Preparation of Ni-P Composite Coatings and Study on the Corrosion Resistance and Antifouling Properties in Low-Temperature Flue Gas Environment
Abstract
1. Introduction
2. Experiments and Methods
2.1. Preparation Method of Coating
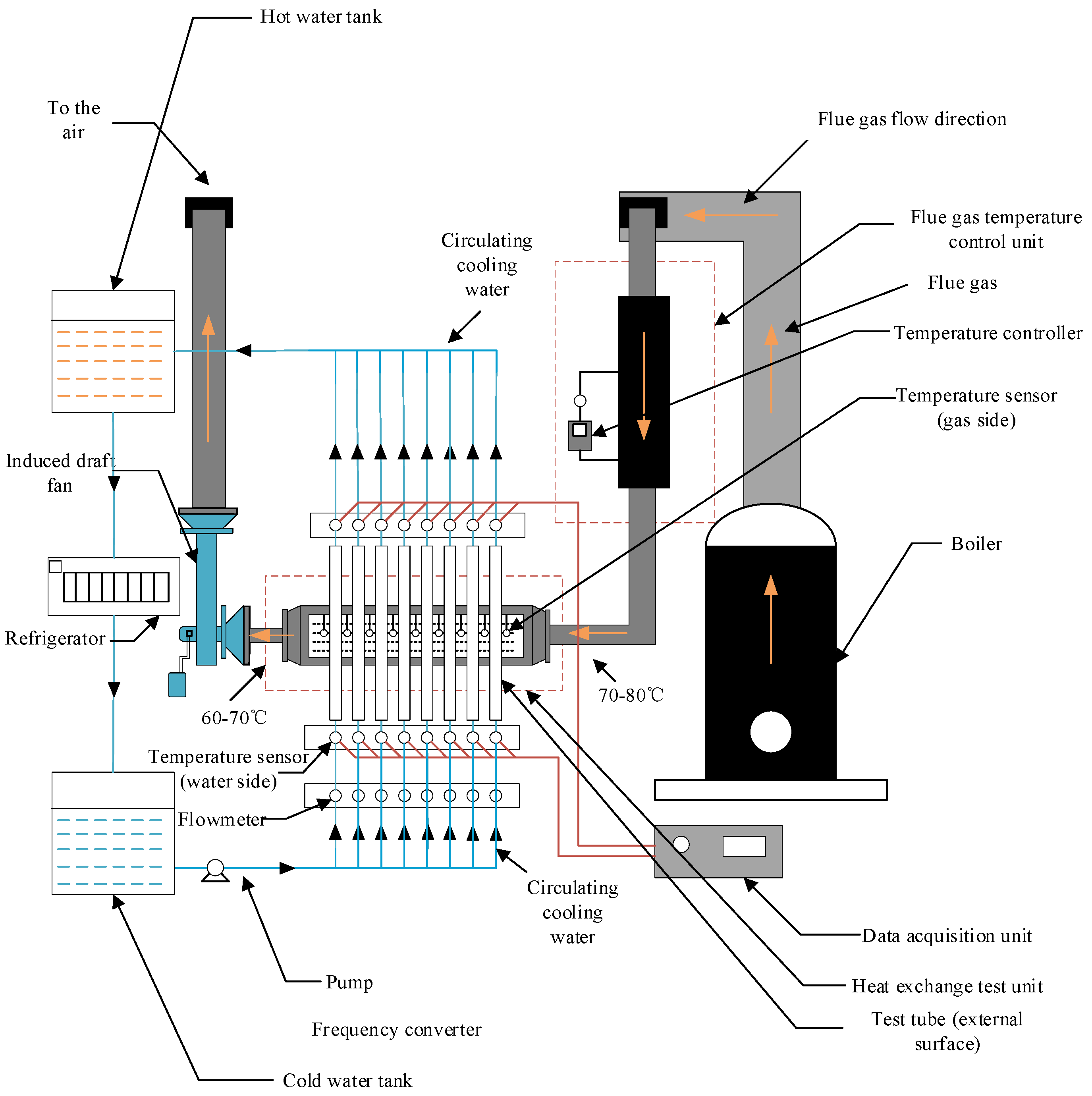
2.2. Experimental System
2.3. Experimental Process
2.4. Analysis Method
3. Results and Discussion
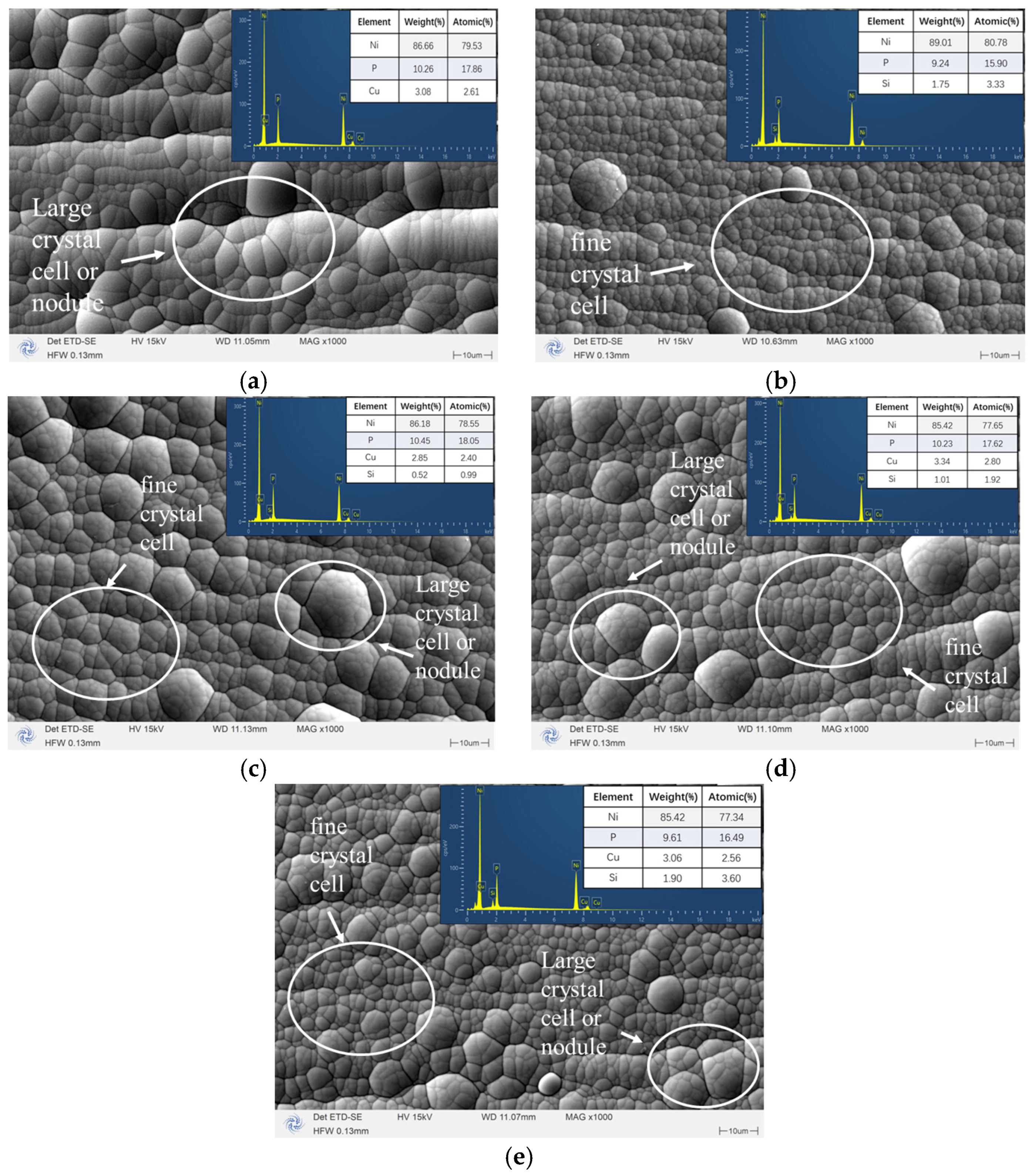
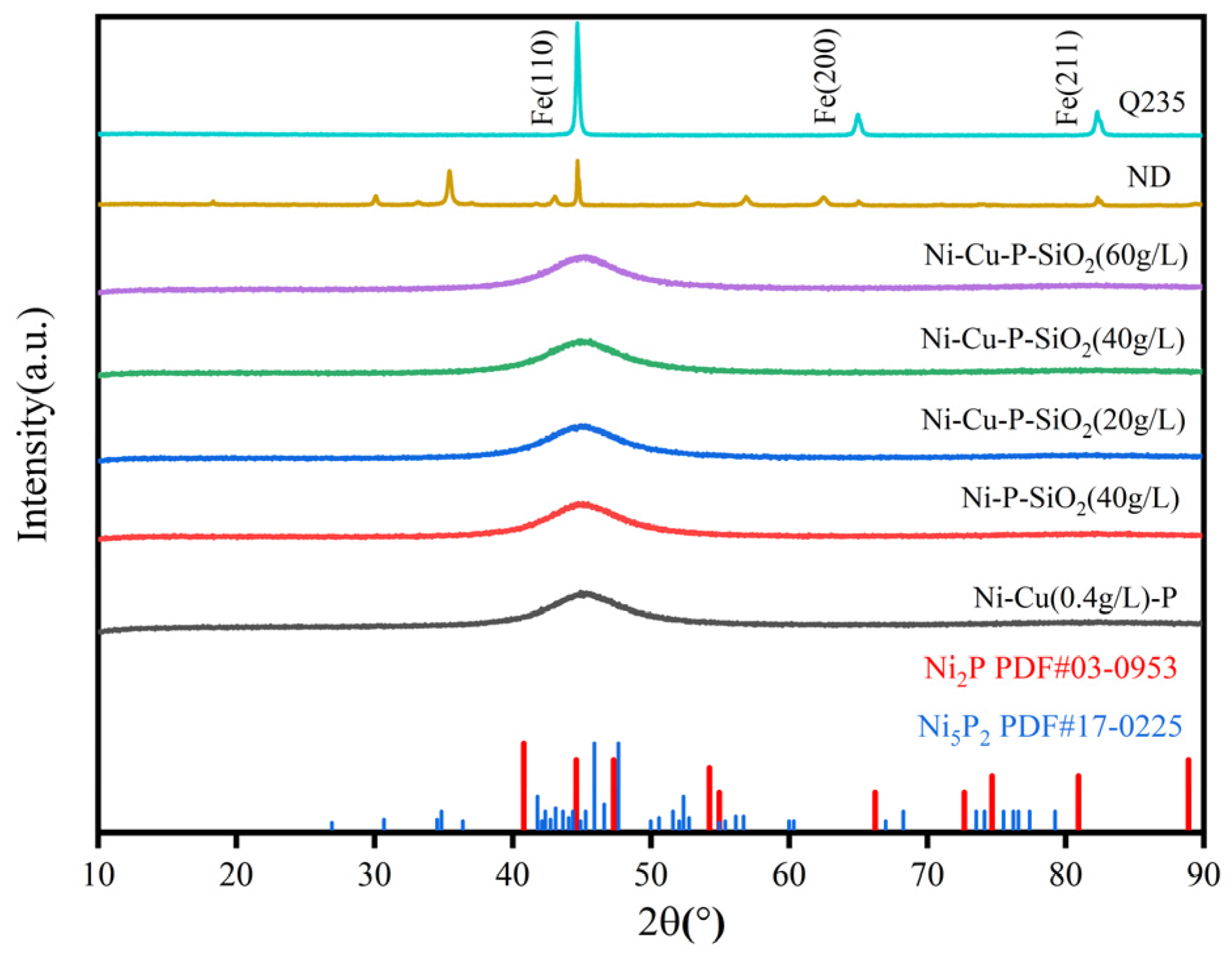
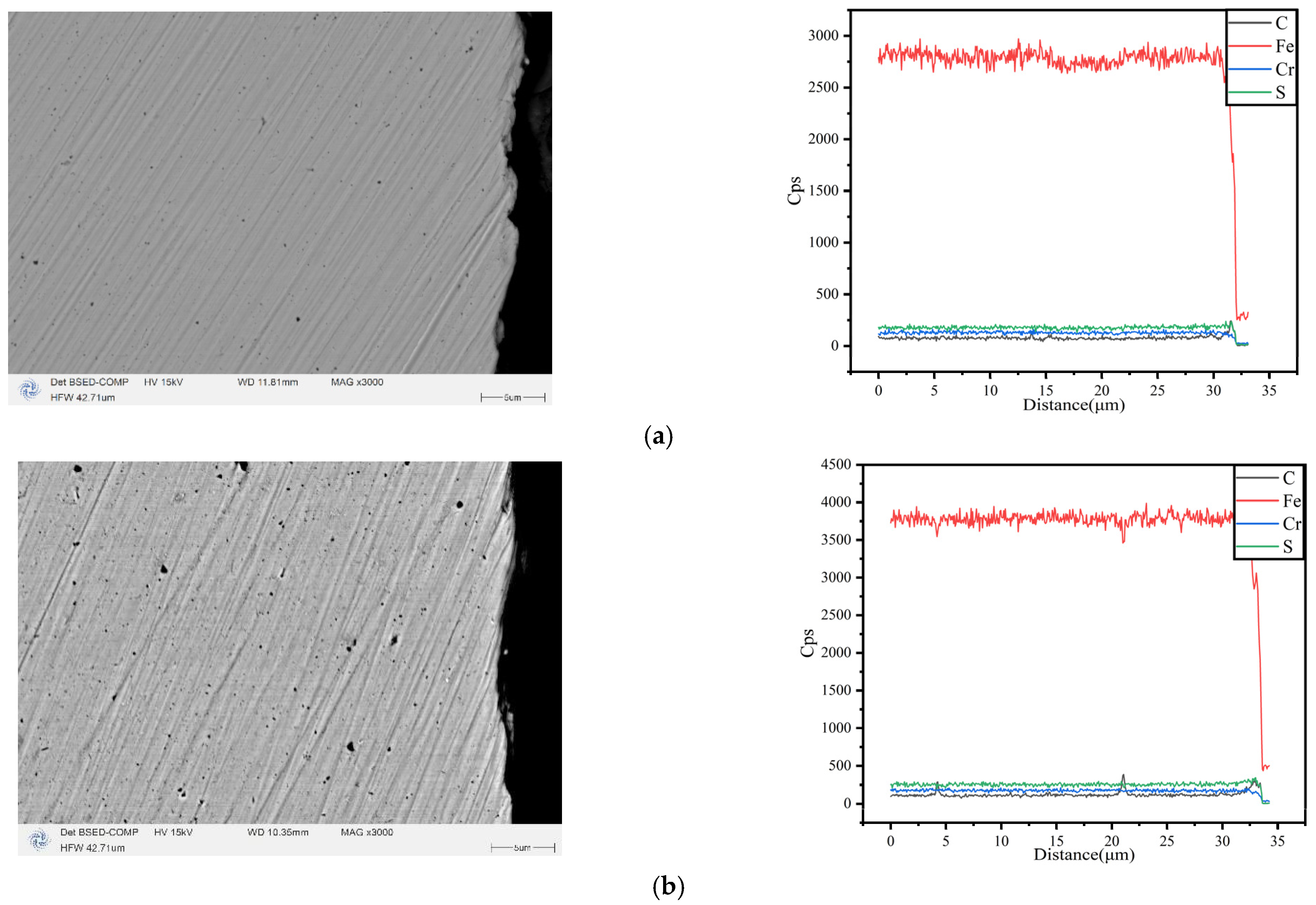
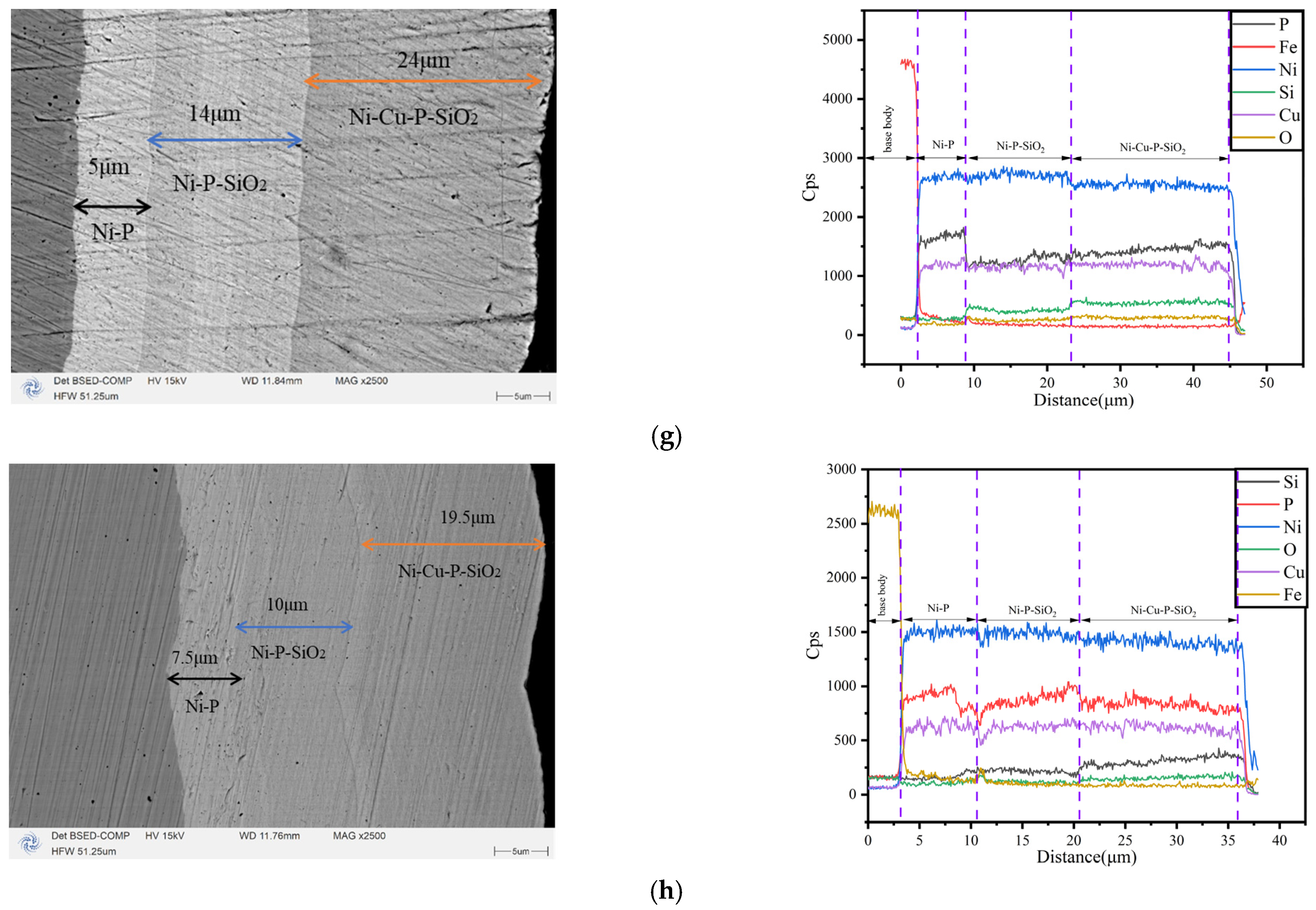
3.1. Characterization of Morphology and Composition
