Green and Efficient Lithium Extraction from Spent NCM Batteries via Electromagnetic Radiation
Abstract
1. Introduction
2. Experimental Section
2.1. Raw and Regents
2.2. Method
2.3. Experimental Procedure
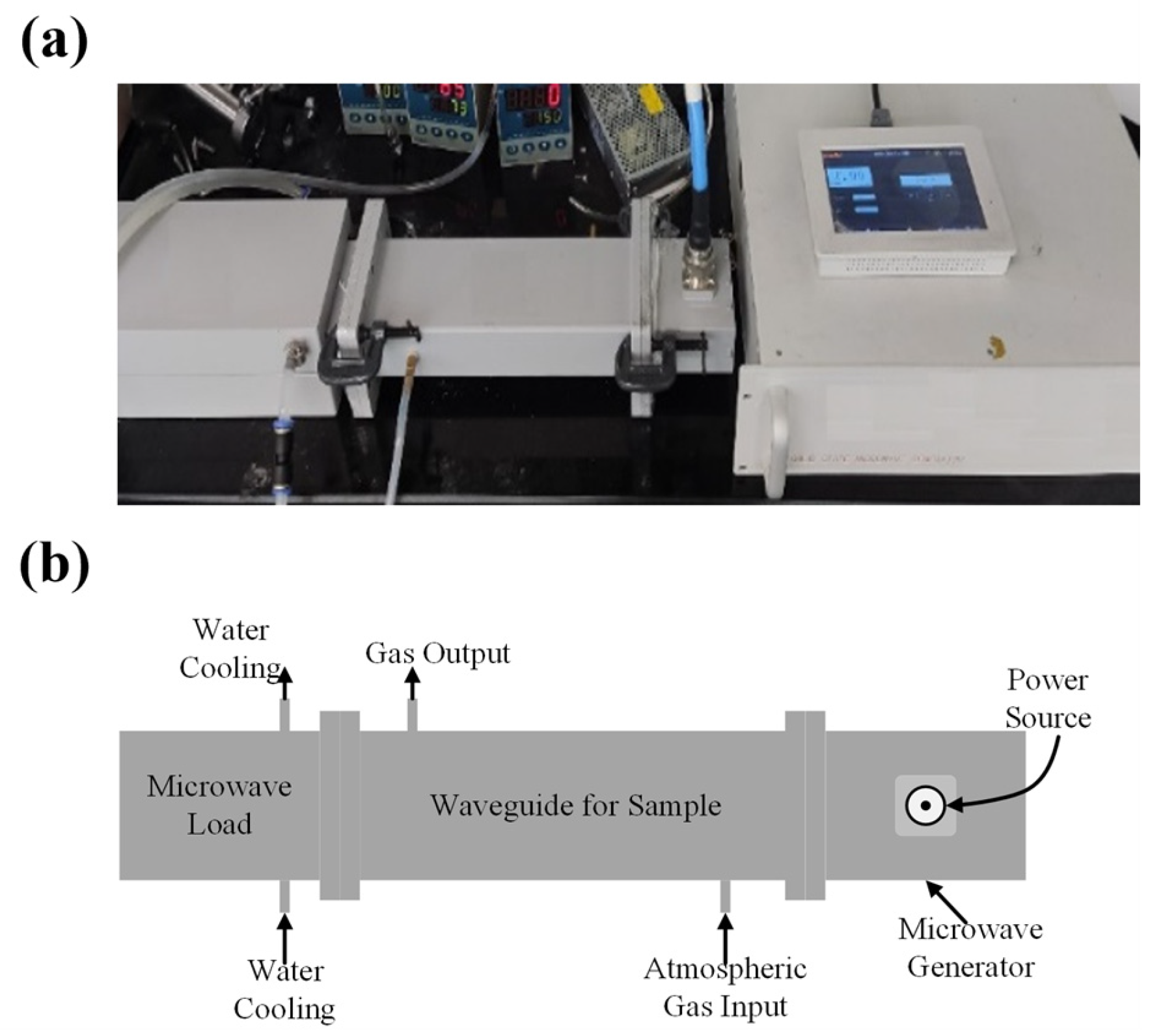
2.4. Experiment Device
2.5. Characterization
3. Results and Discussion
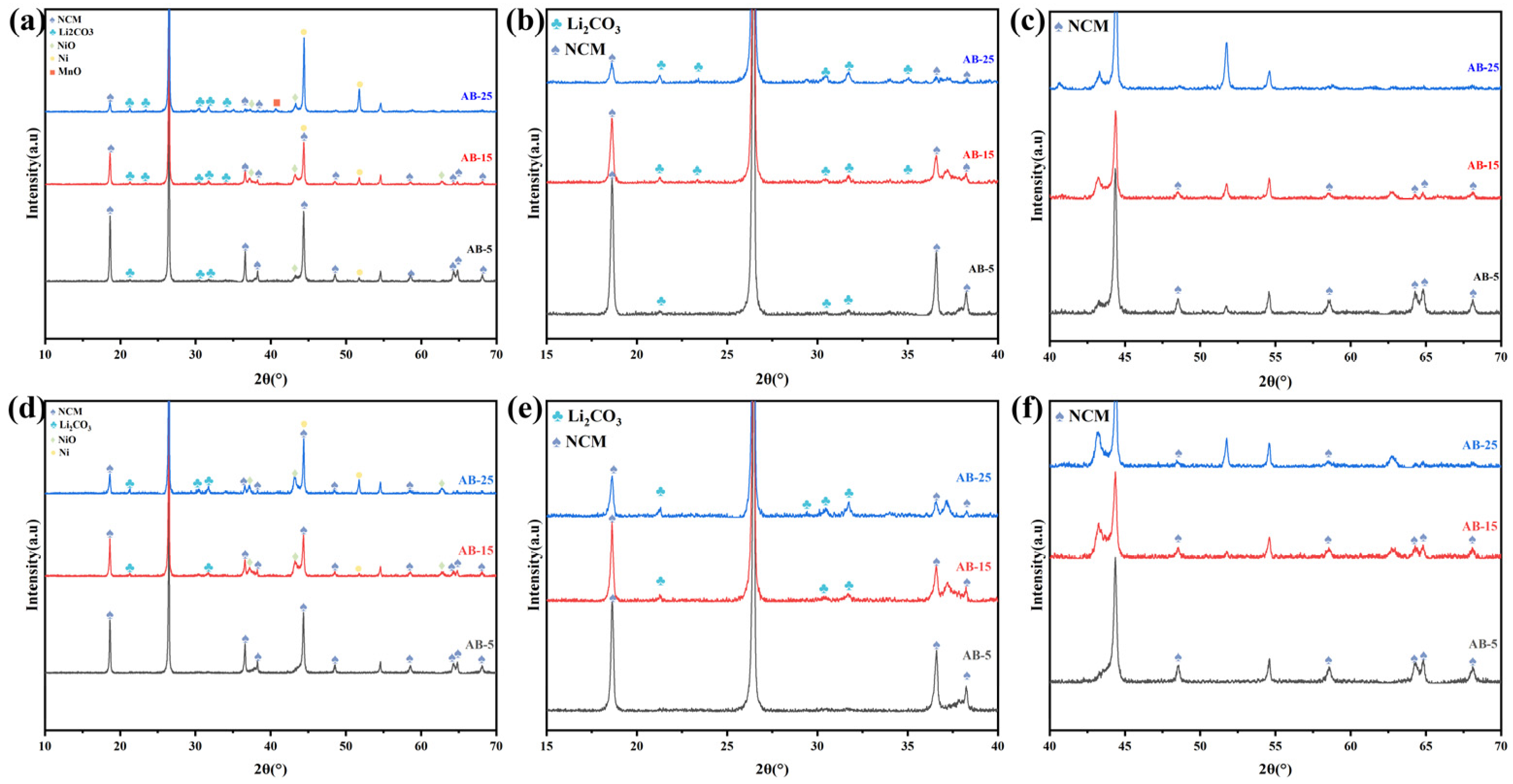
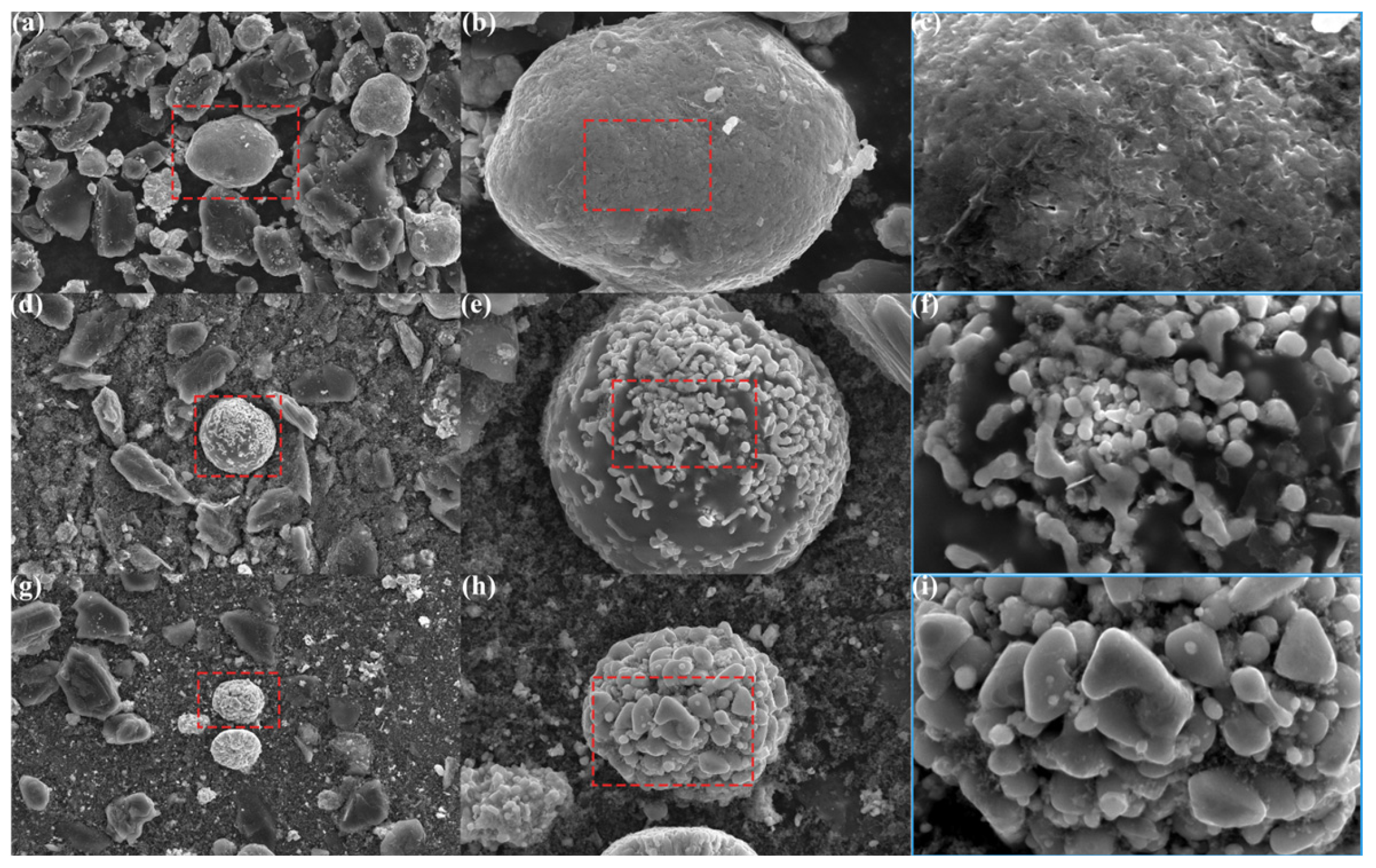
3.1. The Crucial Role of Reducing Atmosphere
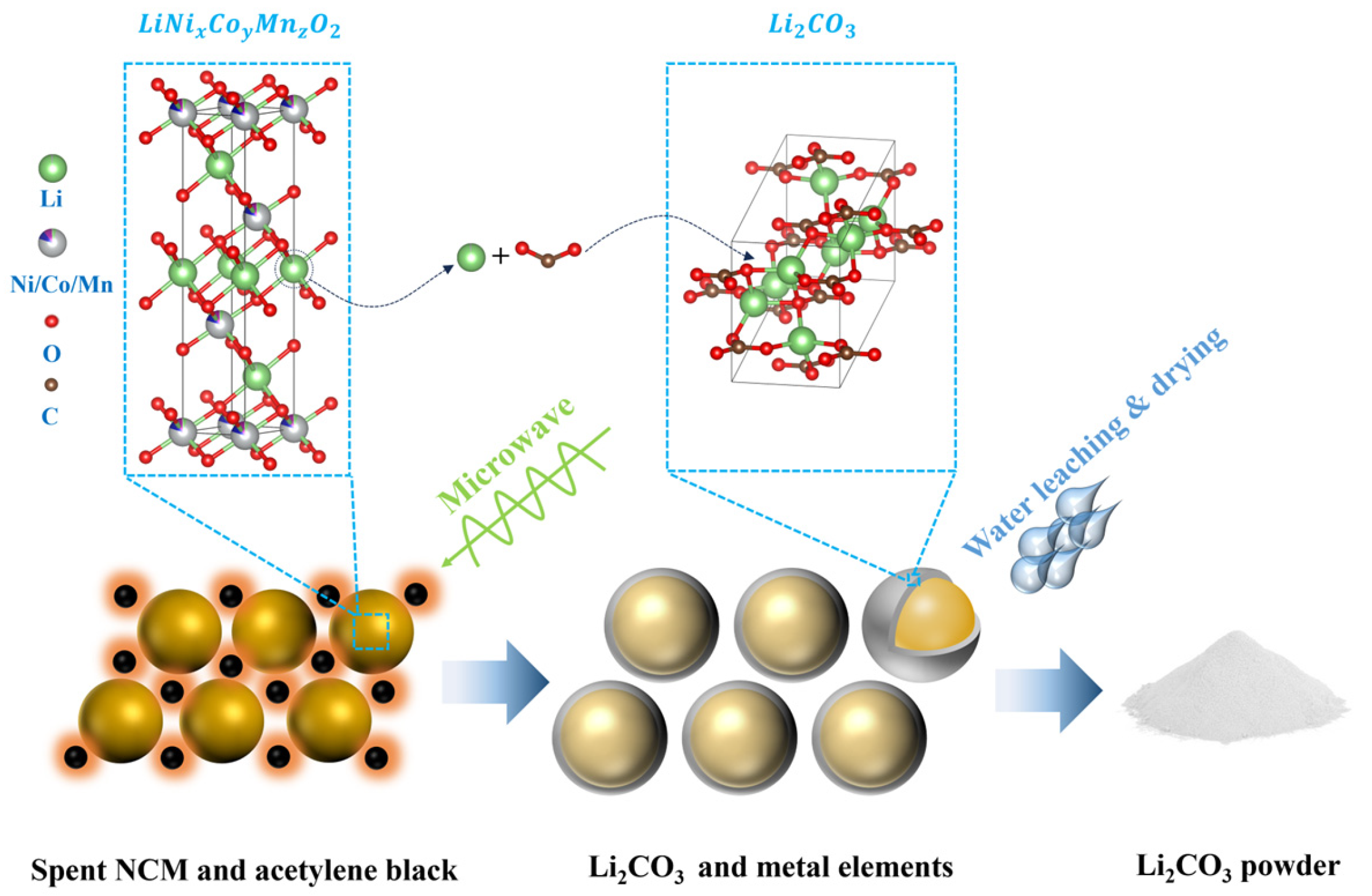
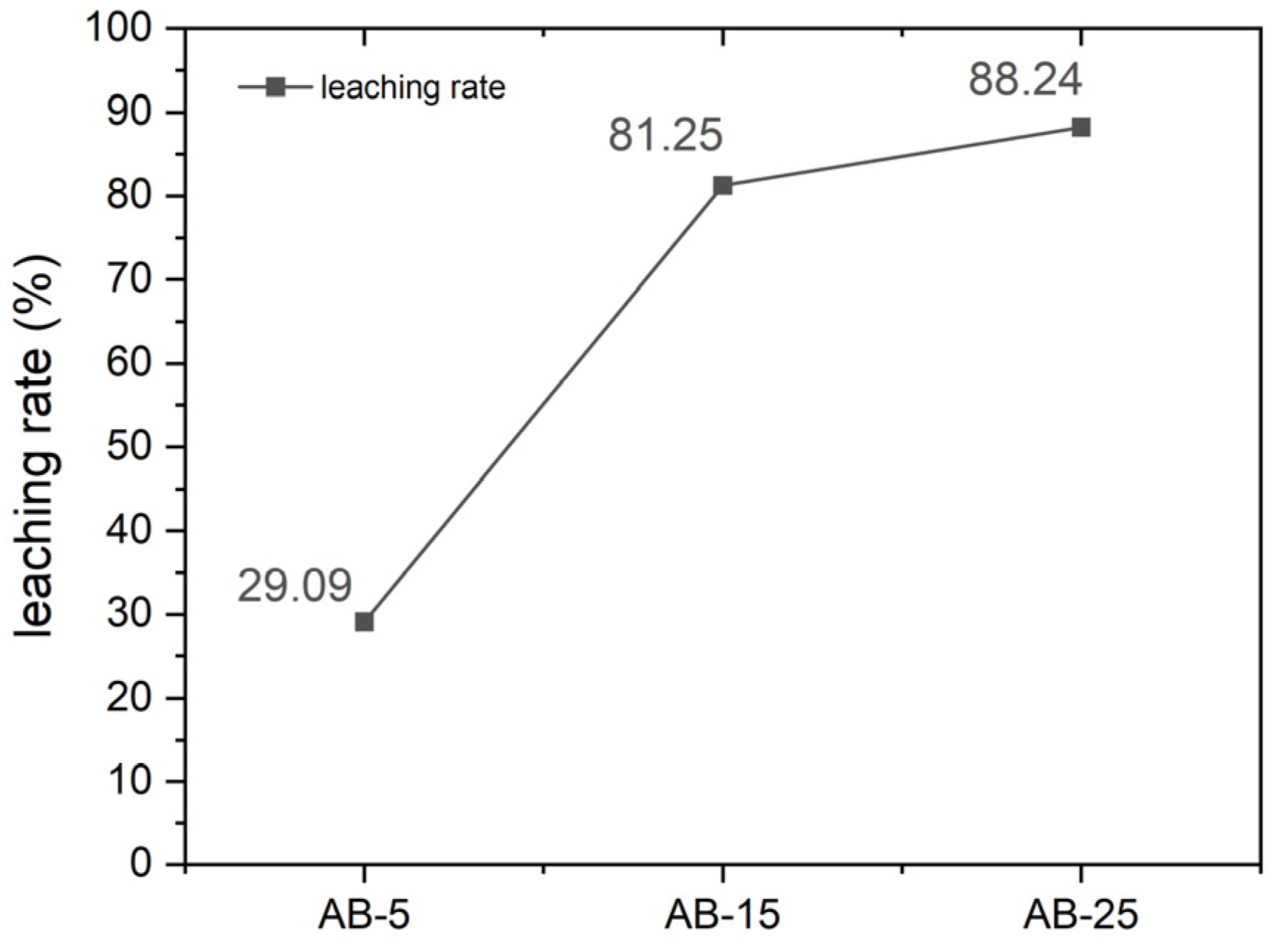
3.2. Formation of Lithium Carbonate and Its Recovery via Water Leaching
3.3. Evaluation of CO2 Emissions and Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornelio, A.; Galli, E.; Scaglia, M.; Zanoletti, A.; Zacco, A.; Bonometti, A.; Magugliani, G.; Mossini, E.; Macerata, E.; Federici, S.; et al. Recovery of NMC-Lithium Battery Black Mass by Microwave Heating Processes. Energy Storage Mater. 2024, 72, 103703. [Google Scholar] [CrossRef]
