Alkali Cation Effects on Compressive Strength of Metakaolin–Low-Calcium Fly Ash-Based Geopolymers
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Preparation of the Activators
2.3. Mixing, Casting, and Curing
2.4. Characterization Methods
3. Results and Discussion
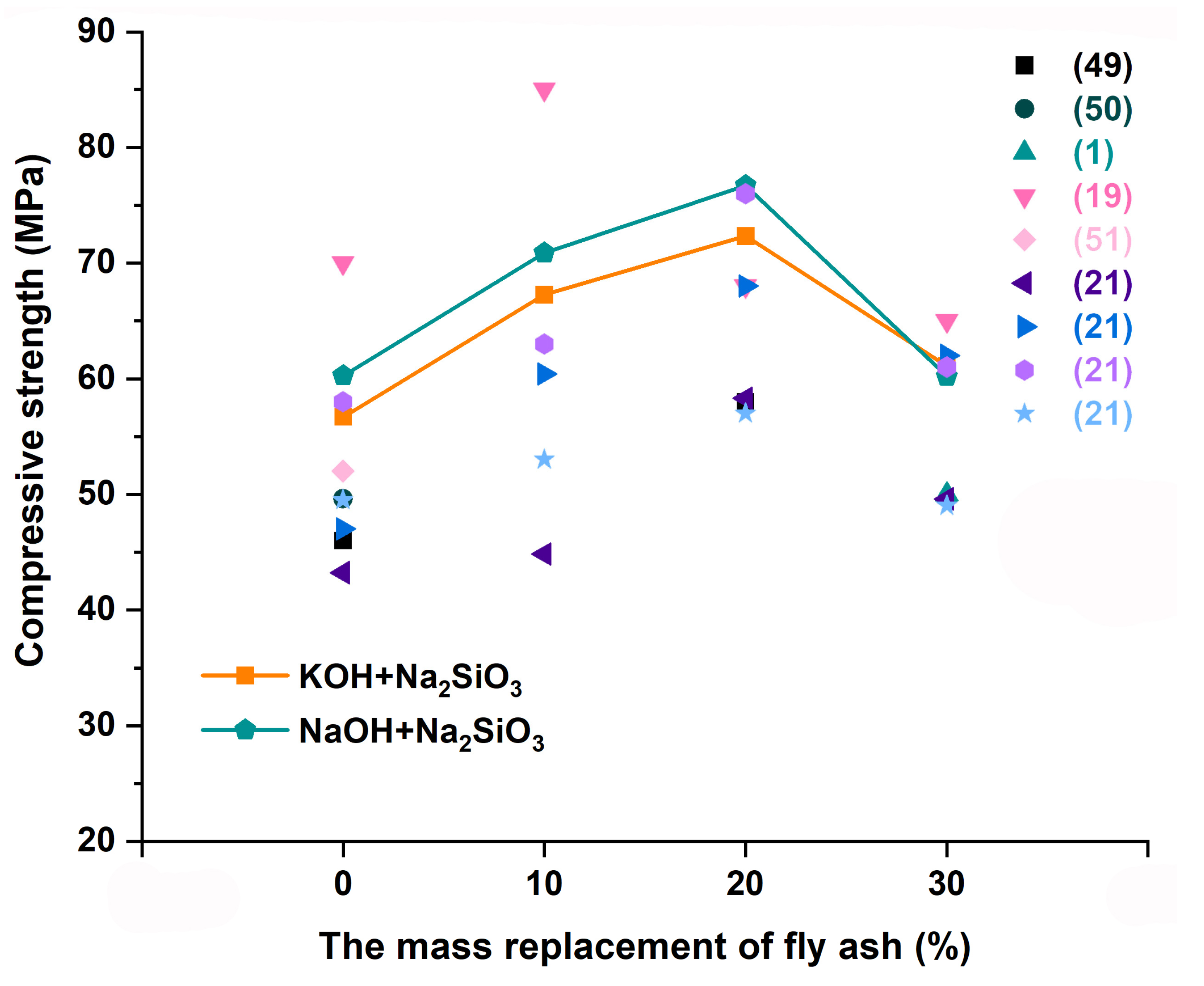
