Assessment of Durability and Degradation Resistance of Geopolymer Composites in Water Environments
Highlights
- The conducted studies showed that slag additions improved mechanical properties.
- The addition of amphibolite has a negative impact on compressive strength.
- The presence of carbon fibers promoted matrix cohesion, but their uneven distribution could lead to local strength differences.
- Water absorption tests have shown that geopolymers reach full water saturation within 24 to 48 h, which indicates rapid establishment of capillary equilibrium and limited further water penetration.
- High strength, low porosity and chemical resistance make the geopolymers suitable for, among others, the construction of marine foundations, protection and structural shields of submerged applications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- Firstly, the metakaolin was mixed with the activator (sodium water glass) and mixed for 5 min.
- In the next step, the short carbon fibers were added to the mixture. The process was continued for 3 min.
- After that time, the proper amount of silicon dioxide was included in the paste. The mixing process was continued for 3 min (for the composition without silica, this step was omitted).
- Then, the mixture was continued for 2 min with the addition of cellulose (for the composition without cellulose, this step was omitted).
- In the last step, the amphibolite and slag were added to the paste. The mixing process was continued next 3 min. After that time, the process was ended.
2.2. Methods
- Distilled water;
- Sodium chloride solution (992 g H2O + 8 g NaCl);
- Hydrochloric acid solution (997.26 g H2O + 2.74 g HCl 38%);
- Acid mixture (994.47 g H2O + 1.54 g HNO 36.5% + 1.25 g CH3COOH 80% + 2.74 g HCl 38%);
- Sodium hydroxide solution (990 g H2O) + 10 g NaOH).
3. Results
3.1. Density
3.2. Chemical Composition
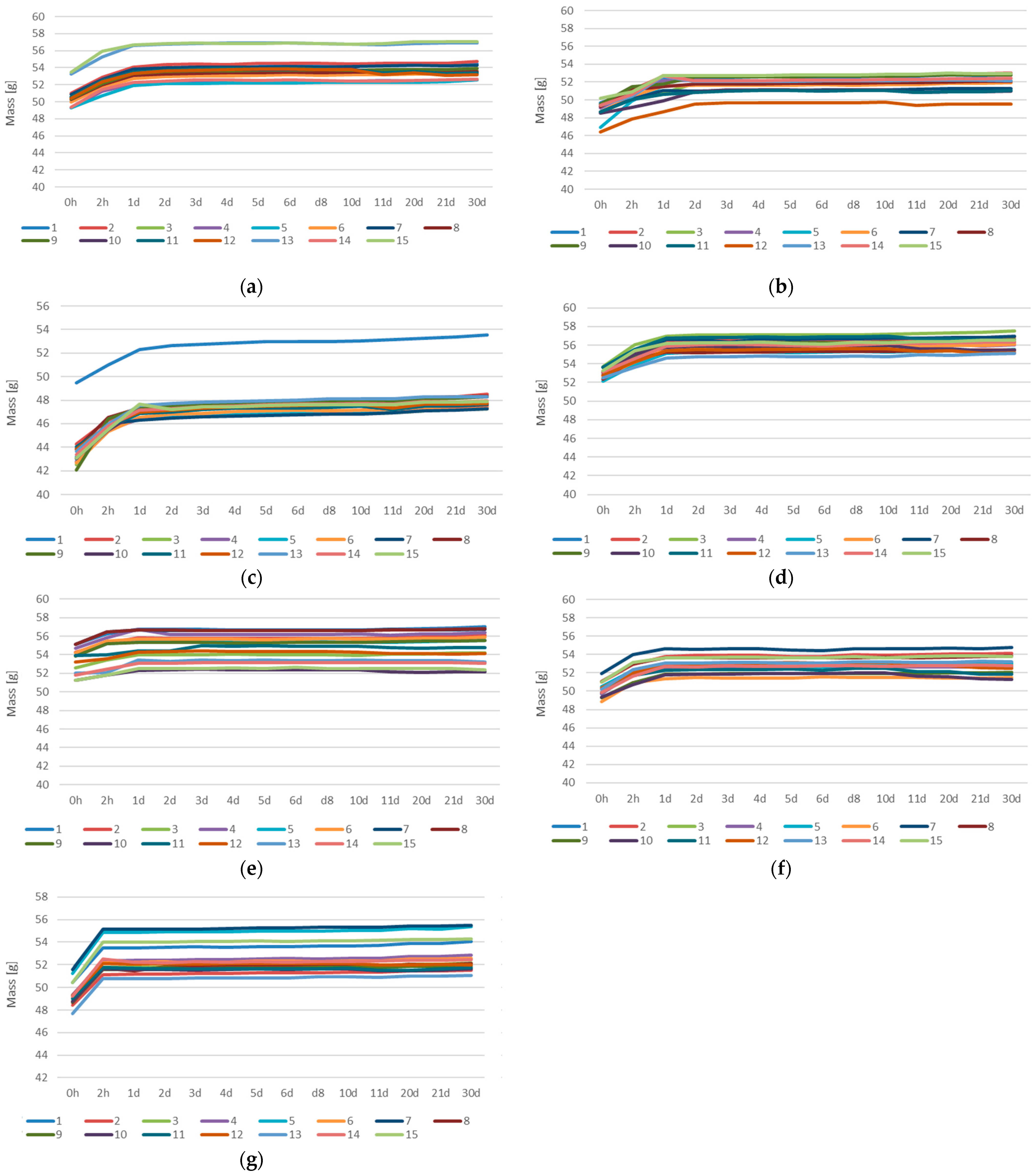
3.3. Absorption Tests
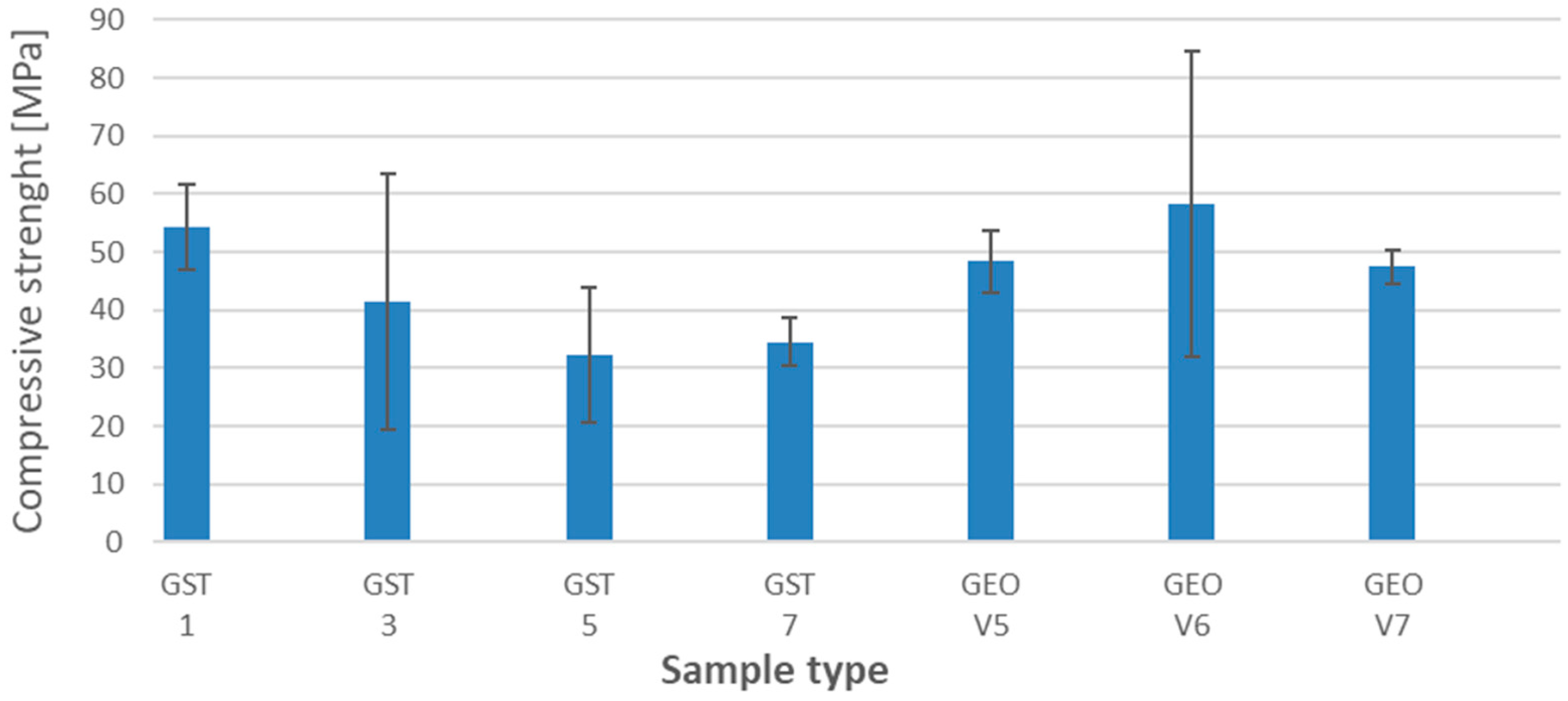
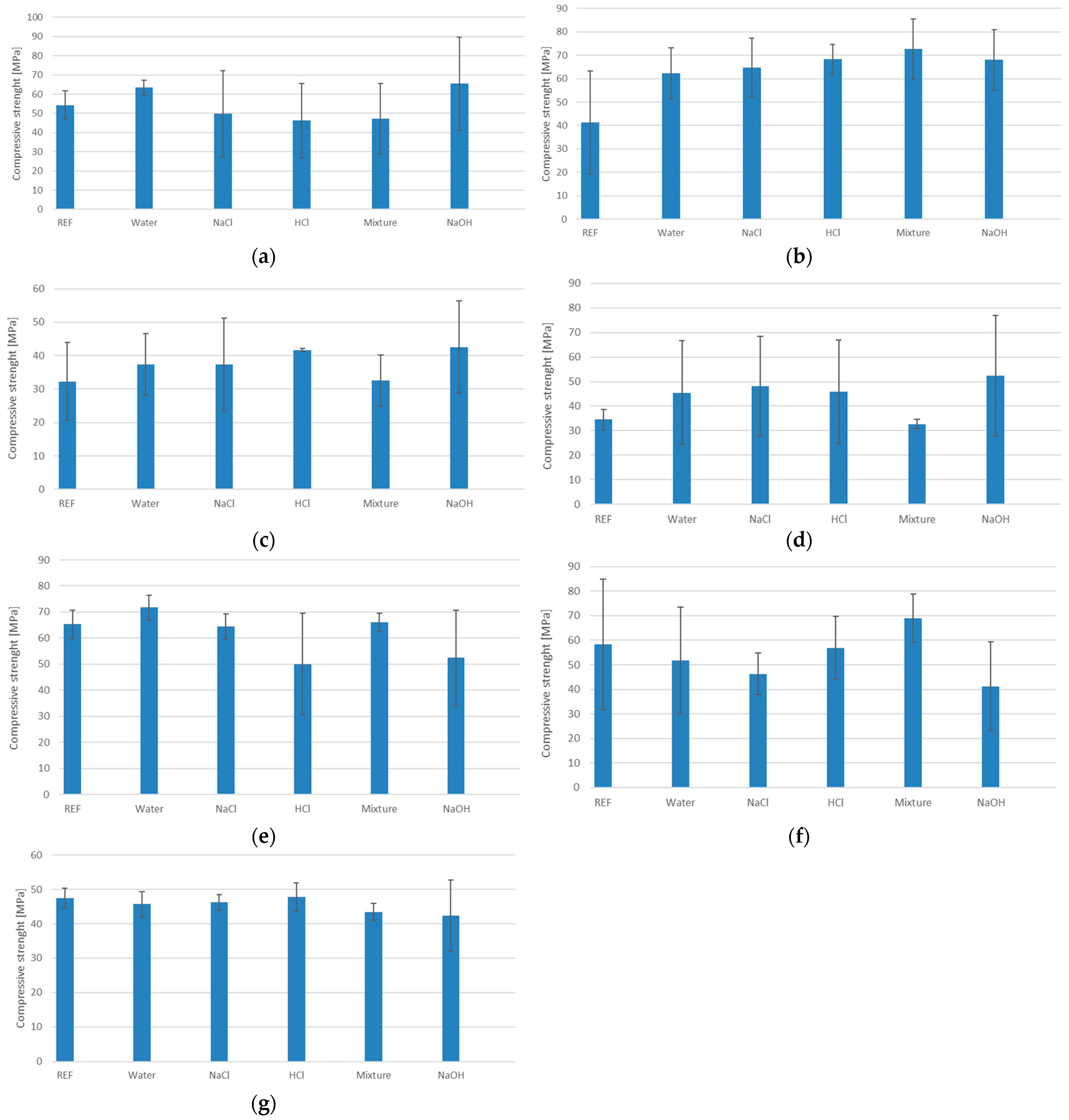
3.4. Compressive Strength
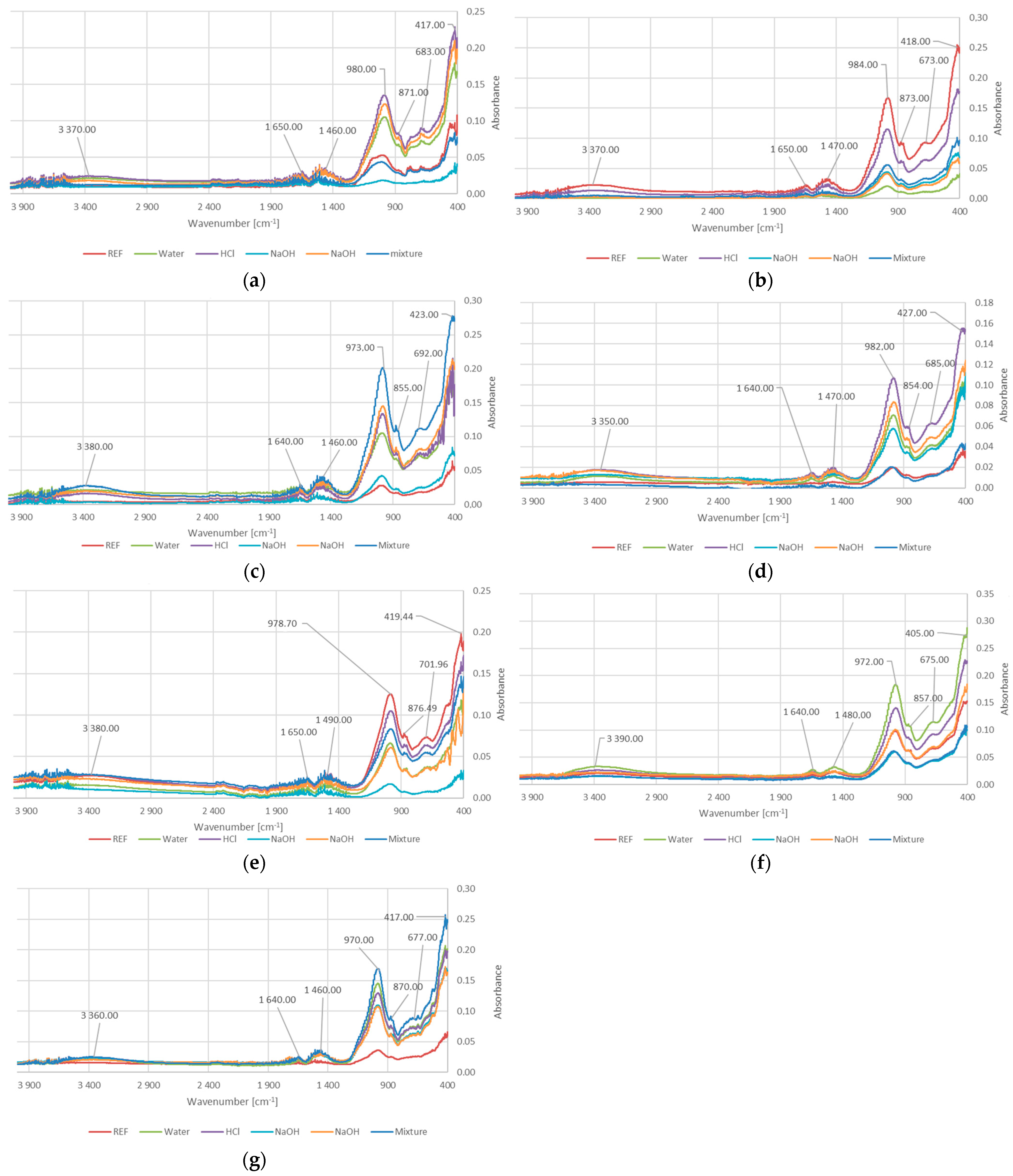
3.5. FTIR Analysis
3.6. Microstructural Analysis
4. Discussion
- The consistent and sustainable sourcing of raw materials, particularly the aluminosilicate precursors, on an industrial scale.
- Geopolymer formulations can be sensitive to mixing procedures, particularly due to their high alkalinity and relatively low water content. It could be hard to control in industrial conditions.
- Effective curing of geopolymers often requires controlled temperature and humidity conditions that are difficult to maintain on-site, especially in marine environments with fluctuating moisture, salinity, and wind exposure.
