Corrosion and Ion Release in 304L Stainless Steel Biomedical Stylets
Abstract
1. Introduction
- -
- In cardiac stimulation, the stylet optimises the placement of the electrodes of implantable pacemakers and defibrillators, thus ensuring the perfect transmission of electrical impulses. Its use is inevitable to avoid complications and ensure effective therapy. Stylets are also used in cardiac catheterisation procedures to guide angiography or angioplasty intervention catheters. They allow for positioning of the electrodes to ensure optimal stimulation [1,2,3,4,5,6,7,8,9,10,11].
- -
- -
- In orthopaedics, stylets are mainly used for implant guidance: they help to precisely position screws, pins, and intramedullary rods. Thus, they grant minimally invasive access and allow for creating a passage for inserting devices without damaging the surrounding tissues. They also allow for surgical navigation associated with imaging systems such as radiography, fluoroscopy or a puncture, and biopsy. Some styli are used to collect bone fragments [17,18,19,20,21].
- -
- In oto-rhino-laryngology (ENT), stylets are used to guide or support other devices during diagnostic or surgical procedures. For example, they are used in difficult naso-tracheal or orotracheal intubation, middle ear surgery (tympanoplasty, ossiculoplasty), nasal endoscopic or sinus and laryngoscopy surgery, and vocal cord interventions [22,23,24,25,26,27,28,29,30,31,32,33,34,35].
- -
- -
- Endodontics for the treatment of root canals. They are used to locate and block root canals before mechanical instrumentation. They also allow for navigation in narrow and curved channels, often in addition to endodontic files [38].
- -
- In apical surgery/microsurgery. They are used to guide very fine instruments or micro-tools in hard-to-reach areas, particularly during apical resection (amputation of the root end) [39].
- -
- In implantology. In some implant guidance techniques, a very thin metal stylus can be used to check the alignment, direction, or depth of the bone drill. They can also help to test bone density before implant insertion [40].
- -
- -
2. Materials and Methods
2.1. Sample Designation
2.2. Manufacturing Process Steps for a Medical Wire Stylet
- The choice of material is crucial and depends on the required flexibility, strength, and biocompatibility. In general, stainless steels of grades 304, 304L, and 316L are used.
- This is an operation used to correct the deformation or curvature of metal materials (wires, bars, tubes, etc.) in order to make them straight and improve their dimensional and mechanical characteristics.
- The straightened raw metal rod is pulled through a series of dies to reduce its diameter, improve the surface finish, and ensure the narrow dimensional tolerances of the wire.
- Degreasing of the wire with non-chlorinated solvents and alcohol-like products.
- Depending on the application, the stylet tips may be bevelled, rounded, or tapered to enhance ease of insertion and minimise tissue trauma.
- Depending on the application, the distal tip may be formed into a straight, J-shape, or spiral profile. Specific geometries enhance navigability and reduce trauma during insertion.
- Wires are annealed to relieve internal stresses, achieve the desired mechanical properties, and create variable flexibility along the wire length.
- Heat treatment at 750 °C.
2.3. Corrosion Assessment by Electrochemical Techniques
- -
- Immersion in the electrolyte for 1 h with potential recording in open circuit.
- -
- Recording of polarisation potentidynamic curves (±400 mV vs. SCE).
- -
- Coulometric analysis in the anodic zone in the range E (i = 0) at +400 mV ECS.
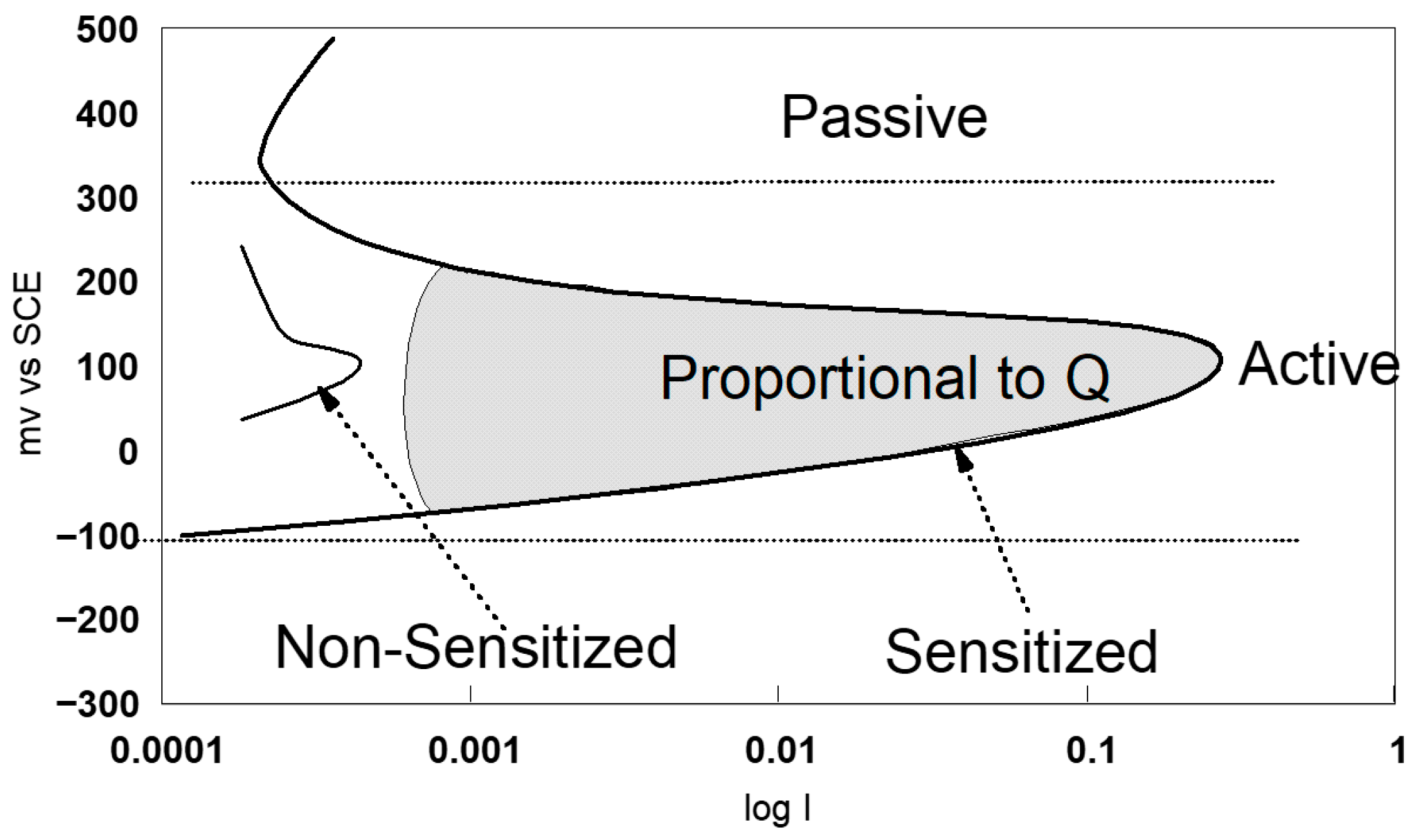
2.4. Evaluation of Intergranular Corrosion Sensitisation
- -
- Chemical test
- -
- Electrochemical tests
2.5. The Release of Cationsi
- -
- Artificial sweat according to standard EN 1811-2023-4 [79], with the following chemical composition: 1 ± 0.001 g/L of urea, 5 ± 0.001 g/L of NaCl ultra-pure, and 940 mL ± 10 µL/L of racemic lactic acid (Merck). The solution is also prepared with ultrapure quality water, conductivity (0.06–0.2 μS/cm), and without silicon (Si). The solution is filtered over a Falcon® 0.22 μm sterilised membrane in order to avoid the risk of developing bacteria during the corrosion tests. The pH of the medium is 4.5.
- -
3. Results and Discussion
3.1. Uniform Corrosion
- -
- The open-circuit potential is a criterion for the analysis of corrosion behaviour, but it is still insufficient. The approach to the results obtained is always qualitative, but gives information on the passivity of the steel surface in our case.
