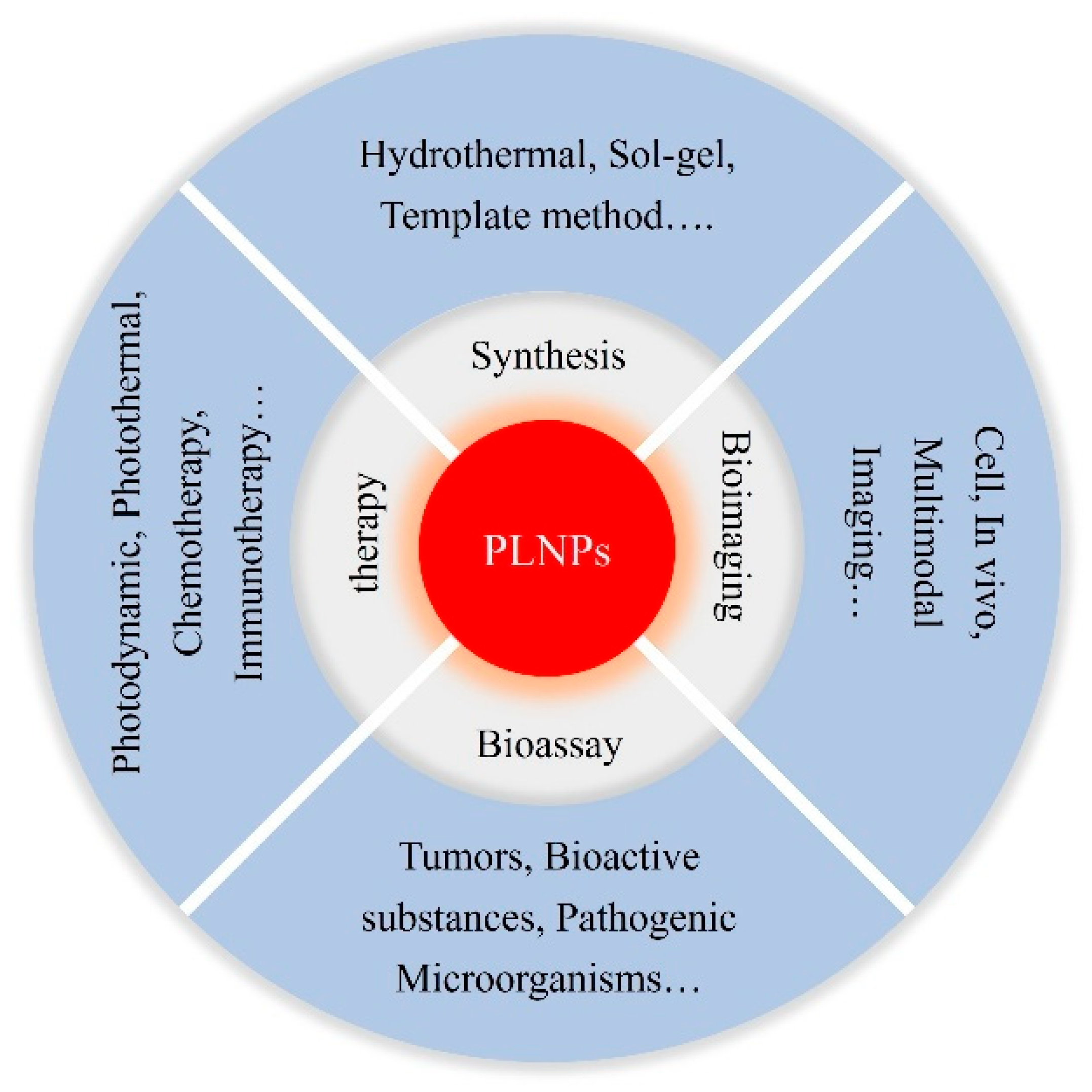
Research Progress of Persistent Luminescence Nanoparticles in Biological Detection Imaging and Medical Treatment
Abstract
1. Introduction
2. Overview of Long Persistent Luminescent Materials
2.1. Classification of LPLMs
2.1.1. Inorganic LPLMs
2.1.2. Organic LPLMs
2.1.3. Organic-Inorganic Hybrid LPLMs
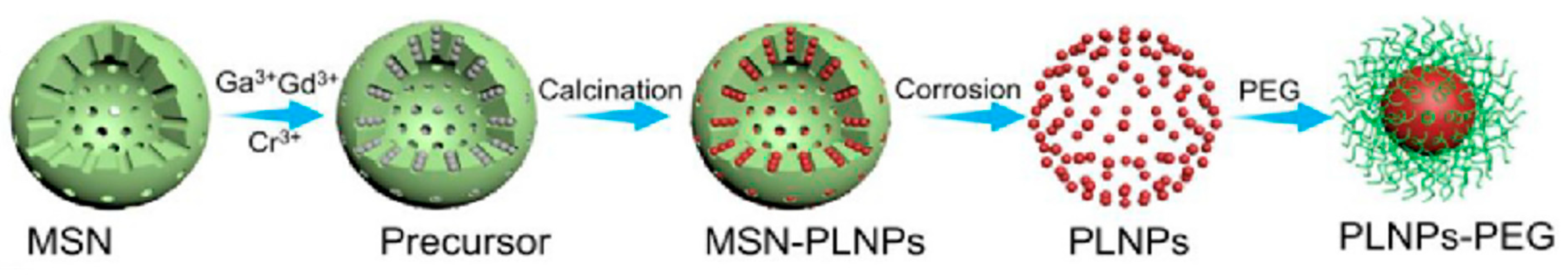
2.2. Synthesis of PLNPs
| Preparation Method | Reaction Condition | Particle Size | Afterglow Time | Advantage | Disadvantage | Ref. | |
|---|---|---|---|---|---|---|---|
| Solid Phase | High-Temperature Solid-State Method | High Temperature (˃1000 °C) | Micron scale | Long | Simple operation, low cost, superior afterglow properties | High temperature, large particles, irregular shape, easily caking, lacks control | [91,92,93] |
| Liquid Phase | Hydrothermal Method | Aqueous solution, high temperature and high pressure | Nano to micron scale | Short | Relatively mild, good dispersion, controllable shape and size | High facility request, low yield, long reaction time, low performance | [94,95,96] |
| Sol-Gel Method | Gel, high-temperature calcination | Nano to micron scale | Mezzo | Small size, good uniformity and performance, tunable | High cost, complex craft, highly reunited irregular shape, limited application | [97,98,99] | |
| Template Method | Using MSNs as templates, room temperature | Nano scale | Mezzo | Mild, small size, avoids reunions, tunable | Single type of template, poor dispersion | [100,101,102] |
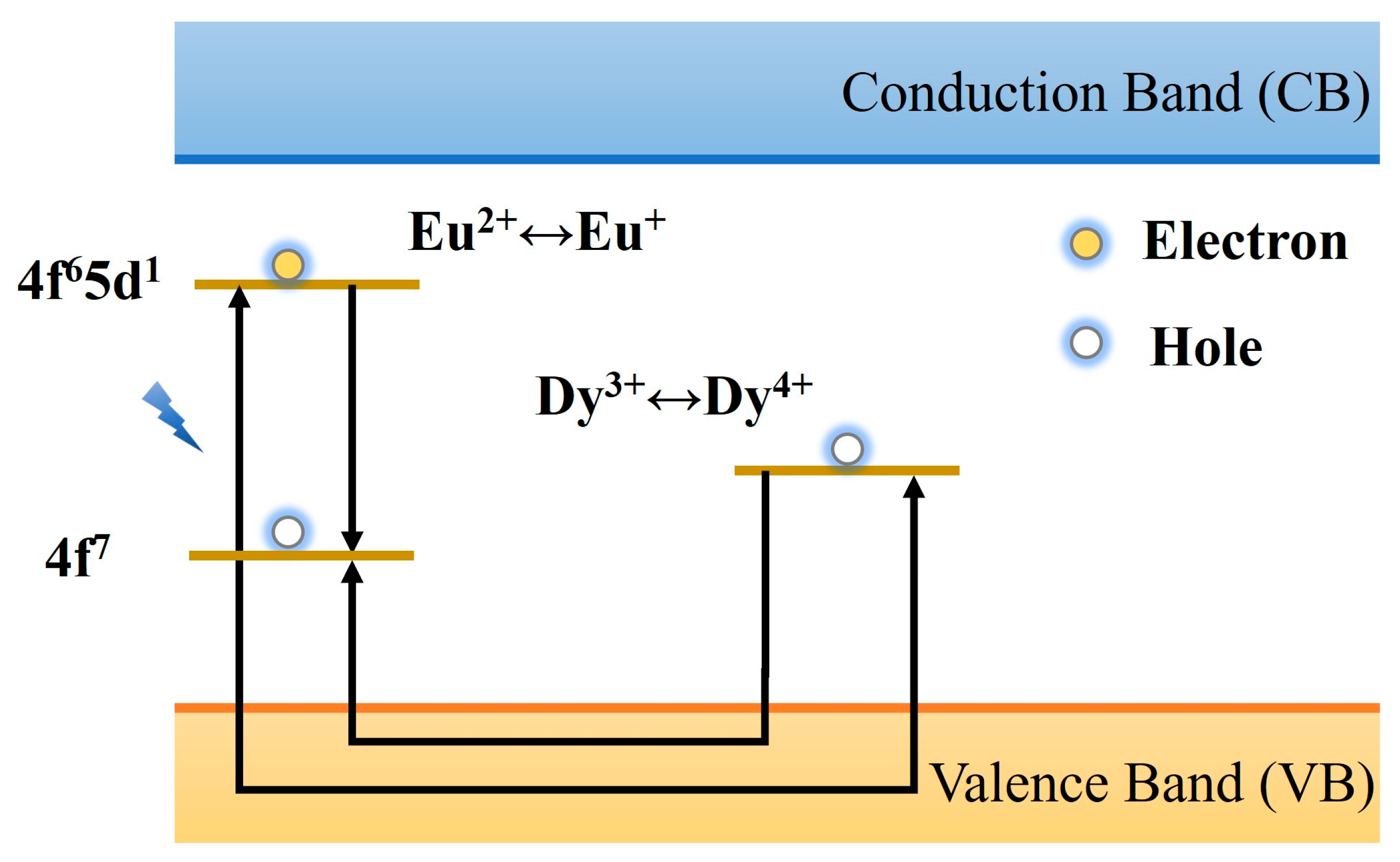
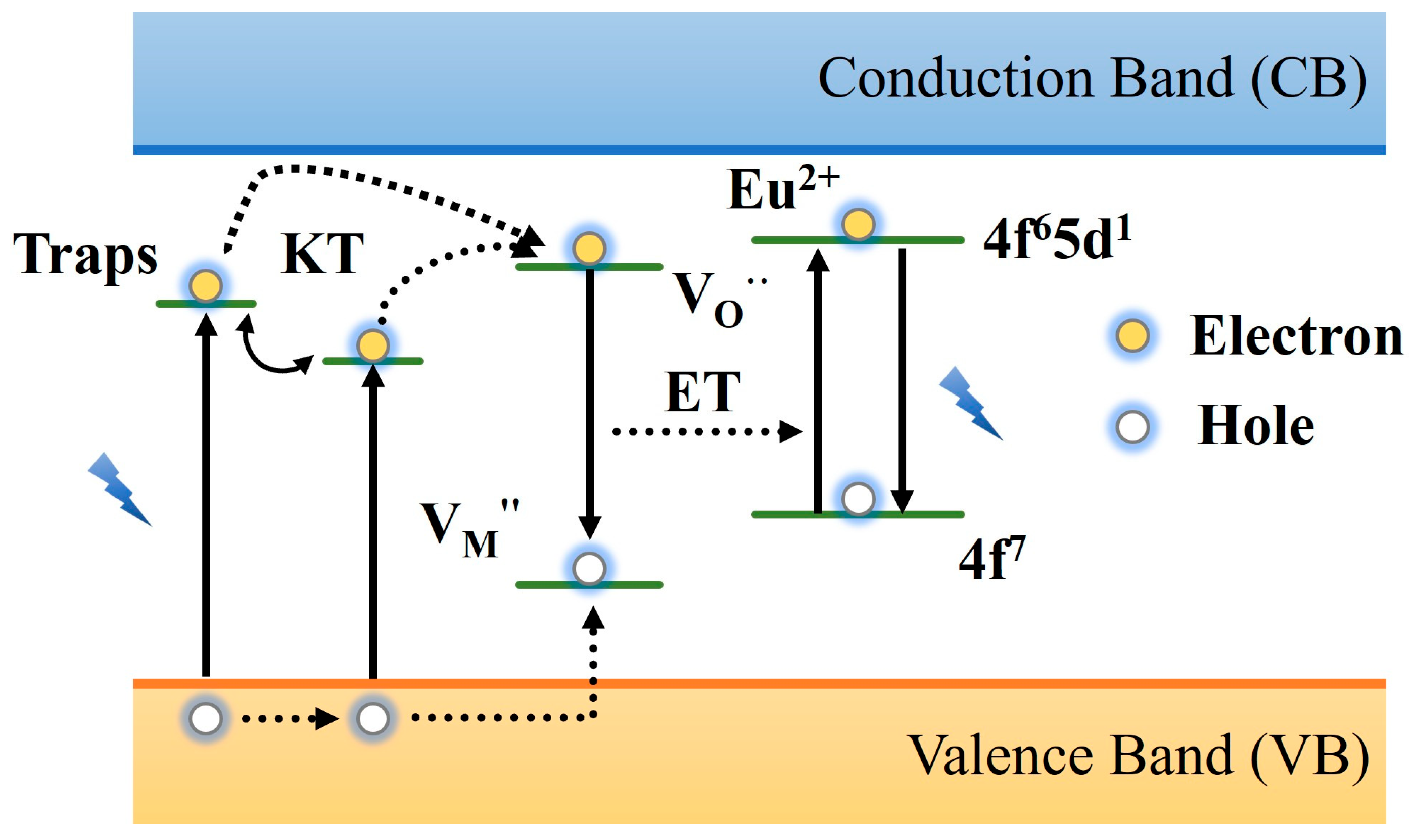
2.3. Luminescence Mechanism of PLNPs
3. Application of PLNPs in Biological Detection
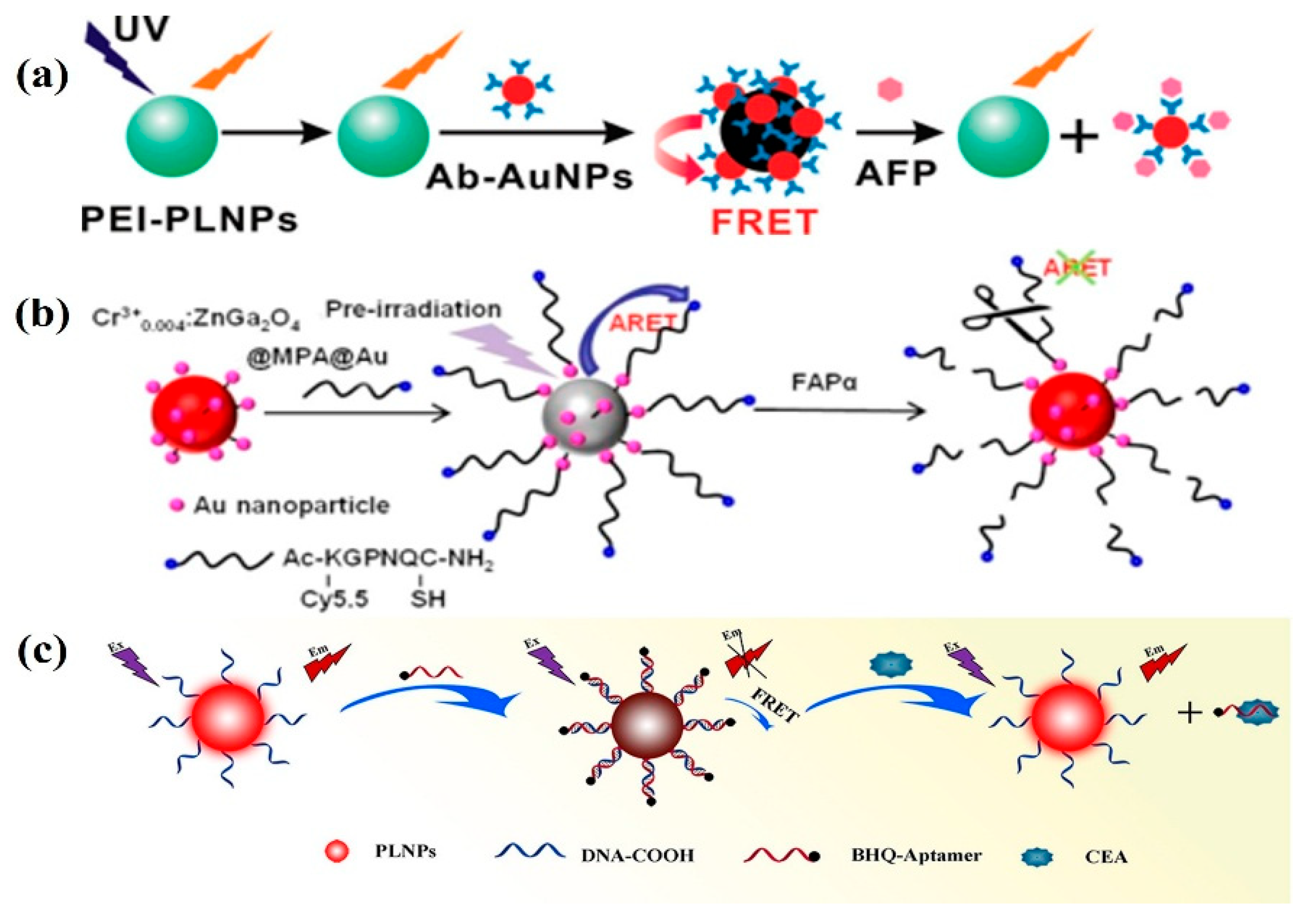
3.1. Tumor Marker Detection
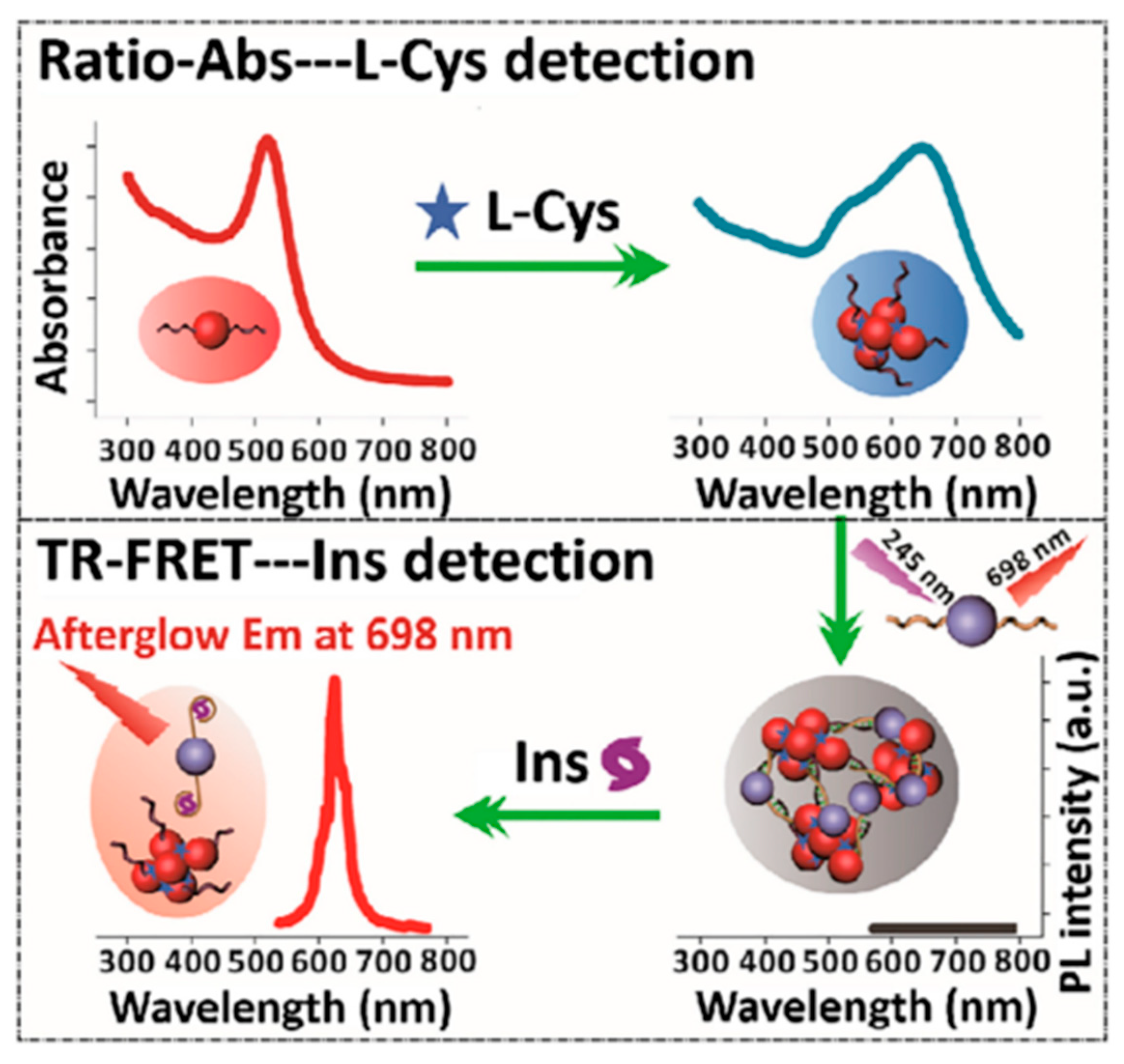
3.2. Detection of Bioactive Substances
3.3. Pathogenic Microorganism Detection
4. Application of PLNPs in Biological Imaging
4.1. Fingerprint Imaging
4.2. Cell Imaging
4.3. In Vivo Imaging
4.4. Multimodal Imaging
5. Application of PLNPs in Medical Treatment
5.1. Photodynamic Therapy
5.2. Photothermal Therapy
5.3. Drug Delivery and Chemotherapy
5.4. Immunotherapy
6. Conclusions and Prospect
- (1)
- Synthesis of materials: Despite the fact that numerous synthetic methods for PLNPs have been reported, none of these methods can be deemed optimal. They cannot simultaneously meet the requirements for the morphological characteristics, homogeneity, and afterglow properties of PLNPs but only enhance one or several of these attributes. Therefore, we need to further develop more advanced and controllable synthesis strategies. On the one hand, the luminescent and biological properties of materials can be enhanced through doping with specific ions or surface modification during synthesis. On the other hand, the limitations of existing synthesis methods can be overcome by optimizing processes, simplifying procedures, integrating multiple synthetic strategies, and incorporating machine learning, thus facilitating the fabrication of high-performance PLNPs.
- (2)
- Excitation and emission wavelengths of the afterglow: The excitation light source is one of the most important factors affecting the biomedical application of PLNPs. Specifically, UV excitation yields an insufficient afterglow duration to support biological applications, necessitating repeated excitation cycles. Consequently, the study of excitation light will focus on NIR, X-rays, and radionuclides. Meanwhile, the emission wavelengths of most PLNPs are concentrated in the NIR-I region, whereas research on NIR-II/III PLNPs remains relatively scarce. It is therefore imperative to strengthen investigations into NIR-II/III PLNPs, as this could enhance tissue penetration depth and imaging performance. In addition, the development of corresponding commercially available imaging instruments is expected to overcome the technical bottlenecks in NIR-II/III region research, thereby further expanding their biomedical applications.
- (3)
- Biological safety of materials: Most existing PLNPs are doped with various transition metal ions or lanthanide ions, both of which may induce potential harmful side effects due to their long-term retention and deposition in the body. Thus, they need to be studied toxicologically. For example, through genomics, proteomics, and metabolomics approaches, we can further examine the long-term toxicity, possible immunotoxicity, metabolic pathways, and biostructural distribution of PLNPs in vivo, which can lead to in-depth biosafety assessment.
- (4)
- Expansion of biomedical applications: Although there has been a significant breakthrough in the application of PLNPs, their full potential remains to be exploited. Notably, PLNPs exhibit relatively limited application scenarios in biodetection, highlighting the necessity of developing multi-wavelength emissive PLNPs to facilitate the assay of diverse substances. Beyond focusing on the diagnostic and therapeutic dimensions of tumors, we hope to develop PLNPs oriented to special applications, such as deep tissue imaging in vivo, transfer tracking of nerve or cell signals, and diagnosis of special diseases. Furthermore, smart imaging-guided therapy systems, as the emerging and urgent trend, may lead to a more intelligent response to PLNPs, which is of great significance for the development of PLNPs.
- (5)
- Clinical transformation: The research on PLNPs at this stage mostly stays in the laboratory stage, and achieving a leap from basic research to clinical trials is a major challenge in this field. In the future, it is necessary to strengthen multi-party cooperation, improve the pre-clinical research data of materials, establish a standardized production process and quality control system, and formulate evaluation criteria in line with clinical application norms so as to promote the early clinical transformation of PLNPs and benefit patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, S.; Zhou, M.; Wang, Y.; Lu, C.; Yin, B.; Zhang, Y.; Liu, H.; Yin, X.; Song, G. Emerging biomedical imaging-based companion diagnostics for precision medicine. iScience 2023, 26, 107277. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Yang, X.; Huang, K.; Yang, D.; Zhao, W.; Zhou, X. Biomedical Big Data Technologies, Applications, and Challenges for Precision Medicine: A Review. Glob. Chall. 2024, 8, 2300163. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Brighi, C.; Glide-Hurst, C.; Liney, G.; Liu, P.Z.Y.; Lydiard, S.; Paganelli, C.; Trang, P.; Shan, S.; Tree, A.C.; et al. Integrated MRI-guided radiotherapy—Opportunities and challenges. Nat. Rev. Clin. Oncol. 2022, 19, 458–470. [Google Scholar] [CrossRef]
