Tailoring the Electronic and Structural Properties of Lead-Free A2ZrX6 “Defect” Perovskites: A DFT Study on A-Site Cation and Halogen Substitutions
Abstract
1. Introduction
2. Computational Methodology
3. Results and Discussion
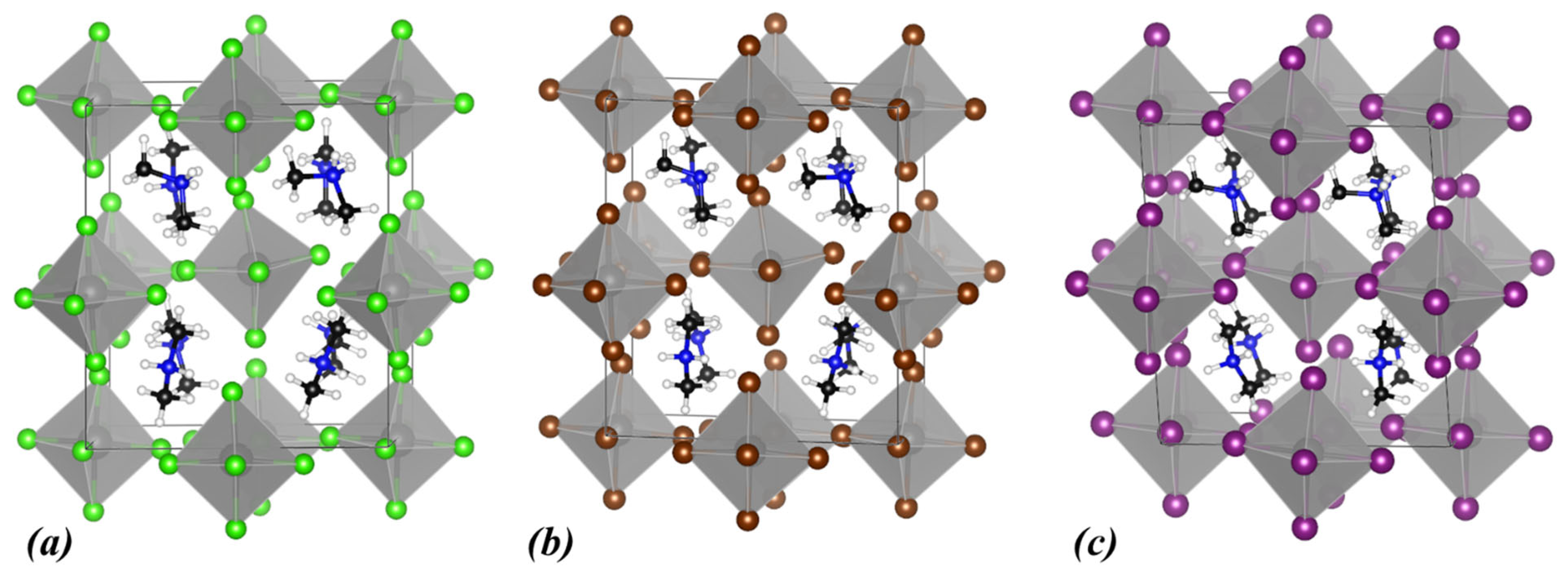
3.1. (NH4)2ZrX6, X: Cl, Br, I
3.2. MA2ZrX6, X: Cl, Br, I
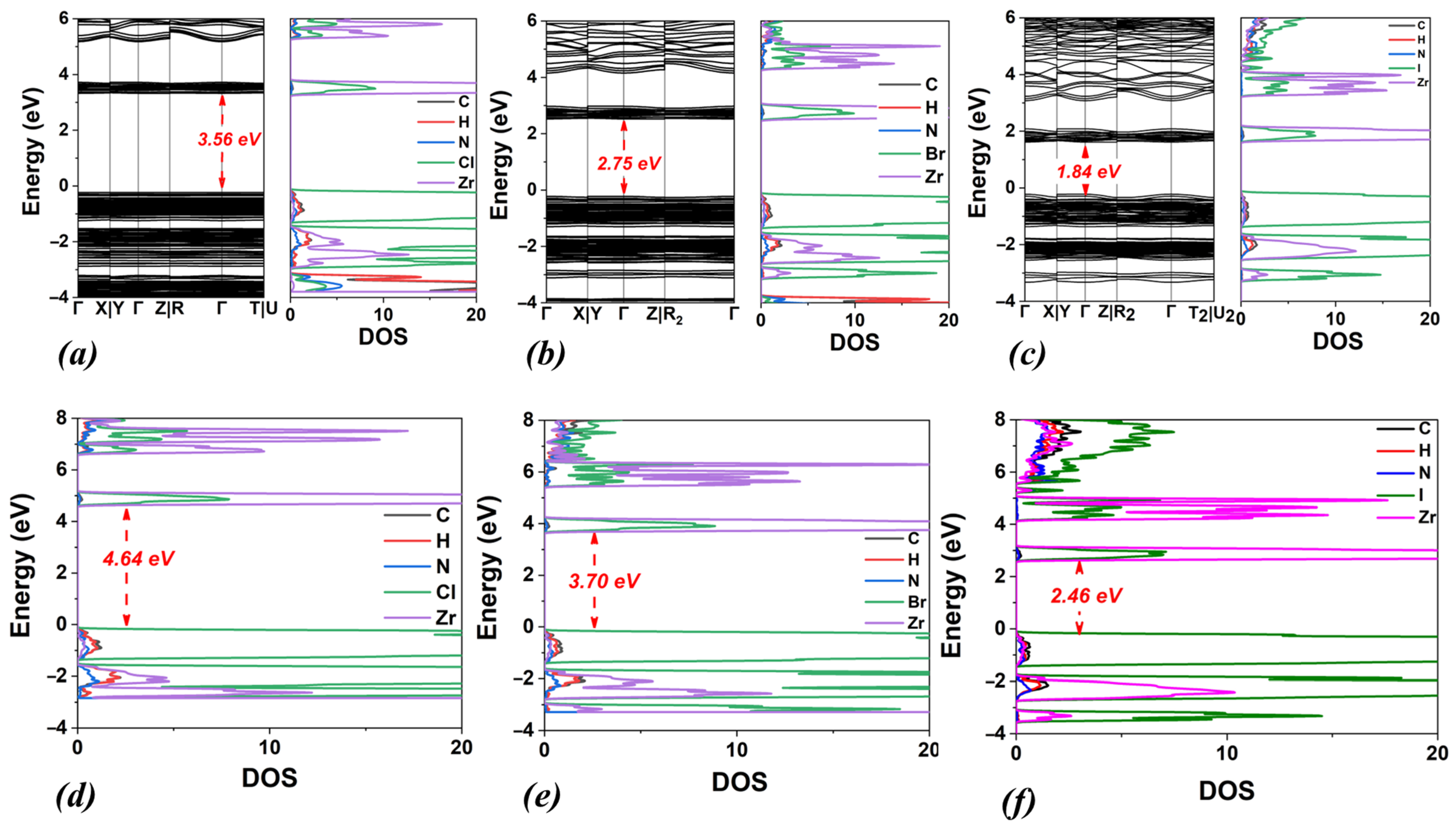
3.3. DMA2ZrX6, X: Cl, Br, I
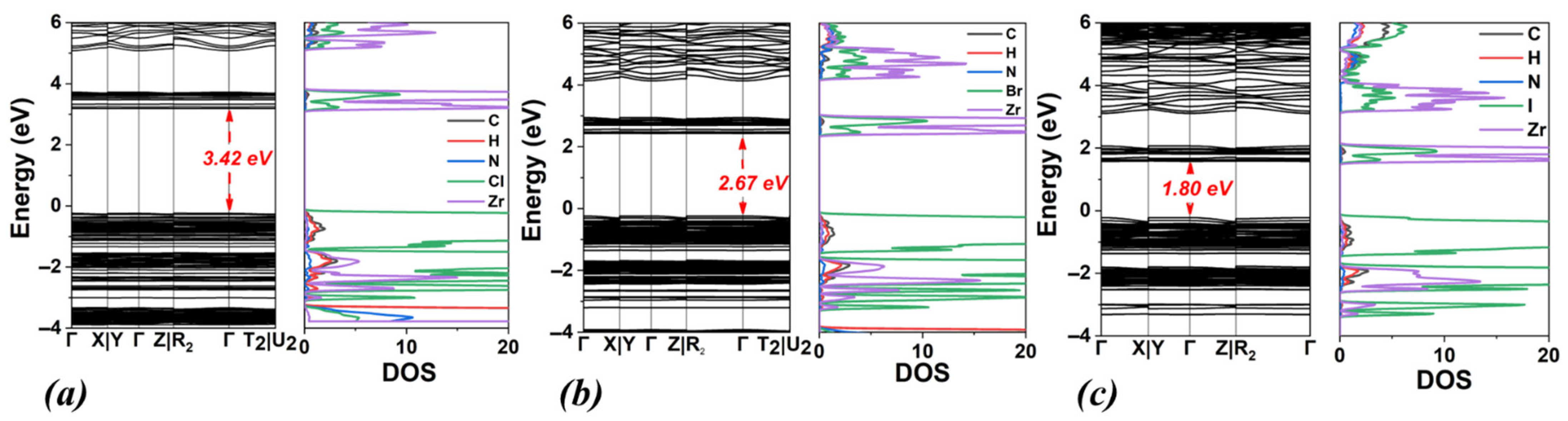
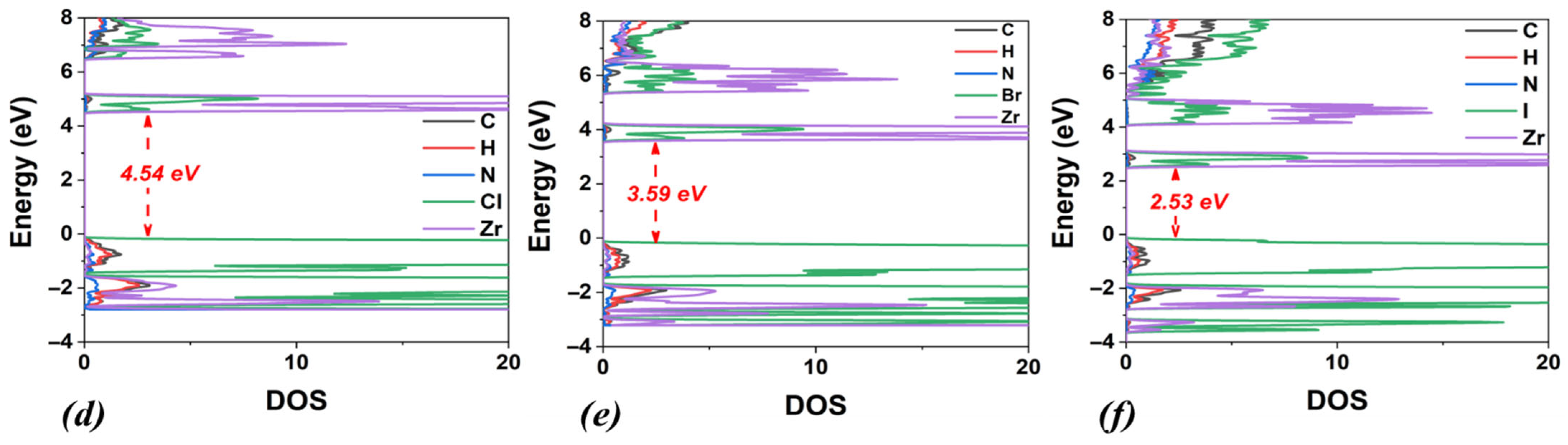
3.4. TMA2ZrX6, X: Cl, Br, I
3.5. (PH4)2ZrX6, X: Cl, Br, I
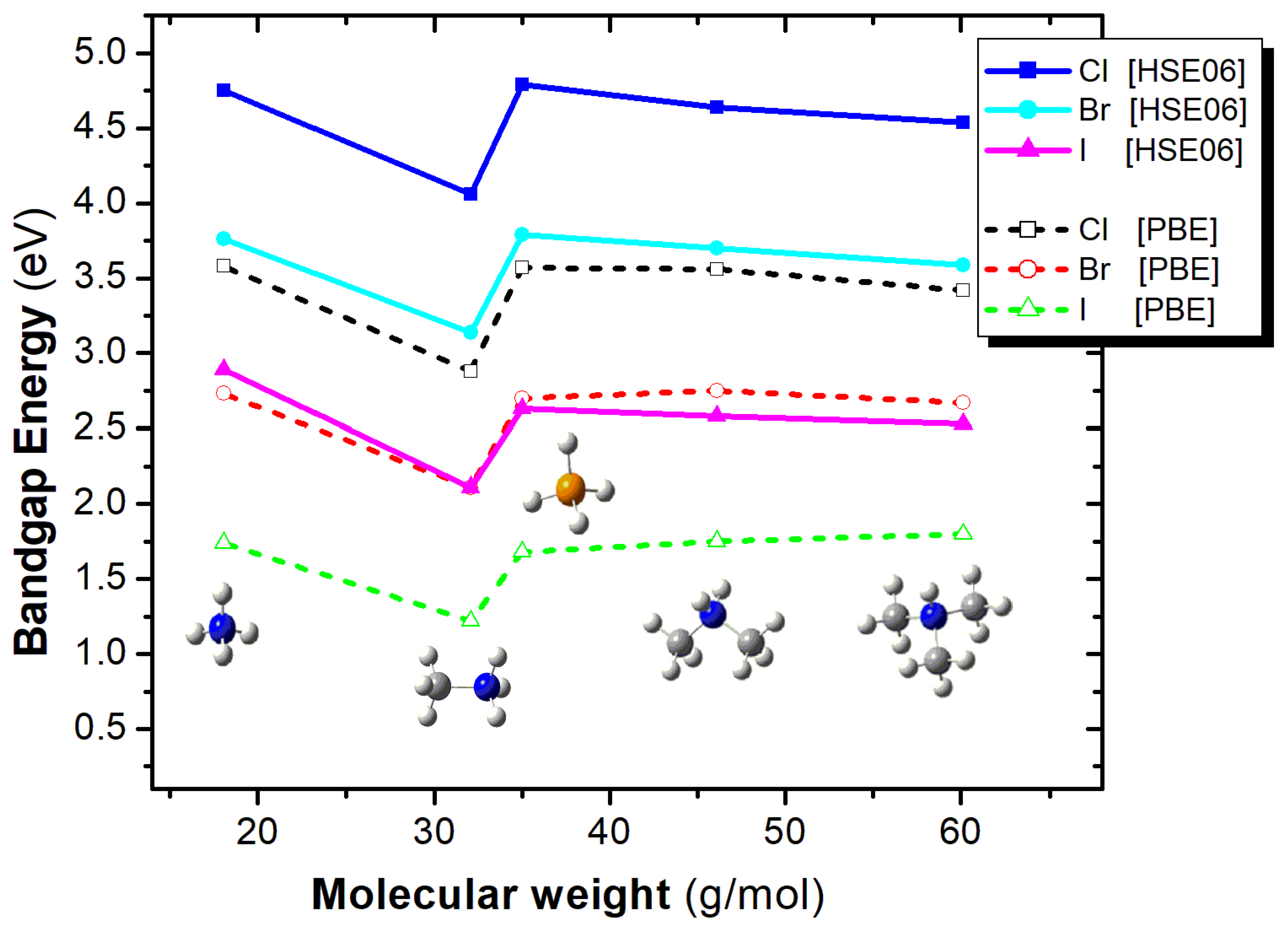
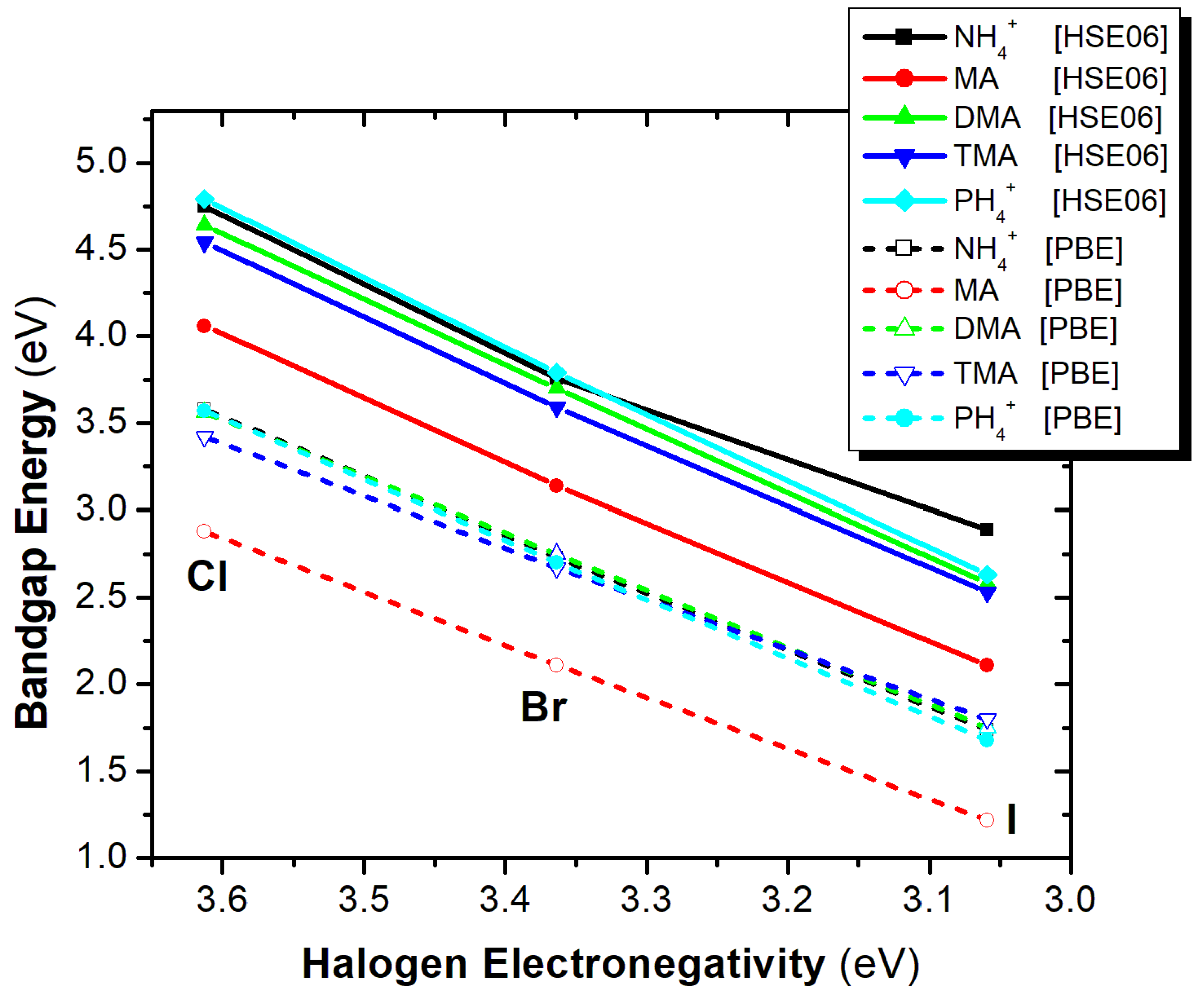
3.6. The Effect of A-Cation and X-Anion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Snaith, H.J. Metal-Halide Perovskites for Photovoltaic and Light-Emitting Devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Deng, Y.; Lin, C.; Fang, Z.; Xiang, C.; Bai, P.; Du, K.; Zuo, X.; Wen, K.; et al. Efficient Light-Emitting Diodes Based on Oriented Perovskite Nanoplatelets. Sci. Adv. 2021, 7, abg8458. [Google Scholar] [CrossRef]
- Wang, C.; Meng, W.; Luo, G.; Xu, G.; Peng, M.; Xu, B.; Nie, S.; Deng, Z. RGB Tri-Luminescence in Organic–Inorganic Zirconium Halide Perovskites. Chem. Sci. 2024, 15, 2954–2962. [Google Scholar] [CrossRef]
- Koutsoubogeras, J.; Tsoureas, N.; Tagiara, N.S.; Kaltzoglou, A. Synthesis, Crystal Structure and Optoelectronic Properties of [(CH3)3S]SnBr3. J. Coord. Chem. 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.-L.; Wu, J.-L.; Zhang, X.-W. Metal Halide Perovskite Photodetectors: Material Features and Device Engineering. Chin. Phys. B 2019, 28, 018502. