Short- and Long-Term Effects of Ca(OH)2/ZnO Heteronanostructure on Photosystem II Function and ROS Generation in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Primary Nanoparticles
2.3. Synthesis of CaZnO Hetero-Nanostructure
2.4. Physicochemical Characterization
2.5. Plant Material and Growth Conditions
2.6. Foliar Spraying of Hetero-Nanostructure on Tomato Plants
2.7. Chlorophyll Fluorescence Measurements
2.8. Imaging of Hydrogen Peroxide Generation
2.9. Statistical Analysis
3. Results
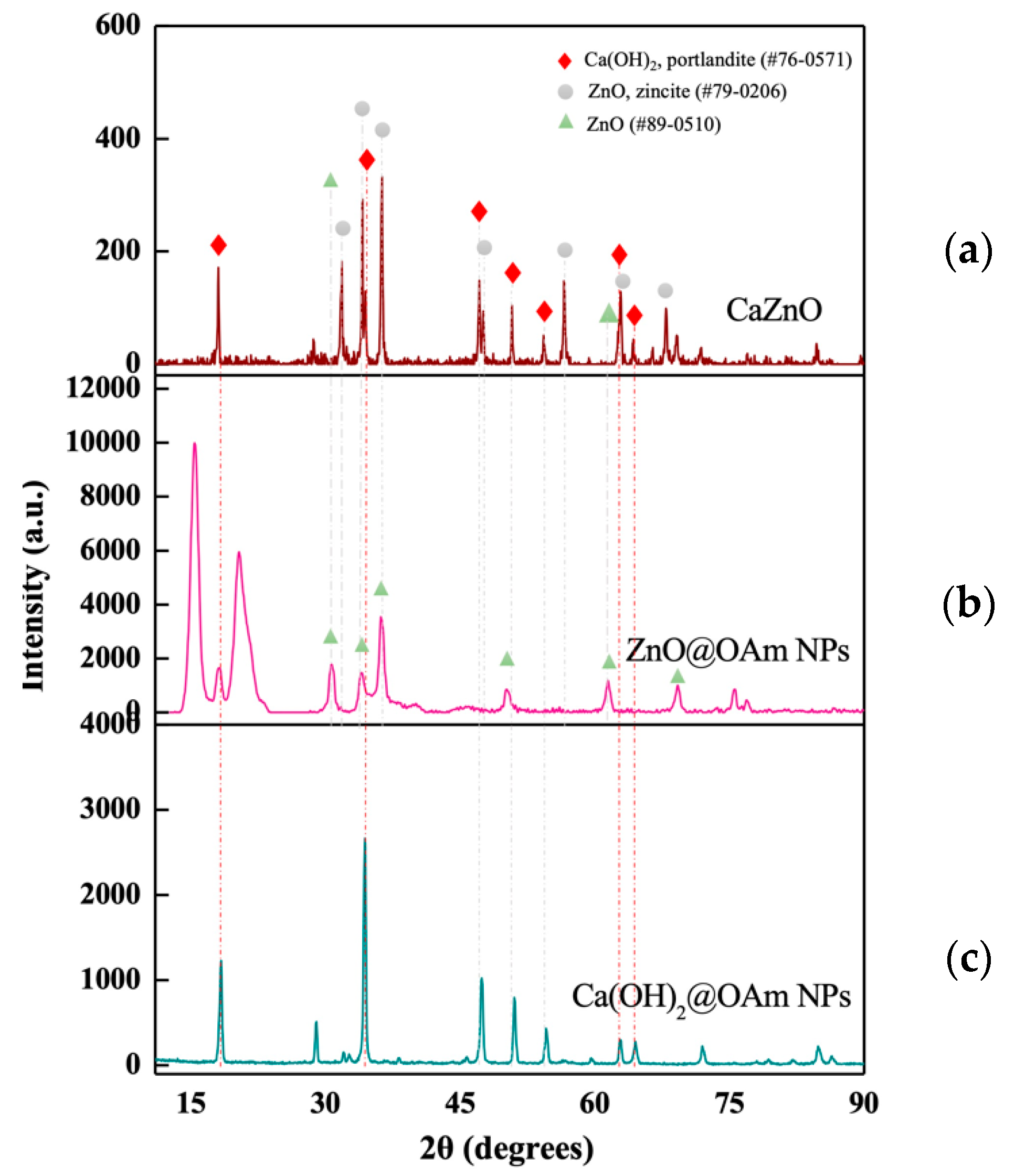
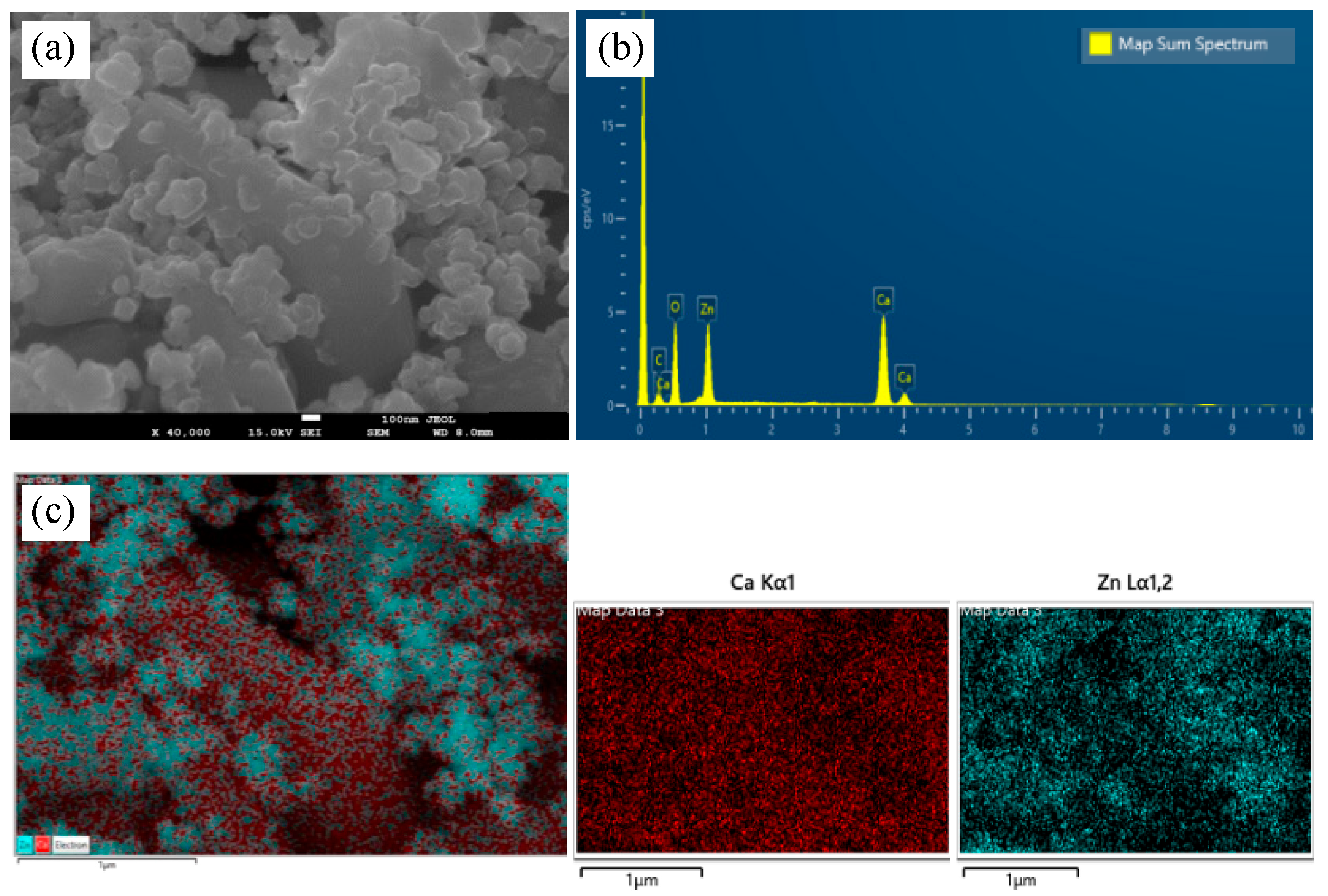