- Lother, D.; Robert, M.; Elwood, E.; Smith, S.; Tunariu, N.; Johnston, S.R.; Parton, M.; Bhaludin, B.; Millard, T.; Downey, K.; et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: Review and new perspectives. Cancer Imaging 2023, 23, 53. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.-T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Molecular Probes for Autofluorescence-Free Optical Imaging. Chem. Rev. 2021, 121, 13086–13131. [Google Scholar] [CrossRef]
- Jaiswal, S.; Das, S.; Kundu, S.; Rawal, I.; Anand, P.; Patra, A. Progress and perspectives: Fluorescent to long-lived emissive multifunctional probes for intracellular sensing and imaging. J. Mater. Chem. C 2022, 10, 6141–6195. [Google Scholar] [CrossRef]
- Dou, W.-T.; Han, H.-H.; Sedgwick, A.C.; Zhu, G.-B.; Zang, Y.; Yang, X.-R.; Yoon, J.; James, T.D.; Li, J.; He, X.-P. Fluorescent probes for the detection of disease-associated biomarkers. Sci. Bull. 2022, 67, 853–878. [Google Scholar] [CrossRef]
- Ojha, A.; Ojha, N.K. Excitation light-induced phototoxicity during fluorescence imaging. J. Biosci. 2021, 46, 78. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X. Chemical Regulation of Fluorescence Lifetime. Chem. Biomed. Imaging 2023, 1, 796–816. [Google Scholar] [CrossRef]
- Kasprzycka, W.; Szumigraj, W.; Wachulak, P.; Trafny, E.A. New approaches for low phototoxicity imaging of living cells and tissues. BioEssays 2024, 46, 2300122. [Google Scholar] [CrossRef]
- Pei, P.; Chen, Y.; Sun, C.; Fan, Y.; Yang, Y.; Liu, X.; Lu, L.; Zhao, M.; Zhang, H.; Zhao, D.; et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 2021, 16, 1011–1018. [Google Scholar] [CrossRef]
- Liang, L.; Chen, J.; Shao, K.; Qin, X.; Pan, Z.; Liu, X. Controlling persistent luminescence in nanocrystalline phosphors. Nat. Mater. 2023, 22, 289–304. [Google Scholar] [CrossRef]
- Xu, J.; Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumin. 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Yang, S.; Dai, W.; Zheng, W.; Wang, J. Non-UV-activated persistent luminescence phosphors for sustained bioimaging and phototherapy. Coord. Chem. Rev. 2023, 475, 214913. [Google Scholar] [CrossRef]
- Huang, K.; Le, N.; Wang, J.S.; Huang, L.; Zeng, L.; Xu, W.C.; Li, Z.; Li, Y.; Han, G. Designing Next Generation of Persistent Luminescence: Recent Advances in Uniform Persistent Luminescence Nanoparticles. Adv. Mater. 2022, 34, 2107962. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors-from fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, Y.; Peng, M. Near-infrared persistent phosphors: Synthesis, design, and applications. Chem. Eng. J. 2020, 399, 125688. [Google Scholar] [CrossRef]
- Vaidyanathan, S. Recent progress on lanthanide-based long persistent phosphors: An overview. J. Mater. Chem. C 2023, 11, 8649–8687. [Google Scholar] [CrossRef]
- Yu, N.; Li, Y.; Li, Z.; Han, G. The “bottom-up” synthesis and applications of persistent luminescence nanoparticles. Sci. China Chem. 2018, 61, 757–758. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Ren, B.; Tu, D.; Wang, Z.; Wang, C.; Zheng, Y.; Li, X.; Wang, D.; Ren, Z.; et al. Smart Mechanoluminescent Phosphors: A Review of Strontium-Aluminate-Based Materials, Properties, and Their Advanced Application Technologies. Adv. Sci. 2023, 10, 2204925. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Ding, W.; Xu, L.; Ma, Y.; Zhang, L. Recent Advances of Persistent Luminescence Nanoparticles in Bioapplications. Nano-Micro Lett. 2020, 12, 70. [Google Scholar] [CrossRef]
- Chan, M.-H.; Chang, Y.-C. Recent advances in near-infrared I/II persistent luminescent nanoparticles for biosensing and bioimaging in cancer analysis. Anal. Bioanal. Chem. 2024, 416, 3887–3905. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-K.; Wang, H.-F.; Yan, X.-P. Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics. Acc. Chem. Res. 2018, 51, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lecuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.; Sun, X.; Sun, X.; Shi, J. Persistent luminescence nanoparticles for cancer theranostics application. J. Nanobiotechnol. 2021, 19, 113. [Google Scholar] [CrossRef]
- Lastusaari, M.; Laamanen, T.; Malkamaki, M.; Eskola, K.O.; Kotlov, A.; Carlson, S.; Welter, E.; Brito, H.F.; Bettinelli, M.; Jungner, H.; et al. The Bologna Stone: history’s first persistent luminescent material. Eur. J. Mineral. 2012, 24, 885–890. [Google Scholar] [CrossRef]
- Hölsä, J. Persistent Luminescence Beats the Afterglow: 400 Years of Persistent Luminescence. Electrochem. Soc. Interface 2009, 18, 42–45. [Google Scholar] [CrossRef]
- Smet, P.F.; Moreels, I.; Hens, Z.; Poelman, D. Luminescence in Sulfides: A Rich History and a Bright Future. Materials 2010, 3, 2834–2883. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- le Masne de Chermont, Q.; Chaneac, C.; Seguin, J.; Pelle, F.; Maitrejean, S.; Jolivet, J.-P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar] [CrossRef]
- Maldiney, T.; Kaikkonen, M.U.; Seguin, J.; de Chermont, Q.L.M.; Bessodes, M.; Airenne, K.J.; Yla-Herttuala, S.; Scherman, D.; Richard, C. In Vitro Targeting of Avidin-Expressing Glioma Cells with Biotinylated Persistent Luminescence Nanoparticles. Bioconjugate Chem. 2012, 23, 472–478. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Hu, Y.; Cai, C.; Li, Y.; Zhang, X.; Liang, B.; Yang, Y.; Qiu, J. Mechanism of the trivalent lanthanides’ persistent luminescence in wide bandgap materials. Light Sci. Appl. 2022, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, E.; Campolucci, M.; Alberti, S.; Locardi, F. From Bulk to Nano: The Effect on the Persistent Luminescence. Cryst. Growth Des. 2024, 24, 4271–4281. [Google Scholar] [CrossRef]
- Yang, L.; Gai, S.; Ding, H.; Yang, D.; Feng, L.; Yang, P. Recent Progress in Inorganic Afterglow Materials: Mechanisms, Persistent Luminescent Properties, Modulating Methods, and Bioimaging Applications. Adv. Opt. Mater. 2023, 11, 2202382. [Google Scholar] [CrossRef]
- Kasim, L.; Li, B.; Abdukayum, A. Preparation and Application of Inorganic Persistent Luminescent Composite Materials. Prog. Chem. 2024, 36, 1254–1268. [Google Scholar] [CrossRef]
- Rodriguez Burbano, D.C.; Sharma, S.K.; Dorenbos, P.; Viana, B.; Capobianco, J.A. Persistent and Photostimulated Red Emission in CaS: Eu2+, Dy3+ Nanophosphors. Adv. Opt. Mater. 2015, 3, 551–557. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W. Enhancement of Afterglow in ZnS: Cu, Co Water-Soluble Nanoparticles by Aging. J. Phys. Chem. C 2011, 115, 8940–8944. [Google Scholar] [CrossRef]