- -
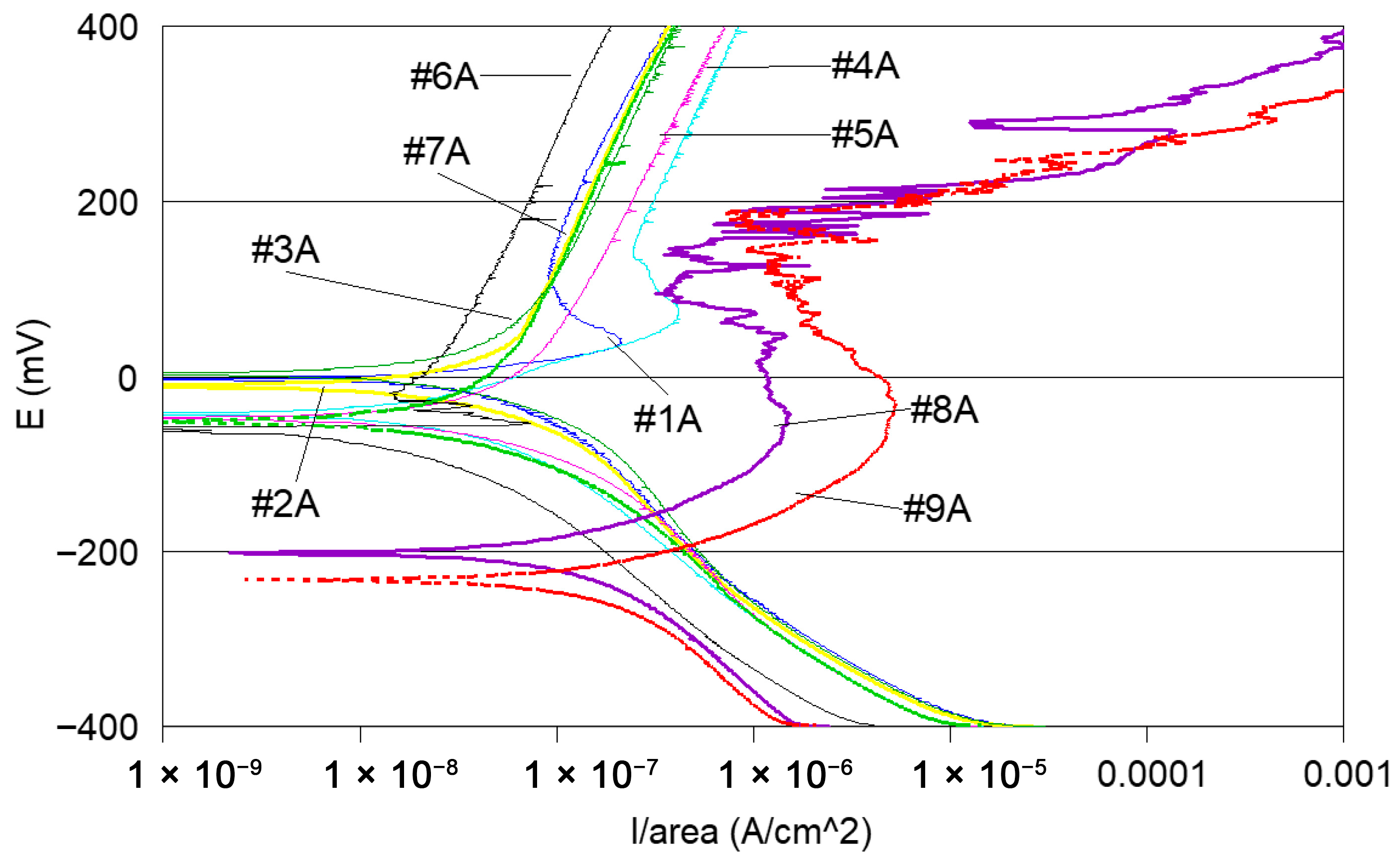
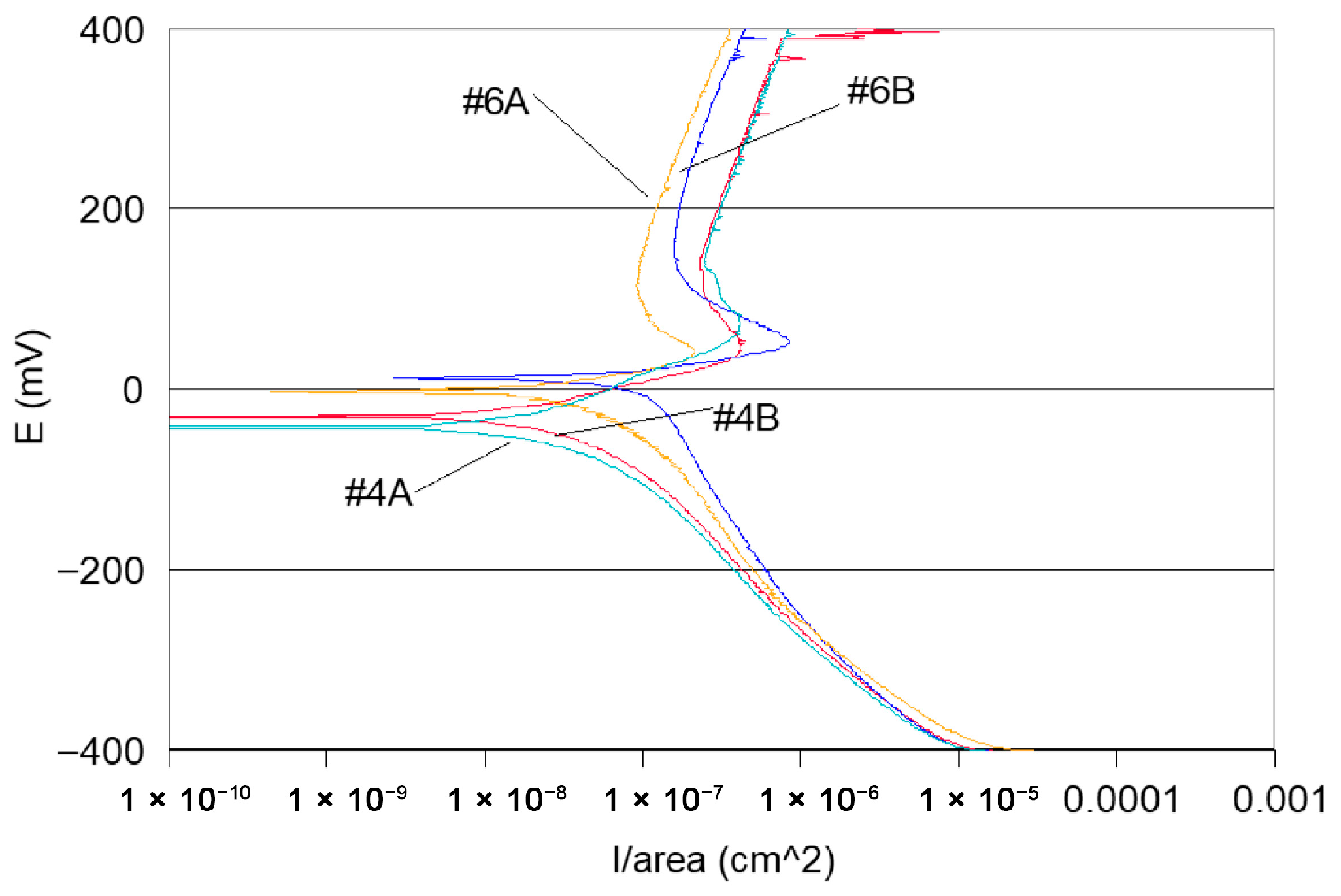
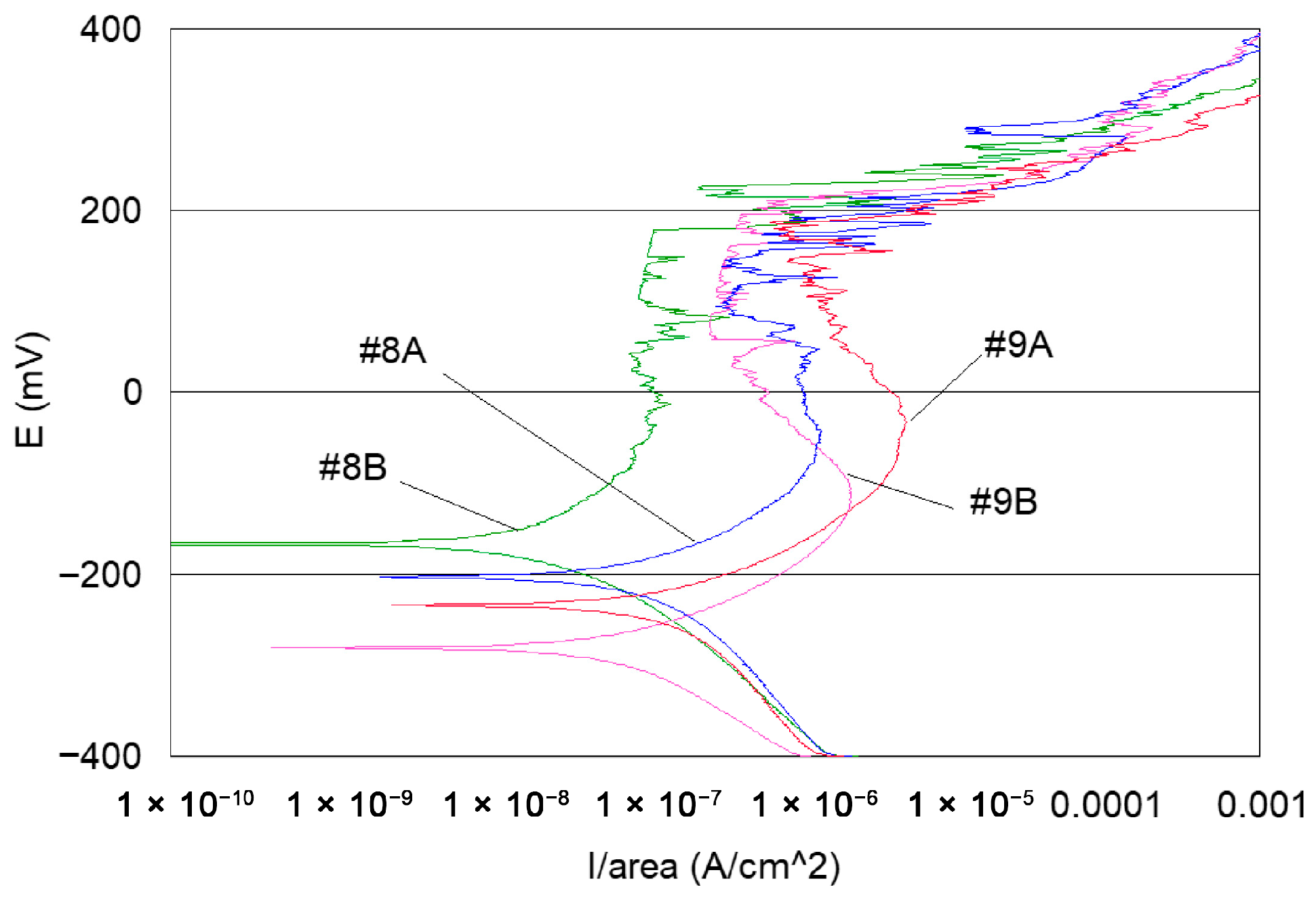
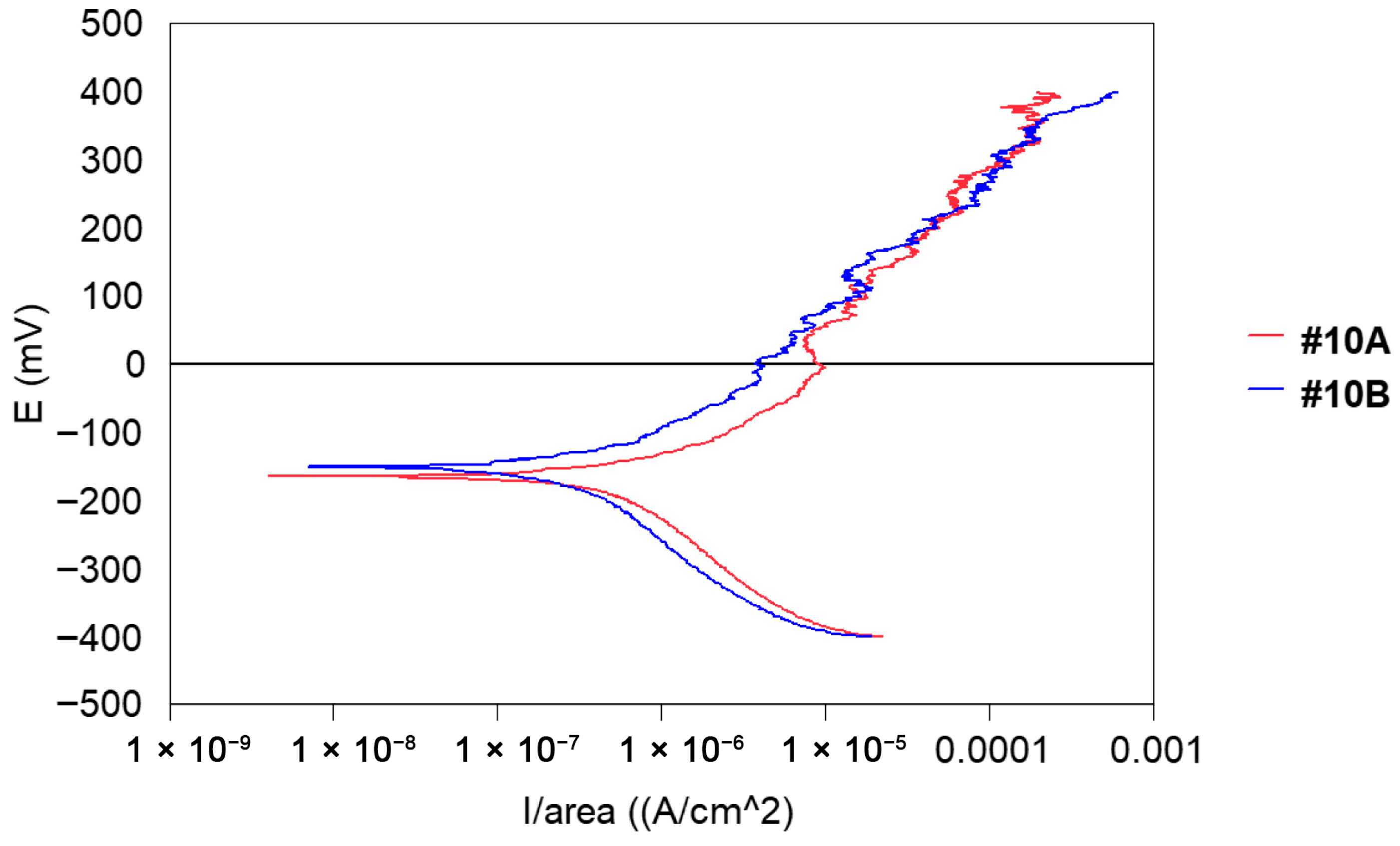
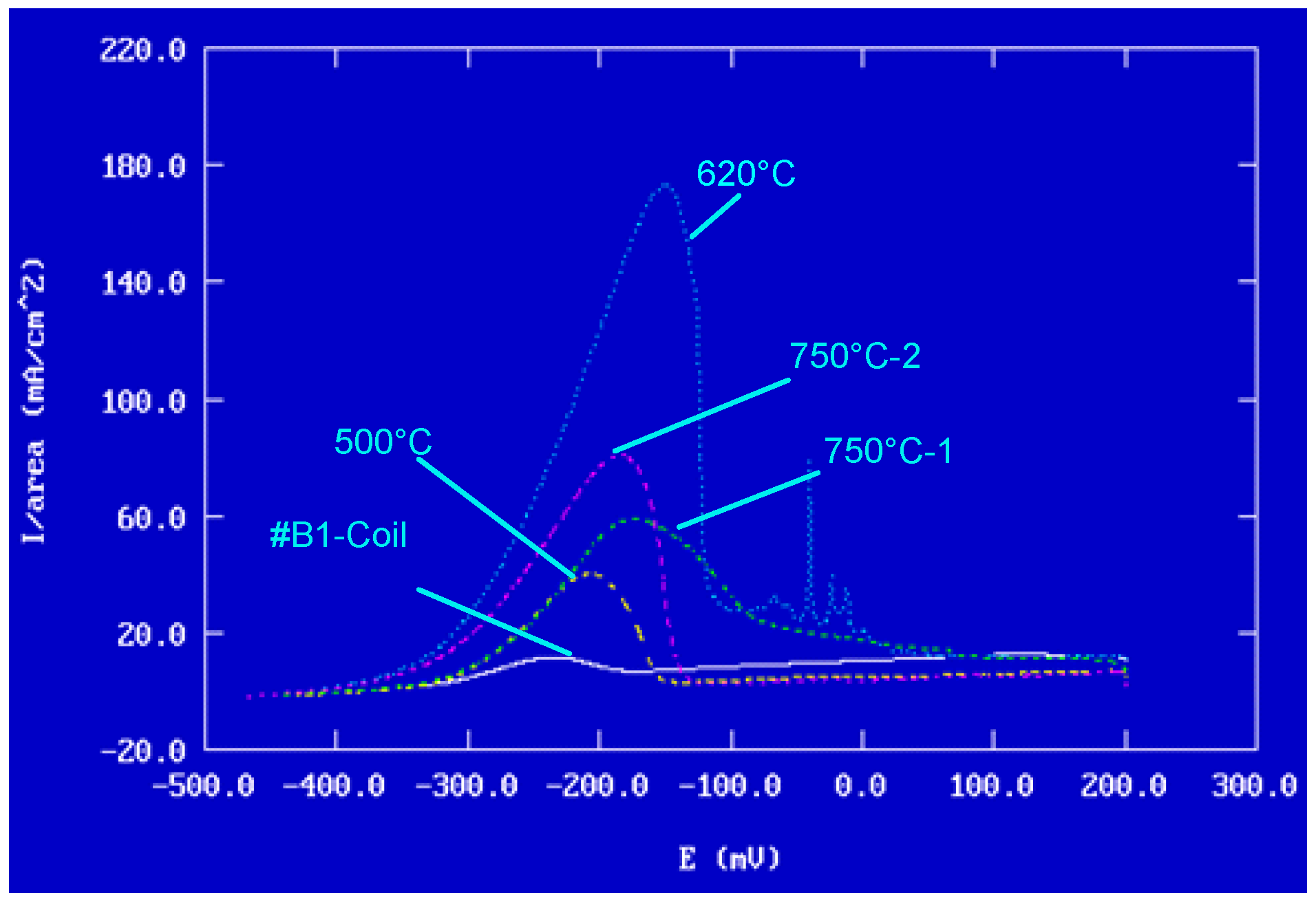
- Potentiodynamic polarisation curves: For the series of samples studied (Table 2), potentiodynamic polarisation curves for each manufacturing step are shown in Figure 7 and Figure 8. Overall, a good reproducibility is observed. However, depending on the stage of manufacture, these polarization curves can be very different. In other words, a manufacturing step can radically change the corrosion behaviour of the wire. Thus, the