Research Progress on Ultrahigh-Temperature Ceramics Modified C/C Composites
Abstract
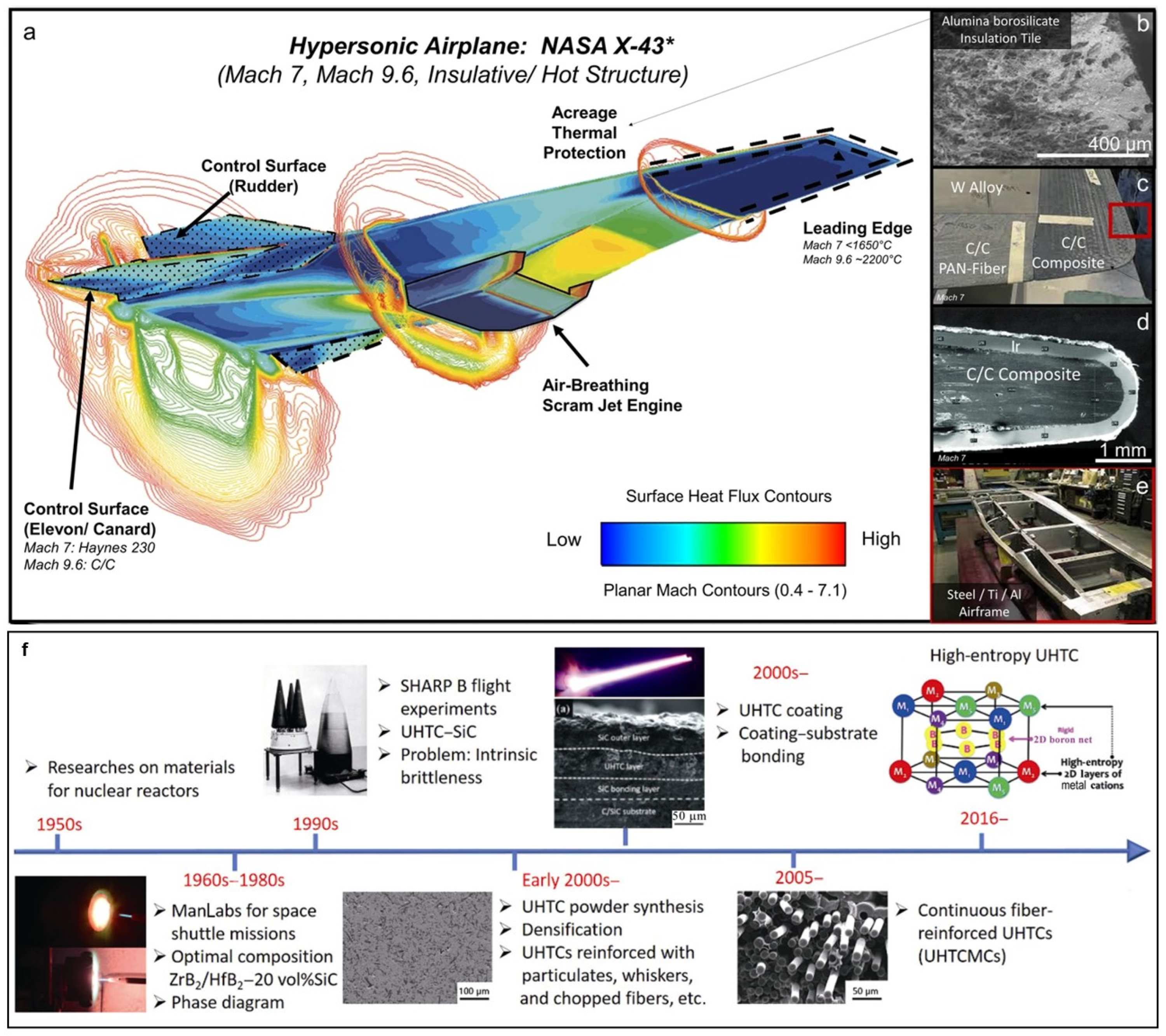
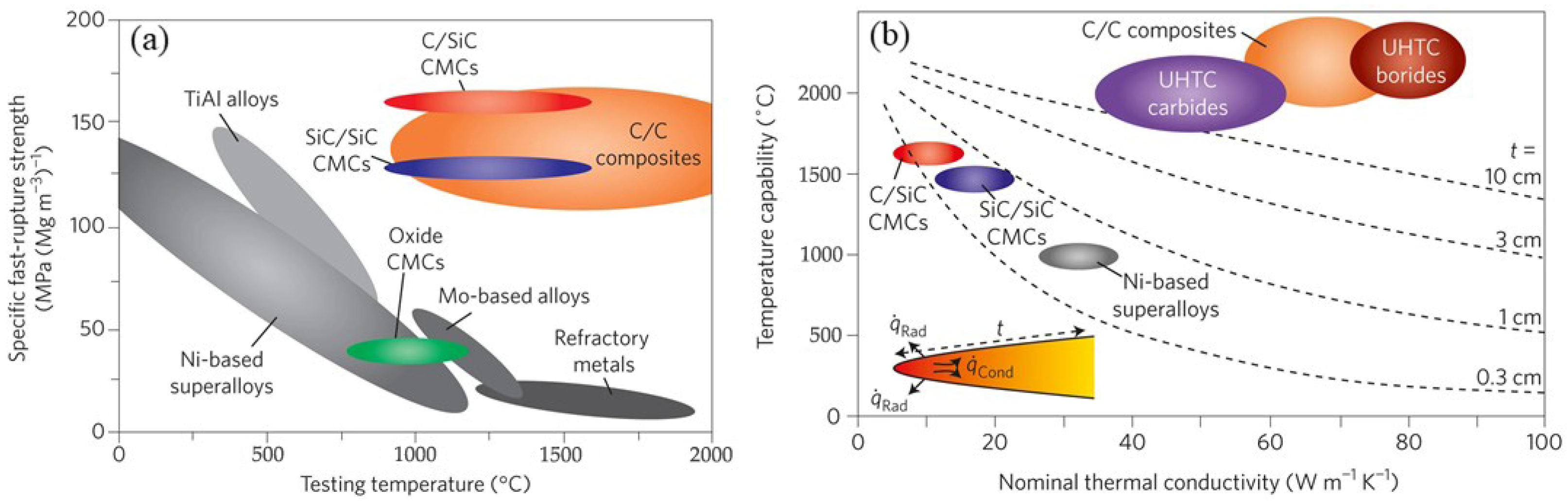
1. Introduction
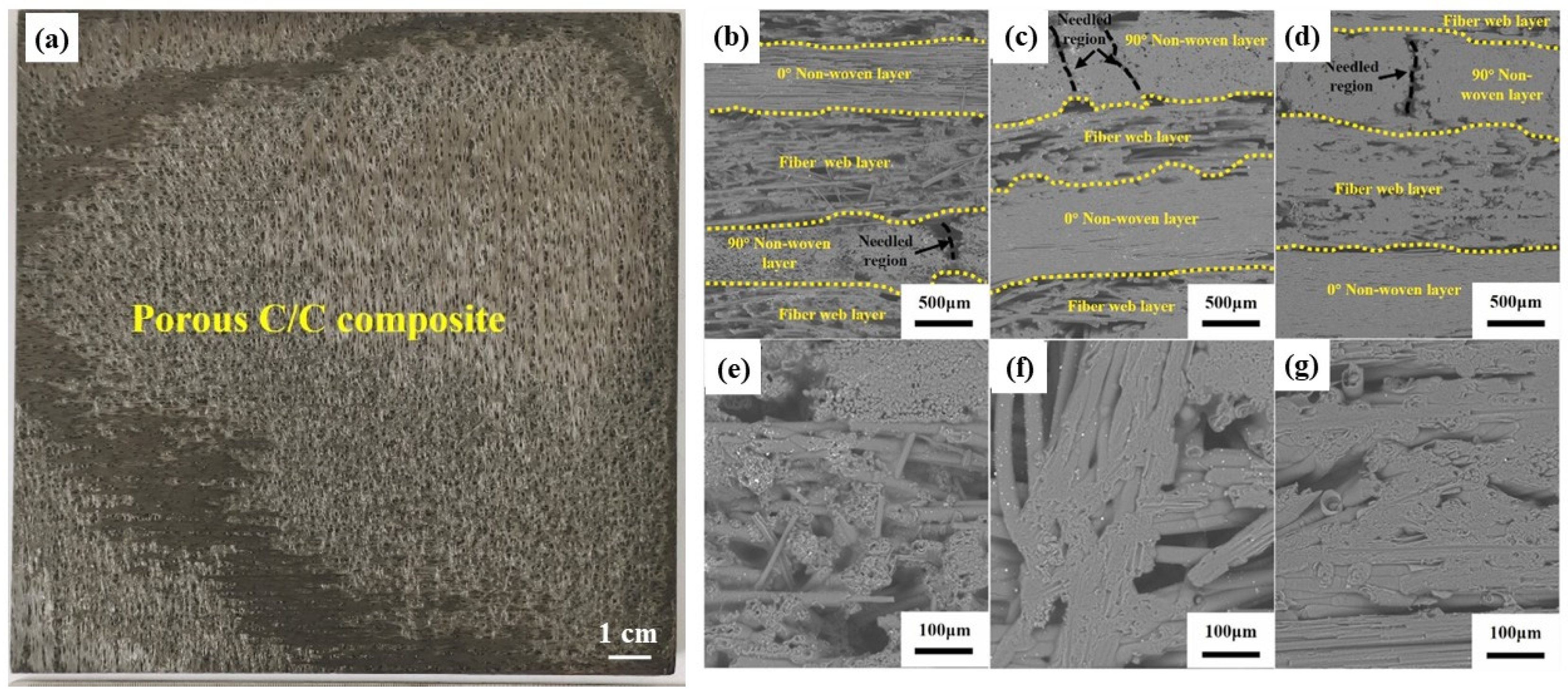
2. Preparation Methods and Microstructure of the UHTCs-Modified C/C Composites
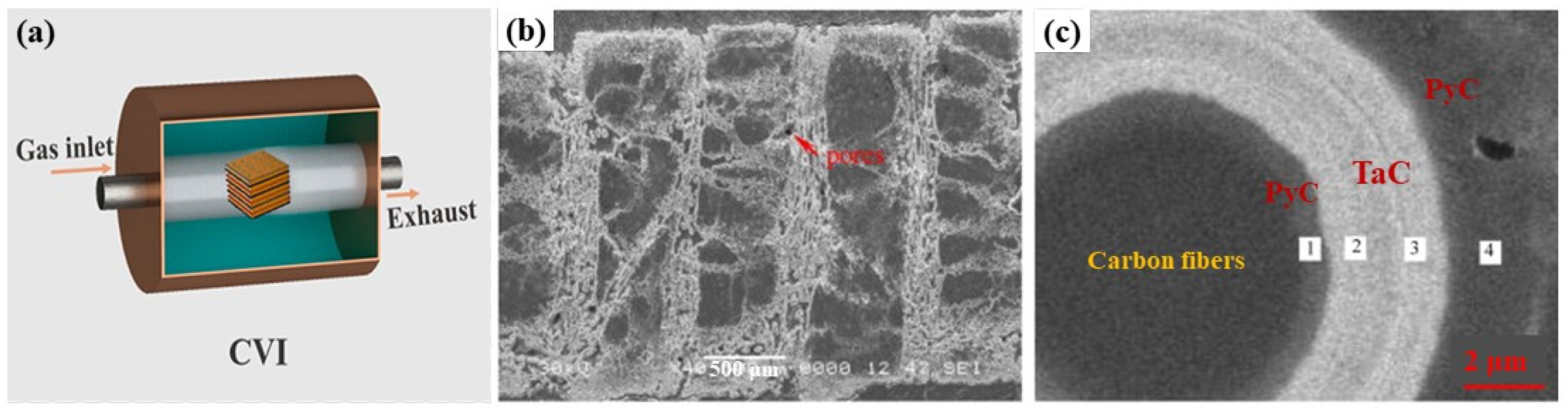
2.1. Chemical Vapor Infiltration
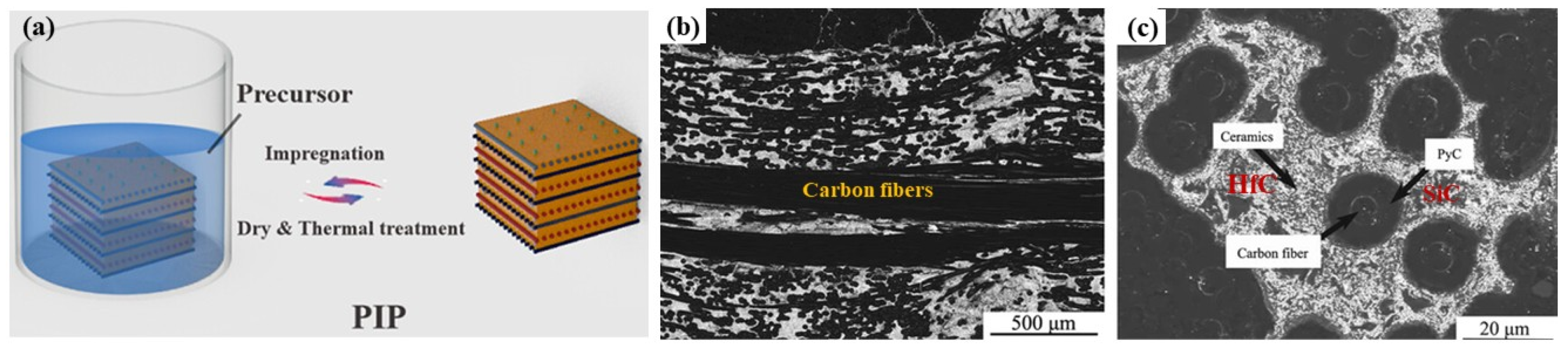
2.2. Precursor Infiltration and Pyrolysis
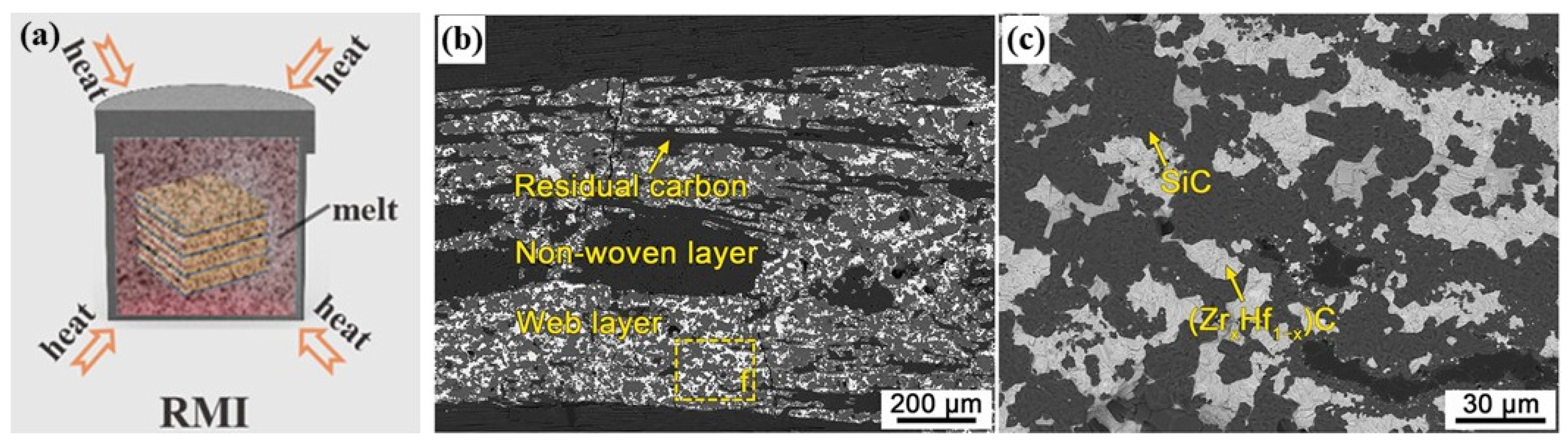
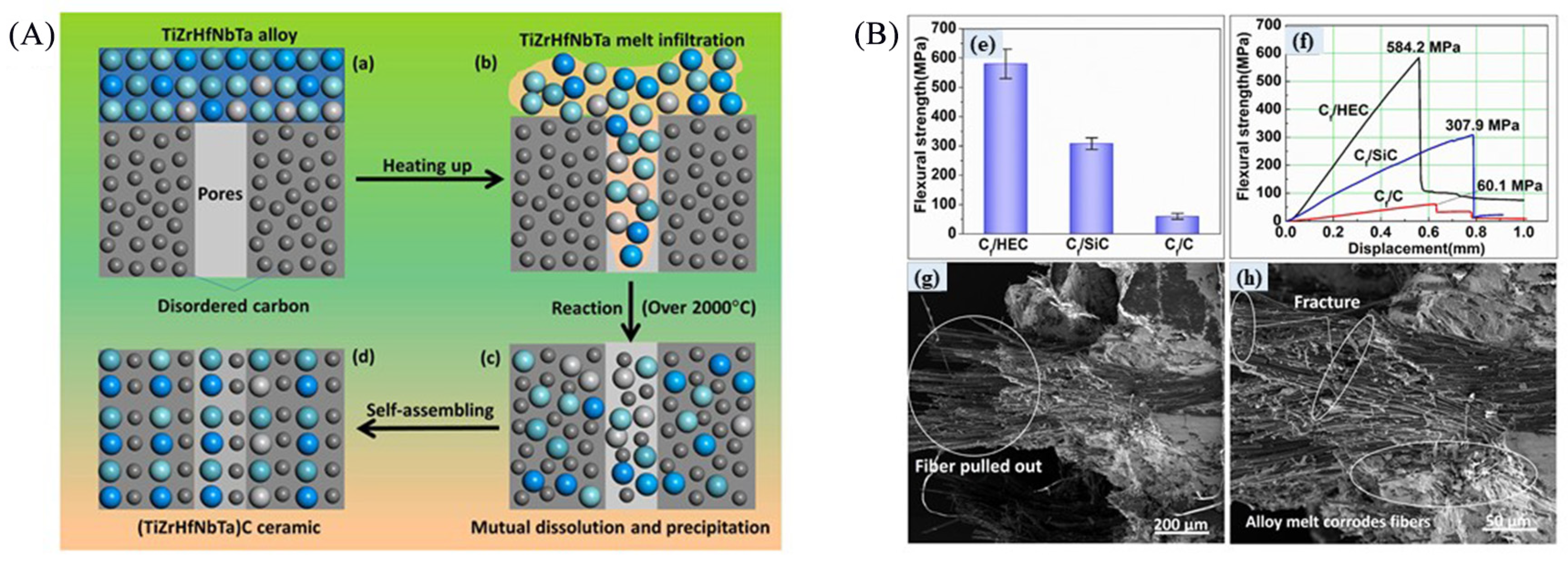
2.3. Reactive Melting Infiltration
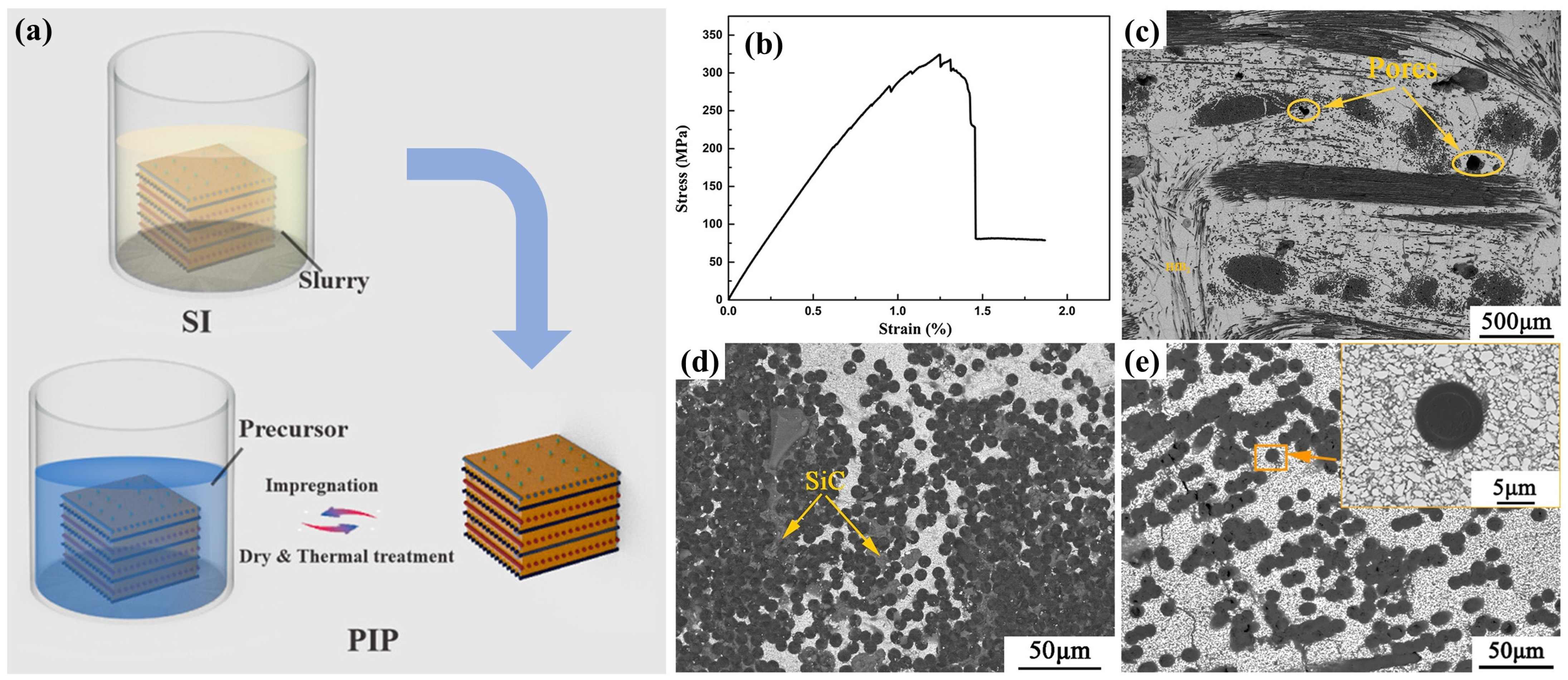
2.4. Combined Preparation Technology
3. Ablation Resistance and Mechanisms of UHTCs-Modified C/C Composites
