Corrosion Failure Analysis of Nickel-Plated Tubing in CO2-Ca2+-SRB Environment of Offshore Oil Fields
Abstract
1. Introduction
2. Failure Analysis
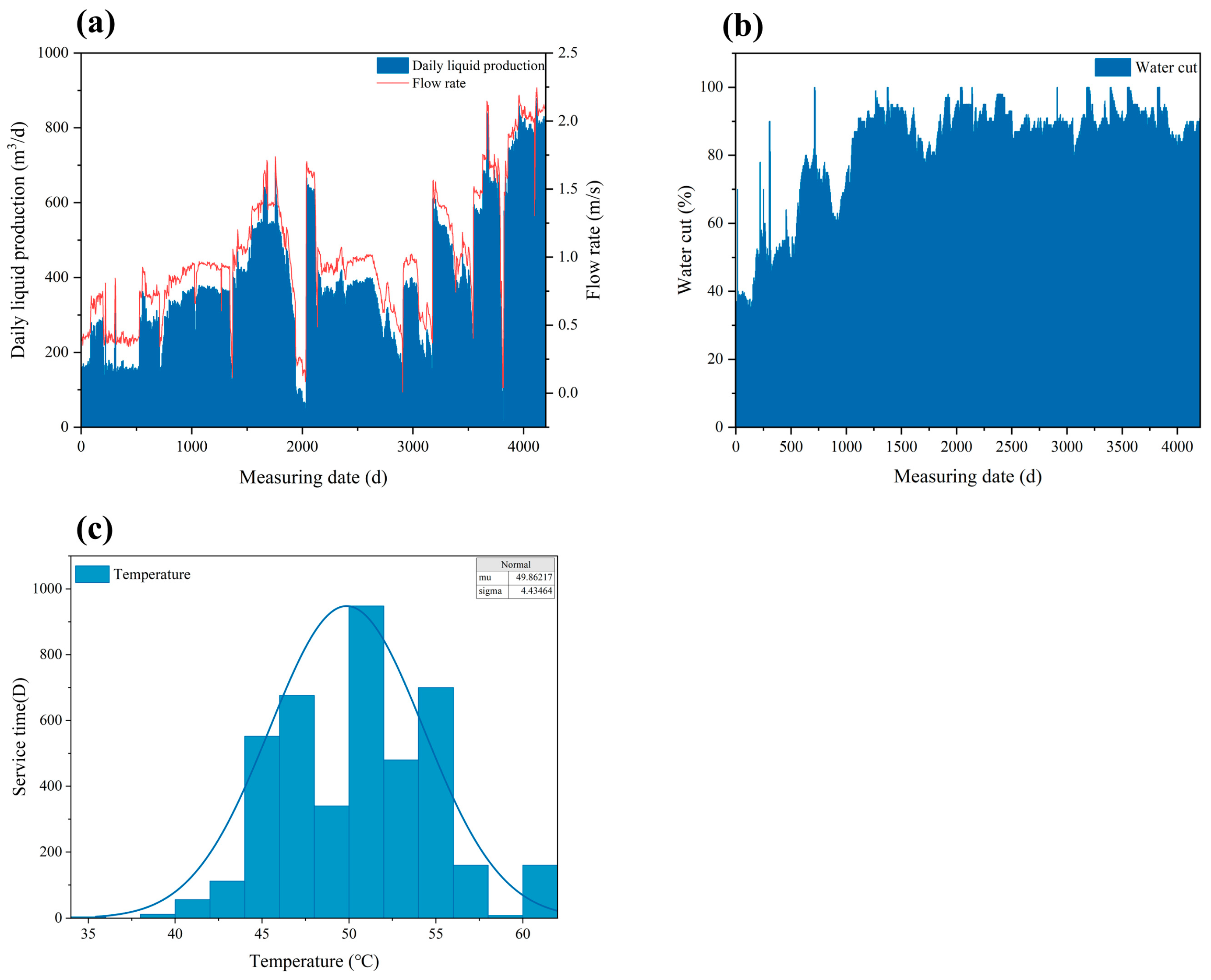
2.1. Service Environment
2.2. Analysis of Water Samples and Microbial Identification
2.2.1. ICP-OES Analysis of Cations