- Kong, M.; Fang, M.; Tan, X.; Liu, M.; Shang, G.L.; Fei, G.T.; Zhang, L. The investigation on the mechanism of the increased decay time in red SrS: Eu2+, Dy3+ phosphor. Mater. Chem. Phys. 2018, 207, 161–166. [Google Scholar] [CrossRef]
- Estefania Rojas-Hernandez, R.; Rubio-Marcos, F.; Angel Rodriguez, M.; Francisco Fernandez, J. Long lasting phosphors: SrAl2O4: Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Qu, B.; Zhang, B.; Wang, L.; Zhou, R.; Zeng, X.C. Mechanistic Study of the Persistent Luminescence of CaAl2O4: Eu, Nd. Chem. Mater. 2015, 27, 2195–2202. [Google Scholar] [CrossRef]
- Demirci, S.; Gultekin, S.; Akalin, S.A.; Oter, O.; Ertekin, K.; Celik, E. Synthesis and spectral characterization of Sr4Al14O25: Eu2+/Dy3+ blue-green phosphorous powders by sol-gel method. Mater. Sci. Semicond. Process. 2015, 31, 611–617. [Google Scholar] [CrossRef]
- Pan, L.; Liu, S.; Zhang, X.; Oderinde, O.; Yao, F.; Fu, G. Optimization method for blue Sr2MgSi2O7: Eu2+, Dy3+ phosphors produced by microwave synthesis route. J. Alloys Compd. 2018, 737, 39–45. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.P.; Tamrakar, R.K.; Shrivastava, R. Enhancement of the photoluminescence and long afterglow properties of Ca2MgSi2O7: Eu2+ phosphor by Dy3+ co-doping. Res. Chem. Intermed. 2016, 42, 1823–1843. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, S.; Yang, X.; Zhang, X.; Liu, X.; Zhou, B.; Jin, X. Preparation and luminescence properties of yellow long-lasting phosphor Ca2ZnSi2O7: Eu2+, Dy3+. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2013, 178, 123–126. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Gupta, S.K.; Mohan, S.; Mao, Y. Molten-Salt-Assisted Annealing for Making Colloidal ZnGa2O4: Cr Nanocrystals with High Persistent Luminescence. Chemistry 2021, 27, 11398–11405. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lu, Y.-Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2012, 11, 58–63. [Google Scholar] [CrossRef]
- Yan, W.; Liu, F.; Lu, Y.-Y.; Wang, X.-J.; Yin, M.; Pan, Z. Near infrared long-persistent phosphorescence in La3Ga5GeO14: Cr3+ phosphor. Opt. Express 2010, 18, 20215–20221. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Yang, X.; Xiao, S. Improving red afterglow properties of CaZnGe2O6: Mn2+ by co-doping Bi3+. Optik 2021, 246, 167799. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Chen, Y.; Chen, Y.; Zhou, J.; Zeng, Q. Long persistent and photo-stimulated luminescence in Pr3+-doped layered perovskite phosphor for optical data storage. J. Am. Ceram. Soc. 2018, 101, 4598–4607. [Google Scholar] [CrossRef]
- Taktak, O.; Souissi, H.; Kammoun, S. Optical properties of the phosphors Zn2SnO4: Cr3+ with near-infrared long-persistence phosphorescence for bio-imaging applications. J. Lumin. 2020, 228, 117563. [Google Scholar] [CrossRef]
- Pekgozlu, I. A Novel Reddish Orange Luminescent Material Sr3B2O6: Sm3+. J. Appl. Spectrosc. 2019, 85, 1136–1139. [Google Scholar] [CrossRef]
- Ju, G.; Hu, Y.; Chen, L.; Wang, X.; Mu, Z. The influence of auxiliary codopants on persistent phosphor Sr2P2O7: Eu2+, R3+ (R = Y, La, Ce, Gd, Tb and Lu). Mater. Res. Bull. 2013, 48, 4743–4748. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940. [Google Scholar] [CrossRef]
- Nidhankar, A.D.; Goudappagouda; Wakchaure, V.C.; Babu, S.S. Efficient metal-free organic room temperature phosphors. Chem. Sci. 2021, 12, 4216–4236. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liao, S.; Li, Z.; Wang, Y.; Huan, S.; Zhang, X.B.; Song, G. Organic Afterglow Nanoparticles in Bioapplications. Chemistry 2023, 29, e202301209. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q.; Li, Z. Afterglow bio-applications by utilizing triplet excited states of organic materials. Sci. China Chem. 2023, 66, 2930–2940. [Google Scholar] [CrossRef]
- Zhao, Z.; Du, R.; Feng, X.; Wang, Z.; Wang, T.; Xie, Z.; Yuan, H.; Tan, Y.; Ou, H. Regulating Triplet Excitons of Organic Luminophores for Promoted Bioimaging. Curr. Med. Chem. 2025, 32, 322–342. [Google Scholar] [CrossRef]
- Gong, Y.; Tan, Y.; Li, H.; Zhang, Y.; Yuan, W.; Zhang, Y.; Sun, J.; Tang, B.Z. Crystallization-induced phosphorescence of benzils at room temperature. Sci. China-Chem. 2013, 56, 1183–1186. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef]
- Xie, Y.; Ge, Y.; Peng, Q.; Li, C.; Li, Q.; Li, Z. How the Molecular Packing Affects the Room Temperature Phosphorescence in Pure Organic Compounds: Ingenious Molecular Design, Detailed Crystal Analysis, and Rational Theoretical Calculations. Adv. Mater. 2017, 29, 1606829. [Google Scholar] [CrossRef]
- Yang, Z.; Ubba, E.; Huang, Q.; Mao, Z.; Li, W.; Chen, J.; Zhao, J.; Zhang, Y.; Chi, Z. Enabling dynamic ultralong organic phosphorescence in molecular crystals through the synergy between intramolecular and intermolecular interactions. J. Mater. Chem. C 2020, 8, 7384–7392. [Google Scholar] [CrossRef]
- Xiao, G.; Fang, X.; Ma, Y.-J.; Yan, D. Multi-Mode and Dynamic Persistent Luminescence from Metal Cytosine Halides through Balancing Excited-State Proton Transfer. Adv. Sci. 2022, 9, 2200992. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Ma, H.; Peng, Q.; Huang, X.; Zheng, X.; Sung, S.H.P.; Shan, G.; Lam, J.W.Y.; Shuai, Z.; et al. A facile strategy for realizing room temperature phosphorescence and single molecule white light emission. Nat. Commun. 2018, 9, 2963. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.J. Halogen bonding in room-temperature phosphorescent materials. Coord. Chem. Rev. 2020, 404, 213107. [Google Scholar] [CrossRef]
- Shi, H.; Yao, W.; Ye, W.; Ma, H.; Huang, W.; An, Z. Ultralong Organic Phosphorescence: From Material Design to Applications. Acc. Chem. Res. 2022, 55, 3445–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, L.; An, W.; Miao, Q. Recent advances and design strategies for organic afterglow agents to enhance autofluorescence-free imaging performance. Chem. Soc. Rev. 2025, 54, 1429–1452. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Ma, H.; Shuai, Z. Theory of Long-Lived Room-Temperature Phosphorescence in Organic Aggregates. Acc. Chem. Res. 2021, 54, 940–949. [Google Scholar] [CrossRef]
- Singh, M.; Liu, K.; Qu, S.; Ma, H.; Shi, H.; An, Z.; Huang, W. Recent Advances of Cocrystals with Room Temperature Phosphorescence. Adv. Opt. Mater. 2021, 9, 2002197. [Google Scholar] [CrossRef]
- Chen, H.; Yao, X.; Ma, X.; Tian, H. Amorphous, Efficient, Room-Temperature Phosphorescent Metal-Free Polymers and Their Applications as Encryption Ink. Adv. Opt. Mater. 2016, 4, 1397–1401. [Google Scholar] [CrossRef]
- Cai, S.; Ma, H.; Shi, H.; Wang, H.; Wang, X.; Xiao, L.; Ye, W.; Huang, K.; Cao, X.; Gan, N.; et al. Enabling long-lived organic room temperature phosphorescence in polymers by subunit interlocking. Nat. Commun. 2019, 10, 4247. [Google Scholar] [CrossRef]