analysis of polarisation curves can provide information if there is an awareness process for each step examined. To obtain a better picture of this, we group the curves by series A and by series B (Figure 8 and Figure 9).
- -
- Curves #4A, #6A and #4 B4, #6B (cleaning step) reveal a peak (Figure 10). The peaks observed in the wash steps are probably due to dissolved deposits or de-passivated–re-passivated oxide layers on the surface of the wire.
- -
- If we examine curves #8A and #9A, compared to the other polarisation curves (Figure 10), we notice a clear difference in the behaviour of the 304L wire. The values of E (i = 0) are −202 mV and −228 mV, respectively, the anode current increases strongly from 10−6 to 10−2 A, and the curves show very important disturbances.
- -
- -
- -
- Coulometric analysis: The surfaces under the polarisation curves are integrated and the results obtained are expressed in μC/cm2. In other words, the amount of current consumed for the electrochemical degradation of steel is integrated into an anodic scanning range between E (i = 0) and +400 mV ECS (Table 5) This is, therefore, a quantitative way of expressing the degree of awareness of steel in its passage through the manufacturing operating range.
- A first group that underwent an operating range without heat treatment.
- A second group that underwent “heat treatment”, but is not deliverable to 620 °C.
- A third group that underwent “heat treatment” at 500 °C and is acceptable for delivery.
3.2. Intergranular Corrosion
- -
- Chemical tests
- -
- Electrochemical tests: EPR method ASTM G108–94(2015) [77].
- -
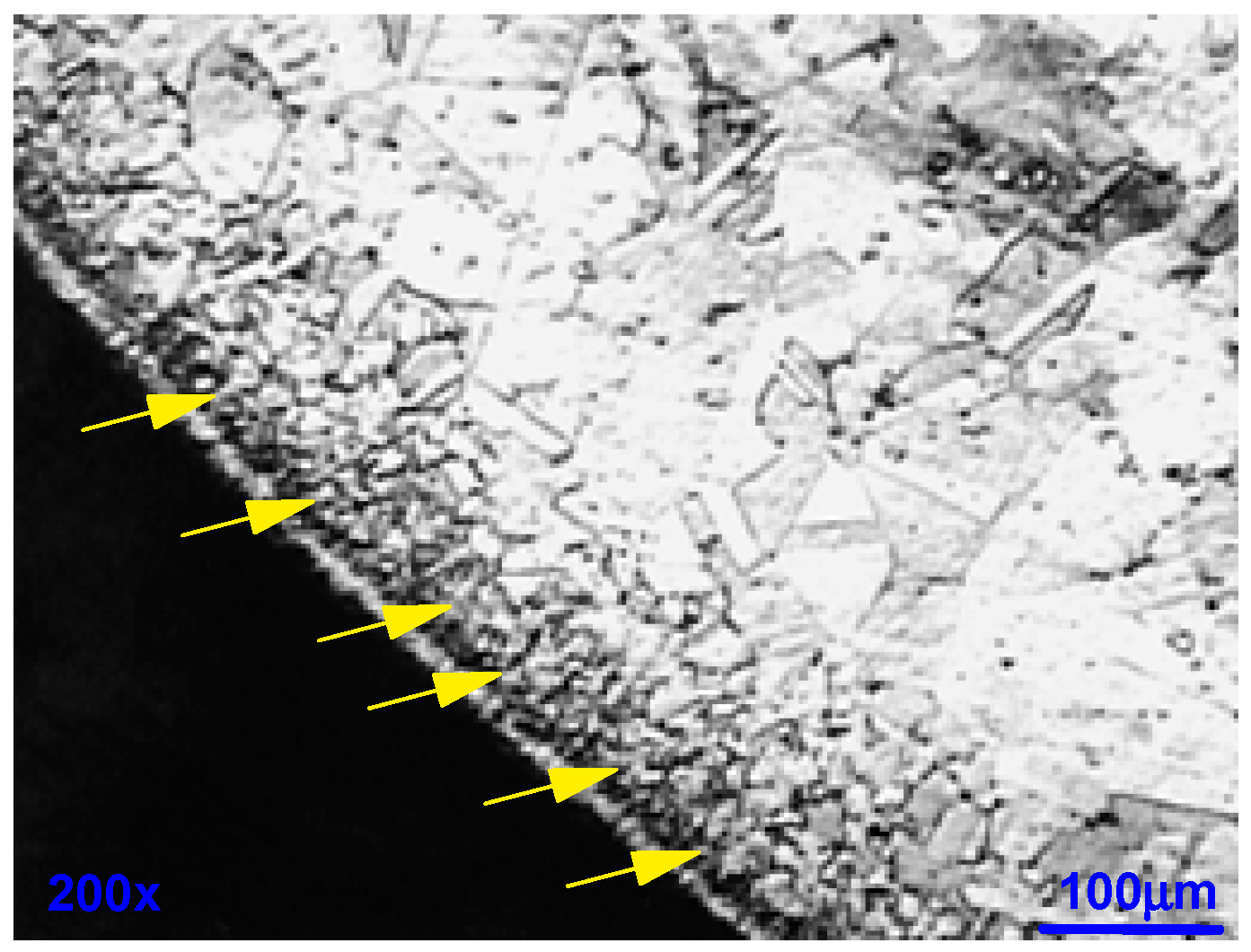
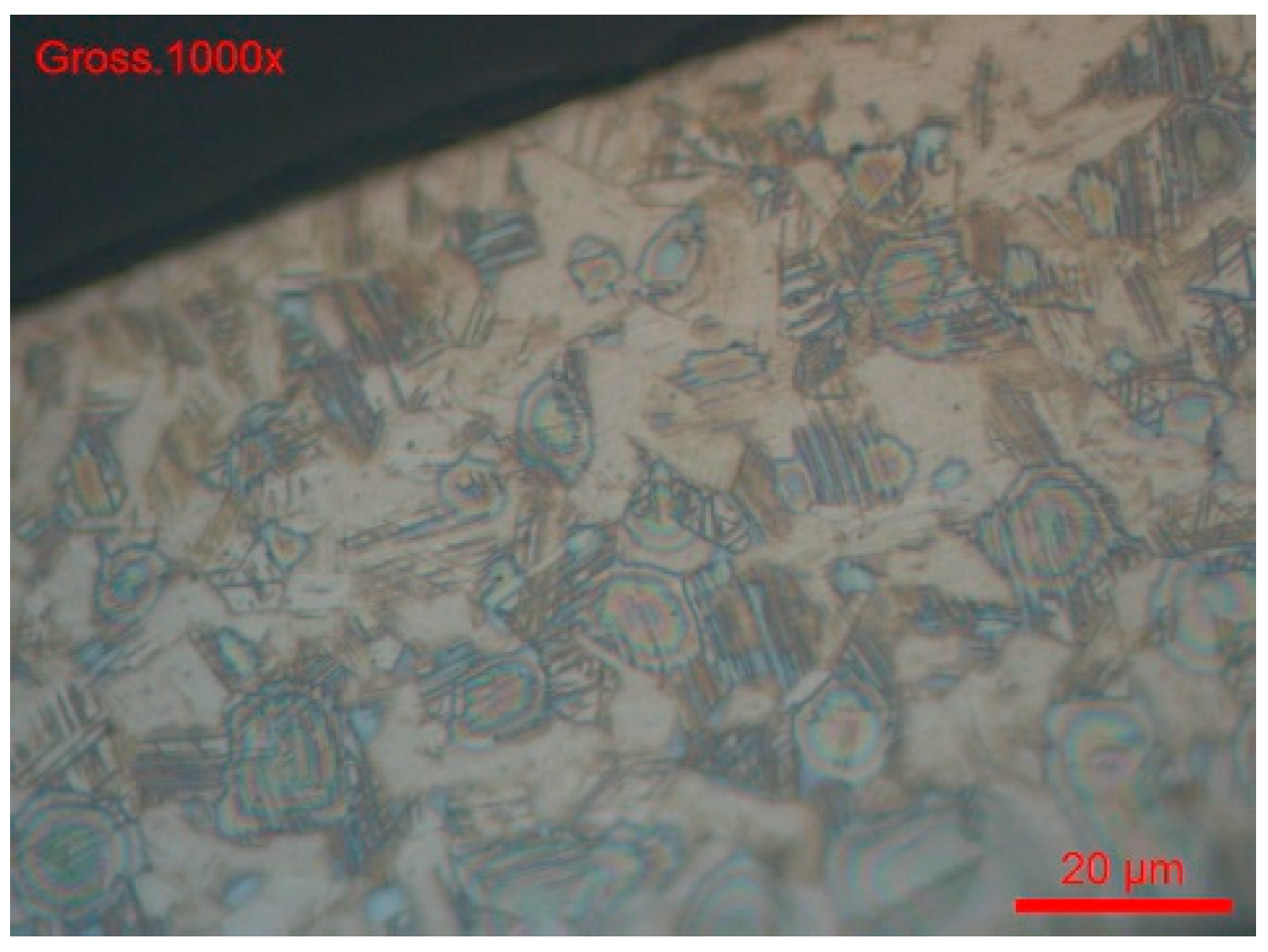
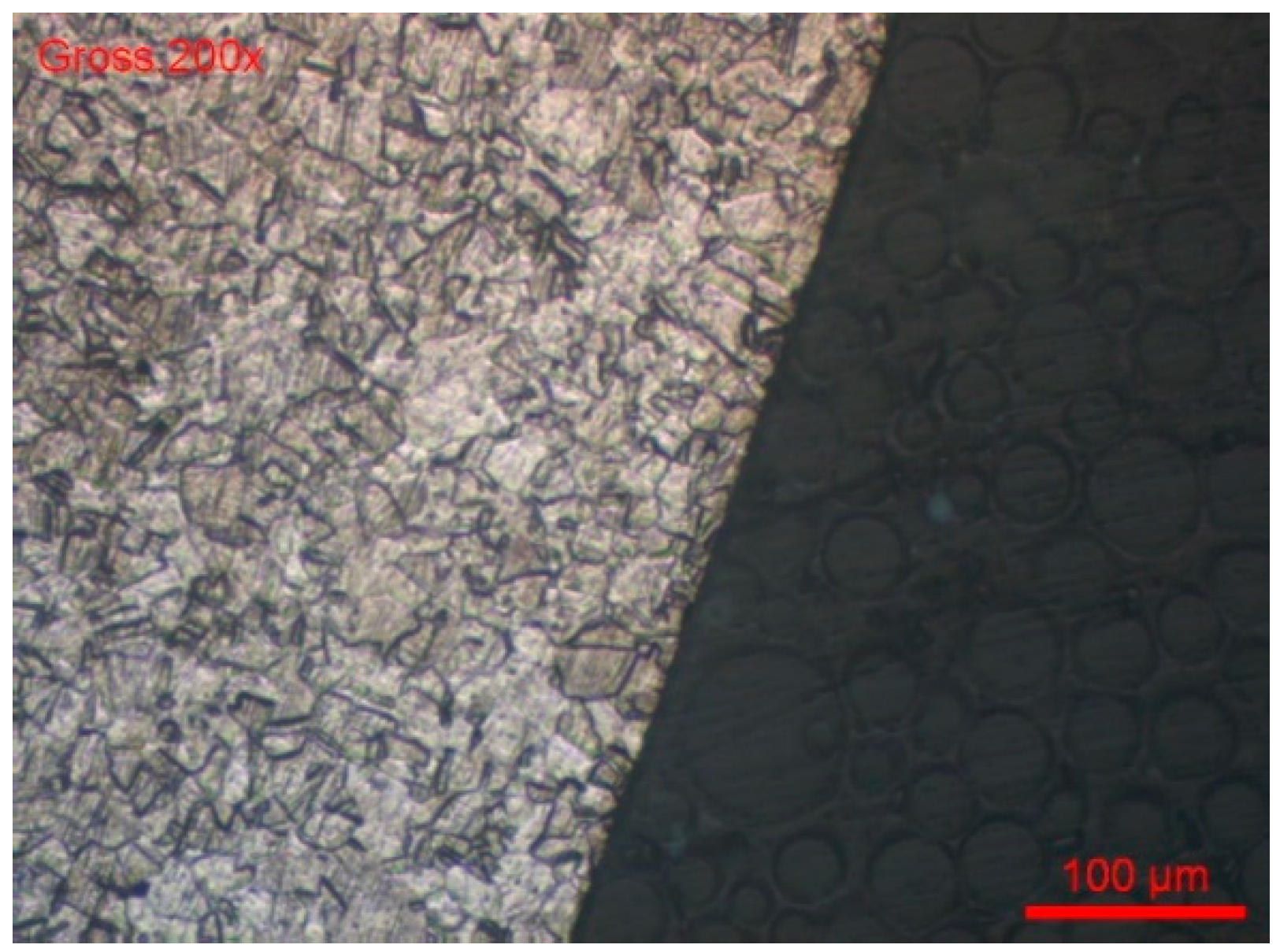
- The sample was cross-sectionally coated and polished. The usual stainless steel attack revealed a normal structure without pronounced surface corrosion.