2.2.2. Ion Chromatographic Analysis of Anions
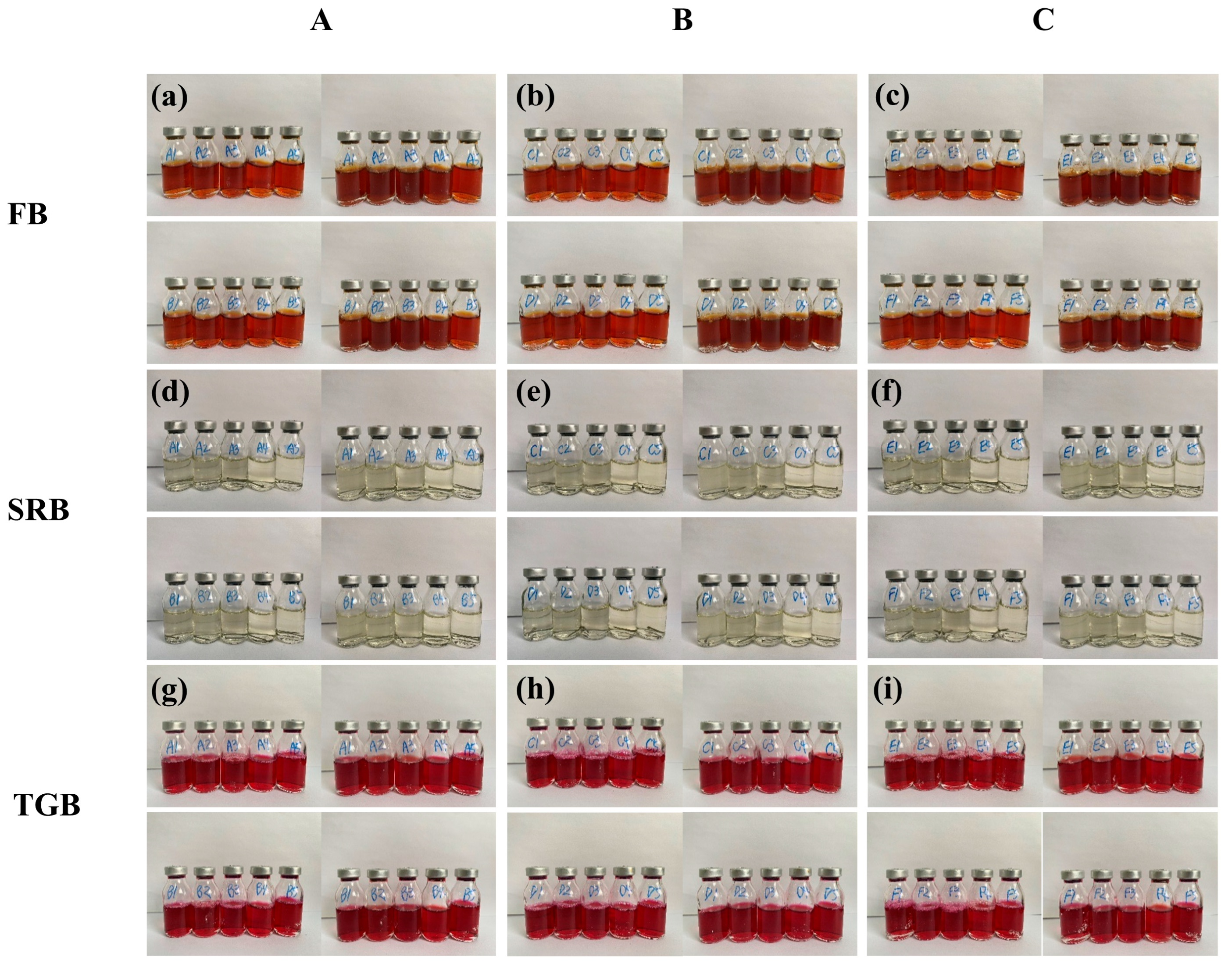
2.2.3. Microbiological Cultivation Analysis
2.3. Microstructural Characterization of the Failed Component
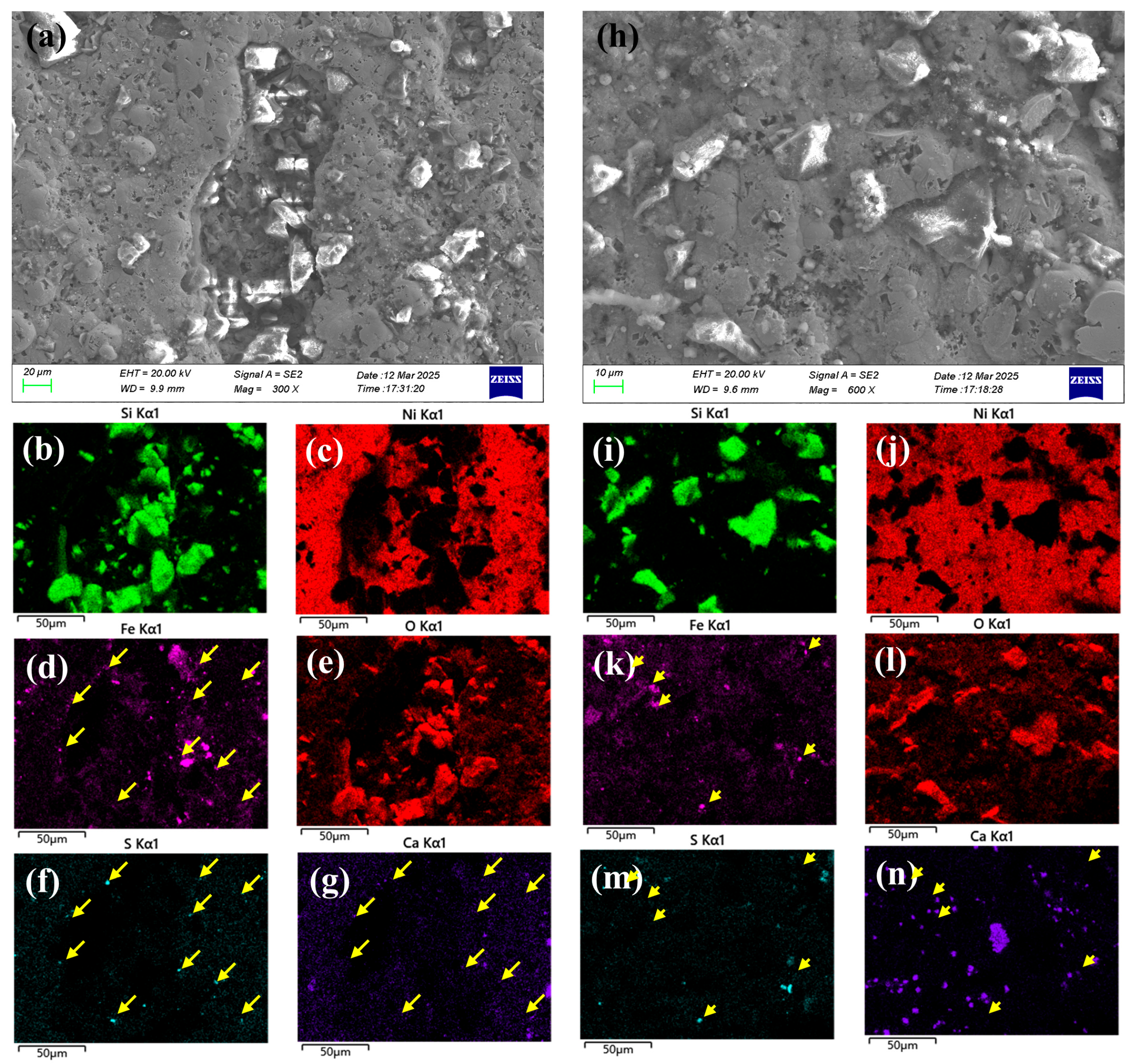
2.3.1. Surface Morphology and Elemental Analysis
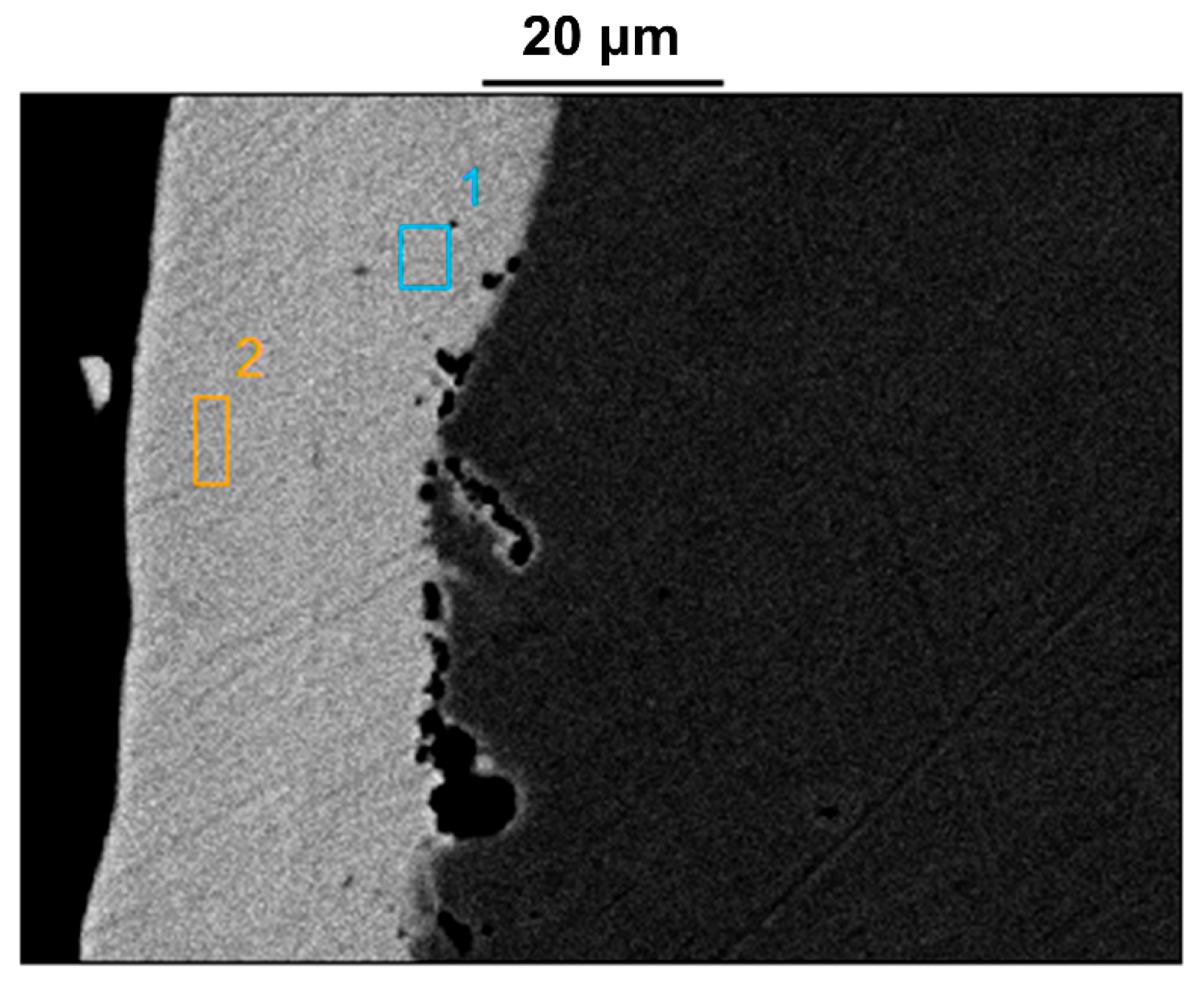
