Synthesis, Characterization, and In Vitro Cytotoxic Evaluation of Neodymium-Doped Cobalt Ferrite Nanoparticles on Human Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis by Combustion Method
2.3. Characterization Methods
2.4. Cell Culture
2.5. Cell Viability Assessment
2.6. Lactate Dehydrogenase (LDH) Assay
2.7. Immunofluorescence Assay
2.8. Statistical Analysis
3. Results
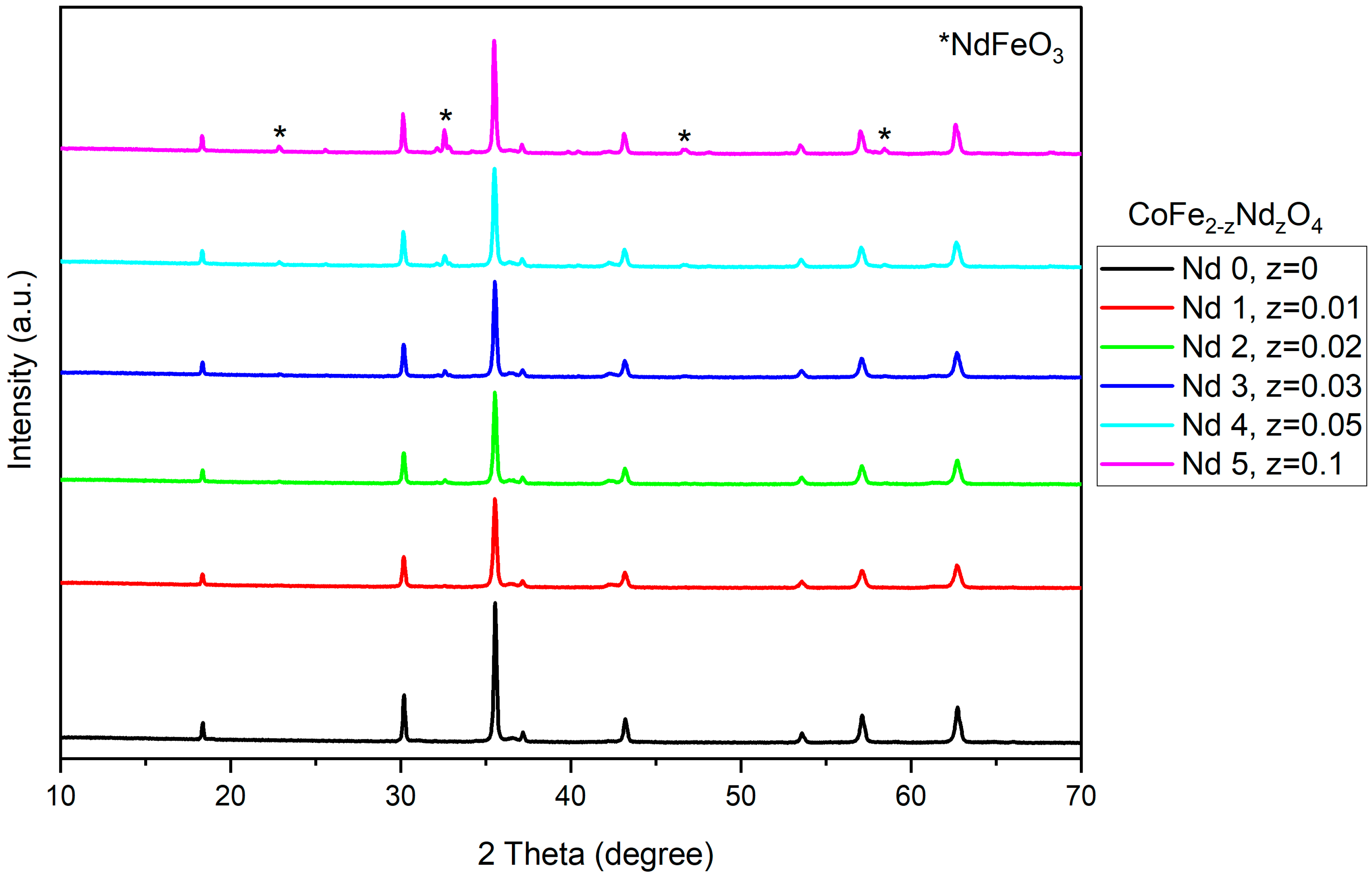
3.1. X-Ray Diffraction
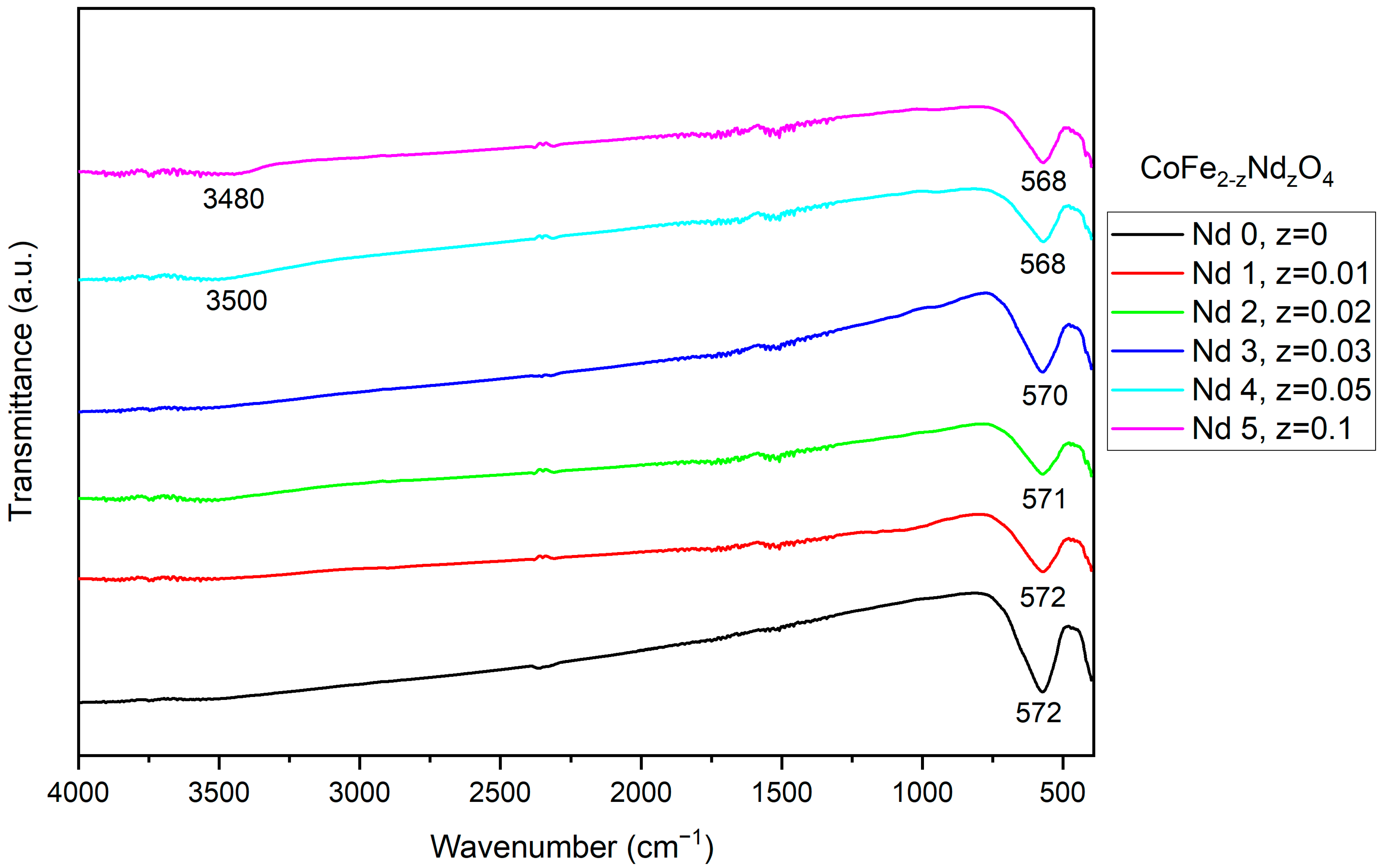
3.2. Fourier-Transform Infrared Spectroscopy
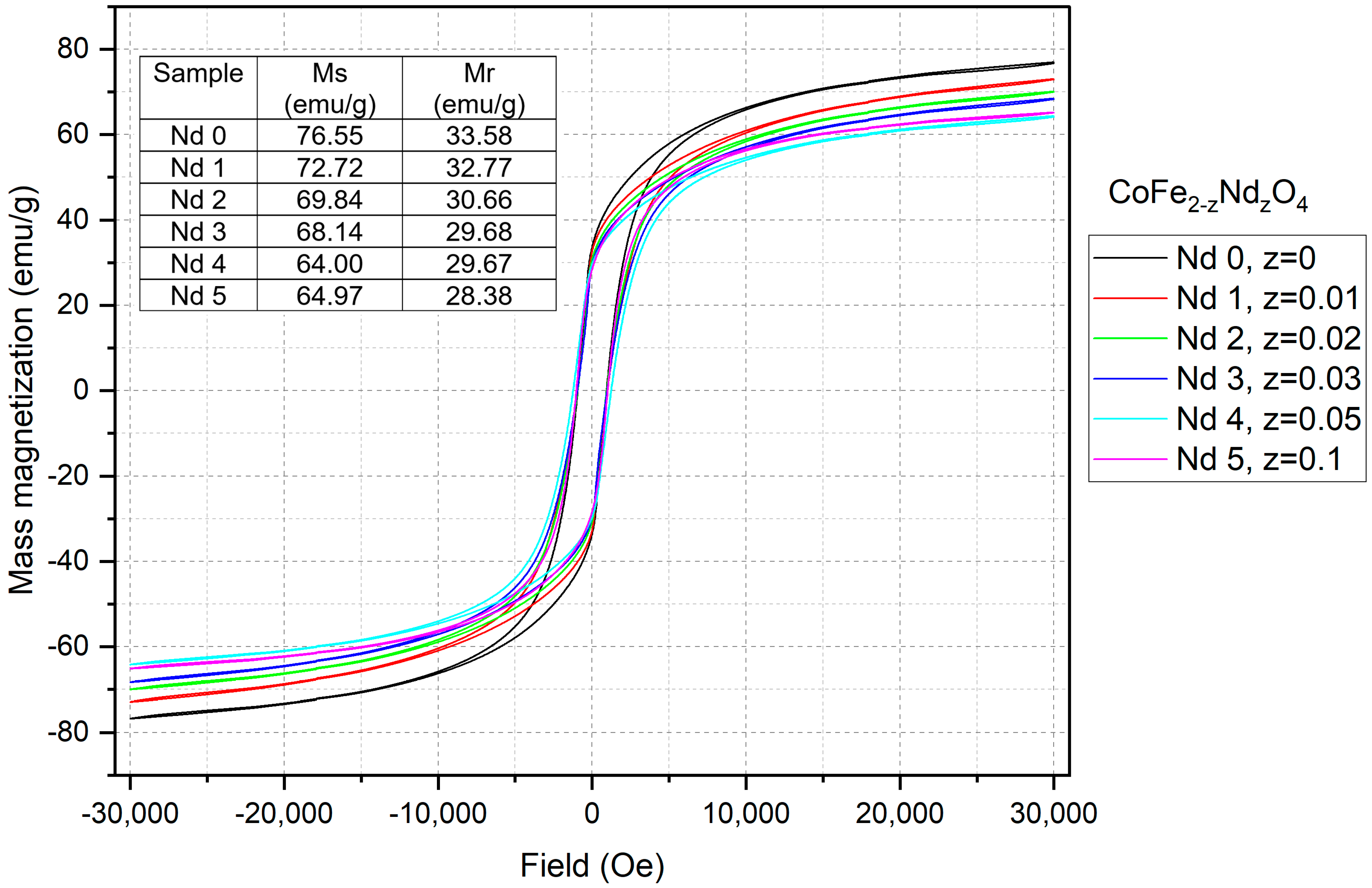
3.3. Vibrational Sample Magnetometry
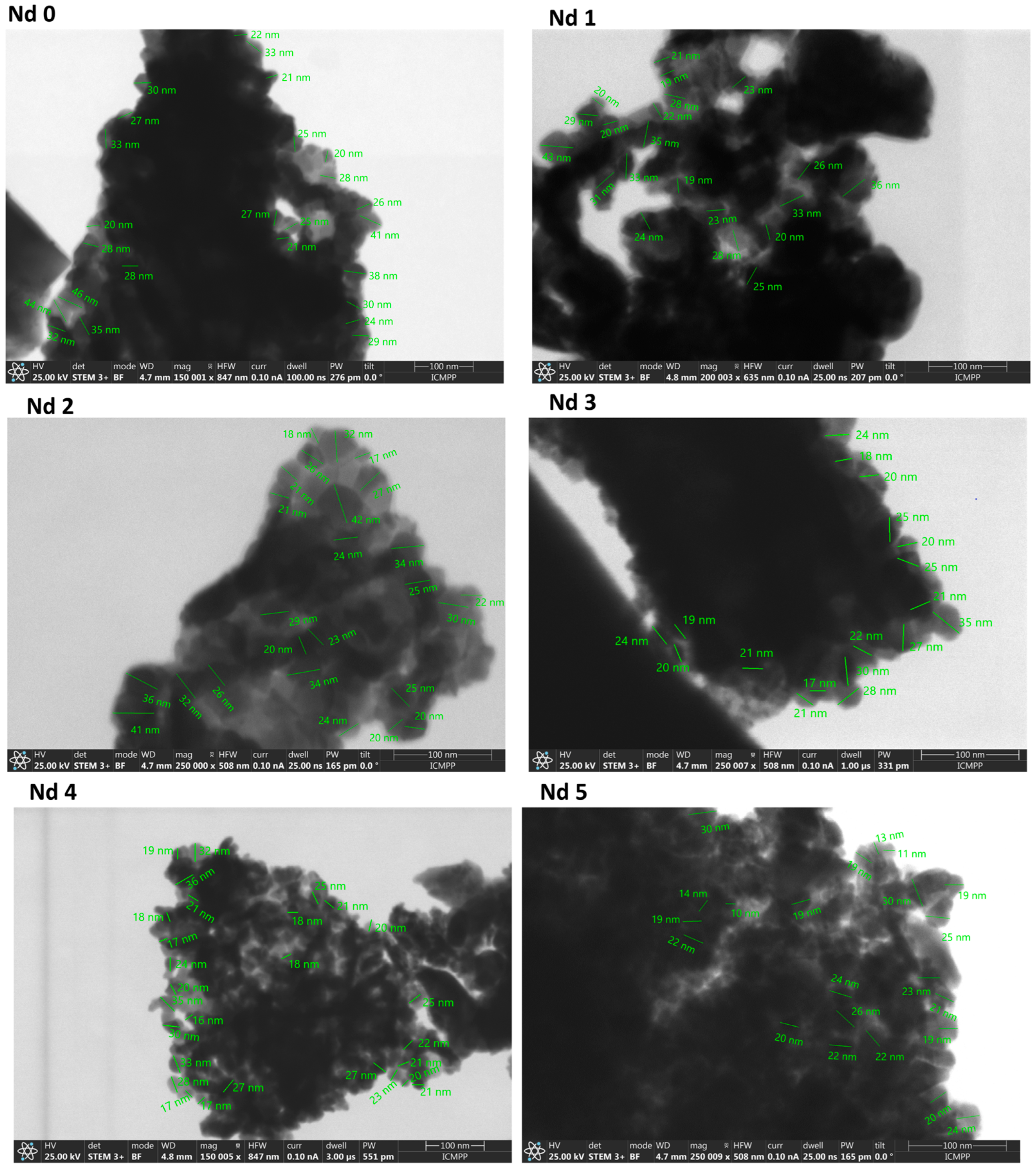
3.4. Scanning Transmission Electron Microscopy
3.5. Energy Dispersive Analysis by X-Ray
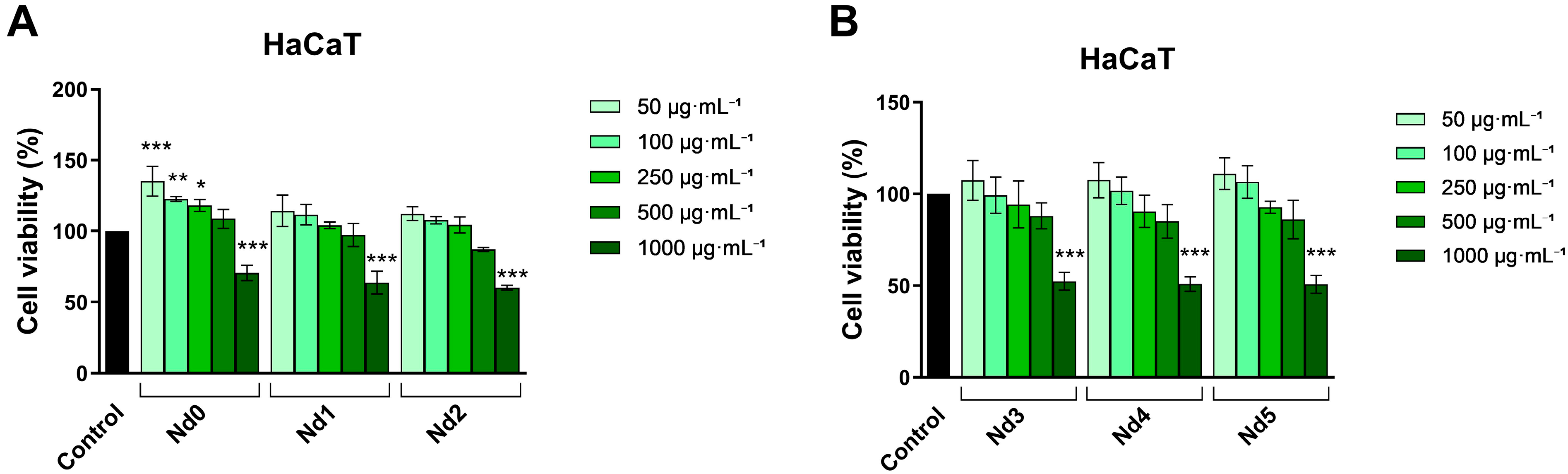
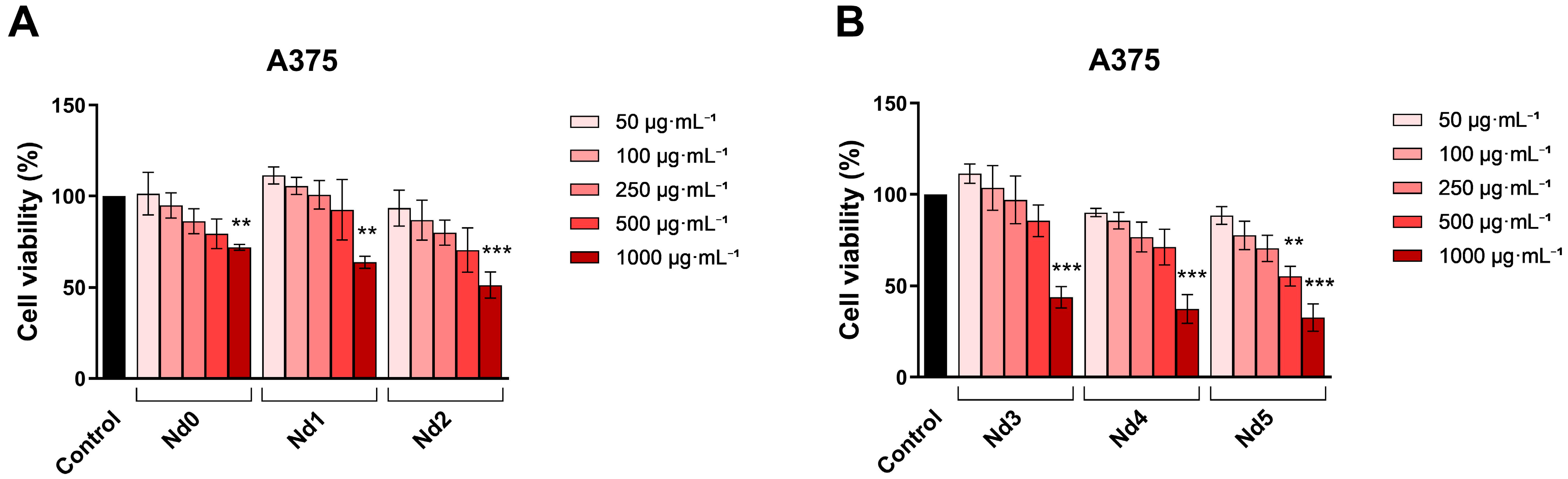
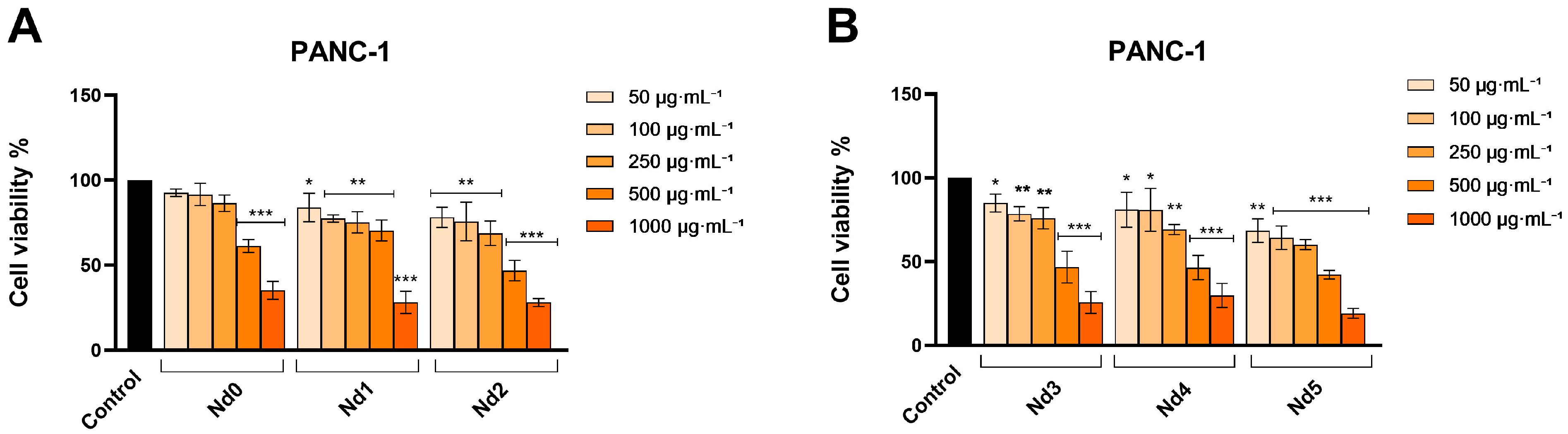
3.6. Nd Compounds Decrease Cancer Cell Viability
3.7. Nd Compounds Increase Lactate Dehydrogenase Release
3.8. Nd Compounds Induce Cell Morphology Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormo-nal Therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef]
- Hazarika, D.; Sarma, S.; Shankarishan, P. Nanotechnology in Cancer Therapeutics, Diagnosis, and Management. BioTechnologia 2024, 105, 287. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Shahalaei, M.; Azad, A.K.; Sulaiman, W.M.A.W.; Derakhshani, A.; Mofakham, E.B.; Mallandrich, M.; Ku-marasamy, V.; Subramaniyan, V. A Review of Metallic Nanoparticles: Present Issues and Prospects Focused on the Preparation Methods, Characterization Techniques, and Their Theranostic Applications. Front. Chem. 2024, 12, 1398979. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhang, W.C.; Guo, Y.W.; Chen, X.Y.; Zhang, Y.N. Metal Nanoparticles as a Promising Technology in Targeted Cancer Treatment. Drug Deliv. 2022, 29, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Saba, I.; Batoo, K.M.; Wani, K.; Verma, R.; Hameed, S. Exploration of Metal-Doped Iron Oxide Nanoparticles as an Antimicrobial Agent: A Comprehensive Review. Cureus 2024, 16, e69556. [Google Scholar] [CrossRef]
- Sahayaraj, R.; Enoch, K.; Pati, S.S.; Somasundaram, A.A. Cobalt Doped Fe3O4 Nanoparticles with Improved Magnetic Anisotropy and Enhanced Hyperthermic Efficiency. Ceram. Int. 2025, 51, 20786–20797. [Google Scholar] [CrossRef]
- Kashid, P.; Suresh, H.K.; Mathad, S.N.; Shedam, R.; Shedam, M.R. A Review on Synthesis, Properties and Ap-plications on Cobalt Ferrite. Int. J. Adv. Sci. Eng 2022, 9, 2567. [Google Scholar] [CrossRef]
- Rauwel, E.; Al-Arag, S.; Salehi, H.; Amorim, C.O.; Cuisinier, F.; Guha, M.; Rosario, M.S.; Rauwel, P. Assessing Cobalt Metal Nanoparticles Uptake by Cancer Cells Using Live Raman Spectroscopy. Int. J. Nanomed. 2020, 15, 7051–7062. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Yang, S.; Wen, D.; Krzyzowska, S.; Taemaitree, F.; Zaman, S.; Fortuni, B.; Rocha, S.; Mulvaney, P.; Uji-i, H.; et al. Neodymium-Doped Nanocrystals for Diffraction-Limited in Vitro Temperature Sensing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Batista, J.A.D.; de Oliveira, R.M.; Lima, C.H.M.; Lana, M.L.; dos Anjos, V.C.; Bell, M.J.V.; Rocha, M.S. Probing the Potential of Rare Earth Elements in the Development of New Anticancer Drugs: Single Molecule Studies. Beilstein J. Nanotechnol. 2025, 16, 187–194. [Google Scholar] [CrossRef]