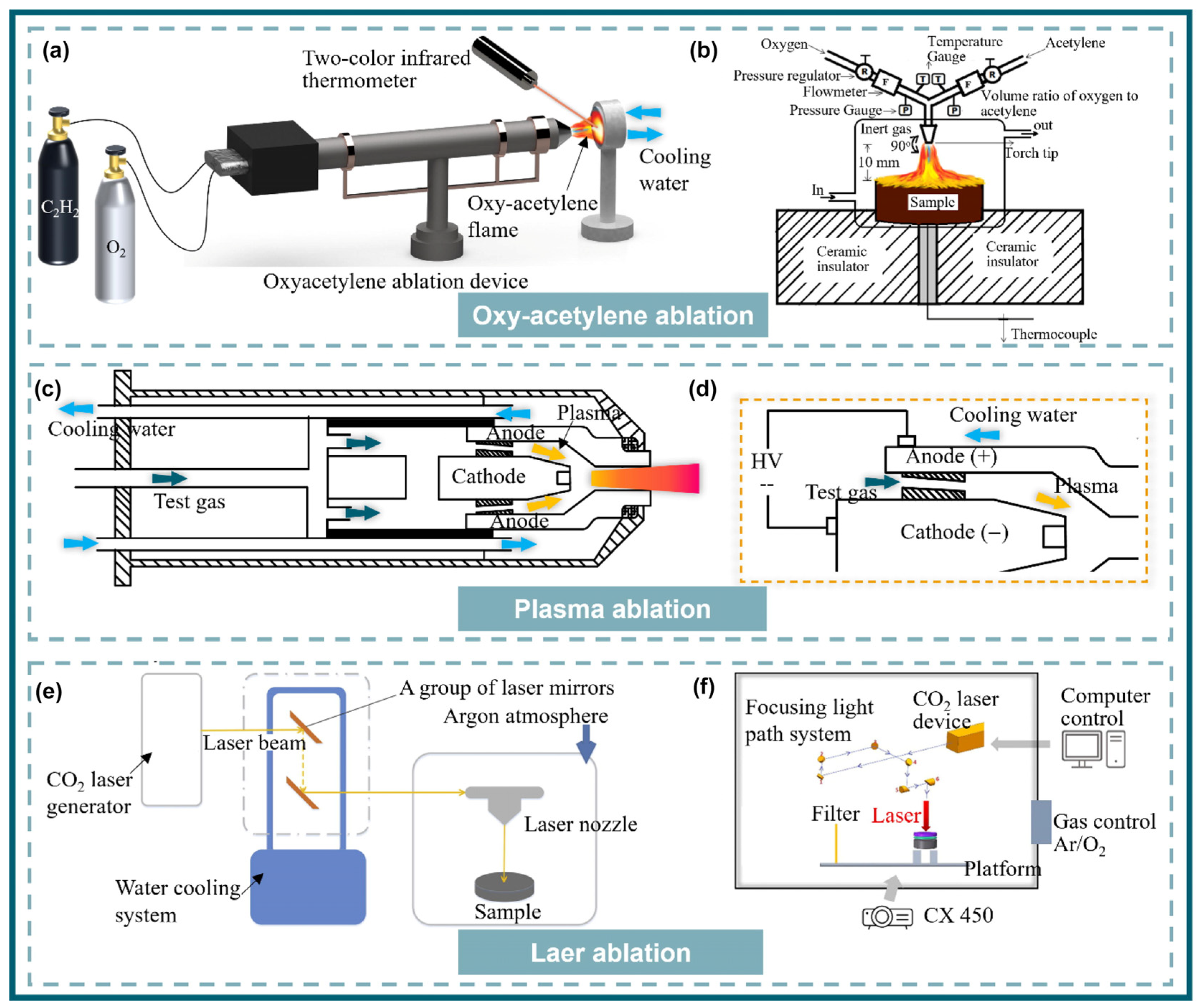
3.1. Testing and Numerical Methods of the Ablation Resistance
3.1.1. Oxygen Acetylene Ablation
3.1.2. Plasma Ablation
3.1.3. Laser Ablation
3.1.4. Wind Tunnel Ablation
3.1.5. Numerical Simulation
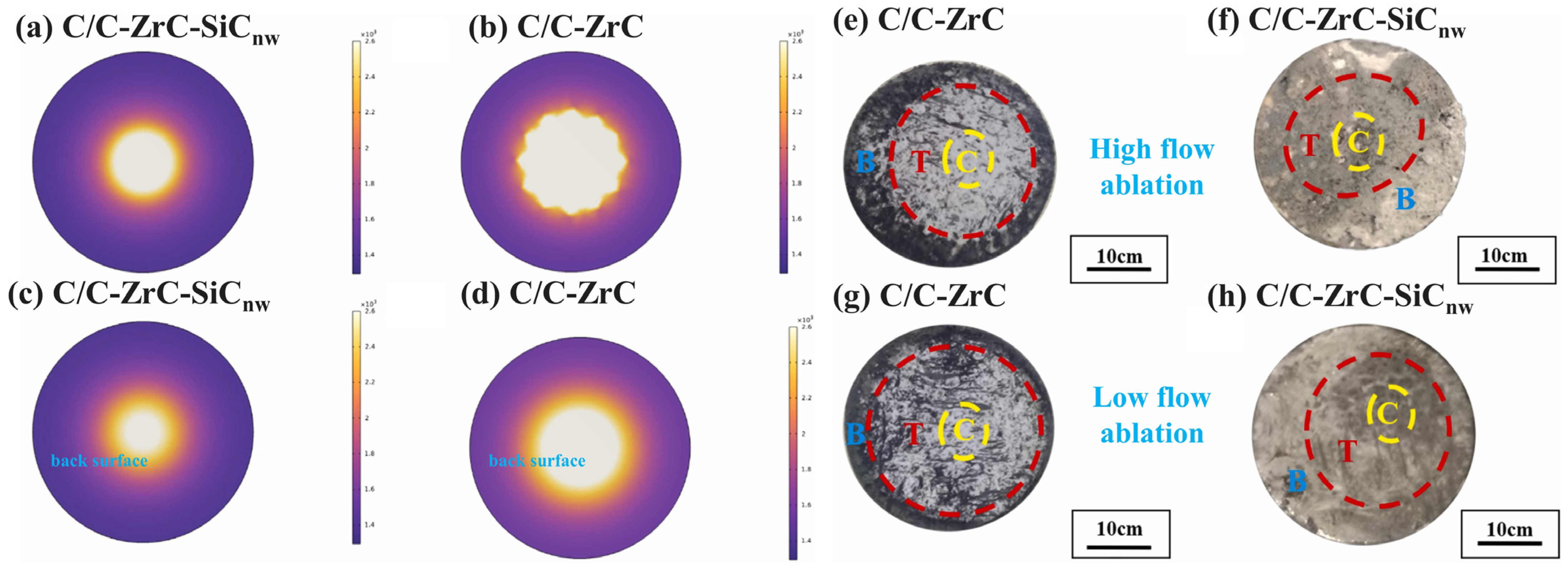
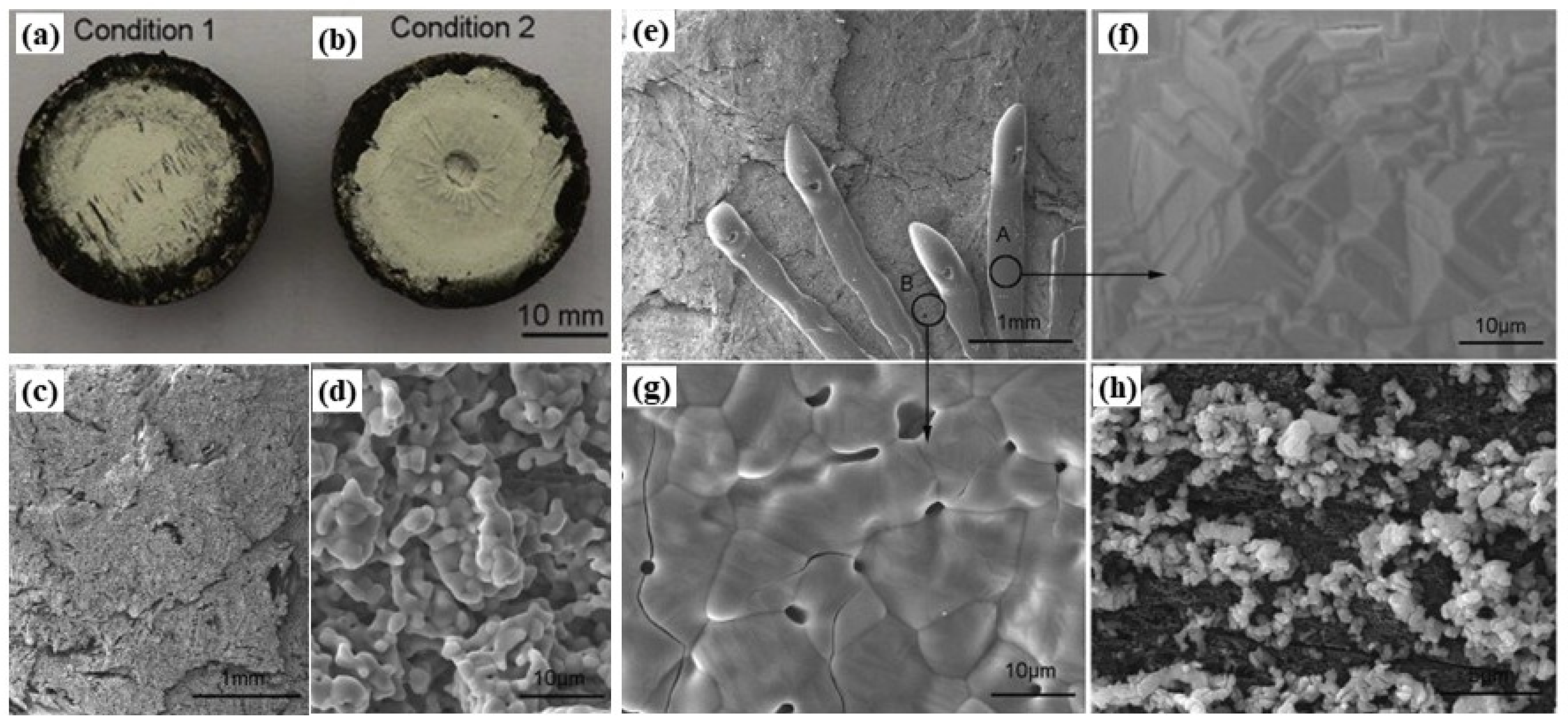
3.2. Evaluation Criteria of Ablation Resistance
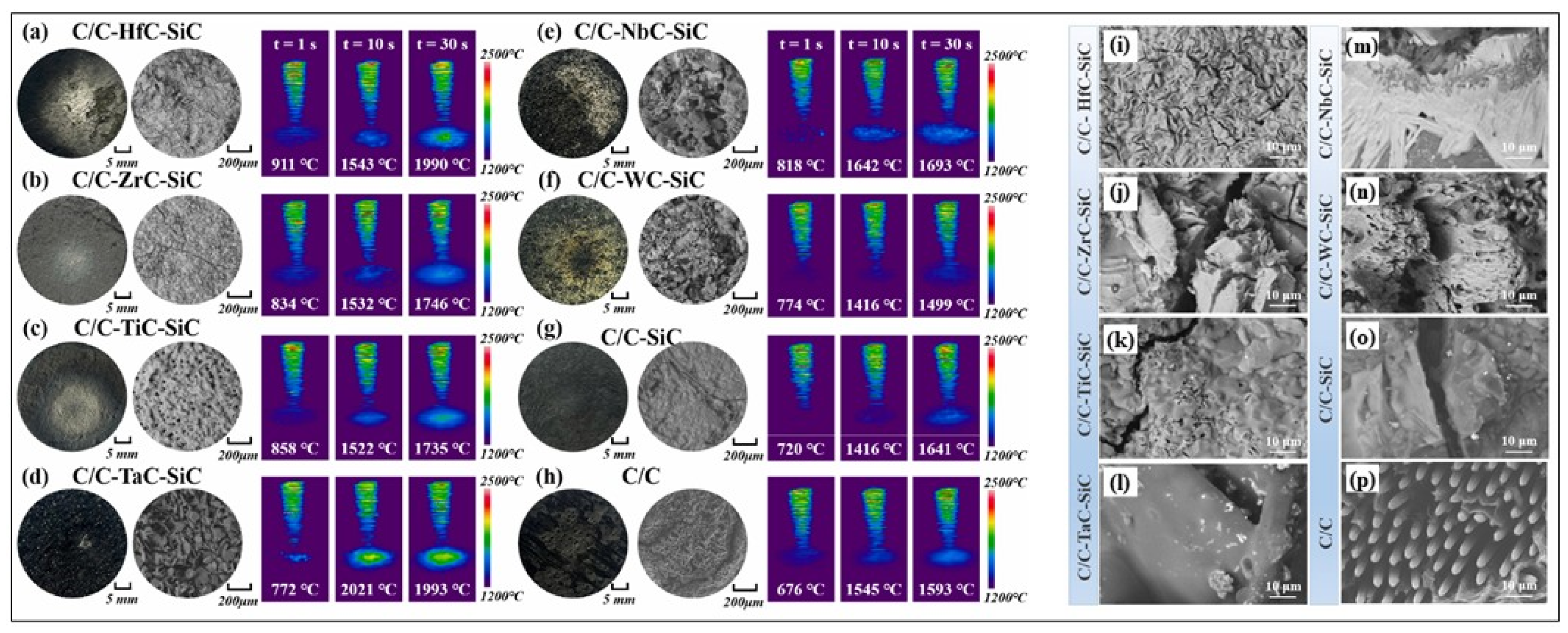
3.3. Single-Phase UHTCs-Modified C/C Composites
3.3.1. Zr-Based UHTCs-Modified C/C Composites
3.3.2. Hf-Based UHTCs-Modified C/C Composites
3.3.3. Ta-Based UHTCs-Modified C/C Composites
3.3.4. Ti-Based UHTCs-Modified C/C Composites
3.4. Multiphase UHTCs-Modified C/C Composites