2.3.2. Plating and Substrate Cross-Sectional Structure Analysis
3. Simulated Corrosion Test
3.1. Material and Solution
3.2. Results
3.2.1. Degradation of Ni-Plating on Substrate
3.2.2. Corrosion of the Substrate Beneath
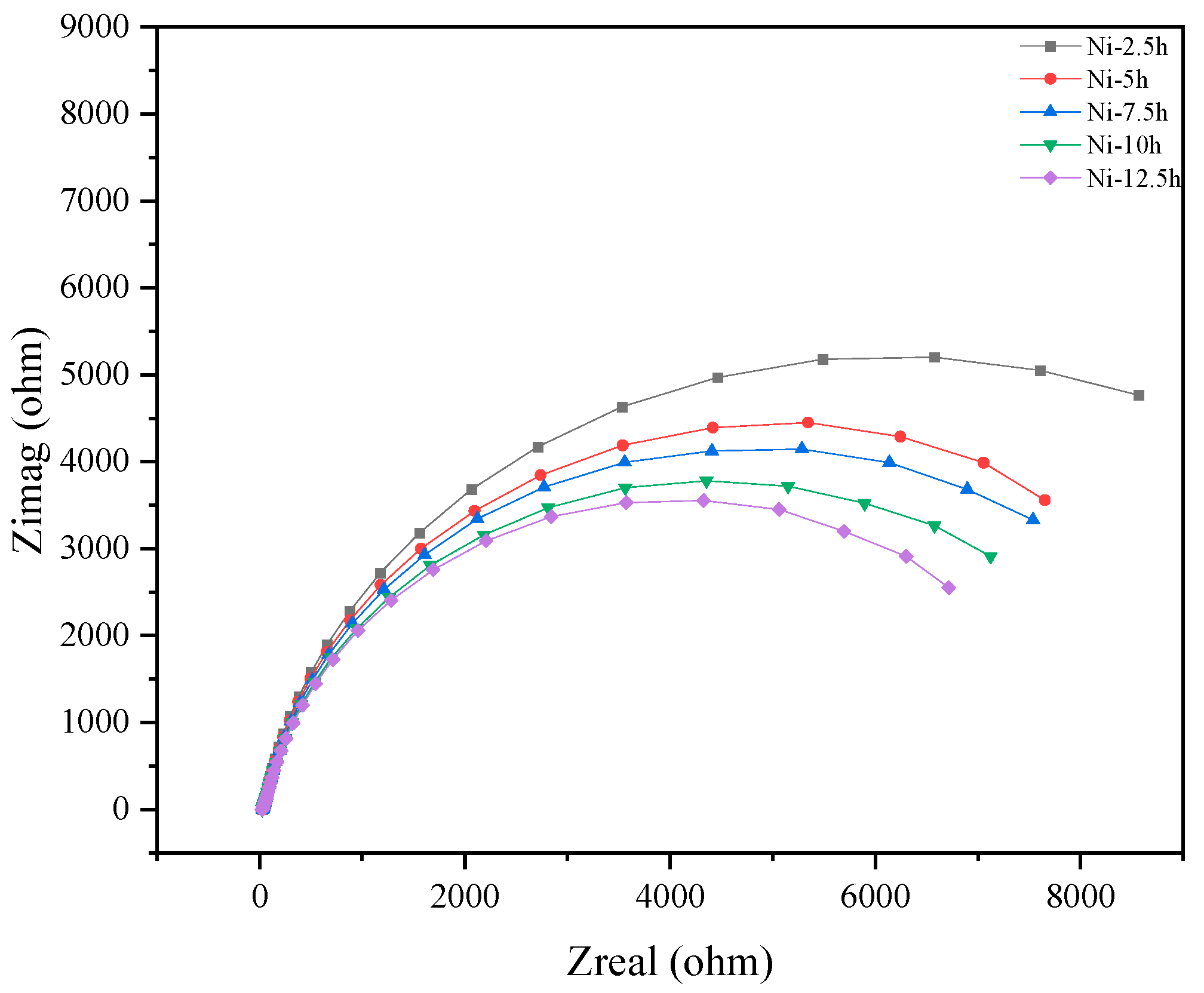
3.2.3. Electrochemical Testing
4. Conclusions
- The coating exhibited certain process-related defects, including non-uniform thickness and poor adhesion, along with the presence of pores penetrating through its structure. Corrosion of the substrate was observed even in the adherent regions of the coating, accompanied by an enrichment of S.