3.1. Compressive Strength
3.2. Microstructural Analysis
3.2.1. Crystalline Phase Analysis
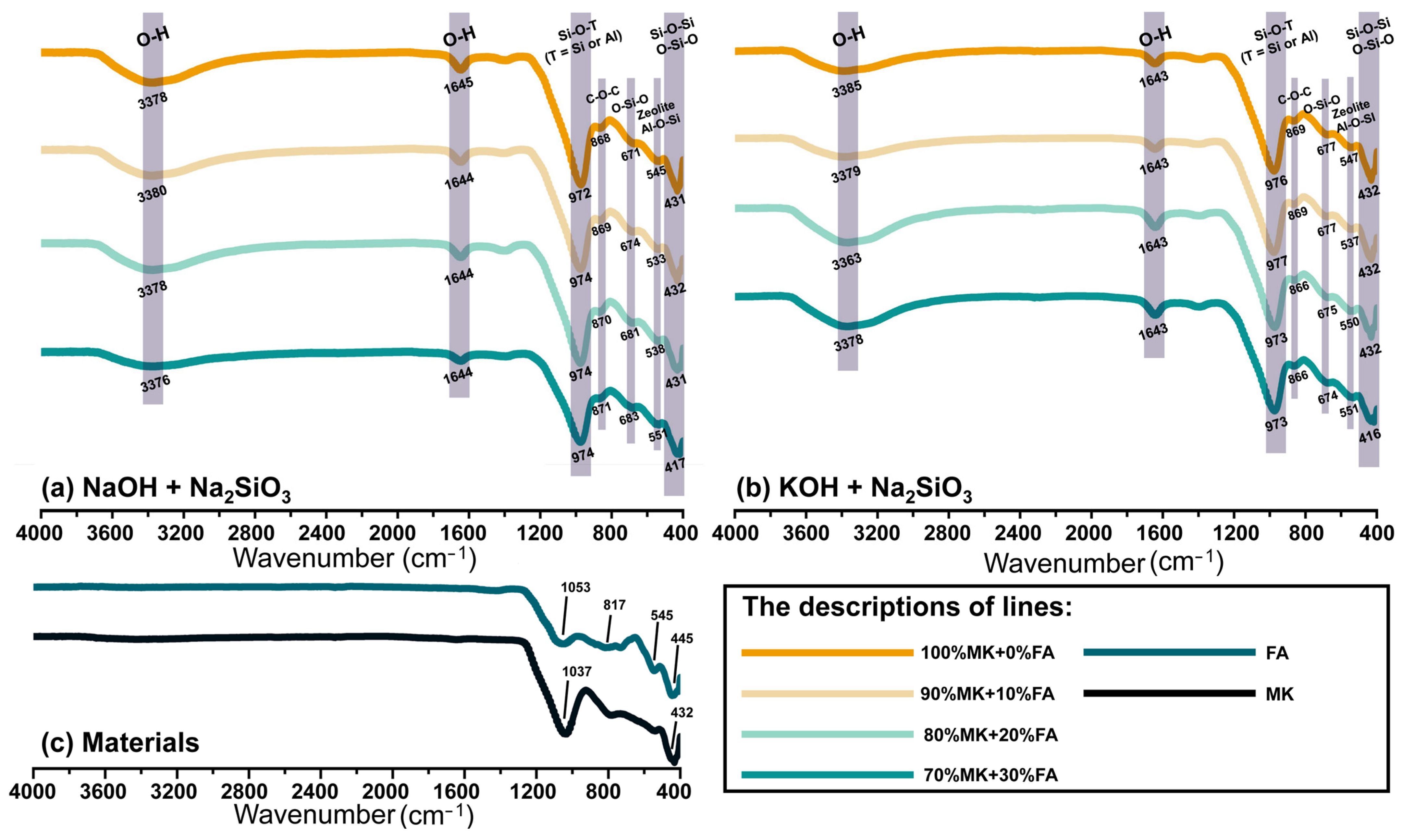
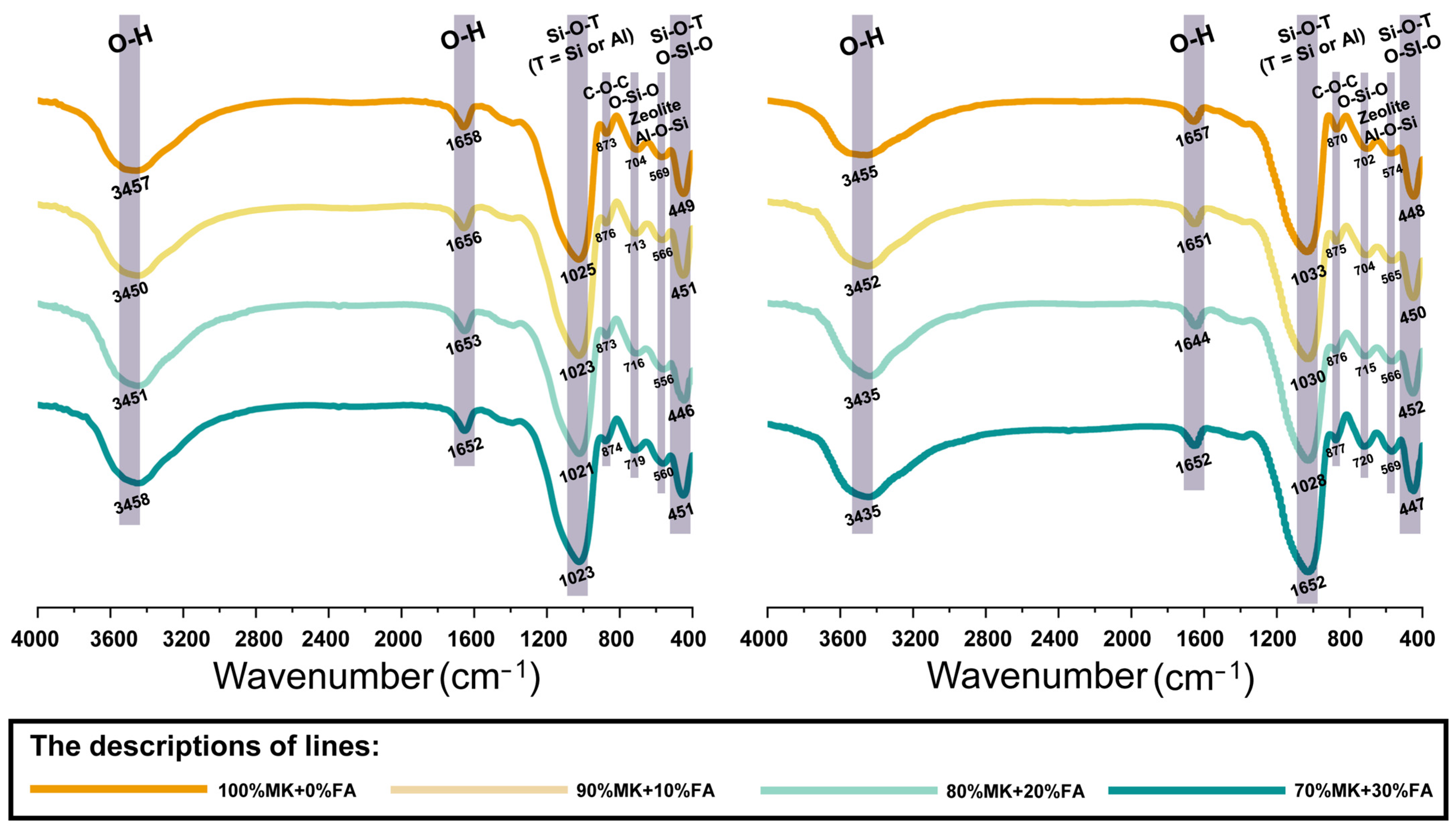
3.2.2. Functional Group Identification
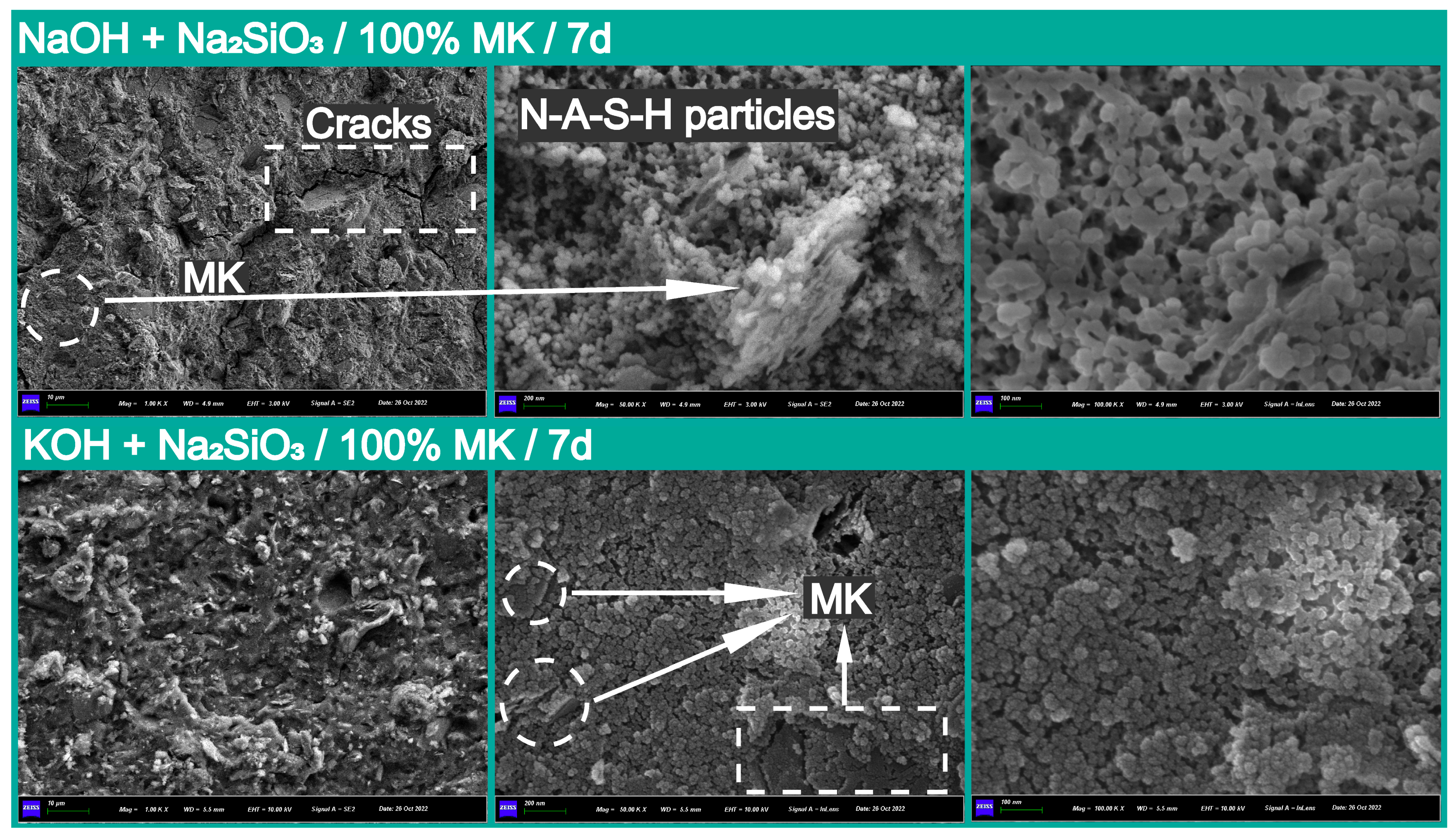
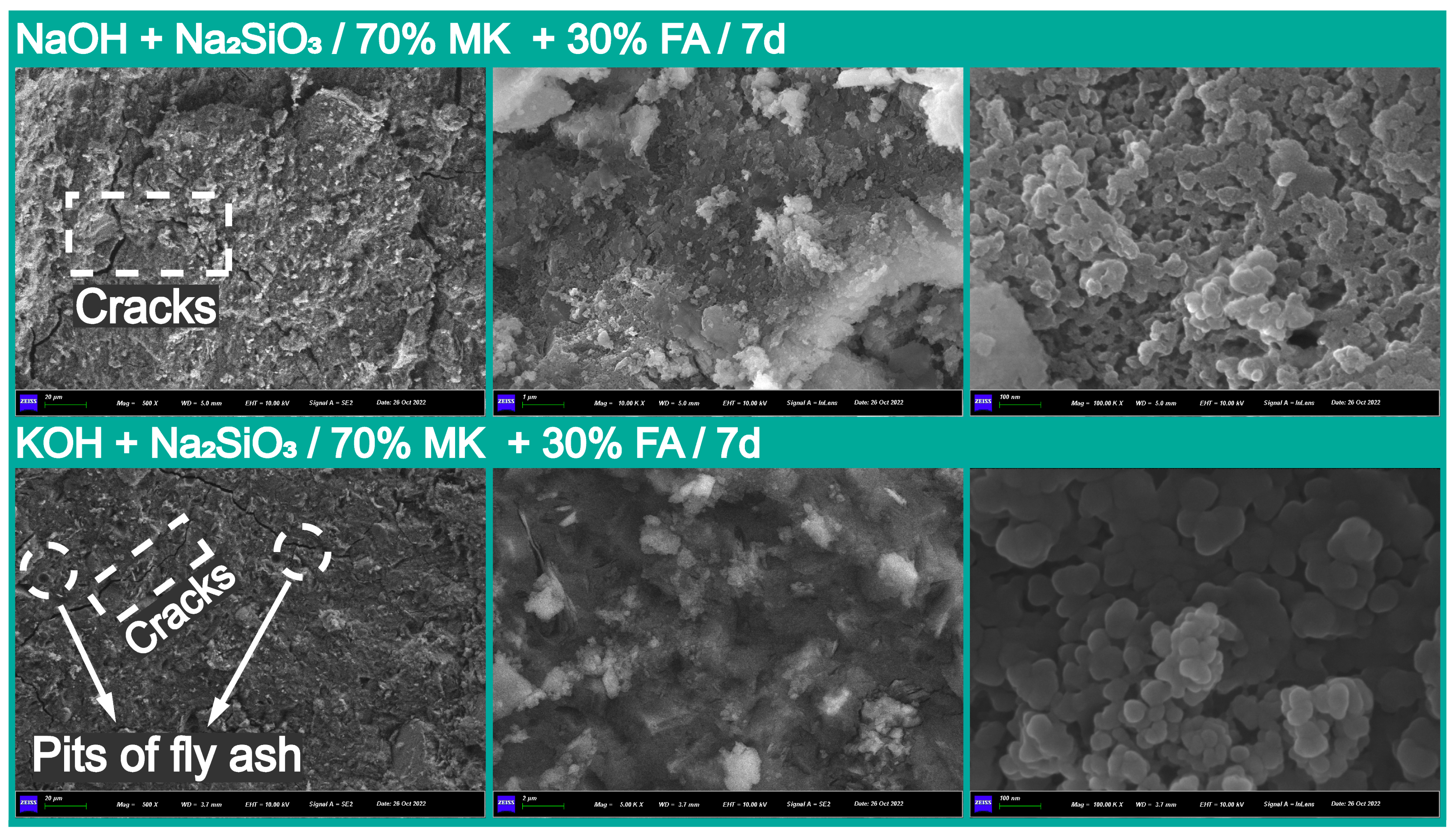
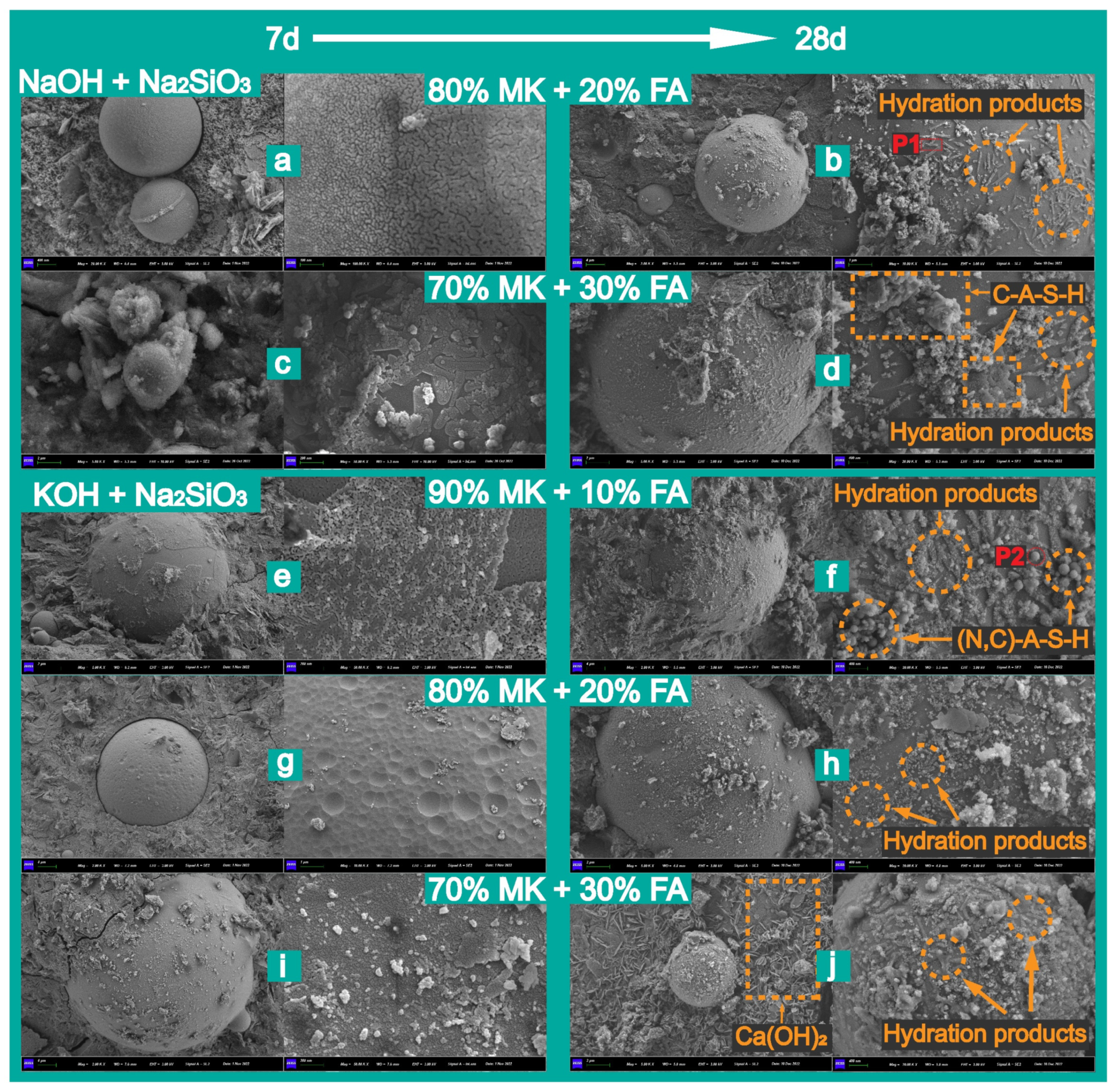
3.2.3. Microstructural Observation and Elemental Analysis
3.3. Solid Solution Reactivity and Inorganic Polymer Phase Evolution
3.4. Mechanism of Compressive Strength Development in the ‘Metakaolin + Low Calcium Fly Ash’ System
4. Conclusions
- (1)
- This study confirmed the feasibility of synthesizing high-compressive-strength composites using metakaolin and low-calcium fly ash at room temperature. The compressive strength of the geopolymer activated by NaOH reached 72.34 MPa. The compressive strength of the geopolymer activated by KOH was up to 76.70 MPa when the admixture of fly ash reached 20%.