- Quality control processes that ensure consistent quality and performance across large volumes of material.
- Standardized procedures for testing geopolymers are still evolving, which can pose regulatory and certification challenges.
5. Conclusions
- Increasing the content of ground granulated blast furnace slag (GGBFS) in geopolymers enhances their compressive strength;
- The highest strength parameters were achieved with a slag content of 50%;
- The addition of amphibolite has a negative impact on compressive strength;
- The presence of slag and amphibolite additives leads to a slight increase in water absorption;
- FTIR analysis revealed characteristic spectra typical of geopolymer materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Additional Test Results
| Element | GST 1 | GST 3 | GST 5 | GST 7 | GEO V5 | GEO V6 | GEO V7 |
|---|---|---|---|---|---|---|---|
| Mg | 3.96% | 6.34% | 6.09% | 7.05% | 4.47% | 7.03% | 3.18% |
| Al | 13.28% | 14.97% | 15.21% | 15.66% | 18.72% | 16.04% | 13.39% |
| Si | 41.83% | 34.72% | 33.27% | 32.60% | 37.45% | 28.74% | 42.59% |
| P | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.01% | 0.00% |
| S | 0.14% | 0.25% | 0.18% | 0.25% | 0.29% | 0.20% | 0.19% |
| Cl | 0.08% | 0.06% | 0.07% | 0.06% | 0.02% | 0.03% | 0.03% |
| K | 7.70% | 11.20% | 10.46% | 9.18% | 11.97% | 8.52% | 10.73% |
| Ca | 19.65% | 23.74% | 23.41% | 22.56% | 20.41% | 23.17% | 22.52% |
| Sc | 0.06% | 0.08% | 0.08% | 0.07% | 0.05% | 0.07% | 0.05% |
| Ti | 1.23% | 1.42% | 1.46% | 1.40% | 2.34% | 1.52% | 1.26% |
| V | 0.15% | 0.10% | 0.13% | 0.15% | 0.08% | 0.19% | 0.04% |
| Cr | 0.36% | 0.12% | 0.14% | 0.16% | 0.23% | 0.22% | 0.10% |
| Mn | 0.41% | 0.50% | 0.49% | 0.47% | 0.41% | 0.49% | 0.48% |
| Fe | 8.10% | 4.74% | 6.45% | 7.40% | 3.00% | 9.78% | 5.09% |
| Ni | 0.07% | 0.02% | 0.02% | 0.02% | 0.07% | 0.03% | 0.03% |
| Cu | 0.07% | 0.05% | 0.06% | 0.07% | 0.00% | 0.09% | 0.00% |
| Zn | 0.02% | 0.01% | 0.02% | 0.02% | 0.03% | 0.02% | 0.02% |
| Sr | 0.10% | 0.10% | 0.11% | 0.12% | 0.08% | 0.14% | 0.08% |
| Y | 0.01% | 0.01% | 0.02% | 0.02% | 0.01% | 0.02% | 0.02% |
| Zr | 2.54% | 1.40% | 2.10% | 2.48% | 0.13% | 3.38% | 0.09% |
| Mo | 0.03% | 0.01% | 0.00% | 0.01% | 0.02% | 0.02% | 0.01% |
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Albayrak, E.; Özen, S. Enhancing Geopolymer Synthesis through Calcination: Increasing the Potential of Natural Material Utilization. Open Ceram. 2025, 22, 100796. [Google Scholar] [CrossRef]
- Peng, J.; Chen, W.; Dong, B.; Wang, Y. Alkali-Activated Sand Washing Slurry-Based Geopolymers: Mechanical Strength, Microstructure and Environmental Impact. Constr. Build. Mater. 2025, 483, 141779. [Google Scholar] [CrossRef]
