Abstract
Protein adsorption on dental zirconia (ZrO2) surfaces plays a crucial role in plaque formation, tissue healing, and bone osseointegration. This study investigated and compared the adsorption behavior of three salivary antimicrobial proteins—peroxidase, lactoferrin, and lysozyme—on a ZrO2 sensor and an Au sensor using a quartz crystal microbalance (QCM) operating at 27 MHz. Protein adsorption was determined from frequency decreases, and the apparent reaction rate constant (kobs) was calculated by fitting frequency–time curves to a kinetic model. The amount of lactoferrin adsorbed on the ZrO2 sensor was significantly higher than that of peroxidase and lysozyme (p < 0.05). Significantly smaller amounts of peroxidase and lysozyme were adsorbed onto the ZrO2 sensor than the Au sensor (p < 0.05). The kobs for lysozyme on the Au sensor was significantly higher than those for lactoferrin on sensors and for peroxidase on the Au sensor (p < 0.05). Differences in salivary antimicrobial protein adsorption between Au and ZrO2 surfaces were influenced, in part, by electrostatic interactions between the proteins and the material surface.
1. Introduction
Over the past two decades, the application of digital technology has expanded to include partially stabilized zirconia (ZrO2), such as dental zirconia, in restorative and prosthetic dentistry. The CAD/CAM systems used in dentistry have enabled the clinical application of ZrO2 and facilitated the advancement of metal-free treatments as alternatives to metals [1,2]. By adjusting stabilizer composition and enhancing transparency, aesthetic restorations using ZrO2 have also become feasible [3]. In particular, ZrO2 is employed in dental implant components, including superstructures, abutments, and implant bodies [4]. Among white ceramics used in dental materials, ZrO2 offers high strength, toughness, and resistance to wear and corrosion, and it does not cause allergic reactions. ZrO2 is also known for its excellent biocompatibility as a biomaterial [5,6,7].
Various oral bacteria form biofilms that contribute to dental caries and periodontal diseases. Therefore, understanding plaque formation and adhesion on prosthetic devices is crucial during their development [8]. Brakel et al. [9] and Re et al. [10] investigated the in vitro growth and adhesion of oral bacteria on Ti and ZrO2, suggesting that plaque formation on ZrO2 surfaces may be lower than on Ti surfaces. Rimondini et al. [11] and Scarano et al. [12] also reported significantly lower bacterial adhesion on ZrO2 than on Ti in an in vivo study. This property is important in controlling inflammation in periodontal tissues and influences the long-term prognosis of dental treatments [13]. Additionally, ZrO2 promotes a higher proliferation rate of fibroblasts in periodontal tissues in vitro compared to Ti, and laminin-5(332) is expressed around ZrO2 implants in vivo [14].
Tokunaga et al. [15] and Hirota et al. [16] created a ZrO2 thin film on roughened Ti implant surfaces using a molecular precursor method without altering the surface topography. In rat models, ZrO2-coated Ti implants supported early-stage bone formation at levels equivalent to or exceeding those of non-coated Ti implants. The chemical composition of the ZrO2 coating was suggested to influence the orientation of collagen fiber bundles in the gingiva. Based on these findings, the mechanisms and significance of plaque formation, tissue healing, and bone integration when using ZrO2—compared to metallic dental materials such as Au, Ag, Co-Cr alloys, and Ti —should be further clarified.
When a material is implanted into the body, proteins are the first to adsorb onto its surface. Kasemo et al. [17] noted that these adsorbed proteins influence cell adhesion. Additionally, Trindade et al. [18] reported that osseointegration is a multi-step process that begins with protein adsorption onto dental implant surfaces. Biofilm formation in the oral cavity also proceeds in a stepwise manner, with salivary protein adsorption marking the initial phase. These adsorbed proteins on biomaterial surfaces play a key role in bacterial adhesion [19]. Therefore, investigating salivary protein adsorption on dental materials is essential for understanding the mechanisms underlying plaque formation.
The quartz crystal microbalance (QCM) technique provides a straightforward method for detecting protein adsorption onto material surfaces by measuring shifts in the oscillation frequency of a quartz sensor [20]. Yoshida et al. [21] previously used the QCM method to examine salivary protein adsorption onto various materials (Au, silica, and Ti) and identified differences in the adsorption behaviors on each surface. They further reported that Ti and stainless steel adsorbed greater amounts of lactoferrin compared to ZrO2 and polymethyl methacrylate [22].