- Oprean, C.; Zambori, C.; Borcan, F.; Soica, C.; Zupko, I.; Minorics, R.; Bojin, F.; Ambrus, R.; Muntean, D.; Dan-ciu, C.; et al. Anti-Proliferative and Antibacterial In Vitro Evaluation of the Polyurethane Nanostructures Incorporating Pentacyclic Triterpenes. Pharm. Biol. 2016, 54, 2714–2722. [Google Scholar] [CrossRef]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium Aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Mioc, M.; Mioc, A.; Gogulescu, A.; Mardale, G.; Avram, Ș.; Maksimović, T.; Mara, B.; Șoica, C. Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines. Molecules 2024, 29, 3399. [Google Scholar] [CrossRef]
- Ansari, S.M.; Bhor, R.D.; Pai, K.R.; Mazumder, S.; Sen, D.; Kolekar, Y.D.; Ramana, C.V. Size and Chemistry Controlled Cobalt-Ferrite Nanoparticles and Their Anti-Proliferative Effect against the MCF-7 Breast Cancer Cells. ACS Biomater. Sci. Eng. 2016, 2, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Neto, J.G.; Viana, J.R.; Abreu, K.R.; Butarelli, A.L.A.; dos Santos, A.P.A.; Lage, M.R.; de Sousa, F.F.; Souto, E.B.; dos Santos, A.O. Antitumor Neodymium(III) Complex with 1,10-Phenanthroline and Nitrate Ligands: A Comprehensive Experimental-Theoretical Study, in Silico Pharmacokinetic and Cytotoxic Prop-erties. J. Mol. Struct. 2025, 1321, 139757. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, B.; Zhao, C.; Zhang, D.; Wu, Q.; Li, Z. Synthesis and in Vitro Anticancer Properties of a Novel Neodymium(III) Complex Containing Tungstogermanate and 5-Fluorouracil. J. Coord. Chem. 2018, 71, 2102–2108. [Google Scholar] [CrossRef]
- Muskan, A.; Kumar, N.; Singh, R.K.; Kumar, P.; Siddique, M.M.H. Rare Earth (Nd3+) Mediated Structural, Magnetic, Ferroelectric Properties of Cobalt Ferrite Nanomaterials for Its Varied Applications. J. Indian Chem. Soc. 2024, 101, 101214. [Google Scholar] [CrossRef]