3.1. Physicochemical Characterization of the Hetero-Nanostructure
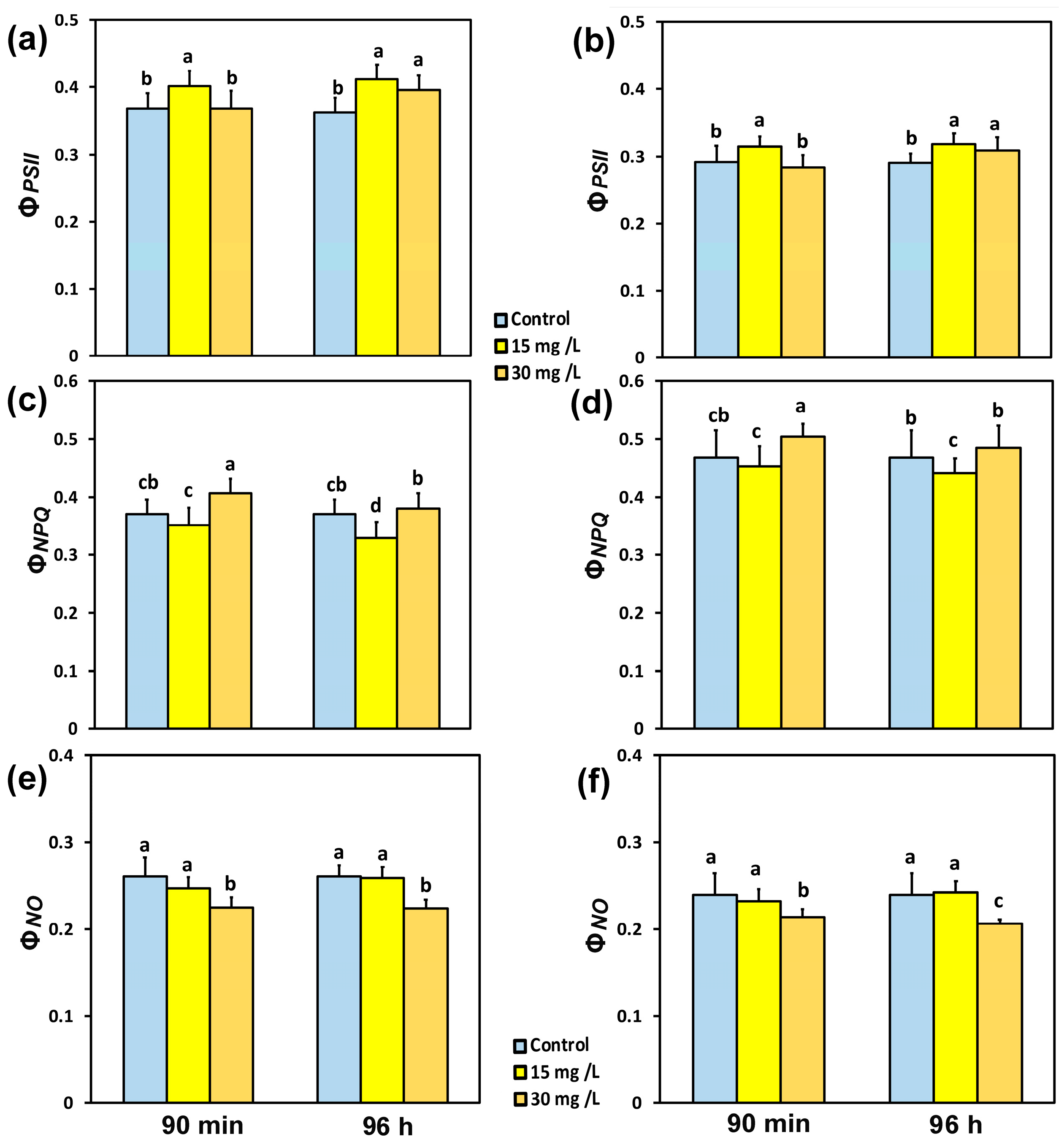
3.2. Impact of the Hetero-Nanostructureon the Allocation of the Absorbed Light Energy in Photosystem II
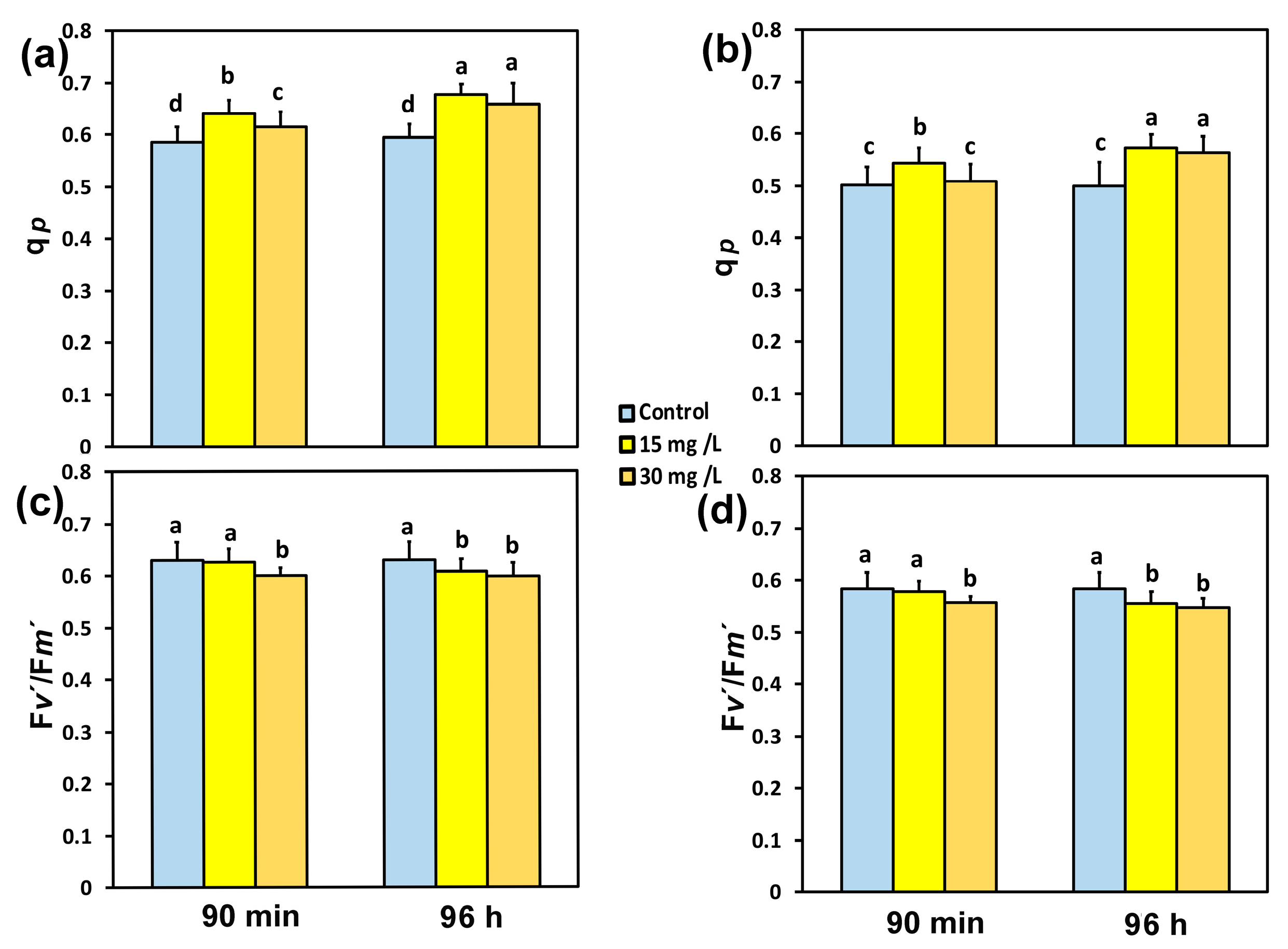
3.3. The Fraction of Open Photosystem II Reaction Centers and the Efficiency of PSII Reaction Centers Before and After Spraying with the Hetero-Nanostructure
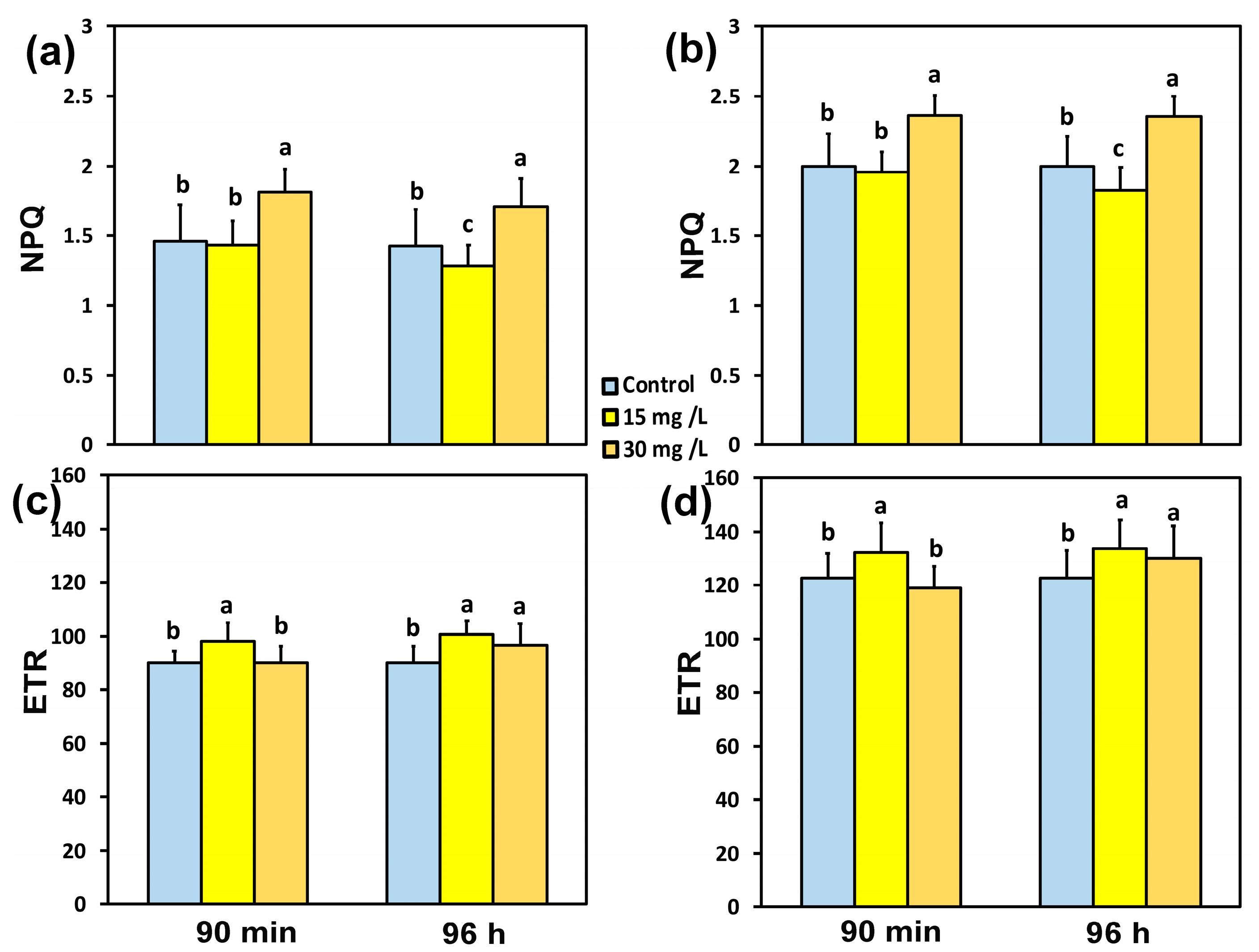
3.4. The Photoprotective Heat Dissipation and the Electron Transport Rate in PSII Before and After Spraying with Hetero-Nanostructure
