Silver Nanoparticles@Zeolite Composites: Preparation, Characterization and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterizations
2.2.1. Preparation of Ag-Modified Zeolite (Ag+-Zeo)
2.2.2. Silver Nanoparticles@zeolite Composites
Synthesis of Ag NPs@zeolite Composite by Thermal Reduction (Ag-Zeo-1)
Synthesis of Ag NPs@zeolite Composite by Chemical Reduction with Na3Cit (Ag-Zeo-2)
Synthesis of Ag NPs@zeolite Composite by Chemical Reduction with NaBH4 (Ag-Zeo-3)
2.2.3. Ag NPs@zeolite Composites’ Characterization
2.2.4. Antibacterial Activity of Ag NPs@zeolite Composites
3. Results and Discussion
Antibacterial Activity Against Escherichia coli of Samples Ag-Zeo-1, Ag-Zeo-2, and Ag-Zeo-3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Goharshadi, E.K.; Azizi-Toupkanloo, H. Silver colloid nanoparticles: Ultrasound-assisted synthesis, electrical and rheological properties. Powder Technol. 2013, 237, 97–101. [Google Scholar] [CrossRef]
- Faraji, N.; Mat Yunus, W.M.; Kharazmi, A.; Saion, E. Third-order nonlinear optical properties of silver nanoparticles mediated by chitosan. Opt. Int. J. Light Electron Opt. 2014, 125, 2809–2812. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 102–108. [Google Scholar] [CrossRef]
- Espeche Turbay, M.B.; Rey, V.; Dorado, R.D.; Sosa, M.C.; Borsarelli, C.D. Silver nanoparticle-protein interactions and the role of lysozyme as an antagonistic antibacterial agent. Colloids Surf. B Biointerfaces 2021, 208, 112030. [Google Scholar] [CrossRef]
- Ramirez, M.; Ben Khalifa, E.; Magnacca, G.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Removal and Recovery of AgNPs from Water by Sustainable Magnetic Nanoflocculants. Polymers 2025, 17, 650. [Google Scholar] [CrossRef]
- Ayadi, T.; Badawi, M.; Cantrel, L.; Lebègue, S. Rational approach for an optimized formulation of silver-exchanged zeolites for iodine capture from first-principles calculations. Mol. Syst. Des. Eng. 2022, 7, 422–433. [Google Scholar] [CrossRef]
- Duran, N.; Marcato, P.; Souza, G.; Alves, O.; Esposito, E. Antibacterial Effect of Silver Nanoparticles Produced by Fungal Process on Textile Fabrics and Their Effluent Treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.; Chen, Y.; Lin, H. Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fibers Polym. 2009, 10, 496–501. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Ittiachen, L.; Babu, S.M. Evaluation of Antibacterial activity of Biosynthesized Silver nanoparticles coated Low Density Polyethylene films. Mater. Today Proc. 2023, 80, 2133–2137. [Google Scholar] [CrossRef]
- Torres-Flores, E.I.; Flores-López, N.S.; Martínez-Núñez, C.E.; Tánori-Córdova, J.C.; Flores-Acosta, M.; Cortez-Valadez, M. Silver nanoparticles in natural zeolites incorporated into commercial coating: Antibacterial study. Appl. Phys. A 2021, 127, 71. [Google Scholar] [CrossRef]
