Research Progress on Laser Additive Manufacturing of Oxide Dispersion-Strengthened Alloys—A Review
Abstract
1. Introduction
2. Introduction of ODS Alloys
2.1. Iron-Based Alloys
2.2. Nickel-Based Alloys
3. Preparation Technology of ODS Alloys
3.1. Traditional Preparation Methods of ODS Alloys
3.1.1. Internal Oxidation Method
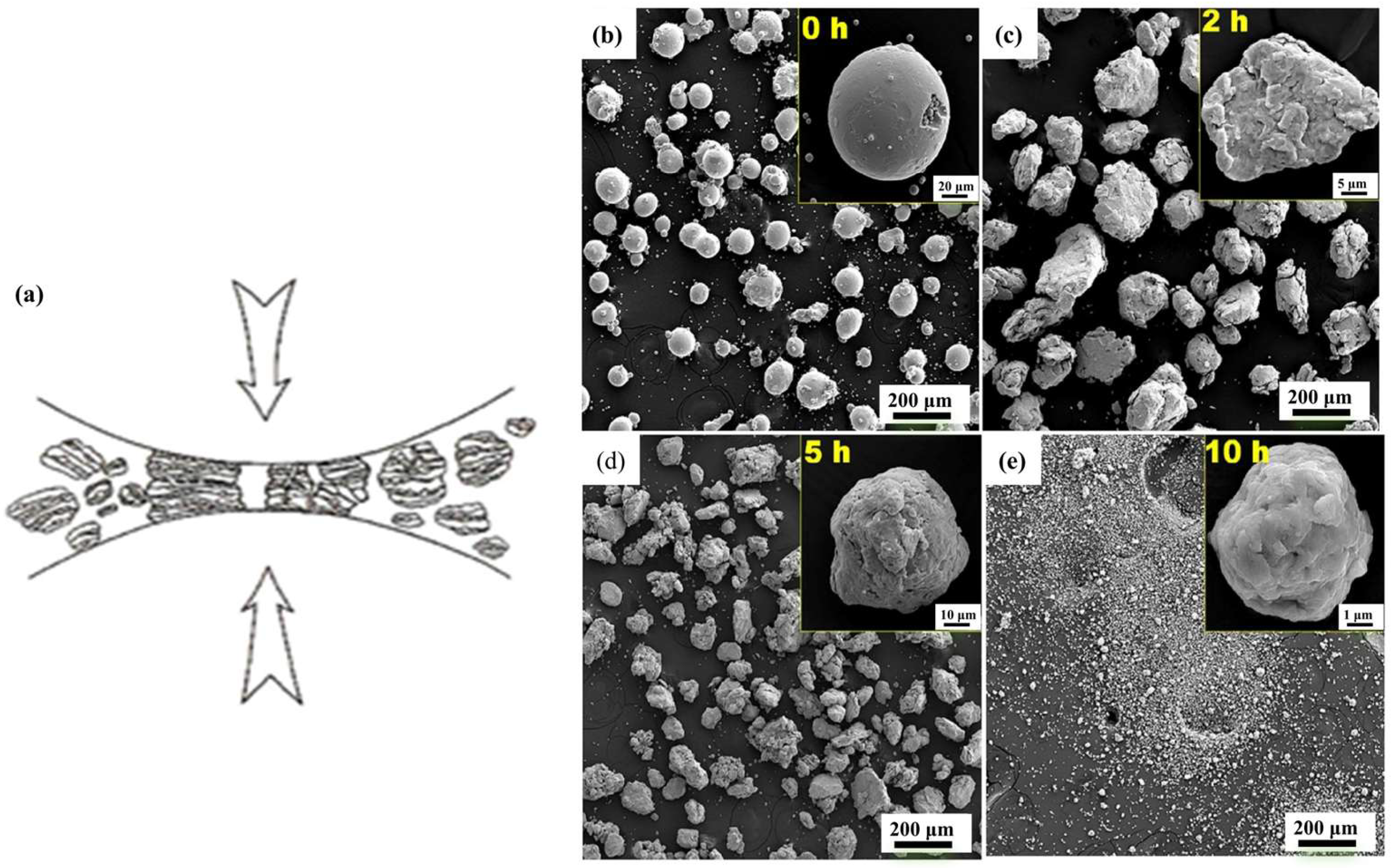
3.1.2. Mechanical Alloying (MA)
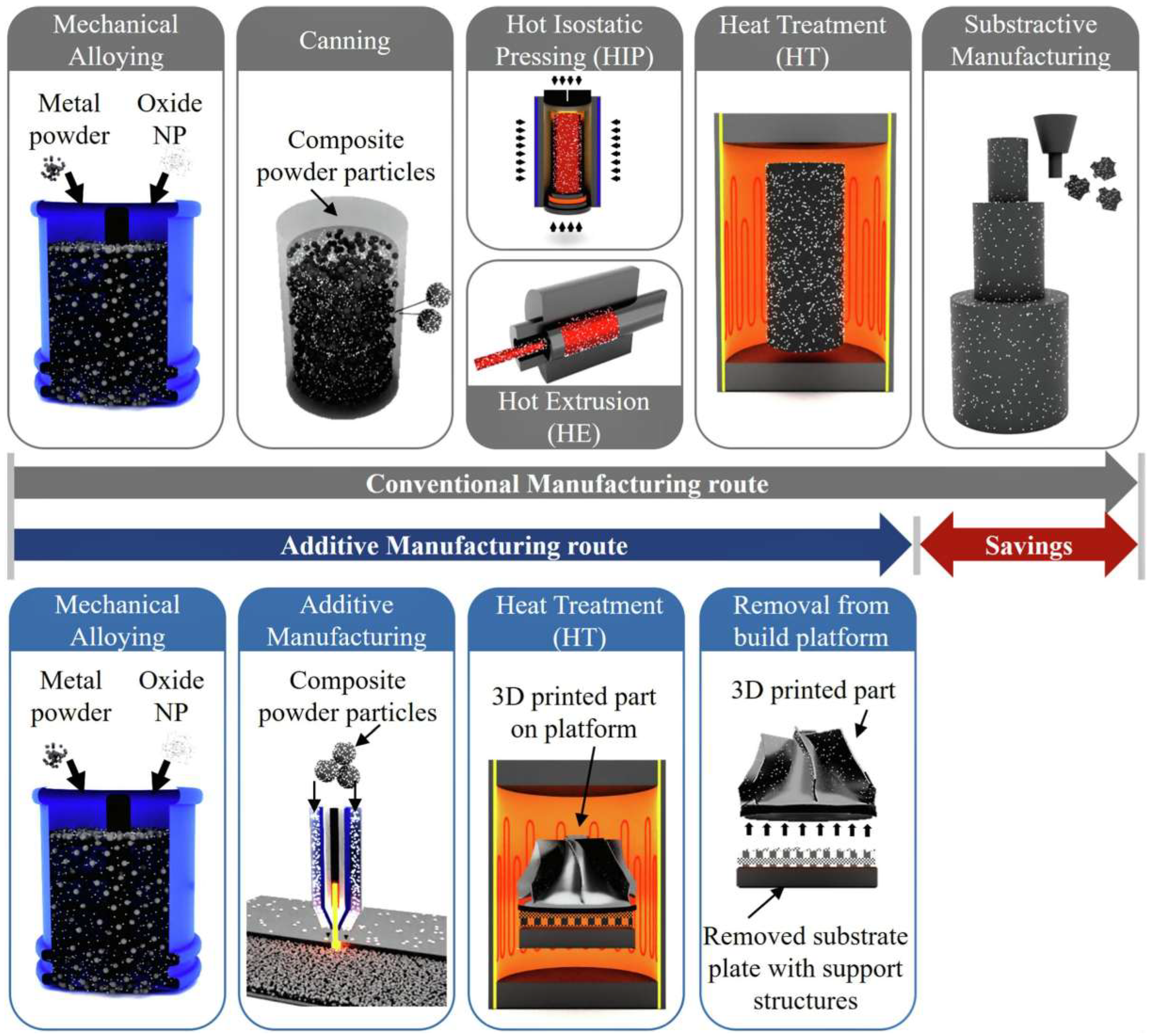
3.2. The Potential of Additive Manufacturing ODS Alloys
4. Research Status of Laser Additive Manufacturing
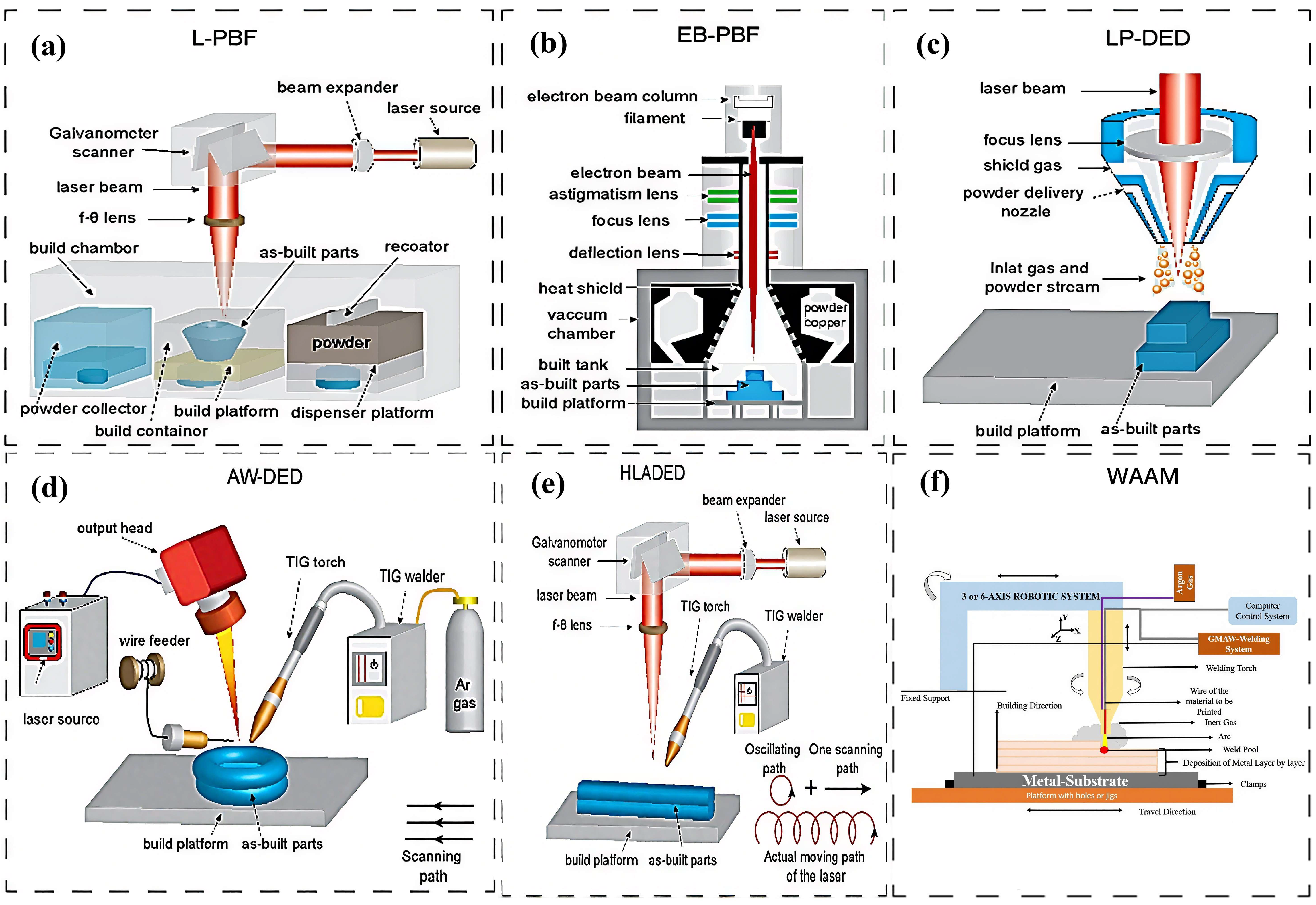
4.1. Powder Bed Fusion
4.2. Direct Energy Deposition
4.3. Wire and Arc Additive Manufacturing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diao, S.Z.; Zhao, Q.; Wang, S.L.; Han, W.T.; Wang, Z.Q.; Liu, P.P.; Chen, Y.H.; Wan, F.R.; Zhan, Q. The microstructure evolution and irradiation hardening in 15Cr-ODS steel irradiated by helium ions. Mater. Charact. 2022, 184, 111699. [Google Scholar] [CrossRef]
- Dong, H.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effect of hafnium addition on the microstructure and tensile properties of aluminum added high-Cr ODS steels. J. Alloys Compd. 2017, 702, 538–545. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Leo, J.R.O.; Moturu, S.R.; Fitzpatrick, M.E. TEM study of the effect of high-temperature thermal cycles on the stability of the Y-Al-O oxides in MA956 ODS steel. J. Mater. Res. Technol. 2019, 8, 3719–3725. [Google Scholar] [CrossRef]
- Liu, D.G.; Zheng, L.; Luo, L.M.; Zan, X.; Song, J.-P.; Xu, Q.; Zhu, X.Y.; Wu, Y.C. An overview of oxidation-resistant tungsten alloys for nuclear fusion. J. Alloys Compd. 2018, 765, 299–312. [Google Scholar] [CrossRef]
- Takaya, S.; Furukawa, T.; Müller, G.; Heinzel, A.; Jianu, A.; Weisenburger, A.; Aoto, K.; Inoue, M.; Okuda, T.; Abe, F.; et al. Al-containing ODS steels with improved corrosion resistance to liquid lead–bismuth. J. Nucl. Mater. 2012, 428, 125–130. [Google Scholar] [CrossRef]
- Alshahrani, H.A. Review of 4D printing materials and reinforced composites: Behaviors, applications and challenges. J. Sci. Adv. Mater. Devices 2021, 6, 167–185. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Kanishka, K.; Acherjee, B. Revolutionizing manufacturing: A comprehensive overview of additive manufacturing processes, materials, developments, and challenges. J. Manuf. Process. 2023, 107, 574–619. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Mujumdar, A.S.; Li, J. AI-based additive manufacturing for future food: Potential applications, challenges and possible solutions. Innov. Food Sci. Emerg. Technol. 2024, 92, 103599. [Google Scholar] [CrossRef]
- Clegg, B.A.; Shrestha, D.R.; Emami, N. Tribo-mechanical properties and bioactivity of additively manufactured PAEK materi als for load bearing medical applications: A systematic review. Biotribology 2023, 35–36, 100263. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Aliyu, A.; Bishop, D.P.; Nasiri, A. Additive manufacturing of copper-based alloys for high-tempera tureaerospace applications: A review. Mater. Today Commun. 2024, 38, 108395. [Google Scholar] [CrossRef]