- The surface morphology of the failed component was characteristic of classic CO2 corrosion, and cross-sectional analysis revealed a high degree of colocalization between Ni and S. Concurrent with the observation of EPS, and considered alongside the sulfur enrichment and bacterial culture results, it was concluded that the synergistic action of SRB and CO2 during service led to the coating’s failure and subsequent substrate corrosion. In light of the challenges associated with chemical biocidal methods, future efforts could be directed toward the development of more sustainable and effective biological control technologies.
- Following the simulated immersion experiment, the cross-sectional morphology of the coating closely resembled that of the failed component, showing partial detachment. EDS analysis detected substrate corrosion, which was accompanied by the co-enrichment of Ca and S. The pores on the coating surface enabled the infiltration of corrosive media, including fluids with a high concentration of Ca2+ ions, to the substrate. Concurrently, an anoxic environment formed at the coating–substrate interface, promoting under-deposit microbiologically influenced corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yue, X.; Zhang, L.; Liu, H.; Zhao, H.; Wang, S.; Hua, Y. Uncoupling chloride and acidification attack on the naturally formed corrosion scales. Corros. Sci. 2022, 199, 110207. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Hua, Y. Fundamental insights into the stabilisation and chemical degradation of the corrosion product scales. Npj Mater. Degrad. 2021, 5, 8. [Google Scholar] [CrossRef]
- Meng, S.; Yue, P.; Zhang, S.; Li, H.; Zhao, Y.; Hua, Y.; Zhang, T.; Wang, F. Synergistic effects of CO2 and H2S on stress corrosion cracking of stainless steel 254SMo in extremely aggressive oilfield environment. Corros. Commun. 2024, 16, 81–95. [Google Scholar] [CrossRef]
- Hu, J.; Lin, G.; Deng, P.; Li, Z.; Tian, Y. Galvanic Corrosion of E690 Offshore Platform Steel in a Simulated Marine Thermocline. Metals 2024, 14, 287. [Google Scholar] [CrossRef]
- Townsend, H.E. Effects of Alloying Elements on the Corrosion of Steel in Industrial Atmospheres. Corrosion 2001, 57, 497–501. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Veeramani, J.; Rahman, M.M.; Nirmal Ram, J.S. Hybrid Polyurethane/Polypyrrole Composite Coatings on Passivated 316L SS for Surface Protective Action Against Corrosion in Saline Medium. Corros. Mater. Degrad. 2022, 3, 612–627. [Google Scholar] [CrossRef]
- Mouez, F.; Halfa, H.; Yahia, A.; Hussien, H.M.; Gaber, M.; Ali, H.H. Development and evaluation of superhydrophobic carbon nanotube coatings onmaraging steel: Corrosion protection, and anti-fouling characteristics. J. Alloys Metall. Syst. 2025, 10, 100188. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Shim, J.-J.; Kim, J.-G. Effects of Cr, Cu, Ni and Ca on the corrosion behavior of low carbon steel in synthetic tap water. J. Alloys Compd. 2005, 391, 162–169. [Google Scholar] [CrossRef]
- Wen, C.; Tian, Y.; Wang, G.; Hu, J.; Deng, P. The Influence of Nickel on Corrosion Behavior of Low Alloy Steel in a Cyclic Wet-dry Condition. Int. J. Electrochem. Sci. 2016, 11, 4161–4173. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a Cl− Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Hua, Y.; Shi, S.; Wang, H.; Wang, L.; Yue, X.; Su, C. Insight into surface microstructure evolution and fracture mechanism of incoloy alloy 800HT under high-temperature conditions. J. Mater. Res. Technol. 2025, 35, 4709–4722. [Google Scholar] [CrossRef]
- Yin, Z.F.; Zhao, W.Z.; Lai, W.Y.; Zhao, X.H. Electrochemical behaviour of Ni-base alloys exposed under oil/gas field environments. Corros. Sci. 2009, 51, 1702–1706. [Google Scholar] [CrossRef]
- deBarbadillo, J.J.; Mannan, S.K. Alloy 718 for Oilfield Applications. JOM 2012, 64, 265–270. [Google Scholar] [CrossRef]
- Foroni, L.; Lherbier, L.; Malara, C. High Performance New Ni-Base Alloy AF955 (AF955) for Oil and Gas Industry. In Proceedings of the 9th International Symposium on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 193–207. [Google Scholar]