3.5. Multicomponent UHTCs-Modified C/C Composites
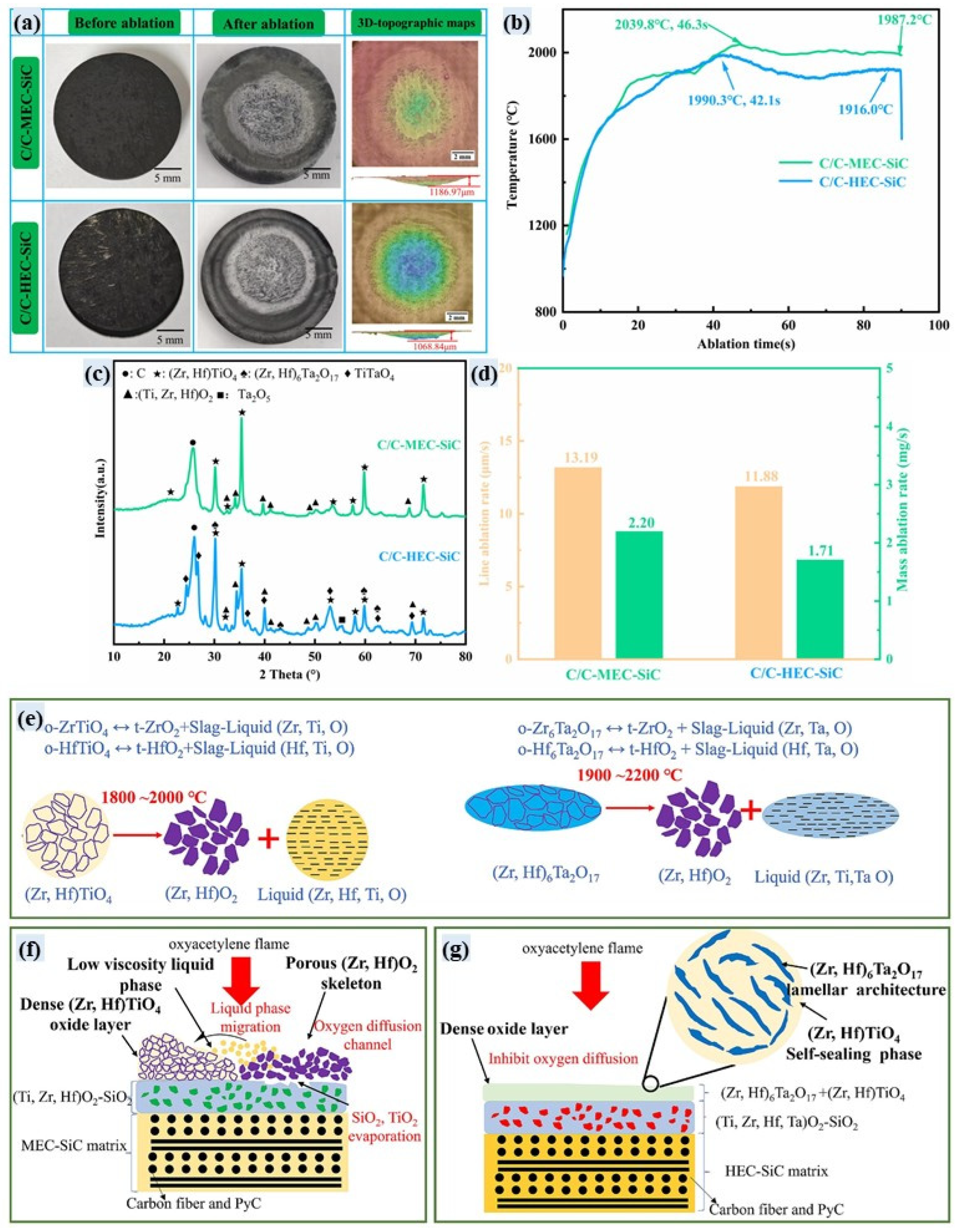
3.6. High-Entropy UHTCs-Modified C/C Composites
4. Summary and Outlook
- (1)
- Systematic compilation and comparison: This work comprehensively compiles and critically compares the fabrication strategies, ablation behaviors, and protective mechanisms across diverse UHTC modification systems, including single-phase (e.g., ZrC and HfC), multiphase (e.g., C/C-ZrC-SiC), multicomponent (e.g., Zr-Ti-C-B solid solutions), and the emerging high-entropy ceramics (e.g., (TiZrHfNbTa)C). This comparison elucidates performance differences and synergistic effects, providing a clear roadmap for material design.
