Effects of Washing Conditions on PAH Removal Effectiveness in Firefighter Protective Clothing Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure of Simulated PAH Exposure
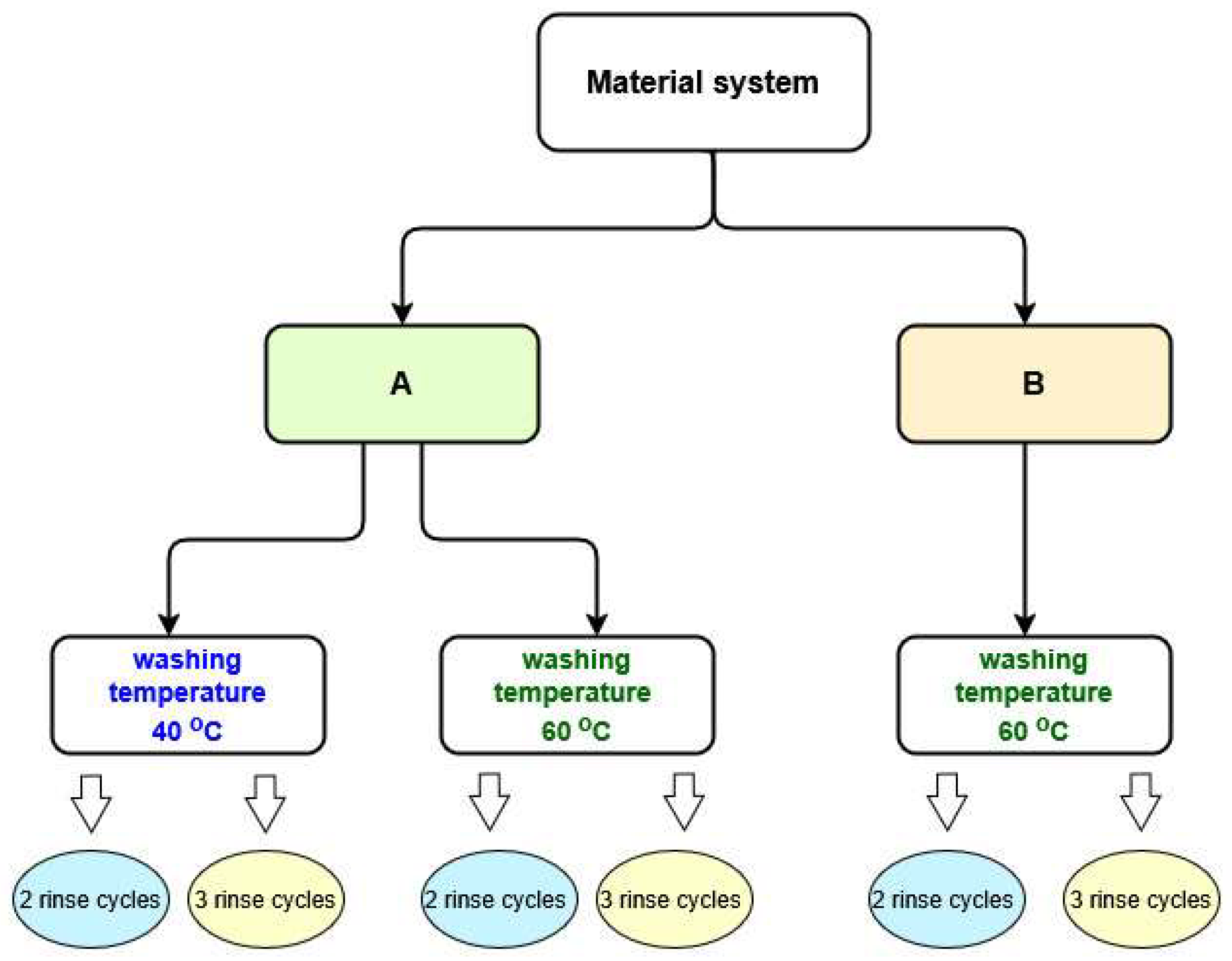
2.3. Washing Procedure
- Temperature 60 °C, 2 rinse cycles—as recommended by the clothing manufacturer.
- Temperature 40 °C, 2 rinse cycles.
- Temperature 60 °C, 3 rinse cycles.
- Temperature 40 °C, 3 rinse cycles.
2.4. Cross-Contamination
- Each set of three packages was washed separately in the washing process under specified conditions (temperature, number of rinse cycles);
- Each set of packages was washed in the same machine;
- The machine was cleaned between each washing cycle by running a standard washing cycle;
- Each day, an empty run was made with water and detergents before washing began.
2.5. Chemicals
2.6. Methods
2.7. Sample Preparation
2.8. Statistical Analyses
- t-test—To test the significance of differences between two groups when the condition of normality of distribution and the condition of homogeneity of variance were met;
- Cochran–Cox test—To test the significance of differences between two groups when the condition of normality of distribution was met but the condition of homogeneity of variance was not;
- Analysis of variance (ANOVA)—To test the significance of differences between at least three groups when the conditions of normality of distribution and homogeneity of variance were met;
- Welch’s F test—To test the significance of differences between at least three groups when the condition of normality of distribution was met but the condition of homogeneity of variance was not.
3. Results and Discussion
3.1. Effect of Washing Temperature
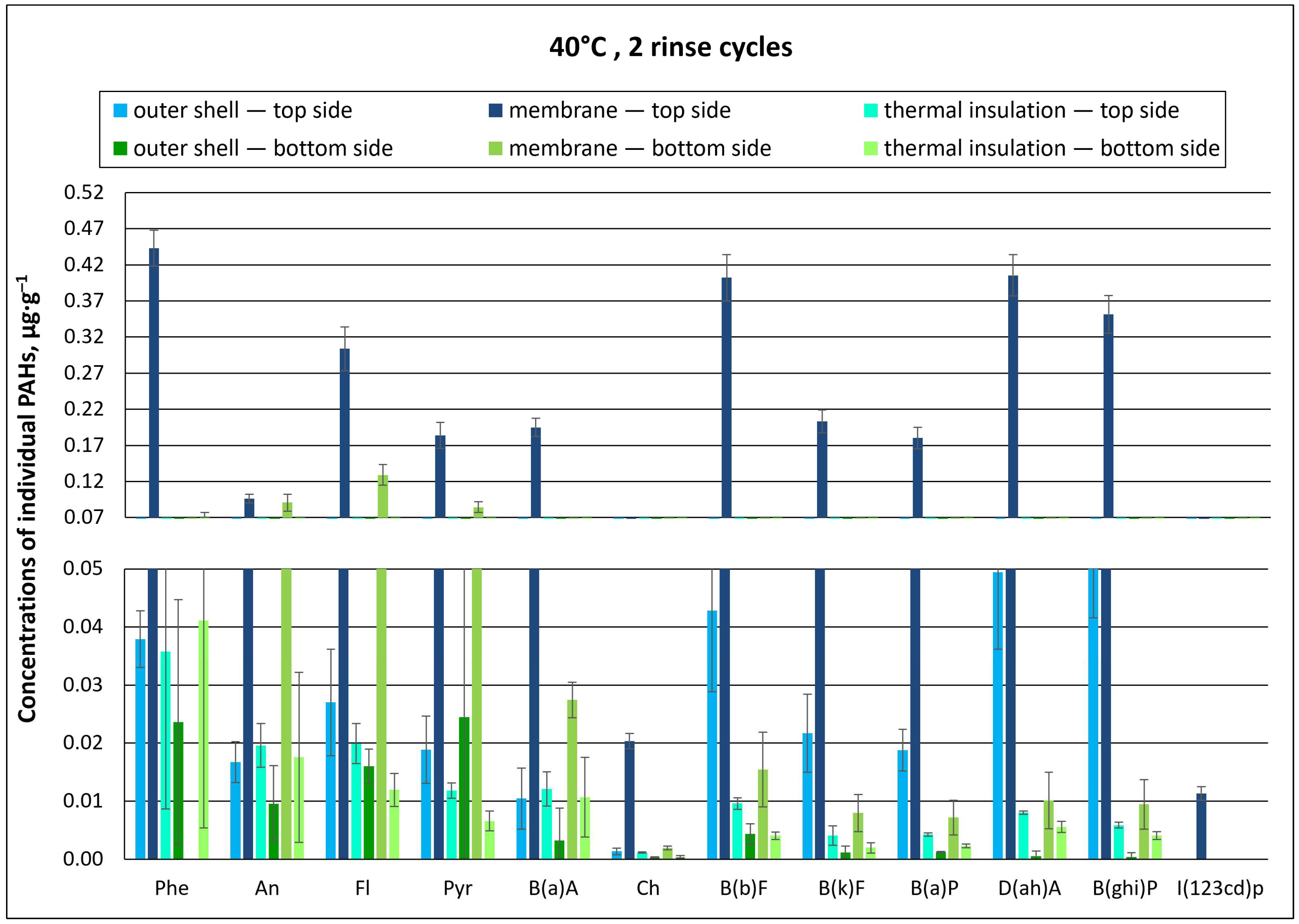
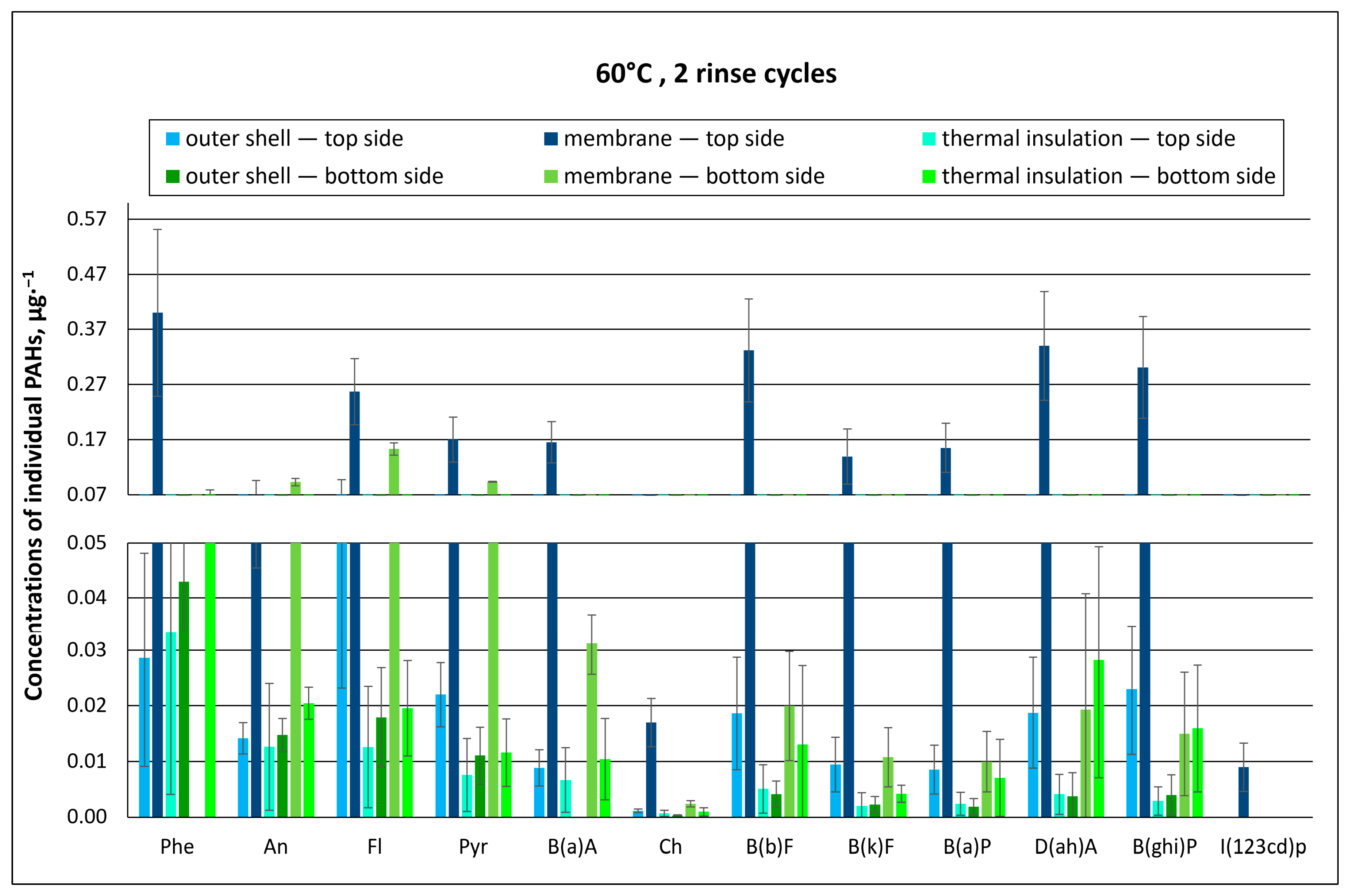
3.1.1. Effect of Material Washing Temperature on Individual PAH Concentrations
