Molecular Adhesion Between Asphalt and Glass Fiber-Reinforced Composites from Recycled Wind Turbine Blades in Dry and Hydrated Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Asphalt Binders
2.1.2. Aggregates
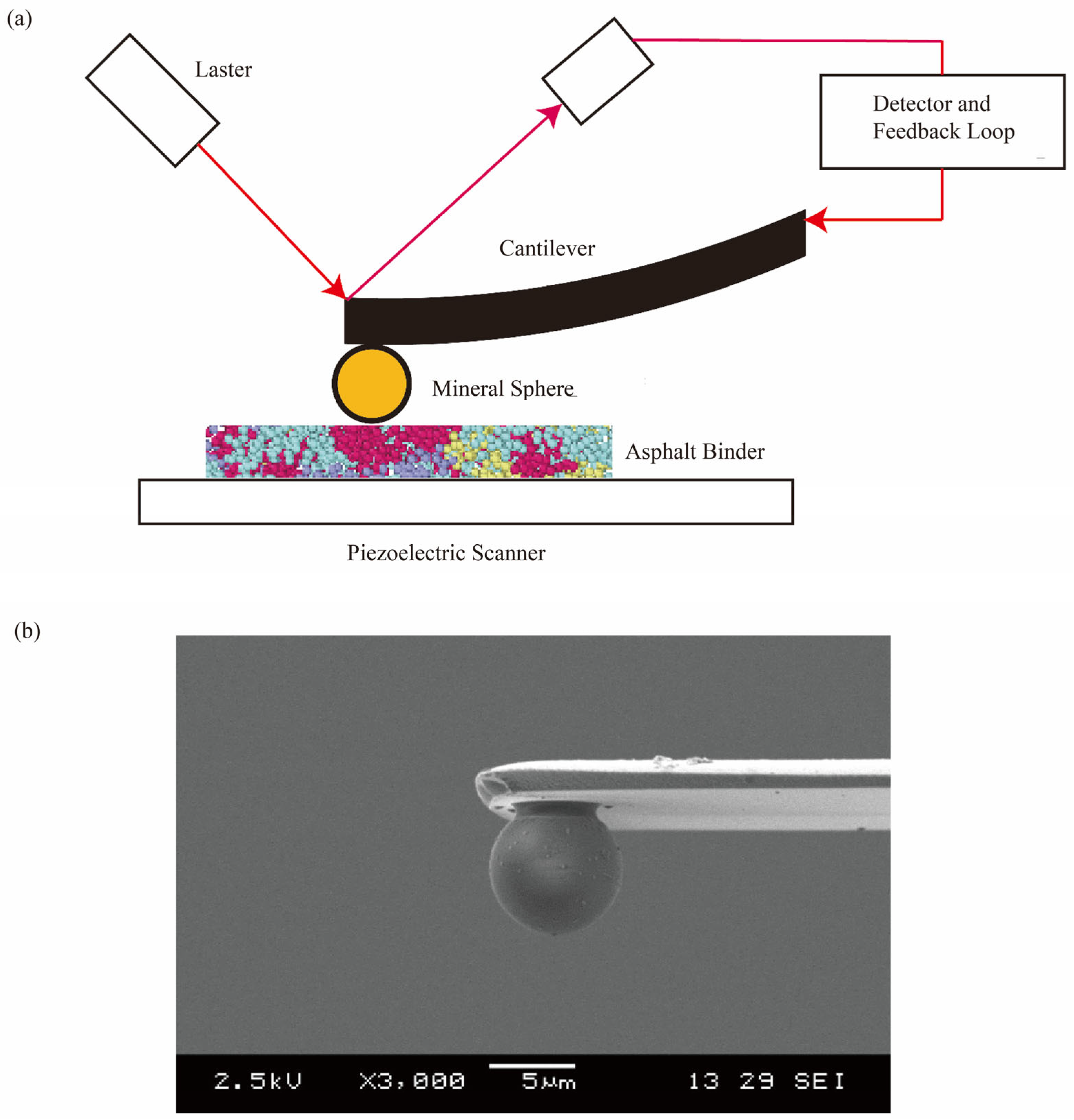
2.2. Experiments
2.3. Molecular Modeling
2.3.1. Forcefields
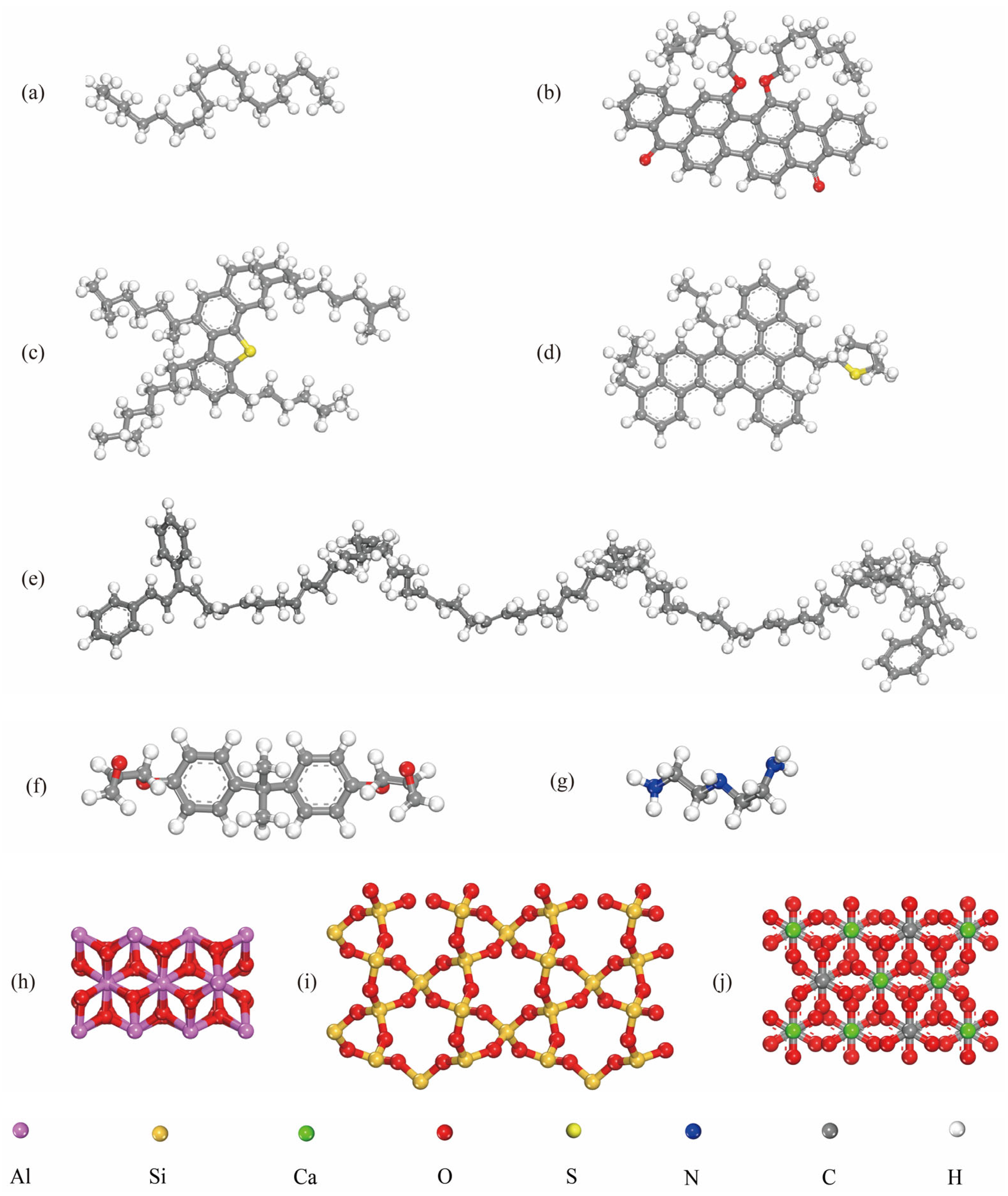
2.3.2. Interfacial Components in Molecular Models
2.3.3. Modeling Setup
3. Analytical Models
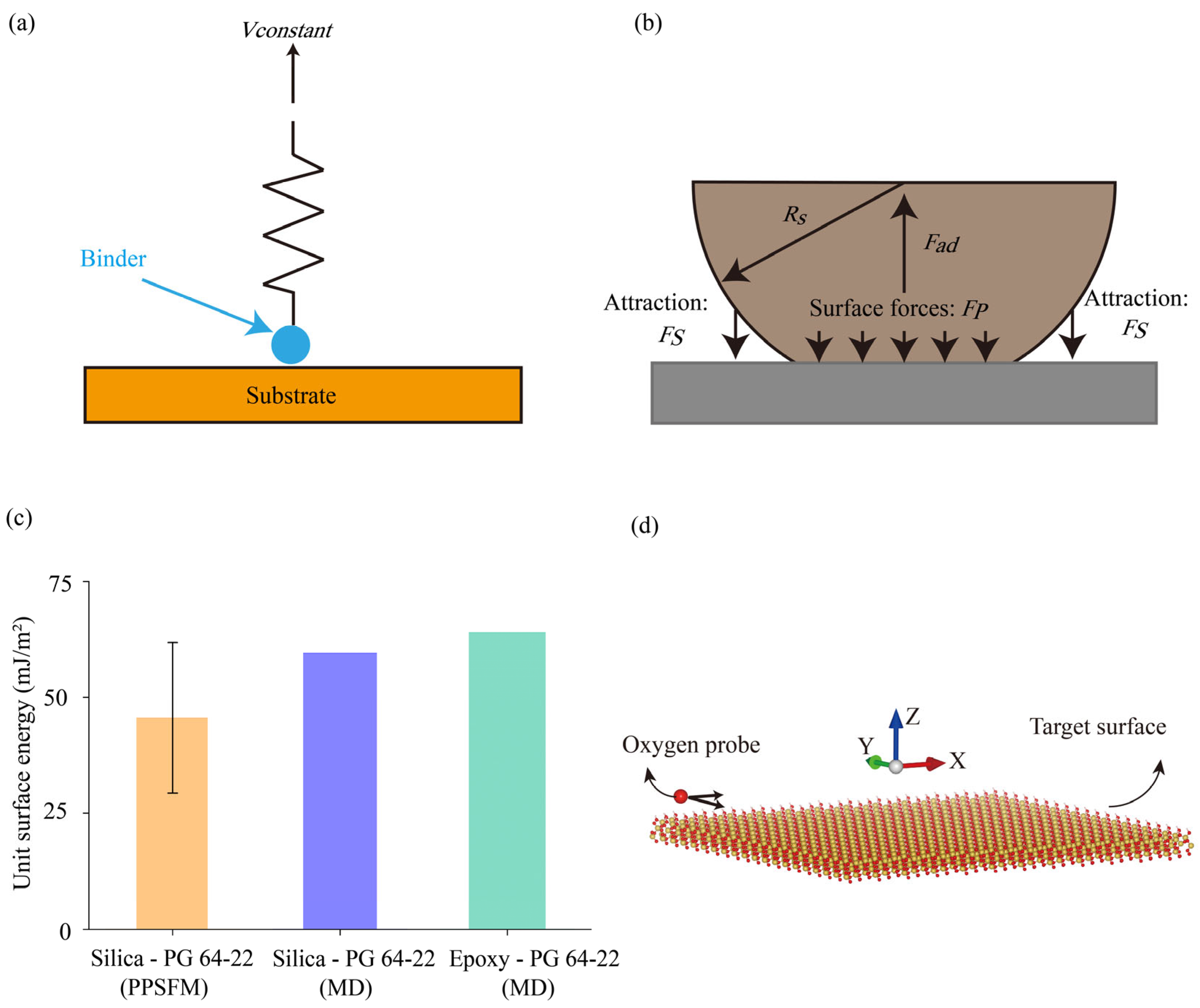
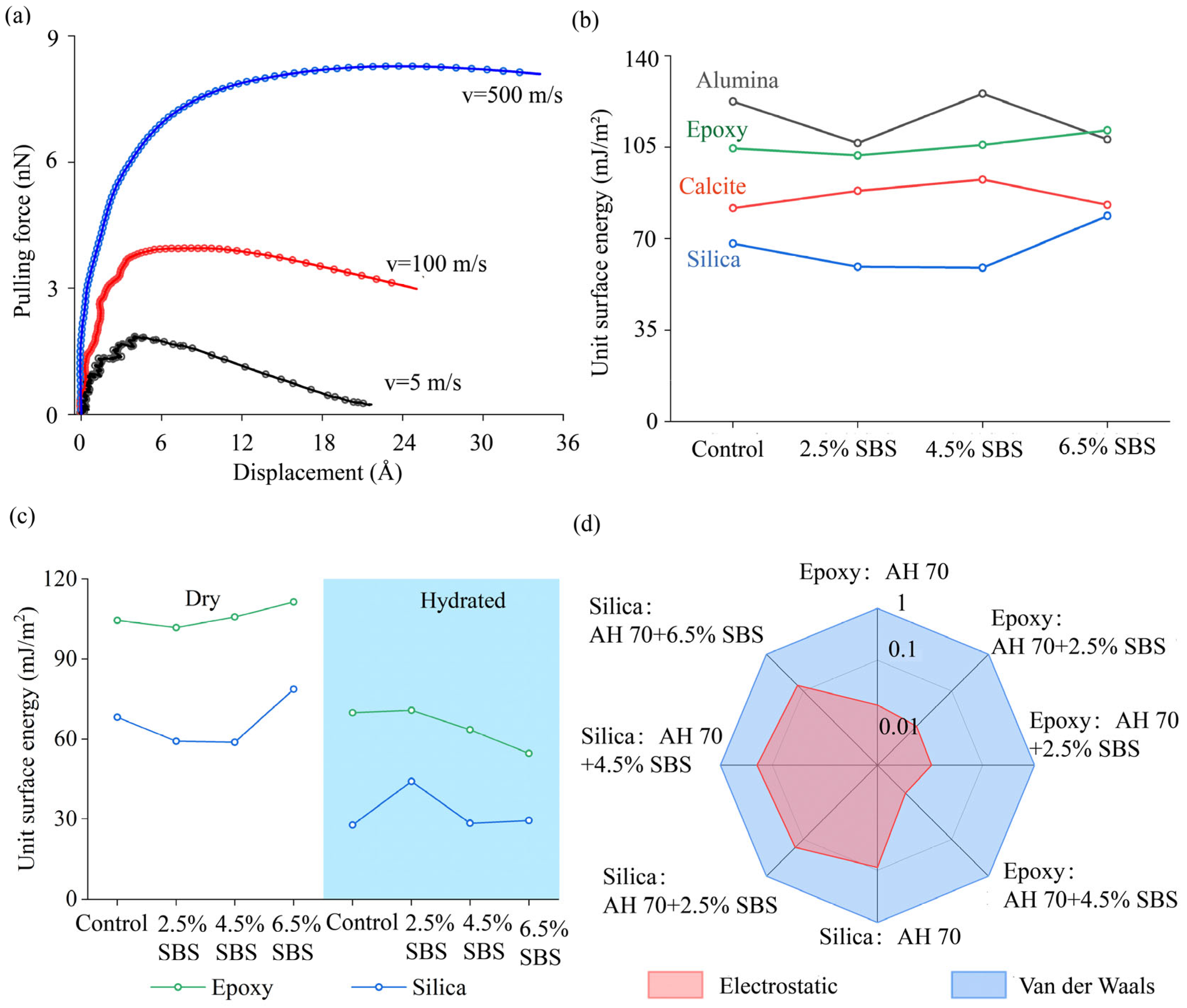
3.1. Unit Surface Energy
3.2. Bell’s Theory
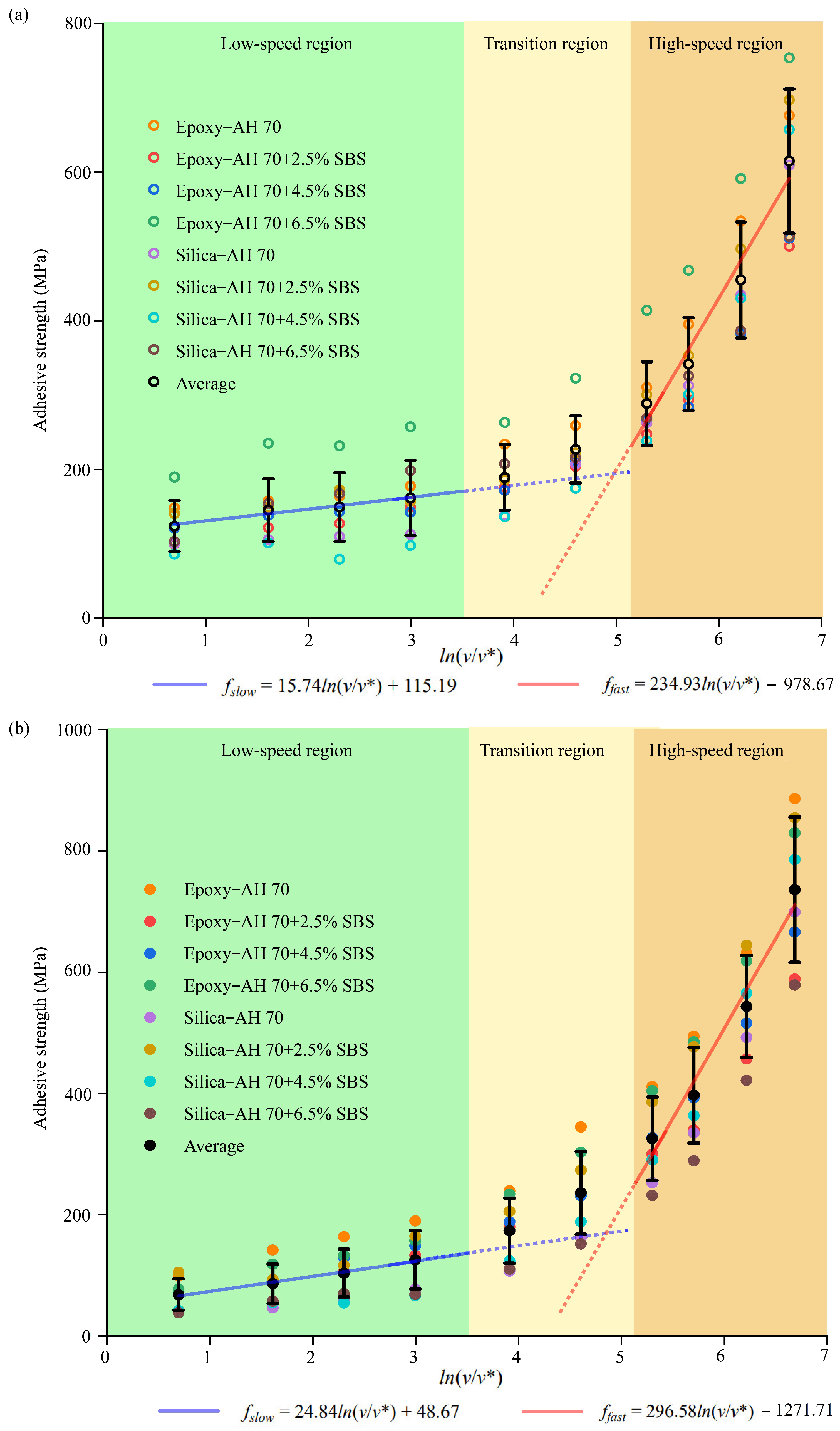
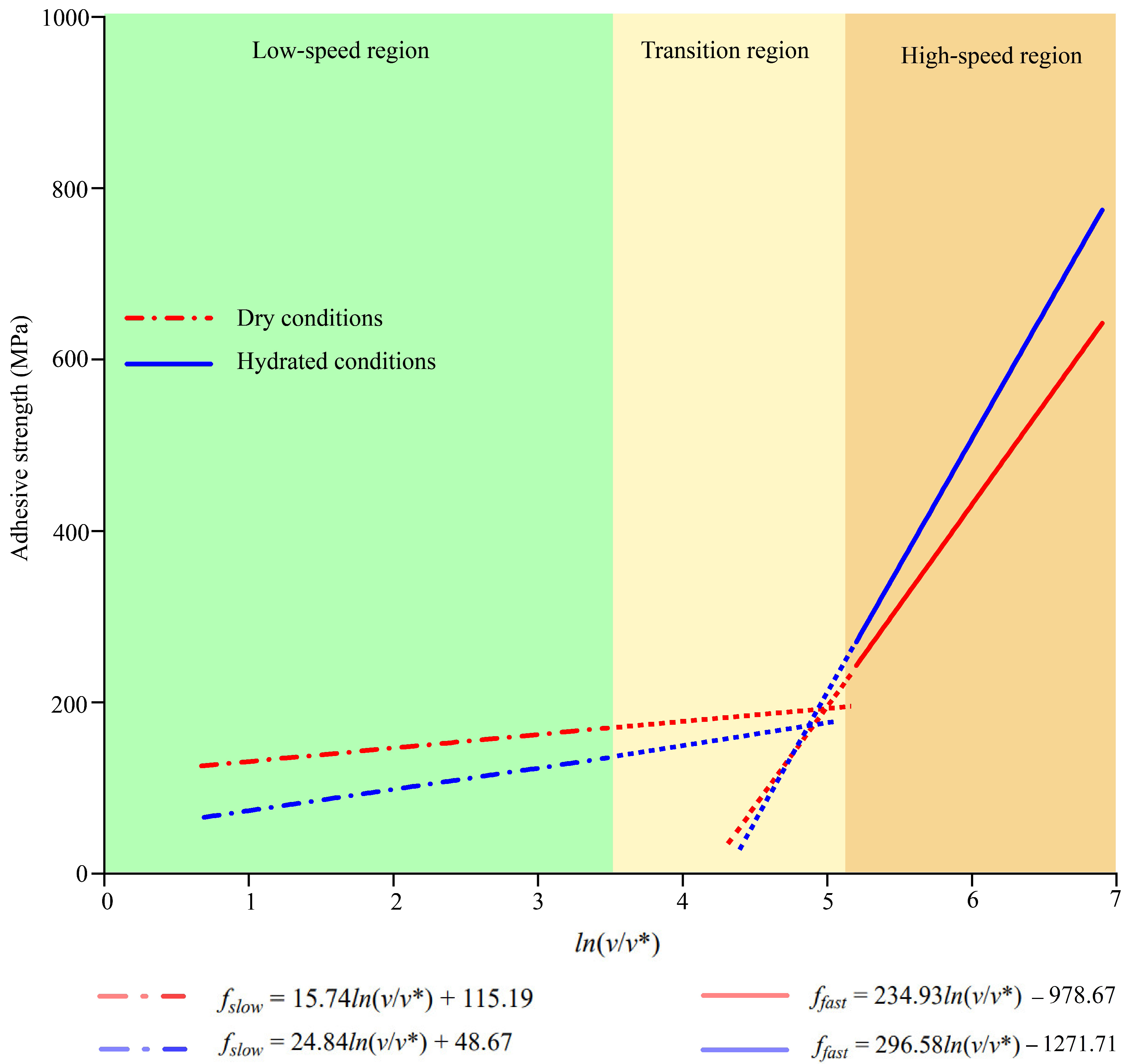
3.3. Adhesive Strength
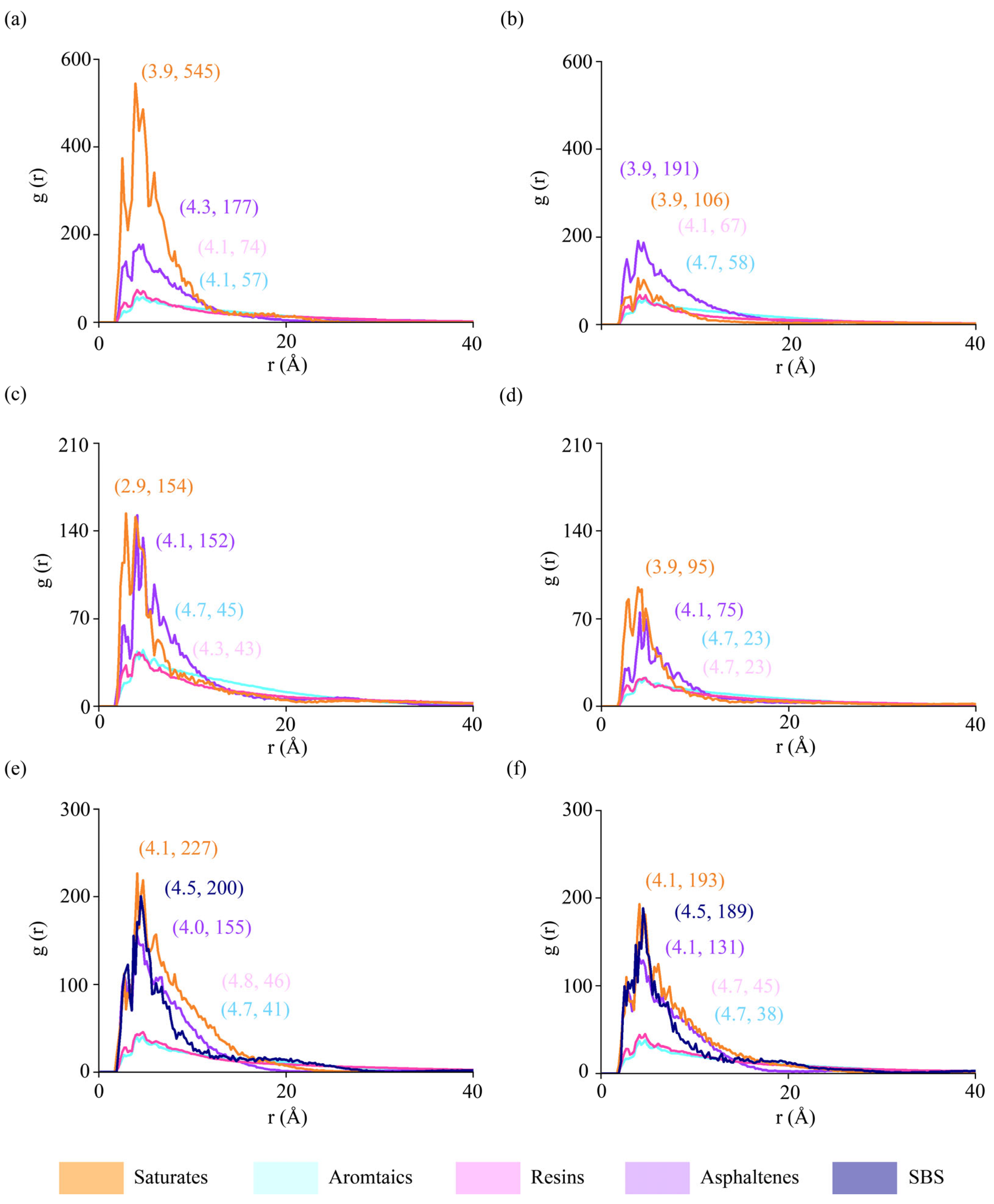
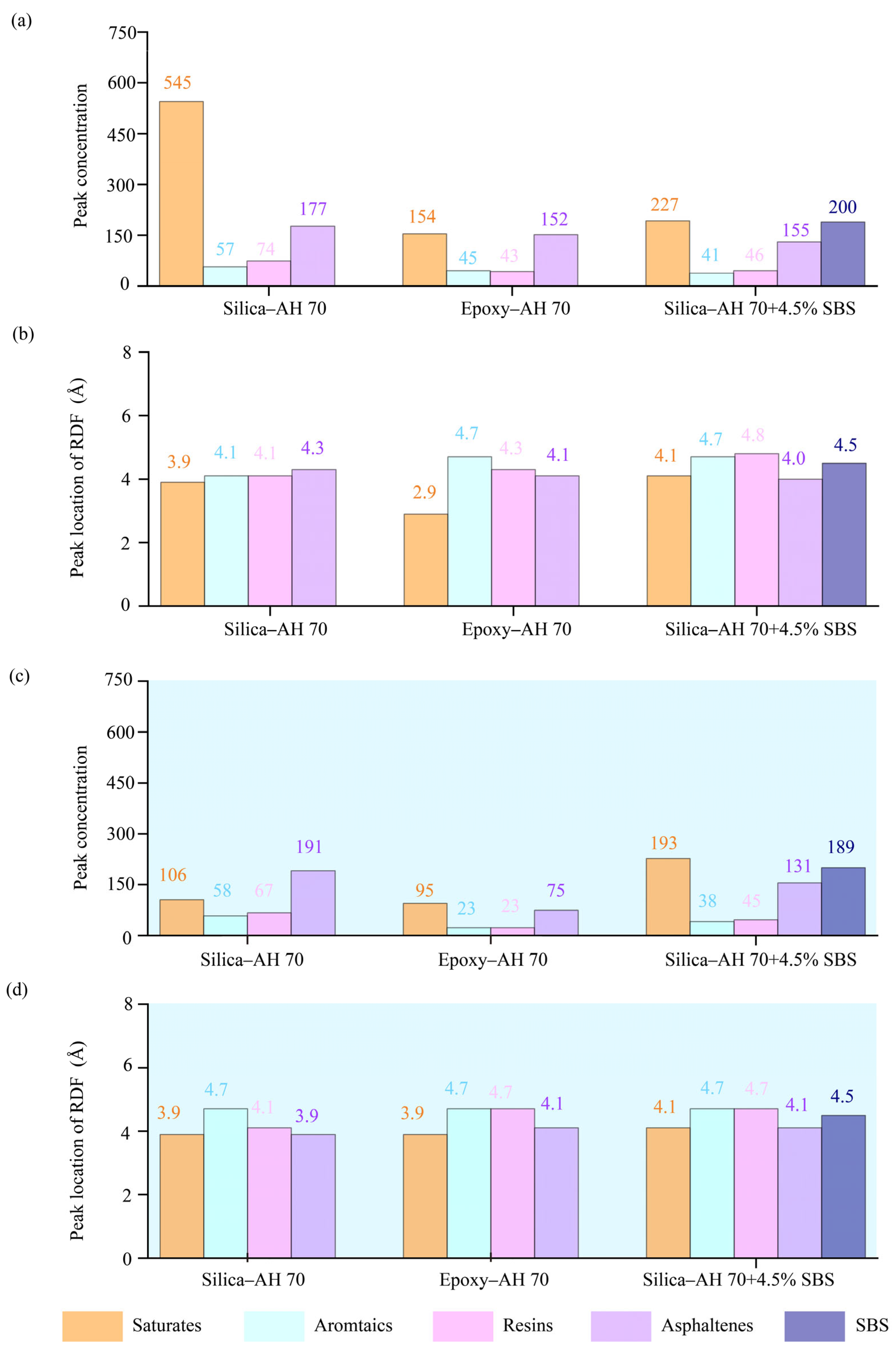
3.4. Radial Distribution Function (RDF)
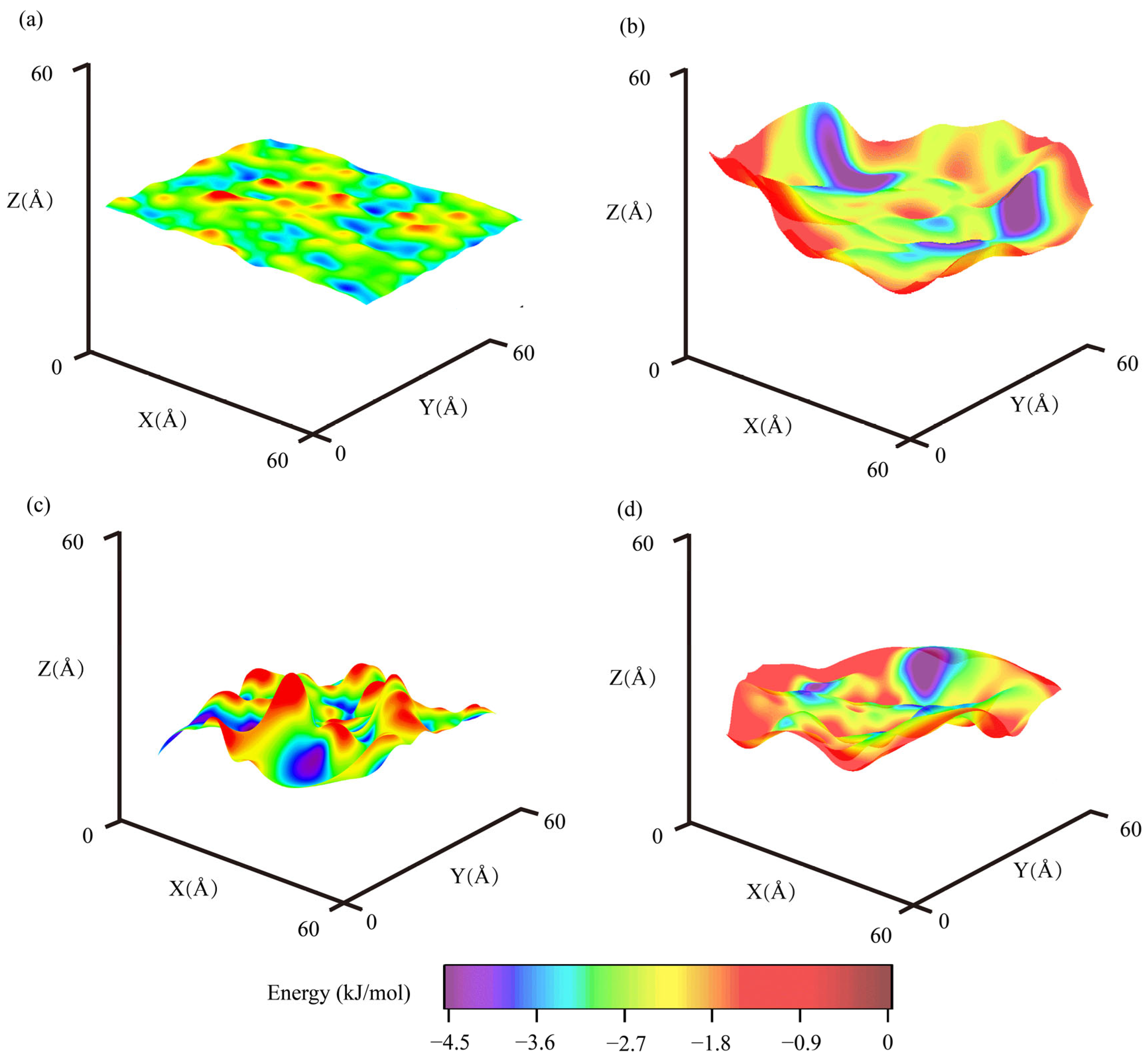
3.5. Interfacial Morphology and Potential Energy Between Asphalt and GFRP
4. Results and Discussions
4.1. Verification of Adhesion Predictions from Molecular Modeling
4.2. Interfacial Behavior from Molecular Modeling
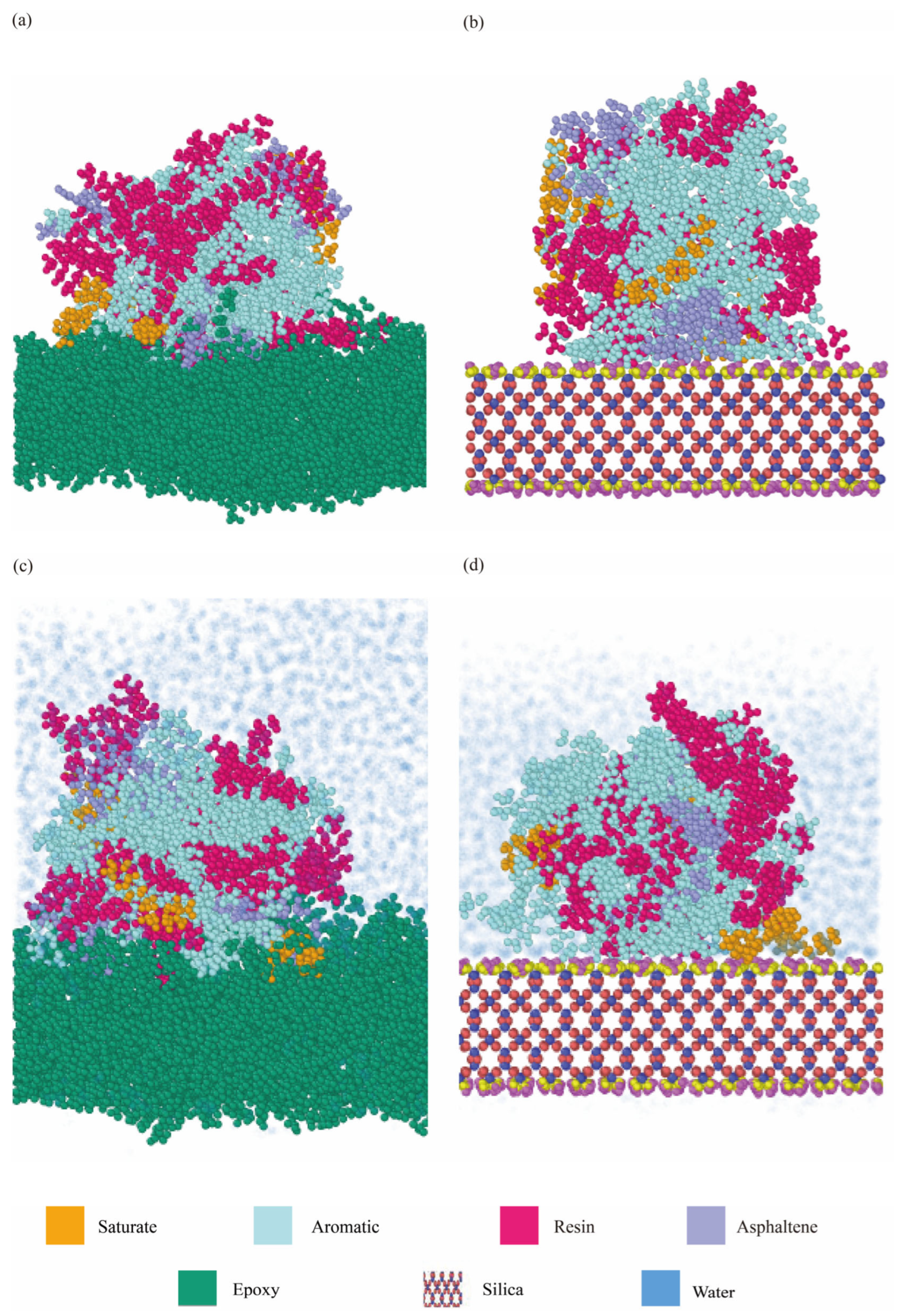
4.2.1. Morphologies of Interfaces Between Aggregates and Binders
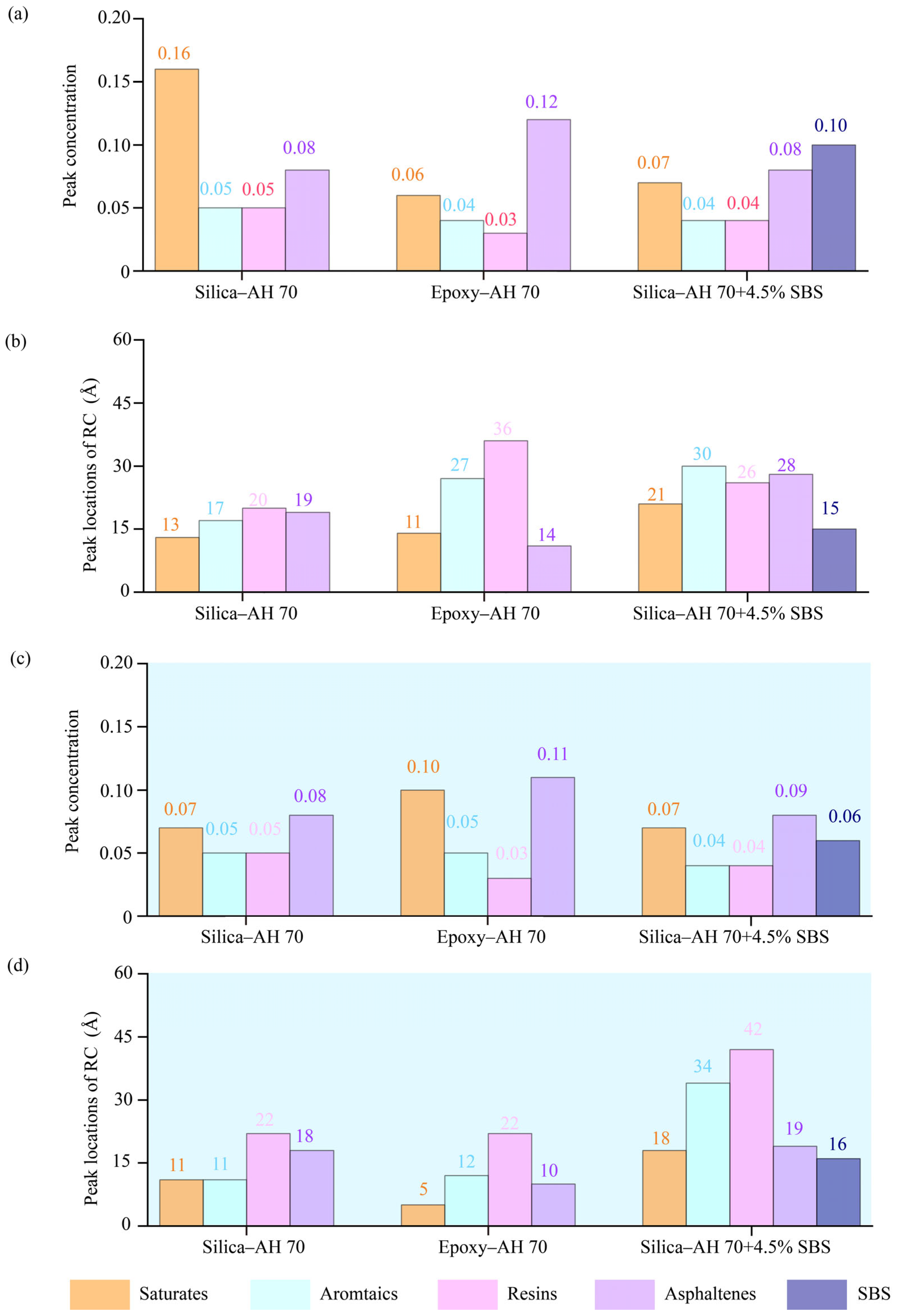
4.2.2. Radial Distribution Functions (RDF) of Binder Components
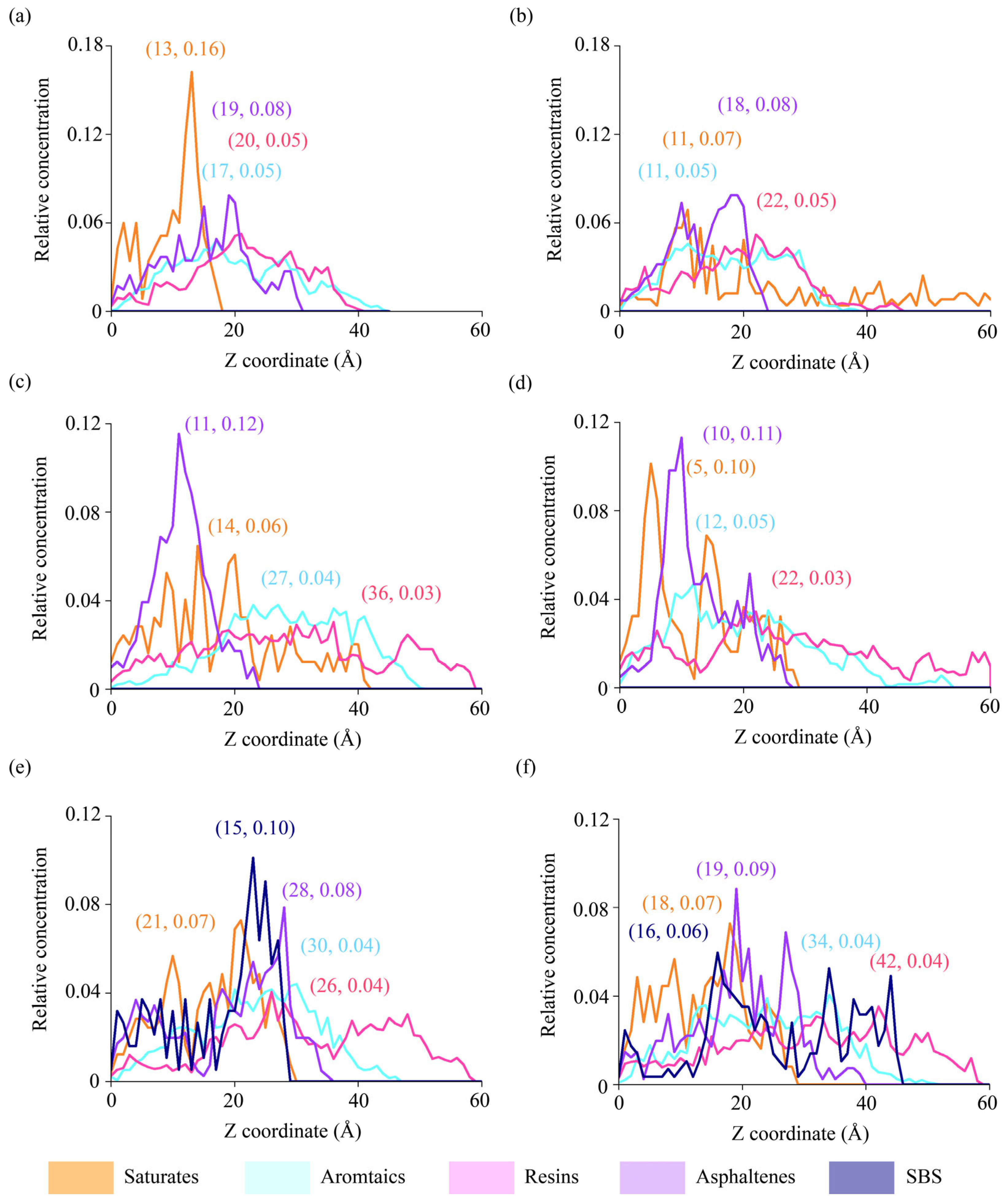
4.2.3. Spatial Distributions of Binder Components
4.2.4. Load–Displacement Curves Between Asphalt Binders and Epoxy
4.2.5. Adhesion Between GFRP Aggregates and Modified Binders
4.2.6. Loading Rates Versus Interfacial Adhesion
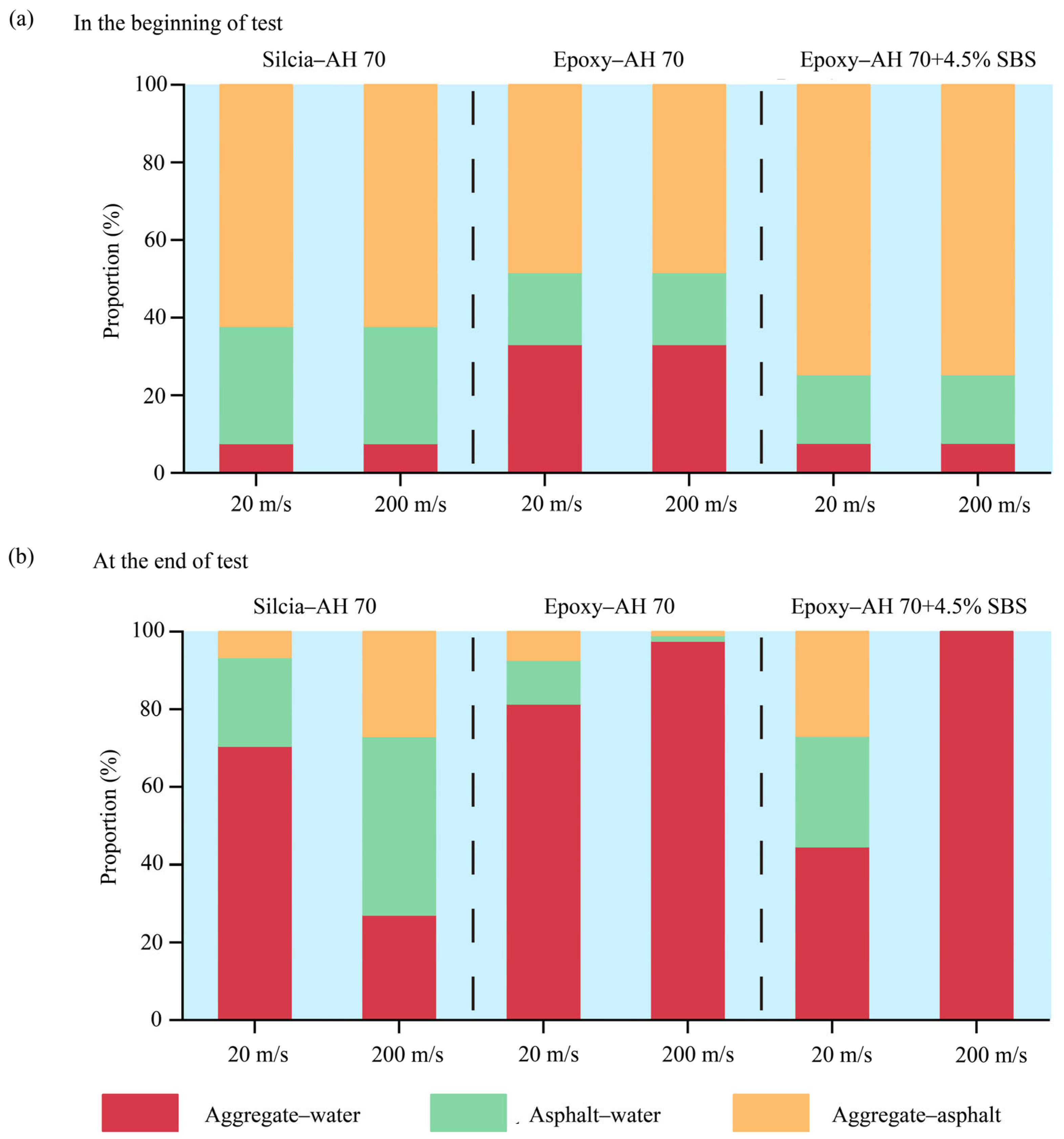