- (2)
- In-depth mechanistic insights: This review offers a profound analysis of the failure mechanisms of C/C-UHTCs composites under extreme thermo-mechano-chemical coupled environments. Crucially, it deciphers the formation, evolution, and failure modes of oxide layers (e.g., ZrO2 and HfO2) and highlights the pivotal role of introducing secondary phases (e.g., SiC) or forming multicomponent/high-entropy oxides (e.g., complex silicates and high-entropy oxides) in improving oxide layer density, stability, self-healing capability, and thermal compatibility. This deepens the fundamental understanding of the protection mechanisms at ultrahigh temperatures.
- (3)
- Critical evaluation of innovative processes: It critically assesses the advantages and limitations of key fabrication techniques—CVI, PIP, RMI, and their hybrids (e.g., SI + PIP and molten salt-assisted RMI)—in controlling ceramic phase content, distribution, interface structure, and ultimately composite performance. The review underscores the potential of process innovations (e.g., molten salt assistance and vacuum vibration-assisted slurry infiltration) in overcoming drawbacks of traditional methods (low efficiency, fiber damage, and metal residue), pointing towards pathways for efficient fabrication of high-performance composites.
- (4)
- Clarification of challenges and future directions: It explicitly identifies the core challenges hindering the practical applications of C/C-UHTCs composites (e.g., precise control of preform pore structure, fabrication of large/complex-shaped components, thorough understanding of ablation mechanisms in multicomponent/high-entropy systems, cost reduction, bridging ground–space simulation gaps). Building on current knowledge, it outlines critical future research priorities, encompassing advanced structural design (e.g., graded structures and nanowire reinforcement), development and application of novel high-entropy/multicomponent ceramics, integration of multi-scale modeling with real-service environment testing, and breakthroughs in low-cost, scalable manufacturing technologies.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Wie, D.M. Hypersonics: Past, present, and potential future. Johns Hopkins APL Tech. Dig. 2021, 35, 335–341. [Google Scholar]
- Peters, A.B.; Zhang, D.; Chen, S.; Ott, C.; Oses, C.; Curtarolo, S.; McCue, I.; Pollock, T.M.; Eswarappa Prameela, S. Materials design for hypersonics. Nat. Commun. 2024, 15, 3328. [Google Scholar] [CrossRef] [PubMed]
- Natali, M.; Kenny, J.M.; Torre, L. Science and technology of polymeric ablative materials for thermal protection systems and propulsion devices: A review. Prog. Mater. Sci. 2016, 84, 192–275. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Fang, D. Research progress on thermal protection materials and structures of hypersonic vehicles. Appl. Math. Mech. 2008, 29, 51–60. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Nemani, S.K.; Hilmas, G.E.; Opila, E.J.; Anasori, B. Ultra-high temperature ceramics for extreme environments. Nat. Rev. Mater. 2024, 9, 773–789. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Yufeng, C.; Changqing, H.; Chenglong, H.; Ping, H.; Ling, L.; Jiachen, L.; Ling, L.; Donghui, L.; Haipeng, Q.; Sufang, T.; et al. Ceramic-based thermal protection materials for aerospace vehicles. Adv. Ceram. 2017, 38, 311–390. [Google Scholar] [CrossRef]
- Nasseri, M.M. Comparison of HfB2 and ZrB2 behaviors for using in nuclear industry. Ann. Nucl. Energy 2018, 114, 603–606. [Google Scholar] [CrossRef]
- Rogl, P.; Potter, P.E. A critical review and thermodynamic calculation of the binary system: Zirconium-Boron. Calphad 1988, 12, 191–204. [Google Scholar] [CrossRef]
- Jin, X.; Fan, X.; Lu, C.; Wang, T. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. J. Eur. Ceram. Soc. 2018, 38, 1–28. [Google Scholar] [CrossRef]
- Sayir, A. Carbon fiber reinforced hafnium carbide composite. J. Mater. Sci. 2004, 39, 5995–6003. [Google Scholar] [CrossRef]
- Tang, S.; Deng, J.; Wang, S.; Liu, W. Fabrication and characterization of an ultra-high-temperature carbon fiber-reinforced ZrB2–SiC matrix composite. J. Am. Ceram. Soc. 2007, 90, 3320–3322. [Google Scholar] [CrossRef]
- Wen, T.; Wen, Q.; Lu, L.; Wang, Y.; Jiang, T.; Hu, J.; Zeng, Y.; Xiong, X. Effects of polymer-derived ZiC interlayer on mechanical properties and ablation performance of C/C-ZiC-ZrC-SiC composites prepared by RMI. J. Eur. Ceram. Soc. 2024, 44, 5623–5638. [Google Scholar] [CrossRef]
- Agarwal, N.; Rangamani, A.; Bhavsar, K.; Virnodkar, S.S.; Fernandes, A.A.A.; Chadha, U.; Srivastava, D.; Patterson, A.E.; Rajasekharan, V. An overview of carbon-carbon composite materials and their applications. Front. Mater. 2024, 11, 1374034. [Google Scholar] [CrossRef]
- Hatta, H.; Goto, K.; Aoki, T. Strengths of C/C composites under tensile, shear, and compressive loading: Role of interfacial shear strength. Compos. Sci. Technol. 2005, 65, 2550–2562. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, G.; Bao, Y.; Sun, D.; Zhang, Q.; Meng, X.; Hu, Y.; Yan, L. Progress in preparation and ablation resistance of ultra-high-temperature ceramics modified C/C composites for extreme environment. Rev. Adv. Mater. Sci. 2023, 62, 20220276. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef]
- Feng, G.; Li, H.; Yao, X.; Chen, M.; Xue, Y. Ablation resistance of TaC-modified HfC coating prepared by supersonic plasma spraying for SiC-coated carbon/carbon composites. Ceram. Int. 2019, 45, 17936–17945. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Gao, L.; Zhang, L.; Cheng, L. Preparation and microstructural evolution of carbon/carbon composites. Mater. Sci. Eng. A 2006, 430, 9–14. [Google Scholar] [CrossRef]
- Farhan, S.; Wang, R.; Li, K.; Wang, C. Sublimation and oxidation zone ablation behavior of carbon/carbon composites. Ceram. Int. 2015, 41, 13751–13758. [Google Scholar] [CrossRef]
- Halbig, M.C.; McGuffin-Cawley, J.D.; Eckel, A.J.; Brewer, D.N. Oxidation kinetics and stress effects for the oxidation of continuous carbon fibers within a microcracked C/SiC ceramic matrix composite. J. Am. Ceram. Soc. 2008, 91, 519–526. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, D.; Xiong, X.; Zhang, X.; Xiao, P. Ablation-resistant carbide Zr0.8Ti0.2C0.74B0.26 for oxidizing environments up to 3,000 °C. Nat. Commun. 2017, 8, 15836. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, X.; Huang, B.; Li, G.; Zheng, F.; Xiao, P.; Zhang, H.; Yin, J. Phase composition and morphology of TaC coating on carbon fibers by chemical vapor infiltration. Thin Solid Film. 2008, 516, 8248–8254. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, preparation and properties of carbon fiber reinforced ultra-high temperature ceramic composites for aerospace applications: A review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, X.; Liu, H.; Fu, Q.; Li, H. Novel structural design strategies in ceramic-modified C/C composites. Acc. Mater. Res. 2023, 4, 1095–1107. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, X. Microstructure, mechanical properties and oxidation behavior of carbon fiber reinforced PyC/C-TaC/PyC layered-structure ceramic matrix composites prepared by chemical vapor infiltration. Mater. Chem. Phys. 2013, 141, 613–619. [Google Scholar] [CrossRef]