- Jiang, X.; Di, X.; Li, C.; Wang, D.; Hu, W. Improvement of mechanical properties and corrosion resistance for wire arc additive manufactured nickel alloy 690 by adding TiC particles. J. Alloys Compd. 2022, 928, 167198. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Mosavi, M.A.; Ramezanzadeh, B. Nickel-aluminium bronze (NiBRAl) casting alloy tribological/corrosion resistance properties improvement via deposition of a Cu-doped diamond-like carbon (DLC) thin film; optimization of sputtering magnetron process conditions. Mater. Chem. Phys. 2023, 296, 127279. [Google Scholar] [CrossRef]
- Cheng, D.; Lv, B.; Chen, C.; Yang, Z.; Hao, X.; Zheng, C.; Zhang, F. Synergistic mechanism of minor Ni and Cr to enhance the corrosion resistance of novel pearlitic rail steel. Constr. Build. Mater. 2025, 484, 141846. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Y.; Cao, R.; Wang, P.; Mao, Y.; Yi, C.; Zhang, S.; Zhang, T.; Liu, X. Phosphorus-containing nickel-based coatings for enhanced corrosion resistance and mechanical performance: A review. FlatChem 2025, 52, 100887. [Google Scholar] [CrossRef]
- Guo, L.; Yu, B.; Zhou, P.; Zhang, T.; Wang, F. Fabrication of low-cost Ni-P composite coating on Mg alloys with a significant improvement of corrosion resistance: Critical role of mitigating the galvanic contact between the substrate and the coating. Corros. Sci. 2021, 183, 109329. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Bathini, L.; Ramakrishna, L.; Rao, D.S.; Padmanabham, G. Pulsed electrodeposition, mechanical properties and wear mechanism in Ni-W/SiC nanocomposite coatings used for automotive applications. Appl. Surf. Sci. 2020, 527, 146896. [Google Scholar] [CrossRef]
- Hwang, H.-K.; Shin, D.-H.; Kim, S.-J. Effect of PVD deposition method on indentation and friction wear characteristics with hydrogen embrittlement of CrN coated stainless steel for electric vehicle hydrogen valves. Surf. Interfaces 2024, 48, 104281. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Rajabalizadeh, Z.; Dikici, B.; Kıranşan, K.D.; Erçarıkcı, E. A review of pretreatment techniques for electroless nickel-phosphorus plating on magnesium alloys with enhanced corrosion resistance and mechanical properties. Mater. Today Commun. 2025, 44, 112171. [Google Scholar] [CrossRef]
- Prajapati, V.; Kumar, Y.; Gupta, D.; Kalam, A.; Dubey, M. Analysis of Pitting Corrosion of Pipelines in a Marine Corrosive Environment Using COMSOL Multiphysics. J. Bio- Tribo-Corros. 2021, 8, 21. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, T.; Yang, S.; Li, J.; Zhao, X.; Lin, X.; Sun, C.; Sun, J. Comparison of corrosion behavior of X52 steel in liquid and supercritical CO2 transport environments with multiple impurities. Corros. Commun. 2025, 18, 44–53. [Google Scholar] [CrossRef]
- Rameshk, M.; Soltanieh, M.; Masoudpanah, S.M. Effects of flow velocity and impact angle on erosion-corrosion of an API-5L X65 steel coated by plasma nitriding of hard chromium underlayer. J. Mater. Res. Technol. 2020, 9, 10054–10061. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Li, X.; Yao, J.; Liu, H. Investigations on the solubleness of carbon dioxide in aqueous and oil-bearing drilling fluids: Mechanisms of temperature and pressure effects. Chem. Eng. J. 2025, 505, 159430. [Google Scholar] [CrossRef]
- Mou, L.; Bian, T.; Zhang, S.; Zhang, J.; Wu, P.; Zhang, J.; Yu, Y.; Zhang, Y.; Liu, B. Understanding the interaction mechanism of chloride ions and carbon dioxide towards corrosion of 3Cr steel. Vacuum 2023, 217, 112571. [Google Scholar] [CrossRef]
- Guo, L.; Chen, P.; Huang, C.; Zhang, J.; Xue, Y.; Zhang, X.; Tang, J.; Wang, S.; Wang, H. Study on under-deposit corrosion behavior of X65 steel in simulated CO2 saturated produced water. Int. J. Electrochem. Sci. 2024, 19, 100731. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Sun, Y.; Gan, Z.; Lu, X.; Lei, X. An atomic scale study of the corrosion mechanism of Fe(100) surface by halogen ions. Phys. B Condens. Matter 2025, 699, 416870. [Google Scholar] [CrossRef]
- Abbas, M.; Rizvi, S.H.M.; Sarfraz, S.; Raza, A.; Khan, A.; Loya, A.; Najib, A. Evaluation of the influence of dissolved nitrates on corrosion behaviour of ship structural steel exposed to seawater environment. Ocean Eng. 2024, 298, 117268. [Google Scholar] [CrossRef]
- SY/T 0532-2012; Analysis Method of the Bacteria for Oilfield Injecting Water—Disappearing Dilution Method. Petroleum and Natural Gas Professional Standards Committee, National Energy Administration of China: Beijing, China, 2012. Available online: https://std.samr.gov.cn/hb/search/stdHBDetailed?id=8B1827F2244FBB19E05397BE0A0AB44A&utm_source=chatgpt.com (accessed on 11 July 2025).