- Gajbhiye, T.S.; Waghmare, S.; Dhande, M.; Gondane, R.; Giripunje, M.; Shelare, S.; Belkhode, P. Polymer composite additive manufacturing: Applications, challenges and opportunities. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Wilms, M.B.; Rittinghaus, S.K.; Goßling, M.; Gökce, B. Additive manufacturing of oxide-dispersion strengthened alloys: Materials, synthesis and manufacturing. Prog. Mater. Sci. 2023, 133, 101049. [Google Scholar] [CrossRef]
- Hayashi, T.; Sarosi, P.M.; Schneibel, J.H.; Mills, M.J. Creep response and deformation processes in nanocluster-strengthened ferritic steels. Acta Mater. 2008, 56, 1407–1416. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Boutard, J.L.; Hoelzer, D.T.; Kimura, A.; Lindau, R.; Odette, G.R.; Rieth, M.; Tan, L.; Tanigawa, H. Development of next generation tempered and ODS reduced activation ferritic/martensitic steels for fusion energy applications. Nucl. Fusion 2017, 57, 092005. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent developments in irradiation-resistant steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Luo, L.; Li, B.; Liu, T.; Zhao, J.; Xu, B.; Wang, L.; Su, Y.; Guo, J.; et al. Laser-based powder bed fusion of pre-alloyed oxide dispersion strengthened steel containing yttrium. Addit. Manuf. 2022, 58, 103018. [Google Scholar] [CrossRef]
- Novotný, R.; Janík, P.; Penttilä, S.; Hähner, P.; Macák, J.; Siegl, J.; Haušild, P. High Cr ODS steels performance under super critical water environment. J. Supercrit. Fluids 2013, 81, 147–156. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Baluc, N. Microstructure and Charpy impact properties of 12–14Cr oxide dispersion-strengthened ferritic steels. J. Nucl. Mater. 2008, 374, 178–184. [Google Scholar] [CrossRef]
- Száraz, Z.; Török, G.; Kršjak, V.; Hähner, P. SANS investigation of microstructure evolution in high chromium ODS steels after thermal ageing. J. Nucl. Mater. 2013, 435, 56–62. [Google Scholar] [CrossRef]
- Turba, K.; Hurst, R.C.; Hähner, P. Anisotropic mechanical properties of the MA956 ODS steel characterized by the small punch testing technique. J. Nucl. Mater. 2012, 428, 76–81. [Google Scholar] [CrossRef]
- Parida, P.K.; Dasgupta, A.; Bajpai, S.; Sakthivel, T.; Mythili, R. Synthesis and characterization of 9Cr ODS F/M steel with optimized Y2O3 and Ti content and its comparison with P91 steel. Powder Technol. 2023, 425, 118564. [Google Scholar] [CrossRef]
- Wang, J.; Long, D.; Yu, L.; Liu, Y.; Li, H.; Wang, Z. Influence of Al addition on the microstructure and mechanical properties of Zr-containing 9Cr-ODS steel. J. Mater. Res. Technol. 2021, 13, 1698–1708. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Zou, J.; Li, X.; Cui, D.; Shen, Z. Oxide dispersion strengthened stainless steel 316L with superior strength and ductility by selective laser melting. J. Mater. Sci. Technol. 2020, 42, 97–105. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, K.; Qian, Z.; Dong, J.; Wu, J.; Ma, Z. Simultaneous enhancement of strength and ductility in selective laser melting manufactured 316L alloy by employing Y2O3 coated spherical powder as precursor. J. Alloys Compd. 2022, 899, 163262. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Liu, L.; Li, Q.; Liu, L.; Liu, F.; Huang, C. Effect of grain size and distribution on the corrosion behavior of Y2O3 dispersion-strengthened 304 stainless steel. Mater. Today Commun. 2022, 31, 103723. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Song, L.; Yang, X.; Zhao, Y.; Mao, X. Effect of silicon on oxidation behavior of 9Cr-ODS steel at 650 °C. Fusion Eng. Des. 2021, 167, 112384. [Google Scholar] [CrossRef]