Few studies have investigated the adsorption of salivary antimicrobial proteins onto ZrO2. Among the various antimicrobial proteins present in saliva, this study focused on three: lactoferrin, peroxidase, and lysozyme [23]. Hydrogen peroxide (H2O2) is produced by bacteria that settle on mucous membranes. Peroxidase is a key component of salivary defense, detoxifying H2O2 in the presence of thiocyanate by converting it into hypothiocyanite, dioxygen, and water [24]. Salivary peroxidase exhibits broad-spectrum antimicrobial activity against oral and non-oral bacteria, including Streptococcus mutans, Fusobacterium nucleatum, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [25]. Lactoferrin is an iron-binding glycoprotein that exhibits antimicrobial activity by depriving bacteria of the iron required for growth. It also shows broad antimicrobial effects against oral pathogens such as Streptococcus mutans and Porphyromonas gingivalis, as well as opportunistic pathogens like Acinetobacter baumannii and Salmonella species [26]. Lysozyme in saliva primarily targets Gram-positive bacteria such as Staphylococcus aureus and Streptococcus mutans by breaking down their cell walls [27]. However, the direct or indirect relationship between ZrO2 and these proteins, as well as their influence on biofilm formation and persistence, remains unclear.
In this study, we investigated the adsorption behavior of three salivary antimicrobial proteins—lactoferrin, peroxidase, and lysozyme—onto ZrO2 surfaces in relation to biofilm formation, using QCM analysis.
2. Materials and Methods
2.1. QCM Device and Sensors
Figure 1 shows the QCM apparatus used in this study (27 MHz, AFFINIX QNμ, Piezo Parts Co., Ltd., Tokyo, Japan). A commercially available Au sensor (Piezo Parts Co., Ltd., Tokyo, Japan) was used as the control. The AT-cut quartz crystal, which is a quartz crystal cut at an angle of 35°15′ from the Z-axis of artificial quartz, was mounted between Au electrodes. The ZrO2 sensor was prepared by sputter-coating the Au electrode using sputtering deposition equipment (CS200, ULVAC, Inc., Kanagawa, Japan). A zirconium target was sputtered at 0.5 Pa in an oxygen atmosphere for 30 min. The film thickness of the sputtered ZrO2 was previously determined to be 115 nm using a profilometer [22,28,29,30].
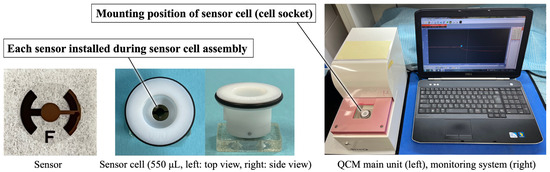
Figure 1.
Appearance of the QCM apparatus, sensor, and sensor cell.
The Au and ZrO2 sensors were assembled into sensor cells. Before QCM measurements, all sensors were exposed to ultraviolet (UV) radiation (BioForce Nanosciences Holdings Inc., Virginia Beach, VA, USA) for 20 min to remove surface contamination [31]. UV irradiation (λ = 254 nm, 15 mW/cm2) was applied vertically from 20 mm above. The ZrO2 sensor was mounted on the cell socket of the main QCM unit following UV irradiation.
2.2. Morphologies and Surface Roughness Values of Au and ZrO2 Sensors
To evaluate the three-dimensional (3D) surface topography of the Au and ZrO2 sensors before protein adsorption, atomic force microscopy (AFM; Nanosurf Easyscan 2, Nanosurf AG, Liestal, Switzerland) was used in air. Tapping-mode measurements were performed using a silicon probe (Budget Sensors Tap190Al-G, force constant 48 N/m, resonance frequency ~190 kHz; Innovative Solutions Bulgaria Ltd., Sofia, Bulgaria). The scanned area for AFM imaging was 5 × 5 μm2. The average diameter of crystalline particles on each sensor surface was determined using an image analysis system (WinROOF, Visual System Division, Mitani Corp., Tokyo, Japan). The 3D arithmetic height (Sa), representing surface roughness, was calculated over the same areas.
Five measurements were performed for each of the Au and ZrO2 sensors.
2.3. Surface Wettability of Au and ZrO2 Sensors
Prior to protein adsorption, the wettability of UV-cleaned Au and ZrO2 sensors was evaluated by measuring their contact angles using double-distilled water. A contact angle meter (DMe-201, Kyowa Interface Science Co., Ltd., Tokyo, Japan) was used. The contact angle was determined 3 s after dispensing a 0.5 µL droplet of water onto each sensor surface.
Ten measurements were performed for each of the Au and ZrO2 sensors at room temperature and humidity.
2.4. QCM Measurement and Procedure
Lactoferrin (from bovine milk), peroxidase (from horseradish), and lysozyme (from egg white) (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) were used as salivary antimicrobial proteins in this study. Each protein was dissolved in phosphate-buffered saline (PBS, pH 7.4) at a final concentration of 0.5 mg/mL.