- Delhaes, P. Chemical vapor deposition and infiltration processes of carbon materials. Carbon 2002, 40, 641–657. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, X.; Huang, Q.; Yi, M.; Huang, B. Densification mechanism of chemical vapor infiltration technology for carbon/carbon composites. Trans. Nonferrous Met. Soc. China 2007, 17, 519–522. [Google Scholar] [CrossRef]
- Kopeliovich, D. Advances in manufacture of ceramic matrix composites by infiltration techniques. In Advances in Ceramic Matrix Composites; Woodhead Publishing: Cambridge, UK, 2018; pp. 93–119. [Google Scholar]
- Li, H.-j.; Yao, X.-y.; Zhang, Y.-l.; Li, K.-z.; Guo, L.-j.; Liu, L. Effect of heat flux on ablation behaviour and mechanism of C/C-ZrB2-SiC composite under oxyacetylene torch flame. Corros. Sci. 2013, 74, 265–270. [Google Scholar] [CrossRef]
- Yan, M.; Li, H.; Fu, Q.; Xie, J.; Liu, L.; Feng, B. Ablative property of C/C-SiC-HfC composites prepared via precursor infiltration and pyrolysis under 3000 °C oxyacetylene torch. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 981–987. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Xiong, X.; Zhang, H.; Ye, Z.; Long, Q.; Wen, Q.; Wang, J.; Li, T.; Liu, C. Structural optimization and air-plasma ablation behaviors of C/C-SiC-(ZrxHf1−x)C composites prepared by reactive melt infiltration method. Corros. Sci. 2023, 222, 111408. [Google Scholar] [CrossRef]
- Tong, Y.; Bai, S.; Liang, X.; Qin, Q.H.; Zhai, J. Reactive melt infiltration fabrication of C/C-SiC composite: Wetting and infiltration. Ceram. Int. 2016, 42, 17174–17178. [Google Scholar] [CrossRef]
- Kütemeyer, M.; Schomer, L.; Helmreich, T.; Rosiwal, S.; Koch, D. Fabrication of ultra high temperature ceramic matrix composites using a reactive melt infiltration process. J. Eur. Ceram. Soc. 2016, 36, 3647–3655. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, M.; Xiao, P.; Li, Y. Influence of graphitization treatment on microstructure and flexural strength of C/C-ZrC-SiC composites fabricated via reactive melt infiltration. Ceram. Int. 2023, 49, 29391–29399. [Google Scholar] [CrossRef]
- Yao, J.; Pang, S.; Hu, C.; Li, J.; Tang, S.; Cheng, H.-M. Mechanical, oxidation and ablation properties of C/(C-SiC)CVI-(ZrC-SiC)PIP composites. Corros. Sci. 2020, 162, 108200. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Ran, L.P.; Peng, K.; Ge, Y.C.; Wu, H.; Yi, M.Z. Microstructure and ablation property of a carbon/carbon–ZrC composite fabricated by reactive melt infiltration with Zr/Cu powder mixture. Adv. Eng. Mater. 2015, 18, 162–168. [Google Scholar] [CrossRef]
- Fang, C.; Hu, P.; Dong, S.; Feng, J.; Xun, L.; Zhang, X. Influence of hydrothermal carbon coating on the properties of Cf/ZrB2/SiBCN prepared by slurry injection. J. Eur. Ceram. Soc. 2021, 41, 84–91. [Google Scholar] [CrossRef]
- Tang, S.; Deng, J.; Wang, S.; Liu, W.; Yang, K. Ablation behaviors of ultra-high temperature ceramic composites. Mater. Sci. Eng. A 2007, 465, 1–7. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Cheng, H.; Chen, S. Microstructure and properties of C/C-ZrC composites with matrix modification by slurry infiltration. Ceram. Int. 2023, 49, 13074–13080. [Google Scholar] [CrossRef]
- Zhou, H.; Ni, D.; He, P.; Yang, J.; Hu, J.; Dong, S. Ablation behavior of C/C-ZrC and C/SiC-ZrC composites fabricated by a joint process of slurry impregnation and chemical vapor infiltration. Ceram. Int. 2018, 44, 4777–4782. [Google Scholar] [CrossRef]
- Tang, Z.; Yi, M.; Yin, H.; Du, Y.; Wu, H.; Peng, K. Ablation behavior of a C/C-ZrC-SiC composite based on high-solid-loading slurry impregnation under oxyacetylene torch. J. Eur. Ceram. Soc. 2022, 42, 4748–4758. [Google Scholar] [CrossRef]
- Tang, Z.; Yi, M.; Xiang, Q.; Du, Y.; Peng, K. Mechanical and ablation properties of a C/C-HfB2-SiC composite prepared by high-solid-loading slurry impregnation combined with precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2021, 41, 6160–6170. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, J.; Hu, P.; Xun, L.; Liu, M.; Dong, S.; Zhang, X. Enhanced mechanical properties and thermal shock resistance of Cf/ZrB2-SiC composite via an efficient slurry injection combined with vibration-assisted vacuum infiltration. J. Eur. Ceram. Soc. 2020, 40, 5059–5066. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, P.; Dong, S.; Liu, X.; Wang, C.; Zhang, Z.; Zhang, X. Oxidation behavior and ablation mechanism of Cf/ZrB2-SiC composite fabricated by vibration-assisted slurry impregnation combined with low-temperature hot pressing. Corros. Sci. 2019, 161, 108181. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, D.y.; Dong, S.; Qu, Q.; Zhang, X.h. A novel vibration-assisted slurry impregnation to fabricate Cf/ZrB2-SiC composite with enhanced mechanical properties. J. Eur. Ceram. Soc. 2019, 39, 798–805. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Liu, H.; Zhang, Y.; Fu, Q.; Yin, X.; Li, H. Advanced anti-ablation C/C composites: Structural design strategies and future perspective. Mater. Today 2024, 80, 710–736. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, S.; Liu, T.; Zhang, Q.; Ma, R. Research progress on antioxidant ablation properties of ultra-high temperature ceramic modified carbon/carbon composites. Mater. Rep. 2024, 38, 112–122. [Google Scholar]
- Shen, X.; Shi, Z.; Zhao, Z.; Wang, X.; Li, C.; Huang, J.; Li, K.; Liu, G. Study of the ablation of a carbon/carbon composite at ∼25 MW/m2 with a nitrogen plasma torch. J. Eur. Ceram. Soc. 2020, 40, 5085–5093. [Google Scholar] [CrossRef]
- Shen, X.; Gao, N.; Shi, Z.; Wang, X.; Zhang, L.; Huang, J.; Li, K. New insight into the ablation behavior of C/C-ZrC composites in a nitrogen plasma torch with a high heat flux of ~25 MW/m2. Corros. Sci. 2021, 185, 109409. [Google Scholar] [CrossRef]
- Teng, L.; Shi, X.H.; Wang, H.H.; Li, W.; Yin, X.M.; Li, H.J. A new method to improve the laser-ablation resistance of Si-SiC coating on C/C composites: Laser cladding. J. Eur. Ceram. Soc. 2022, 42, 6425–6434. [Google Scholar] [CrossRef]
- Tong, Y.; Bai, S.; Hu, Y.; Liang, X.; Ye, Y.; Qin, Q.H. Laser ablation resistance and mechanism of Si-Zr alloyed melt infiltrated C/C-SiC composite. Ceram. Int. 2018, 44, 3692–3698. [Google Scholar] [CrossRef]
- Xu, J.; Guo, L.; Kong, J.; Ma, Y.; Wang, H.; Wang, J. The mechanical properties of C/C-ZrC-SiC composites after laser ablation. J. Eur. Ceram. Soc. 2023, 43, 6732–6745. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R.; Zoli, L.; Silvestroni, L.; Sciti, D. Ultra-high-temperature testing of sintered ZrB2-based ceramic composites in atmospheric re-entry environment. Int. J. Heat Mass Transf. 2020, 156, 119910. [Google Scholar] [CrossRef]
- Cecere, A.; Savino, R.; Allouis, C.; Monteverde, F. Heat transfer in ultra-high temperature advanced ceramics under high enthalpy arc-jet conditions. Int. J. Heat Mass Transf. 2015, 91, 747–755. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Liu, L.; Duan, L.; Wang, G.; Lu, Y. Ablation behavior of C/SiC composites in plasma wind tunnel. Carbon 2016, 103, 73–83. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R.; Silvestroni, L.; Vinci, A.; Zoli, L.; Sciti, D. Arc-jet wind tunnel characterization of ultra-high-temperature ceramic matrix composites. Corros. Sci. 2019, 149, 18–28. [Google Scholar] [CrossRef]