- Getto, E.; Baker, B.; Tobie, B.; Briggs, S.; Hattar, K.; Knipling, K. Effect of friction stir welding and self-ion irradiation on dis persoid evolution in oxide dispersion strengthened steel MA956 up to 25 dpa. J. Nucl. Mater. 2019, 515, 407–419. [Google Scholar] [CrossRef]
- Yao, C.F.; Dai, Y. DBTT shift of Optifer-IX, Eurofer 97 and MA956 steels after irradiation evaluated with small punch tests. J. Nucl. Mater. 2021, 544, 152725. [Google Scholar] [CrossRef]
- Abbasi, M.; Kim, D.-I.; Shim, J.-H.; Jung, W.-S. Effects of alloyed aluminum and titanium on the oxidation behavior of IN CONEL 740 superalloy. J. Alloys Compd. 2016, 658, 210–221. [Google Scholar] [CrossRef]
- He, W.; Liu, F.; Tan, L.; Huang, L.; Nie, Y.; Wang, G.; Zhan, X.; Qin, Z. Bimodal-grained high-strength nickel-base ODS alloy fabricated by mechanical alloying and hot extrusion. Mater. Today Commun. 2021, 26, 101921. [Google Scholar] [CrossRef]
- Li, C.; Lei, G.; Liu, J.; Liu, A.; Ren, C.L.; Huang, H. A potential candidate structural material for molten salt reactor: ODS nickel-based alloy. J. Mater. Sci. Technol. 2022, 109, 129–139. [Google Scholar] [CrossRef]
- Gaikwad, S.D.; Ajay, P.; Dabhade, V.V.; Murty, S.V.S.N.; Manwatkar, S.; Prakash, U. Mechanical properties and microstruc tural analysis of ultra-fine grained Ni-based ODS alloy processed by powder forging. J. Alloys Compd. 2024, 970, 172614. [Google Scholar] [CrossRef]
- Ren, F.; Zhi, A.; Zhang, D.; Tian, B.; Volinsky, A.A.; Shen, X. Preparation of Cu–Al2O3 bulk nano-composites by combining Cu–Al alloy sheets internal oxidation with hot extrusion. J. Alloys Compd. 2015, 633, 323–328. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Shim, S. Nano-scale oxide dispersoids by internal oxidation of Fe–Ti–Y intermetallics. Mater. Sci. Eng. A 2008, 488, 134–138. [Google Scholar] [CrossRef]
- Zhao, S.; Song, G.; Zhang, T. Microstructures, electrical and mechanical properties of an ODS-Cu alloy containing multi-scale complex oxides. Mater. Sci. Eng. A 2024, 890, 145905. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Chung, S.; Lee, T.; Jeong, W.; Kong, B.S.; Ryu, H.J. Additive manufacturing of oxide dispersion strengthened CoCrNi medium entropy alloy by in situ oxide synthesis. J. Alloys Compd. 2023, 965, 171340. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Liu, C.T.; Hoelzer, D.T.; Mills, M.J.; Sarosi, P.; Hayashi, T.; Wendt, U.; Heyse, H. Development of porosity in an oxide dispersion-strengthened ferritic alloy containing nanoscale oxide particles. Scr. Mater. 2007, 57, 1040–1043. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, P.; Xu, J.; Ye, W.; Yin, S.; Zhao, J.; Qiao, Y.; Yan, Y. Optimization of microstructure and tensile properties for a 13Cr-1W ODS steel prepared by mechanical alloying and spark plasma sintering using pre-alloyed powder. Mater. Charact. 2024, 207, 113581. [Google Scholar] [CrossRef]