3.1.2. Effect of Washing Temperature on Total PAH Concentration
3.2. Effect of the Number of Rinse Cycles
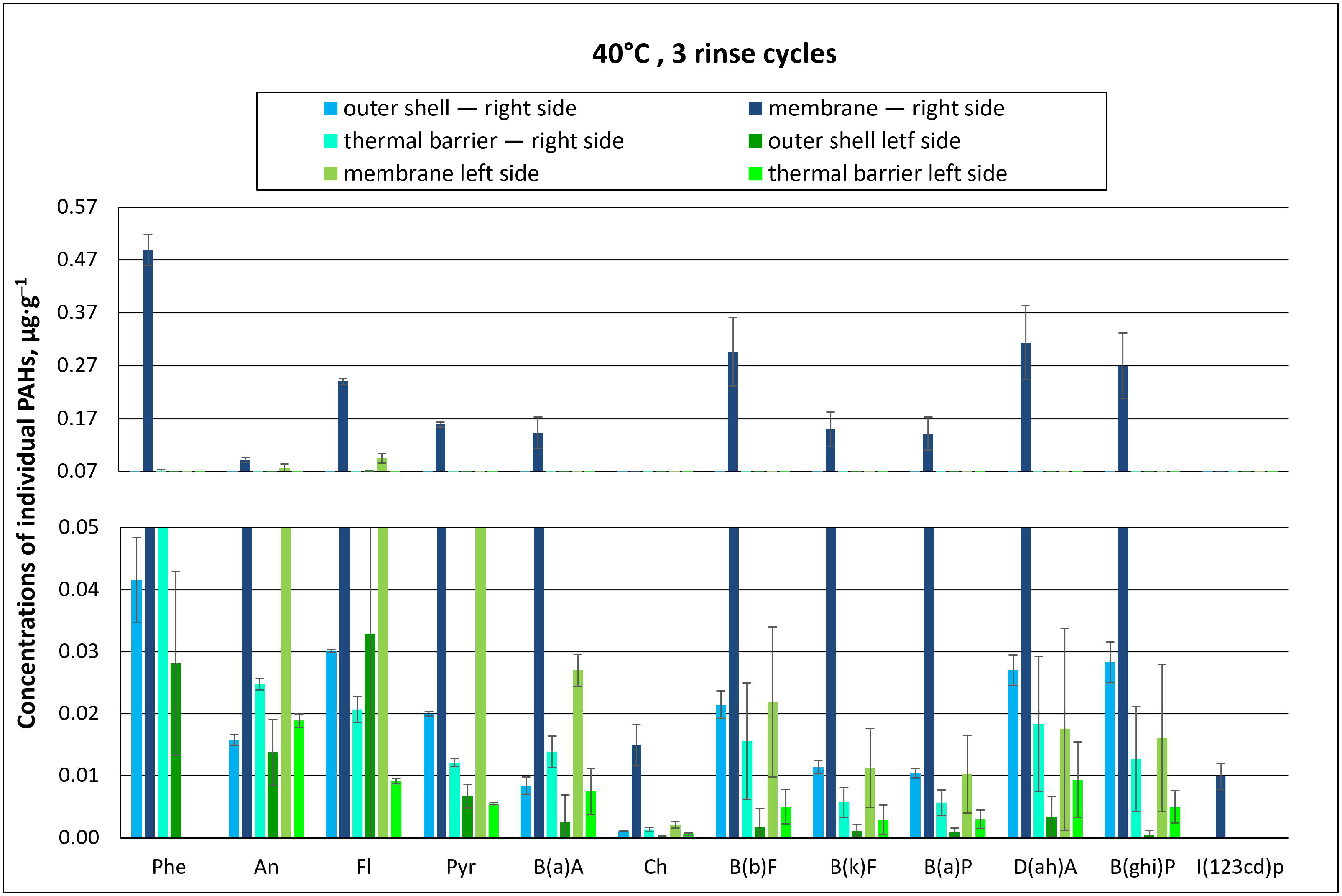
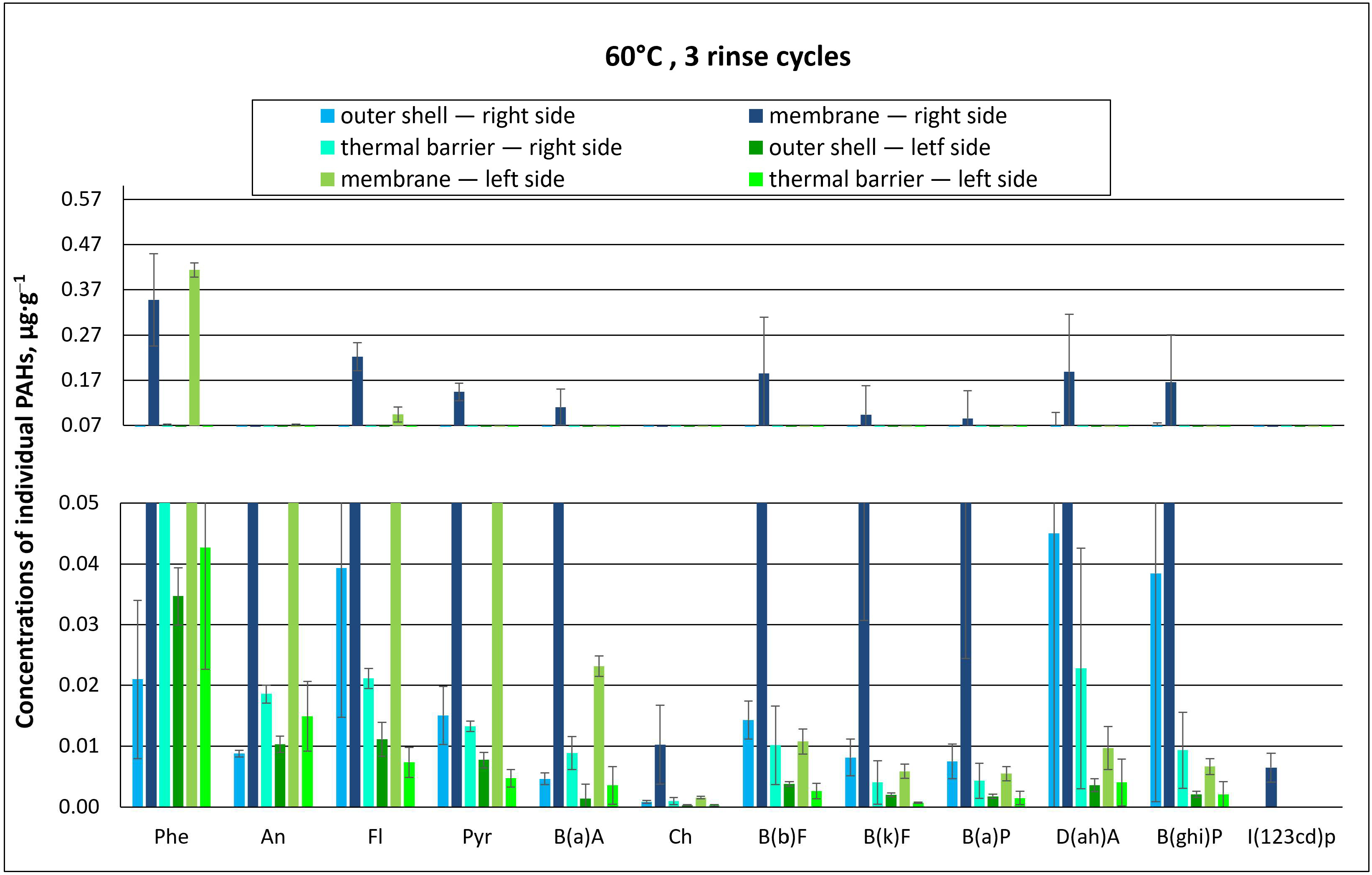
3.2.1. Effect of the Number of Rinse Cycles on the Concentration of Individual PAHs
3.2.2. Effect of the Number of Rinse Cycles on Total PAH Concentration
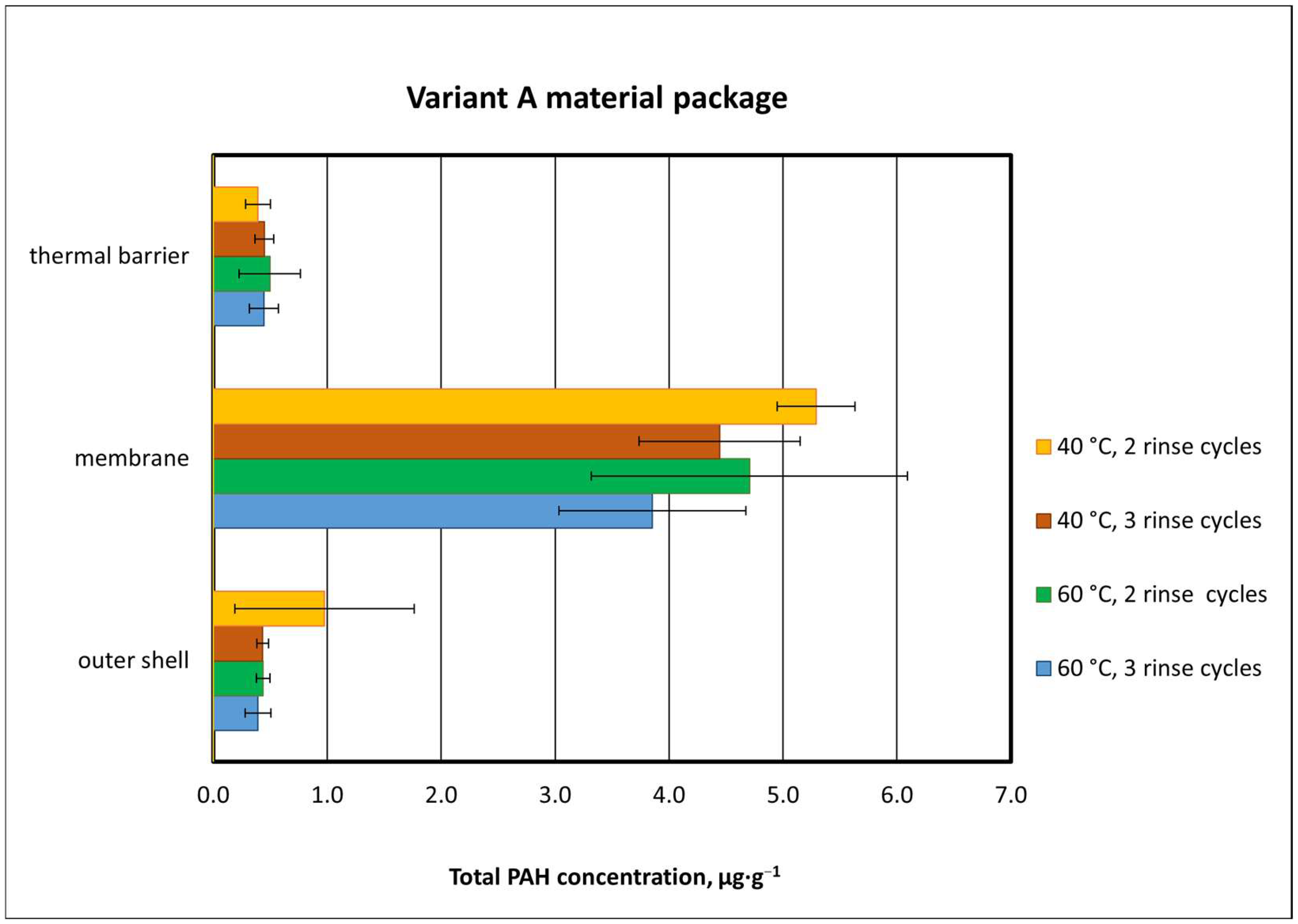
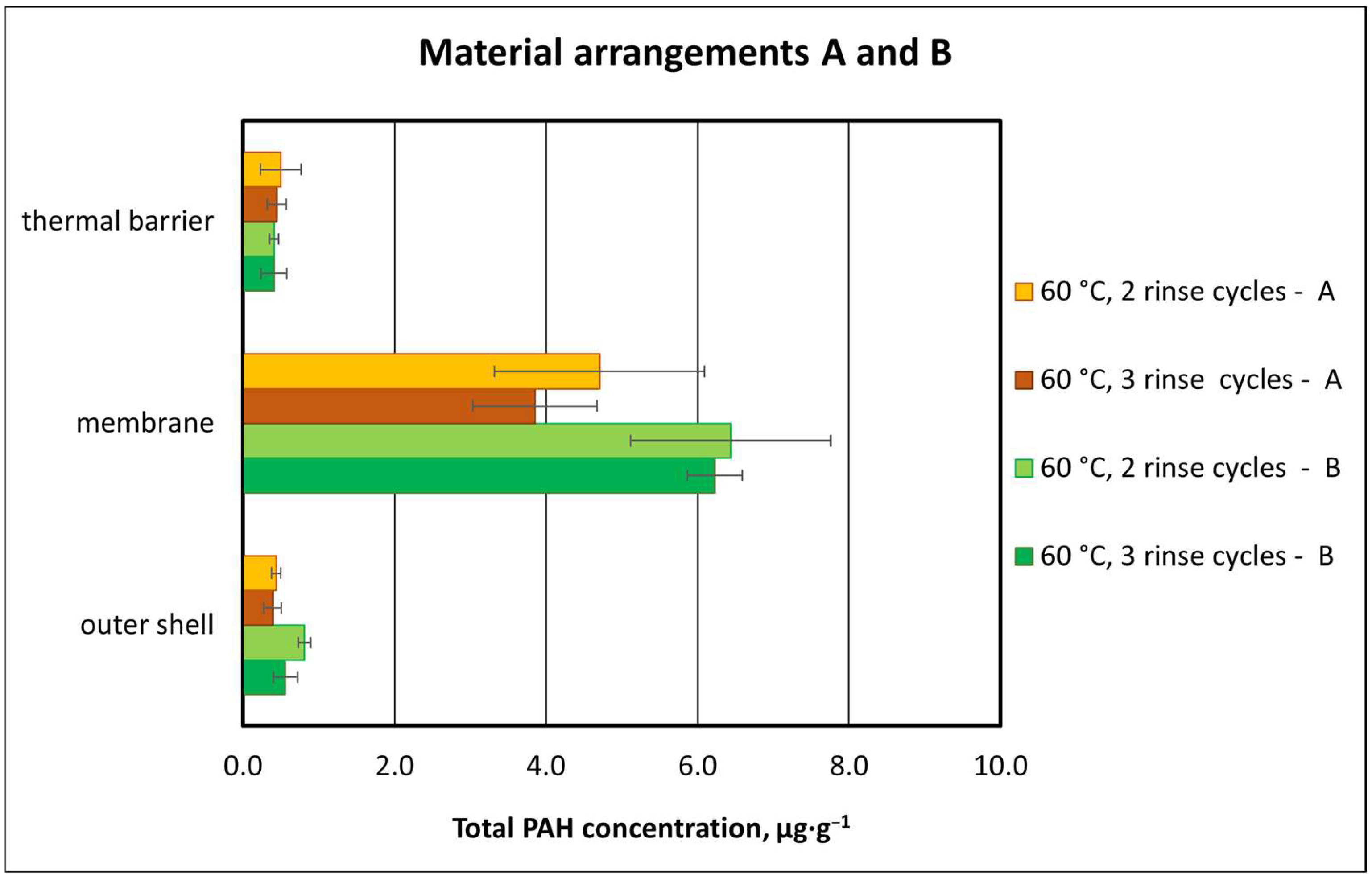
3.2.3. Effect of Material Composition on Total PAH Concentration
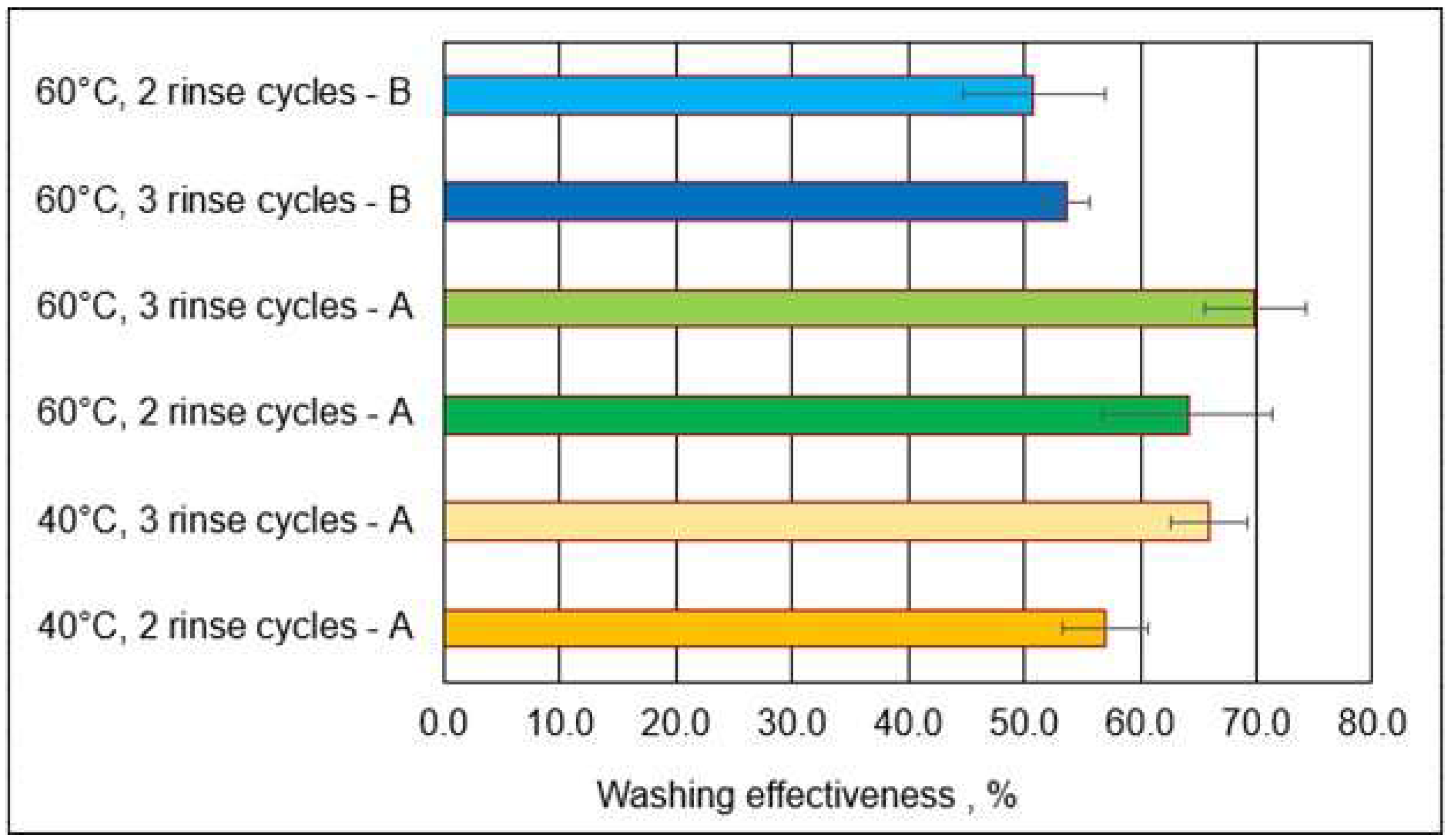
3.3. Washing Effectiveness
- Package A—from 9.5 µg to 2.9 µg;
- Package B—from 9.5 µg to 4.4 µg.
4. Conclusions
5. Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krzemińska, S.; Szewczyńska, M.; Jachowicz, A. Firefighter exposure to chemicals during fires. Occup. Saf. Sci. Pract. 2024, 5, 12–15. [Google Scholar] [CrossRef]
- Krzemińska, S.; Szewczyńska, M.; Kozikowski, P. Analysis of pahs content in materials of firefighter protective clothing and cleaning effectiveness after use. ZN SGSP 2024, 1, 85–110. [Google Scholar] [CrossRef]
- Lee, N.M.; Tadesse, A.W.; Ekpe, O.D.; Lee, S.Y.; Kwon, J.W.; Kim, W.; Cho, Y.H.; Oh, J.-E. Assessment of PAH exposure and health risks among South Korean firefighters based on urinary PAH metabolites. Chemosphere 2024, 353, 141429. [Google Scholar] [CrossRef]
- Casjens, S.; Brüning, T.; Taeger, D. Cancer risks of firefighters: A systematic review and meta-analysis of secular trends and region-specific differences. Int. Arch. Occup. Environ. Health 2020, 93, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Martinsen, J.I.; Weiderpass, E.; Kjaerheim, K.; Lynge, E.; Tryggvadottir, L.; Sparén, P.; Demers, P.A. Cancer incidence among firefighters: 45 years of follow-up in five Nordic countries. Occup. Environ. Med. 2014, 71, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.A.; Robinson, A.; Wolffe, T.A.M.; Bagkeris, E. Scottish