4.2.7. Debonding Between Aggregates and Binders
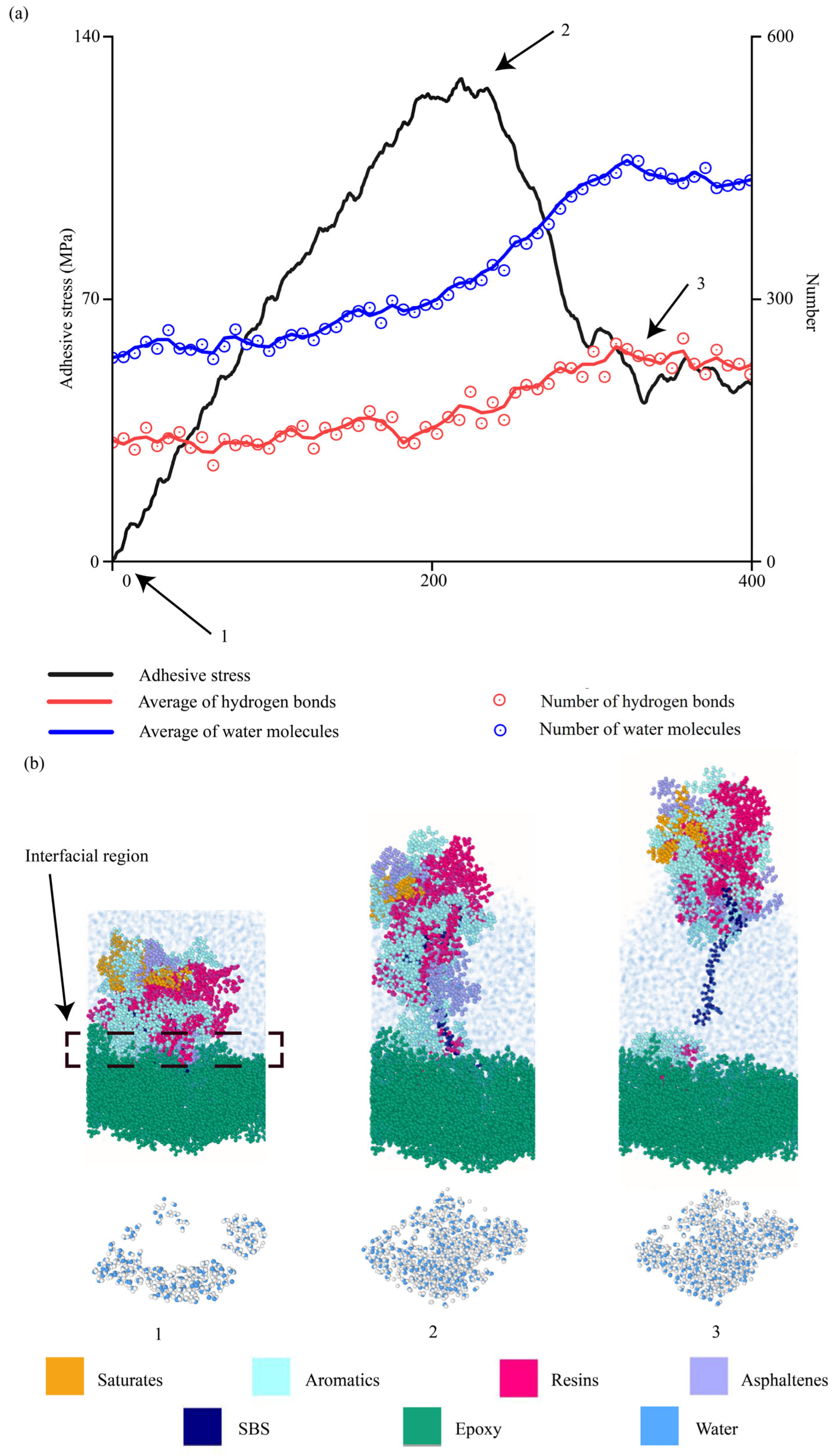
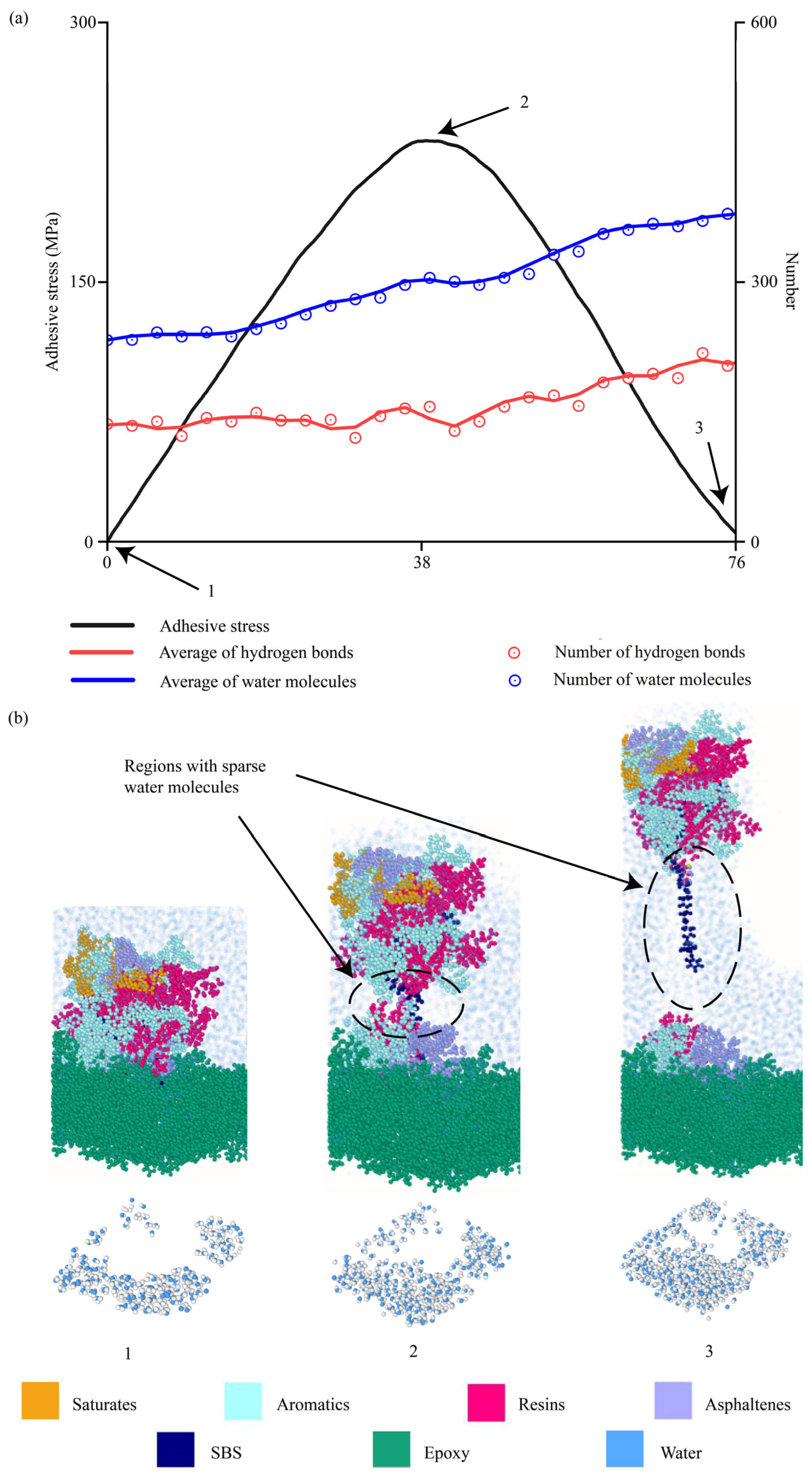
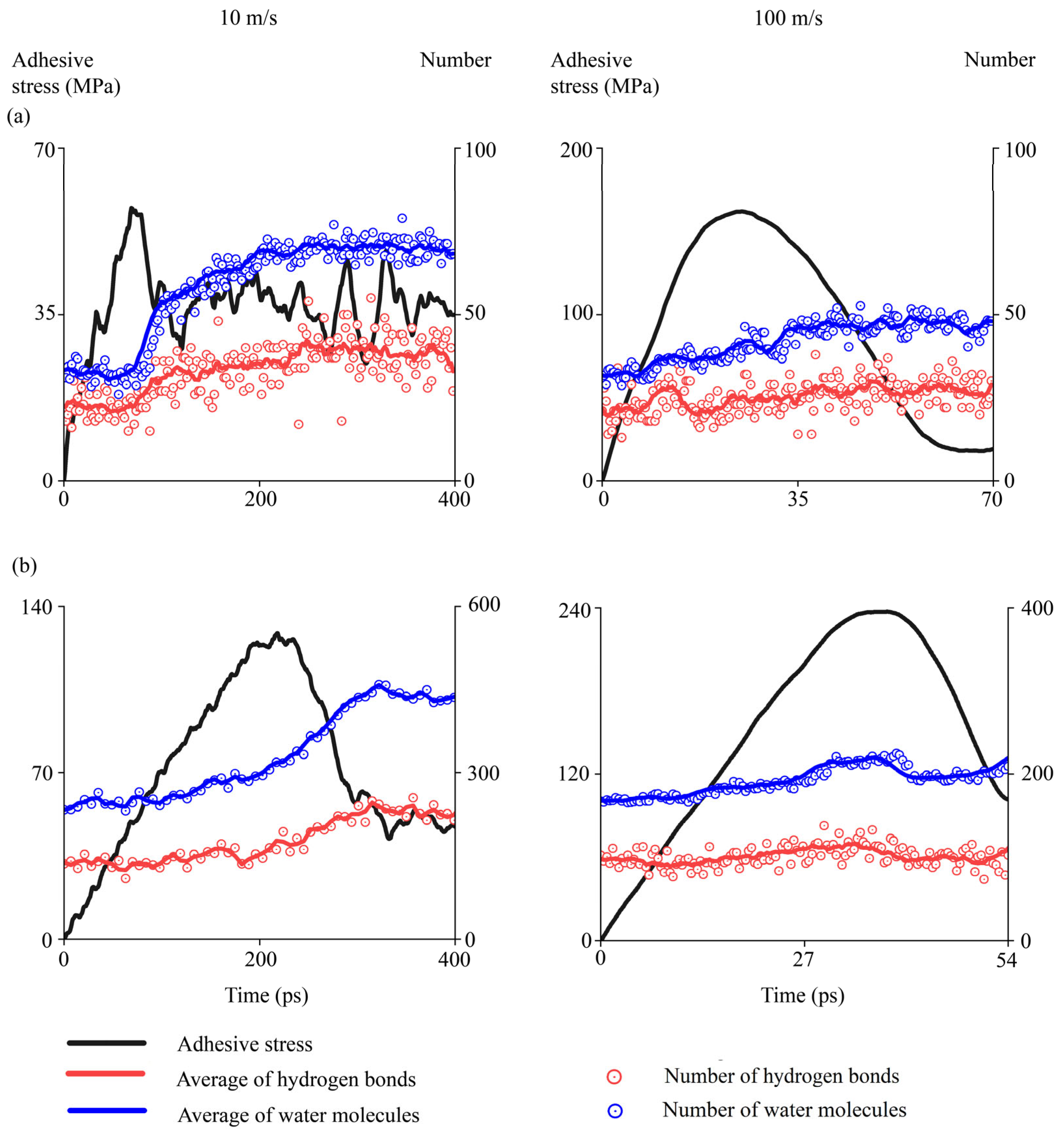
4.2.8. Evolutions of Water Molecules During Interfacial Delamination
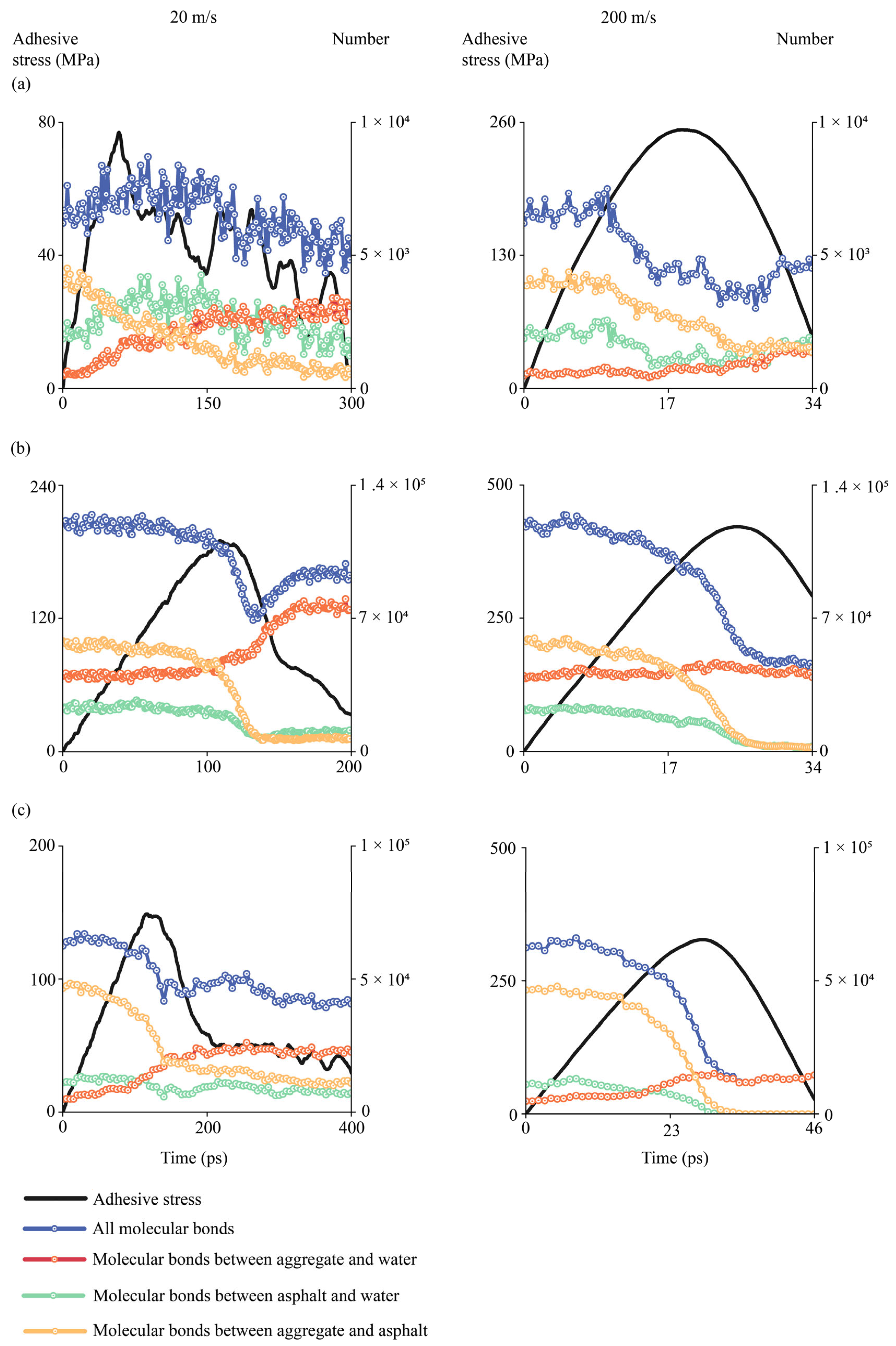
4.2.9. Evolutions of Molecular Bonds During Interfacial Delamination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Dong, S.; Sun, X.; Zhu, M.; Wu, K.; Liu, L. Innovative underwater material from recycled spoil for scour hole remediation in offshore wind foundations. Case Stud. Constr. Mat. 2025, 22, 04522. [Google Scholar] [CrossRef]
- Díaz, H.; Soares, C.G. Review of the current status, technology and future trends of offshore wind farms. Ocean Eng. 2020, 209, 107381. [Google Scholar] [CrossRef]
- Esteban, M.D.; Diez, J.J.; López, J.S.; Negro, V. Why offshore wind energy. Renew. Energy 2011, 36, 444–450. [Google Scholar] [CrossRef]
- Global Wind Energy Council, GWEC Global Wind Report 2024. 2024. Available online: https://www.gwec.net/reports/globalofffshorewindreport (accessed on 29 March 2025).
- Zheng, Y.; Song, Y.; Shen, C.; Chen, N. Aerodynamic and structural analysis for blades of a 15MW floating offshore wind turbine. Ocean. Eng. 2023, 287, 115785. [Google Scholar] [CrossRef]
- Allen, C.; Viscelli, A.; Dagher, H.; Goupee, A.; Gaertner, E.; Abbas, N.; Hall, M.; Barter, G. Definition of the Umaine Volturn US-S Reference Platform Developed for the IEA Wind 15-Megawatt Offshore Reference wind Turbine; National Renewable Energy Laboratory (NREL): Golden, CO, USA; University of Maine: Orono, ME, USA, 2020. [CrossRef]
