The Material Growth and Characteristics of Transition Metal Oxide Thin Films Based on Hot Wire Oxidation Sublimation Deposition Technology
Abstract
1. Introduction
2. Experimental Details
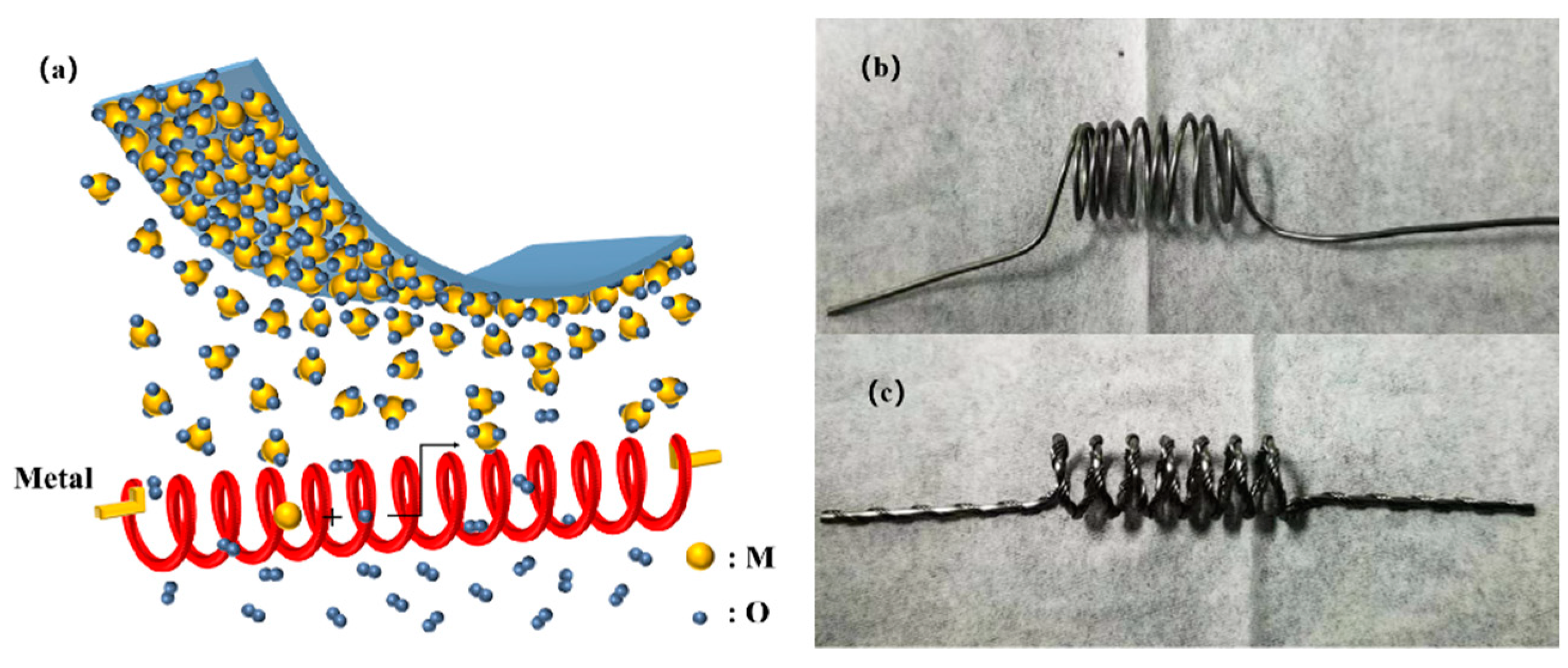
2.1. Hot Wire Oxidation Sublimation Deposition
2.2. Characterization
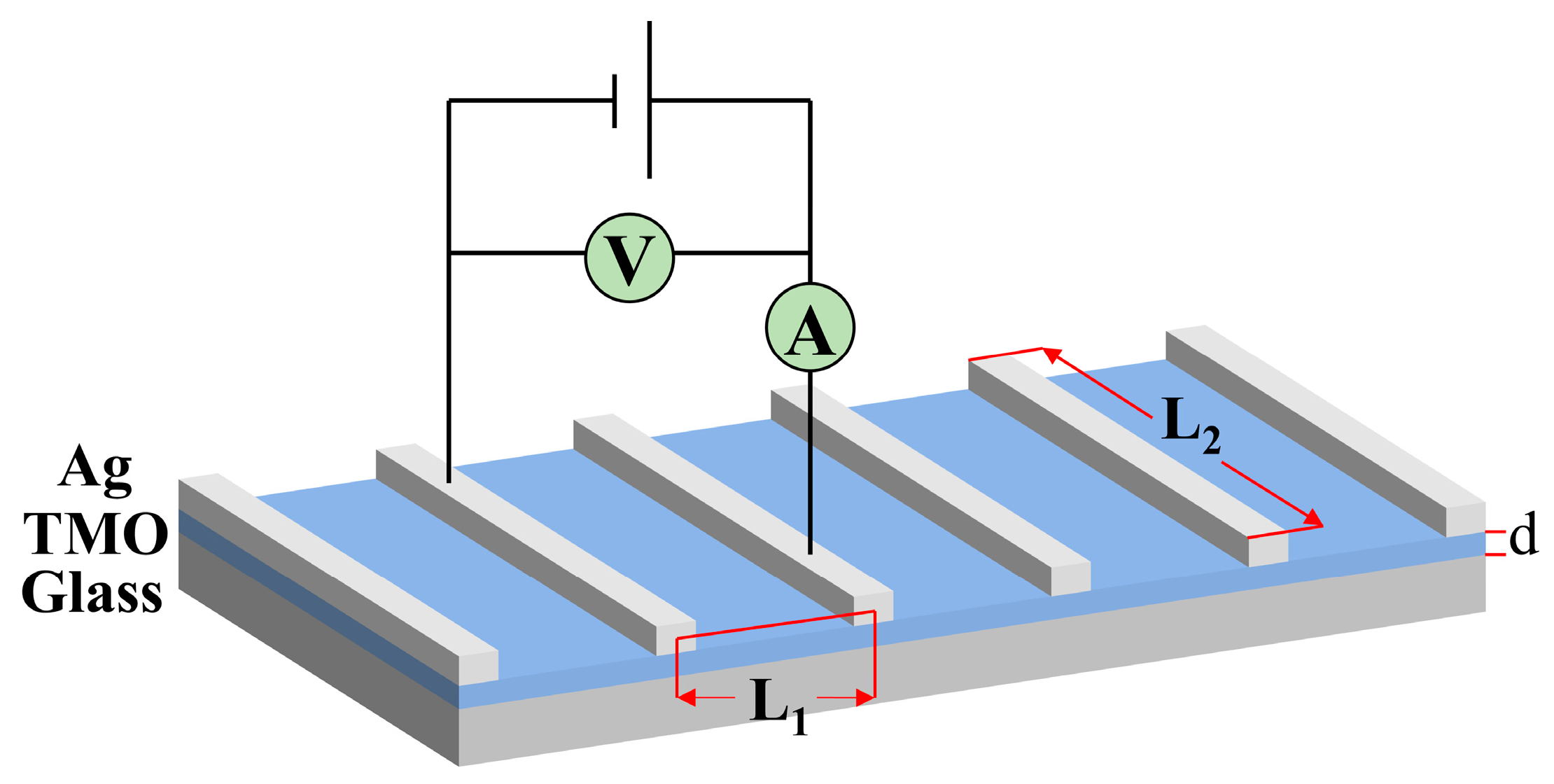
2.3. Fabrication and Characterization of Solar Cells
3. Results and Discussion
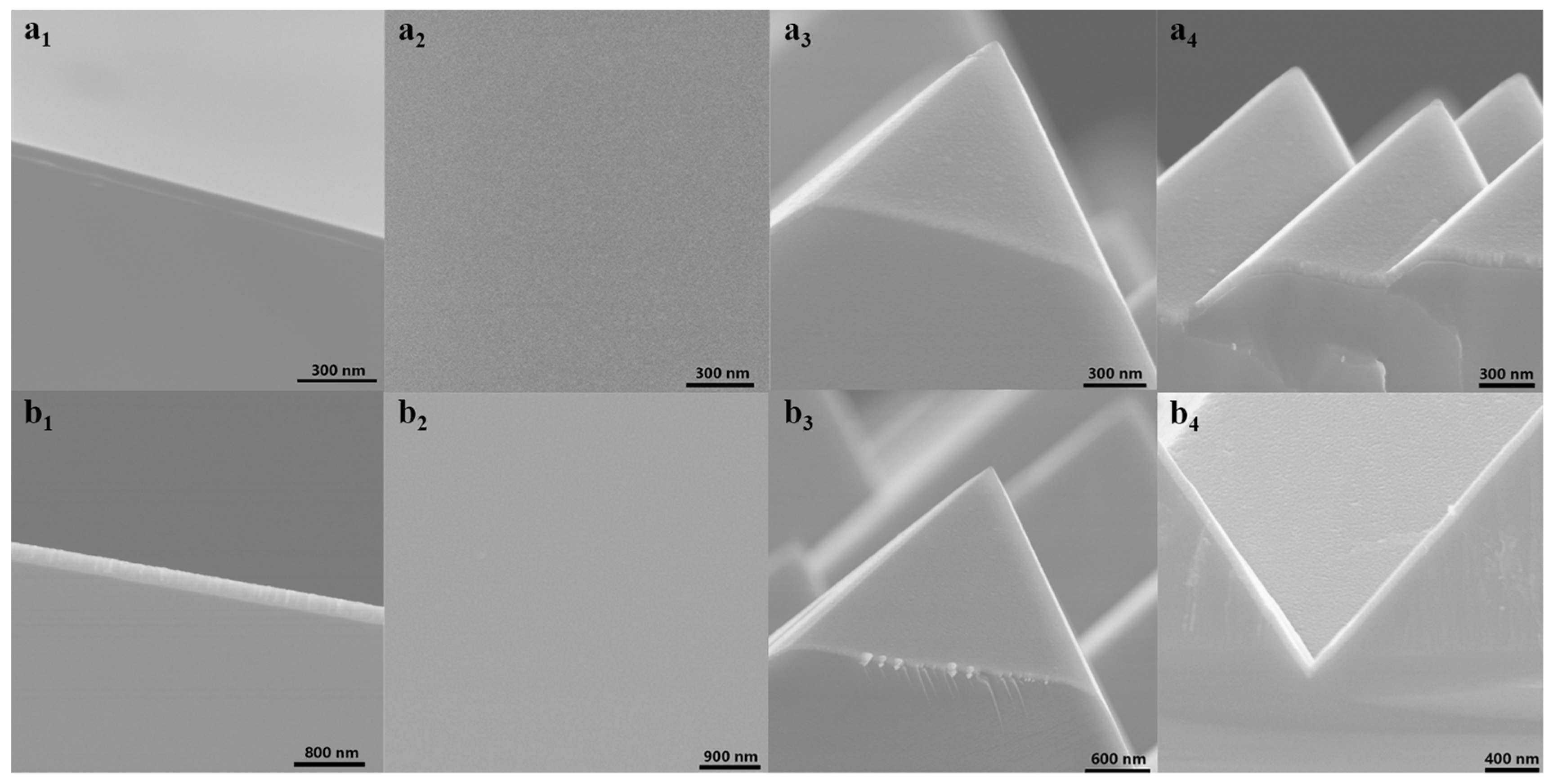
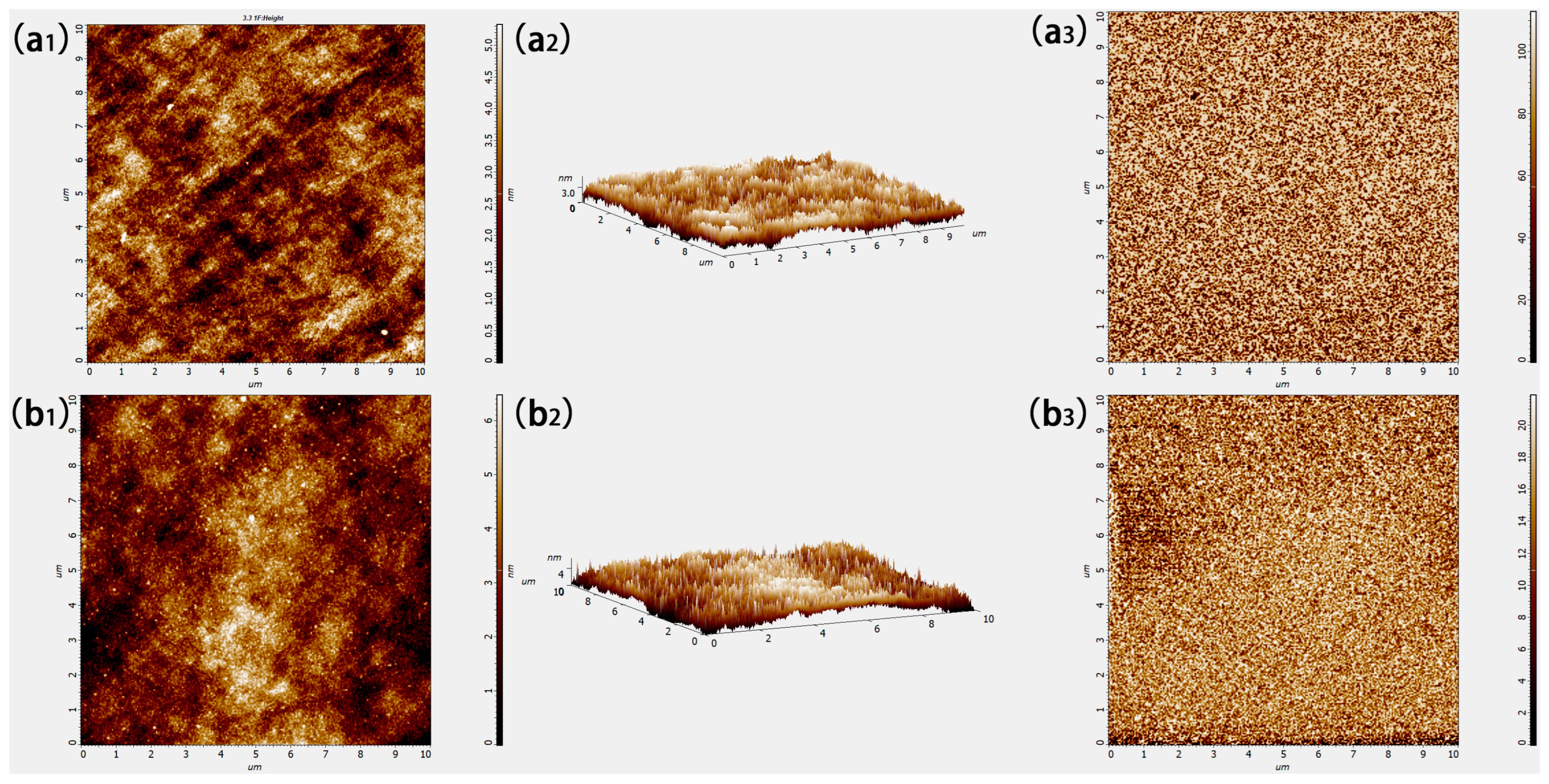
3.1. Morphology of MoOx and WOx Thin Films
3.2. Structural Characteristics of TMO Thin Films
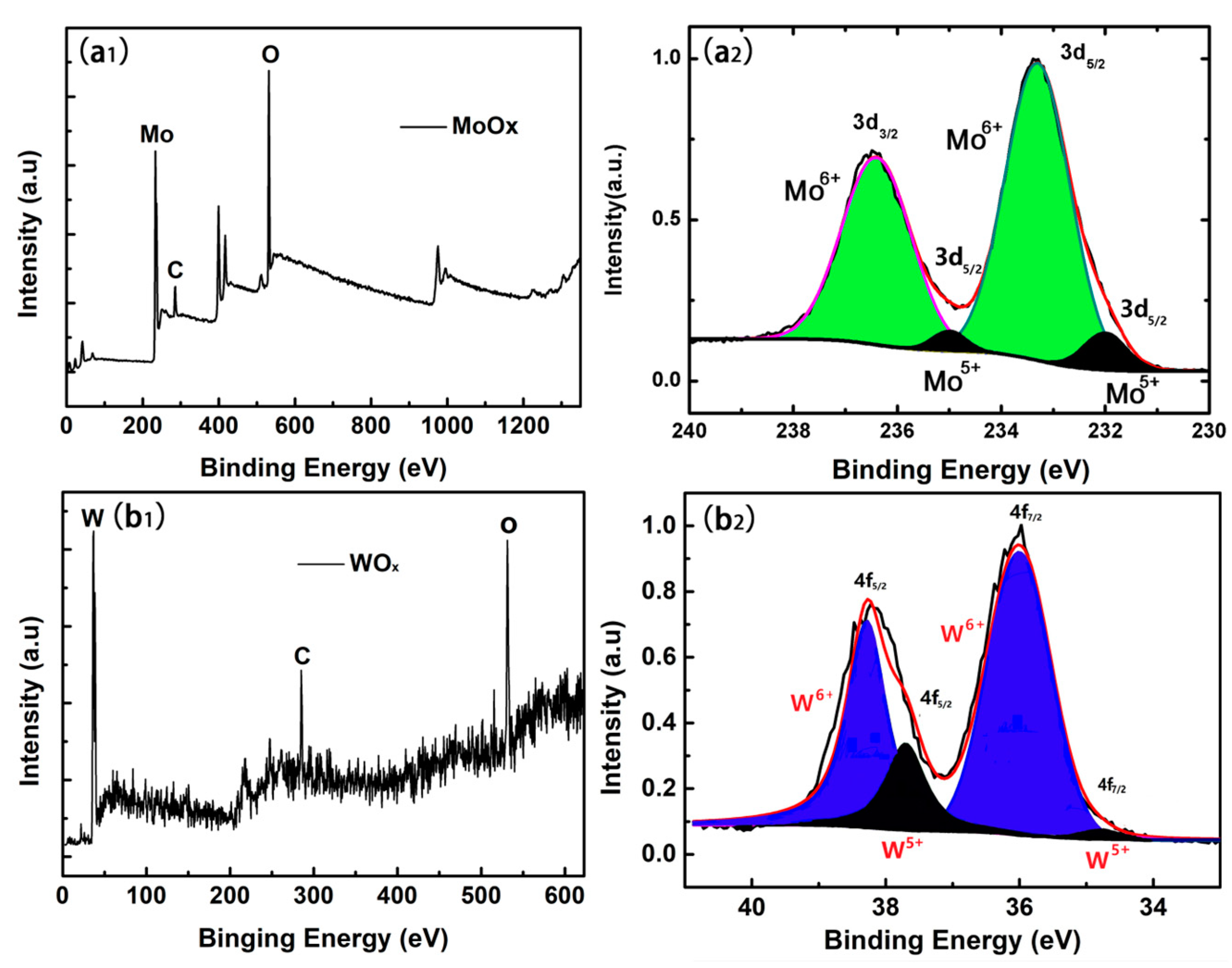
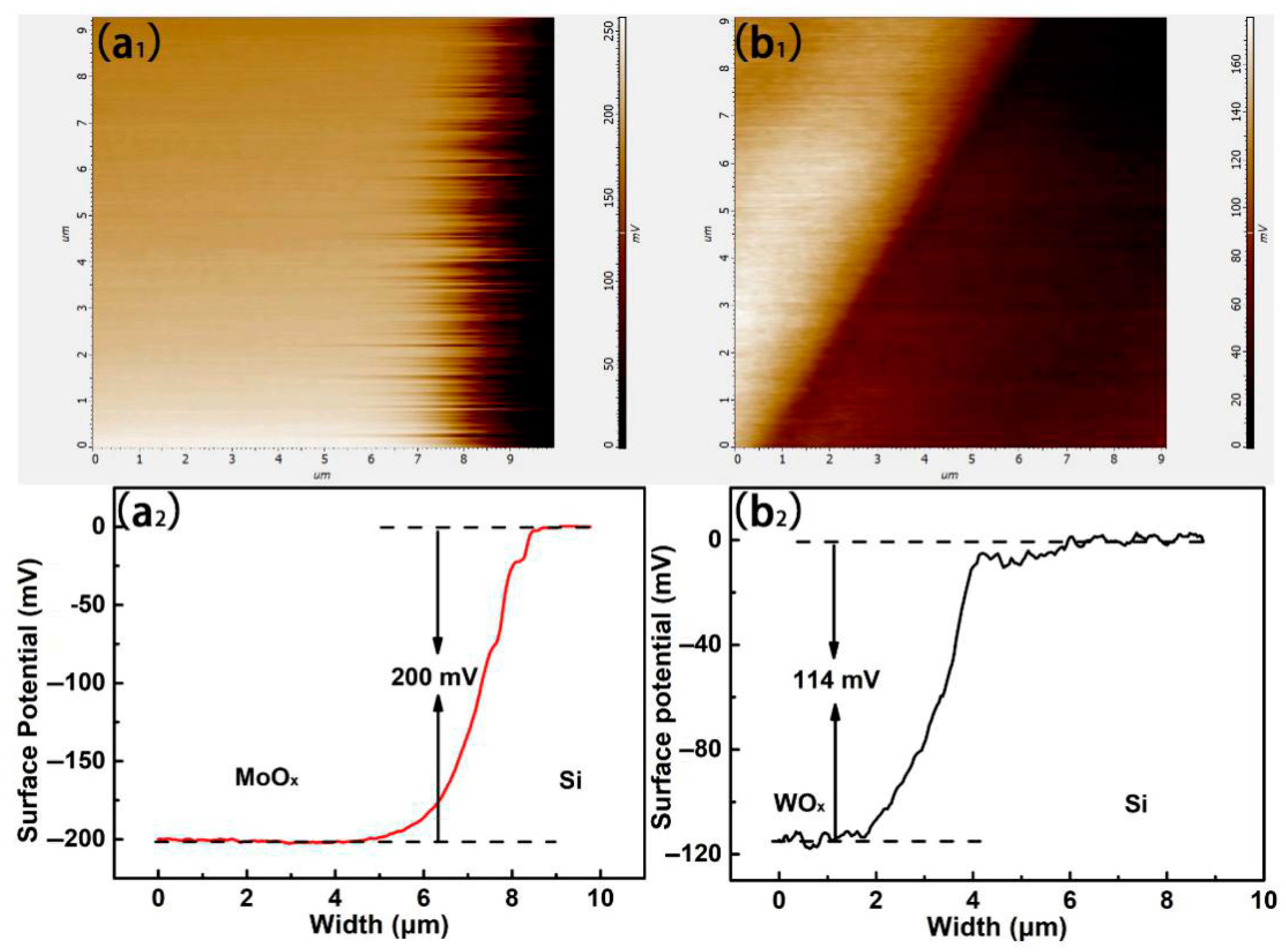
3.3. The Electronic Structure of TMO Thin Films
3.4. Optical Properties of MoOx and WOx
3.5. Electrical Characteristics of MoOx and WOx
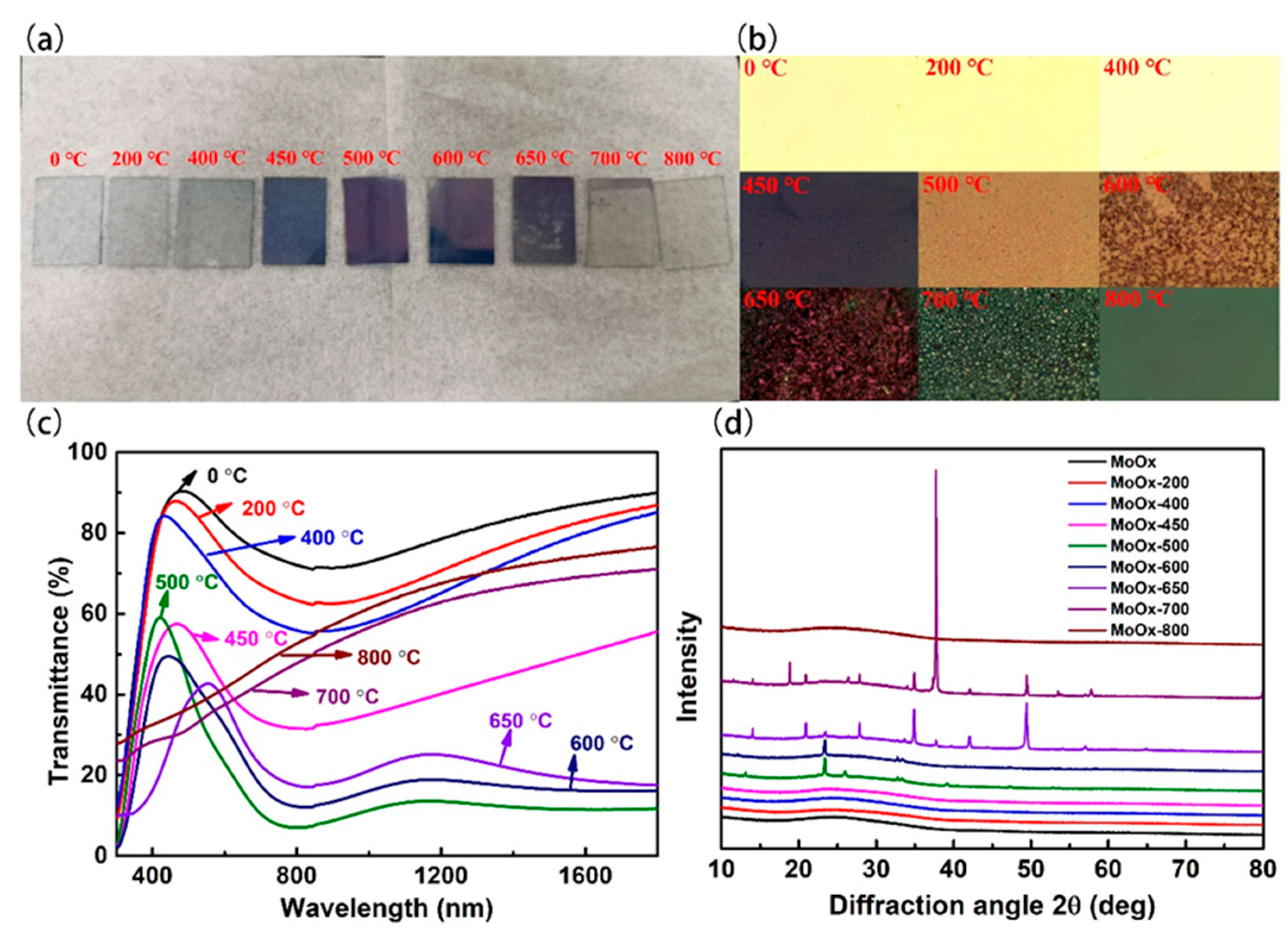
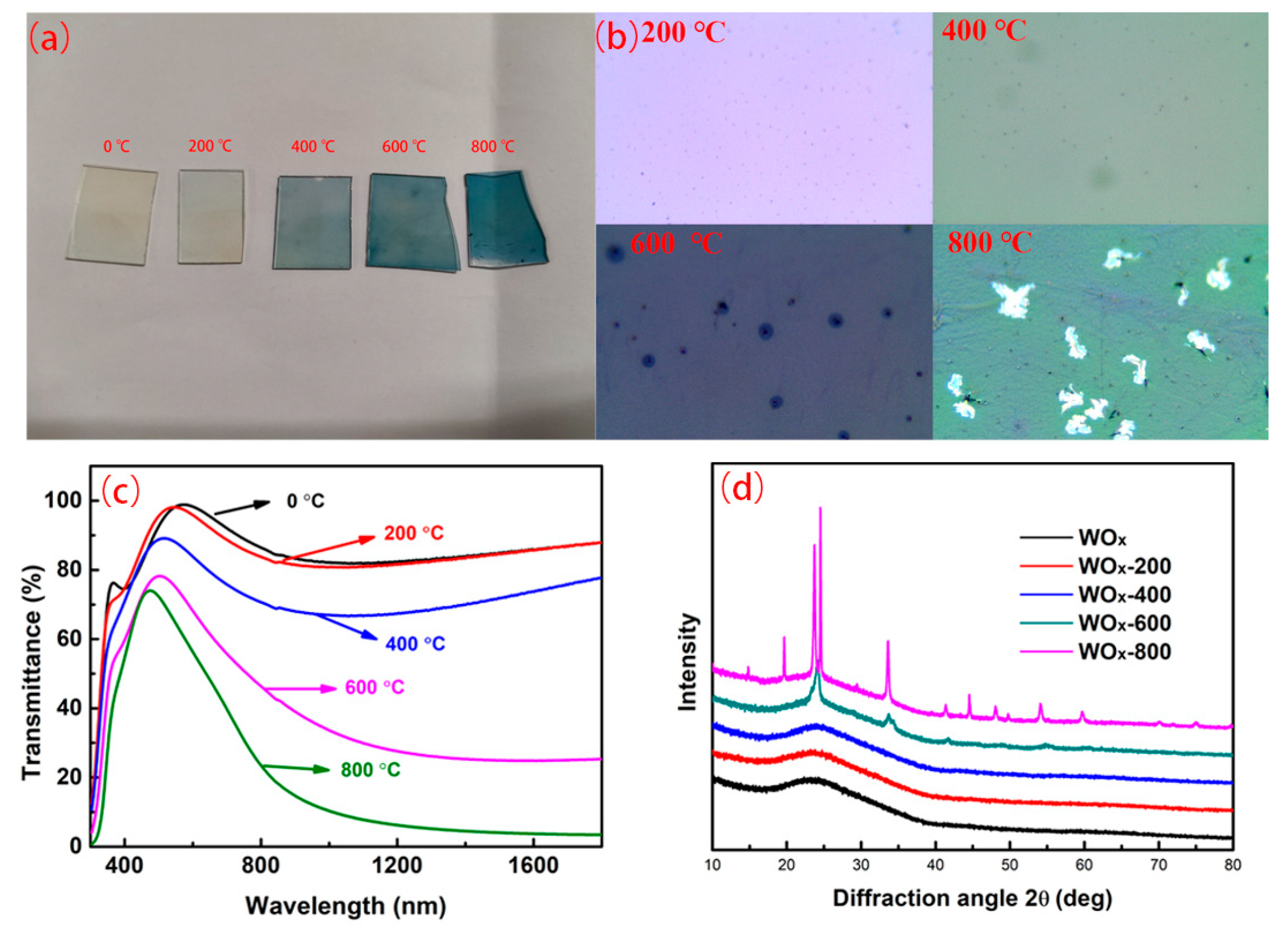
3.6. Effect of Annealing on Optical and Structural Properties of TMO Thin Films
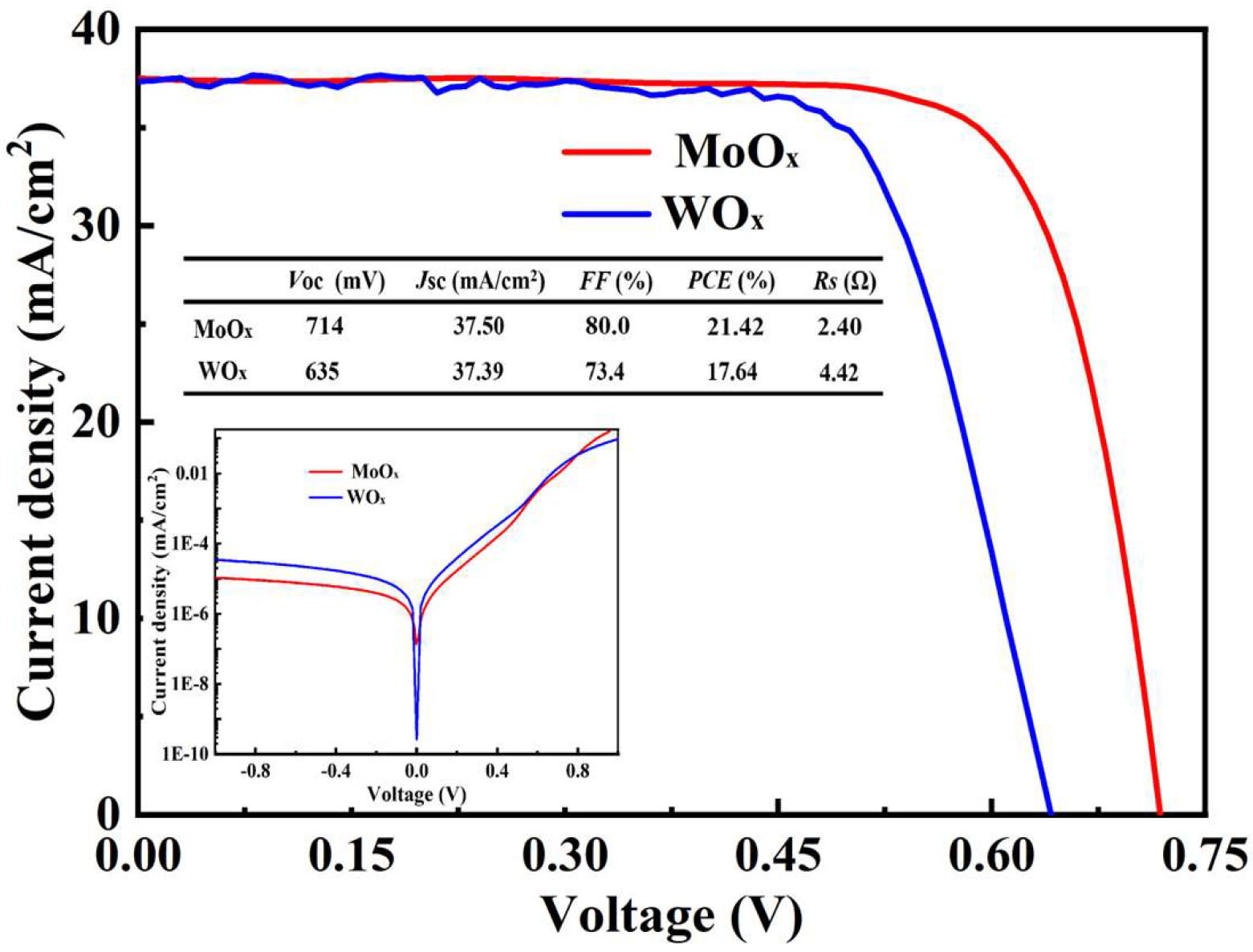
3.7. The Performance of Heterojunction Cells with MoOx and WOx as HTLs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Liu, Y.; Zhang, H.; Liu, Z.; Tian, H.; Sun, Y.; Meng, Z.; Yi, Z.; Sun, J.; Xie, Y. Electric field control of superconductivity at the LaAlO3/KTaO3(111) interface. Science 2021, 372, 721–724. [Google Scholar] [CrossRef]
- Han, Y.; Lao, B.; Zheng, X.; Li, S.; Li, R.-W.; Wang, Z. Transition metal oxides: A new frontier in spintronics driven by novel quantum states and efficient charge-spin interconversion. Front. Mater. 2024, 11, 1444769. [Google Scholar] [CrossRef]