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-Based Resistive Switching Memories—Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Reyren, N.; Thiel, S.; Caviglia, A.D.; Kourkoutis, L.F.; Hammerl, G.; Richter, C.; Schneider, C.W.; Kopp, T.; Rüetschi, A.-S.; Jaccard, D.; et al. Superconducting Interfaces Between Insulating Oxides. Science 2007, 317, 1196–1199. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Y.; Huang, H.; Liu, X.; Li, Q.; Chen, L.; Xu, D. Stable and Highly Efficient Photocatalysis with Lead-Free Double-Perovskite of Cs2AgBiBr6. Angew. Chem. 2019, 131, 7341–7345. [Google Scholar] [CrossRef]
- Sharif, R.; Khalid, A.; Ahmad, S.W.; Rehman, A.; Qutab, H.G.; Akhtar, H.H.; Mahmood, K.; Afzal, S.; Saleem, F. A Comprehensive Review of the Current Progresses and Material Advances in Perovskite Solar Cells. Nanoscale Adv. 2023, 5, 3803–3833. [Google Scholar] [CrossRef] [PubMed]
- Cucco, B.; Katan, C.; Even, J.; Kepenekian, M.; Volonakis, G. Fine Structure of Excitons in Vacancy-Ordered Halide Double Perovskites. ACS Mater. Lett. 2023, 5, 52–59. [Google Scholar] [CrossRef]
- NREL Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency (accessed on 1 July 2025).
- Zhu, H.; Teale, S.; Lintangpradipto, M.N.; Mahesh, S.; Chen, B.; McGehee, M.D.; Sargent, E.H.; Bakr, O.M. Long-Term Operating Stability in Perovskite Photovoltaics. Nat. Rev. Mater. 2023, 8, 569–586. [Google Scholar] [CrossRef]