- Dai, J.; Li, M.; Chen, H.; He, T.; Zhang, F. Progress and challenges on blade load research of large-scale wind turbines. Renew. Energy 2022, 196, 482–496. [Google Scholar] [CrossRef]
- Liu, P.; Barlow, C.Y. Wind turbine blade waste in 2050. Waste Manag. 2017, 62, 229–240. [Google Scholar] [CrossRef]
- Perumal, K.P.S.; Selvarajan, L.; Manikandan, K.P.; Mechanical, C.V. Tribological, and surface morphological studies on the effects of hybrid ilmenite and silicon dioxide fillers on glass fibre reinforced epoxy composites. Biomed. Mater. 2023, 146, 106095. [Google Scholar] [CrossRef]
- Hassan, G.B.; Yaman, S.S.; Kamaki, A.; Azad, A.; Mohammed, A. AlSaad, Long-term exposure of RC columns immersed in seawater or crude oil confined with CFRP fabrics under monotonic or cyclic loading. Case Stud. Constr. Mat. 2023, 18, 01747. [Google Scholar] [CrossRef]
- Alavi, Z.; Khalilpour, K.; Florin, N.; Hadigheh, A.; Hoadley, A. End-of-life wind turbine blade management across energy transition: A life cycle analysis. Resour. Conserv. Recy. 2025, 213, 108008. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, Z.; Zhang, X.; Pang, B. Degradation of GFRP bars in alkaline environments: An experimental and molecular dynamics study. J. Build. Eng. 2023, 77, 107449. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Lin, J.; Liu, W.; Chen, L.; Wang, Z.; Sun, B.; Li, X. Exploring recycling strategies for retired wind turbine blades: The impact of policy subsidies and technological investments using a game-theoretic approach. J. Clean. Prod. 2025, 490, 144628. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, B.; Guo, X.; Zhang, S.; Fei, M.; Shao, L.; Liu, W.; Cao, Y.; Liu, T.; Zhang, J. ild chemical recycling of waste wind turbine blade for direct reuse in production of thermoplastic composites with enhanced performance. Resour. Conserv. Recy. 2025, 215, 108159. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials—Current status. Compos. A Appl. Sci. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Job, S. Recycling glass fibre reinforced composites–history and progress. J. Reinf. Plast. Comp. 2013, 57, 19–23. [Google Scholar] [CrossRef]
- Young, Y.M.; Myung, W.S.; Ho, W.R.; Geon, K.H.; Jeong, S.O.; Sang, J.Y.; Yong, K.K.; Jae, G.L.; Jae, H.K. Pyrolysis characteristics of glass fiber-reinforced plastic (GFRP) under isothermal conditions. J. Anal. Appl. Pyrolysis. 2015, 114, 40–46. [Google Scholar] [CrossRef]
- Larsen, K. Recycling wind turbine blades. Renew. Energy Focus. 2009, 9, 70–73. [Google Scholar] [CrossRef]
- Asokan, P.; Osmani, M.; Price, A.D.F. Assessing the recycling potential of glass fibre reinforced plastic waste in concrete and cement composites. J. Clean. Prod. 2009, 17, 821–829. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Bank, L.C.; Rieder, K.A.; Tian, Y.; Chen, C. Concrete with discrete slender elements from mechanically recycled wind turbine blades. Resour. Conserv. Recycl. 2018, 128, 11–21. [Google Scholar] [CrossRef]