- Li, J.-A.; Zhang, L.; Wu, C.; Huang, Z.; Li, S.; Zhang, H.; Yang, Q.; Mao, Z.; Luo, S.; Liu, C.; et al. Switchable and Highly Robust Ultralong Room-Temperature Phosphorescence from Polymer-Based Transparent Films with Three-Dimensional Covalent Networks for Erasable Light Printing. Angew. Chem.-Int. Ed. 2023, 62, e202217284. [Google Scholar] [CrossRef]
- Li, H.; Xue, X.; Cao, Y.; Cheng, H.; Luo, A.; Guo, N.; Li, H.; Xie, G.; Tao, Y.; Chen, R.; et al. Achieving Stimuli-Responsive Amorphous Organic Afterglow in Single-Component Copolymer through Self-Doping. J. Am. Chem. Soc. 2023, 145, 7343–7351. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Suh, Y.D.; Liu, X.; Huang, W. Rigidity-Mediated Afterglow Tuning of Small Molecules in Polymer Matrix through Photoinitiated Solvent-Free Copolymerization. Adv. Opt. Mater. 2023, 11, 2300240. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Gu, Z.-G.; Zhang, J. Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials. Molecules 2024, 29, 2989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yan, D. Long Persistent Luminescence from Metal-Organic Compounds: State of the Art. Adv. Funct. Mater. 2023, 33, 2300735. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.Y.; Luo, F. A New Zn-Triazole MOF Showing Very Long-Lived Luminescence up to 3 S. J. Solid State Chem. 2021, 301, 122369. [Google Scholar] [CrossRef]
- Yu, X.; Ryadun, A.A.; Pavlov, D.I.; Guselnikova, T.Y.; Potapov, A.S.; Fedin, V.P. Ln-MOF-Based Hydrogel Films with Tunable Luminescence and Afterglow Behavior for Visual Detection of Ofloxacin and Anti-Counterfeiting Applications. Adv. Mater. 2024, 36, 2311939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dong, J.; Lei, S.; Hu, W. Long afterglow MOFs: A frontier study on synthesis and applications. Mater. Chem. Front. 2021, 5, 6824–6849. [Google Scholar] [CrossRef]
- Qin, X.; Wang, J.; Yuan, Q. Synthesis and Biomedical Applications of Lanthanides-Doped Persistent Luminescence Phosphors With NIR Emissions. Front. Chem. 2020, 8, 608578. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Jain, A.; Dhoble, S.J. Persistent phosphors for luminous paints: A review. Luminescence 2022, 37, 524–542. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, U.; Ayoub, I.; Yagoub, M.Y.A.; Som, S.; Swart, H.C.; Kumar, V. Structural and photoluminescent properties of Gd3+ doped barium aluminate phosphor. Phys. B-Condens. Matter 2023, 669, 415296. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Li, B.; Han, L.; Xu, Y. Trap depth engineering in MgGa2O4: Bi3+ for muticolor dynamic anti-counterfeiting, encryption and optical temperature sensing applications. Chem. Eng. J. 2022, 437, 135389. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Liu, F.; Pan, Z. Solar-blind ultraviolet-C persistent luminescence phosphors. Nat. Commun. 2020, 11, 2040. [Google Scholar] [CrossRef]
- Abdukayum, A.; Chen, J.-T.; Zhao, Q.; Yan, X.-P. Functional Near Infrared-Emitting Cr3+/Pr3+ Co-Doped Zinc Gallogermanate Persistent Luminescent Nanoparticles with Superlong Afterglow for in Vivo Targeted Bioimaging. J. Am. Chem. Soc. 2013, 135, 14125–14133. [Google Scholar] [CrossRef]
- Meroni, D.; Porati, L.; Demartin, F.; Poelman, D. Sol–Gel Synthesis of CaTiO3: Pr3+ Red Phosphors: Tailoring the Synthetic Parameters for Luminescent and Afterglow Applications. ACS Omega 2017, 2, 4972–4981. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Onoda, H.; Toyama, T.; Kojima, Y. Synthesis and fluorescence studies of Eu3+-doped SrAl12O19 phosphor. Optik 2019, 180, 183–188. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Q.; Sun, X.; Li, J.-G. Co-doping Zn2+/Sn4+ in ZnGa2O4: Cr3+ for dynamic near-infrared luminescence and advanced anti-counterfeiting. Ceram. Int. 2021, 47, 17000–17007. [Google Scholar] [CrossRef]
- Jin, M.; Li, F.; Xiahou, J.; Zhu, L.; Zhu, Q.; Li, J.-G. A new persistent luminescence phosphor of ZnGa2O4: Ni2+ for the second near-infrared transparency window. J. Alloys Compd. 2023, 931, 167491. [Google Scholar] [CrossRef]
- Ayoub, I.; Mushtaq, U.; Yagoub, M.Y.A.; Som, S.; Swart, H.C.; Kumar, V. Structural and optical characteristics of green-emitting BaGd2ZnO5: Tb3+ phosphor for LED applications. Phys. B-Condens. Matter 2023, 669, 415299. [Google Scholar] [CrossRef]
- Pan, L.; Delaey, M.; Wang, Y.; Poelman, D. Structural and optical properties of Cr ion-doped near-infrared long persistent luminescence silicogermanate phosphors with broad emission bands. J. Alloys Compd. 2024, 983, 173853. [Google Scholar] [CrossRef]
- Li, J.-L.; Shi, J.-P.; Wang, C.-C.; Li, P.-H.; Yu, Z.-F.; Zhang, H.-W. Five-nanometer ZnSn2O4: Cr, Eu ultra-small nanoparticles as new near infrared-emitting persistent luminescent nanoprobes for cellular and deep tissue imaging at 800 nm. Nanoscale 2017, 9, 8631–8638. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Z.; Luo, Q.; Ma, Q.; Chen, N.; Fu, L.; Wang, J.; Yang, R.; Yuan, Q. Facile Synthesis of Luminous Nanoparticles with Tunable Size and Long-Lived Luminescence for Lifetime-Based Biosensing. Cryst. Growth Des. 2019, 19, 2322–2328. [Google Scholar] [CrossRef]
- Fu, L.; Wang, J.; Chen, N.; Ma, Q.; Lu, D.; Yuan, Q. Enhancement of long-lived luminescence in nanophosphors by surface defect passivation. Chem. Commun. 2020, 56, 6660–6663. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tanwar, V.; Simantilleke, A.P.; Kumar, H.; Singh, D. Structural and spectroscopic properties of CaMgSi2O6: RE3+ (Eu3+ and Tb3+) nanophosphors under UV-illumination. Optik 2020, 221, 165364. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wang, Y.; Wu, S.; Shen, Y. Effect of Citric Acid Amount in the Synthesis of LiGa5O8: Cr3+ Nano-Phosphor. Russ. J. Phys. Chem. A 2022, 96, S139–S144. [Google Scholar] [CrossRef]
- Wu, M.Y.; Wang, Y.; Shen, Y.; Li, F.F.; Wang, J.; Liu, Y.; Peng, C. Preparation of Zn3Ga2Ge2O10: Cr3+, Al3+ nanometer phosphors via sol-gel method. Dig. J. Nanomater. Biostruct. 2021, 16, 393–398. [Google Scholar] [CrossRef]
- Zou, R.; Huang, J.; Shi, J.; Huang, L.; Zhang, X.; Wong, K.-L.; Zhang, H.; Jin, D.; Wang, J.; Su, Q. Silica shell-assisted synthetic route for mono-disperse persistent nanophosphors with enhanced in vivo recharged near-infrared persistent luminescence. Nano Res. 2017, 10, 2070–2082. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Yu, J.; Zhang, H.; Zhang, B. Large Hollow Cavity Luminous Nanoparticles with Near-Infrared Persistent Luminescence and Tunable Sizes for Tumor Afterglow Imaging and Chemo-/Photodynamic Therapies. ACS Nano 2018, 12, 4246–4258. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, R.; Zhang, L.; Li, Z.; Su, Y.; Lv, Y. Raspberry-Like Mesoporous Zn1.07Ga2.34Si0.98O6.56: Cr0.01 Nanocarriers for Enhanced Near-Infrared Afterglow Imaging and Combined Cancer Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 44978–44988. [Google Scholar] [CrossRef]