- Bashir, N.; Afzaal, M.; Khan, A.L.; Nawaz, R.; Irfan, A.; Almaary, K.S.; Dabiellil, F.; Bourhia, M.; Ahmed, Z. Green-synthesized silver nanoparticle-enhanced nanofiltration mixed matrix membranes for high-performance water purification. Sci. Rep. 2025, 15, 1001. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Sundaram, S.; Yadav, K.K.; Srivastav, A.L. An overview of silver nano-particles as promising materials for water disinfection. Environ. Technol. Innov. 2021, 23, 101721. [Google Scholar] [CrossRef]
- Pavlović, J.; Rajić, N. Clinoptilolite—An Efficient Carrier for Catalytically Active Nano Oxide Particles. Minerals 2023, 13, 877. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front Pharmacol 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Mehr, F.P.; Khanjani, M.; Vatani, P.J. Synthesis of Nano-Ag particles using sodium borohydride. Orient J Chem 2015, 31, 1831–1833. [Google Scholar] [CrossRef]
- Mavani, K.; Shah, M. Synthesis of Silver Nanoparticles by using Sodium Borohydride as a Reducing Agent. Int. J. Eng. Res. Technol. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Natsuki, J.; Abe, T. Synthesis of pure colloidal silver nanoparticles with high electroconductivity for printed electronic circuits: The effect of amines on their formation in aqueous media. J. Colloid Interface Sci. 2011, 359, 19–23. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.J.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Z.; Zhang, T.; Qin, Y.; Yang, X. Structural transition of synthesized silver nanoparticles under irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 89–95. [Google Scholar] [CrossRef]
- Lin, C.C.; Danaie, M.; Liu, Y.; Mitlin, D.; Kuznicki, S.M.; Eyring, E.M. Thermally stable silver nanoparticles formed on a zeolite surface show multiple crystal twinning. J. Nanosci. Nanotechnol. 2009, 9, 4985–4987. [Google Scholar] [CrossRef] [PubMed]
- Concepción-Rosabal, B.; Rodríguez-Fuentes, G.; Bogdanchikova, N.; Bosch, P.; Avalos, M.; Lara, V.H. Comparative study of natural and synthetic clinoptilolites containing silver in different states. Microporous Mesoporous Mater. 2005, 86, 249–255. [Google Scholar] [CrossRef]
- Gemishev, O.; Panayotova, M.; Mirdzveli, N.; Panayotov, V. Preparation of silver nanoparticles-natural zeolite composite and study of its antibacterial properties. J. Int. Sci. Publ. Ecol. Saf. 2020, 14, 55–64. Available online: https://www.scientific-publications.net/en/article/1002036/ (accessed on 1 August 2025).
- Mintcheva, N.; Panayotova, M.; Gicheva, G.; Gemishev, O.; Tyuliev, G. Effect of Exchangeable Ions in Natural and Modified Zeolites on Ag Content, Ag Nanoparticle Formation and Their Antibacterial Activity. Materials 2021, 14, 4153. [Google Scholar] [CrossRef]
- Kia Kojoori, R.; Sadegh Samiei, S. Synthesis of Silver Nanoparticles Doped in the Zeolite Framework. Asian J. Chem. 2012, 24, 2342–2344. Available online: https://asianpubs.org/index.php/ajchem/article/view/9016 (accessed on 1 August 2025).