- Olhan, S.; Khatkar, V.; Behera, B.K. Novel high-performance textile fibre-reinforced aluminum matrix structural composites fabricated by FSP. Mater. Sci. Eng. B 2023, 289, 116265. [Google Scholar] [CrossRef]
- Rodin, H.; Nassiri, S.; Englund, K.; Fakron, O.; Li, H. Recycled glass fiber reinforced polymer composites incorporated in mortar for improved mechanical performance. Constr. Build. Mater. 2018, 187, 738–751. [Google Scholar] [CrossRef]
- Fu, B.; Liu, K.; Chen, J.; Teng, J. Concrete reinforced with macro fibres recycled from waste GFRP. Constr. Build. Mater. 2021, 310, e125063. [Google Scholar] [CrossRef]
- You, X.; Lin, L.; Fu, B.; Xiang, Y. Ultra-high performance concrete reinforced with macro fibres recycled from waste GFRP composites. Case Stud. Constr. Mater. 2023, 18, e02120. [Google Scholar] [CrossRef]
- Baturkin, D.; Hisseine, O.A.; Masmoudi, R.; Tagnit-Hamou, A.; Massicotte, L. Valorization of recycled FRP materials from wind turbine blades in concrete. Resour. Conserv. Recycl. 2021, 174, e105807. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A review on recycling and reuse methods for carbon fiber/glass fiber composites waste from wind turbine blades. Compos. Part B Eng. 2021, 215, 108768. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Z.; Hong, B.; Xu, J.; Fan, Z.; Lu, G.; Wang, D.; Oeser, M. Using recycled waste glass fibre reinforced polymer (GFRP) as filler to improve the performance of asphalt mastics. J. Clean. Prod. 2022, 336, 130357. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Ortega-López, V.; Manso, J.M. Raw-crushed wind-turbine blade: Waste characterization and suitability for use in concrete production. Resour. Conserv. Recycl. 