- Ma, C.; Ni, M.; Liu, X.; Zhan, K.; Chen, M.; Li, S.; Li, F.; Wang, H.; Dang, Y. Recent Progress in Perovskite Light-Emitting Diodes with High External Quantum Efficiency and Stability. CrystEngComm 2025, 27, 3853–3876. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, C. Recent Progress and Future Opportunities for Optical Manipulation in Halide Perovskite Photodetectors. Nanomaterials 2025, 15, 816. [Google Scholar] [CrossRef]
- Hidalgo, J.; Kaiser, W.; An, Y.; Li, R.; Oh, Z.; Castro-Méndez, A.-F.; LaFollette, D.K.; Kim, S.; Lai, B.; Breternitz, J.; et al. Synergistic Role of Water and Oxygen Leads to Degradation in Formamidinium-Based Halide Perovskites. J. Am. Chem. Soc. 2023, 145, 24549–24557. [Google Scholar] [CrossRef]
- Kore, B.P.; Jamshidi, M.; Gardner, J.M. The Impact of Moisture on the Stability and Degradation of Perovskites in Solar Cells. Mater. Adv. 2024, 5, 2200–2217. [Google Scholar] [CrossRef]
- Ahmed, I.; Prakash, K.; Mobin, S.M. Lead-Free Perovskites for Solar Cell Applications: Recent Progress, Ongoing Challenges, and Strategic Approaches. Chem. Commun. 2025, 61, 6691–6721. [Google Scholar] [CrossRef]
- Meyer, E.; Mutukwa, D.; Zingwe, N.; Taziwa, R. Lead-Free Halide Double Perovskites: A Review of the Structural, Optical, and Stability Properties as Well as Their Viability to Replace Lead Halide Perovskites. Metals 2018, 8, 667. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 Perovskites: A Review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Ghosh, S.; Shankar, H.; Kar, P. Recent Developments of Lead-Free Halide Double Perovskites: A New Superstar in the Optoelectronic Field. Mater. Adv. 2022, 3, 3742–3765. [Google Scholar] [CrossRef]