A schematic diagram of the protein adsorption procedure and a typical frequency-decrease curve for the QCM measurements are shown in Figure 2. QCM is a mass-sensing technique based on a quartz crystal oscillator. The oscillation frequency decreases (ΔF) proportionally to the mass of the adsorbed material, such as a protein, when it binds to the sensor surface. For the 27 MHz oscillator used in the present study, a frequency decrease of 1 Hz corresponds to a mass increase of 0.62 ng/cm2. Therefore, the QCM sensor enables the detection of mass changes at the nanogram scale [32,33].
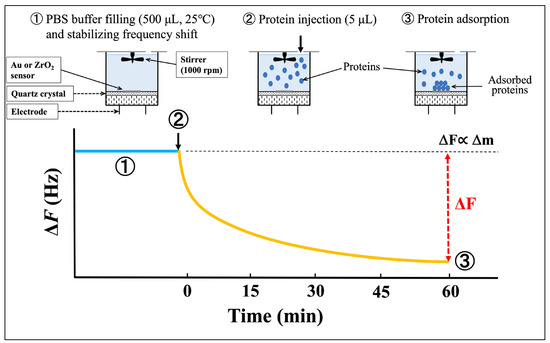
Figure 2.
Schematic diagram of protein adsorption procedure and typical frequency-decrease curve. The blue line indicates the sensor stabilized after PBS injection, while the yellow line indicates the frequency decrease after protein injection.
As described above, UV irradiation was used to clean each sensor before QCM measurement. With the sensor cell mounted on the socket, 500 μL of PBS was introduced into the cell using a micropipette. After the frequency shift stabilized, 5 μL of lactoferrin, peroxidase, or lysozyme solution was injected into the PBS in the cell. The resulting frequency decrease was monitored for 60 min following protein injection. The adsorbed amount (Δm) of each protein at 60 min was calculated using Sauerbrey’s equation [34]:
where:
ΔF: Measured frequency shift (Hz);
Δm: Mass change (g);
F0: Fundamental frequency of the quartz crystal (27 × 106 Hz);
A: Electrode area (0.049 cm2);
ρq: Density of quartz (2.65 g/cm3);
µq: Shear modulus of quartz (2.95 × 1011 dyn/cm2).
The apparent reaction rate constant kobs was determined by fitting the ΔF curve to the following time-dependent adsorption model:
where ΔF∞ is the frequency shift at infinite time.
kobs reflects the combined effect of association and dissociation rates and represents the reciprocal of the relaxation time. The relaxation time is defined as the time required to reach 63% (=1e−1) of the saturated adsorption amount. Thus, a longer relaxation time corresponds to a slower adsorption rate, whereas a larger kobs indicates faster adsorption.
Three runs of QCM measurements were performed.
2.5. Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS Statistics 17.0; IBM Corp., Armonk, NY, USA). The significance level was set at p < 0.05. A non-paired t-test was used to compare the diameters of crystalline particles, surface roughness, and contact angles of Au and ZrO2 sensors. The adsorbed amounts and kobs data from the QCM measurements were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The results were expressed as the mean ± standard deviation (SD).
3. Results
3.1. Characterizations of Au and ZrO2 Sensors
AFM images of the QCM sensors before protein adsorption are shown in Figure 3. Spherical particles formed by sputtering were observed on the surfaces of Au and ZrO2 sensors. The average particle diameter on the Au sensor was 0.12 ± 0.04 μm, while that on the ZrO2 sensor was 0.20 ± 0.07 μm. The particle diameter on the ZrO2 sensor was significantly larger than that on the Au sensor (p < 0.05).
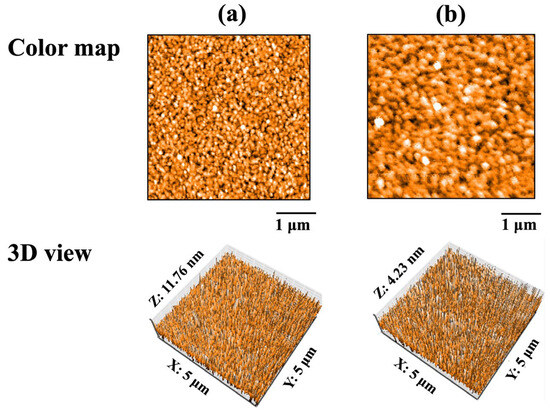
Figure 3.
AFM images of QCM sensors before protein adsorption: (a) Au and (b) ZrO2.
Table 1 lists the contact angles and surface roughness values of the Au and ZrO2 sensors before protein adsorption. No significant differences in surface roughness (Sa) were observed between the Au and ZrO2 sensors (p > 0.05). However, the Au sensor exhibited significantly larger contact angles than the ZrO2 sensor (p < 0.05). Therefore, the surface of the ZrO2 sensor was more hydrophilic than that of the Au sensor.