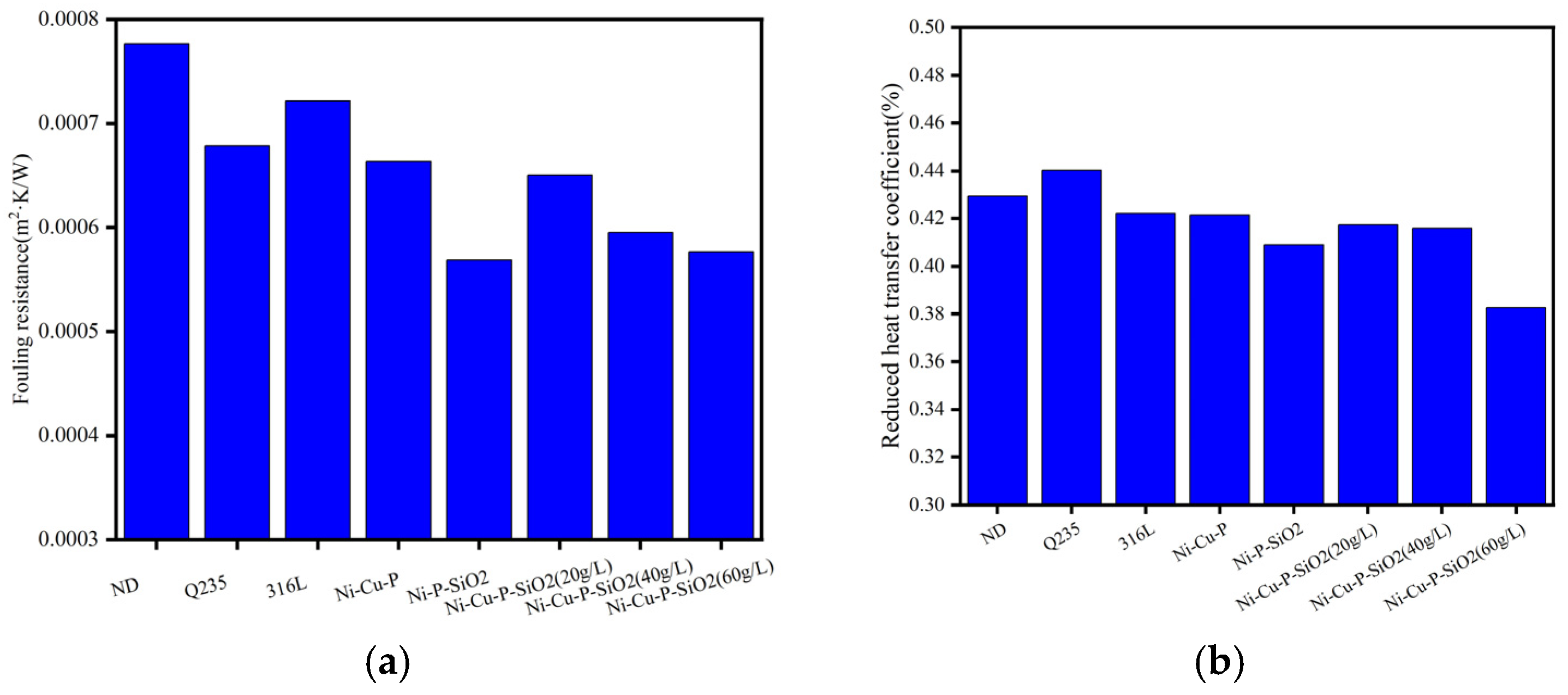
3.2. Antifouling Performance Analysis
3.3. Analysis of Corrosion Resistance
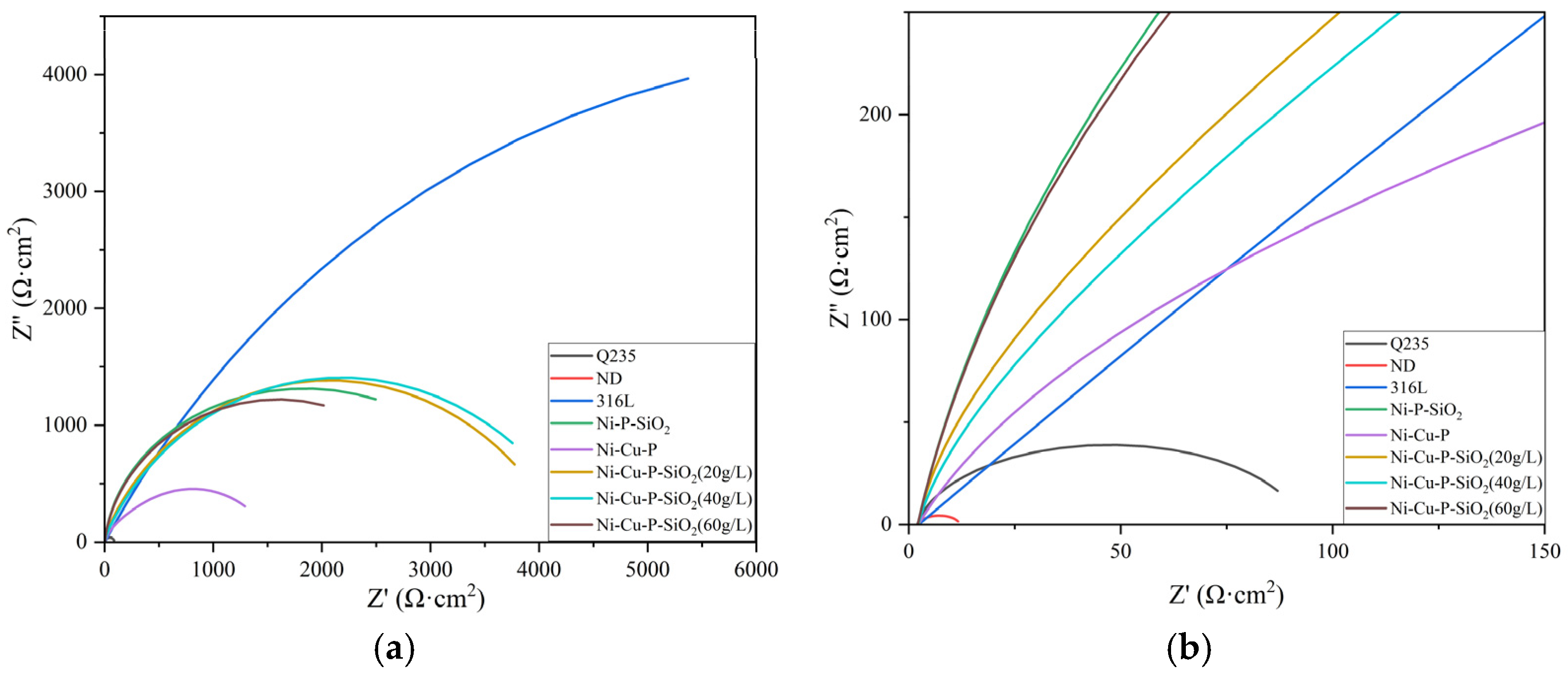
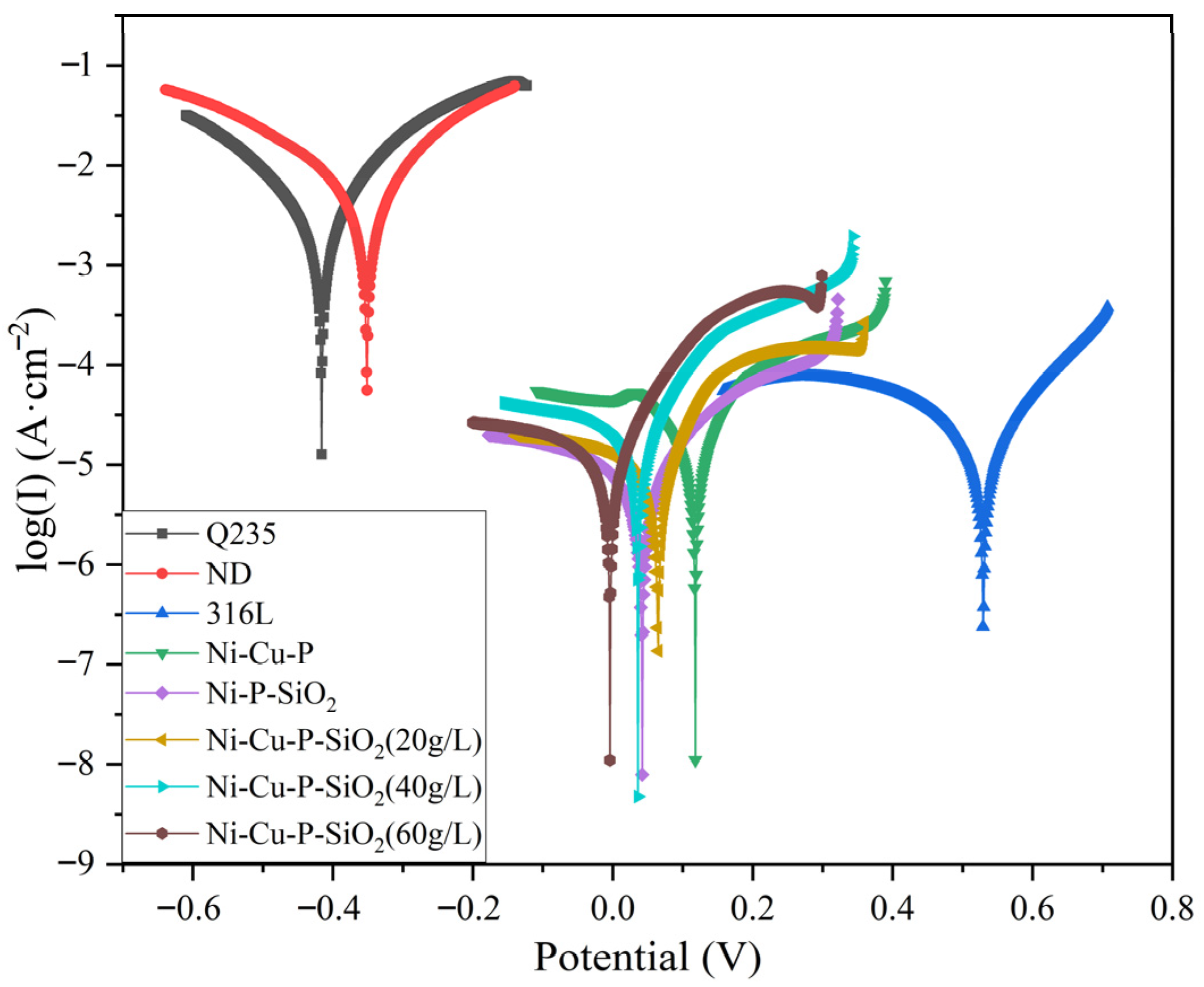
3.3.1. Electrochemical Test
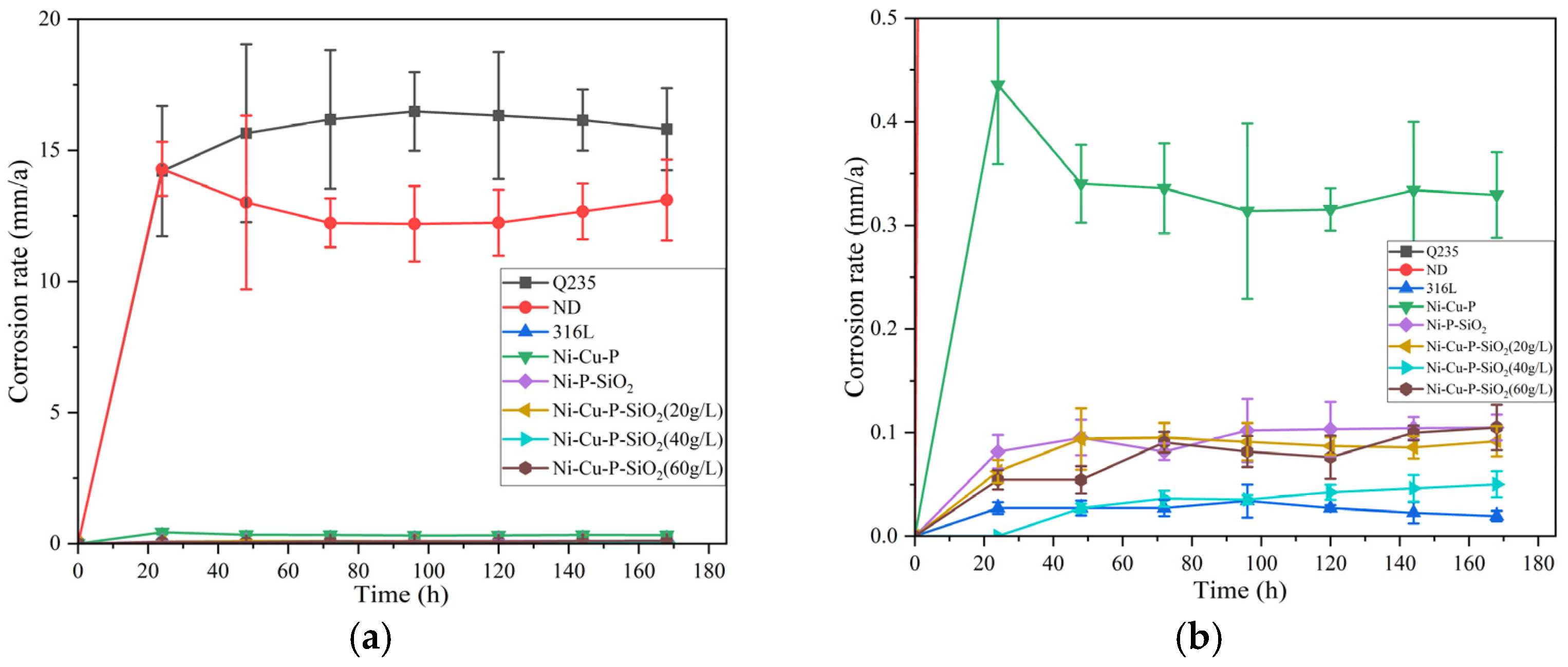
3.3.2. Soaking Experiment
3.3.3. Analysis of Corrosion Resistance Performance in Low-Temperature Flue Gas Environment
3.4. Analysis of the Coatings’ Anti-Corrosion Fouling Mechanisms
4. Conclusions
- The surface morphology and elemental content of the coating were analyzed by SEM and EDS. It can be observed that the surface of the coating is smooth and coherent, the plating solution is well dispersed, and there is no peeling on the whole. By adjusting the concentration of SiO2 in the plating solution, the SiO2 content in the coating can be controlled, indicating that the preparation method is repeatable.
- The corrosion resistance of the coatings was analyzed by means of the polarization curve method, impedance spectroscopy, and hanging corrosion experiments. Among the coating materials, the corrosion voltages and current densities of the three Ni-Cu-P-SiO2 coatings were generally better than those of the other materials; from the AC impedance experiments, the AC impedance of the Ni-Cu-P-SiO2 coatings was larger, which indicated that they could slow down the corrosion more effectively in the face of acidic environments; the hanging chip corrosion experiments yielded the same conclusions. Among the coatings, the Ni-Cu-P-SiO2 (40 g/L) coating has the lowest average corrosion rate, which also indicates that it has the best corrosion resistance among all the coatings.