- Nabi, M.; Padelkar, S.S.; Jasieniak, J.J.; Simonov, A.N.; Alam, A. Lead-Free Magnetic Double Perovskites for Photovoltaic and Photocatalysis Applications. Phys. Rev. Appl. 2024, 21, 014063. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Scanlon, D.O.; Neilson, J.R. Perspectives and Design Principles of Vacancy-Ordered Double Perovskite Halide Semiconductors. Chem. Mater. 2019, 31, 1184–1195. [Google Scholar] [CrossRef]
- Rahim, W.; Cheng, A.; Lyu, C.; Shi, T.; Wang, Z.; Scanlon, D.O.; Palgrave, R.G. Geometric Analysis and Formability of the Cubic A2BX6 Vacancy-Ordered Double Perovskite Structure. Chem. Mater. 2020, 32, 9573–9583. [Google Scholar] [CrossRef]
- Abfalterer, A.; Shamsi, J.; Kubicki, D.J.; Savory, C.N.; Xiao, J.; Divitini, G.; Li, W.; Macpherson, S.; Gałkowski, K.; MacManus-Driscoll, J.L.; et al. Colloidal Synthesis and Optical Properties of Perovskite-Inspired Cesium Zirconium Halide Nanocrystals. ACS Mater. Lett. 2020, 2, 1644–1652. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Zhang, X.; An, K.; Zang, Z.; Li, R. Molecule Design Enabled High Efficiency Flexible Zirconium-Based Lead-Free Perovskite Scintillator. Appl. Phys. Lett. 2025, 126, 081901. [Google Scholar] [CrossRef]
- You, M.-X.; Lyu, Z.; Xu, Q.; Sun, D.; Tan, T.; Luo, P.; Lu, Z.; You, H. Enhanced and Tunable Emission from Organic–Inorganic Metal Halide Perovskites A2ZrCl6:Sb3+ via A-Site Organic Cation Manipulation. ACS Appl. Mater. Interfaces 2025, 17, 35714–35722. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, J.; Huang, Q.; Wu, D.; Qi, F.; Zhang, N.; Pu, Y.; Tian, C.; Chen, W.; Liu, Y.; et al. Ultrahigh PLQY Lead-Free Organic–Inorganic Hybrid Zirconium-Based Perovskites in Anticounterfeiting Applications. Adv. Opt. Mater. 2023, 11, 2300399. [Google Scholar] [CrossRef]