- Koide, N.; Marumo, T.; Arai, Y.; Hasegawa, M.; Nishimura, T.; Inoue, R. Degradation of carbon fiber-reinforced ultra-high-temperature ceramic matrix composites at extremely high temperature using arc-wind tunnel tests. J. Mater. Sci. 2022, 57, 19785–19798. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, D.; Fu, Q. Thermal protection mechanism of UHTCs-modified C/C composites in high temperature gas scouring coupling environments. Compos. Part B Eng. 2025, 302, 112550. [Google Scholar] [CrossRef]
- Tan, Q.; Jiao, X.; He, Q.; Wang, Y.; Yin, X. Effect of mullite content on microstructure and ablation behavior of mullite modified C/C-SiC-HfC composites. Corros. Sci. 2023, 222, 111405. [Google Scholar] [CrossRef]
- Baker, B.; Venkatachalam, V.; Zoli, L.; Vinci, A.; Failla, S.; Sciti, D.; Binner, J. Ablation behaviour of carbon fibre ultra-high temperature composites at oblique angles of attack. Mater. Des. 2021, 212, 110199. [Google Scholar] [CrossRef]
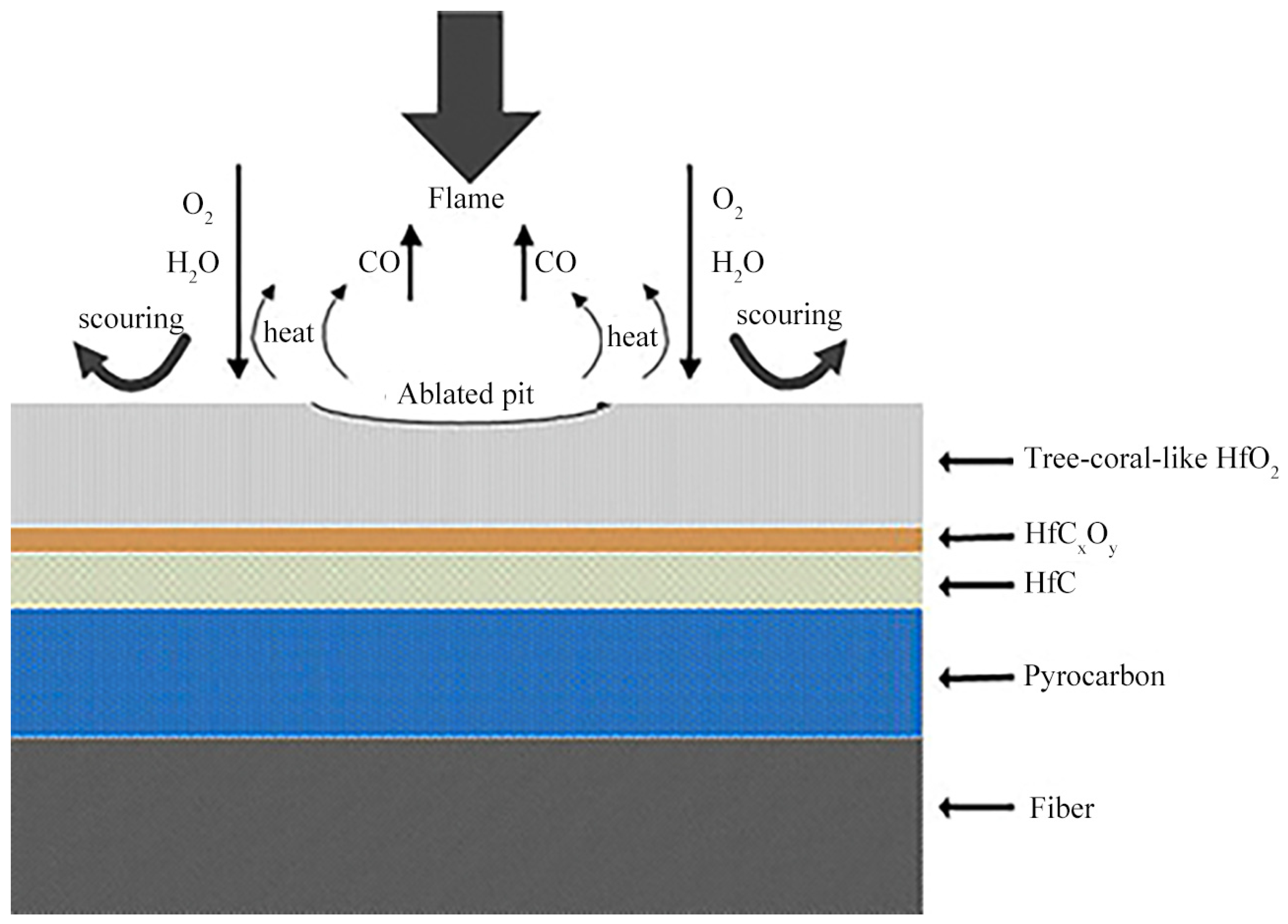
- Zhang, M.; Liu, T.; Hu, D.; Fu, Q. Ablation behavior of UHTCs carbide-modified C/C composites in extreme aerobic environments (3000 °C): Evolution mechanisms of oxides film structure. Corros. Sci. 2025, 253, 113036. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Q.; Zhang, Y.; Ye, C.; Zhu, S.; Fan, Z.; Han, F.; Liu, H.; Liu, J. Ablation behavior and thermal conduction mechanism of 3D ZrC–SiC-modified carbon/carbon composite having high thermal conductivity using mesophase-pitch-based carbon fibers and pyrocarbon as heat transfer channels. Compos. Part B Eng. 2021, 224, 109201. [Google Scholar] [CrossRef]
- Shen, X.; Li, K.; Li, H.; Du, H.; Cao, W.; Lan, F. Microstructure and ablation properties of zirconium carbide doped carbon/carbon composites. Carbon 2010, 48, 344–351. [Google Scholar] [CrossRef]
- Yin, J.; Xiong, X.; Zhang, H.b.; Huang, B. Microstructure and ablation performances of dual-matrix carbon/carbon composites. Carbon 2006, 44, 1690–1694. [Google Scholar] [CrossRef]
- Wen, Q.; Luan, X.; Wang, L.; Xu, X.; Ionescu, E.; Riedel, R. Laser ablation behavior of SiHfC-based ceramics prepared from a single-source precursor: Effects of Hf-incorporation into SiC. J. Eur. Ceram. Soc. 2019, 39, 2018–2027. [Google Scholar] [CrossRef]
- Yu, H.; Li, K.; Lu, J.; Zhao, Z. Effect of SiC nanowires on ablation property of Cu-doped C/C-ZrC composites. Corros. Sci. 2024, 227, 111732. [Google Scholar] [CrossRef]
- Liu, T.; Fu, Q.; Zhang, J. Microstructure and mechanical performance evolution of C/C-ZrC composites exposed to oxyacetylene flame. Ceram. Int. 2021, 47, 22654–22661. [Google Scholar] [CrossRef]
- Li, K.; Xie, J.; Li, H.; Fu, Q. Ablative and Mechanical Properties of C/C-ZrC Composites Prepared by Precursor Infiltration and Pyrolysis Process. J. Mater. Sci. Technol. 2015, 31, 77–82. [Google Scholar] [CrossRef]
- Zhao, Z.g.; Li, K.z.; Kou, G.; Li, W. Comparative research on cyclic ablation behavior of C/C-ZrC-SiC and C/C-ZrC composites at temperatures above 2000 °C. Corros. Sci. 2022, 206, 110496. [Google Scholar] [CrossRef]
- Tian, T.; Sun, W.; Qing, X.; Xiong, X.; Zhang, H.; Chu, Y.; Chen, Z.; Zeng, Y. Intelligent cooling structure design of “Z-pins like” silicon rods to enhance the ablation resistance of C/C-ZrC-SiC composites above 2500 °C. J. Eur. Ceram. Soc. 2020, 40, 3875–3886. [Google Scholar] [CrossRef]
- Xue, L.; Su, Z.; Yang, X.; Huang, D.; Yin, T.; Liu, C.; Huang, Q. Microstructure and ablation behavior of C/C-HfC composites prepared by precursor infiltration and pyrolysis. Corros. Sci. 2015, 94, 165–170. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Li, H.; Ouyang, H.; Zhang, Y.; Guo, L. Ablation resistance and thermal conductivity of carbon/carbon composites containing hafnium carbide. Corros. Sci. 2013, 75, 169–175. [Google Scholar] [CrossRef]
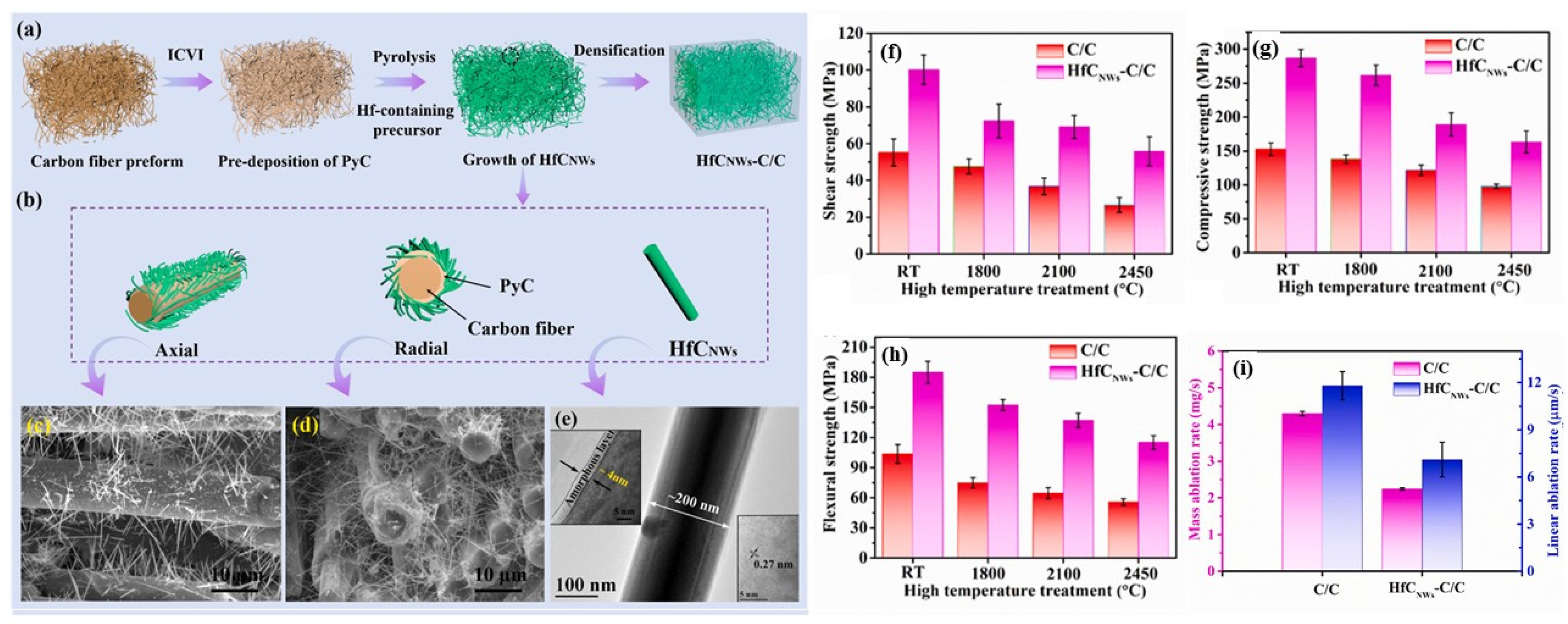
- Fu, Y.; Zhang, Y.; Zhang, J.; Li, T.; Chen, G. Mechanical properties and ablation resistance of HfC nanowire modified carbon/carbon composites. Ceram. Int. 2020, 46, 16142–16150. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Fu, Q.-G.; Tong, M.-D.; Liu, X. Microstructure, ablation behavior and thermal retardant ability of C/C-HfB2 composites prepared by precursor infiltration pyrolysis combined with chemical vapor infiltration. J. Alloys Compd. 2018, 742, 123–129. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, M.; Huang, Q.; Wang, L.; Xue, L.; Tang, X.; He, K. Ablation mechanism of C/C-ZrB2-ZrC-SiC composite fabricated by polymer infiltration and pyrolysis with preform of Cf/ZrB2. Corros. Sci. 2015, 98, 551–559. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Xiong, X.; Chang, Y.; Xu, Y.; Peng, Z.; Tian, T.; Zeng, Y. Microstructure, thermophysical properties, and ablation resistance of C/HfC-ZrC-SiC composites. Ceram. Int. 2019, 45, 4685–4691. [Google Scholar] [CrossRef]
- Lu, J.; Hao, K.; Liu, L.; Li, H.; Li, K.; Qu, J.; Yan, X. Ablation resistance of SiC-HfC-ZrC multiphase modified carbon/carbon composites. Corros. Sci. 2016, 103, 1–9. [Google Scholar] [CrossRef]
- He, H.; Yang, J.; Guo, W.; Zhang, S.; Bai, S. Microstructures and formation mechanism of continuous carbon fiber-reinforced (TiZrHfNbTa)C high-entropy ceramic composites fabricated via high-entropy alloy reactive melt infiltration. Ceram. Int. 2023, 49, 36997–37008. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Hu, J.; Zeng, Y.; Xiong, X. Ablation behaviour of multi-component (Hf, Zr, Ti)C ceramics modified carbon/carbon composites. J. Eur. Ceram. Soc. 2025, 45, 117141. [Google Scholar] [CrossRef]
- Xiong, Y.; He, Q.; Zhang, X.; Wang, Y.; Yin, X. Plasma ablation behavior of C/C-(Hf1−xZrx)C composites prepared by precursor infiltration and pyrolysis. J. Alloys Compd. 2025, 1028, 180733. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Ren, M.; Zuo, Y.; Yang, M.; Zhang, J.; Sun, J. Microstructure and ablation mechanism of C/C-ZrC-SiC composites in a plasma flame. Ceram. Int. 2017, 43, 10661–10667. [Google Scholar] [CrossRef]
- He, Q.; Li, H.; Wang, C.; Li, T.; Lu, J. Microstructure and ablation property of gradient ZrC SiC modified C/C composites prepared by chemical liquid vapor deposition. Ceram. Int. 2019, 45, 13283–13296. [Google Scholar] [CrossRef]
- Rama Rao, G.A.; Venugopal, V. Kinetics and mechanism of the oxidation of ZrC. J. Alloys Compd. 1994, 206, 237–242. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, M.-Y.; Huang, Q.-Z.; Huang, D.; Zhu, P.; Yang, X. Properties of C/C-ZrB2 composites prepared by powder braiding method. Mater. Sci. Eng. Powder Metall. 2015, 20, 907–913. [Google Scholar]
- Huihui, S.; Li, H.; Shen, X.; Cao, G.; Qiang, X.; Ren, X.; Fu, Q. Microstructure and ablation behavior of C/C composites doped with ZrB2. J. Inorg. Mater. 2011, 26, 669–672. [Google Scholar] [CrossRef]
- Yang, G. Preparation and Properties of C/C-ZrB2(ZrC, TaC) Ultra-High Temperature Ceramic Matrix Composites. Master’s Thesis, National University of Defense Technology, Changsha, China, 2008. [Google Scholar]
- Krishnarao, R.V.; Alam, M.Z.; Das, D.K. In-situ formation of SiC, ZrB2-SiC and ZrB2-SiC-B4C-YAG coatings for high temperature oxidation protection of C/C composites. Corros. Sci. 2018, 141, 72–80. [Google Scholar] [CrossRef]
- Bartuli, C.; Valente, T.; Tului, M. Plasma spray deposition and high temperature characterization of ZrB2–SiC protective coatings. Surf. Coat. Technol. 2002, 155, 260–273. [Google Scholar] [CrossRef]
- Patra, N.; Al Nasiri, N.; Jayaseelan, D.D.; Lee, W.E. Thermal properties of Cf/HfC and Cf/HfC-SiC composites prepared by precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2018, 38, 2297–2303. [Google Scholar] [CrossRef]