- Shehata, N.; Mohamed, O.A.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G. Geopolymer Concrete as Green Building Materials: Recent Applications, Sustainable Development and Circular Economy Potentials. Sci. Total Environ. 2022, 836, 155577. [Google Scholar] [CrossRef]
- Philip, S.; Nidhi, M. A Review on the Material Performance of Geopolymer Concrete as Green Building Materials. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Criado, M.; Provis, J.L. Alkali Activated Slag Mortars Provide High Resistance to Chloride-Induced Corrosion of Steel. Front. Mater. 2018, 5, 34. [Google Scholar] [CrossRef]
- Elfadaly, E.; Othman, A.M.; Aly, M.H.; Elgarhy, W.A.; Abdellatief, M. Assessing Performance and Environmental Benefits of High-Performance Geopolymer Mortar Incorporating Pumice and Rice Straw Ash. Sustain. Chem. Pharm. 2025, 44, 101918. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, J.; Gao, X. Sea/Coral Sand in Marine Engineered Geopolymer Composites: Engineering, Mechanical, and Microstructure Properties. Int. J. Appl. Ceram. Tech. 2024, 22, e14874. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Zheng, Z. The Effect of Graphene Oxide on the Mechanical Properties, Impermeability and Corrosion Resistance of Cement Mortar Containing Mineral Admixtures. Constr. Build. Mater. 2021, 288, 123059. [Google Scholar] [CrossRef]
- Huang, T.; Sun, Z. Advances in Multifunctional Graphene-Geopolymer Composites. Constr. Build. Mater. 2021, 272, 121619. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljković, M.; Zuo, Y.; Ye, G. A Review on the Durability of Alkali-Activated Fly Ash/Slag Systems: Advances, Issues, and Perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Craun, Z.; Srubar, W.V. Sulfuric Acid Degradation of Alkali-Activated Metakaolin Cements Supplemented with Brucite. Cem. Concr. Compos. 2021, 121, 104063. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of Geopolymer Materials in Sodium and Magnesium Sulfate Solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Shamsah, M.; Kalfat, R.; Subramaniam, K.V.L. Impact of Low NaOH Molarities on Mechanical and Durability Properties of Ambient and Oven-Cured Fly Ash Geopolymer Concrete. J. Build. Eng. 2025, 105, 112491. [Google Scholar] [CrossRef]
- Frieda, F.S.; Greeshma, S. Effects on the Strength and Durability of Graphene Oxide Modified Geopolymer Concrete Using Industrial Waste Bauxite Tailings. Case Stud. Constr. Mater. 2025, 22, e04726. [Google Scholar] [CrossRef]