- Peng, Y.; Tian, H.; Yao, M.; Li, X.; Tang, X.; Jiao, J.; Zhu, Q.; Cao, J. First-principles prediction of high carrier mobility for β-phase MX2N4 (M = Mo, W, X = Si, Ge) monolayers. Sci. Rep. 2024, 14, 22548. [Google Scholar] [CrossRef]
- Park, K.-W.; Kolpak, A.M. Optimal methodology for explicit solvation prediction of band edges of transition metal oxide photocatalysts. Commun. Chem. 2019, 2, 79. [Google Scholar] [CrossRef]
- Vijayan, A.; Sandhyarani, N. Efficient and sustainable hydrogen evolution reaction: Enhanced photoelectrochemical performance of ReO3-incorporated Cu2Te catalysts. Energy Adv. 2024, 3, 1092–1098. [Google Scholar] [CrossRef]
- Jia, H.; Meng, L.; Lu, Y.; Liang, T.; Yuan, Y.; Hu, Y.; Dong, Z.; Zhou, Y.; Guan, P.; Zhou, L.; et al. Boosting the efficiency of electrocatalytic water splitting via in situ grown transition metal sulfides: A review. J. Mater. Chem. A 2024, 12, 28595–28617. [Google Scholar] [CrossRef]
- Li, M.; Lin, W.; Ji, Y.; Guan, L.; Qiu, L.; Chen, Y.; Lu, Q.; Ding, X. Recent progress in high-voltage P2-NaxTMO2 materials and their future perspectives. RSC Adv. 2024, 14, 24797–24814. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Zhang, J. Conductive Metal–Organic Frameworks and Their Electrocatalysis Applications. ACS Nano 2025, 19, 9484–9512. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Bi, Q.; Ma, Q.; Han, L.; Tao, K. Recent advances in metal–organic framework-based electrode materials for supercapacitors. Dalton Trans. 2021, 50, 11701–11710. [Google Scholar] [CrossRef]
- Long, Z.; Kelly, H.R.; Videla, P.E.; Menzel, J.P.; Lian, T.; Kubiak, C.P.; Batista, V.S. Control of Reversible Oxidative Addition/Reductive Elimination of Surface-Attached Catalysts by External Electric Fields. J. Phys. Chem. Lett. 2025, 16, 2881–2886. [Google Scholar] [CrossRef]