- (2)
- The increase in compressive strength can be attributed to the effective introduction of chemically inert fillers at the matrix interface and the formation of a dense, multidimensional gel structure. Among these inert fillers were mullite, which showed progressively sharper crystal peaks in the XRD spectra with increasing fly ash content, as well as the newly formed CaCO3. The key gel phases, including the granular N-A-S-H, spongy C-A-S-H, and needle-like C-S-H phases and other hydration products, played a role in this process. Additionally, the accelerated hydration of calcium ions and the provision of extra nucleation sites also contributed to the improved performance.
- (3)
- Additionally, it should be noted that the type of alkaline activator did not appear to significantly affect the results in these experiments, as evidenced by the XRD and FTIR data. The combination of sodium hydroxide and sodium silicate remains the preferred choice due to its cost-effectiveness and minimal impact on compressive strength.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahmad, T.; Husain, D.; Majdi, A.; Alam, M.T.; Husain, N.; Wayal, A.K.S. Ecological footprint and economic assessment of conventional and geopolymer concrete for sustainable construction. J. Clean. Prod. 2022, 380, 134910. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Ling, Y. Macroscopic and microscopic analyses on mechanical performance of metakaolin/fly ash based geopolymer mortar. J. Clean. Prod. 2021, 294, 126193. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Qiu, G.H.; Kodur, V.; Yuan, Z.S. Spalling behavior of metakaolin-fly ash based geopolymer concrete under elevated temperature exposure. Cem. Concr. Compos. 2020, 106, 103483. [Google Scholar] [CrossRef]
- Benavent, V.; Lahalle, H.; Patapy, C.; Renaudin, G.; Cyr, M. Microstructural evolution of a sodium metakaolin-based geopolymer paste in neutral and CEM V basic environment. Cem. Concr. Res. 2022, 161, 106897. [Google Scholar] [CrossRef]
- Ridtirud, C.; Chindaprasirt, P.; Pimraksa, K. Factors affecting the shrinkage of fly ash geopolymers. Int. J. Miner. Metall. Mater. 2011, 18, 100–104. [Google Scholar] [CrossRef]
- Fu, C.; Ye, H.; Zhu, K.; Fang, D.; Zhou, J. Alkali cation effects on chloride binding of alkali-activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2020, 114, 103721. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Deng, L.; Li, Y.; Guo, H.; Zhou, J.; Li, L.; Peng, Y. Ion-adsorption type rare earth tailings for preparation of alkali-based geopolymer with capacity for heavy metals immobilization. Cem. Concr. Compos. 2022, 134, 104768. [Google Scholar] [CrossRef]
- Walkley, B.; Ke, X.; Hussein, O.H.; Bernal, S.A.; Provis, J.L. Incorporation of strontium and calcium in geopolymer gels. J. Hazard. Mater. 2020, 382, 121015. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Kang, J.; Um, W. Development of geopolymer waste form for immobilization of radioactive borate waste. J. Hazard. Mater. 2021, 419, 126402. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Cui, J.; Shan, Y.; Niu, Y. Experimental study on calcium carbide residue as a combined activator for coal gangue geopolymer and feasibility for soil stabilization. Constr. Build. Mater. 2021, 312, 125465. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Gopalakrishna, B.; Pasla, D. Development of metakaolin based high strength recycled aggregate geopolymer concrete. Constr. Build. Mater. 2023, 391, 131810. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F. Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci. 2014, 88–89, 194–201. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, Y.; Zhang, J.; Men, J.; Sun, T.; Zhao, H.; Wu, H.; Wang, H.; Dai, S. Orthogonal analysis and mechanism of compressive strength and microstructure of the metakaolin-fly ash geopolymer. Case Stud. Constr. Mater. 2022, 17, e01154. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Hanjitsuwan, S.; Hatanaka, S. The effect of adding nano-SiO2 and nano-Al2O3 on properties of high calcium fly ash geopolymer cured at ambient temperature. Mater. Des. 2014, 55, 58–65. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Wang, Z.; Zhao, X.-L. Fatigue behavior of concrete beams reinforced with glass- and carbon-fiber reinforced polymer (GFRP/CFRP) bars after exposure to elevated temperatures. Compos. Struct. 2019, 229, 111427. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, C.; Li, W.; Zhou, S.; Li, F.; Xiao, J.; Shah, S.P. Effects of carbon nanofibers on hydration and geopolymerization of low and high-calcium geopolymers. Cem. Concr. Compos. 2022, 133, 104695. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Bai, T.; Song, Z.; Wang, H.; Wu, Y.; Huang, W. Performance evaluation of metakaolin geopolymer modified by different solid wastes. J. Clean. Prod. 2019, 226, 114–121. [Google Scholar] [CrossRef]
- Duraisamy, V.; Athira, G.; Bahurudeen, A.; Nanthagopalan, P. Composite cements: Synergistic effects of particle packing and pozzolanicity. Proc. Inst. Civ. Eng.—Eng. Sustain. 2021, 175, 12–21. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Tan, J.; Vandevyvere, B. Thermal and compressive behaviors of fly ash and metakaolin-based geopolymer. J. Build. Eng. 2020, 30, 101307. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef]
- Nuaklong, P.; Janprasit, K.; Jongvivatsakul, P. Enhancement of strengths of high-calcium fly ash geopolymer containing borax with rice husk ash. J. Build. Eng. 2021, 40, 102762. [Google Scholar] [CrossRef]
- Yaseri, S.; Hajiaghaei, G.; Mohammadi, F.; Mahdikhani, M.; Farokhzad, R. The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr. Build. Mater. 2017, 157, 534–545. [Google Scholar] [CrossRef]
- Sithole, N.T.; Mashifana, T. Geosynthesis of building and construction materials through alkaline activation of granulated blast furnace slag. Constr. Build. Mater. 2020, 264, 120712. [Google Scholar] [CrossRef]
- Bagriacik, B. Utilization of alkali-activated construction demolition waste for sandy soil improvement with large-scale laboratory experiments. Constr. Build. Mater. 2021, 302, 124173. [Google Scholar] [CrossRef]
- Alventosa, K.M.L.; White, C.E. The effects of calcium hydroxide and activator chemistry on alkali-activated metakaolin pastes. Cem. Concr. Res. 2021, 145, 106453. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Zhao, Q.; Yang, Y.; Wang, P. Leachability and adverse effects of coal fly ash: A review. J. Hazard. Mater. 2020, 396, 122725. [Google Scholar] [CrossRef]
- 50146-2014 GT; Technical Code for Application of Fly Ash Concrete. China Planning Publishing House: Beijing, China, 2014.