2023, 198, 107160. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Ni, A. Recycling and reuse of composite materials for wind turbine blades: An overview. J. Reinf. Plast. Compos. 2019, 38, 567–577. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, B.; Dai, J.; Lu, G.; Peng, K.; Wang, Y. Value-added recycling strategies for decommissioned wind turbine blades: A review. Renew. Sustain. Energy Rev. 2025, 222, 115950. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J.; Wang, Y.; Hu, T.; Xu, X.; Yin, B. Enhancing the properties and engineering performance of asphalt binders and mixtures with physicochemically treated waste wind turbine blades. Constr. Build. Mater. 2025, 473, 141023. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, Q.; Xiang, Z.; Zhong, S.; Huang, X. Performance and Modification Mechanism of Recycled Glass Fiber of Wind Turbine Blades and SBS Composite-Modified Asphalt. Appl. Sci. 2023, 13, 6335. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Z.; Shi, X.; Sun, X.; Zhou, T.; Yang, H.; Bie, R.; Zhang, M. Experimental Study and Process Simulation on Pyrolysis Characteristics of Decommissioned Wind Turbine Blades. Energies 2024, 17, 3229. [Google Scholar] [CrossRef]
- China Aggregates Association. About 3 Billion Tons of Sand and Gravel Aggregate Are in Demand! National Development and Reform Commission: The Total Planned Scale of the National Highway Network Is About 461000 Kilometers! 2022. Available online: https://www.zgss.org.cn/gongqiuxinxi/2022/14295.html (accessed on 20 August 2025).