- -
- -
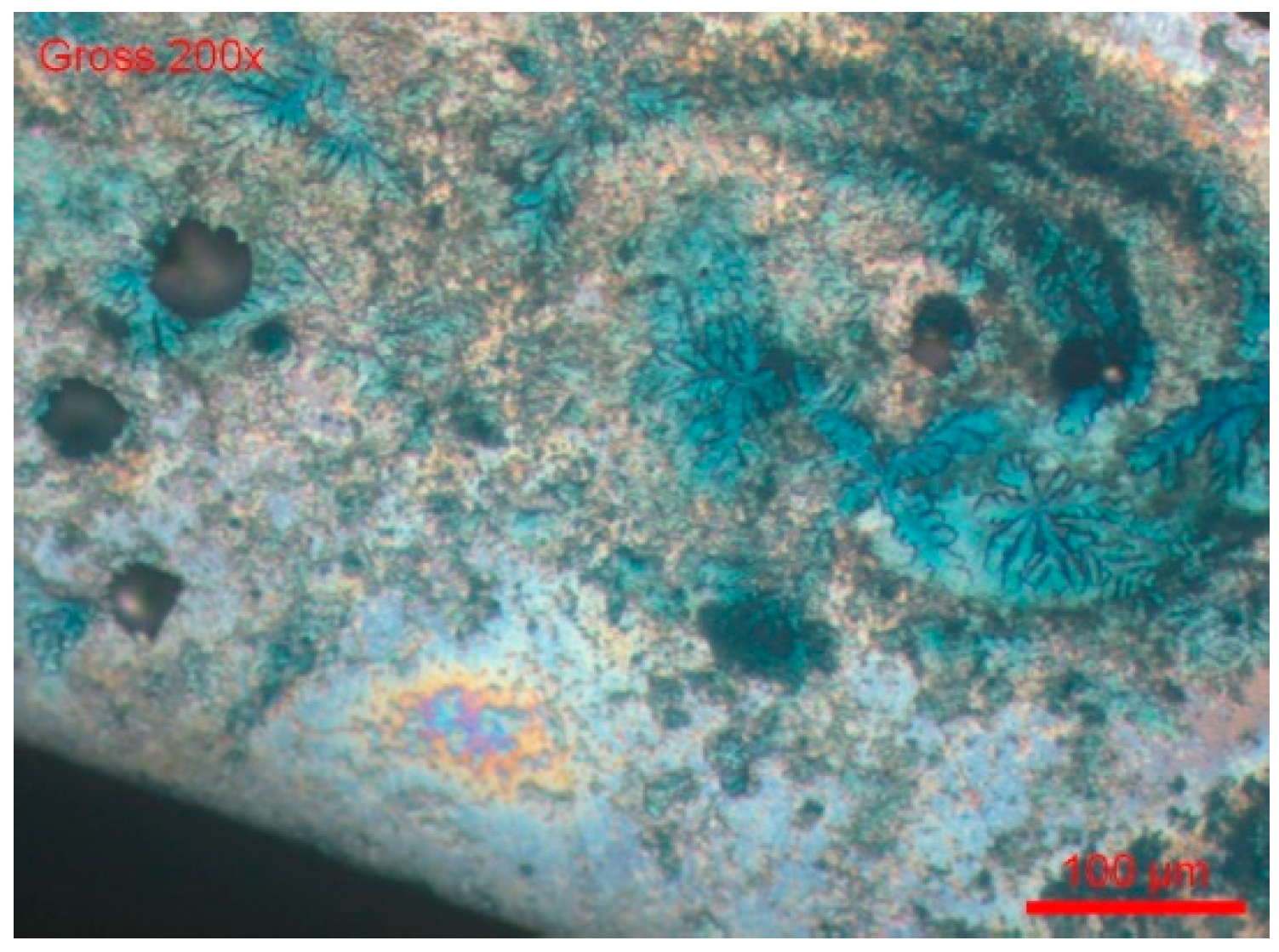
- We dipped the polished sample in a solution of 9 g/L of NaCl and potassium ferrocyanide. Soon, blue spots appeared on the surface of the cut, which assumes that the steel was sensitised in a localised corrosion (pitting, crevice). All the blue zones corresponded to active zones of this form of corrosion. We can also see many pitting instances in Figure 20.
3.3. The Release of Cations
3.4. Nickel Release and the Manufacturing Process
4. Conclusions
- -
- The first difficulty concerns respecting a manufacturing range with very rigorous parameters, in order to respect narrow tolerances and passivation processes to guarantee the chemical inertia of the device surface.
- -
- The second difficulty, even more complex, lies in the heat treatments applied to stainless steel. Indeed, a poor mastery of these treatments can induce an awareness of intergranular corrosion, a phenomenon that compromises the resistance of the material and makes the medical device unusable.
- -
- It is also important to be aware of the biological risks associated with using these materials. During a surgical procedure, the device may release metal cations such as chromium (Cr), nickel (Ni), molybdenum (Mo), manganese (Mn), iron (Fe), and traces of other chemical elements into a patient’s tissues, blood, or plasma. These rejections can have toxic consequences or cause inflammatory reactions.
- -
- The originality of this study lies in its integrated and systematic approach, which scientifically establishes the link between structural sensitisation induced by thermal treatments, electrochemical behaviour, cleaning processes, and surface passivation and the different stages of the industrial process for manufacturing medical devices made with stainless steel 304L. This work brings concrete elements to optimise the manufacturing parameters, in order to guarantee durable resistance to corrosion and the optimal clinical safety.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrd, C.L.; Schwartz, S.J.; Hedin, N.B.; Goode, L.B.; Fearnot, N.E.; Smith, H.J. Intravascular lead extraction using locking stylets and sheaths. PACE 1990, 13, 1871–1875. [Google Scholar] [CrossRef]
- Wilkoff, B.L. Intravascular lead extractions: Details and keys to success. In Interventional Electrophysiology; Singer, I., Ed.; Williams and Wilkins Medical Publishers: Baltimore, MD, USA, 1997; pp. 1055–1084. [Google Scholar]
- Fearnot, N.E.; Smith, H.J.; Goode, L.B.; Byrd, C.L.; Wilkoff, B.L.; Sellers, T.D. Intravascular lead extraction using locking stylets, sheaths, and other techniques. PACE 1990, 13, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Alt, E.; Neuzner, J.; Binner, L.; Göhl, K.; Res, J.C.; Knabe, U.; Zehender, M.; Reinhardt, J. Three-year experience with a stylet for lead extraction: A multicenter study. PACE 1996, 19, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Worley, S.J.; Gohn, D.C.; Pulliam, R.W. Over the wire lead extraction and focused force venoplasty to regain venous access in a totally occluded subclavian vein. J. Interv. Card. Electrophysiol. 2008, 23, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.G.; McGriff, D.; Retel, L.K. Riata implantable cardioverter-defibrillator lead failure: Analysis of explanted leads with a unique insulation defect. Heart Rhythm. 2012, 9, 472–749. [Google Scholar] [CrossRef]
- Love, C.J. Update on indications, techniques, and complications of cardiac implantable device lead extraction. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 565–570. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Segreti, L.; Di Cori, A.; Zucchelli, G.; Viani, S.; Paperini, L.; De Lucia, R.; Boem, A.; Levorato, D.; Soldati, E. Safety and efficacy of internal transjugular approach for transvenous extraction of implantable cardioverter defibrillator leads. Europace 2014, 16, 1356–1362. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Di Cori, A.; Segreti, L.; Zucchelli, G.; Viani, S.; Paperini, L.; De Lucia, R.; Levorato, D.; Boem, A.; Soldati, E. Transvenous extraction profile of Riata leads: Procedural outcomes and technical complexity of mechanical removal. Heart Rhythm. 2015, 12, 580–587. [Google Scholar] [CrossRef]
- Das, A.; Banerjee, S.; Mandal, S.C. A simple method for Bachmann’s bundle pacing with indigenous modification of J-stylet. Indian Heart J. 2016, 68, 678–684. [Google Scholar] [CrossRef]
- Starck, C.T.; Stepuk, A.; Holubec, T.; Steffel, J.; Stark, J.W.; Falk, V. Compression coil provides increased lead control in extraction procedures. Europace 2015, 17, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Paulk, A.C.; Ro, Y.G.; Cleary, D.R.; Tonsfeldt, K.J.; Kfir, Y.; Pezaris, J.S.; Tchoe, Y.; Lee, J.; Bourhis, A.M.; et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 2024, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.P.; Larsen, D.W.; Teitelbaum, G.P. Use of a screw-syringe injector for cement delivery during kyphoplasty: Technical report. Neurosurgery 2003, 53, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Mohyeldin, A.; Zibly, Z.; Ikeda, D.; Deogaonkar, M. Novel tunneling system for implantation of percutaneous nerve field stimulator electrodes: A technical note. Neuromodulation 2015, 18, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Harland, T.A.; Brougham, J.; Gupta, S.; Strahan, J.; Hefner, M.; Wilden, J. A Modified Technique for Interventional MRI-Guided Deep Brain Stimulation Using the ClearPoint System. Oper. Neurosurg. 2023, 25, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sillay, K.; Schomberg, D.; Hinchman, A.; Kumbier, L.; Ross, C.; Kubota, K.; Brodsky, E.; Miranpuri, G.J. Benchmarking the ERG valve tip and MRI Interventions Smart Flow neurocatheter convection-enhanced delivery system’s performance in a gel model of the brain: Employing infusion protocols proposed for gene therapy for Parkinson’s disease. Neural Eng. 2012, 9, 026009. [Google Scholar] [CrossRef] [PubMed]