Table 1.
Contact angle and surface roughness values of Au and ZrO2 sensors before protein adsorption.
3.2. QCM Measurements
Figure 4 shows typical ΔF curves for the adsorption of each salivary antimicrobial protein onto the Au or ZrO2 sensor obtained from QCM measurements. A larger decrease in ΔF corresponds to a higher degree of protein adsorption on the sensor. The ΔF varied depending on the type of sensor and the adsorbed protein. The frequency decrease was recorded immediately after the injection of each protein. However, no decrease in ΔF was observed for the ZrO2 sensor when peroxidase and lysozyme were injected. In other words, the Au sensor exhibited a greater frequency decrease for peroxidase and lysozyme adsorption than the ZrO2 sensor. For lactoferrin adsorption, the frequency decrease did not differ depending on the sensor type.
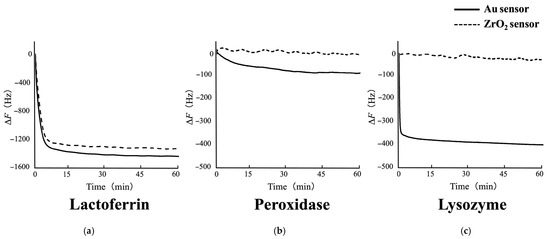
Figure 4.
Typical ΔF curves for the adsorption of each salivary antimicrobial protein onto Au and ZrO2 sensors obtained from QCM measurements: (a) lactoferrin, (b) peroxidase, and (c) lysozyme. The adsorption process was monitored in real time throughout the measurements. The frequency decrease was recorded immediately after the injection of each protein. Lactoferrin adsorption resulted in a similar ΔF decrease on both sensors. Peroxidase and lysozyme caused a ΔF decrease on the Au sensor, while no change was observed on the ZrO2 sensor.
Figure 5 shows the estimated amounts of lactoferrin, peroxidase, and lysozyme adsorbed onto the Au and ZrO2 sensors 60 min after injection, as calculated using the Sauerbrey equation [34]. On the Au and ZrO2 sensors, lactoferrin displayed significantly higher adsorption than peroxidase and lysozyme (p < 0.05). A significant difference was absent between lactoferrin adsorption of the Au and ZrO2 sensors (p > 0.05). The amounts of peroxidase and lysozyme adsorbed on the ZrO2 sensor were significantly lower than those adsorbed on the Au sensor (p < 0.05).
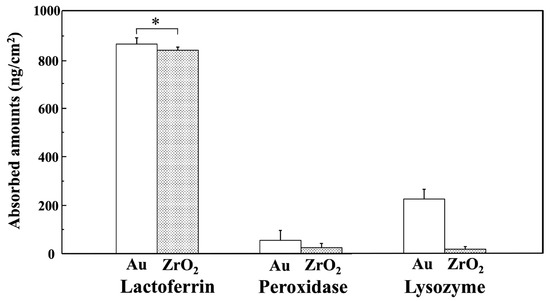
Figure 5.
Estimated amounts of lactoferrin, peroxidase, and lysozyme adsorbed on Au and ZrO2 sensors 60 min after injection, calculated using the Sauerbrey equation. (* Connected bars: no significant difference (p > 0.05)). Lactoferrin adsorption on the Au and ZrO2 sensor was significantly higher than for peroxidase and lysozyme, and peroxidase and lysozyme showed significantly lower adsorption amounts on the ZrO2 sensor compared to the Au sensor (p < 0.05).
Figure 6 shows the kobs values for lactoferrin, peroxidase, and lysozyme adsorption onto the Au and ZrO2 sensors obtained 10 min after injection, based on nonlinear fitting analysis. The kobs values for peroxidase and lysozyme on the ZrO2 sensor were undetectable because curve fitting was not performed. The kobs value for lysozyme on the Au sensor was significantly higher than those for lactoferrin on the Au and ZrO2 sensors and for peroxidase on the Au sensor (p < 0.05).
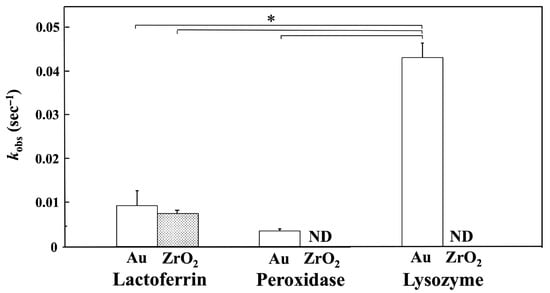
Figure 6.
kobs values for lactoferrin, peroxidase, and lysozyme adsorption onto Au and ZrO2 sensors obtained 10 min after injection by nonlinear fitting analysis. (* Connected bars: significant difference (p < 0.05); ND: not detected). The kobs value for lysozyme on the Au sensor was significantly higher than those for lactoferrin on the Au and ZrO2 sensors and for peroxidase on the Au sensor (p < 0.05).