- Lv, Q.; Chi, K.; Zhang, Y.; Xiao, F.; Xiao, J.; Wang, S.; Loh, K.P. Ultrafast charge/discharge solid-state thin-film supercapacitors via regulating the microstructure of transition-metal-oxide. J. Mater. Chem. A 2017, 5, 2759–2767. [Google Scholar] [CrossRef]
- Irfan, S.; Haleem, Y.A.; Irshad, M.I.; Saleem, M.F.; Arshad, M.; Habib, M. Tunability of the Optical Properties of Transition-Metal-Based Structural Phase Change Materials. Optics 2023, 4, 351–363. [Google Scholar] [CrossRef]
- Wojtasik, K.; Wojtasik, M.; Suchanek, K.; Zięba, M.; Karasiński, P.; Pakieła, W.; Żak, G.; Krasodomski, W. Effect of pyrolytic carbon addition on the structural and optical properties of TiO2 composite thin films. Sci. Rep. 2025, 15, 8078. [Google Scholar] [CrossRef]
- Jung, Y.-C.; Yu, Y.-J.; Kim, Y.-K.; Lee, J.H.; Seo, J.H.; Choi, J.-Y. Asymmetric TMO-Metal-TMO Structure for Enhanced Efficiency and Long-Term Stability of Si-Based Heterojunction Solar Cells. Materials 2023, 16, 5550. [Google Scholar] [CrossRef] [PubMed]
- Fatheema, J.; Shahid, T.; Mohammad, M.A.; Islam, A.; Malik, F.; Akinwande, D.; Rizwan, S. A comprehensive investigation of MoO3 based resistive random access memory. RSC Adv. 2020, 10, 19337–19345. [Google Scholar] [CrossRef]
- Kamoun, O.; Boukhachem, A.; Amlouk, M.; Ammar, S. Physical study of Eu doped MoO3 thin films. J. Alloys Compd. 2016, 687, 595–603. [Google Scholar] [CrossRef]
- Car, T.; Jakovac, I.; Šarić, I.; Bernstorff, S.; Micetic, M. Structural, Optical and Electrical Properties of Al+MoO3 and Au+MoO3 Thin Films Prepared by Magnetron Co-deposition. Materials 2021, 14, 766. [Google Scholar] [CrossRef]