- Zhu, C.; Jin, J.; Wang, Z.; Xu, Z.; Folgueras, M.C.; Jiang, Y.; Uzundal, C.B.; Le, H.K.D.; Wang, F.; Zheng, X.; et al. Supramolecular Assembly of Blue and Green Halide Perovskites with Near-Unity Photoluminescence. Science 2024, 383, 86–93. [Google Scholar] [CrossRef]
- Dai, G.; Ma, Z.; Qiu, Y.; Li, Z.; Fu, X.; Jiang, H.; Ma, Z. Excitation-Dependent Luminescence of 0D ((CH3)4N)2ZrCl6 across the Full Visible Region. J. Phys. Chem. Lett. 2022, 13, 7553–7560. [Google Scholar] [CrossRef]
- Lin, J.-W.; Lu, X.; Liu, Z.; Lin, Y.-P.; Yang, J.; Ge, Y.; Zhou, C.; Wang, X.; Li, J.-R.; Du, K.-Z.; et al. Optical Property Regulation through Host–Guest Interaction in a Zero-Dimensional Zr Chloride. Chem. Mater. 2024, 36, 4600–4606. [Google Scholar] [CrossRef]
- Tagiara, N.S.; Psycharis, V.; Kaltzoglou, A. Synthesis, Crystal Structure and Luminescence of [(CH3)3S]2ZrCl6. J. Coord. Chem. 2024, 77, 286–294. [Google Scholar] [CrossRef]
- Kolokytha, C.; Lathiotakis, N.N.; Kaltzoglou, A.; Petsalakis, I.D.; Tzeli, D. The Effect of A-Cation and X-Anion Substitutions on the Electronic and Structural Properties of A2ZrX6 ‘Defect’ Perovskite Materials: A Theoretical Density Functional Theory Study. Materials 2025, 18, 726. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Feng, X.; Zhou, C.; Liu, Y.; Yu, X.; Zhao, G. Phase Regulation Strategy of Perovskite Nanocrystals from 1D Orthomorphic NH4PbI3 to 3D Cubic (NH4)0.5Cs0.5Pb(I0.5Br0.5)3 Phase Enhances Photoluminescence. Angew. Chem. Int. Ed. 2019, 58, 11642–11646. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab. Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Franchini, C. Hybrid Functionals Applied to Perovskites. J. Phys. Condens. Matter 2014, 26, 253202. [Google Scholar] [CrossRef]
- El-Mellouhi, F.; Brothers, E.N.; Lucero, M.J.; Bulik, I.W.; Scuseria, G.E. Structural Phase Transitions of the Metal Oxide Perovskites SrTiO3, LaAlO3, and LaTiO3 Studied with a Screened Hybrid Functional. Phys. Rev. B 2013, 87, 035107. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Blaudeau, J.-P.; McGrath, M.P.; Curtiss, L.A.; Radom, L. Extension of Gaussian-2 (G2) Theory to Molecules Containing Third-Row Atoms K and Ca. J. Chem. Phys. 1997, 107, 5016–5021. [Google Scholar] [CrossRef]
- Kageshima, Y.; Kumagai, H.; Teshima, K.; Domen, K.; Nishikiori, H. Neutral Buffered Electrolytes Guarantee Ideal Band-Edge Pinning for Semiconductor Photoanodes. Chem. Sci. 2025, 16, 14088–14097. [Google Scholar] [CrossRef]
- Man, G.J.; Kamal, C.; Kalinko, A.; Phuyal, D.; Acharya, J.; Mukherjee, S.; Nayak, P.K.; Rensmo, H.; Odelius, M.; Butorin, S.M. A-Site Cation Influence on the Conduction Band of Lead Bromide Perovskites. Nat. Commun. 2022, 13, 3839. [Google Scholar] [CrossRef]
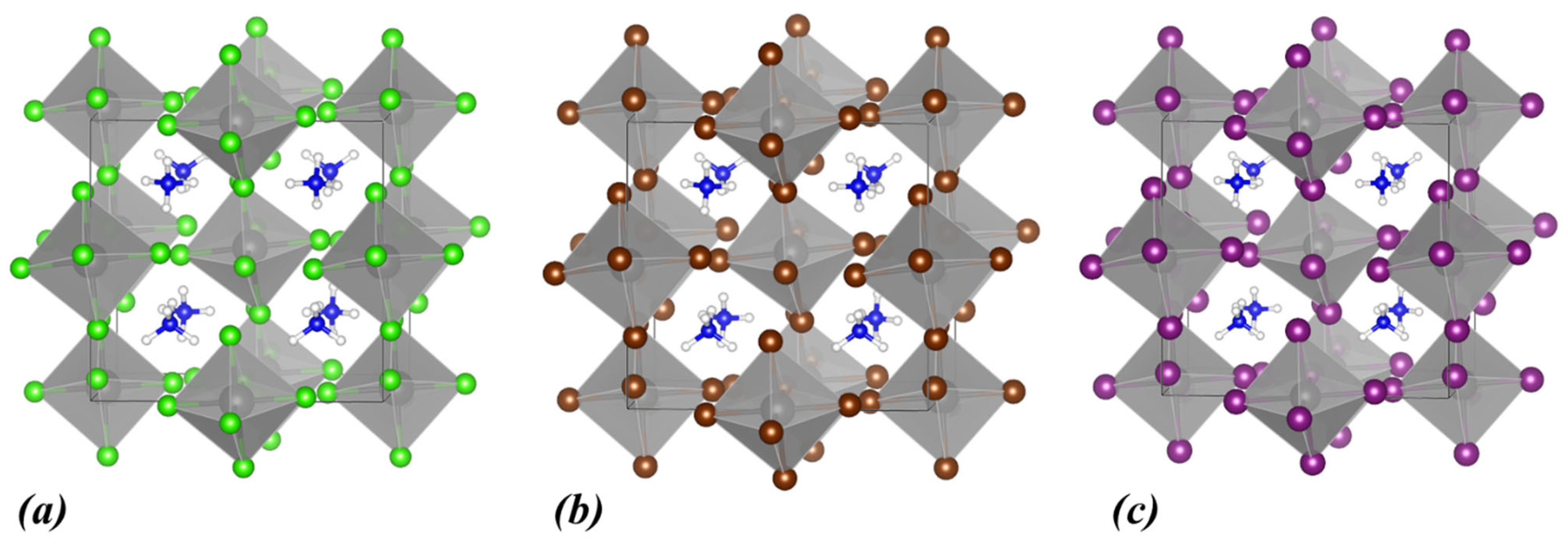

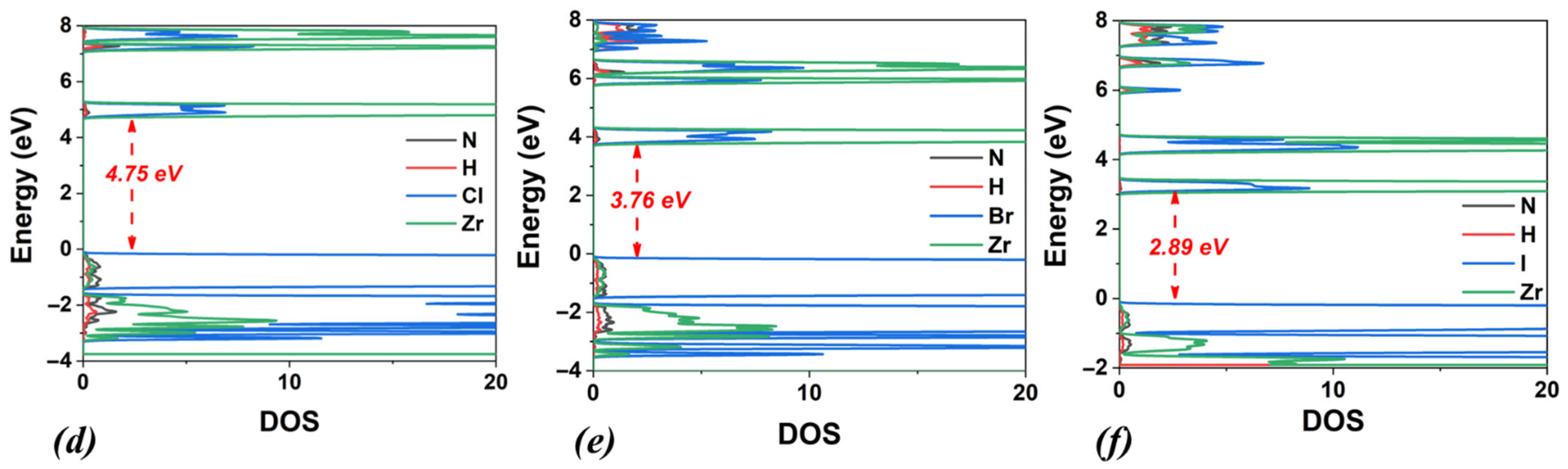
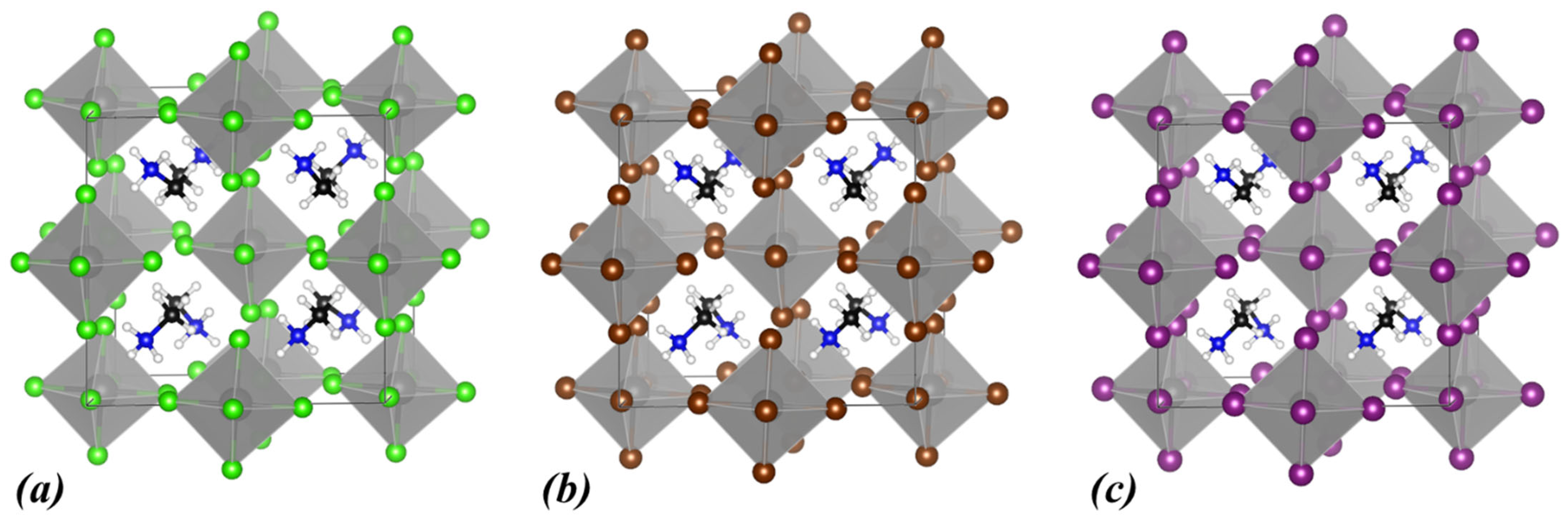
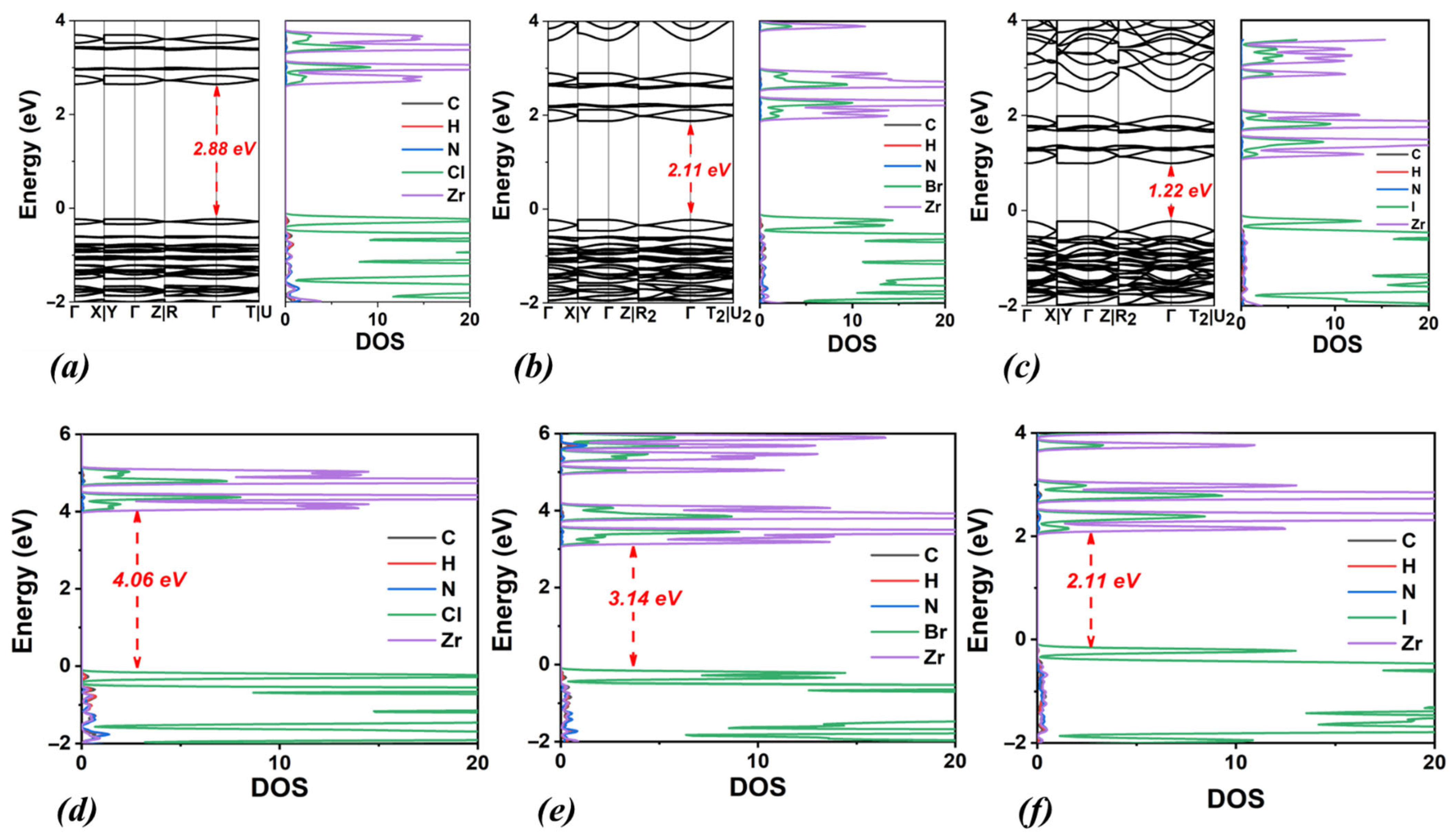

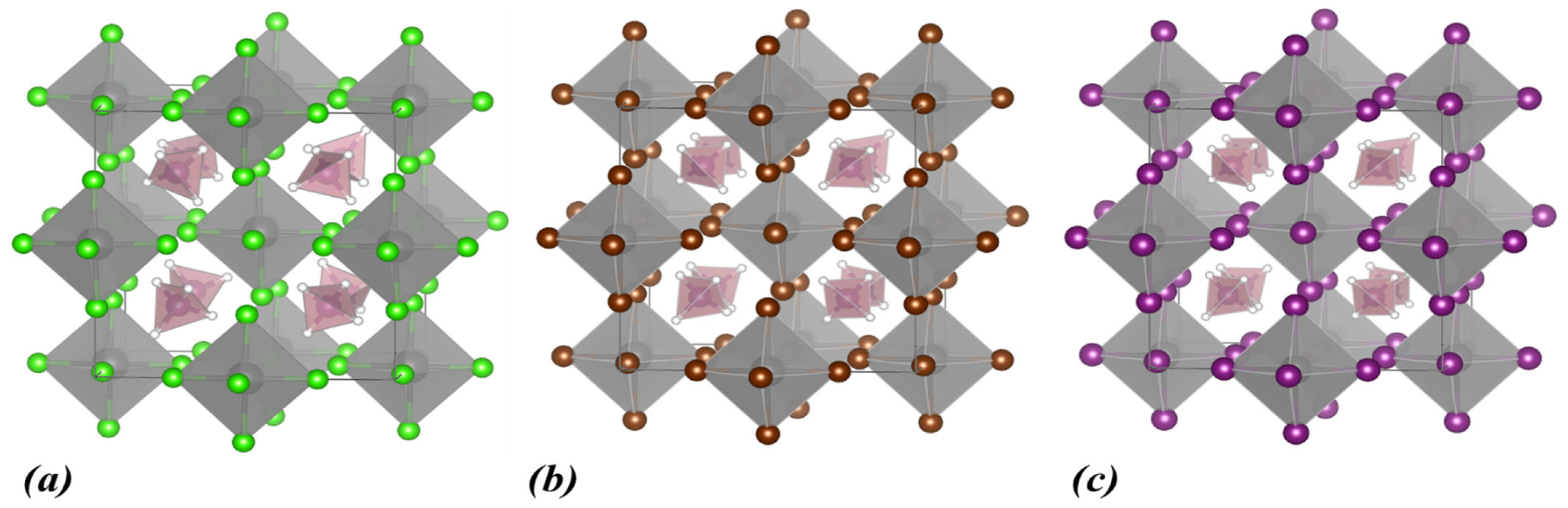