- Liu, X.; Wang, Y.; Song, Y.; Liu, W.; Zhang, J.; Li, N.; Dong, K.; Cai, Y.; Han, E.-H. The respective roles of sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB) in the mixed microbial corrosion process of carbon steel pipelines. Corros. Sci. 2024, 240, 112479. [Google Scholar] [CrossRef]
- Tian, H.M.; Gao, P.K.; Qi, C.; Li, G.Q.; Ma, T. Nitrate and oxygen significantly changed the abundance rather than structure of sulphate-reducing and sulphur-oxidising bacteria in water retrieved from petroleum reservoirs. Environ. Microbiol. Rep. 2024, 16, e13248. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Jiang, J.; Zhang, Y.; Wu, M.; Tang, C.; Wu, T.; Zhao, K. Microbial corrosion behavior of pipeline steels in simulation environment of natural gas transportation pipeline. RSC Adv. 2023, 13, 36168–36180. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.L.; Voordouw, G. Sulfate-Reducing Bacteria Lower Sulfur-Mediated Pitting Corrosion under Conditions of Oxygen Ingress. Environ. Sci. Technol. 2012, 46, 9183–9190. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhong, X.; Liu, H.; Frank Cheng, Y. Microbiologically-enhanced galvanic corrosion of the steel beneath a deposit in simulated oilfield-produced water containing Desulfotomaculum nigrificans. Electrochem. Commun. 2018, 90, 1–5. [Google Scholar] [CrossRef]
- Wu, B.; Li, N.; Xiang, C.; Wang, J.; Ming, H.; Wang, J.; Han, E.-H. Understanding the corrosion behavior of additive manufactured 316L stainless steel in supercritical carbon dioxide at high temperature. J. Supercrit. Fluids 2025, 225, 106697. [Google Scholar] [CrossRef]
- Liu, H.; Frank Cheng, Y. Mechanism of microbiologically influenced corrosion of X52 pipeline steel in a wet soil containing sulfate-reduced bacteria. Electrochim. Acta 2017, 253, 368–378. [Google Scholar] [CrossRef]
- Wei, L.; Ge, Y.; Gao, Q.; Wang, C.; Yu, X.; Zhang, L. Effect of salt-resistant polymer flooding system SRB on corrosion behavior of 20# carbon steel under deposition. J. Electroanal. Chem. 2022, 921, 116714. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Lv, Y.; Asif, M.; Xiong, F.; Zhang, G.; Liu, H. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO2-saturated oilfield produced water. Corros. Sci. 2017, 117, 24–34. [Google Scholar] [CrossRef]