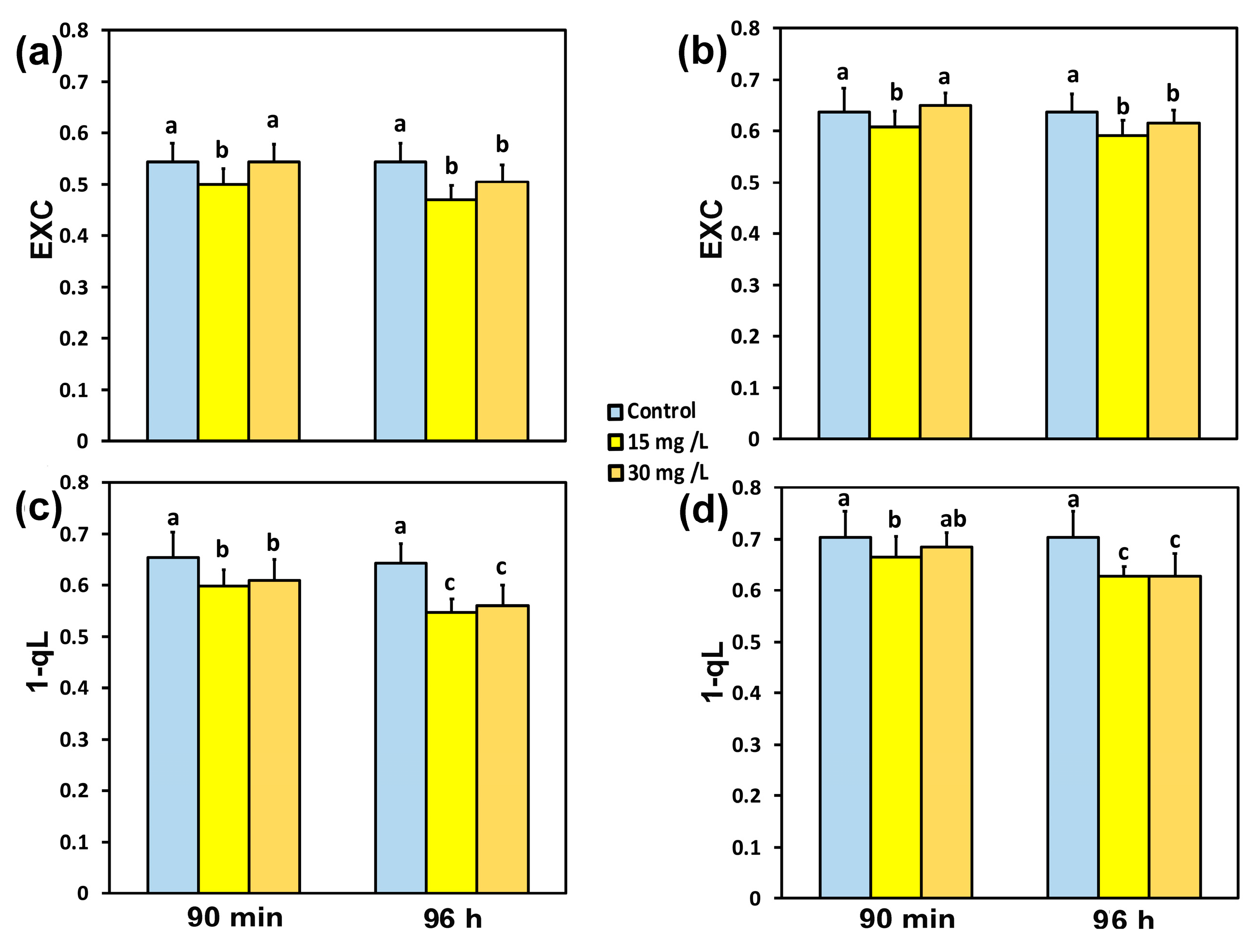
3.5. Impact of the Hetero-Nanostructure on the Excess Excitation Energy and the Excitation Pressure on PSII
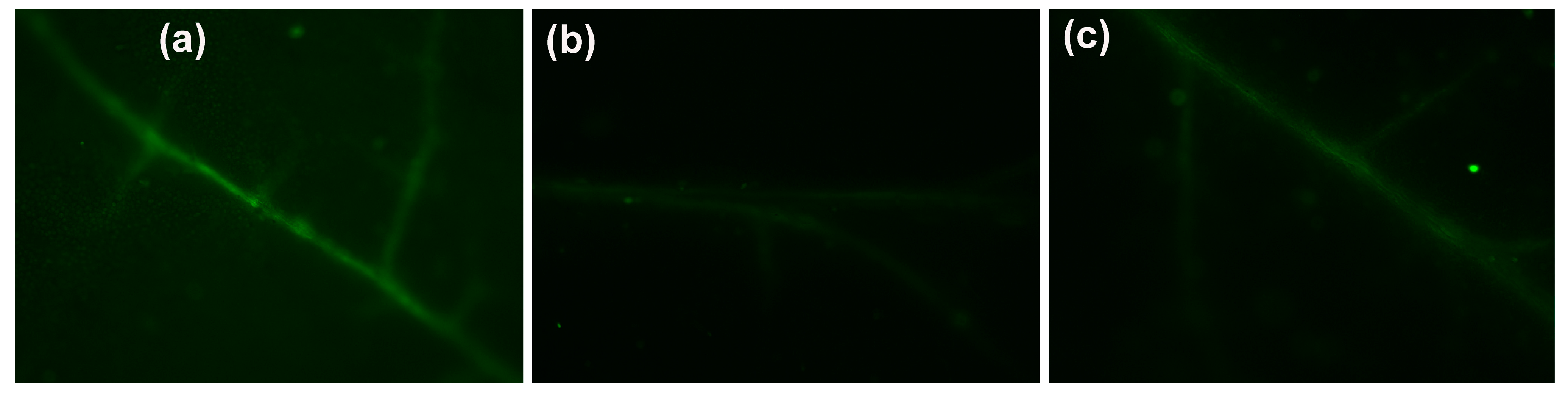
3.6. Impact of the Hetero-Nanostructure on Hydrogen Peroxide Production
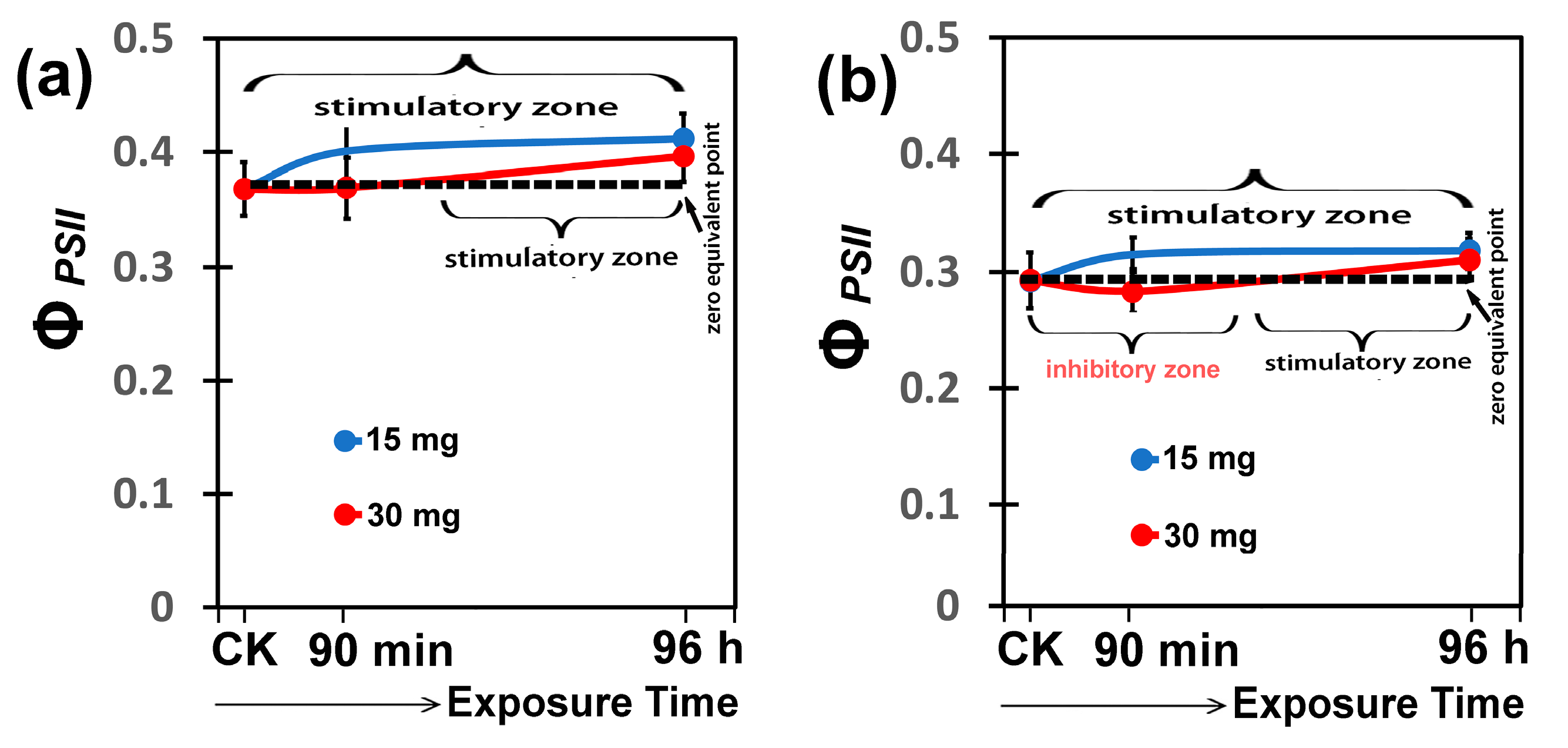
3.7. Hormetic Responses of the Effective Quantum Yield of PSII Photochemistryto Hetero-Nanostructure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, Y.; Hasan, K.; Jalal, A.G.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Jabbari, F.; Cidonio, G.; Babaeipour, V. Revolutionizing agriculture: Harnessing nano-innovations for sustainable farming and environmental preservation. Pestic. Biochem. Physiol. 2024, 198, 105722. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Kanakari, E.; Dendrinou-Samara, C. Fighting phytopathogens with engineered inorganic-based nanoparticles. Materials 2023, 16, 2388. [Google Scholar] [CrossRef]
- Carrillo-Lopez, L.M.; Villanueva-Verduzco, C.; Villanueva-Sánchez, E.; Fajardo-Franco, M.L.; Aguilar-Tlatelpa, M.; Ventura-Aguilar, R.I.; Soto-Hernández, R.M. Nanomaterials for plant disease diagnosis and treatment: A review. Plants 2024, 13, 2634. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F.R. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Outcome of the Consultation with Member States and EFSA on the Basic Substance Application for Approval of Calcium Hydroxide for the Extension of Use in Plant Protection As a Fungicide in Grapevine and Peach, and As Insecticide in Grapevine, Plum, Peach, Apricot, Apple, Pear, Almond and Strawberry; EFSA Publications: Parma, Italy, 2020. [Google Scholar]
- U.S. Food and Drug Administration (FDA). GRAS Notice. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 12 August 2024).