- Shobeiri, V.; Bennett, B.; Xie, T.; Visintin, P. Mix design optimization of waste-based aggregate concrete for natural resource utilization and global warming potential. J. Clean. Prod. 2024, 449, 141756. [Google Scholar] [CrossRef]
- Shobeiri, V.; Bennett, B.; Xie, T.; Visintin, P. A comprehensive data driven study of mechanical properties of concrete with waste-based aggregates: Plastic, rubber, slag, glass and concrete. Case Stud. Constr. Mat. 2024, 20, e02815. [Google Scholar] [CrossRef]
- Xu, L.; Yu, J.; Huang, B.; Lao, J.; Wu, H.; Jiang, X.; Xie, T.; Dai, J. Green and low-carbon matrices for Engineered/Strain-Hardening Cementitious Composites (ECC/SHCC): Toward sustainable and resilient infrastructure. J. Clean. Prod. 2025, 496, 144968. [Google Scholar] [CrossRef]
- Qian, L.; Xu, L.; Huang, B.; Li, Y.; Lan, J.; Gong, F.; Guan, H. Green and low-carbon matrices for Engineered/Strain-Hardening Cementitious Composites (ECC/SHCC): Toward sustainable and resilient infrastructur. Resour. Conserv. Recy. 2025, 212, 107999. [Google Scholar] [CrossRef]
- Mansourkhaki, A.; Ameri, M.; Habibpour, M.; Daryaee, D. The effect of polybutadiene rubber (PBR) on chemical and rheological properties of the binder including RAP. Constr. Build. Mater. 2020, 244, 118320. [Google Scholar] [CrossRef]
- Tan, T.; Meng, J.; Rahbar, N.; Li, H.; Papandreou, G.; Maryanoff, C.A.; Soboyejo, W.O. Effects of silane on the interfacial fracture of a parylene film over a stainless steel substrate. Mater. Sci. Eng. C 2012, 32, 550–557. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Quantification of aggregate-Binder adhesion in asphalt mixtures using particle probe scanning force microscopes. In Proceedings of the 2017 International Multiconference of Engineers and Computer Scientists, IMECS 2017, Hong Kong, 15–17 March 2017; Available online: https://www.iaeng.org/publication/IMECS2017/IMECS2017_pp1063-1067.pdf (accessed on 14 August 2025).
- Li, Y.; Yang, J.; Tan, T. An experimental study on adhesion between steel and cement pastes using particle probe scanning force microscopy. J. Am. Ceram. Soc. 2019, 102, 1347–1361. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Statistical Analyses of Aggregate Mineral–Binder Adhesion: Measuring with Particle Probe Scanning Force Microscopes. Transp. Res. Record. 2017, 2632, 25–31. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Measuring adhesion between steel and early-hydrated Portland cement using particle probe scanning force microscopy. Cem. Concr. Compos. 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Zhou, J.; Li, X.; He, L. Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials 2019, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wang, Q.; Wang, W.; Xiao, Y.; Fang, C. Polyurethane/graphene oxide nanocomposite and its modified asphalt binder: Preparation, properties and molecular dynamics simulation. Mater. Des. 2021, 209, 109994. [Google Scholar] [CrossRef]
- Alam, S.; Hossain, Z. Changes in fractional compositions of PPA and SBS modified asphalt binders. Constr. Build. Mater. 2017, 152, 386–393. [Google Scholar] [CrossRef]
- Xiong, H.; Hou, H.; Lu, Z.; Zhang, H. Study on self-healing performance of microcracks in SBS/waste rubber powder composite-modified asphalt based on molecular dynamics simulation. Chem. Phys. Lett. 2024, 851, 141486. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.; Yin, J.; Zhang, H.; Dou, H.; Li, B. Investigation of Compatibility Mechanisms and Diffusion Behavior of Polymer SBS-Modified Asphalt Compatibilizer Using Molecular Dynamics Simulation. Materials 2025, 18, 2238. [Google Scholar] [CrossRef]
- Alonso-Romero, S.; Medina-Torres, L.; Zitzumbo, R.; Nuñez-Ramirez, D.M. Styrene-butadiene branched star-shaped asphalt modifiers: Synthesis and mechanical characterization. Chem. Phys. Lett. 2019, 207, 933–945. [Google Scholar] [CrossRef]
- Hu, B.; Huang, W.; Yu, J.; Xiao, Z.; Wu, K. Study on the Adhesion Performance of Asphalt-Calcium Silicate Hydrate Gel Interface in Semi-Flexible Pavement Materials Based on Molecular Dynamics. Materials 2021, 14, 4406. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Arab, B. Comparative investigation of thermal and mechanical properties of cross-linked epoxy polymers with different curing agents by molecular dynamics simulation. J. Mol. Graph. Model. 2015, 62, 157–164. [Google Scholar] [CrossRef]
- Wang, X.; Jian, W.; Oral, B.; Leung, C.K.Y.; Denvid, L. Degradation of epoxy/glass interface in hygrothermal environment: An atomistic investigation, Compos. Part B Eng. 2021, 206, 108534. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Adhesion between modified binders and aggregate minerals at ambient conditions measured with particle-probe scanning force microscopes. J. Mater. Civ. Eng. 2017, 29, 04017068. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Measuring adhesion between modified asphalt binders and aggregate minerals: Use of particle probe scanning force microscopes. Transp. Res. Record. 2016, 2574, 117–123. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Study on adhesion between asphalt binders and aggregate minerals under ambient conditions using particle-modified atomic force microscope probes. Constr. Build. Mater. 2015, 101, 159–165. [Google Scholar] [CrossRef]
- Pnina, D.O.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia colidihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins Struct. Funct. Genet. 1988, 1, 31–47. [Google Scholar] [CrossRef]
- Perry, T.D., IV; Cygan, R.T.; Mitchell, R. Molecular models of alginic acid: Interactions with calcium ions and calcite surfaces. Geochim. Cosmochim. Acta 2006, 70, 3508–3532. [Google Scholar] [CrossRef]
- Mishra, R.K.; Fernández-Carrasco, L.; Flatt, R.J.; Heinz, H. A force field for tricalcium aluminate to characterize surface properties, initial hydration, and organically modified interfaces in atomic resolution. Dalton Trans. 2014, 43, 10602–10616. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.; Hui, D.; Lau, D. Molecular dynamics simulations on adhesion of epoxy-silica interface in salt environment. Compos. Part B Eng. Dec. 2017, 15, 165–172. [Google Scholar] [CrossRef]
- Ylikantola, A.; Linnanto, J.; Knuutinen, J.; Oravilahti, A.; Toivakka, M. Molecular modeling studies of interactions between sodium polyacrylate polymer and calcite surface. Appl. Surf. Sci. 2013, 276, 43–52. [Google Scholar] [CrossRef]
- Labora, M.; Rudolph, H. Adsorption of molecular SiO2 on a clean Si(1 0 0) surface. Surf. Sci. 2010, 604, 21–25. [Google Scholar] [CrossRef]
- Effenberger, H.; Mereiter, K.; Zemann, J. Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Z. Krist-Cryst Mater. 1981, 156, 233–244. [Google Scholar] [CrossRef]
- Newnham, E.E.; Haan, Y.M.D. Refinement of the a Al2O3, Ti2O3, V2O3 and Cr2O3 structures. Z. Krist-Cryst. Mater. 1962, 117, 235–237. [Google Scholar] [CrossRef]
- Guo, F.; Pei, J.; Zhang, J.; Li, R.; Liu, P.; Wang, D. Study on Adhesion Property and Moisture Effect between SBS Modified Asphalt Binder and Aggregate Using Molecular Dynamics Simulation. Materials 2025, 15, 6912. [Google Scholar] [CrossRef]
