelectric materials for space explorations. Mater. Adv. 2024, 5, 5351–5364. [Google Scholar] [CrossRef]
- Lange, R.G.; Carroll, W.P. Review of recent advances of radioisotope power systems. Energy Convers. Manag. 2008, 49, 393–401. [Google Scholar] [CrossRef]
- Ambrosi, R.M.; Williams, H.; Watkinson, E.J.; Barco, A.; Mesalam, R.; Crawford, T.; Bicknell, C.; Samara-Ratna, P.; Vernon, D.; Bannister, N.; et al. European Radioisotope Thermoelectric Generators (RTGs) and Radioisotope Heater Units (RHUs) for Space Science and Exploration. Space Sci. Rev. 2019, 215, 55. [Google Scholar] [CrossRef]
- Shen, Z.-G.; Tian, L.L.; Liu, X. Automotive exhaust thermoelectric generators: Current status, challenges and future prospects. Energy Convers. Manag. 2019, 195, 1138–1173. [Google Scholar] [CrossRef]
- Orr, B.; Akbarzadeh, A.; Mochizuki, M.; Singh, R. A review of car waste heat recovery systems utilising thermoelectric generators and heat pipes. Appl. Therm. Eng. 2016, 101, 490–495. [Google Scholar] [CrossRef]
- Saha, M.; Tregenza, O.; Twelftree, J.; Hulston, C. A review of thermoelectric generators for waste heat recovery in marine applications. Sustain. Energy Technol. Assess. 2023, 59, 103394. [Google Scholar] [CrossRef]
- Miller, T.; Durlik, I.; Kostecka, E.; Kozlovska, P.; Jakubowski, A.; Łobodzińska, A. Waste Heat Utilization in Marine Energy Systems for Enhanced Efficiency. Energies 2024, 17, 5653. [Google Scholar] [CrossRef]
- Uyanık, T.; Ejder, E.; Arslanoglu, Y.; Yalman, Y.; Terriche, Y.; Su, C.L.; Guerrero, J.M. Thermoelectric Generators as an Alternative Energy Source in Shipboard Microgrids. Energies 2022, 15, 4248. [Google Scholar] [CrossRef]
- Taskinen, P.; Patana, S.; Kobylin, P.; Latostenmaa, P. Oxidation Mechanism of Copper Selenide. High Temp. Mater. Proc. 2014, 33, 469–476. [Google Scholar] [CrossRef]
- Gospodinov, G.G.; Bogdanov, B.G. Thermal and thermodynamic data concerning the selenites from group IB in the periodic table. Thermochim. Acta 1984, 75, 295–302. [Google Scholar] [CrossRef]
- Zawadzka, K.; Godlewska, E.; Mars, K.; Nocun, M.; Kryshtal, A.; Czyrska-Filemonowicz, A. Enhancement of oxidation resistance of CoSb3 thermoelectric material by glass coating. Mater. Des. 2017, 119, 65–75. [Google Scholar] [CrossRef]
- Leszczyński, J.; Nieroda, P.; Nieroda, J.; Zybała, R.; Król, M.M.; Łącz, A.; Kaszyca, K.; Mikuła, A.; Schmidt, M.; Sitarz, M.; et al. Si-O-C amorphous coatings for high temperature protection of In0.4Co4Sb12 skutterudite for thermoelectric applications. J. Appl. Phys. 2019, 125, 215113. [Google Scholar] [CrossRef]
- Nieroda, P.; Marsa, K.; Nieroda, J.; Leszczyński, J.; Król, M.; Drożdż, E.; Jeleń, P.; Sitarz, M.; Koleżyński, A. New high temperature amorphous protective coatings for Mg2Si thermoelectric material. Ceram. Int. 2019, 45, 10230–10235. [Google Scholar] [CrossRef]
- Tani, J.; Takahashi, M.; Kido, H. Thermoelectric properties and oxidation behaviour of magnesium silicide. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 142013. [Google Scholar] [CrossRef]
- Xiao, X.-X.; Xie, W.J.; Tang, X.-F.; Zhang, Q.-J. Phase transition and high temperature thermoelectric properties of copper selenide Cu2−xSe (0 ≤ x ≤ 0.25). Chin. Phys. B 2011, 20, 087201. [Google Scholar] [CrossRef]