- Zhu, J.; Zhang, P.; Ding, J.; Dong, Y.; Cao, Y.; Dong, W.; Zhao, X.; Li, X.; Camaiti, M. Nano Ca(OH)2: A review on synthesis, properties, and applications. J. Cult. Herit. 2021, 50, 25–42. [Google Scholar] [CrossRef]
- Hamada, A.M.; Radi, A.A.; Al-Kahtany, F.A.; Farghaly, F.A. A review: Zinc oxide nanoparticles: Advantages and disadvantages. J. Plant Nutr. 2024, 47, 656–679. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Verret, F.; Wheeler, G.; Taylor, A.R.; Farnham, G.; Brownlee, C. Calcium channels in photosynthetic eukaryotes: Implications for evolution of calcium-based signalling. New Phytol. 2010, 187, 23–43. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Gupta, S.; Kaur, N.; Kant, K.; Jindal, P.; Ali, A.; Naeem, M. Calcium: A master regulator of stress tolerance in plants. S. Afr. J. Bot. 2023, 163, 580–594. [Google Scholar] [CrossRef]
- Yocum, C.F. The calcium and chloride requirements of the O2 evolving complex. Coord. Chem. Rev. 2008, 252, 296–305. [Google Scholar] [CrossRef]
- Haddy, A.; Beravolu, S.; Johnston, J.; Kern, H.; McDaniel, M.; Ore, B.; Reed, R.; Tai, H. Exploring the interdependence of calcium and chloride activation of O2 evolution in photosystem II. Photosynth. Res. 2024, 162, 385–400. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.J.; Li, X.G.; Wan, S.B. Calcium contributes to photoprotection and repair of photosystem II in peanut leaves during heat and high irradiance. J. Integr. Plant Biol. 2015, 57, 486–495. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Dielectric and antibacterial studies of microwave-assisted calcium hydroxide nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 16509–16518. [Google Scholar] [CrossRef]
- Harish; Kumari, S.; Parihar, J.; Akash; Kumari, J.; Kumar, L.; Debnath, M.; Kumar, V.; Mishra, R.K.; Gwag, J.S. Synthesis, characterization, and antibacterial activity of calcium hydroxide nanoparticles against gram-positive and gram-negative bacteria. ChemistrySelect 2022, 7, e202203094. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Mourdikoudis, S.; Vourlias, G.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Microwave-mediated synthesis and characterization of Ca(OH)2 nanoparticles destined for geraniol encapsulation. Inorganics 2023, 11, 470. [Google Scholar] [CrossRef]
- Tryfon, P.; Antonoglou, O.; Vourlias, G.; Mourdikoudis, S.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Tailoring Ca-based nanoparticles by polyol process for use as nematicidals and pH adjusters in agriculture. ACS Appl. Nano Mater. 2019, 2, 3870–3881. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Samanta, A.; Podder, S.; Ghosh, C.K.; Bhattacharya, M.; Ghosh, J.; Mallik, A.K.; Dey, A.; Mukhopadhyay, A.K. ROS mediated high anti-bacterial efficacy of strain tolerant layered phase pure nano-calcium hydroxide. J. Mech. Behav. Biomed. Mater. 2017, 72, 110–128. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Šebesta, M.; Kurtinová, S.; Kolenčík, M.; Illa, R. Enhancement of stress tolerance of crop plants by ZnO nanoparticles. In Sustainable Agriculture Reviews 53; Faizan, M., Hayat, S., Yu, F., Eds.; Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2021; Volume 53. [Google Scholar] [CrossRef]
- Agarwal, S.; Jangir, L.K.; Rathore, K.S.; Kumar, M.; Awasthi, K. Morphology-dependent structural and optical properties of ZnO nanostructures. Appl. Phys. A 2019, 125, 553. [Google Scholar] [CrossRef]
- Onyszko, M.; Zywicka, A.; Wenelska, K.; Mijowska, E. Revealing the influence of the shape, size, and aspect ratio of ZnO nanoparticles on antibacterial and mechanical performance of cellulose fibers based paper. Part. Part. Syst. Charact. 2022, 39, 2200014. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Pavlou, A.; Mourdikoudis, S.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Nanocapsules of ZnO nanorods and geraniol as a novel mean for the effective control of Botrytis cinerea in tomato and cucumber plants. Plants 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, A.; Arsenov, D.; Borišev, M.; Djordjević, A.; Župunski, M.; Borišev, I. Foliar application of fullerenol and zinc oxide nanoparticles improves stress resilience in drought-sensitive Arabidopsis thaliana. PLoS ONE 2025, 20, e0330022. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Wang, R.; Ju, Q.; Wang, Y.; Xu, J. Comparative physiological and transcriptomic analyses reveal the toxic effects of ZnO nanoparticles on plant growth. Environ. Sci. Technol. 2019, 53, 4235–4244. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Gómez-Ortíz, N.; De la Rosa-García, S.; González-Gómez, W.; Soria-Castro, M.; Quintana, P.; Oskam, G.; Ortega-Morales, B. Antifungal coatings based on Ca(OH)2 mixed with ZnO/TiO2 nanomaterials for protection of limestone monuments. ACS Appl. Mater. Interfaces 2013, 5, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sabri, Y.M.; Bhargava, S.K.; Sunkara, M.V.; Ippolito, S.J. Band offset in calcium hydroxide mediated CaO-ZnO heterointerfaces. Mater. Sci. Eng. B 2021, 265, 115005. [Google Scholar] [CrossRef]