4. Discussion
This study investigated the adsorption behaviors of peroxidase, lactoferrin, and lysozyme onto ZrO2 surfaces using QCM analysis. The adsorption of these antimicrobial proteins on ZrO2 surfaces differed from that on Au surfaces. Au sensors, which are commonly used as metallic dental materials, were employed as controls for comparison with Au alloys.
Numerous methods exist for evaluating protein adsorption on materials, such as infrared reflection spectroscopy, ellipsometry, and surface plasmon resonance [35]. In this study, we used the QCM method, which enables real-time measurement of mass changes at the nanogram level. Additionally, a highly sensitive QCM device with a vibrational frequency of 27 MHz was utilized. Our previous studies have demonstrated the initial behavior of material implantation by accurately investigating the adsorption of various proteins using this QCM system [8,21,22,28,29,30].
An acquired protein film forms on tooth surfaces, and salivary proteins are adsorbed. Oral bacteria specifically bind to various salivary proteins and then form colonies [19]. In a previous study, we investigated the adsorption of bovine serum albumin and mucin onto denture base metals (Au, Ti, and Co–Cr alloy) using the QCM method [8]. We found that significantly lower levels of albumin were adsorbed onto Ti compared to Au and Co–Cr alloys. Au demonstrated significantly greater mucin adsorption than Co–Cr. In this study, we observed the adsorption behaviors of antimicrobial salivary proteins, which have the opposite effect on plaque formation. We conducted basic research to test the hypothesis that ZrO2 has low plaque adhesion. The present study showed no significant difference in the amounts of each protein adsorbed on the Au and ZrO2 sensors, which was an unexpected result. No direct correlation was observed between the adsorption of the antimicrobial proteins on ZrO2 compared to Au and plaque formation. However, a novel finding from this study was that differences were observed depending on the type of protein.
A protein concentration of 0.5 mg/mL has been primarily used in our previous QCM studies involving various proteins [21,22,28,29,30]. Human salivary albumin concentrations have been reported to range from 0.09 to 0.5 mg/mL or higher [36]. Because many of these studies focused on albumin adsorption, the same concentration was applied to lactoferrin, peroxidase, and lysozyme in this study to facilitate meaningful comparisons with past data.
No significant difference was observed in the surface roughness between the Au and ZrO2 sensors. However, the Au sensor was more hydrophobic than the ZrO2 sensor. A comparison of the protein adsorption amounts and kobs rates of each protein onto the Au and ZrO2 sensors revealed either no significant difference or greater adsorption on the Au sensor. These results suggest that surface roughness and wettability have minimal impact on protein adsorption [37]. Electrostatic interactions should be considered as additional influencing factors. The isoelectric point (pI) of peroxidase is 7.3–7.5 [38], whereas those of lactoferrin and lysozyme are 8.2–8.9 [39] and 10.7 [40], respectively. Therefore, peroxidase was neutral, and lactoferrin and lysozyme were positively charged under the pH = 7.4 conditions used in this study. The zeta potential, an important surface electronic characteristic of materials, was measured as the basis for comparison. According to measurements using the streaming potential method [41], the zeta potentials of Au and ZrO2 at pH 7.4 are approximately −20 mV [8,42] and −43 mV [22,29,30], respectively. These results suggest that Au and ZrO2 surfaces were negatively charged under the conditions employed in this study. As a result, electrostatic attraction occurred between the positively charged proteins (lactoferrin and lysozyme) and the negatively charged sensors. Owing to stronger electrostatic attraction, a larger amount of lactoferrin was adsorbed than peroxidase on both sensors. Although lysozyme, like lactoferrin, is positively charged, it showed lower adsorption on both sensors compared to lactoferrin. However, the amount of lysozyme adsorbed and the adsorption rate on the Au sensor were higher than on the ZrO2 sensor. This may be related to the presence of S–S bonds in lysozyme, and possibly Au was chemically influenced and selectively adsorbed [43]. Other factors besides electrostatic interactions may have contributed to the adsorption of peroxidase and lysozyme onto the ZrO2 sensor. No significant difference was observed in the kobs values of lactoferrin between the Au and ZrO2 sensors, which may be due to comparable electrostatic interactions with both surfaces. In contrast, the high kobs value for lysozyme on the Au sensor likely reflects the contribution of S–S bonds, as discussed earlier. In practice, lysozyme adsorbs rapidly onto Au, while lactoferrin and peroxidase are presumed to adsorb subsequently. This difference in the adsorption sequence between Au and ZrO2 may influence subsequent plaque formation.