- Wang, Y. Structural, Magnetic, and Electrical Properties of Nd-Substituted Cobalt Ferrite. J. Mater. Sci. Mater. Electron. 2022, 33, 11017–11024. [Google Scholar] [CrossRef]
- Muskan, A.; Singh, R.K.; Kumar, N.; Kumar, P. Monalisa Analogous Behaviour of Nd3+ Rare Earth Substituted Tunned Structural, Stability, Magnetic and Ferroelectric Properties of CoFe2O4 Ferrite Nanomaterials for Its Multifunctional Application, Synthesized Using Green Approach. J. Mater. Sci. Mater. Electron. 2024, 35, 1597. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Chen, J.; Guan, D.; Hu, Z.; Xu, X.; Lin, Z.; Sun, H.; Sun, X.; Tang, J.; et al. Self-Optimized In-terfacial Co-O-Ru Motifs of Hollow Nanotube Composites Trigger Interfacial Lattice Oxygen Participation and Diffusion. ACS Nano 2025, 19, 25917–25929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, H.; Zhao, X.; Yu, L.; Cui, Y.; Feng, S. Magnetic Properties of CoFe2O4 Ferrite Doped with Rare Earth Ion. Mater. Lett. 2006, 60, 1–6. [Google Scholar] [CrossRef]
- Ahmad, I.; Safdar, M.; Yasmin, N.; Iftikhar, A.; Kalsoom, A.; Khalid, S.; Shams, Z.E.; Mirza, M. Rare Earth Ions (La3+, Nd3+) Substituted Cobalt–Strontium Spinel Ferrites for Photocatalytic Degradation of Textile Dyes. Water Pract. Technol. 2023, 18, 1742–1755. [Google Scholar] [CrossRef]
- Wu, X.; Ding, Z.; Song, N.; Li, L.; Wang, W. Effect of the Rare-Earth Substitution on the Structural, Magnetic and Adsorption Properties in Cobalt Ferrite Nanoparticles. Ceram. Int. 2016, 42, 4246–4255. [Google Scholar] [CrossRef]
- Yunasfi, Y.; Mashadi, M.; Winatapura, D.S.; Setiawan, J.; Taryana, Y.; Gunanto, Y.E.; Adi, W.A. Enhanced Magnetic and Microwave Absorbing Properties of Nd3+ Ion Doped CoFe2O4 by Solid-State Reaction Method. Phys. Status Solidi A Appl. Mater. Sci. 2023, 220, 2200718. [Google Scholar] [CrossRef]