- Aghajani, S.; Wu, C.; Li, Q.; Fang, J. Additively manufactured composite lattices: A state-of-the-art review on fabrications, architecture, constituent materials, mechanical properties, and future directions. Thin Walled Struct. 2024, 197, 111539. [Google Scholar] [CrossRef]
- Kharat, V.J.; Singh, P.; Sharath Raju, G.; Kumar Yadav, D.; Gupta, M.S.; Arun, V.; Hussein Majeed, A.; Singh, N. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Zinatlou Ajabshir, S.; Sofia, D.; Hare, C.; Barletta, D.; Poletto, M. Experimental characterization of the spreading of polymeric powders in powder bed fusion additive manufacturing process at changing temperature conditions. Adv. Powder Technol. 2024, 35, 104412. [Google Scholar] [CrossRef]
- Batalha, G.F.; Silva, L.C.; Coelho, R.S.; Teixeira, M.C.C.; Castro, T.L.; Pereira, M.V.S.; Adamiak, M.; Pawlyta, M.; Krzeminski, L.; Bialas, O.; et al. Mechanical properties characterization of Ti-6Al-4 V grade 5 (recycled) additively manufactured by selective electron beam melting (EB-PBF). Eng. Fail. Anal. 2024, 157, 107892. [Google Scholar] [CrossRef]
- Dejene, N.D.; Lemu, H.G. Current status and challenges of powder bed fusion-based metal additive manufacturing: Literature review. Metals 2023, 13, 424. [Google Scholar] [CrossRef]
- Doñate-Buendia, C.; Streubel, R.; Kürnsteiner, P.; Wilms, M.B.; Stern, F.; Tenkamp, J.; Bruder, E.; Barcikowski, S.; Gault, B.; Durst, K.; et al. Effect of nanoparticle additivation on the microstructure and microhardness of oxide dispersion strengthened steels produced by laser powder bed fusion and directed energy deposition. Procedia CIRP 2020, 94, 41–45. [Google Scholar] [CrossRef]
- Era, I.Z.; Farahani, M.A.; Wuest, T.; Liu, Z. Machine learning in Directed Energy Deposition (DED) additive manufacturing: A state-of-the-art review. Manuf. Lett. 2023, 35, 689–700. [Google Scholar] [CrossRef]
- Yi, H.; Jia, L.; Ding, J.; Li, H. Achieving material diversity in wire arc additive manufacturing: Leaping from alloys to compo sites via wire innovation. Int. J. Mach. Tools Manuf. 2024, 194, 104103. [Google Scholar] [CrossRef]
- Raghavendra Pai, K.; Vijayan, V.; Narayan Prabhu, K. Recent challenges and advances in metal additive manufacturing: A review. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Sun, F.; Liu, P.; Zhou, H.; Fu, S. The current state of CuCrZr and CuCrNb alloys manufactured by additive manufacturing: A review. Mater. Des. 2022, 224, 111419. [Google Scholar] [CrossRef]
- Yan, F.; Xiong, W.; Faierson, E.; Olson, G.B. Characterization of nano-scale oxides in austenitic stainless steel processed by powder bed fusion. Scr. Mater. 2018, 155, 104–108. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Wei, Y.; Zhou, X.; Shen, Y.; Zhou, Y.; Feng, X. Microstructure and mechanical performance of ODS super alloys manufactured by selective laser melting. Opt. Laser Technol. 2021, 144, 107423. [Google Scholar] [CrossRef]
- Xu, R.; Geng, Z.; Wu, Y.; Chen, C.; Ni, M.; Li, D.; Zhang, T.; Huang, H.; Liu, F.; Li, R.; et al. Microstructure and mechanical properties of in-situ oxide-dispersion-strengthened NiCrFeY alloy produced by laser powder bed fusion. Adv. Powder Mater. 2022, 1, 100056. [Google Scholar] [CrossRef]