| Lattice Constants | Lattice Angles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Egap PBE | Egap HSE06 | Symmetry of Crystal | a | b | c | α | β | γ |
| (NH4)2ZrCl6 | 3.58 | 4.75 | triclinic | 10.52 | 10.10 | 10.04 | 90.00 | 90.00 | 89.68 |
| (NH4)2ZrBr6 | 2.73 | 3.76 | triclinic | 11.06 | 10.58 | 10.54 | 90.00 | 90.00 | 89.80 |
| (NH4)2ZrI6 | 1.74 | 2.89 | triclinic | 11.87 | 11.35 | 11.34 | 90.00 | 90.00 | 90.04 |
| Lattice Constants | Lattice Angles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Egap PBE | Egap HSE06 | Symmetry of Crystal | a | b | c | α | β | γ |
| MA2ZrCl6 | 2.88 | 4.06 | triclinic | 11.26 | 10.84 | 11.18 | 90.00 | 90.00 | 89.98 |
| MA2ZrBr6 | 2.11 | 3.14 | triclinic | 11.64 | 11.23 | 11.59 | 90.00 | 90.00 | 89.99 |
| MA2ZrI6 | 1.22 | 2.11 | triclinic | 12.30 | 11.92 | 12.28 | 89.99 | 90.00 | 89.99 |
| Lattice Constants | Lattice Angles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Egap PBE | Egap HSE06 | Symmetry of Crystal | a | b | c | α | β | γ |
| DMA2ZrCl6 | 3.56 | 4.64 | triclinic | 11.21 | 12.68 | 11.41 | 89.21 | 88.68 | 89.07 |
| DMA2ZrBr6 | 2.75 | 3.70 | triclinic | 11.55 | 13.29 | 11.64 | 89.30 | 89.28 | 91.20 |
| DMA2ZrI6 | 1.84 | 2.46 | triclinic | 12.20 | 13.84 | 12.30 | 89.30 | 89.47 | 92.14 |
| Lattice Constants | Lattice Angles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Egap PBE | Egap HSE06 | Symmetry of Crystal | a | b | c | α | β | γ |
| TMA2ZrCl6 | 3.42 | 4.54 | triclinic | 11.64 | 13.21 | 12.04 | 91.07 | 98.32 | 90.12 |
| TMA2ZrBr6 | 2.67 | 3.59 | triclinic | 12.07 | 13.63 | 12.42 | 91.08 | 97.98 | 90.64 |