- Khan, N.U.H.; Gilani, Z.A.; Abid, M.; Samiullah; Hussain, G.; Khalid, M.; Noor Huda Khan Asghar, H.M.; Nawaz, M.Z.; Ali, S.M.; Khan, M.A.; et al. Structural, Dielectric and Magnetic Characteristics of Praseodym-ium Doped Cobalt-Zinc Spinel Ferrites for Communication and Microwave Frequency Applications. Appl. Phys. A 2024, 130, 829. [Google Scholar] [CrossRef]
- Rotunjanu, S.; Racoviceanu, R.; Mioc, A.; Milan, A.; Negrea-Ghiulai, R.; Mioc, M.; Marangoci, N.L.; Şoica, C. Newly Synthesized CoFe2−xDyxO4 (x = 0; 0.1; 0.2; 0.4) Nanoparticles Reveal Promising Anticancer Activity against Melanoma (A375) and Breast Cancer (MCF-7) Cells. Int. J. Mol. Sci. 2023, 24, 15733. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Bakr Mohamed, M.; Arda, L.; Dogan, N. Cation Distribution Correlated with Magnetic Properties of Nanocrystalline Gadolinium Substituted Nickel Ferrite. J. Magn. Magn. Mater. 2015, 391, 195–202. [Google Scholar] [CrossRef]
- Sarkar, T.; Banerjee, S.; Mukherjee, S.; Hussain, A. Mitochondrial Selectivity and Remarkable Photocytotoxi-city of a Ferrocenyl Neodymium(III) Complex of Terpyridine and Curcumin in Cancer Cells. Dalton Trans. 2016, 45, 6424–6438. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Muthulakshmi, V. Green Synthesis of Ionic Liquid Mediated Neodymium Oxide Nanopar-ticles by Andrographis Paniculata Leaves Extract for Effective Bio-Medical Applications. J. Environ. Chem. Eng. 2021, 9, 104716. [Google Scholar] [CrossRef]