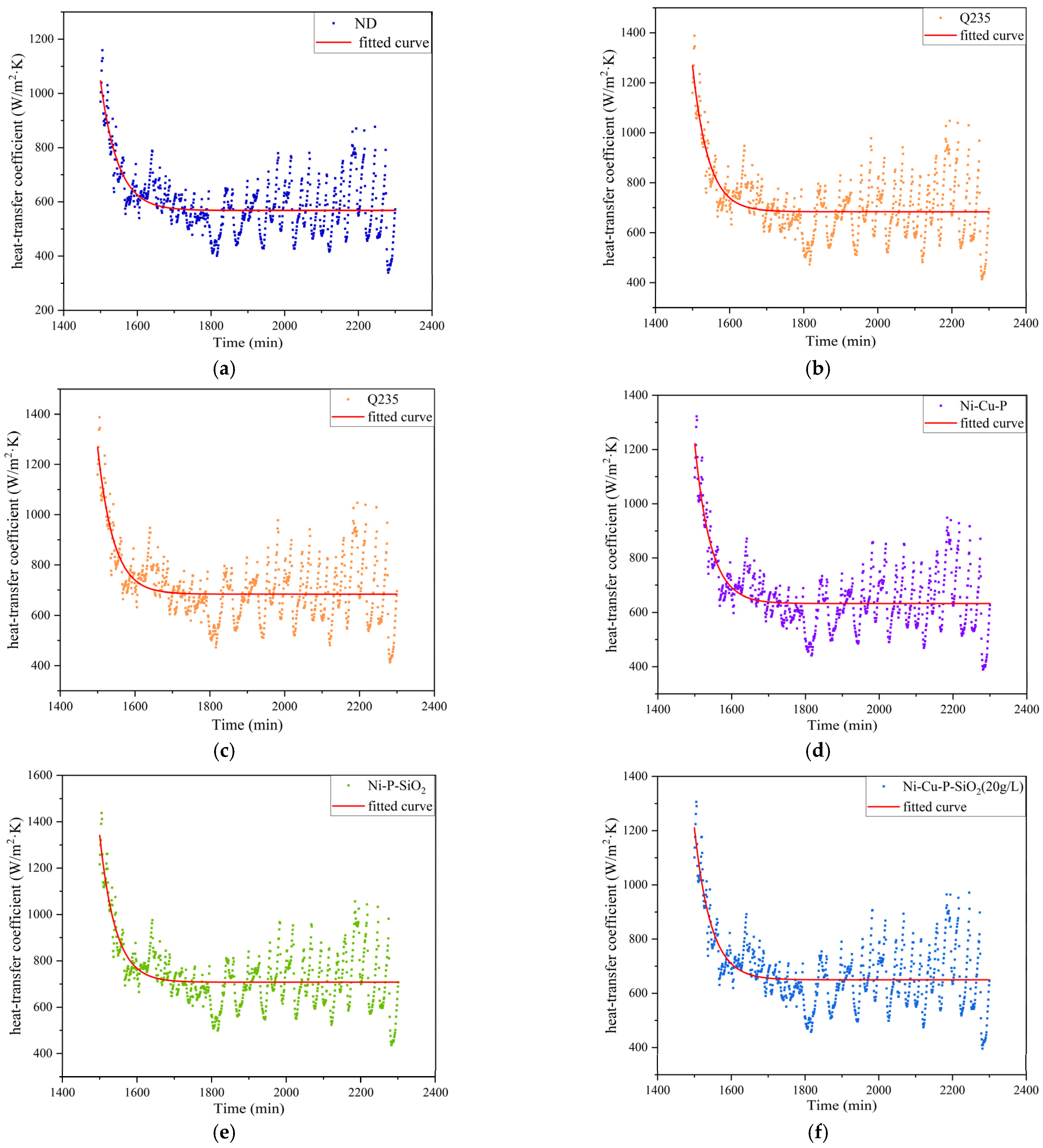
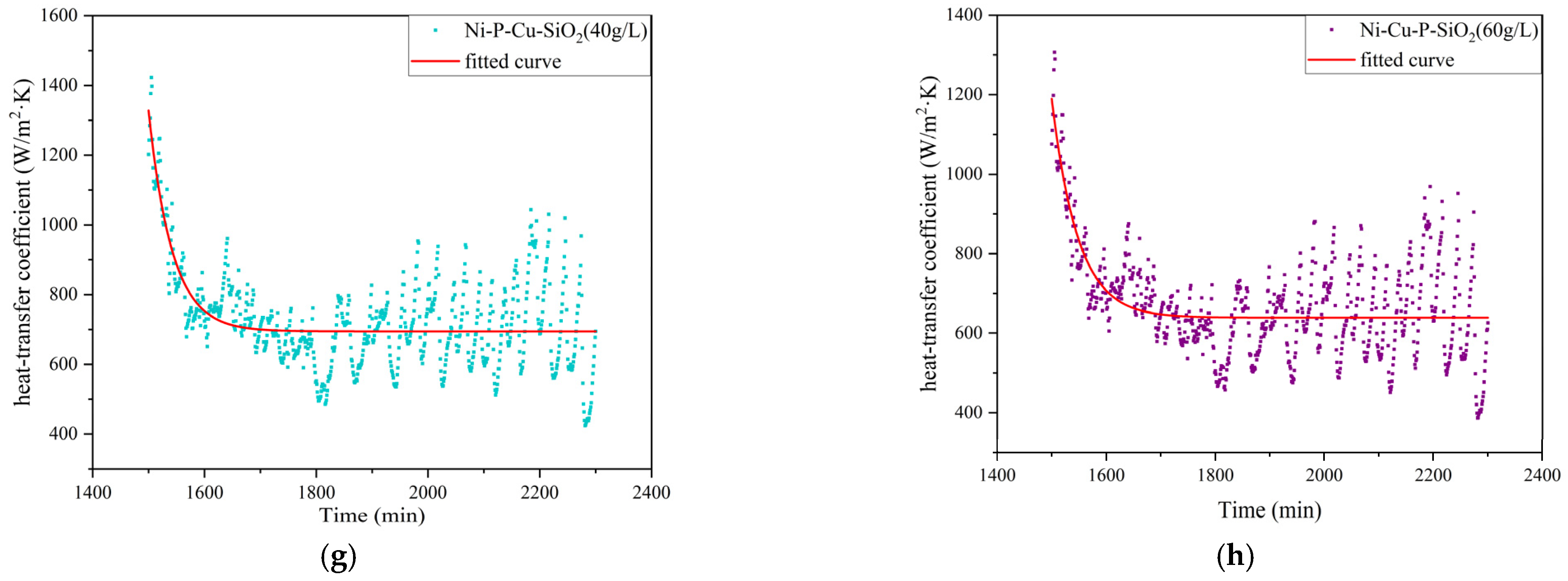
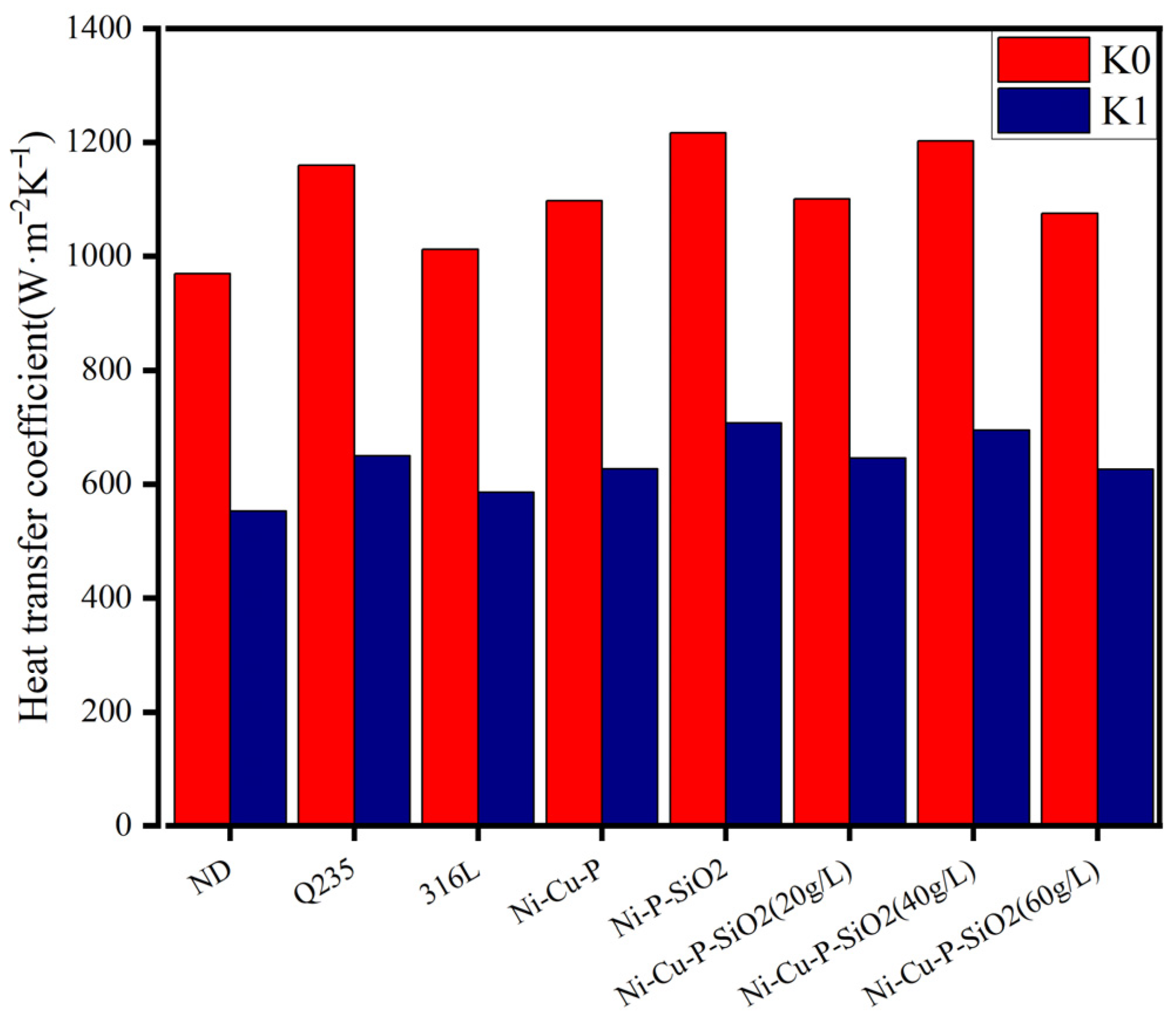
- The performance of the above materials against ash and corrosion was evaluated by building an experimental platform to simulate the flue gas environment. The results show that the thermal resistance and heat transfer attenuation coefficient of the materials without coatings are generally worse than those with coatings; Cu improves heat transfer capacity, and SiO2 particles reduce corrosion and dust deposition. Therefore, the three kinds of Ni-Cu-P-SiO2 coating are superior to the Ni-Cu-P/Ni-P-SiO2 coatings in terms of their thermal resistance and heat transfer attenuation coefficient during stable operation. The results of the profile scanning proved that no significant corrosion occurred below the dew point, which is highly beneficial to the recovery of waste heat from flue gas when the coating contains 3.34 wt% Cu and 2.16 wt% SiO2 (corresponding to 1.01 wt% Si), confirming the synergistic effect of Cu and SiO2 in mitigating acid–ash coupling degradation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Xie, F.; Li, H.; Zheng, C.; Zhao, X. Understanding inter-term fossil energy consumption pathways in China based on sustainable development goals. Geosci. Front. 2024, 15, 101687. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, S. Can “energy saving and emission reduction” demonstration city selection actually contribute to pollution abatement in China? Sustain. Prod. Consum. 2021, 27, 1882–1902. [Google Scholar] [CrossRef]
- Nakaishi, T.; Nagashima, F.; Kagawa, S.; Nansai, K.; Chatani, S. Quantifying the health benefits of improving environmental efficiency: A case study from coal power plants in China. Energy Econ. 2023, 121, 106672. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Wang, Y.; Wang, J. Analysis of an improved economizer system for active control of the coal-fired boiler flue gas temperature. Energy 2019, 170, 185–198. [Google Scholar] [CrossRef]
- Jeong, K.; Levy, E.K. Theoretical prediction of sulfuric acid condensation rates in boiler flue gas. Int. J. Heat Mass Transf. 2012, 55, 8010–8019. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Z.-Y.; Liao, Q.; Gu, Y.-H.; Ding, Y.-D. Heat transfer and pressure drop characteristics of 3-D finned tube heat exchanger in ash-laden flue gas. Int. J. Heat Mass Transf. 2021, 170, 120982. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, Y.; Ding, Y.; Liao, Q.; Zhu, X.; Cheng, M. Experimental research on the effect of acid and ash deposition on heat transfer characteristics of PTFE-coated 3-D finned tube. Int. J. Therm. Sci. 2023, 193, 108544. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, X.; Li, Y. Review of flue gas acid dew-point and related low temperature corrosion. J. Energy Inst. 2020, 93, 1666–1677. [Google Scholar] [CrossRef]
- Wei, W.; Sun, F.; Shi, Y.; Ma, L. Experimental research of fouling layer and prediction of acid condensation outside heat exchanger used in coal-fired boiler. Appl. Therm. Eng. 2018, 131, 486–496. [Google Scholar] [CrossRef]
- Han, H.; He, Y.L.; Tao, W.Q. A numerical study of the deposition characteristics of sulfuric acid vapor on heat exchanger surfaces. Chem. Eng. Sci. 2013, 101, 620–630. [Google Scholar] [CrossRef]
- Chen, H.; Pan, P.; Wang, Y.; Zhao, Q. Field study on the corrosion and ash deposition of low-temperature heating surface in a large-scale coal-fired power plant. Fuel 2017, 208, 149–159. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, Q.; Ding, Y.; Zhu, X.; Fu, Q.; Zhang, J.; Zhao, S. A Review on Ash Deposition on Heat Exchanger Surface in Coal-fired Boilers: Mechanisms and Influence Factors. Resour. Environ. Eng. 2019, 39, 1349–4365. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Chu, D.; Sun, F.; Guo, Z. An experimental study of ash accumulation in flue gas. Adv. Powder Technol. 2016, 27, 1473–1480. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, C.; Ma, Z.; Guo, Z. Experimental investigation of heat transfer with ash deposition in ultra-low temperature WHRS of coal-fired power plant. Appl. Therm. Eng. 2017, 123, 1181–1189. [Google Scholar] [CrossRef]