- Tan, R.; Wu, J.; Wang, C.; Zhao, Z.; Zhang, X.; Zhong, C.; Tang, Z.; Zheng, R.; Du, B.; He, Y.; et al. The develop of persistent luminescence nanoparticles with excellent performances in cancer targeted bioimaging and killing: A review. J. Nanobiotechnol. 2025, 23, 299. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.; Hu, X.-X.; Liu, H.; Zheng, W.; Chen, X.; Yuan, Q.; Tan, W. Autofluorescence-Free Targeted Tumor Imaging Based on Luminous Nanoparticles with Composition-Dependent Size and Persistent Luminescence. ACS Nano 2017, 11, 8010–8017. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wu, X.; Huang, L.; Li, D.; Fan, W.; Han, G. Direct Aqueous-Phase Synthesis of Sub-10 nm “Luminous Pearls” with Enhanced in Vivo Renewable Near-Infrared Persistent Luminescence. J. Am. Chem. Soc. 2015, 137, 5304–5307. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zheng, S.; Li, J.; Fu, X.; Zhang, H. A new near-infrared persistent luminescence nanoparticle as a multifunctional nanoplatform for multimodal imaging and cancer therapy. Biomaterials 2018, 152, 15–23. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Zhao, H.; Yue, W.; Zhang, K.; Jiang, X.; Li, K. Facile and Controllable Synthesis of the Renal-Clearable “Luminous Pearls” for in Vivo Afterglow/Magnetic Resonance Imaging. ACS Nano 2022, 16, 462–472. [Google Scholar] [CrossRef]
- Vitola, V.; Millers, D.; Bite, I.; Smits, K.; Spustaka, A. Recent progress in understanding the persistent luminescence in SrAl2O4: Eu, Dy. Mater. Sci. Technol. 2019, 35, 1661–1677. [Google Scholar] [CrossRef]
- Sun, X.; Song, L.; Liu, N.; Shi, J.; Zhang, Y. Chromium-Doped Zinc Gallate Near-Infrared Persistent Luminescence Nanoparticles in Autofluorescence-Free Biosensing and Bioimaging: A Review. ACS Appl. Nano Mater. 2021, 4, 6497–6514. [Google Scholar] [CrossRef]
- Yang, X.; Waterhouse, G.I.N.; Lu, S.; Yu, J. Recent advances in the design of afterglow materials: Mechanisms, structural regulation strategies and applications. Chem. Soc. Rev. 2023, 52, 8005–8058. [Google Scholar] [CrossRef] [PubMed]
- Abbruscato, V. Optical and Electrical Properties of SrAl2O4: Eu2+ . J. Electrochem. Soc. 1971, 118, 930. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, C.; Liu, M.; Zhou, D.; Luo, X.; Zhang, J.; Xie, Y. The valence of rare earth ions in R2MgSi2O7: Eu, Dy (R = Ca, Sr) long-afterglow phosphors. Phys. Status Solidi (A) 2004, 201, 3109–3112. [Google Scholar] [CrossRef]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.-H.; Garcia, A.; Mercier, T.L. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Aitasalo, T.; Holsa, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Thermoluminescence study of persistent luminescence materials: Eu2+- and R3+-doped calcium aluminates, CaAl2O4: Eu2+, R3+. J. Phys. Chem. B 2006, 110, 4589–4598. [Google Scholar] [CrossRef]
- Dorenbos, P.; Bos, A.J.J.; Poolton, N.R.J. Electron transfer processes in double lanthanide activated YPO4. Opt. Mater. 2011, 33, 1019–1023. [Google Scholar] [CrossRef]
- Dorenbos, P.; Shalapska, T.; Stryganyuk, G.; Gektin, A.; Voloshinovskii, A. Spectroscopy and energy level location of the trivalent lanthanides in LiYP4O12. J. Lumin. 2011, 131, 633–639. [Google Scholar] [CrossRef]
- Lecointre, A.; Bessiere, A.; Bos, A.J.J.; Dorenbos, P.; Viana, B.; Jacquart, S. Designing a Red Persistent Luminescence Phosphor: The Example of YPO4: Pr3+, Ln3+ (Ln = Nd, Er, Ho, Dy). J. Phys. Chem. C 2011, 115, 4217–4227. [Google Scholar] [CrossRef]
- Denis, G.; Deniard, P.; Gautron, E.; Clabau, F.; Garcia, A.; Jobic, S. Structure and white luminescence of Eu-activated (Ba,Sr)13-xAl22-2xSi10+2xO66 materials. Inorg. Chem. 2008, 47, 4226–4235. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Miao, K.; Zhang, X.; Li, W.; Zhao, P.; Sun, P.; Zheng, T.; Zhang, X.; Chen, C. Research progress on near-infrared long persistent phosphor materials in biomedical applications. Nanoscale Adv. 2022, 4, 4972–4996. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.-J.; Zhao, K.-C.; Liu, Y.-S.; Liu, J.-L.; Yan, X.-P. Autofluorescence-free chemo/biosensing in complex matrixes based on persistent luminescence nanoparticles. Trac-Trends Anal. Chem. 2019, 118, 65–72. [Google Scholar] [CrossRef]
- Huang, P.; Tu, D.; Zheng, W.; Zhou, S.; Chen, Z.; Chen, X. Inorganic lanthanide nanoprobes for background-free luminescent bioassays. Sci. China-Mater. 2015, 58, 156–177. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Bradburne, C.E.; Delehanty, J.B.; Gemmill, K.B.; Mei, B.C.; Mattoussi, H.; Susumu, K.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. Cytotoxicity of Quantum Dots Used for In Vitro Cellular Labeling: Role of QD Surface Ligand, Delivery Modality, Cell Type, and Direct Comparison to Organic Fluorophores. Bioconjug. Chem. 2013, 24, 1570–1583. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Stability of fluorescent labels in PLGA polymeric nanoparticles: Quantum dots versus organic dyes. Int. J. Pharm. 2015, 494, 471–478. [Google Scholar] [CrossRef]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.-Y.; Gupta, R.; et al. Photoluminescent Nanoparticles for Chemical and Biological Analysis and Imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mondal, S.; Ghosh, D. Carbon quantum dots in bioimaging and biomedicines. Front. Bioeng. Biotechnol. 2024, 11, 1333752. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Draz, M.S.; Wang, W.; Liao, G.; Xu, Y. The Quality of In Vivo Upconversion Fluorescence Signals Inside Different Anatomic Structures. J. Biomed. Nanotechnol. 2015, 11, 325–333. [Google Scholar] [CrossRef]
- Li, K.; Hong, E.; Wang, B.; Wang, Z.; Zhang, L.; Hu, R.; Wang, B. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sheng, W.; Haruna, S.A.; Hassan, M.M.; Chen, Q. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Q.; Wang, Y.; Shen, H.; Yuan, Q. Recent progress in biomedical applications of persistent luminescence nanoparticles. Nanoscale 2017, 9, 6204–6218. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, C.; Zhao, M.; Zhang, L.; Yang, S.; Li, P.; Ding, Z.; Yuan, Q.; Yang, Y. Recent progress in synthesis of lanthanide-based persistent luminescence nanoparticles. J. Rare Earths 2022, 40, 1333–1342. [Google Scholar] [CrossRef]