- Muslu, E.; Eren, E.; Oksuz, A.U. Research progress on flexible WO3 based thin film electrodes for supercapacitor applications: A comprehensive review. Emergent Mater. 2024, 7, 2205–2236. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Zou, L.; Wang, Q.; Liu, C. A Review on the Properties and Applications of WO3 Nanostructure—Based Optical and Electronic Devices. Nanomaterials 2021, 11, 2136. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Bhosale, M.K.; Jeon, C.-W. PVP-Engineered WO3/TiO2 Heterostructures for High-Performance Electrochromic Applications with Enhanced Optical Modulation and Stability. Polymers 2025, 17, 1683. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, S.; Huang, Q.; Yu, C.; Zhou, J.; Liu, X.; Su, X.; Zhao, Y.; Hou, G. Sub-stochiometric MoOx by radio-frequency magnetron sputtering as hole-selective passivating contacts for silicon heterojunction solar cells. Chin. Phys. B 2022, 31, 098401. [Google Scholar] [CrossRef]
- Borgschulte, A.; Sambalova, O.; Delmelle, R.; Jenatsch, S.; Hany, R.; Nüesch, F. Hydrogen reduction of molybdenum oxide at room temperature. Sci. Rep. 2017, 7, 40761. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, C.; Barberi, R.C.; Castriota, M. Tungsten and Titanium Oxide Thin Films Obtained by the Sol-Gel Process as Electrodes in Electrochromic Devices. Front. Mater. 2022, 9, 912013. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Lin, H.; Ye, F.; Gao, P. Hot wire Oxidatione Sublimation derived work function tunable WOx thin films for building hole-selective contacts. Mater. Today Energy 2023, 38, 101439. [Google Scholar] [CrossRef]