- Wang, J.L.; Tao, Y.B.; Liu, J. Numerical study on acid condensation and corrosion characteristics of three-dimensional finned tube surface. Chem. Eng. Sci. 2021, 238, 116600. [Google Scholar] [CrossRef]
- Tang, S.-Z.; He, Y.-L.; Wang, F.-L.; Tao, Y.-B. Parametric study on fouling mechanism and heat transfer characteristics of tube bundle heat exchangers for reducing fouling considering the deposition and removal mechanisms. Fuel 2018, 211, 301–311. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xiao, J.; Yan, J.; Fan, H.; Sun, L.; Xue, L.; Tang, Z. Time-dependent corrosion behavior of electroless Ni–P coating in H2S/Cl− environment. Int. J. Hydrogen Energy 2021, 46, 11849–11864. [Google Scholar] [CrossRef]
- Cao, S.; Zou, M.; Zhao, B.; Gao, H.; Wang, G. Investigation of corrosion and fouling resistance of Ni–P-nanoparticles composite coating using online monitoring technology. Int. J. Therm. Sci. 2023, 184, 107953. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, D.; Tang, D.; Han, E.; Wang, J. Study on corrosion behavior of Ni–P/Ni–Cu–P superhydrophobic composite coatings preparation on L360 steel by two-step method. J. Mater. Res. Technol. 2023, 23, 3035–3047. [Google Scholar] [CrossRef]
- Zarebidaki, A.; Akbarpour, M. Corrosion and wear behavior of electroless Ni-P-Cu coatings containing 8 and 18 wt% Cu. Surf. Coat. Technol. 2024, 492, 131228. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Y.; Huang, W.; Ji, Y.; Yang, W.; Yin, X.; Liu, Y.; Ling, X. Corrosion behavior of Ni-P-nano-Al2O3 composite coating in the presence of anionic and cationic surfactants. Surf. Coat. Technol. 2017, 310, 122–128. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Gyawali, G.; Kshetri, Y.K.; Choi, J.-H.; Lee, S.W. Influence of SiC and TiC nanoparticles reinforcement on the microstructure, tribological, and scratch resistance behavior of electroless Ni–P coatings. Nanotechnology 2019, 31, 104001. [Google Scholar] [CrossRef]
- Islam, M.; Azhar, M.R.; Fredj, N.; Burleigh, T.D.; Oloyede, O.R.; Almajid, A.A.; Shah, S.I. Influence of SiO2 nanoparticles on hardness and corrosion resistance of electroless Ni–P coatings. Surf. Coat. Technol. 2015, 261, 141–148. [Google Scholar] [CrossRef]
- Dong, D.; Chen, X.; Xiao, W.; Yang, G.; Zhang, P. Preparation and properties of electroless Ni–P–SiO2 composite coatings. Appl. Surf. Sci. 2009, 255, 7051–7055. [Google Scholar] [CrossRef]
- Sassi, W.; Dhouibi, L.; Berçot, P.; Rezrazi, M. The effect of SiO2 nanoparticles dispersion on physico-chemical properties of modified Ni–W nanocomposite coatings. Appl. Surf. Sci. 2015, 324, 369–379. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Sun, B.; Zhang, C.; Zhao, H.; Qu, Z.; Xu, X. Preparation and characterization of Ni-P-Al2O3-PTFE nanocomposite coatings by unidirectional jet electrodeposition. Mater. Today Commun. 2023, 35, 105647. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H.; Zhu, Z.; Jen, T.; Peng, Y. Experimental study on the anti-fouling effects of Ni–Cu–P-PTFE deposit surface of heat exchangers. Appl. Therm. Eng. 2014, 68, 20–25. [Google Scholar] [CrossRef]
- Ardakani, S.R.; Afshar, A.; Sadreddini, S.; Ghanbari, A. Characterization of Ni-P-SiO2-Al2O3 nano-composite coatings on aluminum substrate. Mater. Chem. Phys. 2017, 189, 207–214. [Google Scholar] [CrossRef]
- Monaco, L.; Avramovic-Cingara, G.; Palumbo, G.; Erb, U. Corrosion behaviour of electrodeposited nanocrystalline nickel-iron (NiFe) alloys in dilute H2SO4. Corros. Sci. 2018, 130, 103–112. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Tian, Z.; Yuan, M.; Ma, X. Effect of copper content on the properties of electroless Ni–Cu–P coatings prepared on magnesium alloys. Appl. Surf. Sci. 2015, 356, 289–293. [Google Scholar] [CrossRef]
- Li, M.-J.; Tang, S.-Z.; Wang, F.-L.; Zhao, Q.-X.; Tao, W.-Q. Gas-side fouling, erosion and corrosion of heat exchangers for middle/low temperature waste heat utilization: A review on simulation and experiment. Appl. Therm. Eng. 2017, 126, 737–761. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Yue, M.; Yan, M.; Shi, Y. Heat transfer and ash deposition performance of heat exchange surface in waste incineration flue gas. Int. J. Heat Mass Transf. 2020, 155, 119691. [Google Scholar] [CrossRef]