Firefighters Occupational Cancer and Disease Mortality Rates: 2000–2020. Occup. Med. 2022, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Orysiak, J.; Młynarczyk, M.; Piec, R.; Jakubiak, A. Lifestyle and environmental factors may induce airway and systemic inflammation in frefghters. Environ. Sci. Pollut. Res. 2022, 29, 73741–73768. [Google Scholar] [CrossRef]
- Kirk, K.M.; Logan, M.B. Firefighting instructors’ exposures to polycyclic aromatic hydrocarbons during live fire training scenarios. J. Occup. Environ. Hyg. 2015, 12, 227–234. [Google Scholar] [CrossRef]
- Wolański, R.; Rabajczyk, A. Selected Aspects of Transformation of Textile Elements of Firemen’s Personal Protection. Saf. Fire Technol. 2023, 61, 86–101. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Gniazdowska, J.; Stojek, P.; Bąk, Ł. Sorption Processes of Selected PAHs on Selected Fire Resistant Materials Used in Special Firefighter Clothing. Materials. Materials 2024, 17, 1741. [Google Scholar] [CrossRef]
- Stull, J.O. Evaluation of the Cleaning Effectiveness and Impact of Esporta and Industrial Cleaning Techniques on Firefighter Protective Clothing; Technical Report; International Personnel Protection, Inc.: Austin, TX, USA, 10 May 2006. [Google Scholar]
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.P.; Houldsworth, P.E.; Martin, F.L. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef]
- Stec, A.; Wolffe, T.; Clinton, A. Minimising Firefighters’ Exposure to Toxic Fire Effluents; Interim Best Practice Report; University of Central Lancashire, Fire Brigades Union FBU: Preston, UK, 2020. [Google Scholar]
- Oliveira, M.; Costa, S.; Vaz, J.; Fernandes, A.; Slezakova, K.; Delerue-Matos, C.; Paulo, J.; Carmo, M.; Morais, S. Firefighters exposure to fire emissions: Impact on levels of biomarkers of exposure to polycyclic aromatic hydrocarbons and genotoxic/oxidative-effects. J. Hazard. Mater. 2020, 383, 121179. [Google Scholar] [CrossRef]