- Tay, C.H.; Mazlan, N.; Wayayok, A.; Basri, M.S.; Albakri Abdullah, M.M. Zeolite Based Foamed Geopolymer Concrete Reinforced with Cellulose Nanofibril Prepared in Low Concentration Alkaline Solution: Porosity, Compressive Strength, and Water Permeability. J. Clean. Prod. 2025, 489, 144609. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Van Deventer, J.S.J. Influence of Fly Ash on the Water and Chloride Permeability of Alkali-Activated Slag Mortars and Concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Bernal, S.A. The Resistance of Alkali-Activated Cement-Based Binders to Carbonation. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 319–332. ISBN 978-1-78242-276-1. [Google Scholar]
- Lamaa, G.; Duarte, A.P.C.; Silva, R.V.; De Brito, J. Carbonation of Alkali-Activated Materials: A Review. Materials 2023, 16, 3086. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the Carbonation Mechanism of CH and C-S-H in Terms of Kinetics, Microstructure Changes and Moisture Properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Mejía De Gutiérrez, R.; Van Deventer, J.S.J. Accelerated Carbonation Testing of Alkali-Activated Slag/Metakaolin Blended Concretes: Effect of Exposure Conditions. Mater. Struct. 2015, 48, 653–669. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability Evaluation of Geopolymer and Conventional Concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Luga, E.; Mustafaraj, E.; Corradi, M.; Atiș, C.D. Alkali-Activated Binders as Sustainable Alternatives to Portland Cement and Their Resistance to Saline Water. Materials 2024, 17, 4408. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-Term Durability Properties of Geopolymer Concrete: An in-Depth Review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhong, W.L.; Fan, L.F. Long-Term Durability Investigation of Basalt Fiber-Reinforced Geopolymer Concrete in Marine Environment. J. Mater. Res. Technol. 2024, 31, 593–605. [Google Scholar] [CrossRef]
- Korniejenko, K.; Halyag, N.; Mucsi, G. Fly Ash as a Raw Material for Geopolymerisation-Chemical Composition and Physical Properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012002. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and Mechanical Properties of Alkali-Activated Fly Ash-Slag Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Indhumathi Anbarasan, M.; Leema Margret, A.; Ragavan, V.; Ramprashath, J. Investigation on Corrosion Behaviour of Geopolymer Concrete Using DMS and M−Sand as a Fine Aggregate under Ambient Curing Conditions. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Guo, H.; Chen, J.; Liu, Y.; Yuan, P. Effect of the SiO2/Al2O3 Molar Ratio on the Microstructure and Properties of Clay-Based Geopolymers: A Comparative Study of Kaolinite-Based and Halloysite-Based Geopolymers. Clays Clay Miner. 2022, 70, 882–902. [Google Scholar] [CrossRef]
- Khan, R.; Iqbal, S.; Soliyeva, M.; Ali, A.; Elboughdiri, N. Advanced Clay-Based Geopolymer: Influence of Structural and Material Parameters on Its Performance and Applications. RSC Adv. 2025, 15, 12443–12471. [Google Scholar] [CrossRef]