- Verma, C.; Rhee, K.Y.; Alfantazi, A. Functionalized epoxy resins for enhanced interface properties and corrosion resistance: Tailoring of surface and interface properties and performance. Appl. Surf. Sci. Adv. 2025, 25, 100685. [Google Scholar] [CrossRef]
- Huang, T.; Su, H.; Zhou, Y.; Wang, J.; Zhang, L.; Chen, K. Comparison of the corrosion behavior of four Fe-Cr-Ni austenitic alloys in supercritical water. J. Nucl. Mater. 2025, 603, 155409. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, S.; He, J.; Liu, B.; Chen, C.; Feng, J.; Zhang, X.; Fang, W.; Yin, F. The Effect of Ni Interlayer on the Hot-Rolled and Quenched Stainless Steel Clad Plate. Materials 2020, 13, 5455. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, S.P.; Xavier, J.R. Evaluation of corrosion protection performance and mechanical properties of epoxy-triazole/graphene oxide nanocomposite coatings on mild steel. J. Mater. Sci. 2021, 56, 7094–7110. [Google Scholar] [CrossRef]
- Davoodi, D.; Emami, A.H.; Vaghefi, S.M.M.; Omidi, M.; Bakhsheshi-Rad, H.R. Deposition of electroless Ni–Cu–P coatings on L80 steel substrates and the effects of coatings thickness and heat treatment on the corrosion resistance. Int. J. Press. Vessel. Pip. 2022, 200, 104823. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Matsuda, K.; Zou, Y. Microstructure evolution and corrosion resistance of Ni–Cu–P amorphous coating during crystallization process. Appl. Surf. Sci. 2019, 484, 835–844. [Google Scholar] [CrossRef]
- Aryanto, D.; Hariyati, H.; Karo, P.K.; Wismogroho, A.S.; Widayatno, W.B.; Basyir, A.; Darsono, N.; Herbirowo, S.; Rochman, N.T.; Noviyanto, A. Microstructure and oxidation behavior of an Al–Ni–Cr–Cu–MoO3–SiO2 composite coating on low-carbon steel. Mater. Chem. Phys. 2021, 261, 124250. [Google Scholar] [CrossRef]
- Islam, M.; Azhar, M.R.; Khalid, Y.; Khan, R.; Abdo, H.S.; Dar, M.A.; Oloyede, O.R.; Burleigh, T.D. Electroless Ni-P/SiC Nanocomposite Coatings with Small Amounts of SiC Nanoparticles for Superior Corrosion Resistance and Hardness. J. Mater. Eng. Perform. 2015, 24, 4835–4843. [Google Scholar] [CrossRef]
- GB/T 39534-2020; Corrosion of Metals and Alloys—Method for Determination of the Uniform Corrosion Rate of Stainless Steels and Nickel Based Alloys in Liquids. State Administration for Market Regulation and Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- Sadreddini, S.; Afshar, A.; Jazani, M.A. Tribological properties of Ni–P–SiO2 nanocomposite coating on aluminum. Colloid J. 2015, 77, 628–634. [Google Scholar] [CrossRef]
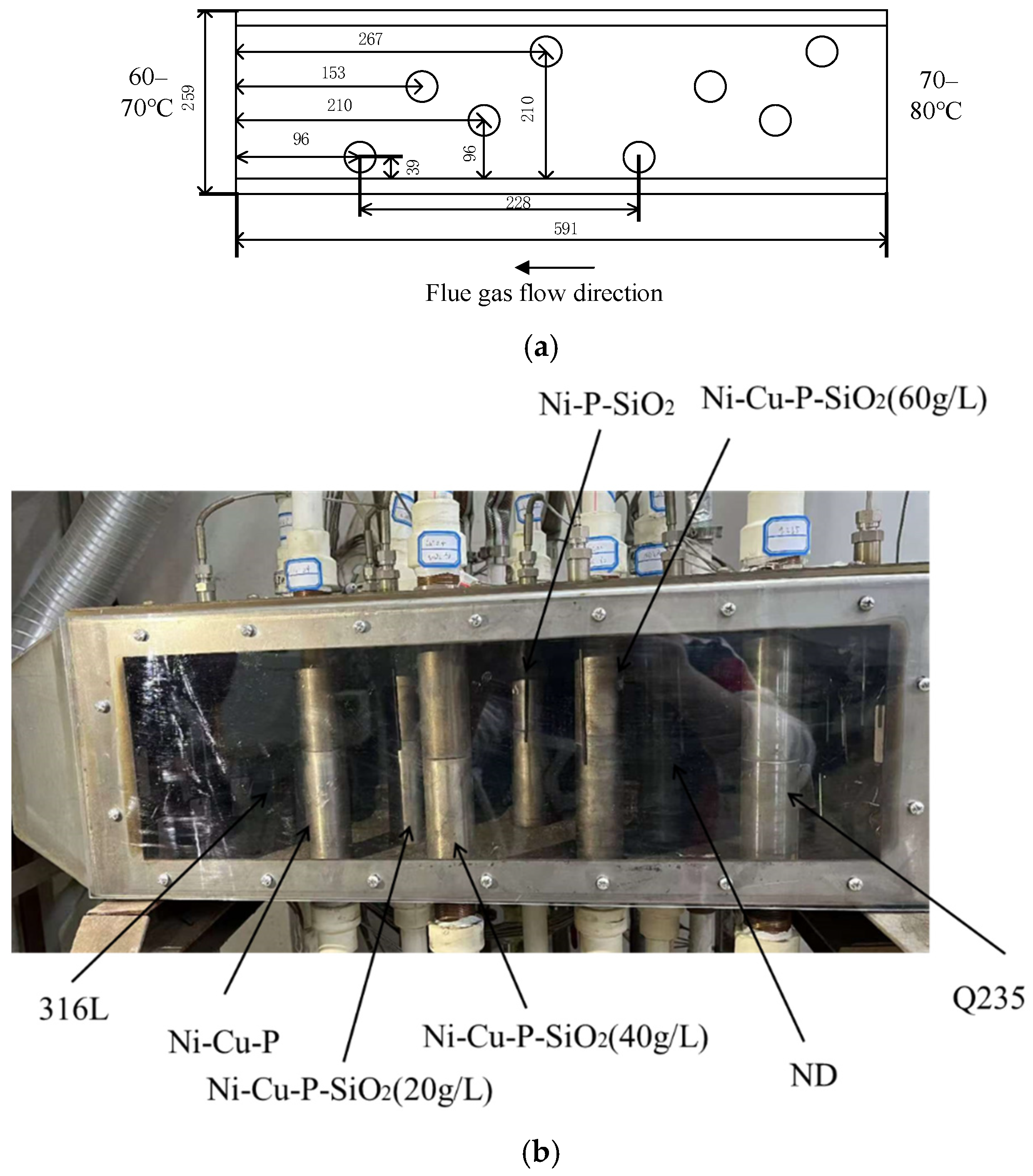




| Material | Q235 | ND | 316L |
|---|---|---|---|
| C | 0.12–0.20 | ≤0.10 | ≤0.030 |
| Si | ≤0.30 | 0.20–0.40 | ≤1.00 |
| Mn | 0.30–0.70 | 0.35–0.65 | ≤2.00 |
| P | ≤0.045 | ≤0.10 | ≤0.045 |
| S | ≤0.045 | ≤0.10 | ≤0.030 |
| Cu | ≤0.30 | 0.25–0.45 | - |
| Ni | ≤0.30 | - | 10.00–14.00 |
| Cr | ≤0.30 | 0.70–1.20 | 16.00–18.00 |
| Mo | - | - | 2.00–3.00 |
| Sb | - | ≤0.10 | - |
| Bath Composition | Concentration | Parameter | Value |
|---|---|---|---|
| NaOH | 15–25 g/L | Temperature | 20–30 °C |
| Na2CO3 | 15–25 g/L | Washing time | 5–10 min |
| Na3PO4 | 20–30 g/L | ||
| Na2SiO3 | 5–10 g/L |
| Ni-Cu-P | Ni-P-SiO2 | Ni-Cu-P-SiO2 SiO2 (20 g/L) | Ni-Cu-P-SiO2 SiO2 (40 g/L) | Ni-Cu-P-SiO2 SiO2 (60 g/L) | |
|---|---|---|---|---|---|
| NiSO4⋅6H2O | 15–25 g/L | 15–25 g/L | 15–25 g/L | 15–25 g/L | 15–25 g/L |
| NaH2PO2⋅H2O | 15–25 g/L | 15–25 g/L | 15–25 g/L | 15–25 g/L | 15–25 g/L |
| C2H3NaO2 | 10–15 g/L | 10–15 g/L | 10–15 g/L | 10–15 g/L | 10–15 g/L |
| C6H5Na3O7·2H2O | 10–15 g/L | 10–15 g/L | 10–15 g/L | 10–15 g/L | 10–15 g/L |
| CuSO4·5H2O | 0.4 g/L | - | 0.4 g/L | 0.4 g/L | 0.4 g/L |
| Silica sol (ω = 30%) | - | 40 g/L | 20 g/L | 40 g/L | 60 g/L |
| pH | 7.0–8.0 | 7.0–8.0 | 7.0–8.0 | 7.0–8.0 | 7.0–8.0 |
| Temperature | 65–75 °C | 65–75 °C | 65–75 °C | 65–75 °C | 65–75 °C |
| Intermediate Ni-P layer | Yes | Yes | Yes | Yes | Yes |