- Yin, Y.-C.; Li, C.; Hu, X.; Zuo, D.; Yang, L.; Zhou, L.; Yang, J.; Wan, J. Rapid, Direct Regeneration of Spent LiCoO2 Cathodes for Li-Ion Batteries. ACS Energy Lett. 2023, 8, 3005–3012. [Google Scholar] [CrossRef]
- Ko, S.; Choi, J.; Hong, J.; Kim, C.; Hwang, U.; Kwon, M.; Lim, G.; Sohn, S.S.; Jang, J.; Lee, U.; et al. Thermodynamically Controlled Chemical Regeneration of Spent Battery Cathodes Using Recyclable Electron Donors under Ambient Conditions. Energy Environ. Sci. 2024, 17, 4064–4077. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward Sustainable and Systematic Recycling of Spent Rechargeable Batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2021, 11, 2003456. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling-Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Hu, X.; Mousa, E.; Tian, Y.; Ye, G. Recovery of Co, Ni, Mn, and Li from Li-Ion Batteries by Smelting Reduction—Part I: A Laboratory-Scale Study. J. Power Sources 2021, 483, 228936. [Google Scholar] [CrossRef]
- Wang, J.; Jia, K.; Ma, J.; Liang, Z.; Zhuang, Z.; Zhao, Y.; Li, B.; Zhou, G.; Cheng, H.-M. Sustainable Upcycling of Spent LiCoO2 to an Ultra-Stable Battery Cathode at High Voltage. Nat. Sustain. 2023, 6, 797–805. [Google Scholar] [CrossRef]
- Ji, G.; Wang, J.; Liang, Z.; Jia, K.; Ma, J.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Direct Regeneration of Degraded Lithium-Ion Battery Cathodes with a Multifunctional Organic Lithium Salt. Nat. Commun. 2023, 14, 584. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical Recovery of Metal Values from Sulfuric Acid Leaching Liquor of Spent Lithium-Ion Batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Zhuang, Z.; Liang, Z.; Jia, K.; Ji, G.; Zhou, G.; Cheng, H.-M. Toward Direct Regeneration of Spent Lithium-Ion Batteries: A next-Generation Recycling Method. Chem. Rev. 2024, 124, 2839–2887. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-Friendly Oxygen-Free Roasting/Wet Magnetic Separation Technology for in Situ Recycling Cobalt, Lithium Carbonate and Graphite from Spent LiCoO2/Graphite Lithium Batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.