- Ashrafi, A. Band offsets at ZnO/SiC heterojunction: Heterointerface in band alignment. Surf. Sci. 2010, 604, L63–L66. [Google Scholar] [CrossRef]
- Patil, B.M.; Patil, V.L.; Bhosale, S.R.; Bhosale, R.R.; Ingavale, D.R.; Patil, S.S.; Kamble, P.D.; Bhosale, A.G.; Mane, S.M.; Lee, J.; et al. Field application of Ca-doped ZnO nanoparticles to maize and wheat plants. Plant Physiol. Biochem. 2024, 210, 108552. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chi, H.Y.; Kim, S.-H. Impact of nanopollution on plant growth, photosynthesis, toxicity, and metabolism in the agricultural sector: An updated review. Plant Physiol. Biochem. 2024, 207, 108370. [Google Scholar] [CrossRef]
- Tripathi, G.; Dutta, S.; Mishra, A.; Basu, S.; Gupta, V.; Kamaraj, C. Nanomaterials impact in phytohormone signaling networks of plants—A critical review. Plant Sci. 2025, 352, 112373. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Moustakas, M.; Dendrinou-Samara, C. Impact of coated zinc oxide nanoparticles on photosystem II of tomato plants. Materials 2023, 16, 5846. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Moustaka, J.; Adamakis, I.-D.S.; Giannousi, K.; Dendrinou-Samara, C.; Moustakas, M. Hormetic Response of Photosystem II Function Induced by Nontoxic Calcium Hydroxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 8350. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical andnon-photochemical components—Calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Giannousi, K.; Menelaou, M.; Arvanitidis, J.; Angelakeris, M.; Pantazaki, A.; Dendrinou-Samara, C. Hetero-nanocomposites of magnetic and antifungal nanoparticles as a platform for magneto mechanical stress induction in Saccharomyces cerevisiae. J. Mater. Chem. B. 2015, 3, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants—A brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release. 2020, 329, 1234–1248. [Google Scholar] [CrossRef]
- Harish; Kumar, P.; Malhotra, B.; Phalswal, P.; Khanna, P.K.; Salim, A.; Singhal, R.; Mukhopadhyay, A.K. Effect of reaction rate on the properties of chemically synthesized calcium hydroxide nanoparticles. Mater. Today Proc. 2020, 28, 2305–2310. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Abdullah, N.; Derawi, D. Synthesis of raspberry-like structure zinc oxide nanoparticles via glycol-solvothermal, low-temperature solvothermal and coprecipitation methods. C. R. Chim. 2021, 24, 33–42. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Vattikuti, S.V.P.; Reddy, G.R.; Kim, K.H. A brief review on Zn-based materials and nanocomposites for supercapacitor applications. J. Energy Storage 2023, 68, 107674. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Ducongé, J.; Márquez, F. Artificial photosynthesis: Current advancements and future prospects. Biomimetics 2023, 8, 298. [Google Scholar] [CrossRef]
- Pinzari, F. Synthesis, photocatalytic and bio activity of ZnO-TiO2 nanocomposites: A review study. Reactions 2024, 5, 680–739. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Panteris, E.; Adamakis, I.-D.S.; Moustakas, M. Aspirin foliar spray-induced changes in light energy use efficiency, chloroplast ultrastructure, and ROS generation in tomato. Int. J. Mol. Sci. 2025, 26, 1368. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams II, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C. Non-photochemical fluorescence quenching and quantum yields in PSI and PSII: Analysis of heat-induced limitations using Maxi-Imaging PAM and Dual-PAM-100. PAM Appl. Notes 2008, 1, 15–18. [Google Scholar]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef]
- Wilson, K.E.; Ivanov, A.G.; Öquist, G.; Grodzinski, B.; Sarhan, F.; Huner, N.P.A. Energy balance, organellar redox status, and acclimation to environmental stress. Can. J. Bot. 2006, 84, 1355–1370. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Huang, X.; Xing, J.; Yao, J.; Yin, T.; Jiang, J.; Wang, P.; Xu, B. STAYGREEN-mediated chlorophyll a catabolism is critical for photosystem stability during heat-induced leaf senescence in perennial ryegrass. Plant Cell Environ. 2022, 45, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Mittler, R.; Berkowitz, G. Hydrogen peroxide, a messenger with too many roles? Redox Rep. 2001, 6, 69–72. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Foyer, C.H. How plant cells sense the outside world through hydrogen peroxide. Nature 2020, 578, 518–519. [Google Scholar] [CrossRef]
- Moustakas, M. Molecular mechanisms of plant abiotic stress tolerance. Int. J. Mol. Sci. 2025, 26, 2731. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Ahmad, P.; Corpas, F.J. Deciphering the interplay between redox switches and signaling networks in plant stress responses. Crit. Rev. Plant Sci. 2025, 44, 180–209. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Wang, A.; Xu, Y.; Wu, T.; Liu, Z.; Shi, G.; Wei, F.; Tian, B. SlGSTU43 and SlERF-B12 genesare involved in SlMYB15-regulated redox homeostasis to enhance cold tolerance in tomato. J. Agric. Food Chem. 2025, 73, 21681–21696. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis can enhance agricultural sustainability in a changing world. Glob. Food Secur. 2019, 20, 150–155. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Agathokleous, E.; Sonne, C.; Benelli, G.; Calabrese, E.J.; Guedes, R.N.C. Low-dose chemical stimulation and pest resistance threaten global crop production. Sci. Total Environ. 2023, 878, 162989. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Kumari, A.; Mandzhieva, S.S.; Sushkova, S.; Prazdnova, E.V.; Zargar, S.M.; Raza, A.; Minkina, T.; Chung, G. Nanobionics in crop production: An emerging approach to modulate plant functionalities. Plants 2022, 11, 692. [Google Scholar] [CrossRef]