- Alexander, A.; Sumohan Pillai, A.; Manikantan, V.; Sri Varalakshmi, G.; Allben Akash, B.; Enoch, I.V.M.V. Magnetic and Luminescent Neodymium-Doped Carbon Dot–Cyclodextrin Polymer Nanocomposite as an Anticancer Drug-Carrier. Mater. Lett. 2022, 313, 131830. [Google Scholar] [CrossRef]
- Bheeram, V.R.; Dadhich, A.S.; Nagumantri, R.; Rentala, S.; Saha, A.; Mukkamala, S.B. Gamma Ray Enhanced Vis-NIR Photoluminescence and Cytotoxicity of Biocompatible Silica Coated Nd3+ Doped GdPO4 Nano-phosphors. Nucl. Instrum. Methods Phys. Res. B 2019, 440, 11–18. [Google Scholar] [CrossRef]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Farshori, N.N.; Saquib, Q.; Ahmad, N.; Al-Khedhairy, A.A. Neodymi-um Oxide Nanostructures and Their Cytotoxic Evaluation in Human Cancer Cells. J. Trace Elem. Med. Biol. 2022, 73, 127029. [Google Scholar] [CrossRef]
- Ghanbari, M.; Davar, F.; Shalan, A.E. Effect of Rosemary Extract on the Microstructure, Phase Evolution, and Magnetic Behavior of Cobalt Ferrite Nanoparticles and Its Application on Anti-Cancer Drug Delivery. Ceram. Int. 2021, 47, 9409–9417. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and Differentiation of HaCaT Keratinocytes. In Epidermal Cells; Springer Nature: London, UK, 2013; pp. 33–41. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; de la Mata, M.; Suár-ez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reor-ganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Leng, J.; Wang, N.; Chang, X.; Zhang, X.; Xu, J.; Yang, Z.; Qian, K.; Zheng, Z.; Tao, G.; Jia, X.; et al. Neodymium Nitrate Promotes the Apoptosis of Mouse Liver Cells via Bcl2l1/Caspase 3 Pathway. Toxicol. Mech. Methods 2025, 1–36. [Google Scholar] [CrossRef]
- Lee, K.H.; Jun, S.; Hur, H.S.; Ryu, J.J.; Kim, J. Candida albicans Protein Analysis during Hyphal Differentiation Using an Integrative HA-Tagging Method. Biochem. Biophys. Res. Commun. 2005, 337, 784–790. [Google Scholar] [CrossRef]