- Benaouda, H.; Bouchiba, N.; Hachemaoui, M.; Abad-López, J.; Bennabi, F.; Mokhtar, A.; Hasnaoui, M.A.; Ismail, I.; Abboud, M.; Boukoussa, B. MNP (M = Zn, Cu, and Ag) Catalyst Embedded onto Zeolite Y Surface for Efficient Dye Reduction and Antimicrobial Activity. Catalysts 2025, 15, 407. [Google Scholar] [CrossRef]
- Guerra, R.; Lima, E.; Viniegra, M.; Guzmán, A.; Lara, V. Growth of Escherichia coli and Salmonella typhi inhibited by fractal silver nanoparticles supported on zeolites. Microporous Mesoporous Mater. 2012, 147, 267–273. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; de Oliveira, A.M.; Beltran, L.B.; Diório, A.; Magalhães-Ghiotto, G.A.V.; de Abreu Filho, B.A.; de Almeida Duarte, E.d.C.N.F.; Bergamasco, R. Antibacterial activity of functionalized natural zeolites (NZ-AgNPs) and its application in bacteriological water treatment and commercial paints. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101001. [Google Scholar] [CrossRef]
- Panayotova, M.; Mintcheva, N.; Gemishev, O.; Tyuliev, G.; Gicheva, G.; Djerahov, L. Preparation and antimicrobial properties of silver nanoparticles supported by natural zeolite clinoptilolite. Bulg. Chem. Commun 2018, 50, 211–218. [Google Scholar]
- Bartolomeu, R.; Bértolo, R.; Casale, S.; Fernandes, A.; Henriques, C.; da Costa, P.; Ribeiro, F. Particular characteristics of silver species on Ag-exchanged LTL zeolite in K and H form. Microporous Mesoporous Mater. 2013, 169, 137–147. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Antúnez-García, J.; Chávez-Rivas, F.; Khramov, E.; Berlier, G.; Moreno-Ruiz, L.; Zubavichus, Y.; Petranovskii, V.; Fuentes-Moyado, S.; et al. Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts 2022, 12, 1453. [Google Scholar] [CrossRef]