- Setlak, K.; Mikuła, J.; Łach, M. Application of Industrial Waste Materials by Alkaline Activation for Use as Geopolymer Binders. Materials 2023, 16, 7651. [Google Scholar] [CrossRef]
- Al-Noaimat, Y.A.; Ghaffar, S.H.; Chougan, M.; Al-Kheetan, M.J. A Review of 3D Printing Low-Carbon Concrete with One-Part Geopolymer: Engineering, Environmental and Economic Feasibility. Case Stud. Constr. Mater. 2023, 18, e01818. [Google Scholar] [CrossRef]
- Asadizadeh, M.; Hedayat, A.; Tunstall, L.; Gonzalez, J.A.V.; Alvarado, J.W.V.; Neira, M.T. The Impact of Slag on the Process of Geopolymerization and the Mechanical Performance of Mine-Tailings-Based Alkali-Activated Lightweight Aggregates. Constr. Build. Mater. 2024, 411, 134347. [Google Scholar] [CrossRef]
- Mohamed, O.; Ahmed, E.; Najm, O.; Al-Aribe, K.; Hijah, E. Water Absorption Characteristics and Rate of Strength Development of Mortar with Slag-Based Alkali-Activated Binder and 25% Fly Ash Replacement. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Gastoł, W.; Shalomieiev, V.A.; Tabunschyk, G.V.; Łach, M.; Kozub, B.; Nykiel, M.; Korniejenko, K. Evaluation of the Possibility of Preparing Geopolymer Materials Based on Slags and Fly Ashes from the Thermal Treatment of Municipal Waste. Mater. Werkst. 2025, 56, 757–769. [Google Scholar] [CrossRef]
- Liu, J.; Lv, C. Durability of Cellulosic-Fiber-Reinforced Geopolymers: A Review. Molecules 2022, 27, 796. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Liu, J.; Liu, J.; Wang, Y.; Shi, L.; Jiang, Q. Property and Microstructure of Waterborne Self-Setting Geopolymer Coating: Optimization Effect of SiO2/Na2O Molar Ratio. Minerals 2018, 8, 162. [Google Scholar] [CrossRef]
- Korniejenko, K.; Kejzlar, P.; Louda, P. The Influence of the Material Structure on the Mechanical Properties of Geopolymer Composites Reinforced with Short Fibers Obtained with Additive Technologies. Int. J. Mol. Sci. 2022, 23, 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, X.; Wang, X. FTIR Analysis of the Functional Group Composition of Coal Tar Residue Extracts and Extractive Residues. Appl. Sci. 2023, 13, 5162. [Google Scholar] [CrossRef]
- Bredács, M.; Barretta, C.; Castillon, L.F.; Frank, A.; Oreski, G.; Pinter, G.; Gergely, S. Prediction of Polyethylene Density from FTIR and Raman Spectroscopy Using Multivariate Data Analysis. Polym. Test. 2021, 104, 107406. [Google Scholar] [CrossRef]
- Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Thomsen, R.M.; Skibsted, J.; Yue, Y. The Charge-Balancing Role of Calcium and Alkali Ions in Per-Alkaline Aluminosilicate Glasses. J. Phys. Chem. B 2018, 122, 3184–3195. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive Reuse of Soda-Lime Waste Glass in Fly Ash-Based Geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Onutai, S.; Osugi, T.; Sone, T. Alumino-Silicate Structural Formation during Alkali-Activation of Metakaolin: In-Situ and Ex-Situ ATR-FTIR Studies. Materials 2023, 16, 985. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Barone, G.; Mazzoleni, P.; Leonelli, C.; Gharzouni, A.; Rossignol, S. FT-IR Study of Early Stages of Alkali Activated Materials Based on Pyroclastic Deposits (Mt. Etna, Sicily, Italy) Using Two Different Alkaline Solutions. Constr. Build. Mater. 2020, 262, 120095. [Google Scholar] [CrossRef]
- Amar, M.; Ladduri, B.; Alloul, A.; Benzerzour, M.; Abriak, N.-E. Microstructure and Mechanical Properties of Geopolymers Utilizing Excavated Soils, Metakaolin and Slags. J. Build. Eng. 2024, 86, 108755. [Google Scholar] [CrossRef]
- Guan, X.; Wu, J.-Q.; Hernandez, A.G.; Li, B.; Do, H. Molecular Dynamic Simulations of Interfacial Interaction Mechanism between the NASH Gels and the Polyethene Fibre. Constr. Build. Mater. 2022, 349, 128769. [Google Scholar] [CrossRef]
- Kai, M.F.; Zhang, L.W.; Liew, K.M. Carbon Nanotube-Geopolymer Nanocomposites: A Molecular Dynamics Study of the Influence of Interfacial Chemical Bonding upon the Structural and Mechanical Properties. Carbon 2020, 161, 772–783. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Residual Water and Interfacial Bonding Effects on the Mechanical Performance of CNT/Fly Ash Geopolymer Binder. Struct. Concr. 2024, 25, 3648–3661. [Google Scholar] [CrossRef]
- Růžek, V.; Dostayeva, A.M.; Walter, J.; Grab, T.; Korniejenko, K. Carbon Fiber-Reinforced Geopolymer Composites: A Review. Fibers 2023, 11, 17. [Google Scholar] [CrossRef]
- Rahman, S.K.; Al-Ameri, R. Long-Term Performance of Basalt Fibre-Reinforced Marine Geopolymer Concrete in Harsh Environment. Mag. Concr. Res. 2023, 75, 1165–1187. [Google Scholar] [CrossRef]
- Wardhono, A.; Risdianto, Y.; Sabariman, B.; Hidajati, N.W.; Andajani, N. The Effect of Sodium Silicate to NaOH Ratio on Strength Development of Fly Ash Geopolymer Mortar in Marine Environment. E3S Web Conf. 2023, 445, 01005. [Google Scholar] [CrossRef]
- Aiken, T.A.; Gu, L.; Kwasny, J.; Huseien, G.F.; McPolin, D.; Sha, W. Acid Resistance of Alkali-Activated Binders: A Review of Performance, Mechanisms of Deterioration and Testing Procedures. Constr. Build. Mater. 2022, 342, 128057. [Google Scholar] [CrossRef]
- Ariyadasa, P.W.; Manalo, A.C.; Lokuge, W.; Aravienthan, V.; Gerdes, A.; Kaltenbach, J. Degradation Mechanisms of Low-Calcium Fly Ash-Based Geopolymer Mortar in Simulated Aggressive Sewer Conditions. Cem. Concr. Res. 2025, 194, 107882. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.-L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.-P. Facile and Fast Determination of Si/Al Ratio of Zeolites Using FTIR Spectroscopy Technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Zhang, B. Durability of Low-Carbon Geopolymer Concrete: A Critical Review. Sustain. Mater. Technol. 2024, 40, e00882. [Google Scholar] [CrossRef]