- Zhao, K.; Blichfeld, A.B.; Chen, H.; Song, Q.; Zhang, T.; Zhu, C.; Ren, D.; Hanus, R.; Qiu, P.; Iversen, B.B.; et al. Enhanced thermoelectric performance through tuning bonding energy in Cu2Se1−xSx liquid-like materials. Chem. Mater. 2017, 29, 6367–6377. [Google Scholar] [CrossRef]
- Liu, F.S.; Huang, M.J.; Gong, Z.N.; Ao, W.Q.; Li, Y.; Li, J.Q. Enhancing the thermoelectric performance of β-Cu2Se by incorporating SnSe. J. Alloys Compd. 2015, 651, 648–654. [Google Scholar] [CrossRef]
- Piyasin, P.; Palaporn, D.; Kurosaki, K.; Pinitsoontorn, S. High-performance thermoelectric properties of Cu2Se fabricated via cold sintering process. Solid State Sci. 2024, 149, 107448. [Google Scholar] [CrossRef]
- Butt, S.; Farooq, M.U.; Mahmood, W.; Salam, S.; Sultan, M.; Basit, M.A.; Ma, J.; Lin, Y.; Nan, C.W. One-step rapid synthesis of Cu2Se with enhanced thermoelectric properties. J. Alloys Compd. 2019, 786, 557–564. [Google Scholar] [CrossRef]
- Moore, W.J.; Selikson, B. The Diffusion of Copper in Cuprous Oxide. J. Chem. Phys. 1951, 19, 1539–1543. [Google Scholar] [CrossRef]
- Grzesik, Z.; Migdalska, M. Oxidation Mechanism of Cu2O and Defect Structure of CuO at High Temperatures. High Temp. Mater. Proc. 2011, 30, 277–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Mimura, K.; Isshiki, M. Oxidation Mechanism of Cu2O to CuO at 600–1050 °C. Oxid. Met. 2004, 62, 2173–2176. [Google Scholar] [CrossRef]
- Rebane, Y.A.; Strelkov, A.V.; Tretyakov, Y.D.; Yakunin, V.G.; Yakubovsky, A.Y. A Study of copper self-diffusion in copper oxide by SNMS technique. Trans. Mat. Res. Soc. Jpn. 1994, 19A, 293–296. [Google Scholar]
- Barchiesi, D.; Grosges, T. New Method to Recover Activation Energy: Application to Copper Oxidation. Metals 2024, 14, 1066–1087. [Google Scholar] [CrossRef]
- Nieroda, P.; Leszczynski, J.; Nieroda, J.; Mars, K.; Mitoraj-Krolikowska, M.; Drożdż, E.; Mikuła, A.; Sitarz, M.; Koleżyński, A. Si–O–C amorphous coatings as a perspective protection against oxidation-caused degradation of Cu2S superionic thermoelectric materials. Ceram. Int. 2021, 47, 12992–12996. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieroda, P.; Rudnik, M.; Mitoraj-Królikowska, M.; Drożdż, E.; Kozień, D.; Leszczyński, J.; Koleżyński, A. Investigating the Stability of Cu2Se Superionic Thermoelectric Material in Air Atmosphere. Materials 2025, 18, 4152. https://doi.org/10.3390/ma18174152
Nieroda P, Rudnik M, Mitoraj-Królikowska M, Drożdż E, Kozień D, Leszczyński J, Koleżyński A. Investigating the Stability of Cu2Se Superionic Thermoelectric Material in Air Atmosphere. Materials. 2025; 18(17):4152. https://doi.org/10.3390/ma18174152
Chicago/Turabian StyleNieroda, Paweł, Małgorzata Rudnik, Marzena Mitoraj-Królikowska, Ewa Drożdż, Dawid Kozień, Juliusz Leszczyński, and Andrzej Koleżyński. 2025. "Investigating the Stability of Cu2Se Superionic Thermoelectric Material in Air Atmosphere" Materials 18, no. 17: 4152. https://doi.org/10.3390/ma18174152
APA StyleNieroda, P., Rudnik, M., Mitoraj-Królikowska, M., Drożdż, E., Kozień, D., Leszczyński, J., & Koleżyński, A. (2025). Investigating the Stability of Cu2Se Superionic Thermoelectric Material in Air Atmosphere. Materials, 18(17), 4152. https://doi.org/10.3390/ma18174152