- Saranteas, T.; Poulogiannopoulou, E.; Riga, M.; Panagouli, K.; Mavrogenis, A.; Papadimos, T. Coiling of echogenic perineural catheters with integral stylet: A proof-of-concept randomized control trial in a sciatic nerve block simulator and a pilot study in orthopaedic-trauma patients. F1000Research 2024, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, T.; Sawai, T.; Komasawa, N.; Shimoyama, Y.; Minami, T.J. Correlation between extraction force during tracheal intubation stylet removal and postoperative sore throat. Clin. Anesth. 2016, 33, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, M.H.; Ata, K.J.; Al-Muqbel, K.M.; Mohaidat, Z.M.; Haddad, W.H.; Rousan, L.A. Radiofrequency ablation of osteoid osteoma using tissue impedance as a parameter of osteonecrosis. J. Med. Imaging Radiat. Oncol. 2012, 56, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Bachmann-M, B.; Biscoping, J.; Hempelmann, G. Initial experiences with a novel nerve stimulator for use in axillary plexus anesthesia. Reg. Anaesthesie 1989, 12, 80–83. [Google Scholar] [PubMed]
- Mortensen, A.J.; Metz, A.K.; Featherall, J.; O’Neill, D.C.; Rosenthal, R.M.; Aoki, S.K. Hip Joint Venting Decreases the Traction Force Required to Access the Central Compartment During Hip Arthroscopy. Arthrosc. Sports Med. Rehabil. 2023, 5, e589–e596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Yang, S.C.; Chien, C.C.; Spielberger, J.; Hung, K.C.; Chung, K.C. Insertion of the ProSeal™ laryngeal mask airway is more successful with the Flexi-Slip™ stylet than with the introducer. Can. J. Anaesth. 2011, 58, 617. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, S.S.; Root, C.W.; Tvede, M.F.; Fedog, T.; Wenger, P.; Gellerfors, M.; Apel, J.; Ünlü, L. Confined space airway management: A narrative review. Scand. J. Trauma. Resusc. Emerg. Med. 2025, 33, 79. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.K.; Rasmussen, N.; Kristensen, M.S.; Bøttger, M.; Fredensborg, B.B.; Hansen, C.M.; Rasmussen, L.S. Laryngeal morbidity after tracheal intubation: The Endoflex(®) tube compared to conventional endotracheal intubation with stylet. Acta Anaesthesiol. Scand. 2013, 57, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Strøm, C.; Barnung, S.; Kristensen, M.S.; Bøttger, M.; Tvede, M.F.; Rasmussen, L.S. Tracheal intubation in patients with anticipated difficult airway using Boedeker intubation forceps and McGrath videolaryngoscope. Acta Anaesthesiol. Scand. 2015, 59, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Tordiglione, P.; Di Lascio, F.; Colagiovanni, D.; Esposito, G.; Quaranta, S.; Gasparetto, A. Effective nasotracheal intubation using a modified transillumination technique. Can. J. Anaesth. 2002, 49, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Nazir, R.; Gulati, S. Mechanism of stylet-facilitated nasotracheal intubation. Can. J. Anaesth. 2014, 61, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Ruetzler, K.; Smereka, J.; Abelairas-Gomez, C.; Frass, M.; Dabrowski, M.; Bialka, S.; Misiolek, H.; Plusa, T.; Robak, O.; Aniolek, O.; et al. Comparison of the new flexible tip bougie catheter and standard bougie stylet for tracheal intubation by anesthesiologists in different difficult airway scenarios: A randomized crossover trial. BMC Anesthesiol. 2020, 20, 90. [Google Scholar] [CrossRef]
- Huang, Y.C.; Ou, S.Y.; Kuo, Y.T.; Chia, Y.Y. Randomized, Active-Controlled, Parallel-Group Clinical Study Assessing the Efficacy and Safety of FKScope® for Nasotracheal Intubation in Patients Scheduled for Oral and Maxillofacial Surgery Under General Anesthesia. Asian J. Anesthesiol. 2021, 59, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.H.; Rowe, N.M.; Hollier, L.H., Jr.; Williams, J.K.; McCormick, S.; Longaker, M.T.; McCarthy, J.G. Development of a device for the delivery of agents to bone during distraction osteogenesis. Craniofac Surg. 2001, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lin, J.C.; Tsai, M.J.; Cheng, K.Y. Retromolar intubation with video intubating stylet in difficult airway: A randomized crossover manikin study. Am. J. Emerg. Med. 2022, 54, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Ucta, C.; Mittmann, P.; Ernst, A.; Seidl, R.; Lauer, G. Minimizing Intracochlear Pressure: Influence of the Insertion Sheath. Audiol. Neurootol. 2021, 26, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hagr, A. Intra-Operative Neural Response Telemetry and Acoustic Reflex Assessment using an Advance-In-Stylet Technique and Modiolus-Hugging: A prospective cohort study. Sultan Qaboos Univ. Med. J. 2011, 11, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Orbach-Zinger, S.; Jadon, A.; Lucas, D.N.; Sia, A.T.; Tsen, L.C.; Van de Velde, M.; Heesen, M. Intrathecal catheter use after accidental dural puncture in obstetric patients: Literature review and clinical management recommendations. Anaesthesia 2021, 76, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Gordin, A.; Papsin, B.; Gordon, K. Otol Packing of the cochleostomy site affects auditory nerve response thresholds in precurved off-stylet cochlear implants. Otol. Neurotol. 2010, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, N.; Barnett, D.; O’Callaghan, M.; Horsell, K.; Gani, J.; Hennessey, D. The impact of catheter-based bladder drainage method on urinary tract infection risk in spinal cord injury and neurogenic bladder: A systematic review. Neurourol. Urodyn. 2020, 39, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Asoglu, H.; Lampmann, T.; Winkelmann, L.M.; Salemdawod, A.; Müller, M.; Vatter, H.; Banat, M.; Eichhorn, L. Pain management with epidural catheter and epidural analgesia after spinal dorsal instrumentation of lumbar spine. Med. Baltim. 2023, 102, e32902. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.C.; Kratchman, S.I. Present status and future directions: Surgical endodontics. Int. Endod. J. 2022, 55 (Suppl. S4), 1020–1058. [Google Scholar] [CrossRef]
- Dibart, S. Practical Advanced Periodontal Surgery; Blackwell Munksgaard, Editorial Offices: Ames, IA, USA, 2020; ISBN 9781119196310, ISBN 9781119196358. [Google Scholar] [CrossRef]
- Chan, H.L.A.; Velasquez-Plata, D. Microsurgery, Book Periodontal and Implant Dentistry. Concepts and Applications; Springer: Cham, Switzerland, 2022; ISBN 978-3-030-96874-8. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Floratos, S.; Kim, S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent. Clin. North. Am. 2017, 61, 81–91. [Google Scholar] [CrossRef]
- Marwah, N.; Vishwanathaiah, S.; Ravi, G.R. Chapr-26 Pit and Fissure Sealants. In Textbook of Pediatric Dentistry; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2024; pp. 252–263. ISBN 9789356961012. [Google Scholar]
- Kher, M.S.; Rao, A. Contemporary Treatment Techniques in Pediatric Dentistry; Springer Nature Switzerland AG: Cham, Switzerland, 2019; ISBN 978-3-030-11859-4, ISBN 978-3-030-11860-0. [Google Scholar] [CrossRef]