| TMA2ZrI6 | 1.80 | 2.53 | triclinic | 12.67 | 14.25 | 13.03 | 91.03 | 97.16 | 91.01 |
| Lattice Constants | ||||||
|---|---|---|---|---|---|---|
| Structure | Egap PBE | Egap HSE06 | Symmetry of Crystal | a | b | c |
| (PH4)2ZrCl6 | 3.57 | 4.79 | cubic | 10.34 | 10.34 | 1034 |
| (PH4)2ZrBr6 | 2.70 | 3.79 | cubic | 10.99 | 10.99 | 10.99 |
| (PH4)2ZrI6 | 1.68 | 2.63 | cubic | 11.73 | 11.73 | 11.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolokytha, C.; Tzeli, D.; Lathiotakis, N.N. Tailoring the Electronic and Structural Properties of Lead-Free A2ZrX6 “Defect” Perovskites: A DFT Study on A-Site Cation and Halogen Substitutions. Materials 2025, 18, 3976. https://doi.org/10.3390/ma18173976
Kolokytha C, Tzeli D, Lathiotakis NN. Tailoring the Electronic and Structural Properties of Lead-Free A2ZrX6 “Defect” Perovskites: A DFT Study on A-Site Cation and Halogen Substitutions. Materials. 2025; 18(17):3976. https://doi.org/10.3390/ma18173976
Chicago/Turabian StyleKolokytha, Christina, Demeter Tzeli, and Nektarios N. Lathiotakis. 2025. "Tailoring the Electronic and Structural Properties of Lead-Free A2ZrX6 “Defect” Perovskites: A DFT Study on A-Site Cation and Halogen Substitutions" Materials 18, no. 17: 3976. https://doi.org/10.3390/ma18173976
APA StyleKolokytha, C., Tzeli, D., & Lathiotakis, N. N. (2025). Tailoring the Electronic and Structural Properties of Lead-Free A2ZrX6 “Defect” Perovskites: A DFT Study on A-Site Cation and Halogen Substitutions. Materials, 18(17), 3976. https://doi.org/10.3390/ma18173976