Nezu et al. [44] reported that lysozyme adsorbs most on Au surfaces, less on SiO2, and least on TiO2, with TiO2 adsorption occurring only at neutral pH by QCM-D (QCM with dissipation monitoring) analysis. Adsorption was reduced by salt, indicating that electrostatic interactions play a key role. ZrO2, like silica, is a ceramic material and may exhibit comparable properties such as plaque formation [45,46]. SiO2-based porcelain prostheses have been reported to show less accumulation of dental biofilm compared to metal prostheses. The markedly lower adsorption of lysozyme on ZrO2 relative to gold is consistent with the present findings and suggests that electrostatic interactions may play a significant role. Teichroeb et al. [47] reported in their QCM-D study that lysozyme adsorbs rapidly and reversibly onto polyHEMA surfaces, whereas lactoferrin adsorbs more slowly but forms a more stable layer. PolyHEMA, a key component of soft contact lenses, presents challenges for protein incorporation. When both proteins are present, they clarified that lactoferrin preferentially adsorbs and displaces lysozyme, thereby altering the overall adsorption behavior. The slower adsorption rate of lactoferrin compared to lysozyme was consistent with our study results. Thus, preferential adsorption of lactoferrin onto ZrO2 surfaces is expected.
We could not determine a clear relationship between the protein adsorption results and characterizations of the Au and ZrO2. Adsorption behavior is influenced by various factors, including the pH conditions of the environment. A PBS solution with a pH of 7.4 was employed in this experiment. Variations in pH may have influenced adsorption owing to biological reactions occurring in the oral environment, where bacterial biofilms form. The concentrations of lactoferrin, peroxidase, and lysozyme may represent another key factor affecting adsorption behavior. Changes in pH and concentration will be explored in future QCM studies. Additionally, proteins derived from saliva do not exist in isolation but interact with each other simultaneously. Therefore, measuring a mixture of multiple proteins is required for QCM analysis. Hirota et al. reported that fibronectin and albumin were adsorbed onto ZrO2 surfaces in two steps using QCM [30]. The proteins were added in two orders: fibronectin followed by albumin, and albumin followed by fibronectin. No significant difference was observed in the total amounts adsorbed between the two sequences. However, the kobs of the second protein was significantly lower. This suggests that the first protein affects the rate of adsorption of the second one. These results indicate that in environments where multiple proteins coexist, adsorption behavior can be altered. Using this approach, the hypothesis that the order of protein adsorption differs between Au and ZrO2 can be evaluated, owing to the faster adsorption of lysozyme, potentially influencing plaque development. Further research is anticipated using sensors coated with materials other than Au and ZrO2. Additionally, this study suggests that controlled electrostatic interactions between material surfaces and antimicrobial proteins could contribute to the development of new dental devices with enhanced antimicrobial properties.
5. Conclusions
Our findings obtained through the QCM method demonstrate that the adsorption behaviors of peroxidase, lactoferrin, and lysozyme differed on Au and ZrO2 surfaces. The adsorption of lactoferrin onto the ZrO2 sensor was significantly higher than that of peroxidase and lysozyme. The adsorption of peroxidase and lysozyme onto the ZrO2 sensor was significantly lower than that onto the Au sensor. This study did not reveal a direct antimicrobial relationship through protein adsorption on Au or ZrO2. However, differences in adsorption behavior between the two materials were observed, highlighting the need to better replicate oral environmental conditions, such as pH and protein concentrations, in future experiments. In the oral cavity, various proteins interact with each other, and acid production by plaque or the presence of inflammation can lead to a decrease in pH, which may affect protein adsorption onto materials by altering their isoelectric points and influencing electrostatic interactions. Under these conditions, protein adsorption behavior may be altered, potentially influencing plaque formation and other biological responses. These differences may affect plaque formation, tissue healing processes, and bone osseointegration when using ZrO2. A deeper understanding of protein adsorption on ZrO2 may reveal its potential as a bio-functional material, contributing to the control of oral diseases. Future work will focus on analyzing mixed protein solutions and exploring sensors coated with materials other than Au and ZrO2.