- Bolina, F.L.; Henn, A.S. Eco-Friendly Reinforced Concrete Beams Exposed to Standardized Fire: A Thermal Finite Element Analysis. Sustainability 2025, 17, 2951. [Google Scholar] [CrossRef]
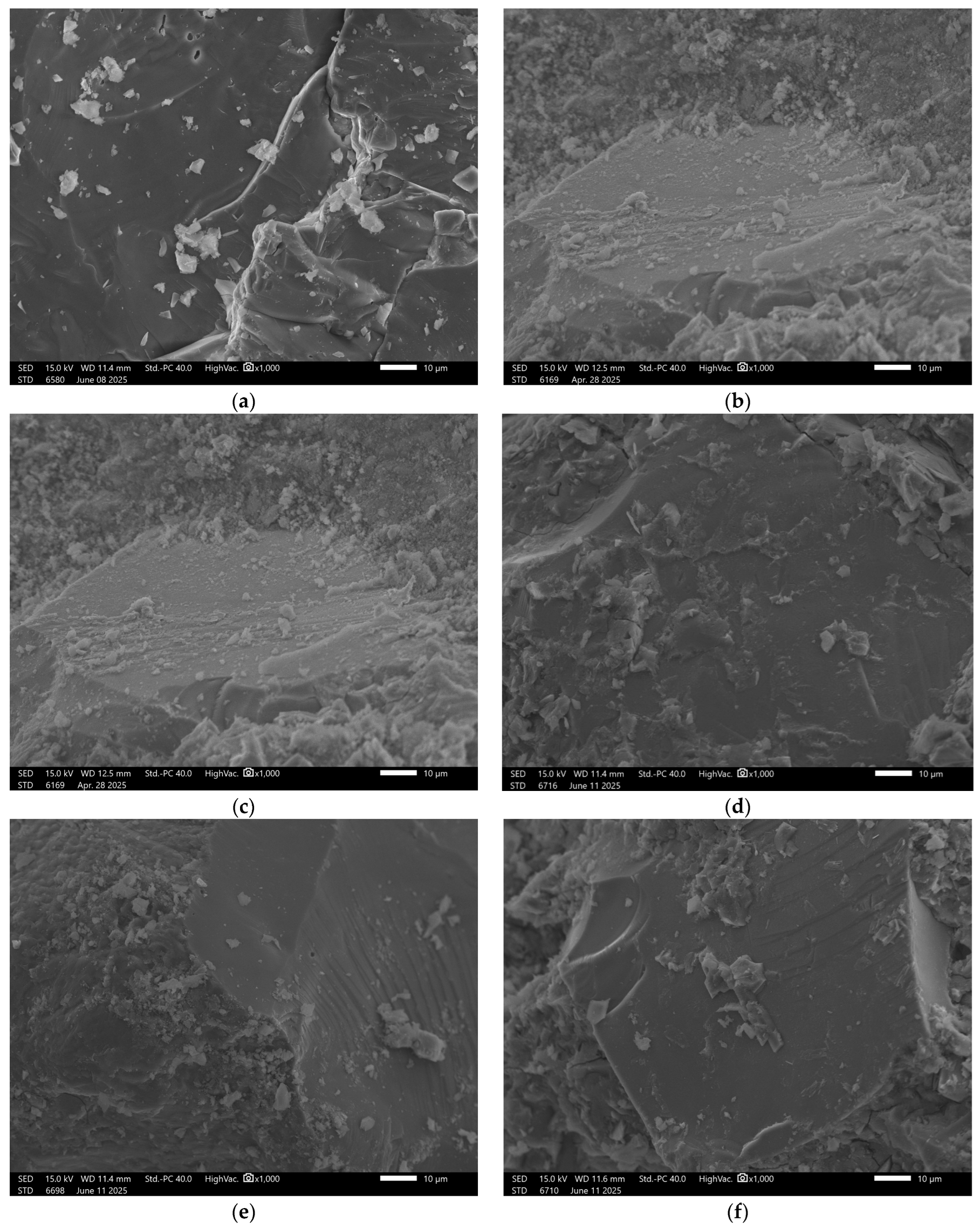
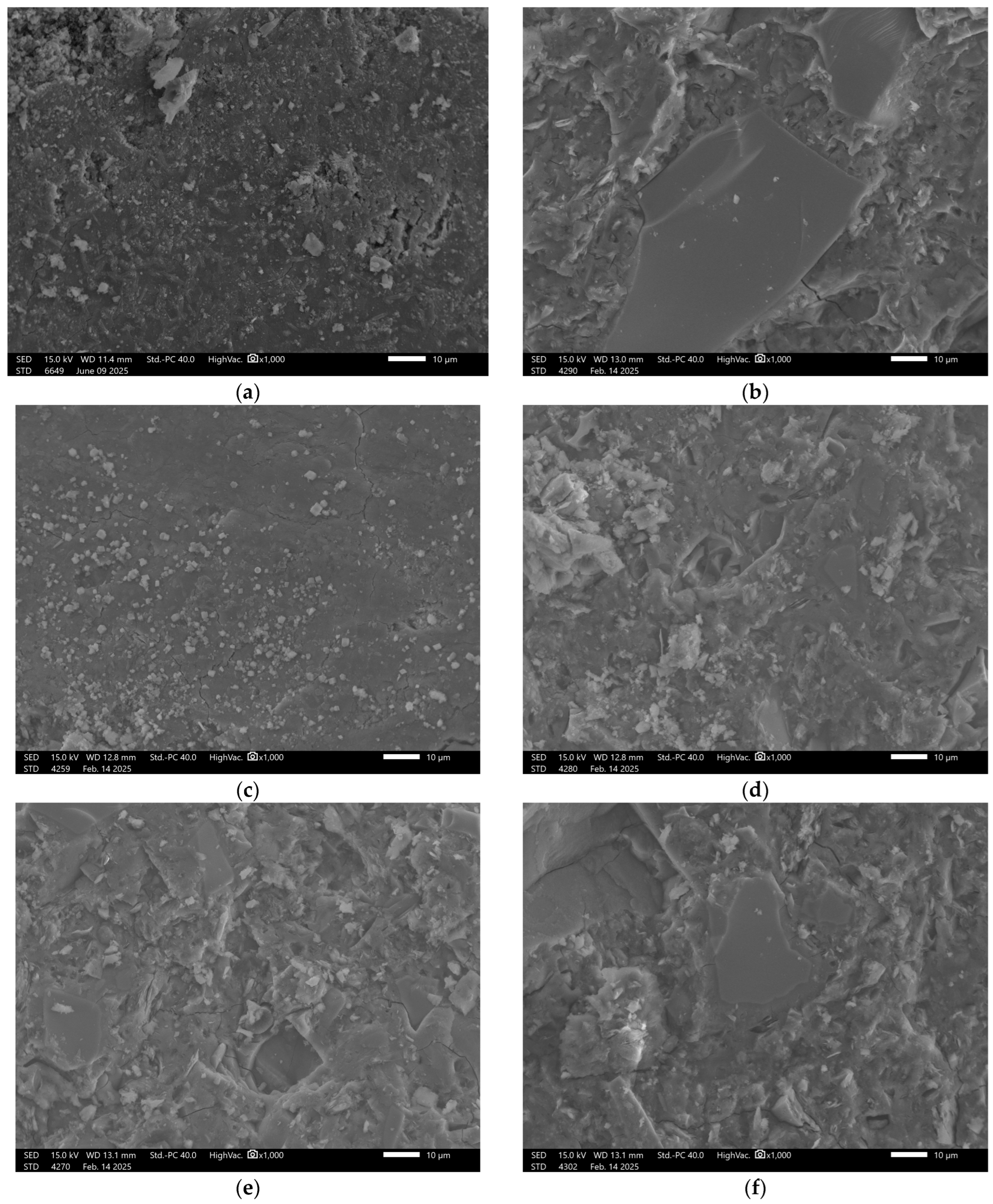
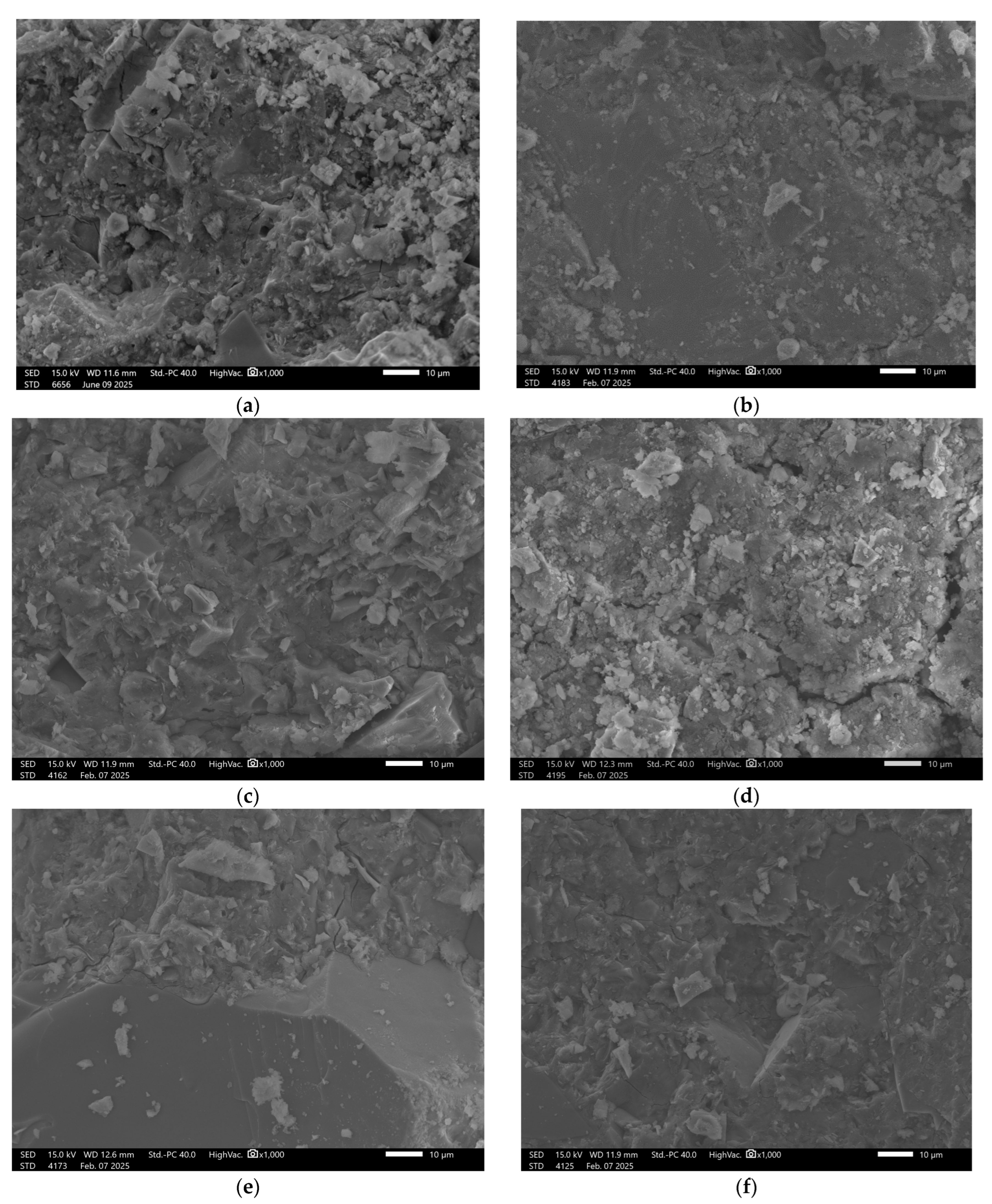

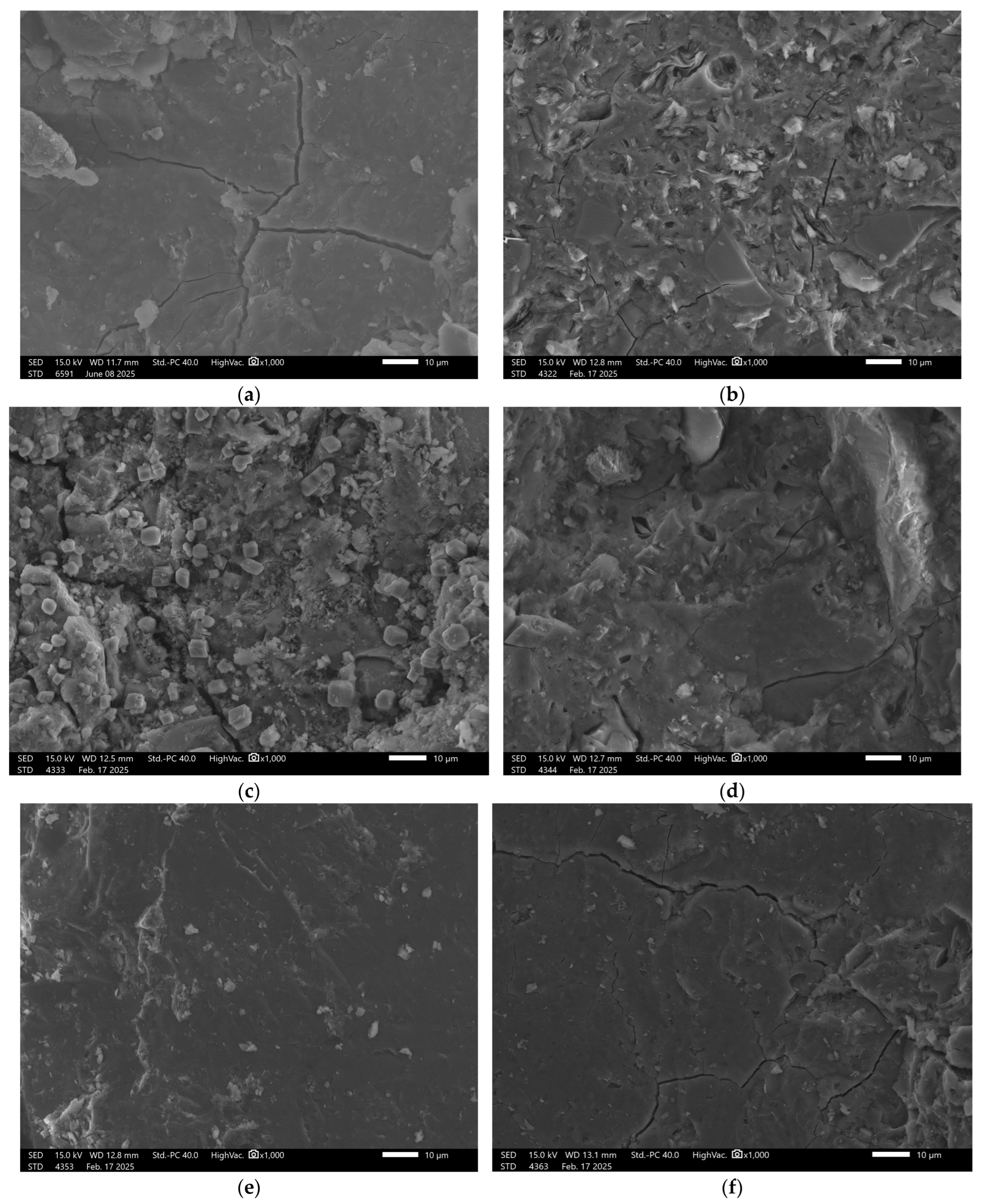


| No. | Sample | Metakaolin [kg] | Amphibolite [kg] | Sodium Water Glass [kg] | Silicon Dioxide [kg] | Carbon Fibers [kg] | Cellulose [kg] | Slag [kg] |
|---|---|---|---|---|---|---|---|---|
| 1 | GST 1 | 1 | 0 | 0.9 | 0.08 | 0.01 | 0.01 | 1 |
| 2 | GST 3 | 1 | 0 | 0.9 | 0.08 | 0.01 | 0.01 | 0.3 |
| 3 | GST 5 | 1 | 0 | 0.9 | 0.08 | 0.01 | 0.01 | 0.5 |
| 4 | GST 7 | 1 | 0 | 0.9 | 0.08 | 0.01 | 0.01 | 0.7 |
| 5 | Geo V5 | 1 | 0 | 0.9 | 0 | 0.01 | 0 | 0 |
| 6 | Geo V6 | 1 | 0 | 0.9 | 0 | 0.01 | 0 | 1 |
| 7 | Geo V7 | 1 | 1 | 0.9 | 0 | 0.01 | 0 | 0 |
| Sample | Density [g/cm3] | Standard Deviation |
|---|---|---|
| GST1 | 1.952 | 0.014 |
| GST3 | 1.756 | 0.036 |
| GST5 | 1.627 | 0.022 |
| GST7 | 1.807 | 0.026 |
| GEOV5 | 1.867 | 0.018 |
| GEOV6 | 1.900 | 0.021 |
| GEOV7 | 1.826 | 0.030 |
| Oxide | GST 1 | GST 3 | GST 5 | GST 7 | GEO V5 | GEO V6 | GEO V7 |
|---|---|---|---|---|---|---|---|
| MgO | 1.95% | 3.42% | 3.02% | 3.65% | 0.18% | 3.38% | 0.00% |
| Al2O3 | 16.69% | 19.56% | 19.88% | 20.27% | 24.47% | 21.31% | 17.02% |
| SiO2 | 55.19% | 47.34% | 46.29% | 45.06% | 49.90% | 40.87% | 56.95% |
| P2O5 | 0.60% | 0.025% | 0.00% | 0.11% | 0.00% | 0.19% | 0.00% |
| SO3 | 0.20% | 0.32% | 0.24% | 0.32% | 0.34% | 0.26% | 0.22% |
| Cl | 0.03% | 0.03% | 0.03% | 0.03% | 0.00% | 0.02% | 0.01% |
| K2O | 4.58% | 7.18% | 6.73% | 6.01% | 7.36% | 5.75% | 6.52% |
| CaO | 12.90% | 16.50% | 16.51% | 16.22% | 13.47% | 17.21% | 14.80% |
| Sc2O3 | 0.03% | 0.05% | 0.05% | 0.05% | 0.02% | 0.04% | 0.03% |
| TiO2 | 0.90% | 1.10% | 1.15% | 1.13% | 1.73% | 1.27% | 0.92% |
| V2O5 | 0.11% | 0.08% | 0.11% | 0.12% | 0.06% | 0.16% | 0.02% |
| Cr2O3 | 0.22% | 0.07% | 0.09% | 0.11% | 0.14% | 0.15% | 0.06% |
| MnO | 0.21% | 0.28% | 0.29% | 0.28% | 0.22% | 0.30% | 0.25% |
| Fe2O3 | 4.82% | 3.03% | 4.18% | 4.91% | 1.81% | 6.70% | 3.04% |
| NiO | 0.03% | 0.01% | 0.01% | 0.01% | 0.04% | 0.01% | 0.01% |
| CuO | 0.04% | 0.02% | 0.03% | 0.04% | 0.00% | 0.05% | 0.00% |
| ZnO | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% |
| SrO | 0.05% | 0.05% | 0.06% | 0.06% | 0.04% | 0.08% | 0.04% |