- Rodríguez Iznaga, I.; Petranovskii, V.; Rodríguez Fuentes, G.; Mendoza, C.; Benítez Aguilar, A. Exchange and reduction of Cu2+ ions in clinoptilolite. J. Colloid Interface Sci. 2007, 316, 877–886. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Vasylechko, V.; Khyzhun, O.; Gryshchouk, G.; Khalameida, S.; Vasylechko, L. Effect of high-energy milling on the structure, some physicochemical and photocatalytic properties of clinoptilolite. Appl. Catal. A Gen. 2021, 610, 117930. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.; Concepción-Rosabal, B. Copper-Silver Bimetallic System on Natural Clinoptilolite: Thermal Reduction of Cu2+ and Ag+ Exchanged. J. Nanosci. Nanotechnol. 2011, 11, 5580–5586. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Metal clusters and nanoparticles assembled in zeolites: An example of stable materials with controllable particle size. Mater. Sci. Eng. C 2002, 19, 327–331. [Google Scholar] [CrossRef]
- Azambre, B.; Chebbi, M.; Hijazi, A. Effects of the cation and Si/Al ratio on CH3I adsorption by faujasite zeolites. Chem. Eng. J. 2020, 379, 122308. [Google Scholar] [CrossRef]
- Li, Z.; Flytzani-Stephanopoulos, M. On the Promotion of Ag–ZSM-5 by Cerium for the SCR of NO by Methane. J. Catal. 1999, 182, 313–327. [Google Scholar] [CrossRef]
- Bartolomeu, R.; Azambre, B.; Westermann, A.; Fernandes, A.; Bértolo, R.; Hamoud, H.I.; Henriques, C.; Da Costa, P.; Ribeiro, F. Investigation of the nature of silver species on different Ag-containing NOx reduction catalysts: On the effect of the support. Appl. Catal. B Environ. 2014, 150–151, 204–217. [Google Scholar] [CrossRef]