- Li, S.-P.; Li, K.-z.; Li, H.-J.; Li, Y.-L.; Yuan, Q.-L. Effect of HfC on the ablative and mechanical properties of C/C composites. Mater. Sci. Eng. A 2009, 517, 61–67. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications; William Andrew: Norwich, NY, USA, 1996; pp. 57–139. [Google Scholar]
- Zhang, T.; Qi, L.; Li, S.; Chao, X.; Tian, W.; Zhou, J. Evaluation of the effect of PyC coating thickness on the mechanical properties of T700 carbon fiber tows. Appl. Surf. Sci. 2019, 463, 310–321. [Google Scholar] [CrossRef]
- Baxter, R.I.; Rawlings, R.D.; Iwashita, N.; Sawada, Y. Effect of chemical vapor infiltration on erosion and thermal properties of porous carbon/carbon composite thermal insulation. Carbon 2000, 38, 441–449. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, Y.; Zhou, L.; Huang, S.; Ren, J.; Ren, J.; Zhang, S.; Li, H. Integrative improvement on thermophysical properties and ablation resistance of laminated carbon/carbon composites modified by in situ grown HfC nanowires onto carbon fiber cloths. J. Eur. Ceram. Soc. 2021, 41, 73–83. [Google Scholar] [CrossRef]
- Xu, C.; Jia, C.; Liu, Q.; Peng, Z.; Chen, S. Microstructure and ablation behavior of C/C-HfC-SiC composites fabricated by reactive melt infiltration with Hf–Si alloy. J. Am. Ceram. Soc. 2025, 108, e20215. [Google Scholar] [CrossRef]
- Feng, T.; Tong, M.; Yao, S.; Li, H.; Wen, S.; Lin, H. Effect of PyC interface phase on the cyclic ablation resistance and flexural properties of two-dimensional Cf/HfC composites. J. Eur. Ceram. Soc. 2021, 41, 158–166. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Chen, H.; Han, L.; Yin, X.; Fu, Q.; Sun, J. Ultra-high temperature performance of carbon fiber composite reinforced by HfC nanowires: A promising lightweight composites for aerospace engineering. Compos. Part B Eng. 2023, 250, 110453. [Google Scholar] [CrossRef]
- Li, H.; Yao, D.; Fu, Q.; Liu, L.; Zhang, Y.; Yao, X.; Wang, Y.; Li, H. Anti-oxidation and ablation properties of carbon/carbon composites infiltrated by hafnium boride. Carbon 2013, 52, 418–426. [Google Scholar] [CrossRef]
- Chen, Z.K.; Wu, Y.; Chen, Y.H.; Wang, H.R.; Zeng, Y.; Xiong, X. Preparation and oxidation behavior of Cf/C–TaC composites. Mater. Chem. Phys. 2020, 254, 123428. [Google Scholar] [CrossRef]
- Djugum, R.; Sharp, K. The fabrication and performance of C/C composites impregnated with TaC filler. Carbon 2017, 115, 105–115. [Google Scholar] [CrossRef]
- Mao, H.; Shen, F.; Zhang, Y.; Wang, J.; Cui, K.; Wang, H.; Lv, T.; Fu, T.; Tan, T. Microstructure and mechanical properties of carbide reinforced tic-based ultra-high temperature ceramics: A review. Coatings 2021, 11, 1444. [Google Scholar] [CrossRef]
- Cai, F.; Ni, D.; Chen, B.; Zou, X.; Liao, C.; Gao, L.; He, P.; Ding, Y.; Zhang, X.; Dong, S. Efficient fabrication and properties of 2D Cf/(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C-SiC high-entropy ceramic matrix composites via slurry infiltration lamination combined with precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2023, 43, 7403–7410. [Google Scholar] [CrossRef]
- Long, Q.; Wang, Y.; Xiong, X.; Zhang, H.; Liu, Z.; Wang, J.; Li, T. Growth behavior of (Hf,Zr,Ti)C multicomponent transition metal carbide during melt infiltrating into porous C/C composites. Ceram. Int. 2025, 51, 10453–10466. [Google Scholar] [CrossRef]
- Xie, J.; Li, K.; Sun, G.; Li, H.; Su, X.; Han, Y.; Li, T. Effects of surface structure unit of 2D needled carbon fiber preform on the microstructure and ablation properties of C/C-ZrC-SiC composites. Ceram. Int. 2019, 45, 11912–11919. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Feng, W.; Shi, X.; Li, K.; Guo, L. Ablation in different heat fluxes of C/C composites modified by ZrB2-ZrC and ZrB2-ZrC-SiC particles. Corros. Sci. 2013, 74, 159–167. [Google Scholar] [CrossRef]
- Li, K.Z.; Duan, T.; Zhang, J.P.; Liu, N.K.; Zhang, M.Y. Ablation Mechanism of Carbon/Carbon Composites Modified by HfC-SiC in Two Conditions Under Oxyacetylene Torch. J. Mater. Sci. Technol. 2017, 33, 71–78. [Google Scholar] [CrossRef]
- Tobe, R.J.; Grandhi, R.V. Hypersonic vehicle thermal protection system model optimization and validation with vibration tests. Aerosp. Sci. Technol. 2013, 28, 208–213. [Google Scholar] [CrossRef]
- Xie, G.; Wang, Q.; Sunden, B.; Zhang, W. Thermomechanical optimization of lightweight thermal protection system under aerodynamic heating. Appl. Therm. Eng. 2013, 59, 425–434. [Google Scholar] [CrossRef]
- Fang, C.; Huang, B.; Yang, X.; Shi, A.; Zhang, Z.; Yi, J.; Huang, Q. Effects of LaB6 on composition, microstructure and ablation property of the HfC-TaC-SiC doped C/C composites prepared by precursor infiltration and pyrolysis. Corros. Sci. 2021, 184, 109347. [Google Scholar] [CrossRef]
- Li, K.-z.; Xie, J.; Fu, Q.-g.; Li, H.-j.; Guo, L.-j. Effects of porous C/C density on the densification behavior and ablation property of C/C-ZrC-SiC composites. Carbon 2013, 57, 161–168. [Google Scholar] [CrossRef]
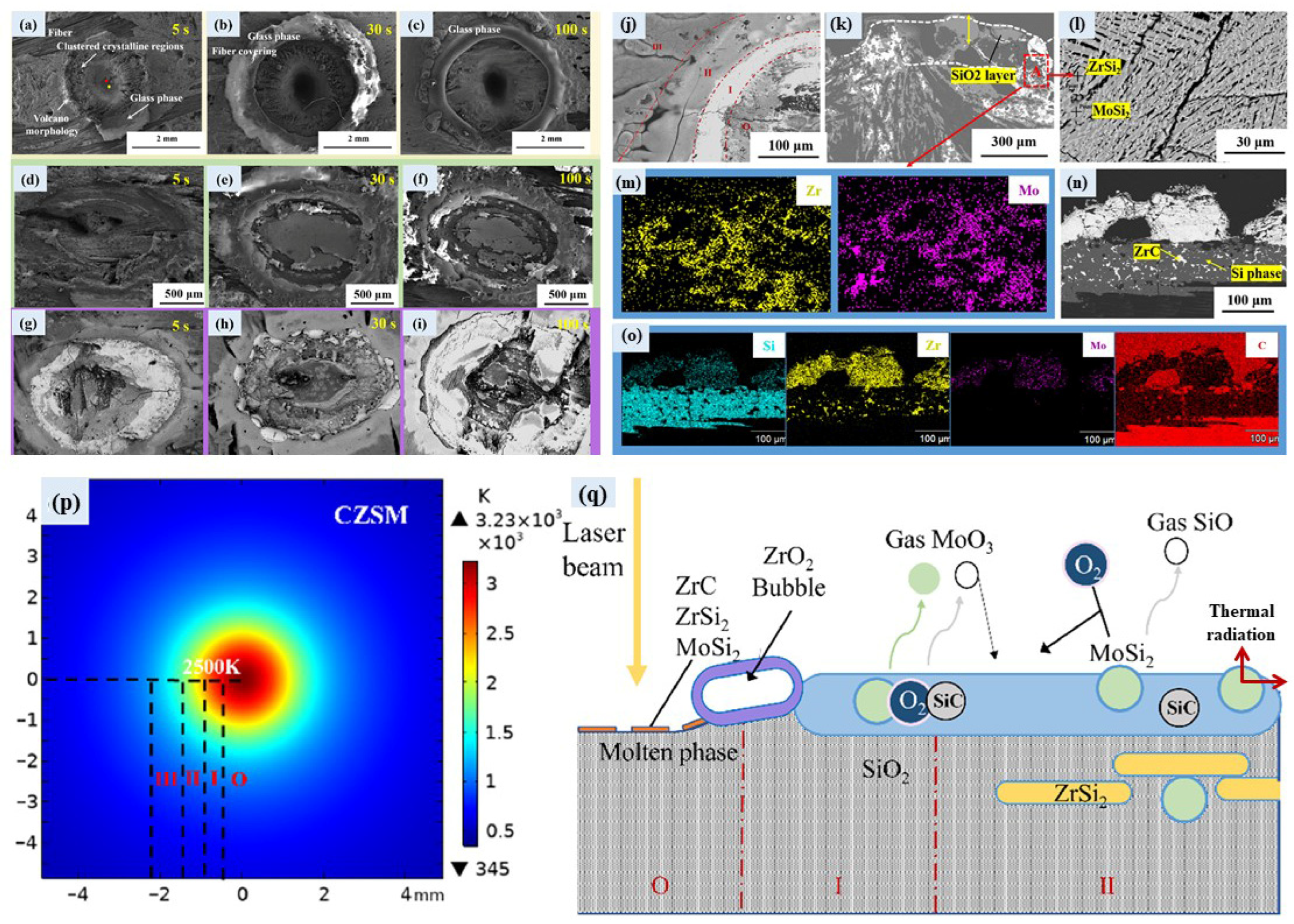
- Geng, L.; Cheng, S.; Liu, G.; Fu, Q.; Li, H. Laser ablation mechanism of ZrC-SiC-MoSi2 ternary ceramic modified C/C. J. Mater. Sci. Technol. 2023, 149, 214–224. [Google Scholar] [CrossRef]
- Fang, C.; Yang, X.; Chai, L.; Zhang, Z.; Weng, Y.; Zheng, L.; Luo, X.; Zhang, X.; Huang, Q. Modifying effects of in-situ grown LaB6 on composition, microstructure and ablation property of C/C-SiC-ZrC composites. Corros. Sci. 2022, 209, 110672. [Google Scholar] [CrossRef]
- Fang, C.; Huang, B.; Yang, X.; He, K.; Chen, L.; Shi, A.; Zhang, Z.; Huang, Q. Effects of LaB6 on the microstructures and ablation properties of 3D C/C-SiC-ZrB2-LaB6 composites. J. Eur. Ceram. Soc. 2020, 40, 2781–2790. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Zhang, J.; Zhu, X. MoSi2 modified HfC coating for the ablation protection of SiC-coated C/C composites: Ablation resistance and behavior. Corros. Sci. 2022, 205, 110418. [Google Scholar] [CrossRef]
- Breede, F.; Hofmann, S.; Jain, N.; Jemmali, R. Design, manufacture, and characterization of a carbon fiber-reinforced silicon carbide nozzle extension. Int. J. Appl. Ceram. Technol. 2016, 13, 3–16. [Google Scholar] [CrossRef]
- Özsoy, M.İ.; Türk, S.; Fındık, F.; Özacar, M. Chapter 7-Carbon–carbon nanocomposites for brake systems and exhaust nozzles. In Nanotechnology in the Automotive Industry; Song, H., Nguyen, T.A., Yasin, G., Singh, N.B., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–154. [Google Scholar]
- Kumar, S.; Kumar, A.; Sampath, K.; Bhanu Prasad, V.V.; Chaudhary, J.C.; Gupta, A.K.; Rohini Devi, G. Fabrication and erosion studies of C–SiC composite Jet Vanes in solid rocket motor exhaust. J. Eur. Ceram. Soc. 2011, 31, 2425–2431. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Mala, R.B.; Mokhasunavisu, R.R. Fabrication and ablation studies of 4D C/SiC composite nozzle under liquid propulsion. Int. J. Appl. Ceram. Technol. 2015, 12, E176–E190. [Google Scholar] [CrossRef]
- Han, J.; Hu, P.; Zhang, X.; Meng, S.; Han, W. Oxidation-resistant ZrB2-SiC composites at 2200 °C. Compos. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- He, Q.; Li, H.; Tan, Q.; Lu, J.; Wang, Y. Influence of carbon preform density on the microstructure and ablation resistance of CLVD-C/C-ZrC-SiC composites. Corros. Sci. 2021, 190, 109648. [Google Scholar] [CrossRef]