| Y2O3 | 0.00% | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% |
| ZrO2 | 1.32% | 0.81% | 1.21% | 1.47% | 0.07% | 2.06% | 0.05% |
| MoO3 | 0.02% | 0.01% | 0.01% | 0.01% | 0.01% | 0.02% | 0.00% |
| Sample | Solution | Initial Mass [g] | Final Mass [g] | Change [%] |
|---|---|---|---|---|
| GST1 | H2O | 50.49 | 54.27 | 7.49 |
| H2O + NaCl | 49.52 | 52.85 | 6.71 | |
| H2O + HCl | 50.48 | 53.93 | 6.84 | |
| H2O + HCl + CH3COOH + HNO3 | 50.50 | 53.31 | 5.56 | |
| H2O + NaOH | 52.01 | 55.54 | 6.78 | |
| GST3 | H2O | 49.19 | 52.64 | 7.01 |
| H2O + NaCl | 48.22 | 52.01 | 7.89 | |
| H2O + HCl | 49.16 | 52.06 | 5.90 | |
| H2O + HCl + CH3COOH + HNO3 | 47.86 | 50.52 | 5.56 | |
| H2O + NaOH | 49.64 | 52.48 | 5.71 | |
| GST5 | H2O | 45.42 | 49.77 | 9.65 |
| H2O + NaCl | 42.90 | 47.53 | 10.81 | |
| H2O + HCl | 42.99 | 47.97 | 11.62 | |
| H2O + HCl + CH3COOH + HNO3 | 43.66 | 47.67 | 9.18 | |
| H2O + NaOH | 43.35 | 48.04 | 10.82 | |
| GST7 | H2O | 53.22 | 56.91 | 6.94 |
| H2O + NaCl | 52.53 | 55.90 | 6.42 | |
| H2O + HCl | 53.06 | 56.02 | 5.57 | |
| H2O + HCl + CH3COOH + HNO3 | 53.15 | 55.76 | 4.90 | |
| H2O + NaOH | 52.95 | 55.99 | 5.73 | |
| GEOV5 | H2O | 53.98 | 55.77 | 3.31 |
| H2O + NaCl | 54.25 | 55.93 | 3.10 | |
| H2O + HCl | 54.69 | 56.33 | 3.01 | |
| H2O + HCl + CH3COOH + HNO3 | 52.79 | 53.69 | 1.70 | |
| H2O + NaOH | 51.69 | 52.89 | 2.31 | |
| GEOV6 | H2O | 50.21 | 53.28 | 6.13 |
| H2O + NaCl | 49.68 | 52.43 | 5.54 | |
| H2O + HCl | 50.77 | 53.55 | 5.46 | |
| H2O + HCl + CH3COOH + HNO3 | 49.86 | 51.87 | 4.04 | |
| H2O + NaOH | 50.35 | 53.27 | 5.79 | |
| GEOV7 | H2O | 49.21 | 52.58 | 6.84 |
| H2O + NaCl | 49.90 | 53.59 | 7.40 | |
| H2O + HCl | 49.63 | 53.19 | 7.16 | |
| H2O + HCl + CH3COOH + HNO3 | 49.01 | 51.8 | 5.69 | |
| H2O + NaOH | 49.11 | 52.61 | 7.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliwa, K.; Kozub, B.; Łoś, K.; Łoś, P.; Korniejenko, K. Assessment of Durability and Degradation Resistance of Geopolymer Composites in Water Environments. Materials 2025, 18, 3892. https://doi.org/10.3390/ma18163892
Oliwa K, Kozub B, Łoś K, Łoś P, Korniejenko K. Assessment of Durability and Degradation Resistance of Geopolymer Composites in Water Environments. Materials. 2025; 18(16):3892. https://doi.org/10.3390/ma18163892
Chicago/Turabian StyleOliwa, Kacper, Barbara Kozub, Katarzyna Łoś, Piotr Łoś, and Kinga Korniejenko. 2025. "Assessment of Durability and Degradation Resistance of Geopolymer Composites in Water Environments" Materials 18, no. 16: 3892. https://doi.org/10.3390/ma18163892
APA StyleOliwa, K., Kozub, B., Łoś, K., Łoś, P., & Korniejenko, K. (2025). Assessment of Durability and Degradation Resistance of Geopolymer Composites in Water Environments. Materials, 18(16), 3892. https://doi.org/10.3390/ma18163892