- Bogdanchikova, N.E.; Petranovskii, V.P.; Machorro, R.; Sugi, Y.; Fuentes, S. Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Appl. Surf. Sci. 1999, 150, 58–64. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Hernandez, M.A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Mater. Sci. Eng. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Pérez, R. Kinetic Adsorption Study of Silver Nanoparticles on Natural Zeolite: Experimental and Theoretical Models. Appl. Sci. 2015, 5, 1869–1881. [Google Scholar] [CrossRef]
- Moulder, J.F.; Sickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Wu, Y.; Li, C.; Bai, J. Preparation of silver supported porous 4A-zeolite through hard template agent combined with heat treatment and study on its catalytic performance. J. Porous Mater. 2018, 25, 1669–1677. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Satayeva, A.; Yagofarova, A.; Tauanov, Z.; Meiramkulova, K.; Farrando-Pérez, J.; Bear, J.C. Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials 2020, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.; Neves, I.C. Study of silver species stabilized in different microporous zeolites. Microporous Mesoporous Mater. 2013, 181, 83–87. [Google Scholar] [CrossRef]
- Hanim, S.A.M.; Malek, N.A.N.N.; Ibrahim, Z. Amine-functionalized, silver-exchanged zeolite NaY: Preparation, characterization and antibacterial activity. Appl. Surf. Sci. 2016, 360, 121–130. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Nenova, E.P.; Yocheva, L.D.; Ivanova, I.A.; Georgiev, P.A. Antimicrobial and Oxidative Activities of Different Levels of Silver-Exchanged Zeolites X and ZSM-5 and Their Ecotoxicity. Pharmaceuticals 2024, 17, 1586. [Google Scholar] [CrossRef]