- Yang, X.; Su, Z.; Huang, Q.; Chang, X.; Fang, C.; Chen, L.; Zeng, G. Effects of oxidizing species on ablation behavior of C/C-ZrB2-ZrC-SiC composites prepared by precursor infiltration and pyrolysis. Ceram. Int. 2016, 42, 19195–19205. [Google Scholar] [CrossRef]
- Meng, X.; Cui, H.; Yan, L.; Zhang, Q.; Song, M.; Zhu, Y. Ablation behaviors of matrix modified C/C-HfC-HfB2-SiC composites. Mater. China 2013, 32, 655–658. [Google Scholar]
- Makurunje, P.; Monteverde, F.; Sigalas, I. Self-generating oxidation protective high-temperature glass-ceramic coatings for Cf/C-SiC-TiC-TaC UHTC matrix composites. J. Eur. Ceram. Soc. 2017, 37, 3227–3239. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, W.; Xu, Y.; Zhang, H.; Xiong, X. Structural characteristics and ablative behavior of YF3 modified C/C-ZrC-SiC composites and their preparation by molten salt assisted reactive melt infiltration. J. Eur. Ceram. Soc. 2023, 43, 1303–1314. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, W.; Qinghua, L.; Xiong, X.; Zhang, H. Effect of different rare earth fluoride doping on microstructure and ablation properties of C/C-ZrC-SiC composites prepared by molten salt assisted reactive melt infiltration. Ceram. Int. 2024, 50, 22209–22219. [Google Scholar] [CrossRef]
- Kaiser, A.; Lobert, M.; Telle, R. Thermal stability of zircon (ZrSiO4). J. Eur. Ceram. Soc. 2008, 28, 2199–2211. [Google Scholar] [CrossRef]
- Hu, Y.; Ni, D.; Chen, B.; Cai, F.; Zou, X.; Zhang, F.; Ding, Y.; Zhang, X.; Dong, S. Cf/(CrZrHfNbTa)C–SiC high-entropy ceramic matrix composites for potential multi-functional applications. J. Mater. Sci. Technol. 2024, 182, 132–140. [Google Scholar] [CrossRef]
- Miao, Q.; Fu, Y.; Chen, H.; Zhang, J.; Zhao, J.; Zhang, Y. Simultaneous enhancement of mechanical and ablation properties of C/C composites modified by (Hf-Ta-Zr)C solid solution ceramics. J. Eur. Ceram. Soc. 2023, 43, 3182–3190. [Google Scholar] [CrossRef]
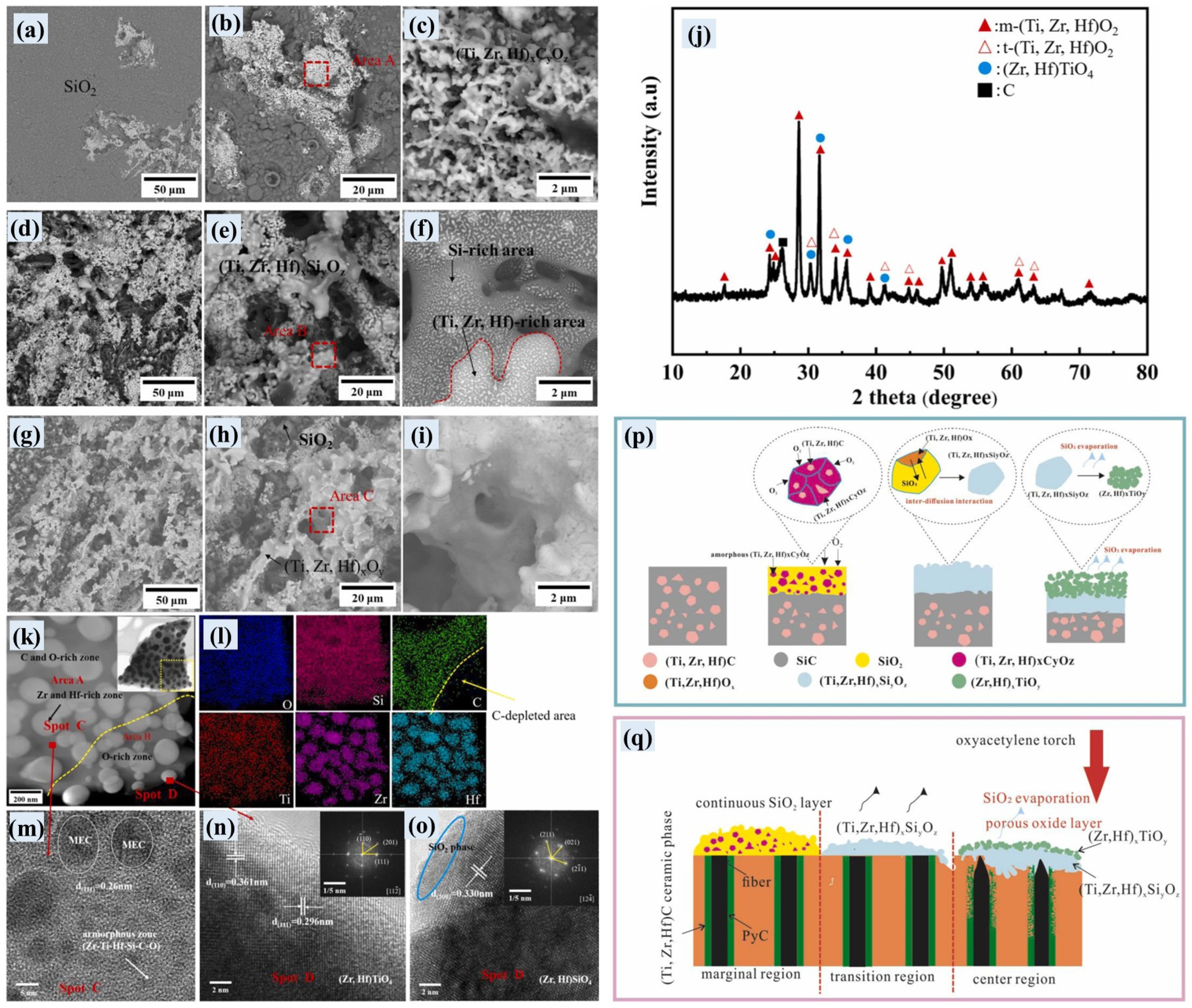
- Zhang, X.; Yang, X.; Luo, X.; Weng, Y.; Fang, C.; Huang, Q. Formation mechanism of (Ti, Zr, Hf)C medium-entropy carbide derived from polymer precursors and its modification on the ablative mechanism of C/C-SiC composites. Corros. Sci. 2024, 230, 111935. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, X.; Liu, Z.; Zuo, Y.; Yang, X.; Huang, Q. Effect of (Ti, Zr, Hf)C medium-entropy and (Ti, Zr, Hf, Ta)C high-entropy modification on microstructure and ablative properties of C/C-SiC composites. Mater. Charact. 2025, 223, 114952. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of nitriding on the microstructure and properties of VAlTiCrMo high-entropy alloy coatings by sputtering technique. J. Alloys Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Xiang, H.M.; Xing, Y.; Dai, F.Z.; Wang, H.J.; Su, L.; Miao, L.; Zhang, G.J.; Wang, Y.G.; Qi, X.W.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, F.; Qiu, W.; Ye, L.; Han, W.; Zhao, W.; Zhou, H.; Zhao, T. Synthesis of rare earth containing single-phase multicomponent metal carbides via liquid polymer precursor route. J. Am. Ceram. Soc. 2020, 103, 6081–6087. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Lv, J.; Li, J.; Chen, H.; Cao, Y.; Li, X.; Li, W.; Ding, J.; Zhang, Y. Insights into high entropy ceramics under extreme conditions: Ablation behavior and microstructure evolution of C/C-(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C composites. J. Eur. Ceram. Soc. 2025, 45, 117616. [Google Scholar] [CrossRef]
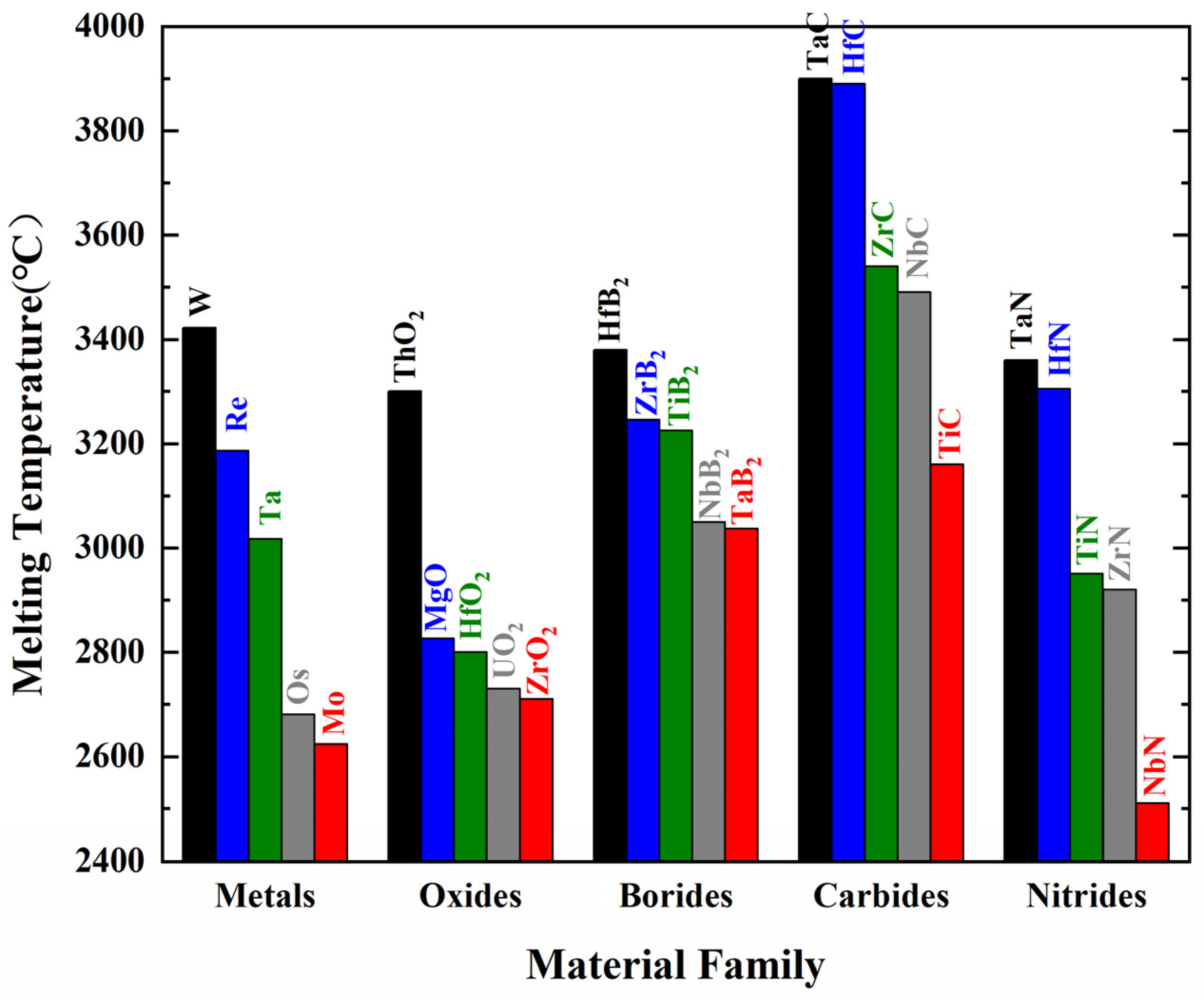

| Material | Crystal Structure | Melting Point (°C) | Density (g/cm3) | CTE, α (10−6 K−1) | Thermal Conductivity (W·m−1·K−1) | Electrical Resistivity (μΩ·cm) | Elastic Modulus (GPa) | Hardness (GPa) | |
|---|---|---|---|---|---|---|---|---|---|
| Cal. | Exp. | ||||||||
| Boride | |||||||||
| TaB2 | HCP | 3040 | 12.5 | 8.2–8.8 | 10.9–16.0 | 33 | 497 | 551 | 19.6 |
| TiB2 | HCP | 3225 | 4.5 | 7.6–8.6 | 64.4 | 16–28.4 | 583 | 575 | 24.0 |
| ZrB2 | HCP | 3245 | 6.1 | 5.5–8.3 | 57.9 | 9.2 | 523 | 489 | 23.0 |
| HfB2 | HCP | 3380 | 11.2 | 6.3–7.6 | 51.6 | 8.8–11 | 535 | 451 | 28.0 |
| Carbide | |||||||||
| TiC | FCC | 3100 | 4.9 | 7.5–7.7 | 17–21 | 52.5 | 455 | 437 | 30.0 |
| ZrC | FCC | 3530 | 6.6 | 6.82 | 20.61 | 68.0 | 436 | 387 | 25.0 |
| TaC | FCC | 3800 | 14.5 | 6.6–8.4 | 22.2 | 30–42.1 | 550 | 537 | 17.0 |
| HfC | FCC | 3900 | 12.8 | 6.3 | 22.2 | 45.0 | 537 | 461 | 24.2 |
| Nitride | |||||||||
| TaN | FCC | 2900 | 13.4 | 3.2 | 8.3 | 128–135 | 490 | 490 | 10.8 |
| TiN | FCC | 2950 | 5.4 | 9.35 | 29.1 | 21.7 | 463 | 400 | 18.6 |
| ZrN | FCC | 2950 | 7.3 | 7.24 | 20.9 | 13.6 | 390 | 384 | 15 |
| HfN | FCC | 3385 | 13.9 | 6.5 | 21.6 | 33 | 411 | 398 | 16.1 |
| Materials | Method | Ablation Method | Time (s) | Heat Flow (MW/m2) | Linear Ablation Rate (μm/s) | Mass Ablation Rate (mg/s/cm2) | Ref. |
|---|---|---|---|---|---|---|---|
| C/C-ZrC | SI + TCVI | Oxygen–acetylene | 60 | 4.200 | 0.510 | 0.620 | [65] |
| C/C-ZrC | ICVI + RMI | Oxygen–acetylene | 60 | 2.380 | 0.264 | 0.422 | [69] |
| C/C-ZrC | PIP | Oxygen–acetylene | 120 | 2.380 | −1.230 | −1.640 | [70] |
| C/C-ZrC-SiC | RMI | Oxygen–acetylene | 120 | 2.380 | −0.028 | 0.545 | [71] |
| C/C-ZrC-SiC | RMI | Oxygen–acetylene | 300 | - | −0.280 | 0.870 | [72] |
| C/C-ZrB2-SiC | CVI + PIP | Oxygen–acetylene | 120 | 2.400 | 2.450 | 0.0095 | [31] |
| C/C-HfC | PIP | Air plasma | 240 | - | 5.310 | 0.550 | [73] |
| C/C-HfC | SI | Oxygen–acetylene | 60 | - | 0.720 | 0.12 | [74] |
| C/C-HfCnw | PIP + ICVI | Oxygen–acetylene | 20 | - | 7.500 | 3.200 | [75] |
| C/C-HfB2 | PIP + CVI | Oxygen–acetylene | 90 | 4.180 | 6.560 | 0.275 | [76] |
| C/C-ZrC-SiC | RMI | Oxygen–acetylene | 120 | 3.860 | 0.415 | 0.500 | [44] |
| C/C-ZrB2-ZrC-SiC | PPI | Air plasma | 180 | - | 2.610 | 5.09 | [77] |
| C/C-SiC-ZrC-HfC | RMI | Oxygen–acetylene | 120 | - | −1.100 | 1.500 | [78] |
| C/C-SiC-ZrC-HfC | PIP | Oxygen–acetylene | 120 | 2.380 | 0.255 | 0.151 | [79] |
| C/C-Zr0.8Ti0.2C0.74B0.26-SiC | RMI | Oxygen–acetylene | 60 | - | −0.3 | 0.01 | [23] |
| C/C-ZiC-ZrC-SiC | CVI + PIP + RMI | Air plasma | 120 | - | 11.00 | 4.17 | [14] |
| Cf/(TiZrHfNbTa)C | RMI | Oxygen–acetylene | 180 | - | 0.600 | 0.900 | [80] |
| C/C-(Hf0.5, Zr0.3, Ti0.2)C | RMI | Air plasma | 240 | 5.520 | −1.860 | 1.14 | [81] |
| C/C-(Hf0.5Zr0.5)C | PIP | Air plasma | 60 | 2.300 | 3.15 | −0.25 | [82] |
| C/C-ZrC-SiC | CVI + PIP | Air plasma | 180 | - | 0.194 | 1.73 | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wang, S.; Zhou, T.; Jiang, T.; Lu, L.; Wen, Q.; Tao, S.; Xiong, X. Research Progress on Ultrahigh-Temperature Ceramics Modified C/C Composites. Materials 2025, 18, 3891. https://doi.org/10.3390/ma18163891
Gao R, Wang S, Zhou T, Jiang T, Lu L, Wen Q, Tao S, Xiong X. Research Progress on Ultrahigh-Temperature Ceramics Modified C/C Composites. Materials. 2025; 18(16):3891. https://doi.org/10.3390/ma18163891
Chicago/Turabian StyleGao, Ruize, Shuibin Wang, Tianci Zhou, Tianxing Jiang, Li Lu, Qingbo Wen, Shasha Tao, and Xiang Xiong. 2025. "Research Progress on Ultrahigh-Temperature Ceramics Modified C/C Composites" Materials 18, no. 16: 3891. https://doi.org/10.3390/ma18163891
APA StyleGao, R., Wang, S., Zhou, T., Jiang, T., Lu, L., Wen, Q., Tao, S., & Xiong, X. (2025). Research Progress on Ultrahigh-Temperature Ceramics Modified C/C Composites. Materials, 18(16), 3891. https://doi.org/10.3390/ma18163891