- Guo, C.; Li, J.; Liu, R.; Zhang, D.; Qiu, J.; Zhuang, Z.; Chen, Y.; Qiu, Q.; Liu, W.; Huang, Y.; et al. In-situ deposition of tungsten oxide hole-contact by Hot-Wire CVD and its application in dopant-free heterojunction solar cells. Semicond. Sci. Technol. 2023, 38, 015007. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Yang, Y.; Dong, G.; Zhou, Y.; Liu, F.; Yu, D. Silicon Heterojunction Solar Cells with MoOx Hole-Selective Layer by Hot Wire Oxidation–Sublimation Deposition. Sol. RRL 2020, 4, 1900514. [Google Scholar] [CrossRef]
- Tan, G.-L.; Tang, D.; Dastan, D.; Jafar, A.; Shi, Z.C.; Chu, Q.-Q.; Silva, J.P.B.; Yin, X.-T. Structures, morphological control, and antibacterial performance of tungsten oxide thin films. Ceram. Int. 2021, 47, 17153–17160. [Google Scholar] [CrossRef]
- Mehmood, H.; Bektaş, G.; Yildiz, İ.; Tauqeer, T.; Nasser, H.; Turan, R. Electrical, optical and surface characterization of reactive RF magnetron sputtered molybdenum oxide films for solar cell applications. Mater. Sci. Semicond. Process. 2019, 101, 46–56. [Google Scholar] [CrossRef]
- Khanlary, M.R.; Rahmani, E.; Pasalari, Z.; Reyhani, A. Post-annealing effect on the structural and optical properties of tungsten trioxide thin films prepared by thermal evaporation. Mater. Chem. Phys. 2025, 338, 130670. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Nasher, M.A.; Makhlouf, M.M. Structural, electrical, and linear/nonlinear optical characteristics of thermally evaporated molybdenum oxide thin films. Ceram. Int. 2022, 48, 8069–8080. [Google Scholar] [CrossRef]