- Jiraroj, D.; Tungasmita, S.; Tungasmita, D.N. Silver ions and silver nanoparticles in zeolite A composites for antibacterial activity. Powder Technol. 2014, 264, 418–422. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Kubo, A.-L.; Capjak, I.; Vrček, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial potency of differently coated 10 and 50 nm silver nanoparticles against clinically relevant bacteria Escherichia coli and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef]
- Missengue, R.N.M.; Musyoka, N.M.; Madzivire, G.; Babajide, O.; Fatoba, O.O.; Tuffin, M.; Petrik, L.F. Leaching and antimicrobial properties of silver nanoparticles loaded onto natural zeolite clinoptilolite by ion exchange and wet impregnation. J. Environ. Sci. Health Part A 2016, 51, 97–104. [Google Scholar] [CrossRef]
- Top, A.; Ülkü, S. Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity. Appl. Clay Sci. 2004, 27, 13–19. [Google Scholar] [CrossRef]
- Akhigbe, L.; Ouki, S.; Saroj, D.; Lim, X.M. Silver-modified clinoptilolite for the removal of Escherichia coli and heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. 2014, 21, 10940–10948. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kanzaki, Y. The mechanism of antibacterial activity of silver-loaded zeolite. J. Inorg. Biochem. 1997, 67, 377. [Google Scholar] [CrossRef]
- Inoue, Y.; Hoshino, M.; Takahashi, H.; Noguchi, T.; Murata, T.; Kanzaki, Y.; Hamashima, H.; Sasatsu, M. Bactericidal activity of Ag–zeolite mediated by reactive oxygen species under aerated conditions. J. Inorg. Biochem. 2002, 92, 37–42. [Google Scholar] [CrossRef] [PubMed]