- Silva, P.; Oliveira, S.H.; Vinhas, G.M.; Carvalho, L.J.; Baraúna, O.S.; Urtiga Filho, S.L.; Lima, M.A.G.A. Tetrakis hydroxymethyl phosphonium sulfate (THPS) with biopolymer as strategy for the control of microbiologically influenced corrosion in a dynamic system. Chem. Eng. Process.—Process Intensif. 2021, 160, 108272. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B.; Xiang, J.; Chen, X.; Gu, T.; Liu, H. The performance and mechanism of bifunctional biocide sodium pyrithione against sulfate reducing bacteria in X80 carbon steel corrosion. Corros. Sci. 2019, 150, 296–308. [Google Scholar] [CrossRef]
- Ortega Morente, E.; Fernández-Fuentes, M.A.; Grande Burgos, M.J.; Abriouel, H.; Pérez Pulido, R.; Gálvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef] [PubMed]








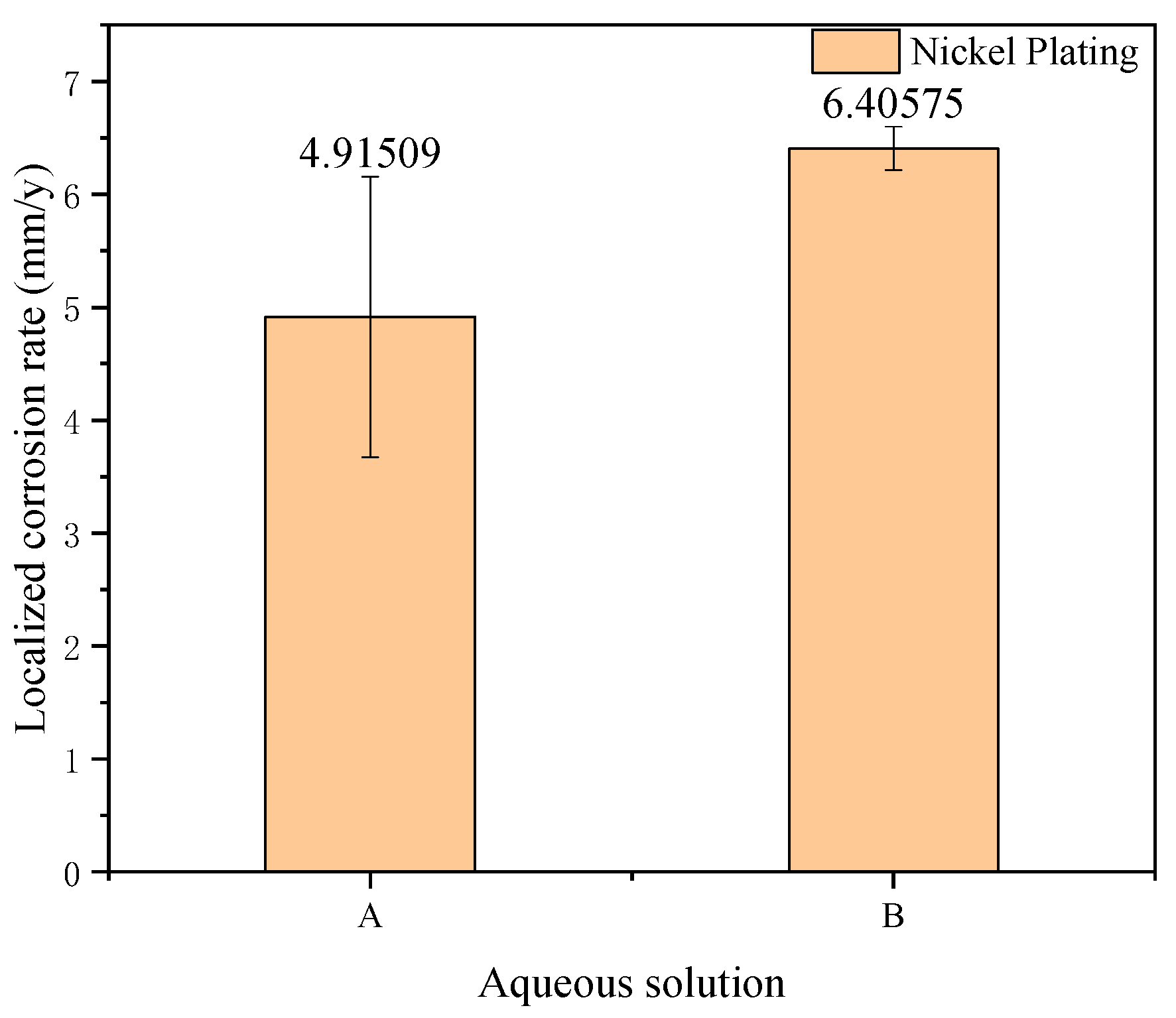


| Elements | C | Si | Mn | P | S | Cr | Al | Cu |
|---|---|---|---|---|---|---|---|---|
| Comp/wt% | 0.31 | 0.26 | 1.53 | 0.01 | 0.004 | 0.04 | 0.04 | 0.077 |
| Cation Species | Content (mg/kg) |
|---|---|
| Ca2+ | 975.50 |
| Mg2+ | 160.15 |
| Na+ | 995.70 |
| K+ | 27.83 |
| Anionic Species | Content (mg/kg) |
|---|---|
| Cl− | 5990.40 |
| HCO3− | 152.66 |
| Br− | 137.78 |
| SO42− | 45.27 |
| NO3− | 42.00 |
| Atom (%) | O-K | Na-K | Mg-K | Al-K | Si -K | P -K | Cl-K | K-K | Ca-K | Fe-K | Ni-K | C-K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt1 | 3.0 | 2.4 | 1.1 | 0.2 | 0.6 | 14.4 | 0.2 | 0.1 | 0.9 | 3.3 | 73.8 | |
| Pt2 | 2.1 | 0.3 | 0.2 | 9.5 | 0.3 | 2.4 | 85.1 | |||||
| Pt3 | 0.0 | 8.6 | 2.3 | 89.2 | ||||||||
| Pt4 | 0.2 | 7.8 | 5.6 | 86.4 | ||||||||
| Pt5 | 42.8 | 20.6 | 0.17 | 0.44 | 0.39 | 35.6 |
| Atom (%) | P-K | Fe-K | Ni-K |
|---|---|---|---|
| Pt1 | 9.8 | 2.9 | 87.3 |
| Pt2 | 7.1 | 1.6 | 91.3 |
| Cation Species | Content (mg/kg) |
|---|---|
| Ca2+ | 131.94 |
| Cl− | 2382.5 |
| HCO3− | 730.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, S.; Wang, K.; Song, C.; Hu, J.; Yue, X. Corrosion Failure Analysis of Nickel-Plated Tubing in CO2-Ca2+-SRB Environment of Offshore Oil Fields. Materials 2025, 18, 4006. https://doi.org/10.3390/ma18174006
Zhang H, Yang S, Wang K, Song C, Hu J, Yue X. Corrosion Failure Analysis of Nickel-Plated Tubing in CO2-Ca2+-SRB Environment of Offshore Oil Fields. Materials. 2025; 18(17):4006. https://doi.org/10.3390/ma18174006
Chicago/Turabian StyleZhang, Hui, Shuo Yang, Kongyang Wang, Chuang Song, Jinyang Hu, and Xiaoqi Yue. 2025. "Corrosion Failure Analysis of Nickel-Plated Tubing in CO2-Ca2+-SRB Environment of Offshore Oil Fields" Materials 18, no. 17: 4006. https://doi.org/10.3390/ma18174006
APA StyleZhang, H., Yang, S., Wang, K., Song, C., Hu, J., & Yue, X. (2025). Corrosion Failure Analysis of Nickel-Plated Tubing in CO2-Ca2+-SRB Environment of Offshore Oil Fields. Materials, 18(17), 4006. https://doi.org/10.3390/ma18174006