- Zhang, H.; Li, H.; Guo, H.; Li, Y.; Wei, L. Mechanical properties of alkali activated geopolymer cement mortar for non vibratory compacted trench backfilling. Sci. Rep. 2025, 15, 12347. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Knyazev, Y.V.; Semenov, S.V.; Bayukov, O.A.; Anshits, A.G. Magnetic Fractions of PM2.5, PM2.5–10, and PM10 from Coal Fly Ash as Environmental Pollutants. ACS Omega 2021, 6, 20076–20085. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.M.; Meseguer, S.; Pardo, F.; Montero, M.A. High-Temperature Mineral Formation after Firing Clay Materials Associated with Mined Coal in Teruel (Spain). Appl. Sci. 2020, 10, 3114. [Google Scholar] [CrossRef]
- Niu, M.; Yao, Y.; Zi, M.; Dong, P.; Chen, D. Clay mineral mediated dynamics of CO2 hydrate formation and dissociation: Experimental insights for carbon sequestration. Energy 2024, 311, 133375. [Google Scholar] [CrossRef]
- Hattaf, R.; Aboulayt, A.; Samdi, A.; Lahlou, N.; Touhami, M.O.; Gomina, M.; Moussa, R. Metakaolin and Fly Ash-based Matrices for Geopolymer Materials: Setting Kinetics and Compressive Strength. Silicon 2021, 14, 6993–7004. [Google Scholar] [CrossRef]
- Ferraro, G.; Romei, L.; Fratini, E.; Chen, S.-H.; Jeng, U.S.; Baglioni, P. Functionalised nanoclays as microstructure modifiers for calcium and magnesium silicate hydrates. Phys. Chem. Chem. Phys. 2021, 23, 2630–2636. [Google Scholar] [CrossRef]
- Yu, J.; Ji, F.; Lv, Q.; Li, W.; Lin, Z.; Peng, Y. Mechanical property and microstructure of fly ash-based geopolymer by calcium activators. Case Stud. Constr. Mater. 2024, 21, e03811. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: A review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Xi, X.; Zheng, Y.; Zhuo, J.; Zhang, P.; Golewski, G.L.; Du, C. Influence of water glass modulus and alkali content on the properties of alkali-activated thermally activated recycled cement. Constr. Build. Mater. 2024, 452, 138867. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline activation of metakaolin: Effect of calcium hydroxide in the products of reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Chu, S.H.; Wang, S.; Zeng, L.; Ji, G. Utilization of BOF steel slag aggregate in metakaolin-based geopolymer. Constr. Build. Mater. 2021, 300, 124024. [Google Scholar] [CrossRef]
- Ariyanti, D.; Sugianto, D.N.; Purbasari, A.; Lesdantina, D.; Handayani, E.P. Synthesis of environmental-friendly fouling release coating, PDMS-X, to prevent the attachment of marine biofouling. J. Indian Chem. Soc. 2025, 102, 101768. [Google Scholar] [CrossRef]
- Singh, A.; Anand, K.; Liu, Q.; Tam, V.Y.; Goyal, S.; Reddy, M.S. Enhancing interlayer bonding in 3-dimensional printed concrete using bacteria-based biomineralization. Cem. Concr. Compos. 2025, 164, 106258. [Google Scholar] [CrossRef]
- Malaiškienė, J.; Antonovič, V.; Boris, R.; Stonys, R. Improving the physical and mechanical properties and alkali resistance of fireclay-based castables by modifying their structure with SiO2 sol. Ceram. Int. 2022, 48, 22534–22544. [Google Scholar] [CrossRef]