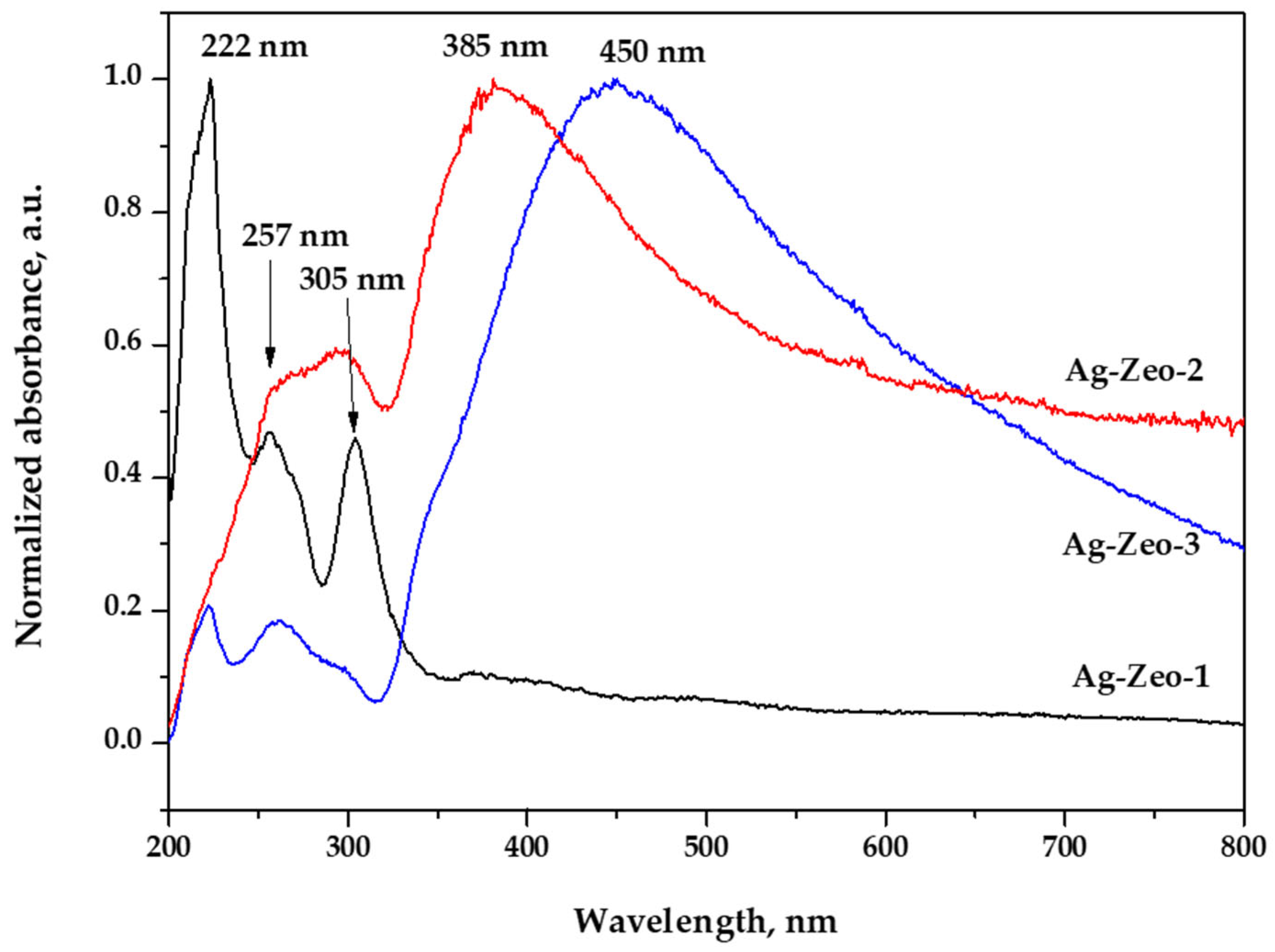
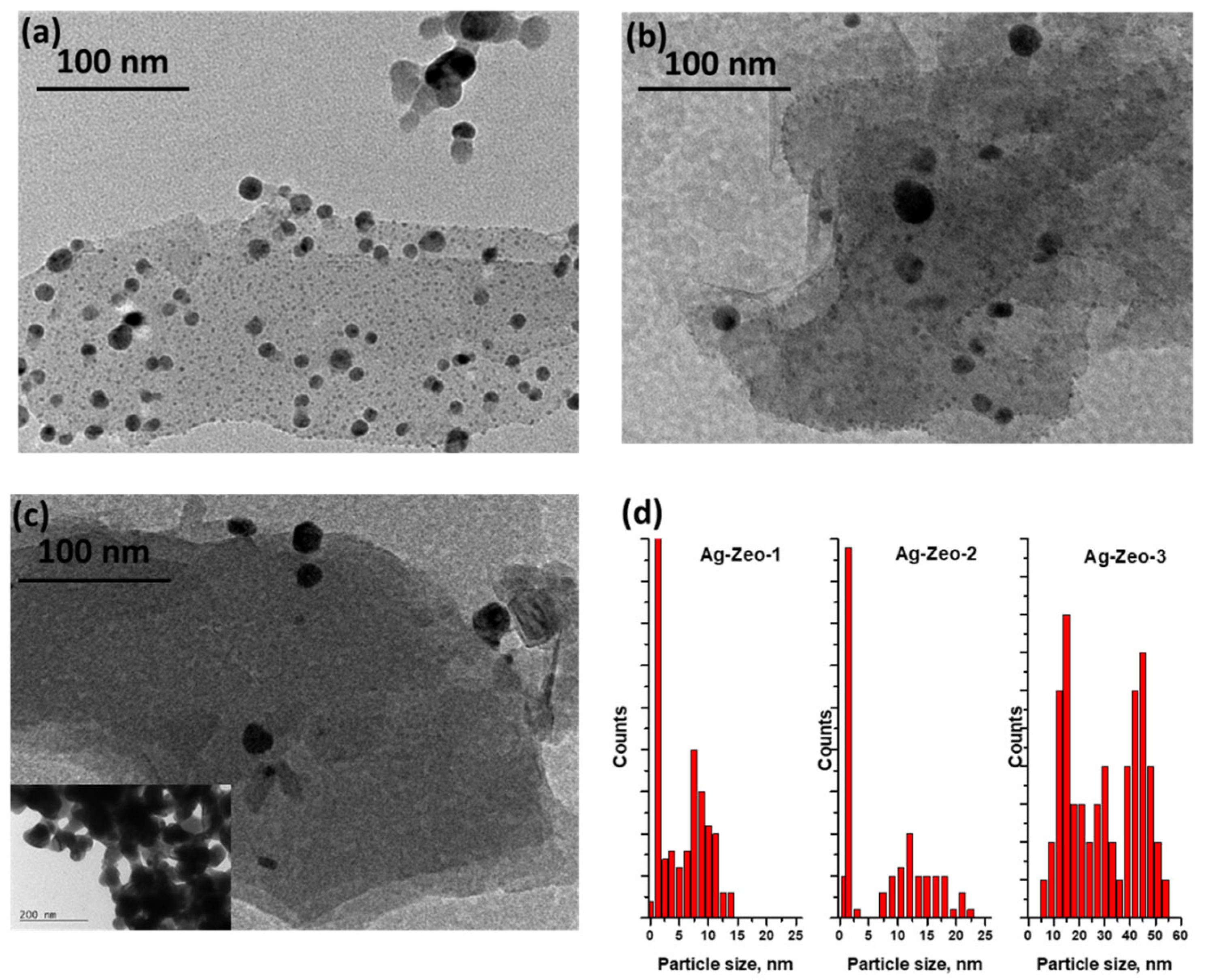
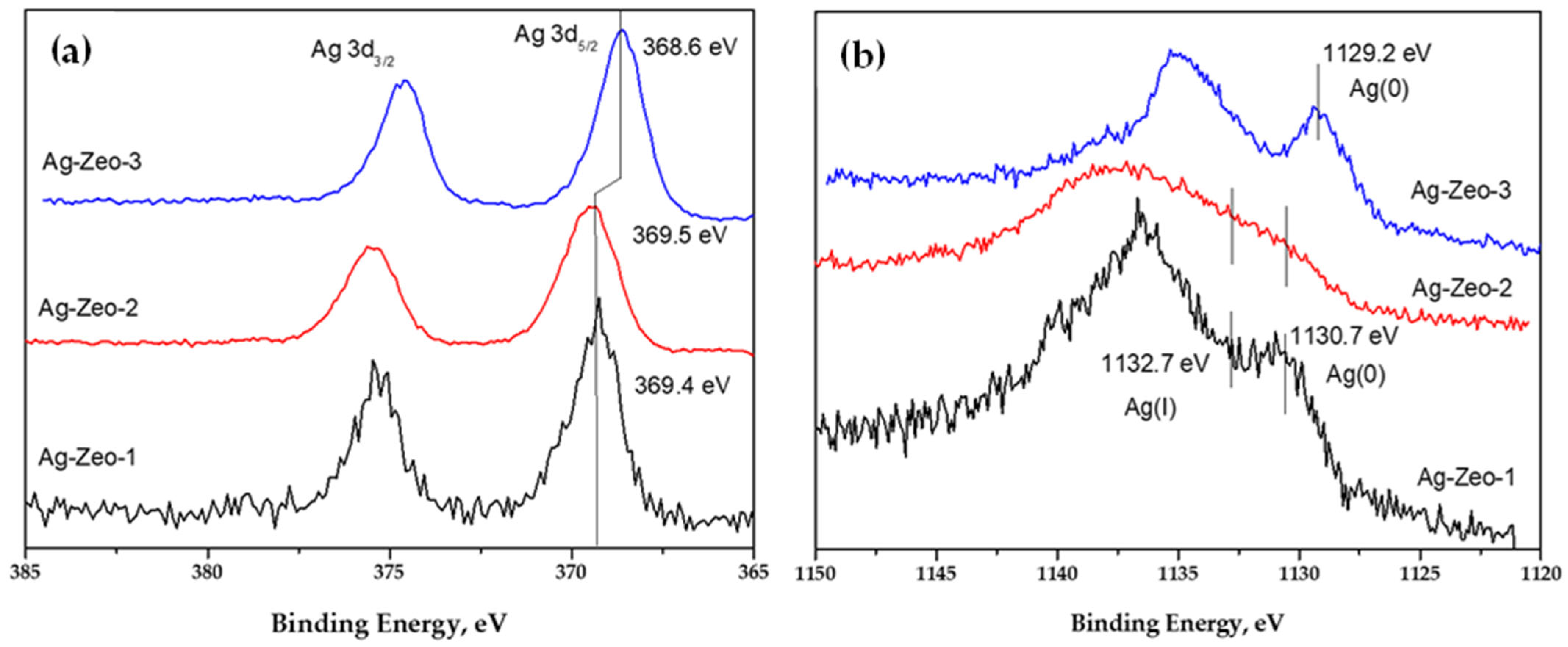

| Ag-Zeo-1 | Ag-Zeo-2 | Ag-Zeo-3 | Assignment | |
|---|---|---|---|---|
| UV–Vis | 222 (s) | 222 (sh) | 222 (w) | Ag+ ions in zeolite |
| band | 257 (m) | 257 (w) | 257 (w) | Zeolite network |
| (nm) | 305 (m) | 305 (w) | 305 (sh) | Ag clusters |
| 380 (sh) | 385 (s, br) | Ag NPs 1–3 nm | ||
| 450 (s, br) | Ag NPs > 15 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gicheva, G.; Panayotova, M.; Gemishev, O.; Kulinich, S.A.; Mintcheva, N. Silver Nanoparticles@Zeolite Composites: Preparation, Characterization and Antibacterial Properties. Materials 2025, 18, 3964. https://doi.org/10.3390/ma18173964
Gicheva G, Panayotova M, Gemishev O, Kulinich SA, Mintcheva N. Silver Nanoparticles@Zeolite Composites: Preparation, Characterization and Antibacterial Properties. Materials. 2025; 18(17):3964. https://doi.org/10.3390/ma18173964
Chicago/Turabian StyleGicheva, Gospodinka, Marinela Panayotova, Orlin Gemishev, Sergei A. Kulinich, and Neli Mintcheva. 2025. "Silver Nanoparticles@Zeolite Composites: Preparation, Characterization and Antibacterial Properties" Materials 18, no. 17: 3964. https://doi.org/10.3390/ma18173964
APA StyleGicheva, G., Panayotova, M., Gemishev, O., Kulinich, S. A., & Mintcheva, N. (2025). Silver Nanoparticles@Zeolite Composites: Preparation, Characterization and Antibacterial Properties. Materials, 18(17), 3964. https://doi.org/10.3390/ma18173964