- Rossbach, B.; Wollschl, D.; Letzel, S.; Muttray, A. Internal exposure of firefighting instructors to polycyclic aromatic hydrocarbons (PAH) during live firetraning. Toxicol. Lett. 2020, 331, 102–111. [Google Scholar] [CrossRef]
- Rosenfeld, P.E.; Spaeth, K.R.; Remy, L.L.; Byers, V.; Muerth, S.A.; Hallman, R.C.; Summers-Evans, J.; Barker, S. Perfluoroalkyl substances exposure in firefighters: Sources and implications. Environ. Res. 2023, 220, 115164. [Google Scholar] [CrossRef] [PubMed]
- Paris-Davila, T.; Gaines, L.G.T.; Lucas, K.; Nylander-French, L.A. Occupational exposures to airborne per- and polyfluoroalkyl substances (PFAS)—A review. Am. J. Ind. Med. 2023, 66, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Gaines, L.G.T.; Paris-Davila, T.; Nylander-French, L.A. Occupational exposure and serum levels of per- and polyfluoroalkyl substances (PFAS): A review. Am. J. Ind. Med. 2023, 66, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Peaslee, G.F.; Wilkinson, J.T.; McGuinness, S.R.; Tighe, M.; Caterisano, N.; Lee, S.; Gonzales, A.; Roddy, M.; Mills, S.; Mitchell, K. Another pathway for firefighter exposure to per- and polyfluoroalkyl substances: Firefighter textiles. Environ. Sci. Technol. Lett. 2020, 7, 594–599. [Google Scholar] [CrossRef]
- Řiháčková, K.; Pindur, A.; Komprdová, K.; Pálešová, N.; Kohoutek, J.; Šenk, P.; Navrátilová, J.; Andrýsková, L.; Šebejová, L.; Hůlek, R.; et al. The exposure of Czech firefighters to perfluoroalkyl substances and polycyclic aromatic hydrocarbons: CELSPAC–FIREexpo case-control human biomonitoring study. Sci. Total Environ. 2023, 881, 163298. [Google Scholar] [CrossRef]
- Krzemińska, S.; Szewczyńska, M. PAH contamination of firefighter protective clothing and cleaning effectiveness. Fire Saf. J. 2022, 131, 103610. [Google Scholar] [CrossRef]