- Pandey, K.; Dasgupta, C.N. Role of nanobionics to improve the photosynthetic productivity in plants and algae: An emerging approach. 3 Biotech 2025, 15, 74. [Google Scholar] [CrossRef]
- Pu, S.; Yan, C.; Huang, H.; Liu, S.; Deng, D. Toxicity of nano-CuO particles to maize and microbial community largely depends on its bioavailable fractions. Environ. Pollut. 2019, 255, 113248. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Zhan, X.; Li, A.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Role of charge and size in the translocation and distribution of zinc oxide particles in wheat cells. ACS Sustain. Chem. Eng. 2021, 9, 11556–11564. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Dendrinou-Samara, C.; Moustakas, M. Modification of tomato photosystem II photochemistry with engineered zinc oxide nanorods. Plants 2023, 12, 3502. [Google Scholar] [CrossRef]
- He, Q.; Zhan, J.; Liu, X.; Dong, C.; Tian, D.; Fu, Q. Multispectral polarimetric bidirectional reflectance research of plant canopy. Opt. Lasers Eng. 2025, 184, 108688. [Google Scholar] [CrossRef]
| Parameter | Definition | Calculation |
|---|---|---|
| Fo | Minimum chlorophyll a fluorescence in the dark-adapted leaf (PSII centers open) | Obtained by applying measuring photon irradiance of 1.2 μmol photons m−2 s−1 |
| Fm | Maximum chlorophyll a fluorescence in the dark-adapted leaf (PSII centers closed) | Obtained with a saturating pulse (SP) of 6000 μmol photons m−2 s−1 |
| Fo′ | Minimum chlorophyll a fluorescence in the light-adapted leaf | It was computed by the Imaging Win software V2.41a (Heinz Walz GmbH, Effeltrich, Germany) as Fo′ = Fo/(Fv/Fm + Fo/Fm′) |
| Fm′ | Maximum chlorophyll a fluorescence in the light-adapted leaf | Measured with saturating pulses (SPs) every 20 s for 5 min after application of the actinic light (AL) of 580 μmol photons m−2 s−1 or 1000 μmol photons m−2 s−1 |
| Fs | Steady-state photosynthesis | Measured after 5 min illumination time before switching off the actinic light (AL) of 580 μmol photons m−2 s−1 or 1000 μmol photons m−2 s−1 |
| ΦPSII | Effective quantum yield of PSII photochemistry | (Fm′ − Fs)/Fm′ |
| ΦNPQ | Quantum yield of regulated non-photochemical energy loss in PSII | Fs/Fm′ − Fs/Fm |
| ΦNO | Quantum yield of non-regulated energy loss in PSII | Fs/Fm |
| Fv′/Fm′ | Efficiency of the open PSII reaction centers | (Fm′ − Fo′)/Fm′ |
| ETR | Electron transport rate | ΦPSII × PAR × c × abs, where PAR is the photosynthetically active radiation, c is 0.5, and abs is the total light absorption of the leaf taken as 0.84 |
| qp | Photochemical quenching, representing the redox state of quinone A (QA), or in other words the fraction of open PSII reaction centers based on the “puddle” model for the photosynthetic unit | (Fm′ − Fs)/(Fm′ − Fo′) |
| NPQ | Non-photochemical quenching reflecting the dissipation of excitation energy as heat | (Fm − Fm′)/Fm′ |
| EXC | Excess excitation energy | (1 − qp) × Fv′/Fm′ |
| 1-qL | The fraction of closed PSII reaction centers based on the “lake” model for the photosynthetic unit | 1 − (qp × Fo′/Fs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tryfon, P.; Moustaka, J.; Sperdouli, I.; Papoulia, C.; Pavlidou, E.; Vourlias, G.; Adamakis, I.-D.S.; Moustakas, M.; Dendrinou-Samara, C. Short- and Long-Term Effects of Ca(OH)2/ZnO Heteronanostructure on Photosystem II Function and ROS Generation in Tomato. Materials 2025, 18, 4078. https://doi.org/10.3390/ma18174078
Tryfon P, Moustaka J, Sperdouli I, Papoulia C, Pavlidou E, Vourlias G, Adamakis I-DS, Moustakas M, Dendrinou-Samara C. Short- and Long-Term Effects of Ca(OH)2/ZnO Heteronanostructure on Photosystem II Function and ROS Generation in Tomato. Materials. 2025; 18(17):4078. https://doi.org/10.3390/ma18174078
Chicago/Turabian StyleTryfon, Panagiota, Julietta Moustaka, Ilektra Sperdouli, Chrysanthi Papoulia, Eleni Pavlidou, George Vourlias, Ioannis-Dimosthenis S. Adamakis, Michael Moustakas, and Catherine Dendrinou-Samara. 2025. "Short- and Long-Term Effects of Ca(OH)2/ZnO Heteronanostructure on Photosystem II Function and ROS Generation in Tomato" Materials 18, no. 17: 4078. https://doi.org/10.3390/ma18174078
APA StyleTryfon, P., Moustaka, J., Sperdouli, I., Papoulia, C., Pavlidou, E., Vourlias, G., Adamakis, I.-D. S., Moustakas, M., & Dendrinou-Samara, C. (2025). Short- and Long-Term Effects of Ca(OH)2/ZnO Heteronanostructure on Photosystem II Function and ROS Generation in Tomato. Materials, 18(17), 4078. https://doi.org/10.3390/ma18174078