Author Contributions
Conceptualization, M.H.; methodology, M.H.; software, M.H.; validation, M.H.; formal analysis, M.H.; investigation, M.H.; resources, M.H.; data curation, M.H.; writing—original draft preparation, M.H.; writing—review and editing, M.H. and T.Y.; visualization, M.H.; supervision, T.Y.; project administration, T.Y.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grants-in-Aid for Scientific Research (C) (24K12990) from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to especially acknowledge the support of Tohru Hayakawa, Tsurumi University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ZrO2 | Partially stabilized zirconia |
| QCM | Quartz crystal microbalance |
| UV | Ultraviolet |
| AFM | Atomic force microscope |
| Sa | Surface roughness parameter (the 3D arithmetic height) |
| PBS | Phosphate-buffered saline |
References
- Casar, P.F.; de Paula Miranda, R.B.; Santos, K.F.; Scherrer, S.S.; Zhang, Y. Recent advances in dental zirconia: 15 years of material and processing evolution. Dent. Mater. 2024, 40, 824–836. [Google Scholar] [CrossRef]
- Miura, S.; Fujita, T.; Fujisawa, M. Zirconia in fixed prosthodontics: A review of the literature. Odontology 2025, 113, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Kui, A.; Manziuc, M.; Petruțiu, A.; Buduru, S.; Labuneț, A.; Negucioiu, M.; Chisnoiu, A. Translucent zirconia in fixed prosthodontics—An integrative overview. Biomedicines 2023, 11, 3116. [Google Scholar] [CrossRef] [PubMed]
- Ban, S. Classification and properties of dental zirconia as implant fixtures and superstructures. Materials 2021, 14, 4879. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, M.; Wang, J.; Zhang, X. Advances in zirconia-based dental materials: Properties, classification, applications, and future prospects. J. Dent. 2024, 147, 105111. [Google Scholar] [CrossRef]
- Matsui, K.; Hosoi, K.; Feng, B.; Yoshida, H.; Ikuhara, Y. Ultrahigh toughness zirconia ceramics. Proc. Natl. Acad. Sci. USA 2023, 120, e2304498120. [Google Scholar] [CrossRef]
- Yoshinari, M. Future prospects of zirconia for oral implants—A review. Dent. Mater. J. 2020, 39, 37–45. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T. Adsorption behaviors of salivary pellicle proteins onto denture base metals using 27-MHz quartz crystal microbalance. Biomed. Mater. Eng. 2022, 33, 1–11. [Google Scholar] [CrossRef]
- Van Brakel, R.; Cune, M.S.; van Winkelhoff, A.J.; de Putter, C.; Verhoeven, J.W.; van der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral. Implants Res. 2011, 22, 571–577. [Google Scholar] [CrossRef]
- Re, D.; Pellegrini, G.; Francinetti, P.; Augusti, D.; Rasperini, G. In vivo early plaque formation on zirconia and feldspathic ceramic. Minerva. Stomatol. 2011, 60, 339–348. [Google Scholar]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. Oral. Maxillofac. Implants 2002, 17, 793–798. [Google Scholar]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Ryu, J.S.; Shimono, M.; Lee, K.W.; Lee, J.M.; Jung, H.S. Differential healing patterns of mucosal seal on zirconia and titanium implant. Front. Physiol. 2019, 10, 796. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Hirota, M.; Hayakawa, T. Influence of the surface chemical composition differences between zirconia and titanium with the similar surface structure and roughness on bone formation. Nanomaterials 2022, 12, 2478. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Osawa, K.; Sakurai, T.; Nagai, H.; Ohkubo, C.; Hayakawa, T. Bone and soft-tissue compatibility of thin ZrO2 film coated implant with same surface topography as titanium substrate. J. J. Dent. Mater. 2025, 44, 29. [Google Scholar]
- Kasemo, B.; Lausmaa, J. The biomaterial-tissue interface and its analogues in surface science and technology. In The Bone-Biomaterial Interface; Davis, J.E., Ed.; University of Toronto Press: Toronto, ON, Canada, 1991; pp. 19–32. [Google Scholar]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign body reaction to biomaterials: On mechanisms for buildup and breakdown of osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Pohanka, M. Quartz crystal microbalance (QCM) sensing materials in biosensors development. Int. J. Electrochem. Sci. 2021, 16, 211220. [Google Scholar] [CrossRef]
- Yoshida, E.; Hayakawa, T. Adsorption study of pellicle proteins to gold, silica and titanium by quartz crystal microbalance method. Dent. Mater. J. 2013, 32, 883–887. [Google Scholar] [CrossRef]
- Yoshida, E.; Hayakawa, T. Adsorption analysis of lactoferrin to titanium, stainless steel, zirconia, and polymethyl methacrylate using the quartz crystal microbalance method. Biomed. Res. Int. 2016, 2016, 3961286. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Courtoris, P. Oral peroxidases: From antimicrobial agents to ecological actors (Review). Mol. Med. Rep. 2021, 24, 500. [Google Scholar] [CrossRef]