- Banks, A.P.W.; Wang, X.; Engelsman, M.; He, C.; Osorio, A.F.; Mueller, J.F. Assessing decontamination and laundering processes for the removal of polycyclic aromatic hydrocarbons and flame retardants from firefighting uniforms. Environ. Res. 2021, 194, 110616. [Google Scholar] [CrossRef]
- Hossain, M.T.; Ormond, R.B. Assessing the impact of pre-soaking to enhance laundering efficacy of firefighter turnout gear. Toxics 2024, 12, 544. [Google Scholar] [CrossRef]
- Pośniak, M.; Skowroń, J. Harmful Factors in the Work Environment. Permissible Values, 14th ed.; Inter-ministerial Commission for the Maximum Permissible Concentrations and Intensities of Factors Harmful to Health in the Work Environment: Warsaw, Poland, 2024. [Google Scholar]
- Sapota, A. Polycyclic aromatic hydrocarbons (cyclohexane-soluble tar substances). Documentation of proposed occupational exposure limit values. Fundam. Methods Occup. Environ. Assess. 2002, 18, 179–208. [Google Scholar]
- Forester, C.D.; Tarley, J. Effects of Temperature and Advanced Cleaning Practices on the Removal of Select Organic Chemicals from Structural Firefighter Gear. Fire Technol 2023, 59, 2127–2145. [Google Scholar] [CrossRef]
- AfPS GS 2019:01 PAK.; GS-Spezifikation Prüfung und Bewertung von Polyzyklischen Aromatischen Kohlenwasserstoffen (PAK) bei der Zuerkennung des GS-Zeichens–Spezifikation gemäß § 21 Abs. 1 Nr. 3 ProdSG. Ausschuss für Produktsicherheit (AfPS): Dortmund, Germany, 2020. Available online: https://www.sgs.com/en/news/2020/05/safeguards-05720-german-afps-publishes-latest-version-of-pah-specifications-under-gs-mark (accessed on 25 March 2025).
- Oeko-Tex Tables with Limit Values, Annex 4 dated 04 February 2025. Available online: https://www.oeko-tex.com/importedmedia/downloadfiles/OEKO-TEX_STANDARD_100_Limit_Values_and_Individual_Substances_According_to_Appendices_4_5_EN_DE.pdf (accessed on 25 March 2025).