- Evaristo, L.; Silveira, R.; Tissot, M.; Hippler, G.; Moulton, B.; Buchner, S. Effect of high pressure in barium disilicate glass investigated by DTA and Raman spectroscopy. Int. J. Appl. Glass Sci. 2023, 14, 240–246. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Utilization of OPC and FA to enhance reclaimed lime-fly ash macadam based geopolymers cured at ambient temperature. Constr. Build. Mater. 2021, 303, 124378. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and characterization of geopolymer from metakaolin and sugarcane bagasse ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Influence of iron making slags on strength and microstructure of fly ash geopolymer. Constr. Build. Mater. 2013, 38, 924–930. [Google Scholar] [CrossRef]
- Allali, F.; Joussein, E.; Kandri, N.I.; Rossignol, S. The influence of calcium content on the performance of metakaolin-based geomaterials applied in mortars restoration. Mater. Des. 2016, 103, 1–9. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Kan, L.-l.; Wang, W.-s.; Liu, W.-d.; Wu, M. Development and characterization of fly ash based PVA fiber reinforced Engineered Geopolymer Composites incorporating metakaolin. Cem. Concr. Compos. 2020, 108, 103521. [Google Scholar] [CrossRef]
- Lin, R.-S.; Lee, H.-S.; Han, Y.; Wang, X.-Y. Experimental studies on hydration–strength–durability of limestone-cement-calcined Hwangtoh clay ternary composite. Constr. Build. Mater. 2021, 269, 121290. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, K.; Sun, W.; Zeng, Y.; Zou, Z. Mechanics and microstructure analysis of geopolymer utilizing ilmenite tailing and metakaolin powder as alkali-activated materials. Case Stud. Constr. Mater. 2024, 21, e03567. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, B.; Wu, W.; Yang, H.; Ning, Y.; Lai, Y.; Bradley, R. Low Cost, Robust, Environmentally Friendly Geopolymer–Mesoporous Carbon Composites for Efficient Solar Powered Steam Generation. Adv. Funct. Mater. 2018, 28, 1803266. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, J.; Ye, G.; De Schutter, G. A 3D reactive transport model for simulation of the chemical reaction process of ASR at microscale. Cem. Concr. Res. 2022, 151, 106640. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.u.A.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Pascual, A.B.; Tognonvi, T.M.; Tagnit-Hamou, A. Optimization study of waste glass powder-based alkali activated materials incorporating metakaolin: Activation and curing conditions. J. Clean. Prod. 2021, 308, 127435. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Fernandezjimenez, A.; Delatorre, A.; Palomo, A.; Lopezolmo, G.; Alonso, M.; Aranda, M. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Du, C.; Yang, Q. Experimental study of the feasibility of using calcium carbide residue as an alkaline activator for clay-plant ash geopolymer. Constr. Build. Mater. 2021, 301, 124351. [Google Scholar] [CrossRef]
- Khater, H.M.; Abd el Gawaad, H.A. Characterization of alkali activated geopolymer mortar doped with MWCNT. Constr. Build. Mater. 2016, 102, 329–337. [Google Scholar] [CrossRef]
- Saloni; Parveen; Yan Lim, Y.; Pham, T.M. Influence of Portland cement on performance of fine rice husk ash geopolymer concrete: Strength and permeability properties. Constr. Build. Mater. 2021, 300, 124321. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of calcium additions on N–A–S–H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