- Hu, K.; Chen, Y.; Qin, M.; Hu, R.; Hu, X.; Tao, X. An eco-friendly crack sealant approach for asphalt pavement by using laboratory tests and molecular dynamic simulation. Mater. Today Sustain. 2024, 27, 100893. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Lu, Y.; Zhou, X.; Hu, Y.; Sun, J.; Fu, G. Effect of dynamic water pressure on the adhesion behavior of recycled asphalt-aggregate interface by molecular dynamics method. Constr. Build. Mater. 2024, 382, 131296. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Raos, G.D.; Ma, F.; Zhao, P.; Hou, Y. Molecular dynamics simulation of adhesion at the asphalt-aggregate interface: A review. Surf. Interfaces 2024, 44, 103706. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Izrailev, S.; Stepaniants, S.; Balsera, M.; Oono, Y.; Schulten, K. Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 1997, 72, 1568–1581. [Google Scholar] [CrossRef]
- Park, S.; Schulten, K. Calculating potentials of mean force from steered molecular dynamics simulations. J. Chem. Phys. 2004, 120, 5946–5961. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 1997, 78, 2690. [Google Scholar] [CrossRef]
- Ackbarow, T.; Chen, X.; Keten, S.; Buehler, M.J. Hierarchies, multiple energy barriers, and robustness govern the fracture mechanics of α-helical and β-sheet protein domains. Proc. Natl. Acad. Sci. USA 2007, 104, 16410–16415. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.; Hu, X.; Zhang, X. Flexible pavement response analysis under dynamic loading at different vehicle speeds and pavement surface roughness conditions. J. Transp. Eng. B-Pavements 2020, 146, 04020040. [Google Scholar] [CrossRef]
- Stewart, M.G.; Netherton, M.D. Reliability-based design load factors for explosive blast loading. J. Perform. Constr. Facil. 2015, 29, B4014010. [Google Scholar] [CrossRef]
- Wei, G.; Hao, C.; Jin, H.; Deng, Y. Experimental investigation of high-velocity impact response and compression after impact behavior of continuous carbon fiber thermoplastic composites. Thick. Wall. Struct. 2024, 205, 112578. [Google Scholar] [CrossRef]
- Nossek, M.M.M.; Petrinic, N.; Hiermaier, S.; Thoma, K. Adaptive multi-scale modeling of high velocity impact on composite panels. Compos. Part A Appl. Sci. Manuf. 2014, 58, 56–64. [Google Scholar] [CrossRef]
- Blair, D.P. Blast vibration dependence on charge length, velocity of detonation and layered media. Int. J. Rock Mech. Min. 2014, 65, 29–39. [Google Scholar] [CrossRef]
- Aldas, G.G.U. Explosive charge mass and peak particle velocity (PPV)-frequency relation in mining blast. J. Geophys. Eng. 2010, 7, 223. [Google Scholar] [CrossRef]
- Wang, S.; Du, F.; Alghamdi, S.; Feng, J.; Chen, F.; Wang, Z.; Wu, C.; Xiong, H.; Liu, K.; Zheng, Y.; et al. Effects of loading rates on interfacial adhesion between aggregates and asphalt binders. Constr. Build. Mater. 2023, 408, 133454. [Google Scholar] [CrossRef]
- Al-Rawashdeh, A.S.; Sargand, S. Performance assessment of a warm asphalt binder in the presence of water by using surface free energy concepts and nanoscale techniques. J. Mater Civil Eng. 2014, 26, 803–811. [Google Scholar] [CrossRef]
- Cheng, D.; Dallas, L.; Robert, L.L.; James, C.H. Moisture damage evaluation of asphalt mixtures by considering both moisture diffusion and repeated-load conditions. Transport. Res. Rec. 2003, 1832, 42–49. [Google Scholar] [CrossRef]
- Bell, G.I. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; He, F.; Feng, J.; Wu, C.; Wang, Z.; Chen, F.; Alghamdi, S.; Zheng, Y.; Du, F.; et al. Rate-dependent interfacial adhesive strength and toughness between aggregates and modified asphalt binders in ambient conditions. J. Mol. Liq. 2025, 429, 127679. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Zhao, D.; Hou, J.; Wu, C.; Denvid, L.; Lik-ho, T. Degradation of fiber/matrix interface under various environmental and loading conditions: Insights from molecular simulations. Constr. Build. Mater. 2023, 309, 131101. [Google Scholar] [CrossRef]
- Baykara, M.Z.; Schwendemann, T.C.; Altman, E.I.; Schwarz, U.D. Three-dimensional atomic force microscopy-taking surface imaging to the next level. Adv. Mater. 2010, 22, 26–27. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Hasz, K.; Zhao, M.; Johnson, A.T.C.; Carpick, R.W.; Martini, A. Nanoscale friction behavior of transition-metal dichalcogenides: Role of the chalcogenide. ACS Nano 2020, 14, 16013–16021. [Google Scholar] [CrossRef] [PubMed]
- Vazirisereshk, M.R.; Hasz, K.; Carpick, R.W.; Martini, A. Friction anisotropy of MoS2: Effect of tip-sample contact quality. J. Phys. Chem. Lett. 2020, 11, 6900–6906. [Google Scholar] [CrossRef]
- Xu, W.; Yin, Z.; Zheng, Y. FRP-soil interfacial mechanical properties withmolecular dynamics simulations: Insights into friction and creep behavior. Int. J. Numer. Anal. Methods Geomech. 2023, 47, 2951–2967. [Google Scholar] [CrossRef]
- Tan, T.; Wu, C.; Wang, Z.; Chen, F.; Feng, J. A lightweight asphalt mixture prepared using recycled wind turbine blades. Patent CN202311011194.0, 11 August 2023. under review. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Hbcalculator: A tool for hydrogen bond distribution calculations in molecular dynamics simulations. J. Chem. Inf. Model. 2024, 64, 1772–1777. [Google Scholar] [CrossRef]
- Sangiorgio, V.; Uva, G.; Fatiguso, F.; Adam, J.M. A new index to evaluate exposure and potential damage to RC building structures in coastal areas. Eng. Fail. Anal. 2019, 100, 439–455. [Google Scholar] [CrossRef]
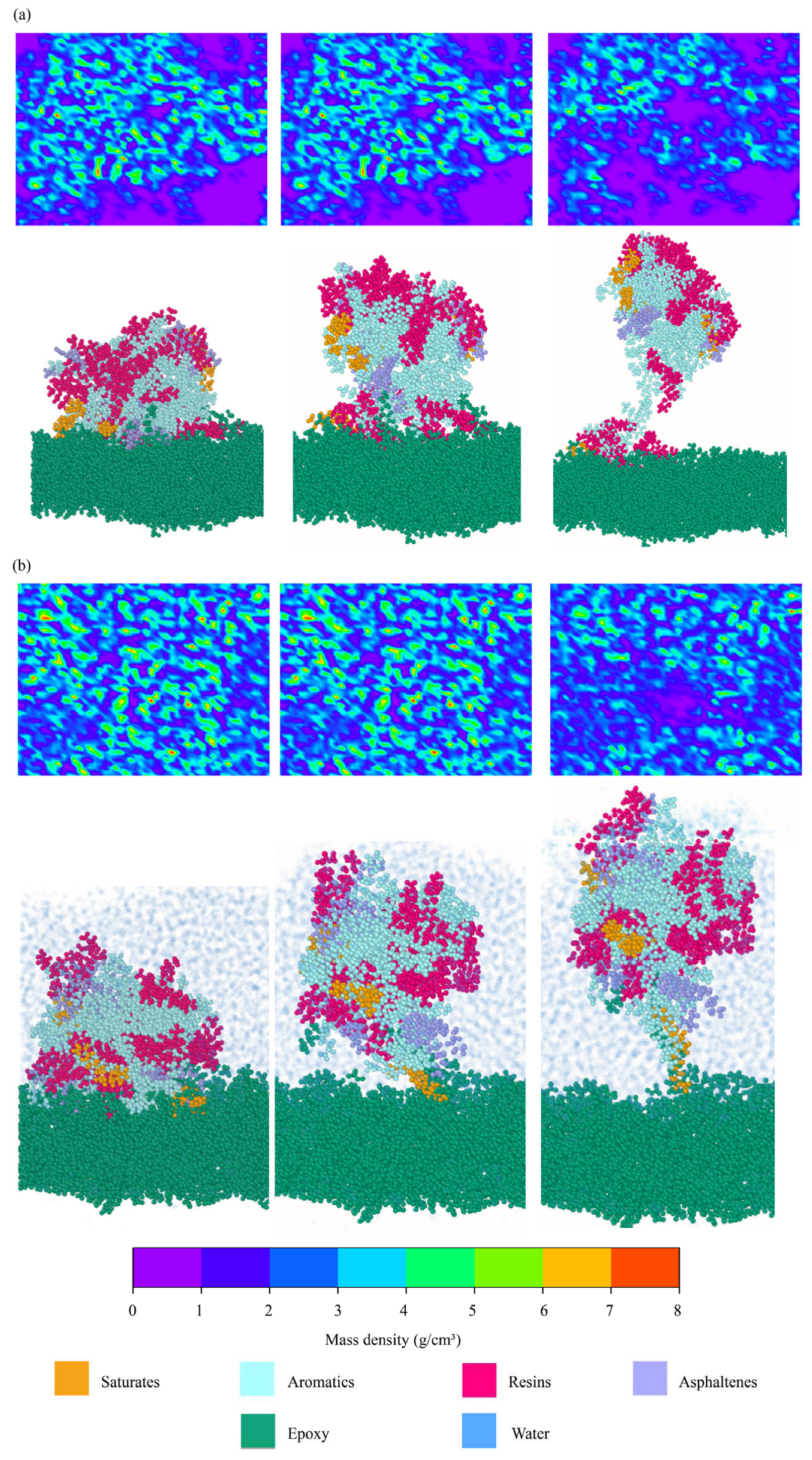
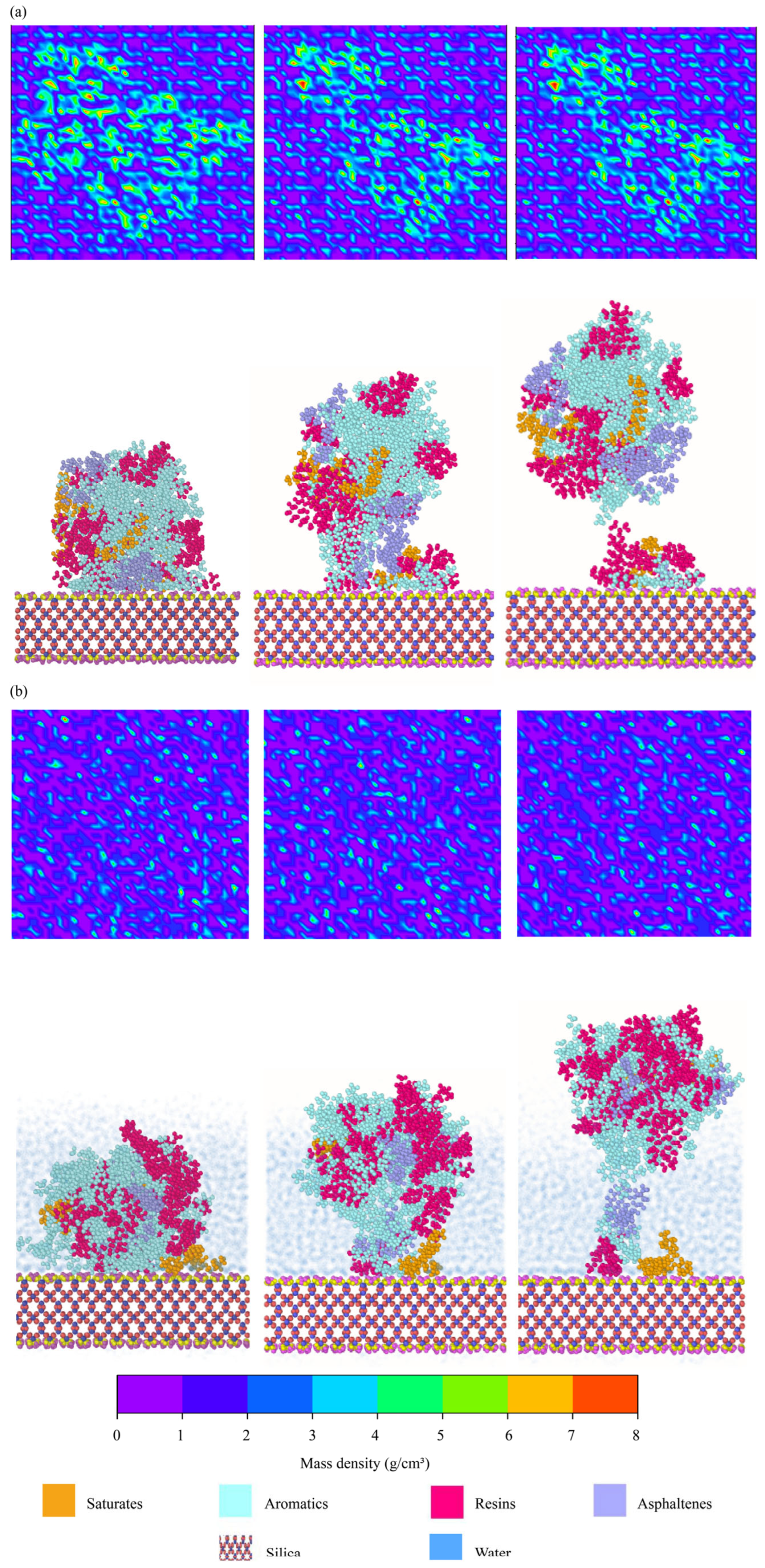
| Saturates | Aromatics | Resins | Asphaltenes | ||
|---|---|---|---|---|---|
| AH 70 | wt% | 4.3% | 52.8% | 31.6% | 11.3% |
| PG 64-22 | wt% | 10.0% | 38.3% | 31.8% | 19.9% |
| (Å) | (Å) | (Å) | |
|---|---|---|---|
| Epoxy | 85.05 | 85.05 | 25.00 |
| Alumina | 98.91 | 99.94 | 20.13 |
| Silica | 93.55 | 88.38 | 17.63 |
| Calcite | 115.66 | 119.76 | 19.77 |
| Interfaces | Morphology RMS Rh (Å) | Energy Avg ± RMS Ea ± Re (kJ/mol) | ||
|---|---|---|---|---|
| Aggregate | Binder | Aggregate | Binder | |
| Silica–AH 70 in dry condition | 0.44 | 1.44 | −2.62 ± 0.84 | −2.23 ± 0.96 |
| Epoxy–AH 70 in dry condition | 3.30 | 1.98 | −2.49 ± 1.16 | −1.99 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, S.; He, F.; Wu, C.; Wang, Z.; Du, F.; Huston, D.; Dewoolkar, M.; Tan, T. Molecular Adhesion Between Asphalt and Glass Fiber-Reinforced Composites from Recycled Wind Turbine Blades in Dry and Hydrated Conditions. Materials 2025, 18, 3936. https://doi.org/10.3390/ma18173936
Feng J, Wang S, He F, Wu C, Wang Z, Du F, Huston D, Dewoolkar M, Tan T. Molecular Adhesion Between Asphalt and Glass Fiber-Reinforced Composites from Recycled Wind Turbine Blades in Dry and Hydrated Conditions. Materials. 2025; 18(17):3936. https://doi.org/10.3390/ma18173936
Chicago/Turabian StyleFeng, Jiehao, Shuliang Wang, Fan He, Chuanhai Wu, Zhixiang Wang, Fen Du, Dryver Huston, Mandar Dewoolkar, and Ting Tan. 2025. "Molecular Adhesion Between Asphalt and Glass Fiber-Reinforced Composites from Recycled Wind Turbine Blades in Dry and Hydrated Conditions" Materials 18, no. 17: 3936. https://doi.org/10.3390/ma18173936
APA StyleFeng, J., Wang, S., He, F., Wu, C., Wang, Z., Du, F., Huston, D., Dewoolkar, M., & Tan, T. (2025). Molecular Adhesion Between Asphalt and Glass Fiber-Reinforced Composites from Recycled Wind Turbine Blades in Dry and Hydrated Conditions. Materials, 18(17), 3936. https://doi.org/10.3390/ma18173936