- Engelsman, M.; Toms, L.-M.L.; Wang, X.; Banks, A.P.W. Firefighter undergarments: Assessing contamination and laundering efficacy. Environ. Res. 2023, 216, 114344. [Google Scholar] [CrossRef]
- ISO 23616; Cleaning, inspection and repair of firefighters’ personal protective equipment (PPE). ISO copyright office: Geneva, Switzerland, 2022.
- Denchev, Z.; Dencheva, N. Manufacturing and Properties of Aramid Reinforced Composites. In Synthetic Polymer-Polymer Composites; Bhattacharyya, D., Fakirov, S., Eds.; Hanser Publishers: Munich, Germany, 2012; Chapter 8; pp. 251–280. [Google Scholar] [CrossRef]
- Alhendal, A.; Almoaeen, R.A.; Rashad, M.; Husain, A.; Mouffouk, F.; Ahmad, Z. Aramid-wrapped CNT hybrid sol–gel sorbent for polycyclic aromatic hydrocarbons. RSC Adv. 2022, 12, 18077–18083. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; López-Ruiz, R.; Arrebola Liébanas, F.J.; Peralta, M.L.V.; Frenich, A.G. From flames to lab: A robust non-destructive sampling method for evaluating PAH exposure in firefighters’ personal protection equipment. Microchem. J. 2024, 207, 111909. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhao, B.; Hu, S.; Xue, G.; Xia, J. Contamination and removal of polycyclic aromatic hydrocarbons in multilayered assemblies of firefighting protective clothing. J. Ind. Text. 2022, 52. [Google Scholar] [CrossRef]
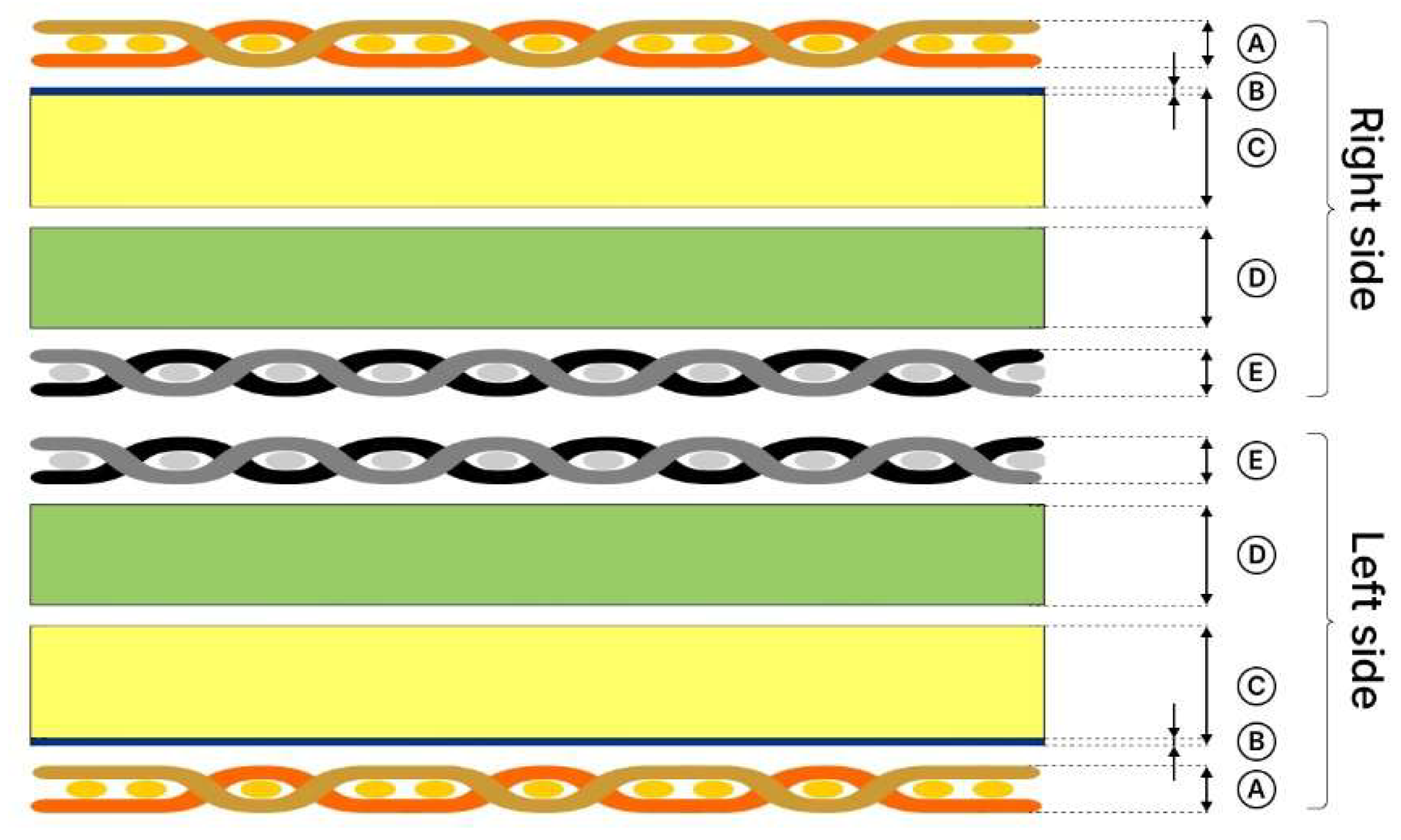
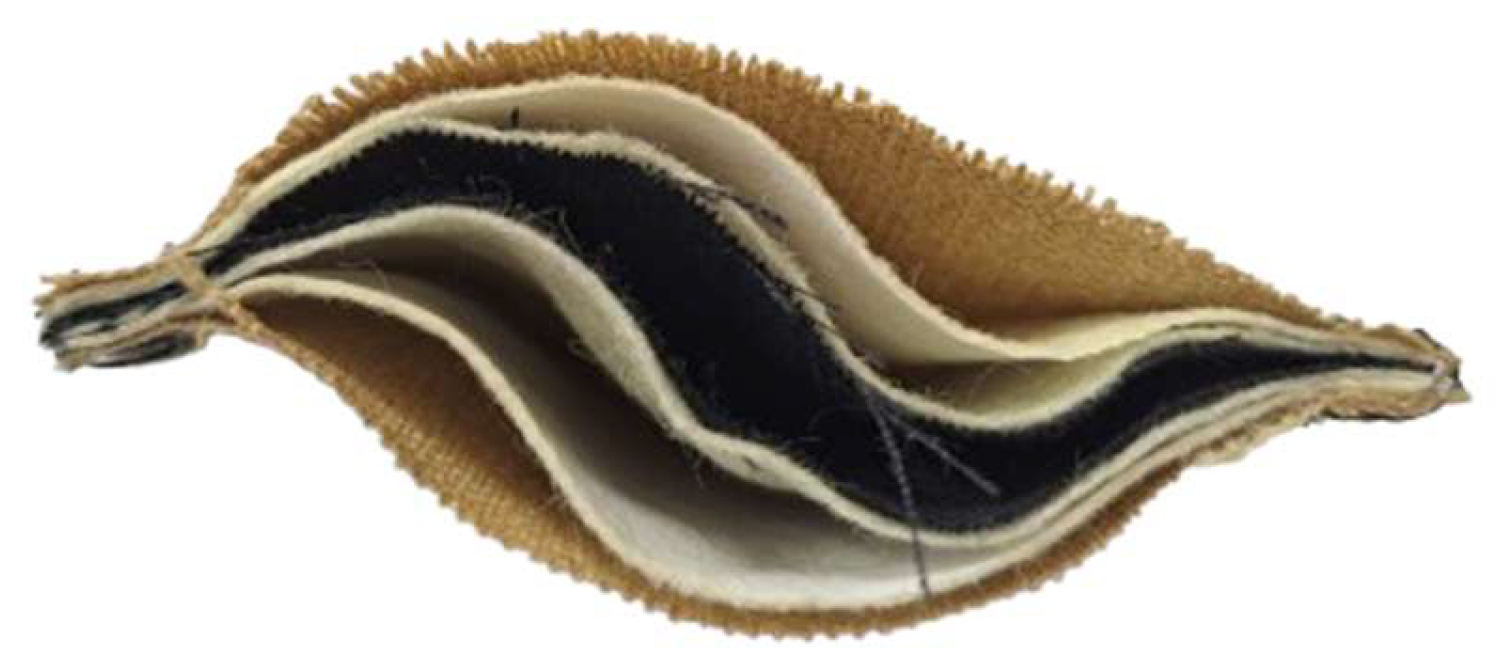
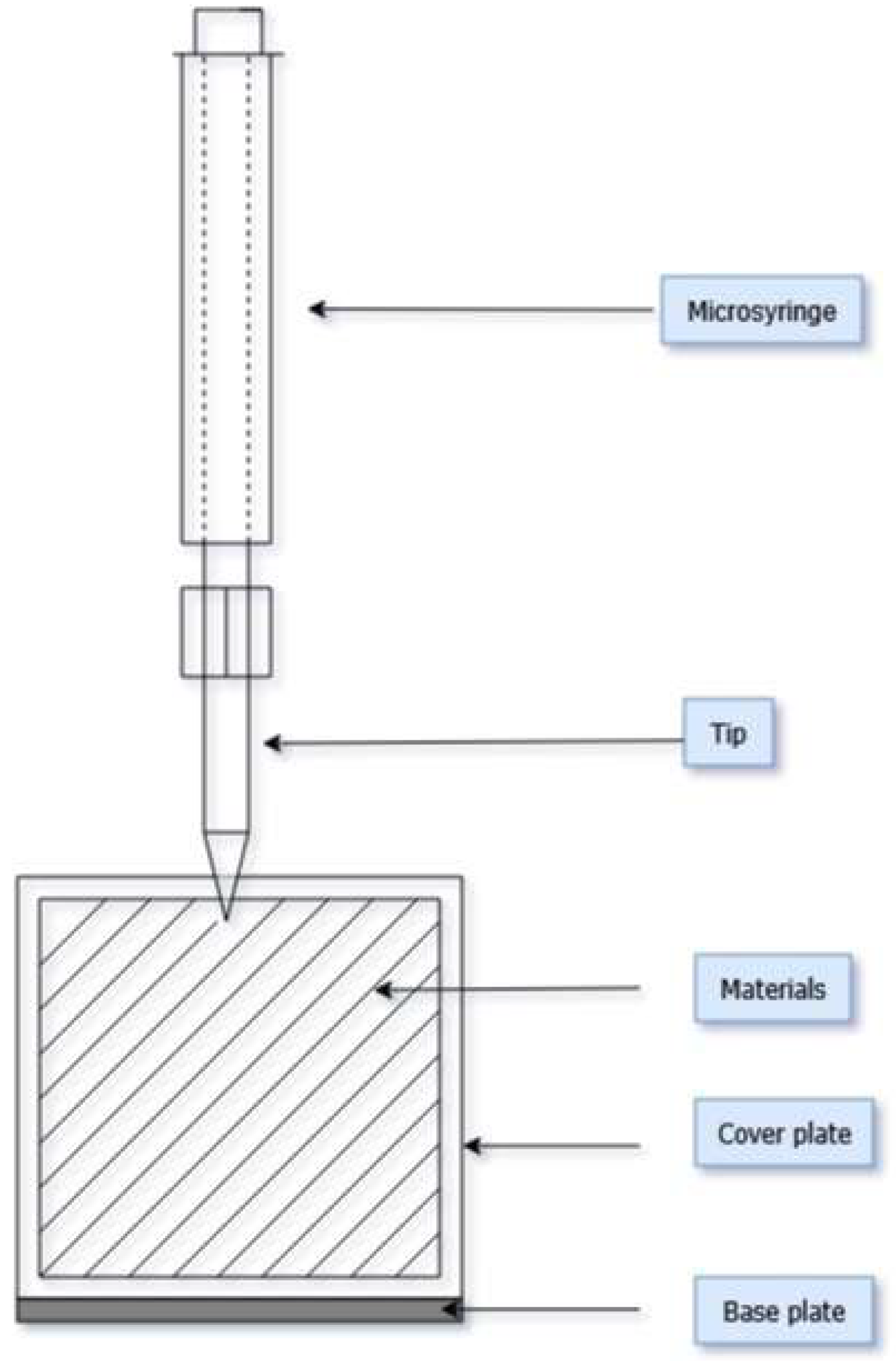

| Type of Clothing | Composition | Mass Per Unit Area, g∙m−2 | Thickness, mm | Sample Weight (1), g |
|---|---|---|---|---|
| A | Outer shell: 98% meta-aramid, 2% antistatic fiber | 218.6 ± 2.5 | 0.50 ± 0.01 | 0.35 |
| Moisture membrane: 50% meta-aramid, 25% para-aramid, 25% polytetrafluoroethylene (PTFE) laminate | 176.5 ± 1.0 | 1.14 ± 0.02 | 0.28 | |