- Sun, M.; Chen, M.; Wang, J. Perspective and Prospects on Persistent Luminescent Nanoparticles for Biological Imaging and Tumor Therapy. Curr. Med. Chem. 2024, 31, 938–951. [Google Scholar] [CrossRef]
- Lecuyer, T.; Teston, E.; Ramirez-Garcia, G.; Maldiney, T.; Viana, B.; Seguin, J.; Mignet, N.; Scherman, D.; Richard, C. Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics 2016, 6, 2488–2524. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Chu, H.; Mi, Y.; Zhou, Z.; Di, Z.; Zhao, M.; Li, L. Light-Activated Nanoprobes for Biosensing and Imaging. Adv. Mater. 2019, 31, 1804745. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Zhou, J.; Zheng, L.; Wang, Y. Fluorescence-based quantitative platform for ultrasensitive food allergen detection: From immunoassays to DNA sensors. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3343–3364. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Yu, L.; He, S.; Zhu, R.; Meng, Z. Constructing “off-on” luminescence biosensor based on fluorescence resonance energy transfer for autofluorescence-free detection of uric acid and glucose. Inorg. Chem. Commun. 2025, 174, 113889. [Google Scholar] [CrossRef]
- Dang, Q.; Jiang, Y.; Wang, J.; Wang, J.; Zhang, Q.; Zhang, M.; Luo, S.; Xie, Y.; Pu, K.; Li, Q.; et al. Room-Temperature Phosphorescence Resonance Energy Transfer for Construction of Near-Infrared Afterglow Imaging Agents. Adv. Mater. 2020, 32, 2006752. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.-Y. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-Y.; Wang, H.-F.; Chen, J.-T.; Yan, X.-P. Fluorescence Resonance Energy Transfer Inhibition Assay for α-Fetoprotein Excreted during Cancer Cell Growth Using Functionalized Persistent Luminescence Nanoparticles. J. Am. Chem. Soc. 2011, 133, 686–688. [Google Scholar] [CrossRef]
- Feng, F.; Chen, X.; Li, G.; Liang, S.; Hong, Z.; Wang, H.-F. Afterglow Resonance Energy Transfer Inhibition for Fibroblast Activation Protein-α Assay. ACS Sens. 2018, 3, 1846–1854. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, D.; Lin, J.; Shao, K.; Shi, S.; Teng, Y.-J.; Liu, H.; She, Y. Autofluorescence free detection of carcinoembryonic antigen in pleural effusion by persistent luminescence nanoparticle-based aptasensors. Anal. Chim. Acta 2022, 1194, 339408. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Pang, Y.; Fu, K.; Shang, Q.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics 2022, 12, 1557–1569. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Yan, X.-P. Bioconjugated persistent luminescence nanoparticles for Föster resonance energy transfer immunoassay of prostate specific antigen in serum and cell extracts without in situ excitation. Chem. Commun. 2015, 51, 3903–3906. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, S.; Fu, P.; Lin, Y.; Ye, S.; Long, Y.; Gao, G.; Zheng, J. A persistent luminescence resonance energy transfer-based molecular beacon probe for the highly sensitive detection of microRNA in biological samples. Biosens. Bioelectron. 2022, 198, 113849. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, L.; Liu, R.; Lv, Y. Modulating near-infrared persistent luminescence of core-shell nanoplatform for imaging of glutathione in tumor mouse model. Biosens. Bioelectron. 2019, 144, 111671. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Han, Y.; Pan, W.; Zhang, T.; Tang, B. A Highly Selective and Instantaneous Nanoprobe for Detection and Imaging of Ascorbic Acid in Living Cells and in Vivo. Anal. Chem. 2014, 86, 3924–3930. [Google Scholar] [CrossRef]
- Pavao, M.L.; Ferin, R.; Lima, A.; Baptista, J. Cysteine and related aminothiols in cardiovascular disease, obesity and insulin resistance. Adv. Clin. Chem. 2022, 109, 75–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Wang, W.-L.; Yan, X.-P. Functionalized gold and persistent luminescence nanoparticle-based ratiometric absorption and TR-FRET nanoplatform for high-throughput sequential detection of L-cysteine and insulin. Nanoscale 2018, 10, 14931–14937. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Jiang, K.; Sun, S.; Qian, S.; Wu, Q.; Lin, H. A persistent luminescence-based label-free probe for the ultrasensitive detection of hemoglobin in human serum. Talanta 2020, 206, 120206. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef]
- Yao, T.; Dong, G.; Qian, S.; Cui, Y.; Chen, X.; Tan, T.; Li, L. Persistent luminescence nanoparticles/hierarchical porous ZIF-8 nanohybrids for autoluminescence-free detection of dopamine. Sens. Actuators B-Chem. 2022, 357, 131470. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Zhang, X.-X.; Wang, W.-L.; Yang, C.; Shi, X.; Feng, Y.-W.; Abdurahman, R. NIR persistent luminescence nanoparticles based turn-on aptasensor for autofluorescence-free determination of 17β-estradiol in milk. Food Chem. 2022, 373, 131432. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-X.; Pan, L.-M.; Wang, M.-C.; Chen, L.-J.; Zhao, X. Exogenous interference and autofluorescence-free ratiometric aptasensor for detection of OTA based on dual-colored persistent luminescence nanoparticles. Food Chem. 2023, 413, 135611. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Zhang, Z.; Sun, Z.; Tang, Z.; Xie, X.; Chen, Q.; Cao, H.; Yu, X.; Xu, Y.; Liu, X.; et al. Fluonanobody-based nanosensor via fluorescence resonance energy transfer for ultrasensitive detection of ochratoxin A. J. Hazard. Mater. 2022, 422, 126838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Liang, L.; Chen, N.; Jia, Y.; Ma, Q.; Wang, J.; Yuan, Q.; Tan, W. Recent progress in engineering near-infrared persistent luminescence nanoprobes for time-resolved biosensing/bioimaging. Nano Res. 2019, 12, 1279–1292. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, J.; Liu, J.; Ma, F.; Ju, H. Persistent luminescence nanoprobe for biosensing and lifetime imaging of cell apoptosis via time-resolved fluorescence resonance energy transfer. Biomaterials 2015, 67, 323–334. [Google Scholar] [CrossRef]