- Tonoyan, L.; Montagner, D.; Friel, R.; O’Flaherty, V. Antimicrobials offered from nature: Peroxidase-catalyzed systems and their mimics. Biochem. Pharmacol. 2020, 182, 114281. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Kobayashi, T.; Yaeshima, T.; Iwatsuki, K.; Yoshie, H. Inhibitory Effects of Lactoferrin on Growth and Biofilm Formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents. Chemother. 2009, 53, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Takeuchi, H.; Sato, M.; Sumitomo, S. Antimicrobial peptides in saliva and salivary glands: Their roles in the oral defense system. Oral. Med. Pathol. 2006, 11, 1–17. [Google Scholar] [CrossRef]
- Yoshida, E.; Hayakawa, T. Quantitive analysis of apatite formation on titanium and zirconia in a simulated body fluid solution using the quartz crystal microbalance method. Adv. Mater. Sci. Eng. 2017, 2017, 7928379. [Google Scholar] [CrossRef]
- Kusakawa, Y.; Yoshida, E.; Hayakawa, T. Protein adsorption to titanium and zirconia using a quartz crystal microbalance method. Biomed. Res. Int. 2017, 2017, 1521593. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T. QCM analysis of stepwise adsorption of albumin and fibronectin onto the zirconia surface. Adv. Mater. Sci. Eng. 2021, 2021, 2492387. [Google Scholar] [CrossRef]
- Suzumura, T.; Matsuura, T.; Komatsu, K.; Sugita, Y.; Maeda, H.; Ogawa, T. Vacuum ultraviolet (VUV) light photofunctionalization to induce human oral fibroblast transmigration on zirconia. Cells 2023, 12, 2542. [Google Scholar] [CrossRef]
- Kumagami, H.; Furusawsa, H. Real-time monitoring of a nucleic acid amplification reaction using a mass sensor based on a quartz-crystal microbalance. Biosensors 2024, 14, 155. [Google Scholar]
- Yoshimine, H.; Sasaki, K.; Furusawsa, H. Pocketable biosensor based on quartz-crystal microbalance and its application to DNA detection. Sensors 2023, 23, 281. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Stachiv, I.; Kuo, C.Y.; Li, W. Protein adsorption by nanomechanical mass spectrometry: Beyond the real-time molecular weighting. Front. Mol. Biosci. 2023, 9, 1058441. [Google Scholar] [CrossRef]
- Mulki, S.; Prakash, G.P.; Pushparaj, S. Salivary protein concentration, flow rate, buffer capacity and pH estimation: A comparative study among young and elderly subjects, both normal and with gingivitis and periodontitis. J. Indian Soc. Periodontol. 2013, 17, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Lüders, A.; Hoth-Hanning, W.; Hanning, M.; Ziegler, C. Initial bioadhesion on dental materials as a function of contact time, pH, surface wettability, and isoelectric point. Langmuir 2010, 26, 4136–4141. [Google Scholar] [CrossRef] [PubMed]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Renal. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. Physico-chemical properties of different forms of bovine lactoferrin. Food Chem. 2013, 141, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, X.; Tang, Q.; Ma, M.; Jin, Y.; Sheng, L. Functional properties and extraction techniques of chicken egg white proteins. Foods 2022, 11, 2434. [Google Scholar] [CrossRef]
- Salgin, S.; Salgin, U.; Soyer, N. Streaming potential measurements of polyethersulfone ultrafiltration membranes to determine salt effects on membrane zeta potential. Int. J. Electrochem. Sci. 2013, 8, 4073–4084. [Google Scholar] [CrossRef]
- Giesbers, M.; Kleijn, J.M.; Stuart, M.A.C. The electrical double layer on gold probed by electrokinetic and surface force measurements. J. Colloid. Interface. Sci. 2002, 248, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Awotunde, O.; Okyem, S.; Chikoti, R.; Driskell, J.D. Role of free thiol on protein adsorption to gold nanoparticles. Langmuir 2020, 36, 9241–9249. [Google Scholar] [CrossRef] [PubMed]
- Nezu, T.; Masuyama, T.; Sasaki, K.; Saitoh, S.; Taira, M.; Araki, Y. Effect of pH and addition of salt on the adsorption behavior of lysozyme on gold, silica, and titania surfaces observed by quartz crystal microbalance with dissipation monitoring. Dent. Mater. J. 2008, 27, 573–580. [Google Scholar] [CrossRef]
- Kawai, K.; Urano, M. Adherence of plaque components to different restorative materials. Oper. Dent. 2001, 26, 396–400. [Google Scholar]
- Pradhan, A.; Shrestha, K.; Aryal, S.; Shrestha, S. Dental biofilm accumulation and gingival health of teeth with fixed single prosthesis fabricated by various prosthetic materials. Kathmandu Univ. Med. J. 2024, 22, 27–30. [Google Scholar]
- Teichroeb, J.H.; Forrest, J.A.; Jones, L.W.; Chan, J.; Dalton, K. Quartz crystal microbalance study of protein adsorption kinetics on poly(2-hydroxyethylmethacrylate). J. Colloid. Interface Sci. 2008, 325, 157–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).