- Li, A.; Chen, Q.; Wang, P.; Mao, J.; Wu, X.; Xin, H.; Fang, Z.; Teng, C.; Wu, L.; Tang, J. Laser powder bed fusion of oxide dispersion strengthened FeCrAl alloy: Processing and microstructural evolution. Mater. Charact. 2024, 208, 113590. [Google Scholar] [CrossRef]
- Kumar, S. Comprehensive review on high entropy alloy-based coating. Surf. Coat. Technol. 2024, 477, 130327. [Google Scholar] [CrossRef]
- Kim, Y.K.; Baek, M.S.; Yang, S.; Lee, K.A. In-situ formed oxide enables extraordinary high-cycle fatigue resistance in additively manufactured CoCrFeMnNi high-entropy alloy. Addit. Manuf. 2021, 38, 101832. [Google Scholar] [CrossRef]
- Han, B.; Feng, K.; Li, Z.; Wang, Z.; Yu, Y.; Du, S. Oxide dispersion strengthening in CrCoNi medium entropy alloy built by selective laser melting with oxygen-rich powder. J. Alloys Compd. 2024, 973, 172798. [Google Scholar] [CrossRef]
- Velişa, G.; Fan, Z.; Crespillo, M.L.; Bei, H.; Weber, W.J.; Zhang, Y. Temperature effects on damage evolution in ion-irradiated NiCoCr concentrated solid-solution alloy. J. Alloys Compd. 2020, 832, 154918. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, H.; Wang, Z.; Gludovatz, B.; Zhang, Z.; George, E.P.; Yu, Q.; Mao, S.X.; Ritchie, R.O. Dislocation mechanisms and 3D twin architectures generate exceptional strength-ductility-toughness combination in CrCoNi medium-entropy alloy. Nat. Commun. 2017, 8, 14390. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhu, S.; Wang, T.; Chai, L.; Li, Q.; Luo, J. Effects of laser power on microstructures and mechanical properties of CoCrFeNiMn high entropy alloy with the addition of Y2O3 by directed energy deposition. Opt. Laser Technol. 2024, 169, 110122. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Luo, L.; Oliveira, J.P.; Li, B.; Yan, H.; Liu, T.; Zhao, J.; Wang, L.; Su, Y.; et al. Effects of process atmosphere on additively manufactured FeCrAl oxide dispersion strengthened steel: Printability, microstructure and tensile properties. Mater. Sci. Eng. A 2023, 882, 145438. [Google Scholar] [CrossRef]
- Bertsch, K.M.; Meric de Bellefon, G.; Kuehl, B.; Thoma, D.J. Origin of dislocation structures in an additively manufactured austenitic stainless steel 316L. Acta Mater. 2020, 199, 19–33. [Google Scholar] [CrossRef]
- Grandhi, M.; Ma, C.; Liu, Z.; Kang, B. Microstructural characterization and properties of laser-DED built oxide dispersion strengthened SS 316 L. Manuf. Lett. 2022, 33, 758–764. [Google Scholar] [CrossRef]
- Li, H.; Shi, X.; Wu, B.; Corradi, D.R.; Pan, Z.; Li, H. Wire arc additive manufacturing: A review on digital twinning and visual ization process. J. Manuf. Process. 2024, 116, 293–305. [Google Scholar] [CrossRef]
- Kumar, N.; Bhavsar, H.; Mahesh, P.V.S.; Srivastava, A.K.; Bora, B.J.; Saxena, A.; Dixit, A.R. Wire Arc Additive Manufacturing—A revolutionary method in additive manufacturing. Mater. Chem. Phys. 2022, 285, 126144. [Google Scholar] [CrossRef]
- Singh Tanwar, R.; Jhavar, S. Ti based alloys for aerospace and biomedical applications fabricated through wire arc additive manufacturing (WAAM). Mater. Today Proc. 2024, 98, 226–232. [Google Scholar] [CrossRef]