- Abudayyak, M.; Altincekic Gurkaynak, T.; Özhan, G. In Vitro Toxicological Assessment of Cobalt Ferrite Nanoparticles in Several Mammalian Cell Types. Biol. Trace Elem. Res. 2017, 175, 458–465. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Cobalt-Based Nanoparticles Strongly Diminish CalceinAM Fluores-cence Independently of Their Cytotoxic Potential in Human Lung Cell Line. J. King Saud Univ. Sci. 2024, 36, 102987. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of Reactive Oxygen Species in Inflammation and Cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, H. PH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Ghaemi, B.; Javad Hajipour, M. Tumor Acidic Environment Directs Nanoparticle Impacts on Cancer Cells. J. Colloid Interface Sci. 2023, 634, 684–692. [Google Scholar] [CrossRef]
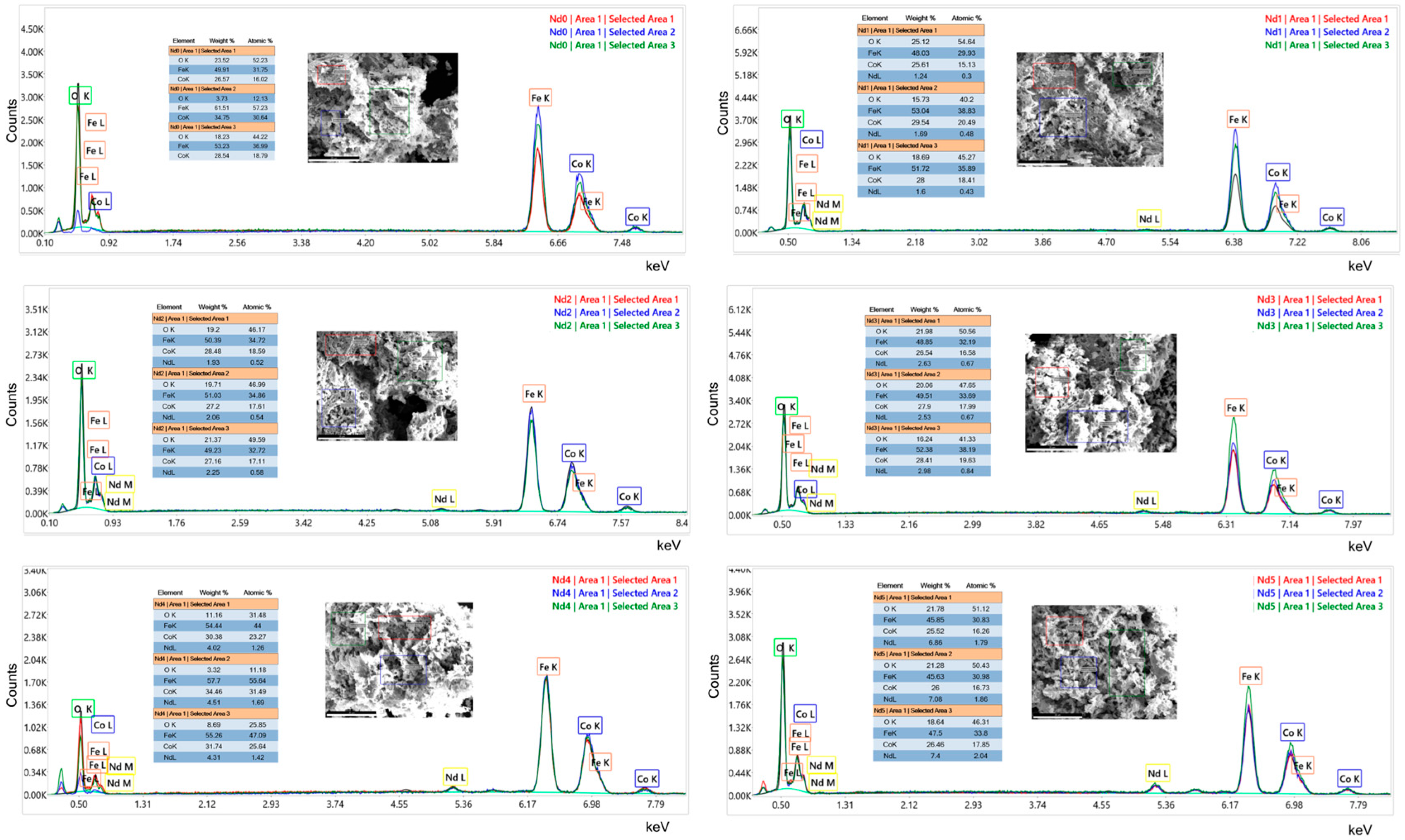

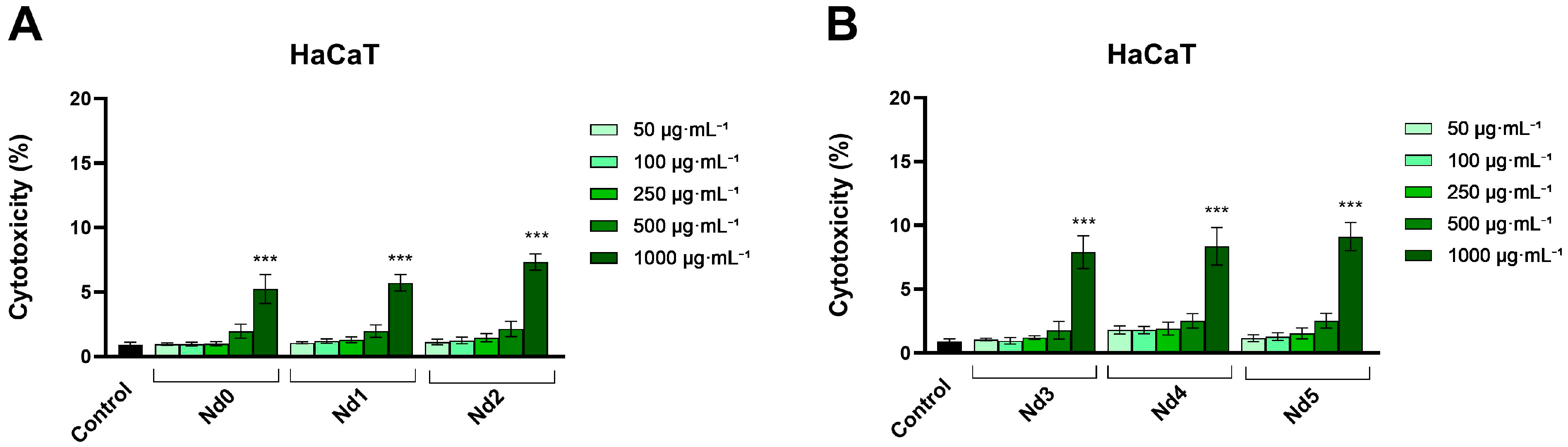
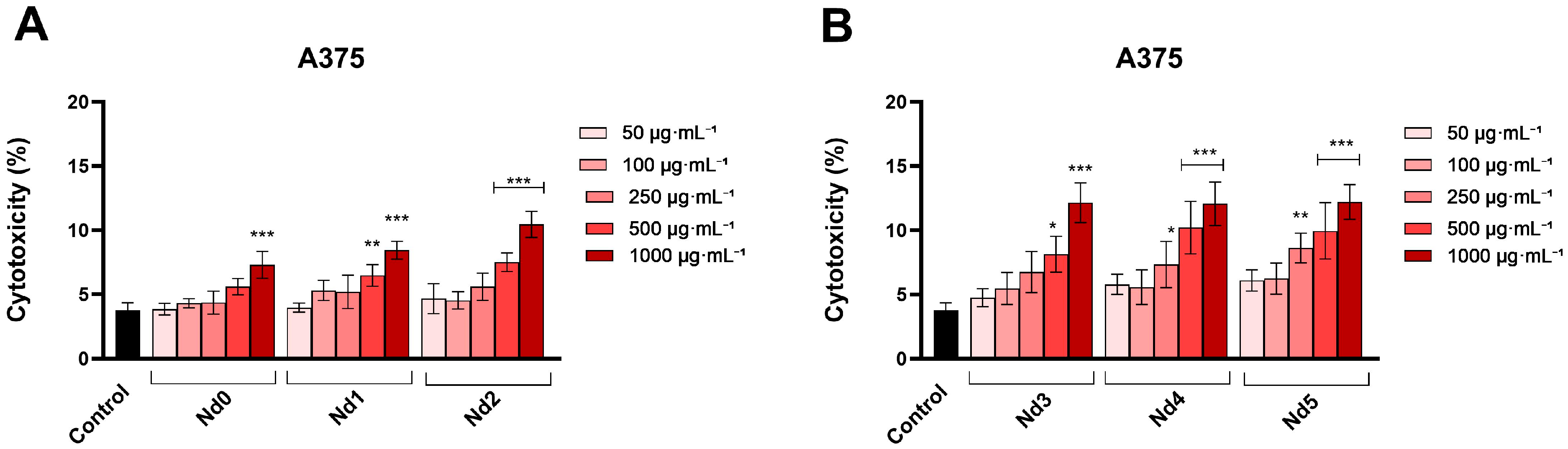
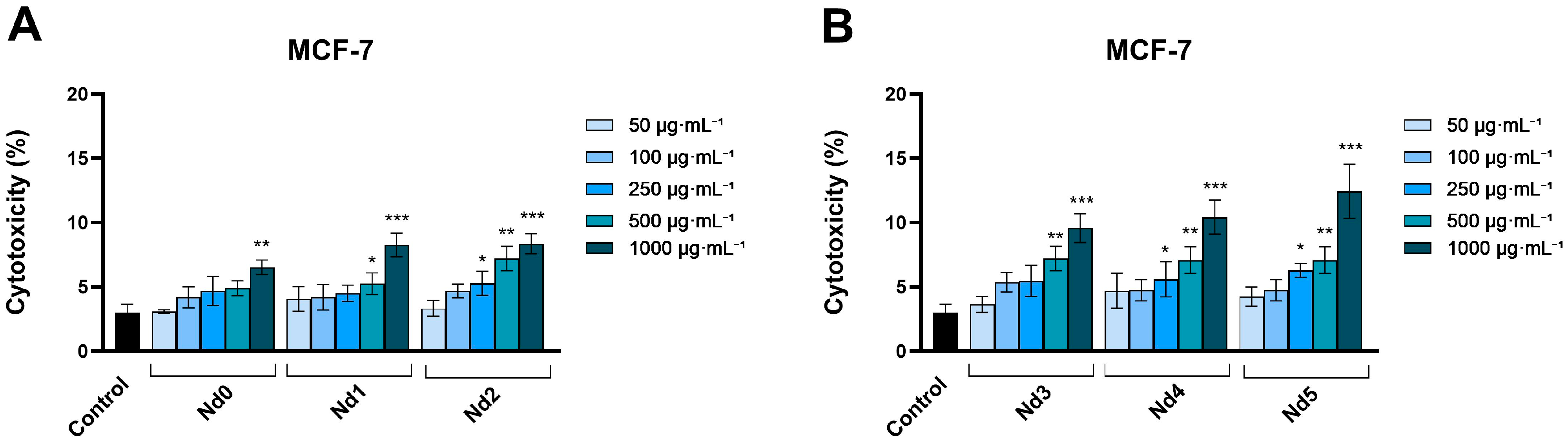
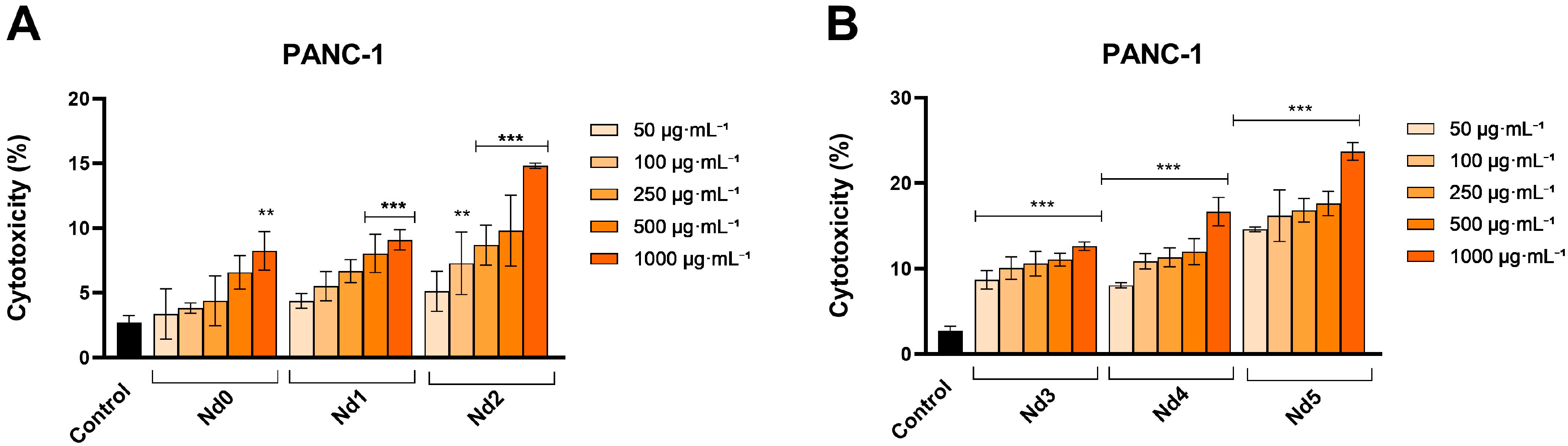
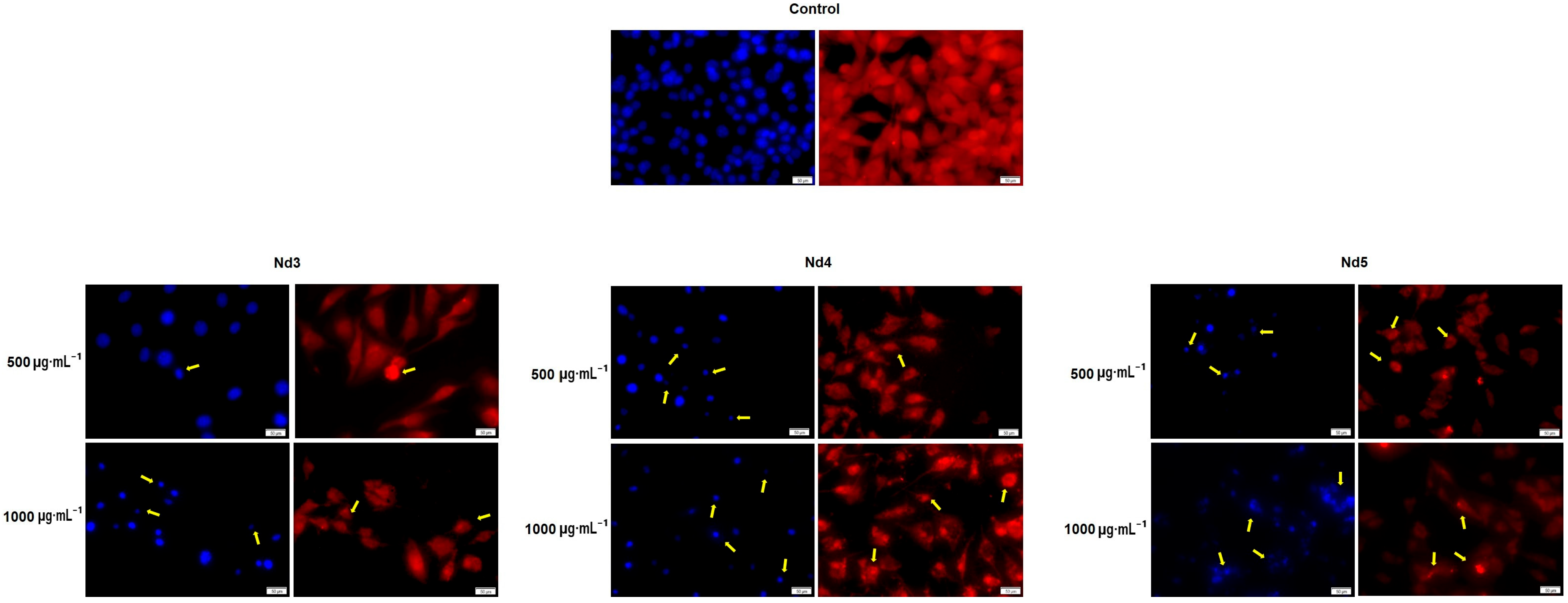
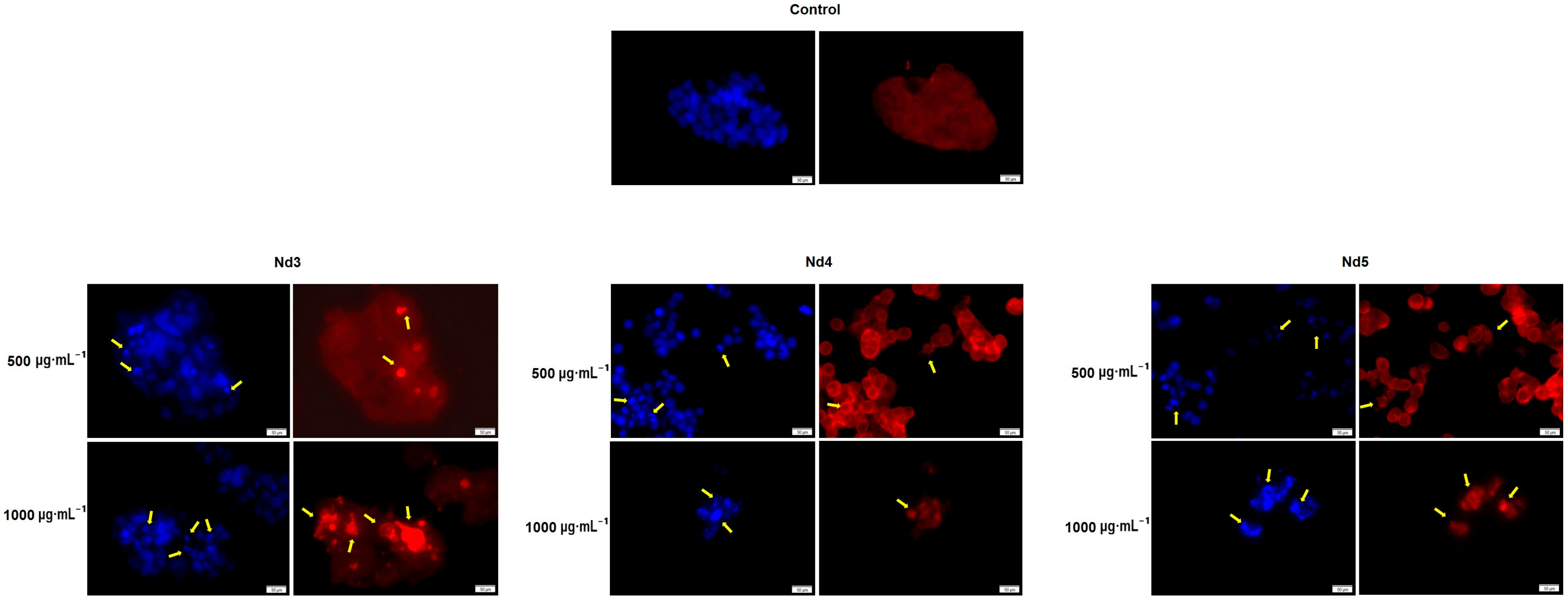
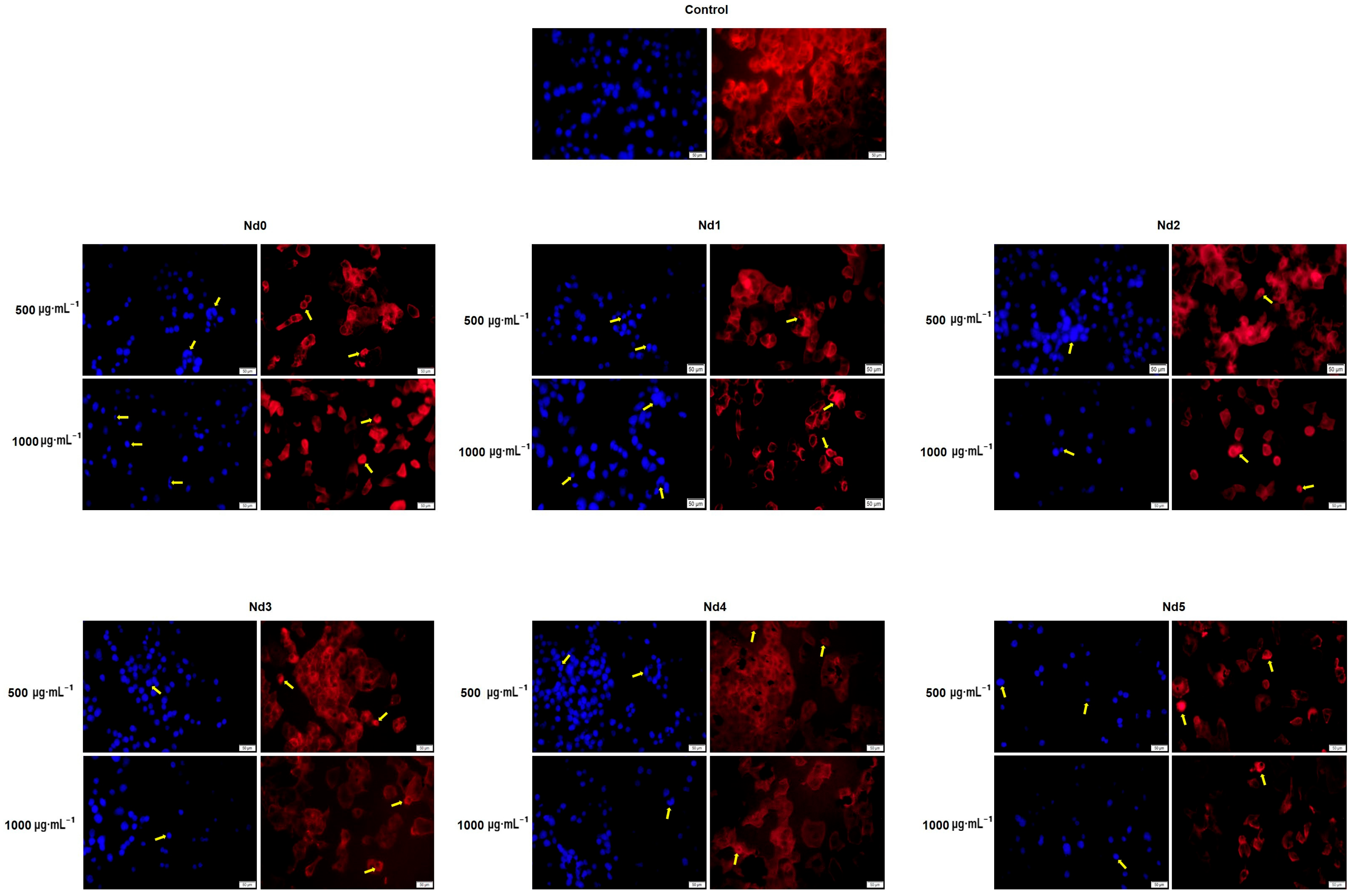
| Sample | NdCl3·6H2O (Moles) | Fe(NO3)3·9H2O (Moles) |
|---|---|---|
| Nd0 | - | 0.0400 |
| Nd1 | 0.0002 | 0.0398 |
| Nd2 | 0.0004 | 0.0396 |
| Nd3 | 0.0006 | 0.0394 |
| Nd4 | 0.0010 | 0.0390 |
| Nd5 | 0.0020 | 0.0380 |
| Sample | Lattice Parameter a (Å) |
|---|---|
| Nd0 | 8.3762 ± 0.0003 |
| Nd1 | 8.3781 ± 0.0004 |
| Nd2 | 8.3796 ± 0.0004 |
| Nd3 | 8.3799 ± 0.0004 |
| Nd4 | 8.3830 ± 0.0004 |
| Nd5 | 8.3873 ± 0.0002 |
| HaCaT | A375 | MCF-7 | PANC-1 | |
|---|---|---|---|---|
| Nd0 | >1000 | >1000 | >1000 | 748.7 |