-M.; Petranikova, M. Incineration of EV Lithium-Ion Batteries as a Pretreatment for Recycling—Determination of the Potential Formation of Hazardous by-Products and Effects on Metal Compounds. J. Hazard. Mater. 2020, 393, 122372. [Google Scholar] [CrossRef]
- Kelly, J.C.; Wang, M.; Dai, Q.; Winjobi, O. Energy, Greenhouse Gas, and Water Life Cycle Analysis of Lithium Carbonate and Lithium Hydroxide Monohydrate from Brine and Ore Resources and Their Use in Lithium Ion Battery Cathodes and Lithium Ion Batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the Reduction Roasting of Spent LiNixCoyMnzO2 Lithium-Ion Battery Cathode Materials. J. Therm. Anal. Calorim. 2018, 136, 1323–1332. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A Promising Approach for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy. Environ. Sci. Technol. 2017, 51, 11960–11966. [Google Scholar] [CrossRef]


| Ni | Co | Mn | Li | Al | Cu |
|---|---|---|---|---|---|
| 24.38 | 4.19 | 3.42 | 3.42 | 0.46 | 0.41 |
| Sample | Li | Ni | Co | Mn | Al | Cu |
|---|---|---|---|---|---|---|
| AB-5 | 0.2330 | ND [a] | ND | ND | 0.0062 | ND |
| AB-15 | 0.6383 | ND | ND | ND | 0.0055 | ND |
| AB-25 | 0.6984 | ND | ND | ND | 0.0086 | ND |
| Sample | Li | Ni | Co | Mn | Al | Cu |
|---|---|---|---|---|---|---|
| AB-5 | 2.89 | 25.30 | 4.62 | 3.58 | 0.48 | 0.35 |
| AB-15 | 0.83 | 26.85 | 4.86 | 3.77 | 0.56 | 0.45 |
| AB-25 | 0.50 | 25.92 | 4.69 | 3.63 | 0.48 | 0.50 |
| Sample | Original Mass (mg) | Mass Loss (mg) | Mass Loss Rate |
|---|---|---|---|
| AB-5 | 2094.46 | 45.96 | 2.2% |
| AB-15 | 2304.19 | 186.83 | 8.1% |
| AB-25 | 2498.15 | 254.81 | 10.2% |
| Reference | Original BM | Method | Time (min) | Energy [a] (MWh/t) |
|---|---|---|---|---|
| [20] | NCM | furnace-based | 30 | ~1000 |
| [21] | NCM | furnace-based | 180 | ~6000 |
| [22] | NCM | furnace-based | 30 | ~1000 |