- Krishna Prasad, A.; Kim, J.-Y.; Kang, S.-H.; Ahn, K.-S. Molybdenum induced defective WO3 multifunctional nanostructure as an electrochromic energy storage device: Novel assembled photovoltaic-electrochromic Mo–WO3 film. J. Ind. Eng. Chem. 2024, 135, 388–396. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, J.; Shi, C.; Ma, D. Room-Temperature Sol–Gel Derived Molybdenum Oxide Thin Films for Efficient and Stable Solution-Processed Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2013, 5, 6024–6029. [Google Scholar] [CrossRef]
- Anwar, M.; Hogarth, C.A. Optical properties of amorphous thin films of MoO3 deposited by vacuum evaporation. Phys. Status solidi (a) 1988, 109, 469–478. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Anders, A. Structural, optical and electrical properties of WOxNy films deposited by reactive dual magnetron sputtering. Surf. Coat. Technol. 2006, 201, 2977–2983. [Google Scholar] [CrossRef]
- Mohamed, S.; Mohamed, H.; El Ghani, H.A. Development of structural and optical properties of WOx films upon increasing oxygen partial pressure during reactive sputtering. Phys. B Condens. Matter 2011, 406, 831–835. [Google Scholar] [CrossRef]
- Sindhu, S.; Sanghi, S.; Agarwal, A.; Seth, V.P.; Kishore, N. Structural, optical, physical and electrical properties of V2O5.SrO.B2O3 glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Singh, K.; Singh, P.; Kumar, P.; Thakur, A. Thickness dependent structural, morphological and optical properties of molybdenum oxide thin films. SN Appl. Sci. 2020, 2, 1439. [Google Scholar] [CrossRef]