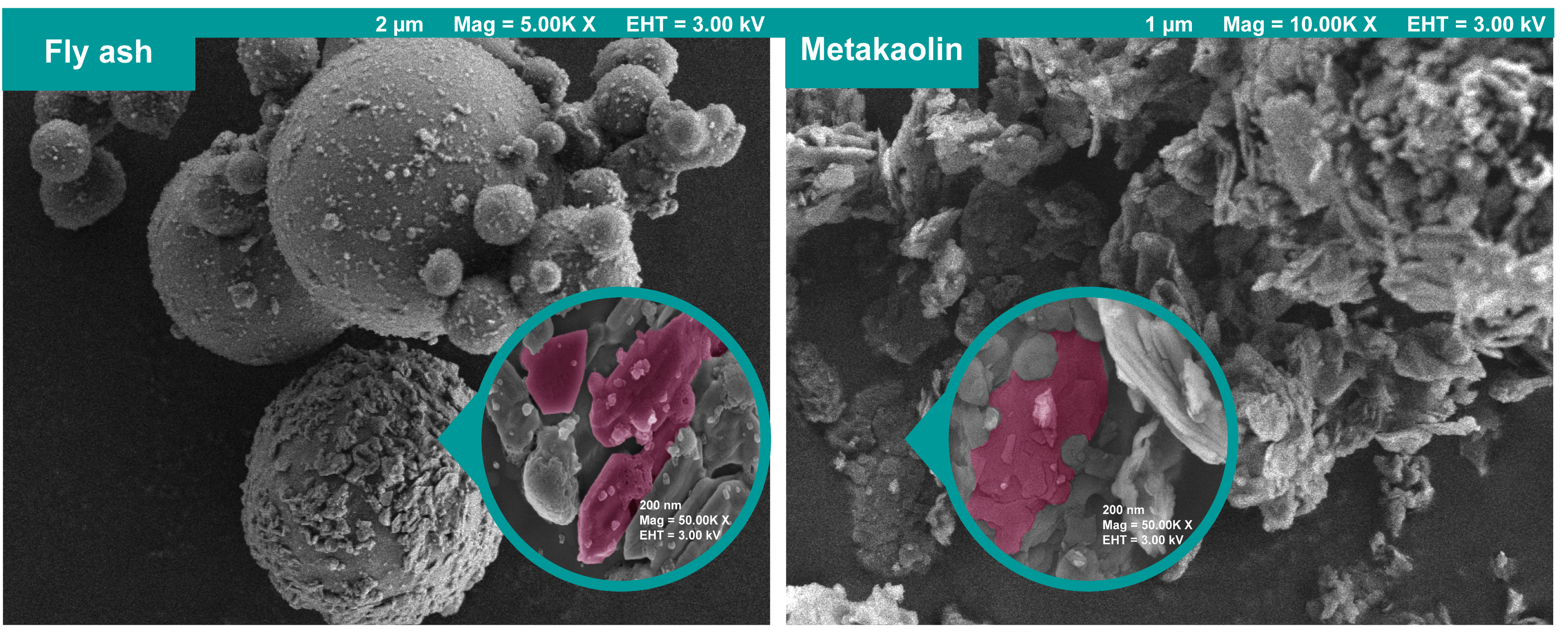

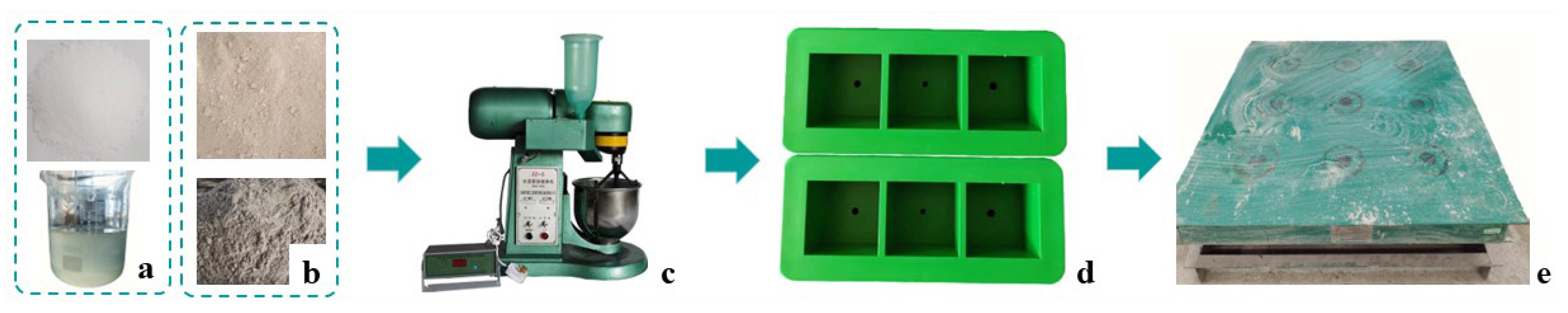



| Chemical Composition | Quantity (% by Mass) | |
|---|---|---|
| Fly Ash | Metakaolin | |
| Al2O3 | 36.50 | 43.50 |
| SiO2 | 43.70 | 47.90 |
| CaO | 5.19 | 0.86 |
| Fe2O3 | 3.89 | 3.72 |
| SO3 | 5.70 | 0.74 |
| P2O5 | 1.63 | 0.41 |
| TiO2 | 1.47 | 2.17 |
| Others | 1.92 | 0.70 |
| D (10 nm) | D (50 nm) | D (90 nm) | |
|---|---|---|---|
| Fly ash | 302 | 357 | 422 |
| Metakaolin | 353 | 428 | 519 |
| Mix No. | Replacement Rate of Fly Ash (%) | Mass Distribution (g) | Alkali Exciter (g) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|---|---|
| Metakaolin | Fly Ash | Na2SiO3 | NaOH | KOH | 7 Days | 28 Days | ||
| 1 | 0 | 1500 | 0 | 900 | 450 | -- | 34.90 | 56.71 |
| 2 | 10 | 1350 | 150 | 900 | 450 | -- | 38.73 | 67.26 |
| 3 | 20 | 1200 | 300 | 900 | 450 | -- | 40.51 | 72.34 |
| 4 | 30 | 1050 | 450 | 900 | 450 | -- | 37.24 | 61.09 |
| 5 | 0 | 1500 | 0 | 900 | -- | 450 | 26.34 | 60.27 |
| 6 | 10 | 1350 | 150 | 900 | -- | 450 | 30.92 | 70.89 |
| 7 | 20 | 1200 | 300 | 900 | -- | 450 | 36.07 | 76.70 |
| 8 | 30 | 1050 | 450 | 900 | -- | 450 | 27.41 | 60.21 |
| Bands, cm−1 | Assignments | References |
|---|---|---|
| 3360–3460 | Stretching vibration of O-H | [13,16] |
| 1640–1660 | Bending vibration of O-H | [13] |
| 1053 | Stretching vibration of Si-O-Si | [13] |
| 1037 | Asymmetric vibration of Si–O | [55] |
| 970–1035 | Asymmetric stretching vibrations of the Si-O-T (T = Si or Al) | [16,58,59] |
| 865–870 | The C-O-C bonds in the formation of calcite (CaCO3) | [52,60] |
| 817 | Symmetric stretching vibration of Si-O-Si bonds and stretching vibration of Al-O in mullite-like structures | [51] |
| 670–685 | The O-Si-O bands that correspond to the species of quartz | [58,61] |
| 530–555 | Symmetric stretching vibration of Al-O-Si | [52,59] |
| 430–445 | Bending vibration of Si-O-Si and O-Si-O | [53,54] |
| 416 | Bending vibrations of O-Si-O | [9] |
| O K | Na K | Al K | Si K | S K | K K | Ca K | ||
|---|---|---|---|---|---|---|---|---|
| P1 | Weight% | 53.71 | 5.69 | 17.52 | 20.35 | 0.04 | 2.36 | 0.33 |
| Atomic% | 66.50 | 4.90 | 12.87 | 14.35 | 0.07 | 1.20 | 0.16 | |
| P2 | Weight% | 45.97 | 2.22 | 24.72 | 24.37 | 0.02 | 0.53 | 2.16 |
| Atomic% | 59.59 | 2.00 | 19.00 | 17.99 | 0.07 | 0.28 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, H. Alkali Cation Effects on Compressive Strength of Metakaolin–Low-Calcium Fly Ash-Based Geopolymers. Materials 2025, 18, 4080. https://doi.org/10.3390/ma18174080
Li Y, Wang H. Alkali Cation Effects on Compressive Strength of Metakaolin–Low-Calcium Fly Ash-Based Geopolymers. Materials. 2025; 18(17):4080. https://doi.org/10.3390/ma18174080
Chicago/Turabian StyleLi, Yan, and Hongguang Wang. 2025. "Alkali Cation Effects on Compressive Strength of Metakaolin–Low-Calcium Fly Ash-Based Geopolymers" Materials 18, no. 17: 4080. https://doi.org/10.3390/ma18174080
APA StyleLi, Y., & Wang, H. (2025). Alkali Cation Effects on Compressive Strength of Metakaolin–Low-Calcium Fly Ash-Based Geopolymers. Materials, 18(17), 4080. https://doi.org/10.3390/ma18174080