- Chen, S.; Cai, G.; Gong, X.; Wang, L.; Cai, C.; Gong, H. Non-autofluorescence Detection of H5N1 Virus Using Photochemical Aptamer Sensors Based on Persistent Luminescent Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 46964–46971. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Shen, R.; Li, Z.; Yang, Y.; Yuan, Q. Point-of-Care Pathogen Testing Using Photonic Crystals and Machine Vision for Diagnosis of Urinary Tract Infections. Nano Lett. 2021, 21, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, T.; Rao, Q.; Xie, X.; Lv, Y.; Zhang, L. Time-Resolved Persistent Luminescence Encoding for Multiplexed Severe Acute Respiratory Syndrome Coronavirus 2 Detection. Anal. Chem. 2022, 94, 16967–16974. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, F.; Liu, F.; Noh, H.M.; Lee, B.R.; Choi, B.C.; Park, S.H.; Jeong, J.H.; Du, P. Designing ultra-highly efficient Mn2+-activated Zn2GeO4 green-emitting persistent phosphors toward versatile applications. Mater. Today Chem. 2022, 23, 100693. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Zhang, R.; Fan, K.; Ding, W.; Xu, L.; Zhang, L. Persistent luminescence-polypyrrole nanocomposite for dual-modal imaging and photothermal therapy of mammary cancer. Talanta 2021, 221, 121435. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, K.; Li, Y.; Nan, F.; Li, J.; Zhang, H.; Deng, W.; Ding, W.; Li, K.; Jarhen, N.; et al. High resolution osteoclast-targeted imaging-guided osteoporosis alleviation via persistent luminescence nanocomposite. Chem. Eng. J. 2024, 484, 149468. [Google Scholar] [CrossRef]
- Luo, X.; Shi, J.; Wang, R.; Cao, L.; Gao, Y.; Wang, J.; Hong, M.; Sun, X.; Zhang, Y. Near-Infrared Persistent Luminescence Nanoprobe for Early Detection of Atherosclerotic Plaque. ACS Nano 2024, 18, 6500–6512. [Google Scholar] [CrossRef]
- Maldiney, T.; Bessiere, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Li, J.; Hu, J.; Cheng, L.; Yang, X. ROS-based dynamic therapy synergy with modulating tumor cell-microenvironment mediated by inorganic nanomedicine. Coord. Chem. Rev. 2021, 437, 213828. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J. X-ray/γ-ray/Ultrasound-Activated Persistent Luminescence Phosphors for Deep Tissue Bioimaging and Therapy. ACS Appl. Mater. Interfaces 2024, 16, 56519–56544. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, X.; Xu, M.; Yuan, W.; Feng, W.; Li, F. Hybrid Nanoclusters for Near-Infrared to Near-Infrared Upconverted Persistent Luminescence Bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 32583–32590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Huang, K.; Huang, L.; Tian, X.; Dong, H.; Kang, R.; Hu, Y.; Nie, J.; Qiu, J.; et al. Trap Energy Upconversion-Like Near-Infrared to Near-Infrared Light Rejuvenateable Persistent Luminescence. Adv. Mater. 2021, 33, 2008722. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Wang, X.; Li, Y.; Li, T.; Kang, H.; Zhang, H.; Yang, Y. Greatly enhanced persistent luminescence of YPO4: Sm3+ phosphors via Tb3+ incorporation for in vivo imaging. Opt. Express 2020, 28, 2649–2660. [Google Scholar] [CrossRef]
- Liu, B.-M.; Zou, R.; Lou, S.-Q.; Gao, Y.-F.; Ma, L.; Wong, K.-L.; Wang, J. Low-dose X-ray-stimulated LaGaO3: Sb, Cr near-infrared persistent luminescence nanoparticles for deep-tissue and renewable in vivo bioimaging. Chem. Eng. J. 2021, 404, 127133. [Google Scholar] [CrossRef]
- Liu, N.; Shi, J.; Wang, Q.; Guo, J.; Hou, Z.; Su, X.; Zhang, H.; Sun, X. In Vivo Repeatedly Activated Persistent Luminescence Nanoparticles by Radiopharmaceuticals for Long-Lasting Tumor Optical Imaging. Small 2020, 16, 2001494. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, J.; Guo, Y.; Zou, Q.; Chen, D.; Xu, K.; Liang, S.; Yi, X.; Lu, H.; Wang, S.-B.; et al. Enhanced persistent luminescence of MgGeO3: Yb3+ nanoparticles via substitution of Ge4+ by Ga3+ ions for biological applications. J. Lumin. 2023, 257, 119741. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Li, H.; Qu, J.; Song, J. Simultaneous realization of persistent luminescence and CT dual-mode imaging by X-ray recharged Bi2Ga4O9: Cr nanoprobes in depth-independent tumors. Chem. Eng. J. 2021, 406, 126008. [Google Scholar] [CrossRef]
- Zou, R.; Li, J.; Yang, T.; Zhang, Y.; Jiao, J.; Wong, K.-L.; Wang, J. Biodegradable manganese engineered nanocapsules for tumor-sensitive near-infrared persistent luminescence/magnetic resonance imaging and simultaneous chemotherapy. Theranostics 2021, 11, 8448–8463. [Google Scholar] [CrossRef]
- Wu, S.; Qian, Z.; Li, Y.; Hu, S.; Ma, Y.; Wei, S.; Zhang, L. Persistent Luminescence Nanoplatform with Fenton-like Catalytic Activity for Tumor Multimodal Imaging and Photoenhanced Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 25572–25580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.-Q.; Nan, F.; Deng, W.; Li, K.; Jarhen, N.; Zhou, Y.; Ma, Q.; Qu, Y.; Chen, C.; et al. Single-Atom Iridium Nanozyme-Based Persistent Luminescence Nanoparticles for Multimodal Imaging-Guided Combination Tumor Therapy. Adv. Healthc. Mater. 2024, 13, 2402544. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Meng, Y.; Zhu, H.; Yan, D.; Liu, C.; Xu, C.; Zhang, H.; Xu, L.; Li, Y.; et al. Irradiation-free photodynamic therapy in vivo induced by enhanced deep red afterglow within NIR-I bio-window. Chem. Eng. J. 2020, 387, 124067. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zheng, S.; Song, L.; Zhang, F.; Madl, T.; Zhang, Y.; Zhang, H.; Hong, M. Tin-Doped Near-Infrared Persistent Luminescence Nanoparticles with Considerable Improvement of Biological Window Activation for Deep Tumor Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 5995–6004. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-M.; Gan, W.-J.; Lou, S.-Q.; Zou, R.; Tang, Q.; Wang, C.-X.; Jiao, J.; Wang, J. X-ray-activated, UVA persistent luminescent materials based on Bi-doped SrLaAlO4 for deep-Seated photodynamic activation. J. Appl. Phys. 2021, 129, 120901. [Google Scholar] [CrossRef]
- Lin, P.; Shi, J.; Ming, L.; Sheng, Y.; Song, L.; Hong, M.; Zhang, Y. An intelligent persistent luminescence nanoplatform with high-efficiency O2 utilization for continuous hypoxic tumors treatment. Chem. Eng. J. 2022, 442, 135638. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, N.; Hou, Z.; Shi, J.; Su, X.; Sun, X. Radioiodinated Persistent Luminescence Nanoplatform for Radiation-Induced Photodynamic Therapy and Radiotherapy. Adv. Healthc. Mater. 2021, 10, e2000802. [Google Scholar] [CrossRef]
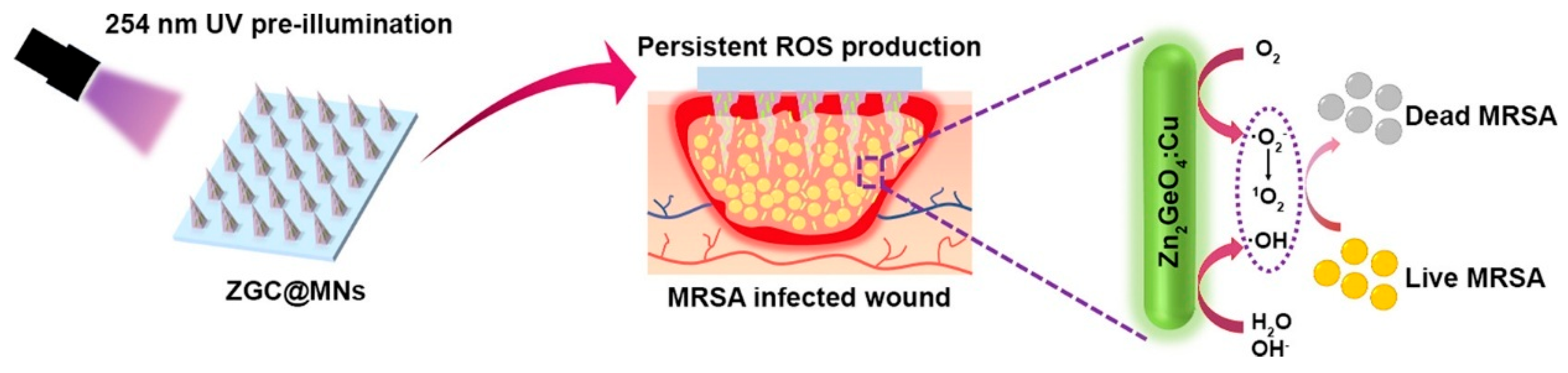
- Gong, J.-H.; Chen, L.-J.; Zhao, X.; Yan, X.-P. Persistent Production of Reactive Oxygen Species with Zn2GeO4: Cu Nanorod-Loaded Microneedles for Methicillin-Resistant Staphylococcus Aureus Infectious Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 17142–17152. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, J.-M.; Zhao, N.; Li, C.-Y.; Lv, S.-W.; Hu, Y.; Lv, H.; Wang, D.; Wang, S. Cancer Cell Macrophage Membrane Camouflaged Persistent Luminescent Nanoparticles for Imaging-Guided Photothermal Therapy of Colorectal Cancer. ACS Appl. Nano Mater. 2020, 3, 7105–7118. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, J.; Jiang, R.; Wang, S.; Zheng, L.; Wang, G.; Tian, X.; Zhu, H.; Yan, D.; Liu, C.; et al. Biocompatible PLNP-GNR composite nanoplatforms for monitoring deep-tissue photothermal therapy process. Appl. Surf. Sci. 2021, 562, 150189. [Google Scholar] [CrossRef]