- Battaglia, C.; Yin, X.; Zheng, M.; Sharp, I.D.; Chen, T.; McDonnell, S.; Azcatl, A.; Carraro, C.; Ma, B.; Maboudian, R.; et al. Hole Selective MoOx Contact for Silicon Solar Cells. Nano Lett. 2014, 14, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, C.; de Nicolás, S.M.; De Wolf, S.; Yin, X.; Zheng, M.; Ballif, C.; Javey, A. Silicon heterojunction solar cell with passivated hole selective MoOx contact. Appl. Phys. Lett. 2014, 104, 113902. [Google Scholar] [CrossRef]
- Borah, D.J.; Mostako, A.; Saikia, P.; Dutta, P. Effect of thickness and post deposition annealing temperature on the structural and optical properties of thermally evaporated molybdenum oxide films. Mater. Sci. Semicond. Process. 2019, 93, 111–122. [Google Scholar] [CrossRef]
- Cody, G.D.; Tiedje, T.; Abeles, B.; Moustakas, T.D.; Brooks, B.; Goldstein, Y. Disorder and the Optical Absorption Edge of Hydrogenated Amorphous Silicon. Phys. Rev. Lett. 1981, 42, C4-301. [Google Scholar]
- Al-Kuhaili, M.F.; Mekki, M.B. Laser-induced photocoloration in molybdenum oxide thin films. J. Alloys Compd. 2021, 885, 161043. [Google Scholar] [CrossRef]
- García-Hernansanz, R.; García-Hemme, E.; Montero, D.; Olea, J.; del Prado, A.; Mártil, I.; Voz, C.; Gerling, L.G.; Puigdollers, J.; Alcubilla, R. Transport mechanisms in silicon heterojunction solar cells with molybdenum oxide as a hole transport layer. Sol. Energy Mater. Sol. Cells 2018, 185, 61–65. [Google Scholar] [CrossRef]
- Mahato, S.; Voz, C.; Biswas, D.; Bhunia, S.; Puigdollers, J. Defect states assisted charge conduction in Au/MoO3–x/n-Si Schottky barrier diode. Mater. Res. Express 2019, 6, 036303. [Google Scholar] [CrossRef]