| Thermal insulation: lining: 50% aramid, 50% FR viscose; felt: 85% meta-aramid, 15% para-aramid | 177.7 ± 1.6 | 0.96 ± 0.04 | 0.28 | |
| B | Outer shell: 58% para-aramid, 40% polybenzimidazole (PBI), 2% antistatic fiber | 200.2 ± 1.0 | 0.45 ± 0.01 | 0.32 |
| Moisture membrane: 25% meta-aramid, 25% para-aramid, 50% polytetrafluoroethylene (PTFE) laminate | 107.9 ± 1.9 | 0.61 ± 0.03 | 0.17 | |
| Thermal insulation: lining: 93% meta-aramid, 5% para-aramid, 2% antistatic fiber; felt: 67% meta-aramid, 33% para-aramid | 167.9 ± 1.7 | 0.84 ± 0.02 | 0.27 |
| PAH | Abbreviation | PAH Content Dripped Onto Package, µg | Molecular Weight, g·mol−1 | Number of Aromatic Rings | Solubility in Water, mg·L−1 |
|---|---|---|---|---|---|
| Naphthalene | Naph | 2.5 | 128.17 | 2 | 31.000 |
| Acenaphthylene | Acn | 2.5 | 152.20 | 3 | 3.800 |
| Fluorene | Flu | 0.5 | 166.20 | 3 | 1.900 |
| Phenanthrene | Phe | 0.25 | 178.23 | 3 | 1.100 |
| Anthracene | An | 0.25 | 178.23 | 3 | 0.045 |
| Fluoranthene | Fl | 0.5 | 202.25 | 4 | 0.260 |
| Pyrene | Pyr | 0.25 | 202.26 | 4 | 0.132 |
| Benz(a)anthracene | B(a)A | 0.25 | 228.29 | 4 | 0.011 |
| Chrysene | Ch | 0.25 | 228.29 | 4 | 0.001 |
| Benzo(b)fluoranthene | B(b)F | 0.5 | 252.32 | 5 | 0.001 |
| Benzo(k)fluoranthene | B(k)F | 0.25 | 252.32 | 5 | 0.001 |
| Benzo(a)pyrene | B(a)P | 0.25 | 252.32 | 5 | 0.004 |
| Dibenzo[a,h]anthracene | D(ah)A | 0.5 | 278.35 | 5 | 0.001 |
| Benzo(g,h,i)perylene | B(ghi)P | 0.5 | 276.33 | 6 | 0.0002 |
| Indeno(1,2,3-cd)pyrene | I(123cd)p | 0.25 | 276.33 | 6 | 0.062 |
| Total | 9.5 | ||||
| Type of Clothing | Washing Conditions | Package Number | Total PAH Content, µg (µg PAH per g of Package) | |
|---|---|---|---|---|
| Dripped Onto the Package Before Washing | In the Package After Washing | |||
| A | 40 °C, 2 rinse cycles | 1 | 9.5 | 4.5 |
| 2 | 9.5 | 3.9 | ||
| 3 | 9.5 | 3.9 | ||