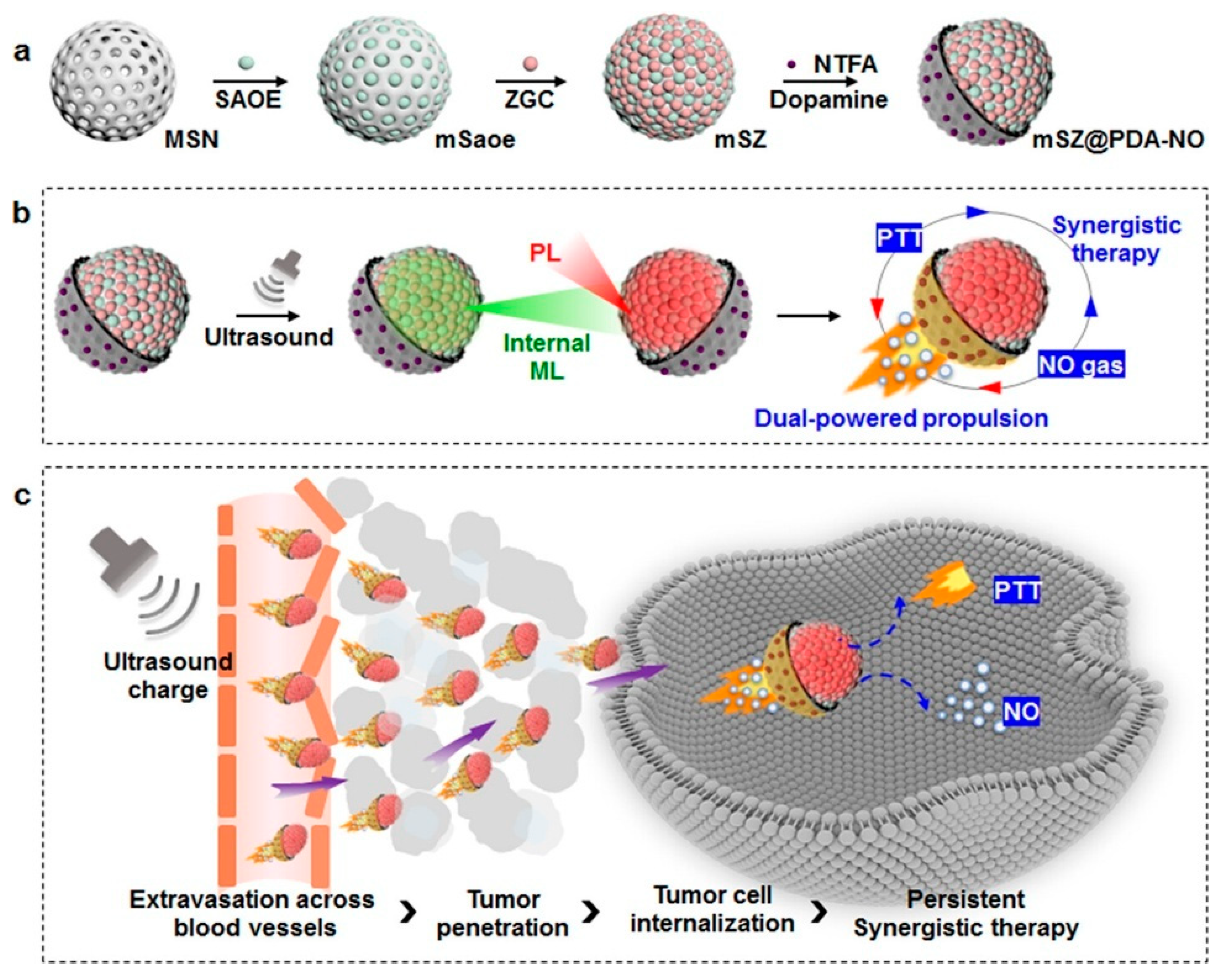
- Zhang, Z.; Yan, H.; Cao, W.; Xie, S.; Ran, P.; Wei, K.; Li, X. Ultrasound-Chargeable Persistent Luminescence Nanoparticles to Generate Self-Propelled Motion and Photothermal/NO Therapy for Synergistic Tumor Treatment. ACS Nano 2023, 17, 16089–16106. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, L.; Mo, F.; Zhong, Y.; Xu, L.; Fu, F. Persistent luminescent multifunctional drug delivery nano-platform based on nanomaterial ZnGa2O4: Cr3+, Sn4+ for imaging-guided cancer chemotherapy. J. Mater. Chem. B 2019, 7, 3019–3026. [Google Scholar] [CrossRef]
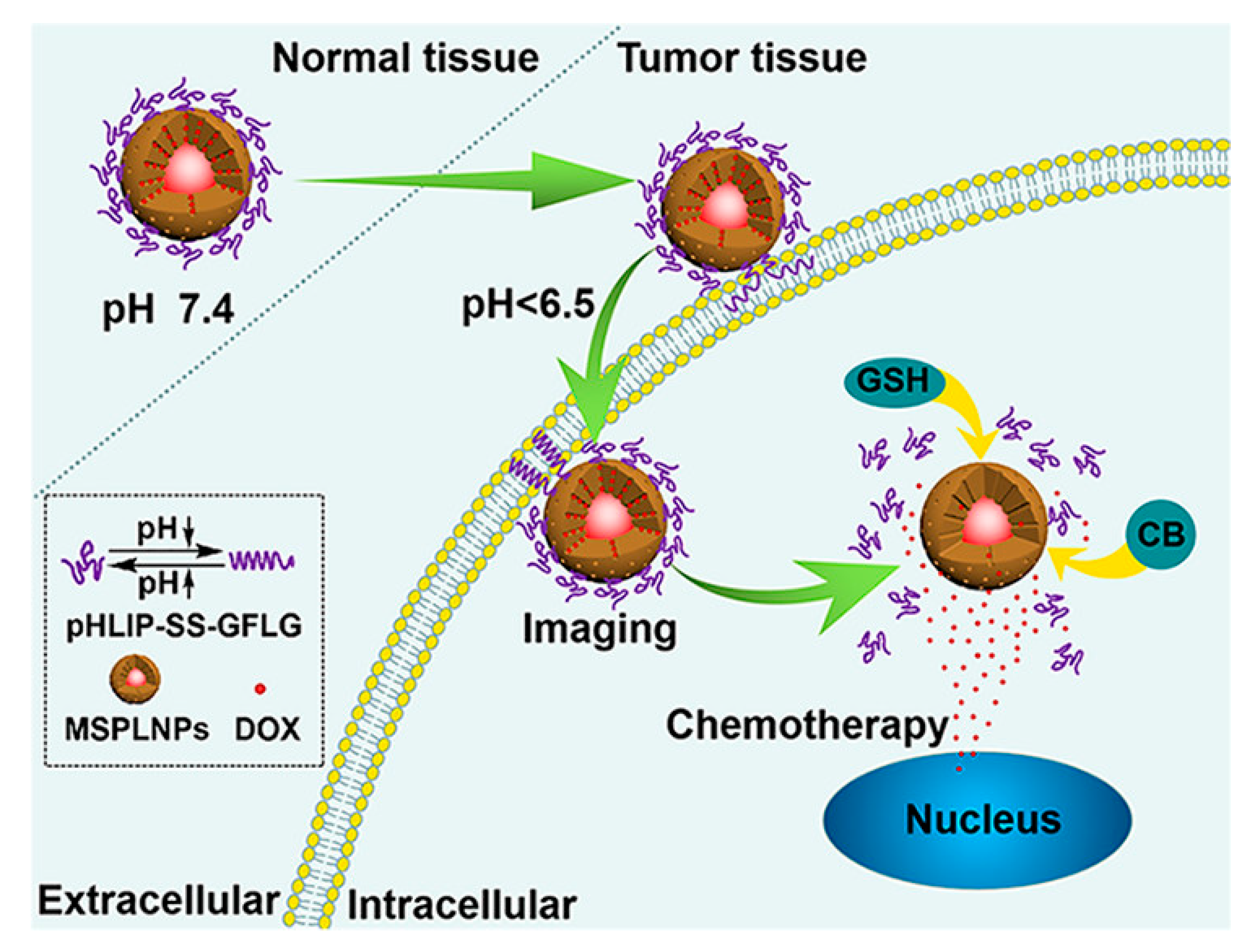
- Zhang, H.-J.; Zhao, X.; Chen, L.-J.; Yang, C.-X.; Yan, X.-P. pH-Driven Targeting Nanoprobe with Dual-Responsive Drug Release for Persistent Luminescence Imaging and Chemotherapy of Tumor. Anal. Chem. 2020, 92, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhao, X.; Chen, L.-J.; Ma, P.; Liu, T.; Yan, X.-P. Mesoporous polyacrylic acid/calcium phosphate coated persistent luminescence nanoparticles for improved afterglow bioimaging and chemotherapy of bacterial infection. Biomater. Sci. 2023, 11, 5186–5194. [Google Scholar] [CrossRef] [PubMed]
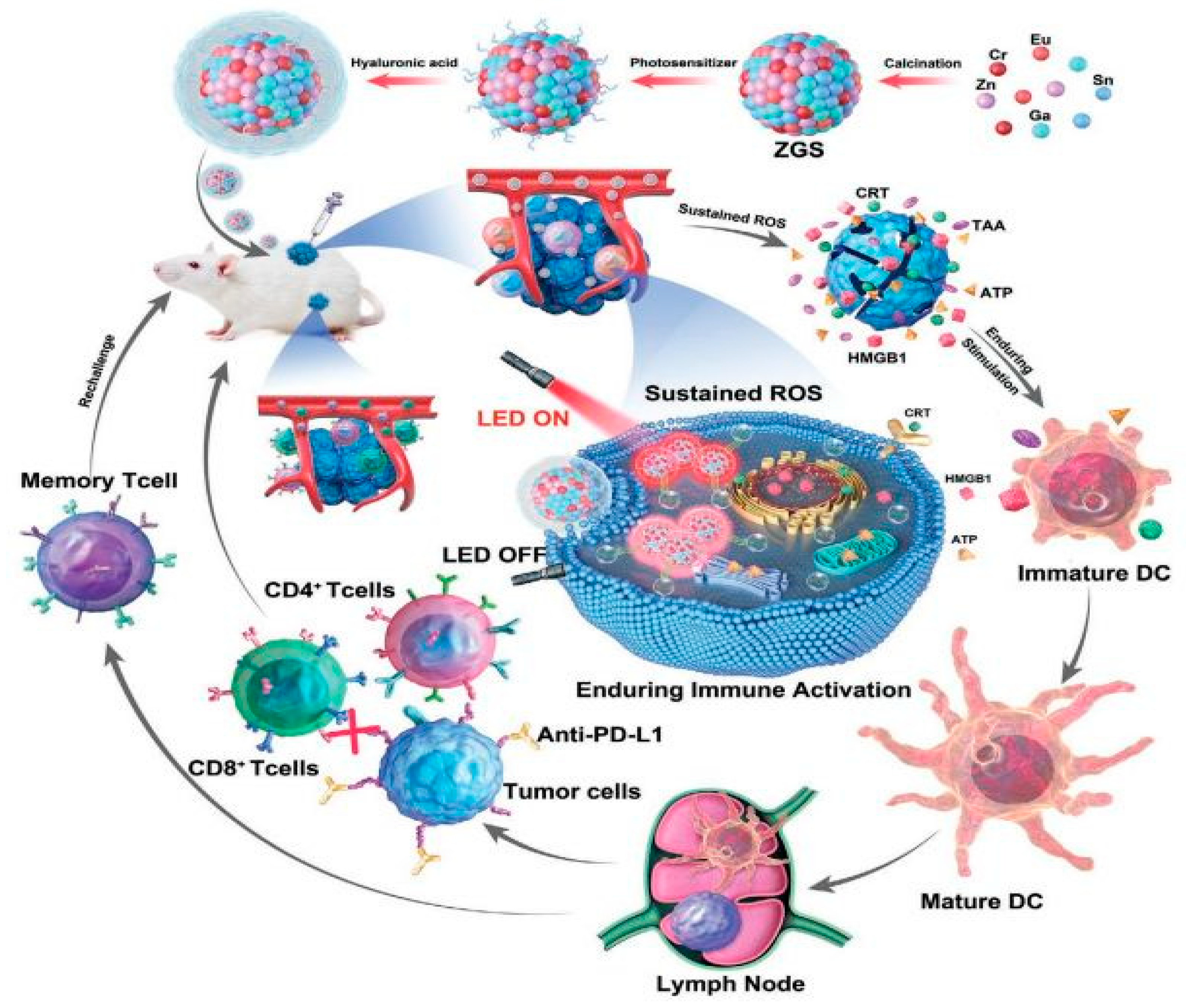
- Wang, R.; Shi, J.; Song, L.; Zheng, S.; Liu, X.; Hong, M.; Zhang, Y. Sustained Antitumor Immunity Based on Persistent Luminescence Nanoparticles for Cancer Immunotherapy. Adv. Funct. Mater. 2021, 31, 2106884. [Google Scholar] [CrossRef]
- Shu, G.; Zhu, W.; Jiang, Y.; Li, X.; Pan, J.; Zhang, X.; Zhang, X.; Sun, S.-K. Persistent Luminescence Immune Hydrogel for Photodynamic-Immunotherapy of Tumors In Vivo. Adv. Funct. Mater. 2021, 31, 2104472. [Google Scholar] [CrossRef]
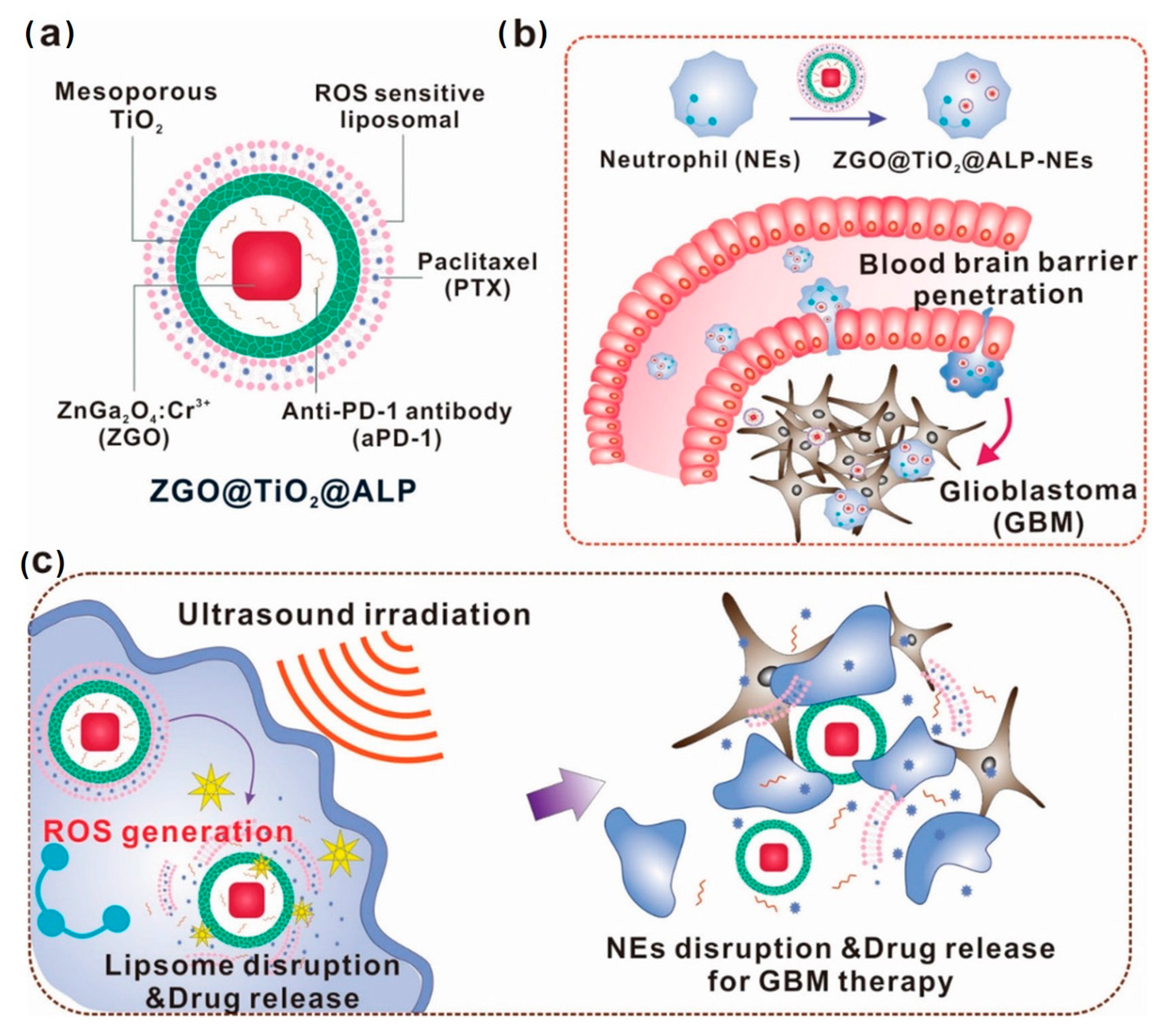
- Li, Y.; Teng, X.; Wang, Y.; Yang, C.; Yan, X.; Li, J. Neutrophil Delivered Hollow Titania Covered Persistent Luminescent Nanosensitizer for Ultrosound Augmented Chemo/Immuno Glioblastoma Therapy. Adv. Sci. 2021, 8, 2004381. [Google Scholar] [CrossRef]



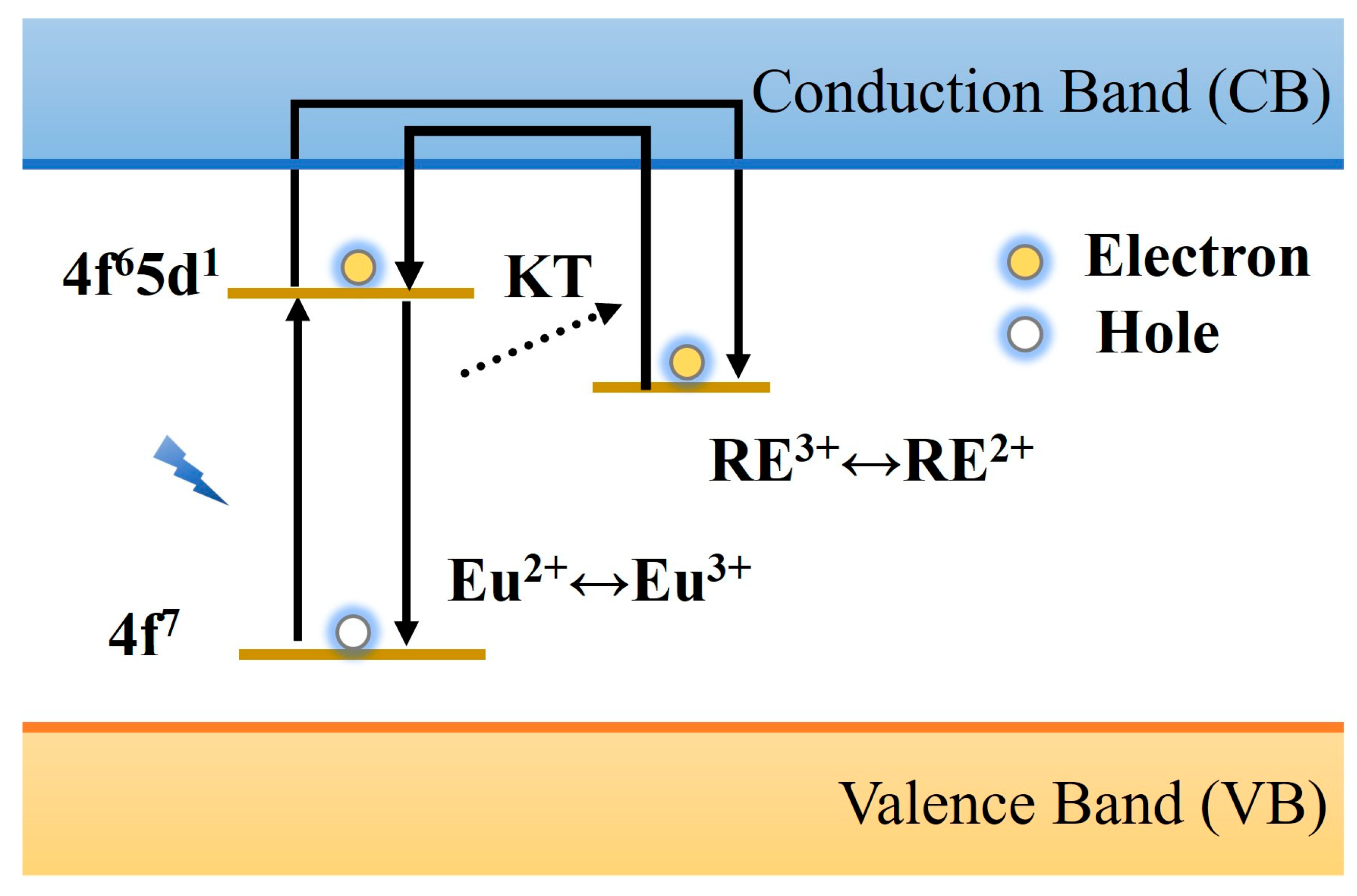
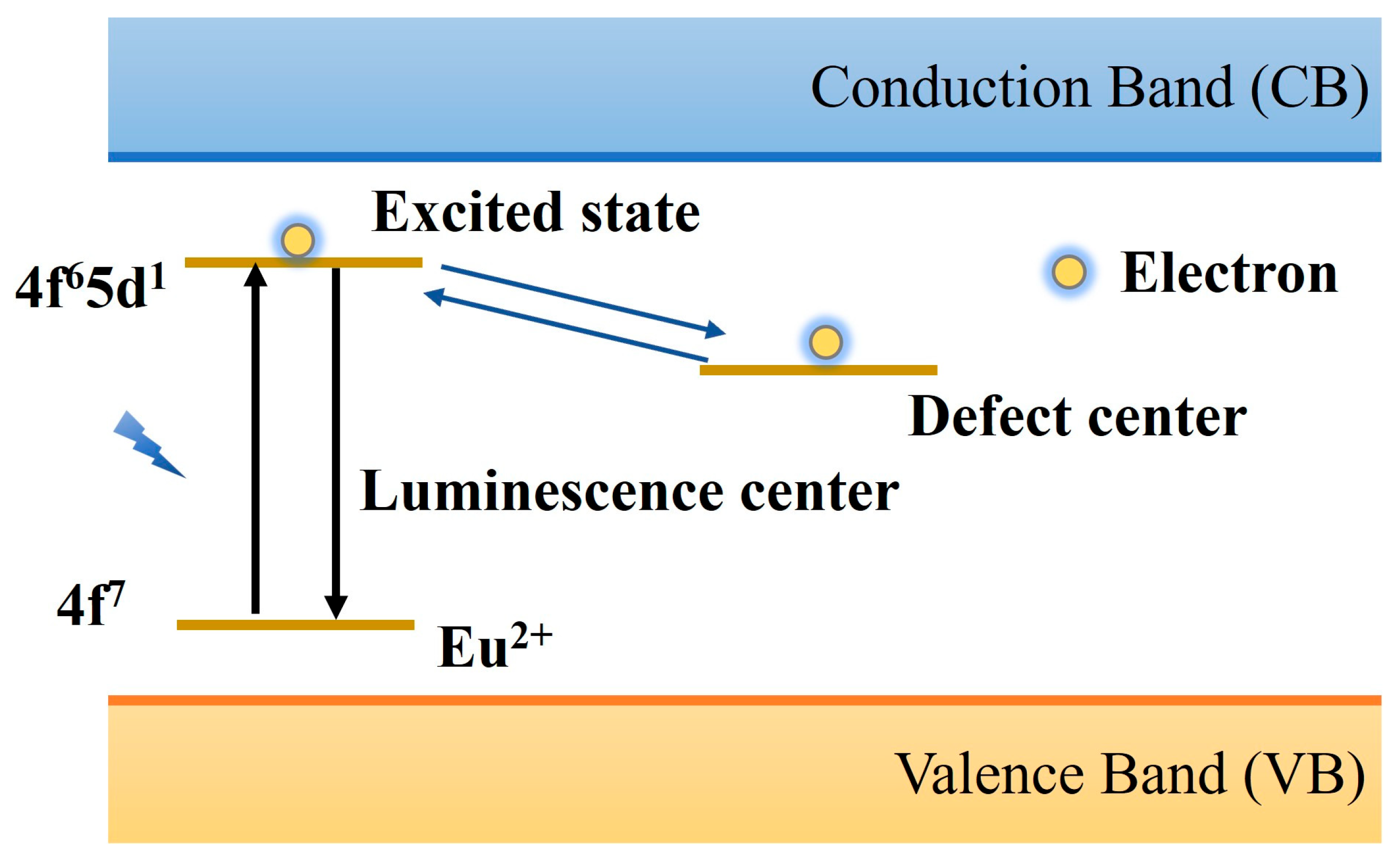
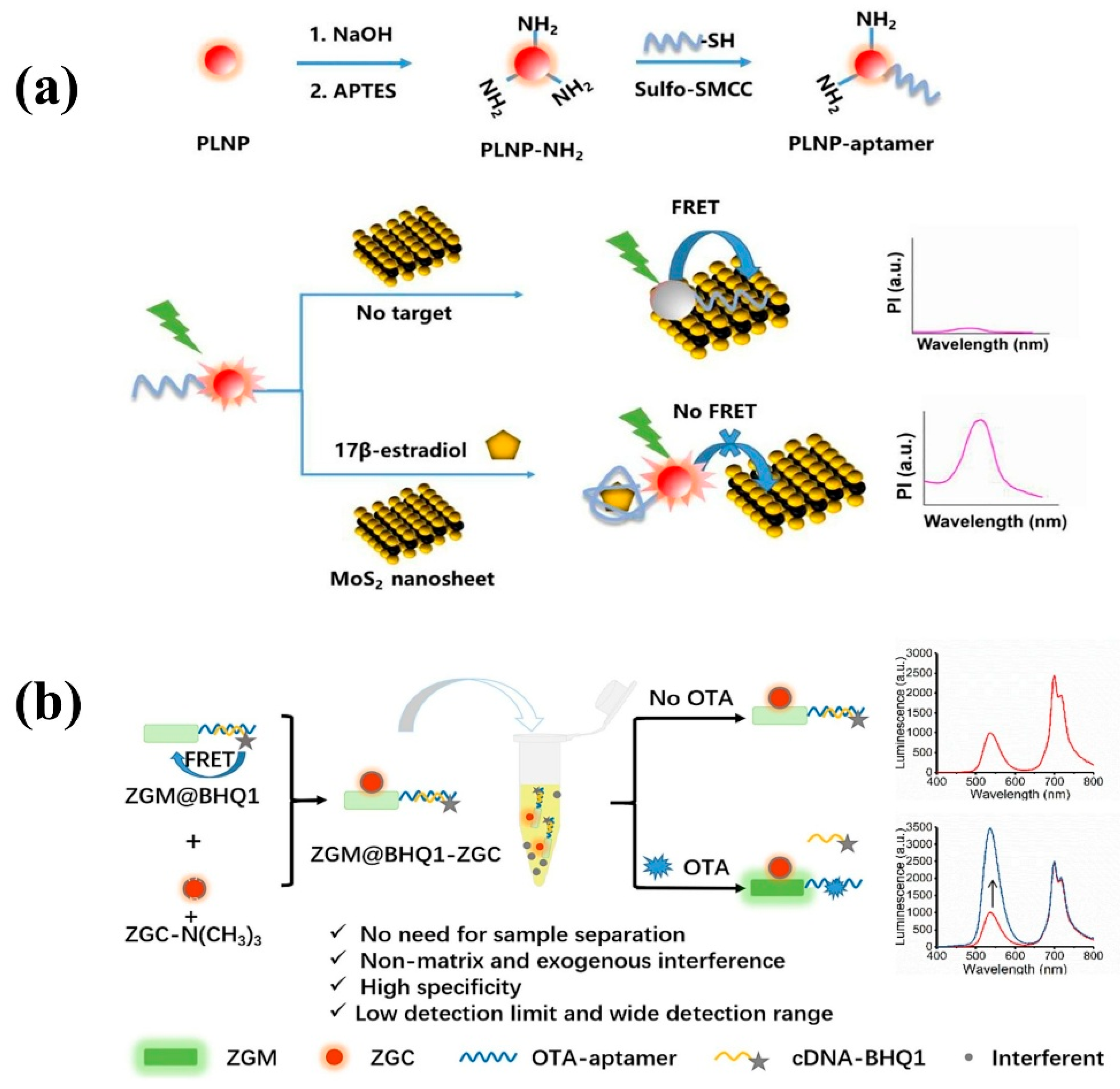


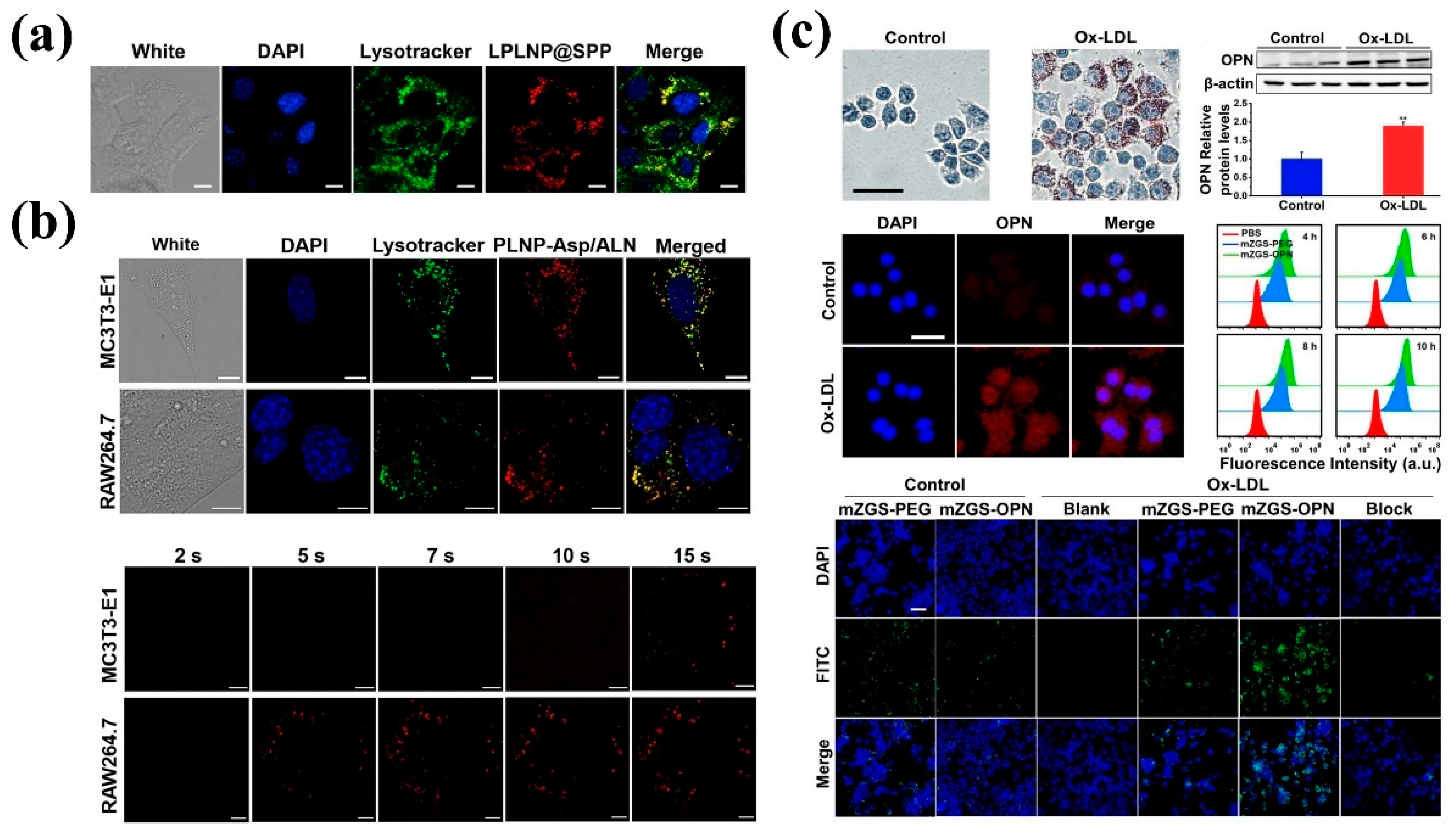


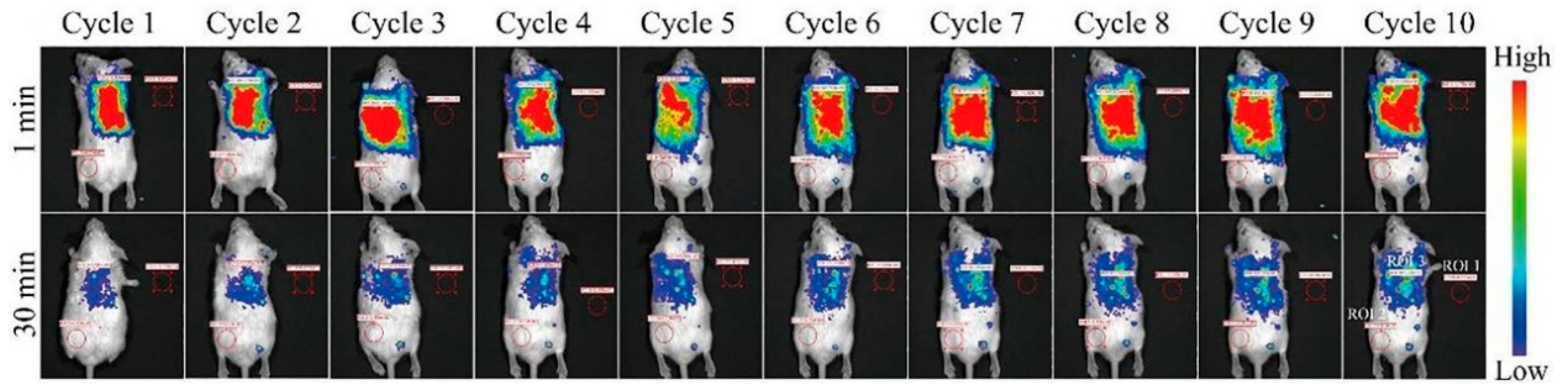
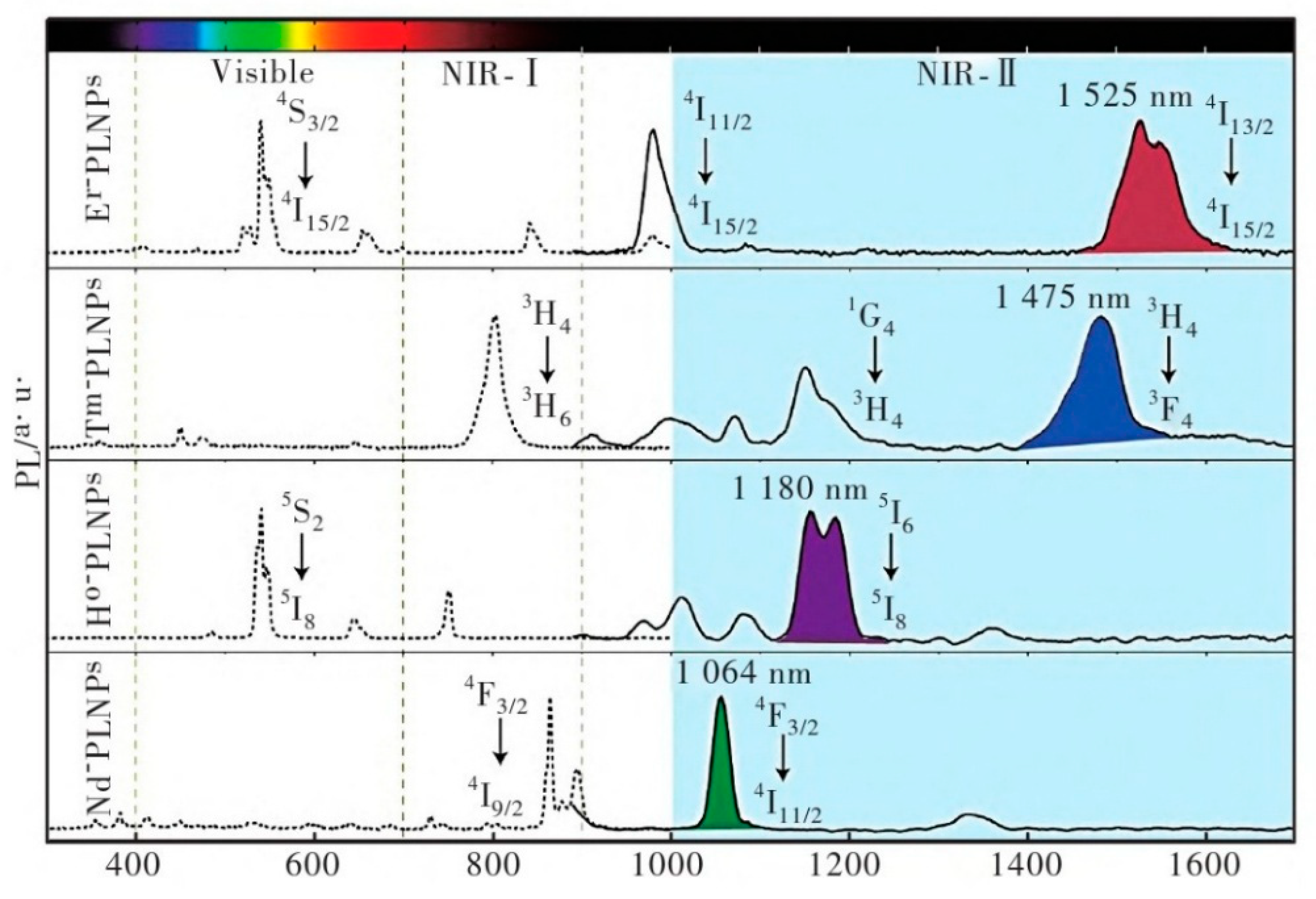

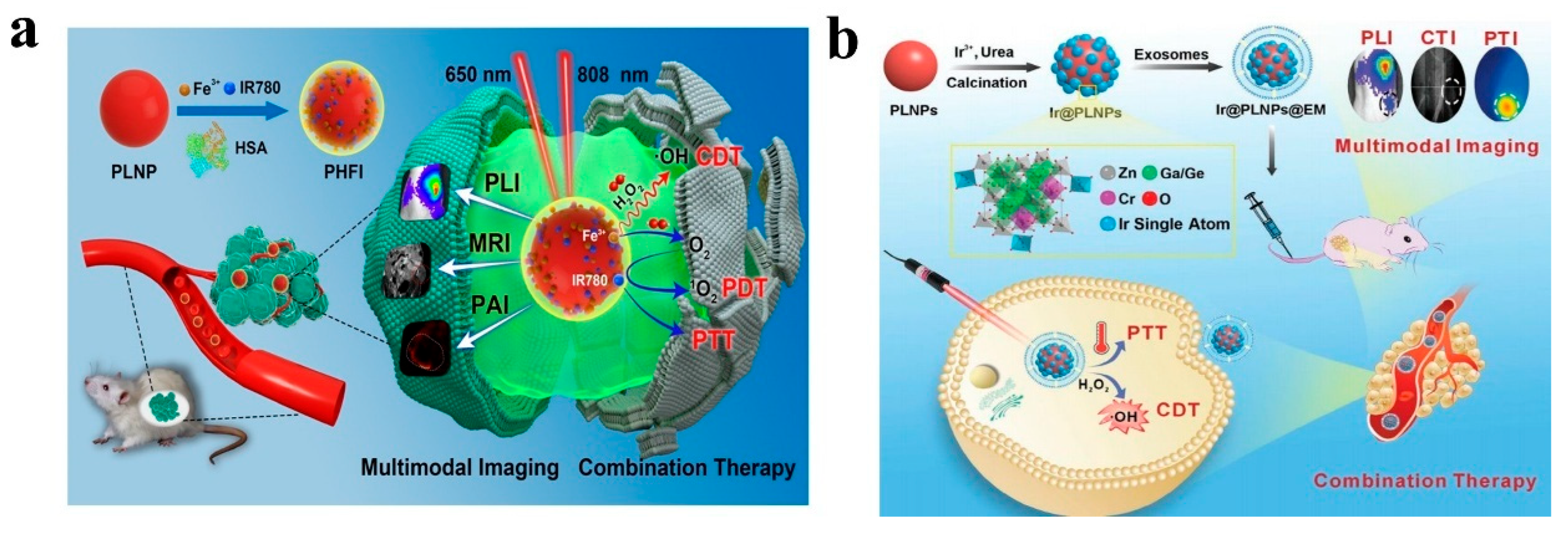
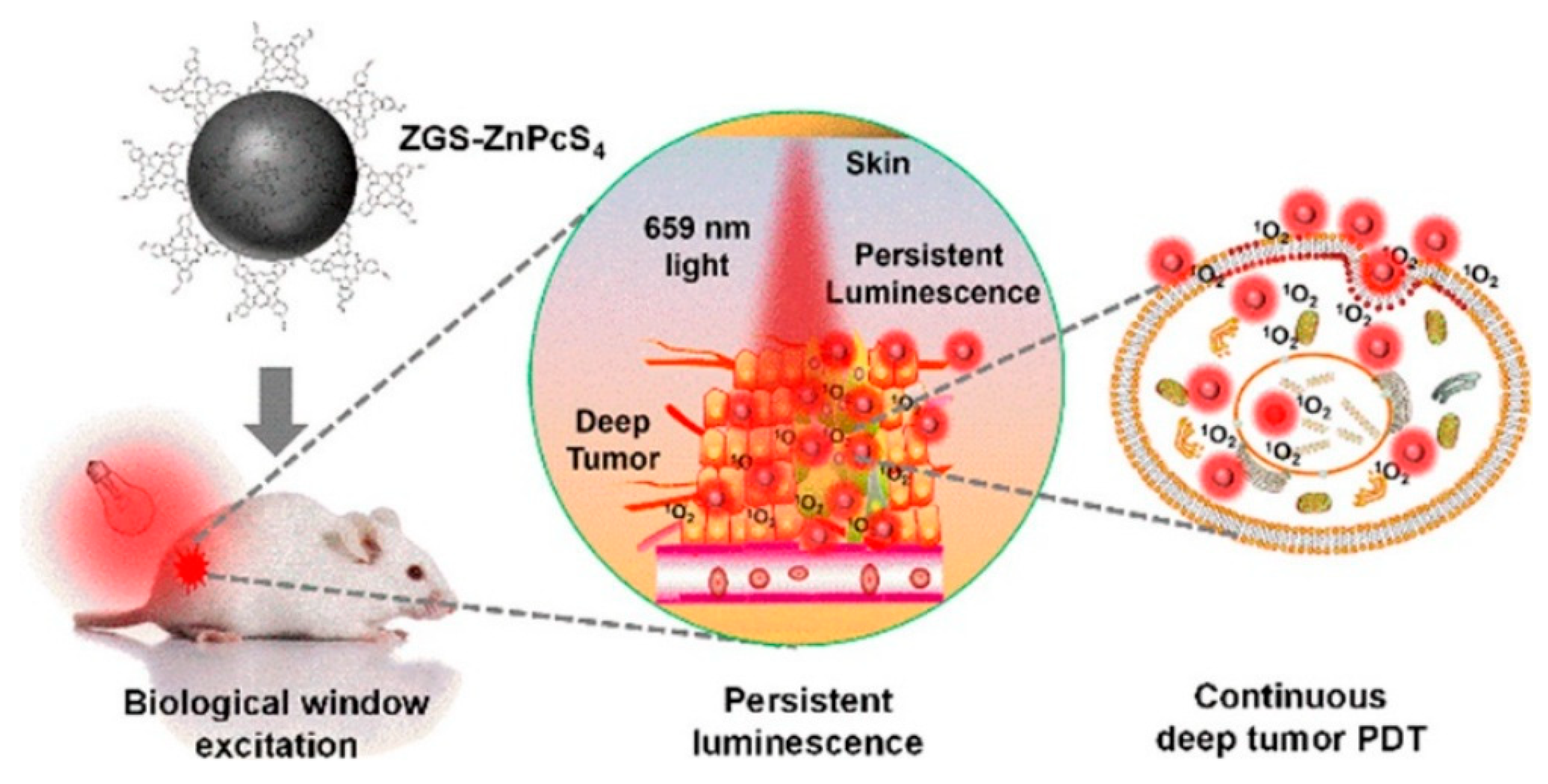
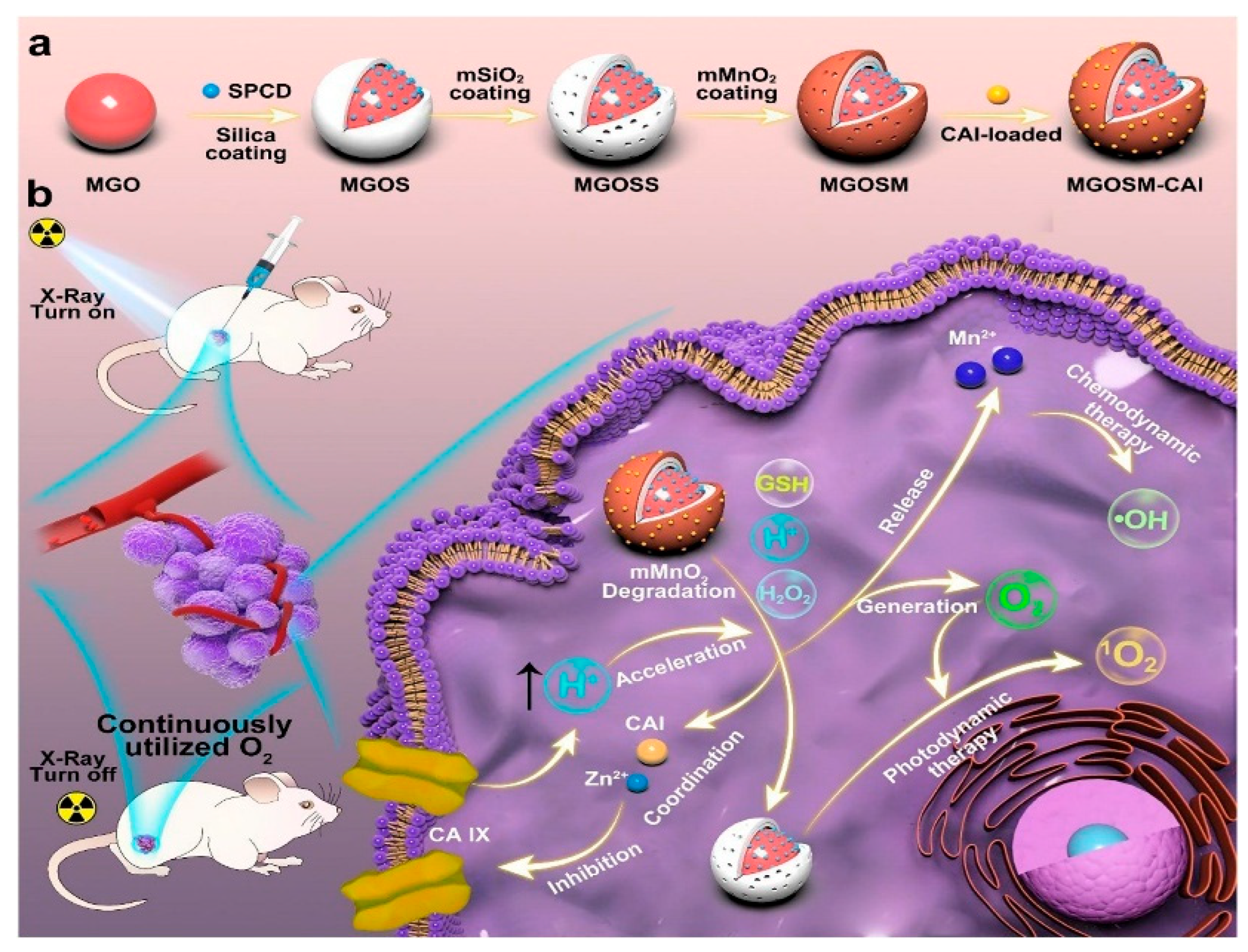
| Matrix | Typical Material | Afterglow Colors | Emission Peak (nm) | Persistence | Feature | Ref. |
|---|---|---|---|---|---|---|
| Sulfide | CaS: Eu, Dy ZnS: Cu, Co SrS: Eu, Dy | Red Green Orange | 650 525 607 | 5 h 3 h ˃60 min | Various emission color, Poor stability, short persistency | [39,40,41] |
| Aluminate | SrAl2O4: Eu, Dy CaAl2O4: Eu, Nd Sr4Al14O25: Eu, Dy | Green Blue Cyan | 520 450 490 | 60 h 20 h 20 h | High light, long lasting time, low water resistance and high cost | [42,43,44] |
| Silicate | Sr2MgSi2O7: Eu, Dy Ca2MgSi2O7: Eu, Dy Ca2ZnSi2O7: Eu, Dy | Blue Green Yellow | 470 535 580 | 10 h 12 h 12 h | Cheap, good stability and water resistance, slightly low luminescence time and efficiency | [45,46,47] |
| Gallium and Germanate | ZnGa2O4: Cr Zn3Ga2Ge2O10: Cr La3Ga5GeO14: Cr CaZnGe2O6: Mn, Bi | Red-near infrared | 600–800 650–1000 700–1300 648 | 12 h 360 h 8 h 3 h | Good chemical and thermal stability, strong penetrability, widely used in biomedicine | [48,49,50,51] |
| Others | Ca4Ti3O10: Pr Zn2SnO4: Cr Sr3B2O6: Sm Sr2P2O7: Eu, Y | Red NIR Orange Violet | 612 800 598 420 | 3 h ˃2 min ˃2 h ˃8 h | Diversification, complementarity of different matrix materials, wider application | [52,53,54,55] |
| Material | Excitation | Emission | Quantum Yield | Lifetime | Biological Characteristics | SNR | Refs. |
|---|---|---|---|---|---|---|---|
| Organic dyes | UV-Vis-NIR | Vis-NIR-II | Medium, dependence structure | Nanosecond | Low toxicity, poor stability, low penetration ability | Low | [122,123,124] |
| Quantum dots | UV-Vis-NIR | UV-Vis-NIR-III | High, strong absorption | Nanosecond | High toxicity, good stability, middle biocompatibility | Middle | [125,126,127] |
| Upconversion nanoparticles | NIR | UV-Vis-NIR-II | Low, dependence on doped ions | Nanosecond | Low toxicity, continuous external excitation | High | [128,129,130] |
| Long persistent luminescent nanoparticles | X-rays-UV-Vis-NIR | UV-Vis-NIR-III | Medium | Up to tens of hours | Low toxicity, no continuous external excitation | Ultra-high | [131,132,133] |
| Therapies | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Photodynamic Therapy | Photosensitizers generate reactive oxygen species (such as singlet oxygen) under light to destroy tumor cells | High specificity, non-invasive, little injury, low toxicity, repeatable treatment | Dependent on oxygen and external light source, limited penetrability, restricted photosensitizer selection |
| Photothermal Therapy | Conversion of absorbed light energy into heat by photothermal conversion agents to generate high temperature and destroy tumor cells | Non-invasiveness, precise heating, no drug resistance | Limited penetration depth, high-temperature-induced damage to surrounding normal tissues |
| Chemotherapy | Nanomaterials loaded with chemotherapeutic drugs for passive or active targeting delivery to tumor sites | Systemic therapy, combination with other therapies | High side effects, prone to drug resistance, uncontrolled drug release |
| Immunotherapy | Specific activation of the immune system for recognition and attack of tumor cells | Safety, persistence, long-term immune memory, potential curative effect | Slow onset, immune escape, high interindividual variability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, K.; Chen, K.; Huang, S.; Li, J.; Liu, Z. Research Progress of Persistent Luminescence Nanoparticles in Biological Detection Imaging and Medical Treatment. Materials 2025, 18, 3937. https://doi.org/10.3390/ma18173937
Deng K, Chen K, Huang S, Li J, Liu Z. Research Progress of Persistent Luminescence Nanoparticles in Biological Detection Imaging and Medical Treatment. Materials. 2025; 18(17):3937. https://doi.org/10.3390/ma18173937
Chicago/Turabian StyleDeng, Kunqiang, Kunfeng Chen, Sai Huang, Jinkai Li, and Zongming Liu. 2025. "Research Progress of Persistent Luminescence Nanoparticles in Biological Detection Imaging and Medical Treatment" Materials 18, no. 17: 3937. https://doi.org/10.3390/ma18173937
APA StyleDeng, K., Chen, K., Huang, S., Li, J., & Liu, Z. (2025). Research Progress of Persistent Luminescence Nanoparticles in Biological Detection Imaging and Medical Treatment. Materials, 18(17), 3937. https://doi.org/10.3390/ma18173937