- Mahjabin, S.; Haque, M.M.; Sobayel, K.; Selvanathan, V.; Jamal, M.S.; Bashar, M.S.; Sultana, M.; Hossain, M.I.; Shahiduzzaman, M.; Algethami, M.; et al. Investigation of Morphological, Optical, and Dielectric Properties of RF Sputtered WOx Thin Films for Optoelectronic Applications. Nanomaterials 2022, 12, 3467. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Li, J.; Lin, Y.; Xue, Z.; Di, Z.; Yin, M.; Wang, J.; Lu, L.; Yang, L.; Li, D. Structural and optical studies of molybdenum oxides thin films obtained by thermal evaporation and atomic layer deposition methods for photovoltaic application. J. Mater. Sci. Mater. Electron. 2021, 32, 3475–3486. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Kang, Q.; Kang, Z.; Li, T.; Yu, J.; Qiu, H.; Liang, T.; Lei, C. The Material Growth and Characteristics of Transition Metal Oxide Thin Films Based on Hot Wire Oxidation Sublimation Deposition Technology. Materials 2025, 18, 4083. https://doi.org/10.3390/ma18174083
Li F, Kang Q, Kang Z, Li T, Yu J, Qiu H, Liang T, Lei C. The Material Growth and Characteristics of Transition Metal Oxide Thin Films Based on Hot Wire Oxidation Sublimation Deposition Technology. Materials. 2025; 18(17):4083. https://doi.org/10.3390/ma18174083
Chicago/Turabian StyleLi, Fengchao, Qingguo Kang, Zhenwei Kang, Tengteng Li, Jiangang Yu, Haibing Qiu, Ting Liang, and Cheng Lei. 2025. "The Material Growth and Characteristics of Transition Metal Oxide Thin Films Based on Hot Wire Oxidation Sublimation Deposition Technology" Materials 18, no. 17: 4083. https://doi.org/10.3390/ma18174083
APA StyleLi, F., Kang, Q., Kang, Z., Li, T., Yu, J., Qiu, H., Liang, T., & Lei, C. (2025). The Material Growth and Characteristics of Transition Metal Oxide Thin Films Based on Hot Wire Oxidation Sublimation Deposition Technology. Materials, 18(17), 4083. https://doi.org/10.3390/ma18174083