| Mean | 9.5 | 4.1 | ||
| Standard deviation | 0.00 | 0.35 (1) | ||
| 40 °C, 3 rinse cycles | 1 | 9.5 | 3.5 | |
| 2 | 9.5 | 2.9 | ||
| 3 | 9.5 | 3.3 | ||
| Mean | 9.5 | 3.2 | ||
| Standard deviation | 0.00 | 0.31 (1) | ||
| 60 °C, 2 rinse cycles | 1 | 9.5 | 4.0 | |
| 2 | 9.5 | 2.7 | ||
| 3 | 9.5 | 3.5 | ||
| Mean | 9.5 | 3.4 | ||
| Standard deviation | 0.00 | 0.70 (1) | ||
| 60 °C, 3 rinse cycles | 1 | 9.5 | 3.0 | |
| 2 | 9.5 | 3.2 | ||
| 3 | 9.5 | 2.4 | ||
| Mean | 9.5 | 2.9 | ||
| Standard deviation | 0.00 | 0.41 (1) | ||
| B | 60 °C, 2 rinse cycles | 1 | 9.5 | 5.3 |
| 2 | 9.5 | 4.6 | ||
| 3 | 9.5 | 4.1 | ||
| Mean | 9.5 | 4.7 | ||
| Standard deviation | 0.00 | 0.58 (1) | ||
| 60 °C, 3 rinse cycles | 1 | 9.5 | 4.4 | |
| 2 | 9.5 | 4.2 | ||
| 3 | 9.5 | 4.6 | ||
| Mean | 9.5 | 4.4 | ||
| Standard deviation | 0.00 | 0.19 (1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzemińska, S.M.; Szewczyńska, M.; Miśkiewicz, P.; Sygocki, W. Effects of Washing Conditions on PAH Removal Effectiveness in Firefighter Protective Clothing Materials. Materials 2025, 18, 4073. https://doi.org/10.3390/ma18174073
Krzemińska SM, Szewczyńska M, Miśkiewicz P, Sygocki W. Effects of Washing Conditions on PAH Removal Effectiveness in Firefighter Protective Clothing Materials. Materials. 2025; 18(17):4073. https://doi.org/10.3390/ma18174073
Chicago/Turabian StyleKrzemińska, Sylwia Maria, Małgorzata Szewczyńska, Pamela Miśkiewicz, and Witold Sygocki. 2025. "Effects of Washing Conditions on PAH Removal Effectiveness in Firefighter Protective Clothing Materials" Materials 18, no. 17: 4073. https://doi.org/10.3390/ma18174073
APA StyleKrzemińska, S. M., Szewczyńska, M., Miśkiewicz, P., & Sygocki, W. (2025). Effects of Washing Conditions on PAH Removal Effectiveness in Firefighter Protective Clothing Materials. Materials, 18(17), 4073. https://doi.org/10.3390/ma18174073