- Evans, S.I.; Wang, J.; Qin, J.; He, Y.; Shepherd, P.; Ding, J. A review of WAAM for steel construction—Manufacturing, material and geometric properties, design, and future directions. Structures 2022, 44, 1506–1522. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z.; Chi, P.; Zhang, B.; Ding, T.; Zhang, Q. Double pulsed current adopted in local dry underwater WAAM to simultaneously enhanced strength and ductility of 308 L multi-layer component. J. Manuf. Process. 2024, 118, 389–406. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, S.; Zhou, Q.; Peng, J.; Luo, H.; Guo, L.; Li, H.; Yan, Y. Preliminary study on the microstructure and hardness of wire and arc additively manufactured ODS-RAFM steel subjected to 2.5 MeV Fe ion irradiation. Nucl. Mater. Energy 2023, 36, 101490. [Google Scholar] [CrossRef]
- Shi, S.; Liu, X.; Xie, G.; Chen, X. Enhanced cyclic stress response and low-cycle fatigue life of modified 9Cr-1Mo steel by wire-arc additive manufacturing and post-heat treatment. Int. J. Fatigue 2024, 184, 108333. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, S.; Jiang, Y.; Zhou, Q.; Peng, J.; Yan, Y. Wire and arc additive manufacturing fabrication of ODS-RAFM steels and preliminary evaluation on microstructures and mechanical properties. J. Nucl. Mater. 2022, 572, 154068. [Google Scholar] [CrossRef]
- Dong, Z.; Torbati-Sarraf, H.; Huang, C.; Xu, K.; Gu, X.-L.; Fu, C.; Liu, X.; Meng, Z. Microstructure and corrosion behaviour of structural steel fabricated by wire arc additive manufacturing (WAAM). Mater. Des. 2024, 244, 113158. [Google Scholar] [CrossRef]
- Michel, L.; Sanchez, A.M.A.; Silvestru, V.A.; Ariza, I.; Taras, A.; Angst, U. Corrosion behaviour of point-by-point wire and arc additively manufactured steel bars. Mater. Corros. 2022, 73, 996–1014. [Google Scholar] [CrossRef]
- Mannan, M.S.; Jang, C. High temperature corrosion of wrought and wire arc additively manufactured 316L stainless steel in a simulated boiler environment. J. Mater. Res. Technol. 2024, 32, 4278–4292. [Google Scholar] [CrossRef]
- Scipioni Bertoli, U.; Guss, G.; Wu, S.; Matthews, M.J.; Schoenung, J.M. In-situ characterization of laser-powder interaction and cooling rates through high-speed imaging of powder bed fusion additive manufacturing. Mater. Des. 2017, 135, 385–396. [Google Scholar] [CrossRef]
- Thampy, V.; Fong, A.Y.; Calta, N.P.; Wang, J.; Martin, A.A.; Depond, P.J.; Kiss, A.M.; Guss, G.; Xing, Q.; Ott, R.T.; et al. Subsurface cooling rates and microstructural response during laser based metal additive manufacturing. Sci. Rep. 2020, 10, 1981. [Google Scholar] [CrossRef]
- Shen, N.; Chou, K. Thermal modeling of electron beam additive manufacturing process: Powder sintering effects. In Proceedings of the International Manufacturing Science and Engineering Conference, Notre Dame, IN, USA, 4–8 June 2012; pp. 287–295. [Google Scholar] [CrossRef]
- Vasquez, E.; Giroux, P.-F.; Lomello, F.; Chniouel, A.; Maskrot, H.; Schuster, F.; Castany, P. Elaboration of oxide dispersion strengthened Fe-14Cr stainless steel by selective laser melting. J. Mater. Process. Technol. 2019, 267, 403–413. [Google Scholar] [CrossRef]