| Our work | NCM and Graphite | EM irradiation | 10 | ~2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Zhang, G.-R.; Li, D.-S.; Huang, X.-Y.; Liu, Y.-L.; Cheng, Y.-Q. Green and Efficient Lithium Extraction from Spent NCM Batteries via Electromagnetic Radiation. Materials 2025, 18, 3975. https://doi.org/10.3390/ma18173975
Tong L, Zhang G-R, Li D-S, Huang X-Y, Liu Y-L, Cheng Y-Q. Green and Efficient Lithium Extraction from Spent NCM Batteries via Electromagnetic Radiation. Materials. 2025; 18(17):3975. https://doi.org/10.3390/ma18173975
Chicago/Turabian StyleTong, Ling, Gui-Rong Zhang, Da-Shuai Li, Xing-Yu Huang, Yuan-Long Liu, and Yan-Qing Cheng. 2025. "Green and Efficient Lithium Extraction from Spent NCM Batteries via Electromagnetic Radiation" Materials 18, no. 17: 3975. https://doi.org/10.3390/ma18173975
APA StyleTong, L., Zhang, G.-R., Li, D.-S., Huang, X.-Y., Liu, Y.-L., & Cheng, Y.-Q. (2025). Green and Efficient Lithium Extraction from Spent NCM Batteries via Electromagnetic Radiation. Materials, 18(17), 3975. https://doi.org/10.3390/ma18173975