| Intermediate Ni-P-SiO2 layer | No | No | Yes | Yes | Yes |
| Ni-Cu-P | Ni-P-SiO2 | Ni-Cu-P-SiO2 (20 g/L) | Ni-Cu-P-SiO2 (40 g/L) | Ni-Cu-P-SiO2 (60 g/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
| Ni | 86.66 | 79.53 | 89.01 | 80.78 | 86.18 | 78.55 | 85.42 | 77.65 | 85.42 | 77.34 |
| P | 10.26 | 17.86 | 9.24 | 15.90 | 10.45 | 18.05 | 10.23 | 17.62 | 9.61 | 16.49 |
| Cu | 3.08 | 2.61 | 2.85 | 2.40 | 3.34 | 2.80 | 3.06 | 2.56 | ||
| Si | 1.75 | 3.33 | 0.52 | 0.99 | 1.01 | 1.92 | 1.90 | 3.60 | ||
| C | H | N | O | S |
|---|---|---|---|---|
| 80.46 | 4.16 | 0.76 | 13.97 | 0.65 |
| K2O | Na2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 0.36 | 0.12 | 50.93 | 32.72 | 7.76 | 2.36 | 0.85 | 2.16 | 0.85 | 0.14 |
| Sample | Rs (Ω·cm2) | Rf (Ω·cm2) | CPEf | Rct (Ω·cm2) | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·Sn) | n | Y0 (Ω−1·cm−2·Sn) | n | ||||
| Q235 | 2.016 | / | / | / | 90.97 | 0.000151 | 0.901 |
| ND | 2.370 | / | / | / | 9.641 | 0.00104 | 0.942 |
| 316L | 2.339 | / | / | / | 14,357 | 0.000653 | 0.669 |
| Ni-Cu-P | 2.251 | 442.8 | 0.000744 | 0.823 | 1112 | 0.0155 | 0.656 |
| Ni-P-SiO2 | 2.104 | 2106 | 0.000484 | 0.932 | 1710 | 0.001430 | 0.701 |
| Ni-Cu-P-SiO2 (20 g/L) | 2.351 | 351.5 | 0.000064 | 0.952 | 3918 | 0.000155 | 0.642 |
| Ni-Cu-P-SiO2 (40 g/L) | 2.213 | 336.5 | 0.000207 | 0.918 | 4130 | 0.000337 | 0.633 |
| Ni-Cu-P-SiO2 (60 g/L) | 2.105 | 1916 | 0.002207 | 0.932 | 1324 | 0.003435 | 0.710 |
| Sample | Rs | Rf | CPEf | Rct | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 | n | Y0 | n | ||||
| Q235 | 0.875 | / | / | / | 1.382 | 0.935 | 0.556 |
| ND | 0.396 | / | / | / | 0.585 | 2.453 | 0.431 |
| 316L | 2.416 | / | / | / | 8.812 | 1.333 | 0.605 |
| Ni-Cu-P | 0.656 | 5.986 | 0.777 | 0.591 | 3.661 | 3.267 | 2.213 |
| Ni-P-SiO2 | 0.642 | 8.419 | 0.681 | 0.396 | 8.855 | 8.226 | 15.178 |
| Ni-Cu-P-SiO2 (20 g/L) | 0.879 | 4.606 | 0.994 | 0.802 | 3.222 | 3.179 | 1.663 |
| Ni-Cu-P-SiO2 (40 g/L) | 0.508 | 2.732 | 0.577 | 0.432 | 1.028 | 1.843 | 1.874 |
| Ni-Cu-P-SiO2 (60 g/L) | 0.533 | 10.851 | 0.671 | 0.404 | 9.552 | 9.032 | 4.904 |
| Sample | Ecorr (V, Mean) | Standard Deviation | Icorr (A·cm−2, Mean) | Standard Deviation |
|---|---|---|---|---|
| ND | −0.343 | 5.888 × 10−3 | 5.776 × 10−3 | 4.360 × 10−4 |
| Q235 | −0.424 | 5.907 × 10−3 | 3.562 × 10−3 | 4.825 × 10−4 |
| 316L | 0.527 | 1.70 × 10−3 | 1.707 × 10−5 | 2.704 × 10−6 |
| Ni-Cu-P | 0.106 | 9.031 × 10−3 | 4.220 × 10−5 | 8.300 × 10−6 |
| Ni-P-SiO2 | 0.036 | 5.888 × 10−3 | 1.038 × 10−5 | 6.341 × 10−7 |
| Ni-Cu-P-SiO2 (20 g/L) | 0.049 | 1.167 × 10−2 | 1.894 × 10−5 | 5.628 × 10−6 |
| Ni-Cu-P-SiO2 (40 g/L) | 0.033 | 2.494 × 10−3 | 2.287 × 10−5 | 8.523 × 10−6 |
| Ni-Cu-P-SiO2 (60 g/L) | −0.023 | 1.476 × 10−2 | 1.417 × 10−5 | 2.278 × 10−6 |
| Sample | Corrosion Rate (mm/a) | Standard Deviation |
|---|---|---|
| ND | 12.82 | 0.69 |
| Q235 | 15.83 | 0.71 |
| 316L | 0.03 | 0.01 |
| Ni-Cu-P | 0.34 | 0.04 |
| Ni-P-SiO2 | 0.1 | 0.01 |
| Ni-Cu-P-SiO2 (20 g/L) | 0.09 | 0.01 |
| Ni-Cu-P-SiO2 (40 g/L) | 0.03 | 0.02 |
| Ni-Cu-P-SiO2 (60 g/L) | 0.08 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Cao, S.; Zhao, B.; Yu, X. Preparation of Ni-P Composite Coatings and Study on the Corrosion Resistance and Antifouling Properties in Low-Temperature Flue Gas Environment. Materials 2025, 18, 3939. https://doi.org/10.3390/ma18173939
Lv C, Cao S, Zhao B, Yu X. Preparation of Ni-P Composite Coatings and Study on the Corrosion Resistance and Antifouling Properties in Low-Temperature Flue Gas Environment. Materials. 2025; 18(17):3939. https://doi.org/10.3390/ma18173939
Chicago/Turabian StyleLv, Changqi, Shengxian Cao, Bo Zhao, and Xingdong Yu. 2025. "Preparation of Ni-P Composite Coatings and Study on the Corrosion Resistance and Antifouling Properties in Low-Temperature Flue Gas Environment" Materials 18, no. 17: 3939. https://doi.org/10.3390/ma18173939
APA StyleLv, C., Cao, S., Zhao, B., & Yu, X. (2025). Preparation of Ni-P Composite Coatings and Study on the Corrosion Resistance and Antifouling Properties in Low-Temperature Flue Gas Environment. Materials, 18(17), 3939. https://doi.org/10.3390/ma18173939