- Horn, T.; Rock, C.; Kaoumi, D.; Anderson, I.; White, E.; Prost, T.; Rieken, J.; Saptarshi, S.; Schoell, R.; DeJong, M.; et al. Laser powder bed fusion additive manufacturing of oxide dispersion strengthened steel using gas atomized reaction synthesis powder. Mater. Des. 2022, 216, 110574. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Chen, M.; Zuo, L.; Hou, B.; Wang, Z. Microstructure and mechanical property of in-situ nano-particle strengthened ferritic steel by novel internal oxidation. Mater. Sci. Eng. A 2014, 609, 293–299. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, Y.; Qian, Z.; Wu, J.; Dong, J.; Ma, Z.; Liu, Y. Crack elimination and mechanical properties enhancement in additive manufactured Hastelloy X via in-situ chemical doping of Y2O3. Mater. Sci. Eng. A 2021, 824, 141867. [Google Scholar] [CrossRef]
- Kenel, C.; De Luca, A.; Joglekar, S.S.; Leinenbach, C.; Dunand, D.C. Evolution of Y2O3 dispersoids during laser powder bed fusion of oxide dispersion strengthened Ni-Cr-Al-Ti γ/γ’ superalloy. Addit. Manuf. 2021, 47, 102224. [Google Scholar] [CrossRef]
- Doñate Buendia, C.; Kürnsteiner, P.; Stern, F.; Wilms, M.B.; Streubel, R.; Kusoglu, I.M.; Tenkamp, J.; Bruder, E.; Pirch, N.; Bacikowski, S.; et al. Microstructure formation and mechanical properties of ODS steels built by laser additive manufacturing of nanoparticle coated iron-chromium powders. Acta Mater. 2021, 206, 116566. [Google Scholar] [CrossRef]
- Ou, W.; Mukherjee, T.; Knapp, G.L.; Wei, Y.; DebRoy, T. Fusion zone geometries, cooling rates and solidification parameters during wire arc additive manufacturing. Int. J. Heat Mass Transf. 2018, 127, 1084–1094. [Google Scholar] [CrossRef]
- Streubel, R.; Wilms, M.B.; Doñate-Buendía, C.; Weisheit, A.; Barcikowski, S.; Schleifenbaum, J.H.; Gökce, B. Depositing laser-generated nanoparticles on powders for additive manufacturing of oxide dispersed strengthened alloy parts via laser metal deposition. Jpn. J. Appl. Phys. 2018, 57, 040310. [Google Scholar] [CrossRef]
- Wang, A.; Yin, Y.; Lu, C.; Zheng, Q.; He, H.; Lin, L.; Shi, W.; Zhang, R.; Tie, D. Selective laser melting of 2507 duplex stainless steel: Effect of energy density on microstructure and corrosion resistance. J. Mater. Res. Technol. 2024, 33, 431–440. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Gupta, R.K. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
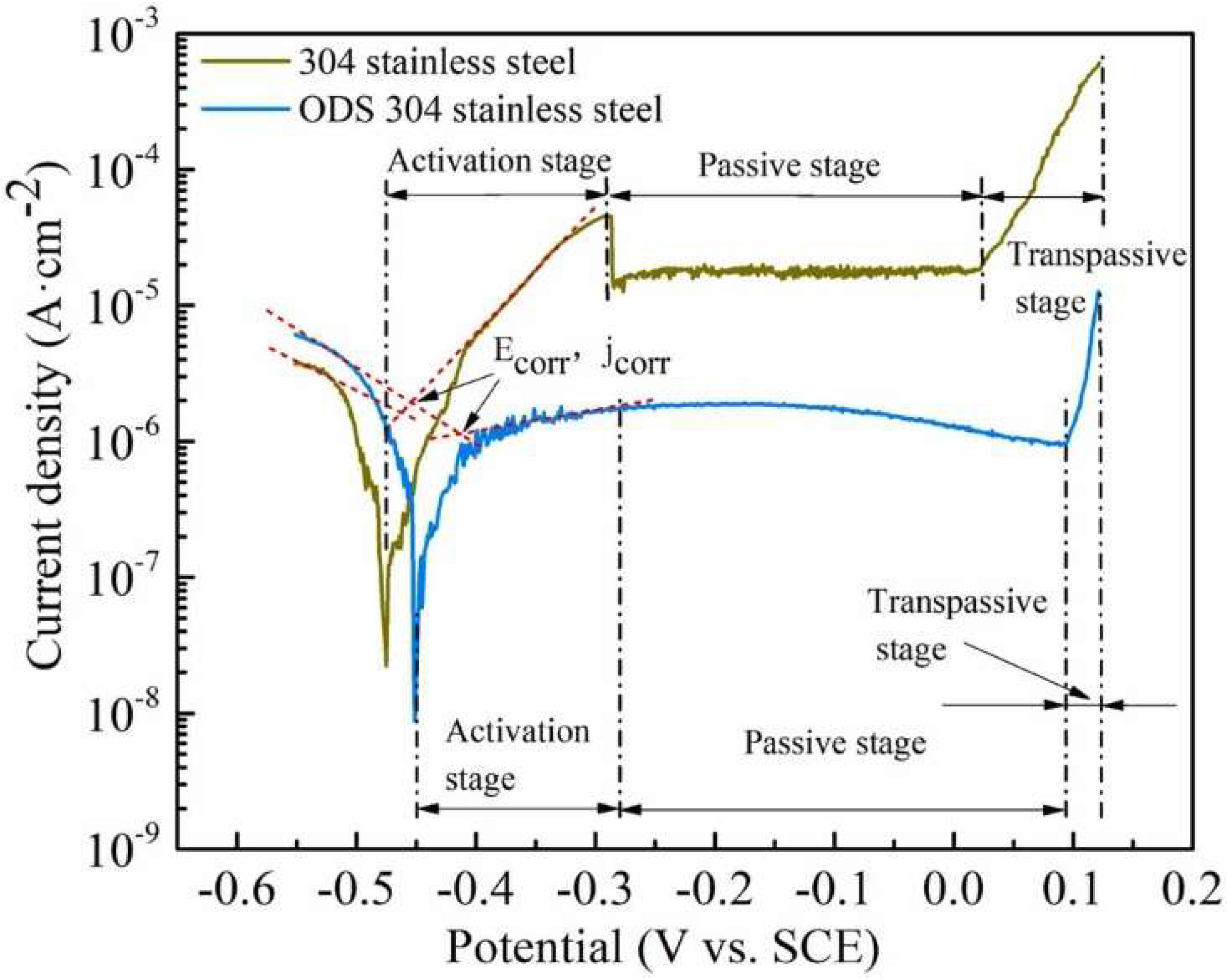
- Wang, Z.; Paschalidou, E.M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo enrichments in the passive oxide film on 316L austenitic stainless steel. Front. Mater. 2019, 6, 00232. [Google Scholar] [CrossRef]
- Voisin, T.; Forien, J.; Perron, A.; Aubry, S.; Bertin, N.; Samanta, A.; Baker, A.; Wang, Y.M. New insights on cellular structures strengthening mechanisms and thermal stability of an austenitic stainless steel fabricated by laser powder-bed-fusion. Acta Mater. 2021, 203, 116476. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, R.; Wei, X.; Xiao, M.; Yin, Y.; Qu, Y.; Li, H.; Liu, P.; Qiu, X.; Guo, T. An investigation on the oxidation behavior of spatters generated during the laser powder bed fusion of 316L stainless steel. Appl. Surf. Sci. 2022, 586, 152796. [Google Scholar] [CrossRef]
- Rott, S.; Ladewig, A.; Friedberger, K.; Casper, J.; Full, M.; Schleifenbaum, J.H. Surface roughness in laser powder bed fusion—Interdependency of surface orientation and laser incidence. Addit. Manuf. 2020, 36, 101437. [Google Scholar] [CrossRef]