| Nd1 | >1000 | >1000 | >1000 | 687.9 |
| Nd2 | >1000 | >1000 | >1000 | 593.5 |
| Nd3 | >1000 | 943.5 | >1000 | 581.8 |
| Nd4 | >1000 | 798.8 | 956.3 | 572.3 |
| Nd5 | >1000 | 650.7 | 877.2 | 397.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotunjanu, S.; Gogulescu, A.; Marangoci, N.L.; Dascălu, A.-I.; Mioc, M.; Racoviceanu, R.; Mioc, A.; Maksimović, T.; Eșanu, O.; Antal, G.; et al. Synthesis, Characterization, and In Vitro Cytotoxic Evaluation of Neodymium-Doped Cobalt Ferrite Nanoparticles on Human Cancer Cell Lines. Materials 2025, 18, 3911. https://doi.org/10.3390/ma18163911
Rotunjanu S, Gogulescu A, Marangoci NL, Dascălu A-I, Mioc M, Racoviceanu R, Mioc A, Maksimović T, Eșanu O, Antal G, et al. Synthesis, Characterization, and In Vitro Cytotoxic Evaluation of Neodymium-Doped Cobalt Ferrite Nanoparticles on Human Cancer Cell Lines. Materials. 2025; 18(16):3911. https://doi.org/10.3390/ma18163911
Chicago/Turabian StyleRotunjanu, Slaviţa, Armand Gogulescu, Narcisa Laura Marangoci, Andrei-Ioan Dascălu, Marius Mioc, Roxana Racoviceanu, Alexandra Mioc, Tamara Maksimović, Oana Eșanu, Gabriela Antal, and et al. 2025. "Synthesis, Characterization, and In Vitro Cytotoxic Evaluation of Neodymium-Doped Cobalt Ferrite Nanoparticles on Human Cancer Cell Lines" Materials 18, no. 16: 3911. https://doi.org/10.3390/ma18163911
APA StyleRotunjanu, S., Gogulescu, A., Marangoci, N. L., Dascălu, A.-I., Mioc, M., Racoviceanu, R., Mioc, A., Maksimović, T., Eșanu, O., Antal, G., & Șoica, C. (2025). Synthesis, Characterization, and In Vitro Cytotoxic Evaluation of Neodymium-Doped Cobalt Ferrite Nanoparticles on Human Cancer Cell Lines. Materials, 18(16), 3911. https://doi.org/10.3390/ma18163911






.jpg)