- Bagehorn, S.; Wehr, J.; Maier, H.J. Application of mechanical surface finishing processes for roughness reduction and fatigue improvement of additively manufactured Ti-6Al-4V parts. Int. J. Fatigue 2017, 102, 135–142. [Google Scholar] [CrossRef]
- Uzan, N.E.; Ramati, S.; Shneck, R.; Frage, N.; Yeheskel, O. On the effect of shot-peening on fatigue resistance of AlSi10Mg specimens fabricated by additive manufacturing using selective laser melting (AM-SLM). Addit. Manuf. 2018, 21, 458–464. [Google Scholar] [CrossRef]
- Ali, U.; Fayazfar, H.; Ahmed, F.; Toyserkani, E. Internal surface roughness enhancement of parts made by laser powder-bed fusion additive manufacturing. Vacuum 2020, 177, 109314. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Gibson, I.; Ghasemi, A.; Ghaderi, A. Modelling of laser powder bed fusion process and analysing the effective parameters on surface characteristics of Ti-6Al-4V. Int. J. Mech. Sci. 2020, 168, 105299. [Google Scholar] [CrossRef]
- Bai, B.; Zheng, Q.; Ren, S.; Zhang, C.; Yang, W.; Hu, C. Composition design of high strength ODS alloy based on machine learning. At. Energy Sci. Technol. 2020, 54, 678–682. [Google Scholar]

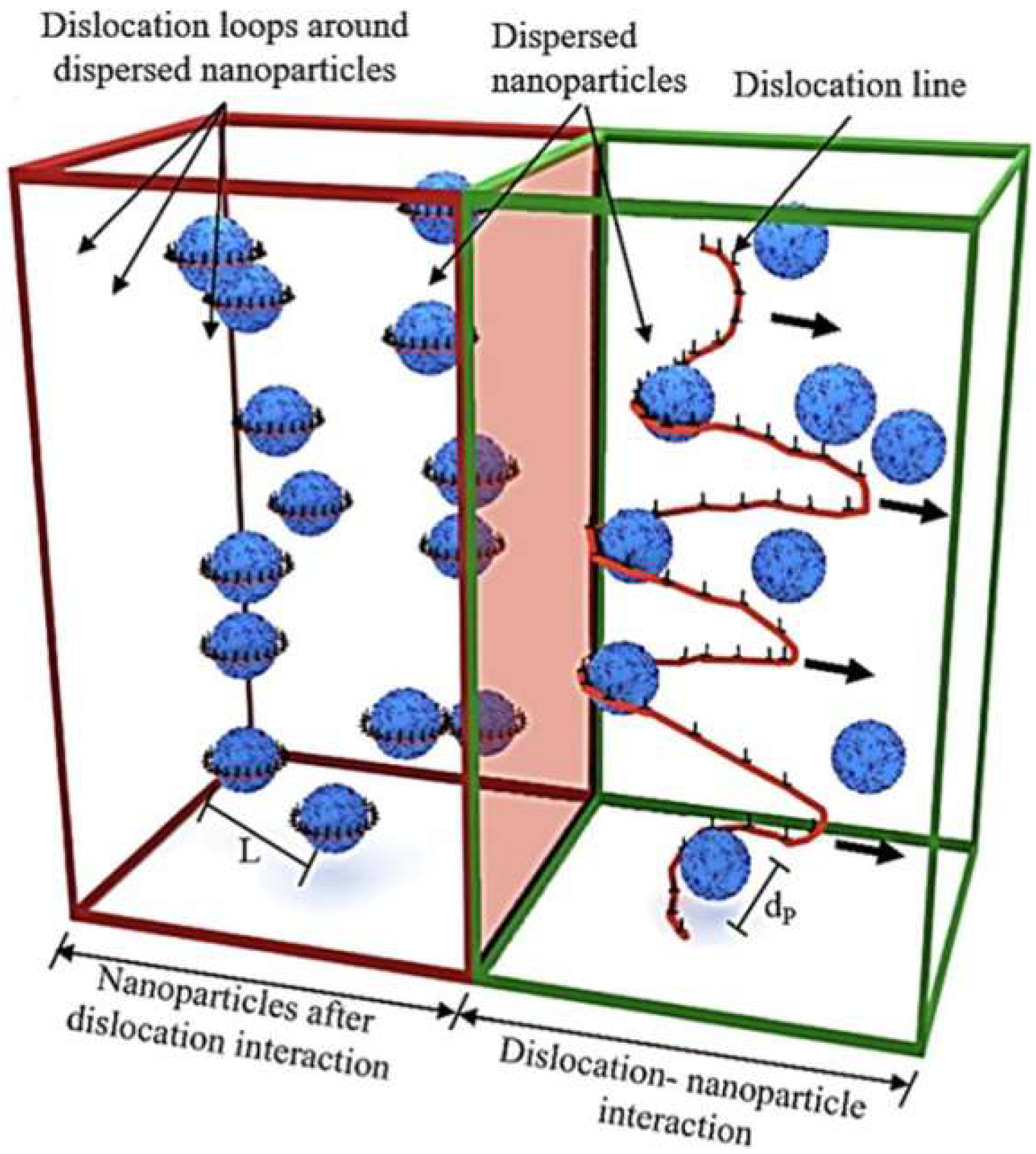
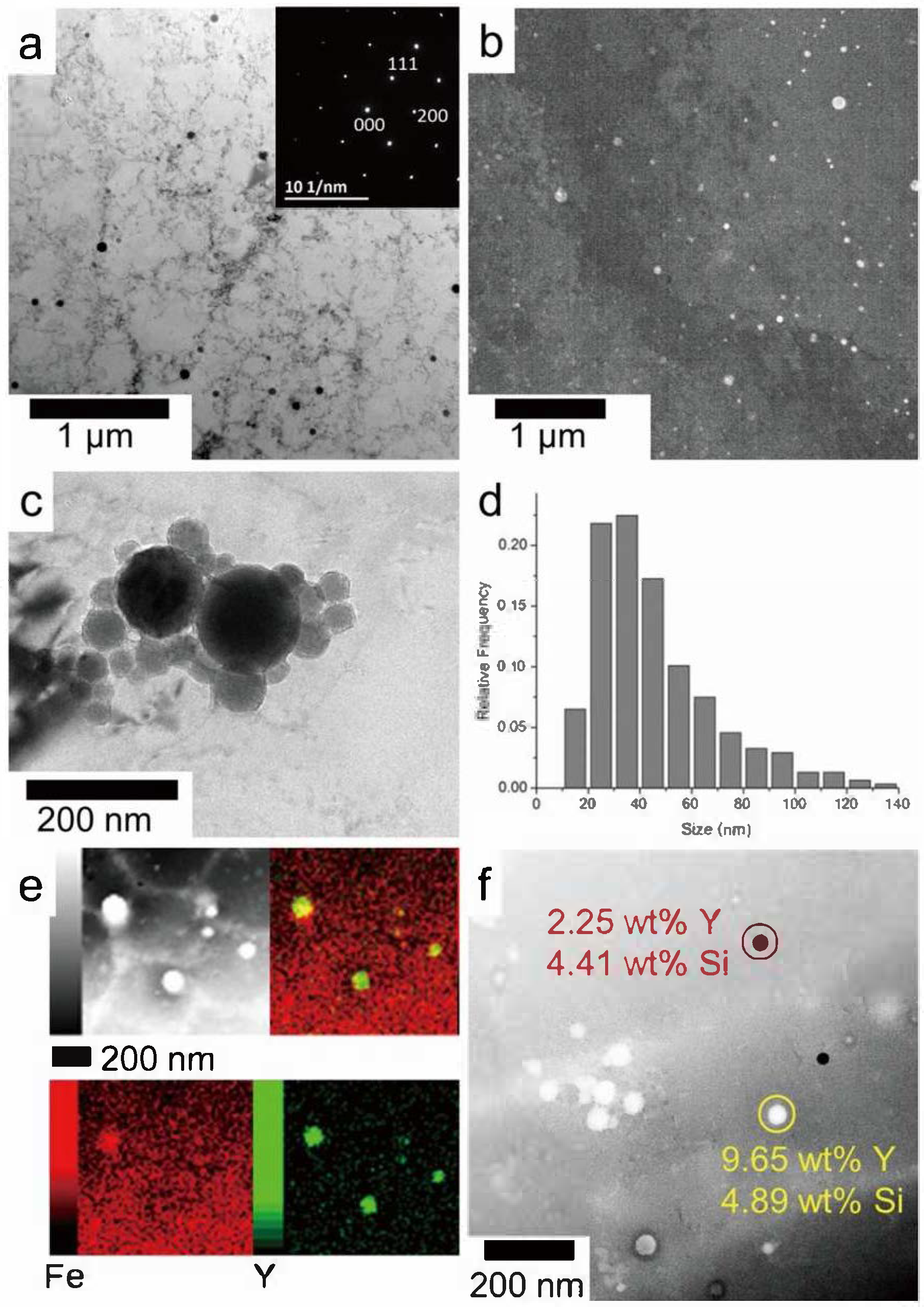
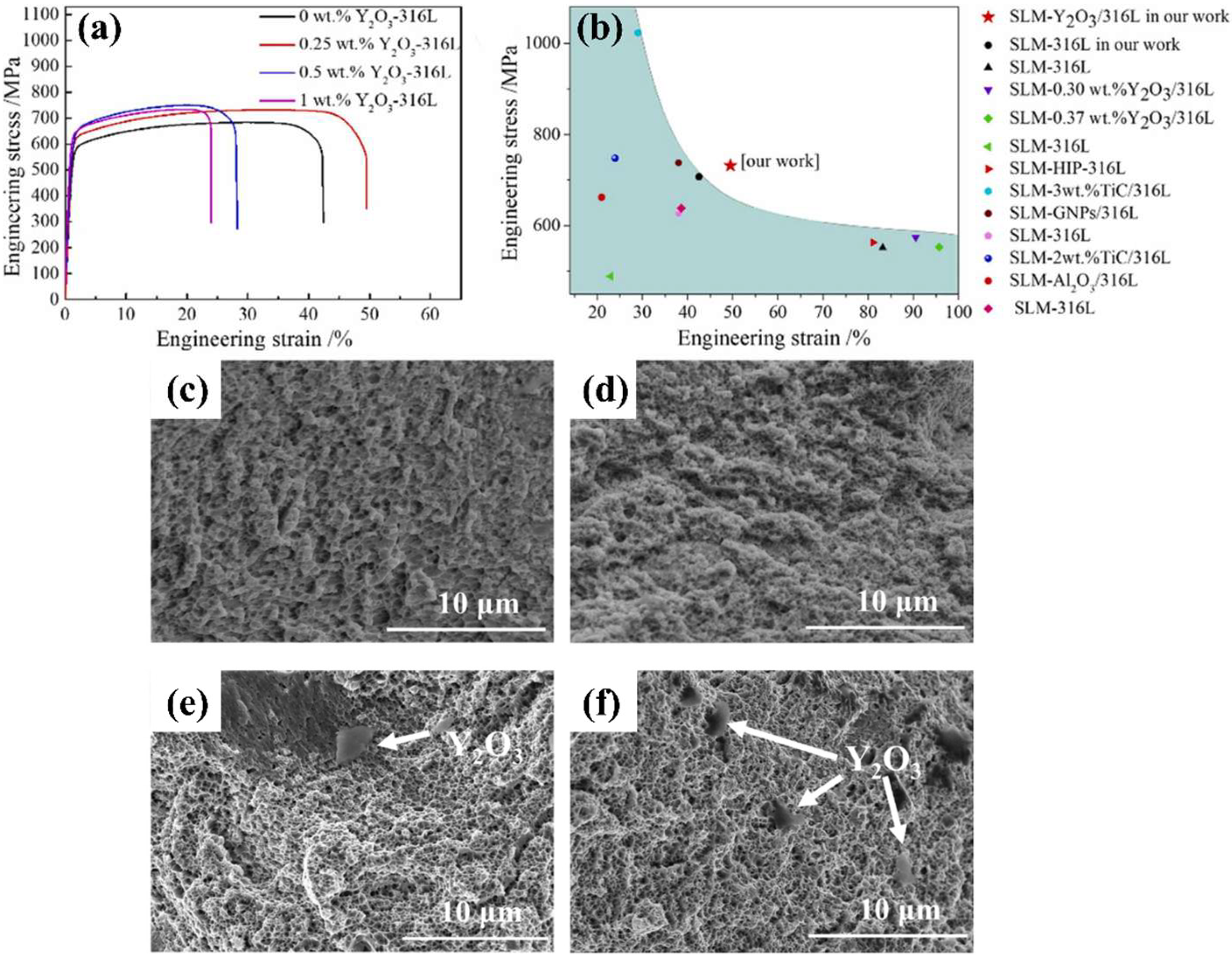
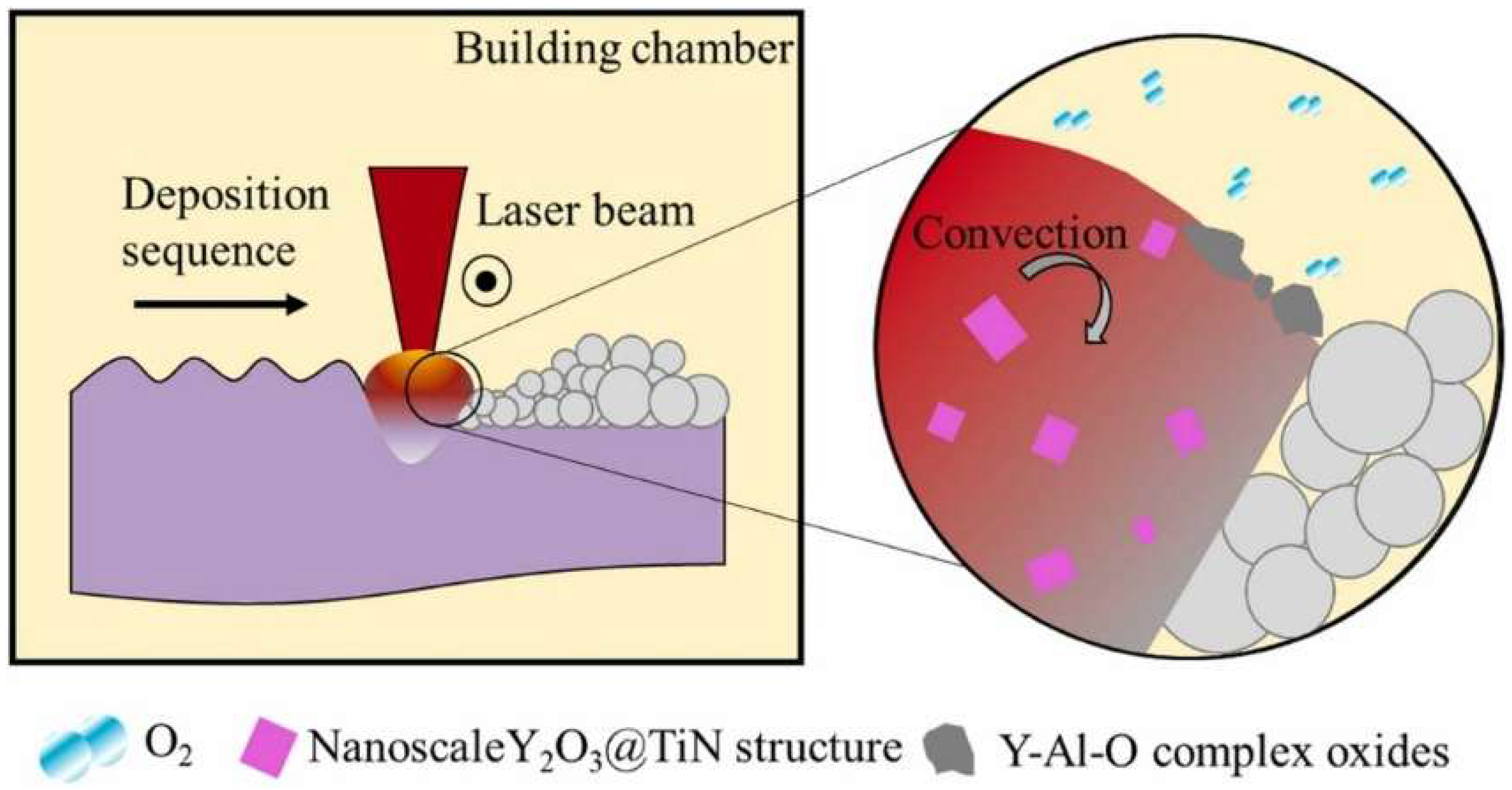
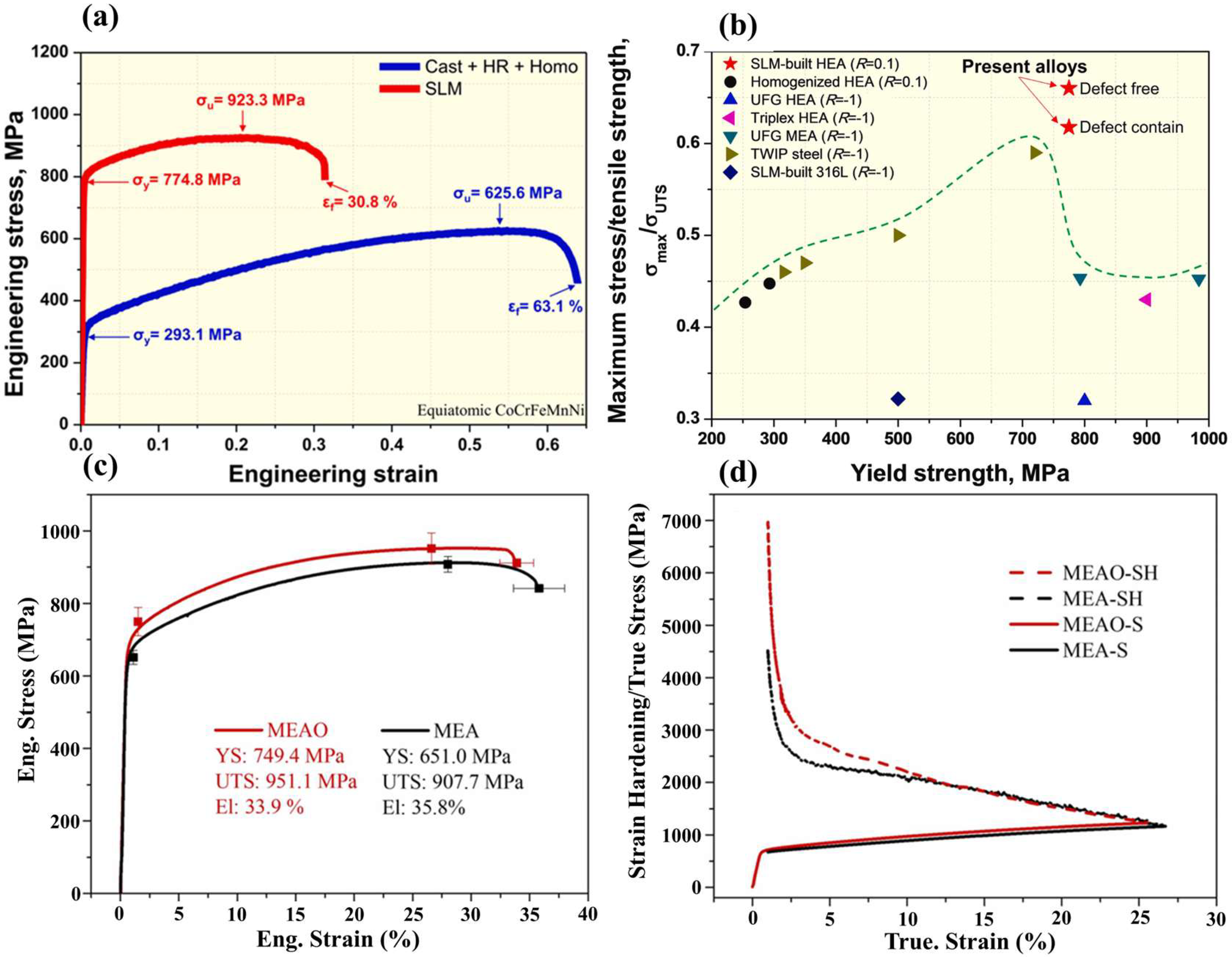
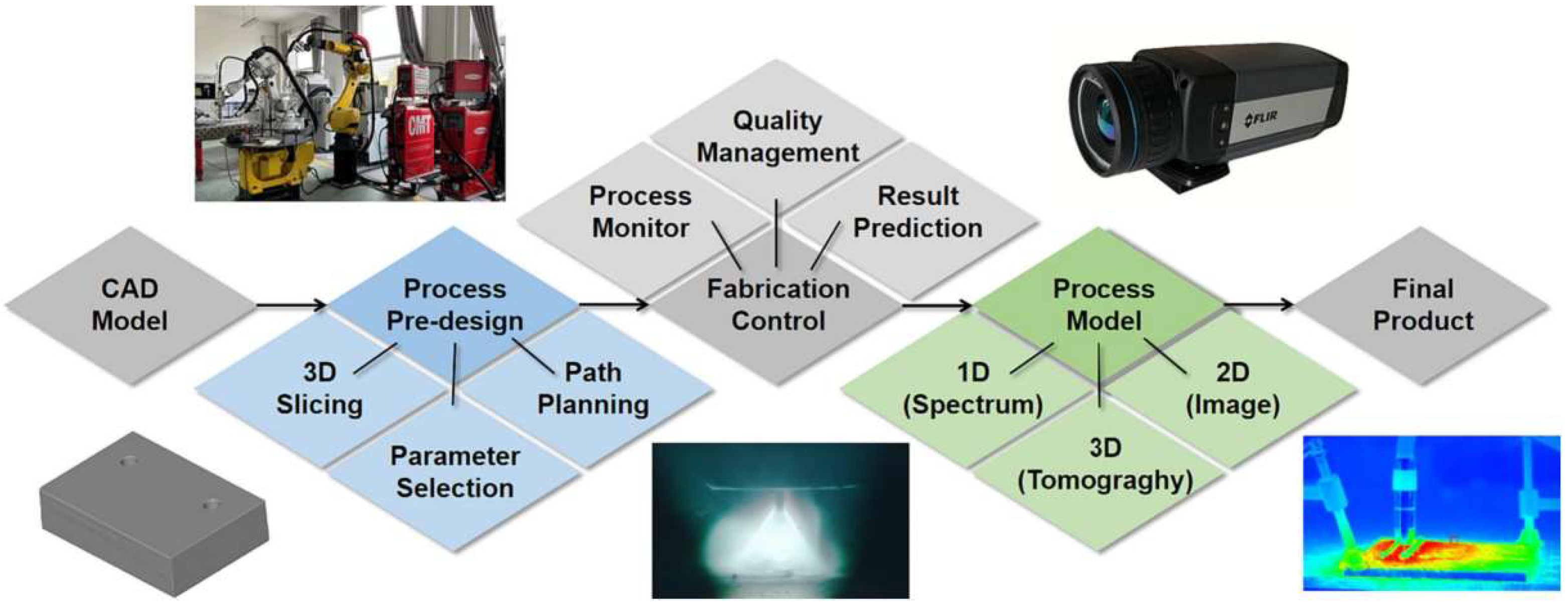
| Aspects | Advantage |
|---|---|
| Resolution | Micro-scale (μm) fine feature formation can be achieved. |
| Material Cost | It features extremely an high material utilization rate and lower comprehensive costs for small-batch and complex components. |
| Design Limitations | It has no minimum wall thickness limitation, enables free formation of complex internal structures, and breaks the “geometric constraints” of traditional manufacturing. |
| Processing Speed | Printing can be initiated directly from a digital model, eliminating the time required for mold design, manufacturing, and debugging in traditional manufacturing, thus achieving faster processing speed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Yin, Y.; Lu, C.; Cui, X.; Gao, Y.; Zhu, H.; Li, Z.; Shi, J.; Shi, W.; Tie, D. Research Progress on Laser Additive Manufacturing of Oxide Dispersion-Strengthened Alloys—A Review. Materials 2025, 18, 4094. https://doi.org/10.3390/ma18174094
Zheng Q, Yin Y, Lu C, Cui X, Gao Y, Zhu H, Li Z, Shi J, Shi W, Tie D. Research Progress on Laser Additive Manufacturing of Oxide Dispersion-Strengthened Alloys—A Review. Materials. 2025; 18(17):4094. https://doi.org/10.3390/ma18174094
Chicago/Turabian StyleZheng, Qian, Yan Yin, Chao Lu, Xiaoli Cui, Yutong Gao, Heng Zhu, Zhong Li, Junwei Shi, Wenqing Shi, and Di Tie. 2025. "Research Progress on Laser Additive Manufacturing of Oxide Dispersion-Strengthened Alloys—A Review" Materials 18, no. 17: 4094. https://doi.org/10.3390/ma18174094
APA StyleZheng, Q., Yin, Y., Lu, C., Cui, X., Gao, Y., Zhu, H., Li, Z., Shi, J., Shi, W., & Tie, D. (2025). Research Progress on Laser Additive Manufacturing of Oxide Dispersion-Strengthened Alloys—A Review. Materials, 18(17), 4094. https://doi.org/10.3390/ma18174094