- Zhang, K.Z.; Wu, Q.H.; Wang, Y.X.; Duan, J.T.; Guo, W.H.; Zang, Q.L. A dual analysis of bougie and stylet development trend and impact of Chinese regulations on medical devices innovation, Review Article. Am. J. Transl. Res. 2024, 16, 4071–4082. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Revie, R.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 84. [Google Scholar]
- Tiamiyu, A.A.; Eduok, U.; Szpunar, J.A.; Odeshi, A.G. Corrosion behavior of metastable AISI 321 austenitic stainless steel: Investigating the effect of grain size and prior plastic deformation on its degradation pattern in saline media. Sci. Rep. 2019, 9, 12116. [Google Scholar] [CrossRef]
- Gupta, R.K.; Birbilis, N. The influence of nanocrystalline structure and processing route on corrosion of stainless steel: A review. Corros. Sci. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Reclaru, L.; Ziegenhagen, R.; Eschler, P.Y.; Blatter, A.; Lemaître, J. Comparative corrosion study of ”Ni-free” austenitic stainless steels in view of medical applications. Acta Biomater. 2006, 2, 433–444. [Google Scholar] [CrossRef]
- Baker, M. European Standards Developed in Support of the European Union Nickel Directive. In Metal Allergy; Chen, J.K., Thyssen, J.P., Eds.; Springer: Berlin, Germany, 2018; pp. 23–29. [Google Scholar] [CrossRef]
- Liden, C. Nickel in jewelry and associated products. Contact Dermat. 1992, 26, 73–75. [Google Scholar] [CrossRef]
- Belsito, D.V. The diagnostic evaluation, treatment, and prevention of allergic contact dermatitis in the new millennium. J. Allergy Clin. Immunol. 2000, 105, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Schafer, T.; Bohler, E.; Ruhdorfer, S.; Weigl, L.; Wessner, D.; Filipiak, B.; Wichmann, H.E.; Ring, J. Epidemiology of contact allergy in adults. Allergy 2001, 56, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.; Basketter, D. Metal Exposure Regulations and Their Effect on Allergy Prevention. In Metal Allergy; Chen, J.K., Thyssen, J.P., Eds.; Springer: Berlin, Germany, 2018; pp. 39–54. [Google Scholar]
- Commission Communication in the framework of the implementation of Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Euro Union. 2012, C142, 8.
- European Parliament and Council directive 94/27/EC of 30 June 1994: Amending for the 12th time Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations. Off. J. Eur. Commun. 1994, L188, 1–2.
- Eliaz, N. Degradation of Implant Materials; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-3941-7, ISBN 978-1-4614-3942-4. [Google Scholar] [CrossRef]
- Kamachi Mudali, U.; Sridhar, T.M.; Eliaz, N.; Raj, B. Failures of stainless steel orthopaedic devices—Causes and remedies. Corros. Rev. 2003, 21, 231–267. [Google Scholar] [CrossRef]
- Pound, B.G. Corrosion behavior of metallic materials in biomedical applications. Ti and its alloys. Corros. Rev. 2014, 32, 1–20. [Google Scholar] [CrossRef]
- Technical Sheet Steel 304L. Available online: https://pxgroup.com/sites/default/files/304-L-1.4306.pdf (accessed on 30 April 2025).
- Key to Steel-Stahlschlüssel, 25th ed.; Verlag Stahlschlüssel Wegst GmbH: Marbach, Germany, 2019; Available online: http://www.online.stahlschluessel.de/BrowseProducts.aspx (accessed on 30 April 2025).
- Streicher, M.A. Intergranular. In Corrosion Tests and Standards: Application and Interpretation, 2nd ed.; Baboian, R., Ed.; ASTM International: West Conshohocken, PA, USA, 2004; pp. 244–266. [Google Scholar]
- Henthorne, M. Localized Corrosion. ASTM Tech. Publ. 1972, 518, 108. [Google Scholar]
- Desestret, A.; Froment, M.; Guiraldenq, P. Intergranular corrosion of austenitic stainless steels in the sensitized or solution-annealed condition. In Extended Abstracts, 23rd ed.; Meeting of I.S.E.: Stockholm, Sweden, 1972; p. 87. [Google Scholar]
- ASTM A262-15; Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels. ASTM International: West Conshohocken, PA, USA, 2015.
- Cihal, V.; Stefec, R. On the development of the electrochemical potentiokinetic method. Electrochim. Acta 2001, 46, 3867–3877. [Google Scholar] [CrossRef]
- Iacoviello, F.; Di Cocco, V.; D’Agostino, L. Analysis of the intergranular corrosion susceptibility in stainless steel by means of potentiostatic reactivation tests. Procedia Struct. Integr. 2017, 3, 269–275. [Google Scholar] [CrossRef]
- Clarke, W.L.; Romero, V.M.; Danko, J.C. Detection of Sensitization in Stainless Steel Using Electrochemical Techniques. In Corrosion 77; International Corrosion Forum, National Association of Corrosion Engineers: San Francisco, CA, USA, 1977; p. 180. [Google Scholar]
- Novak, P.; Stefec, R.; Franz, F. Testing the susceptibility of stainless steels to intergranular corrosion by a reactivation method. Corrosion 1975, 31, 344–347. [Google Scholar] [CrossRef]
- Aydoğdu, G.H.; Aydinol, K. Determination of susceptibility to intergranular corrosion and electrochemical reactivation behaviour of AISI 316L type stainless steel. Corr. Sci. 2006, 48, 3565–3583. [Google Scholar] [CrossRef]
- Cíhal, V.; Lasek, S.; Blahetová, M.; Kalabisová, E.; Krhutová, Z. Trends in the Electrochemical Polarization Potentiodynamic Reactivation Method—EPR. Chem. Biochem. Eng. Q. 2007, 21, 47–54. [Google Scholar]
- Parvathavarthini, N.; Dayal, R.K.; Seshadri, S.K.; Gnanamoorthy, J.B. Influence of Prior Deformation on the Sensitisation of AISI Type 304 Stainless Steel and Applicability of EPR Technique. Br. Corr. J. 1991, 26, 67–76. [Google Scholar] [CrossRef]
- Majidi, A.P.; Streicher, M.A. The Double Loop Reactivation Method for Detecting Sensitization in AISI 304 Stainless Steels. Corrosion 1984, 40, 584–593. [Google Scholar] [CrossRef]
- ASTM G108-94(2015); Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM E112-13; Standard Test Methods for Determining Average Grain Size. ASTM International: West Conshohocken, PA, USA, 2013.
- DIN EN 1811-223-4; Reference Test Method for Release of Nickel from All Post Assemblies which are Inserted into Pierced Parts of the Human Body and Articles Intended to Come Into Direct or Prolonged Contact with the Skin. DIN: Berlin, Germany, 2011.
- Burks, J.K.; Peck, W.A. Bone cells: A serum free medium supports proliferation in primary culture. Science 1978, 199, 542–544. [Google Scholar] [CrossRef]
- Stonawská, Z.; Svoboda, M.; Sozańska, M.; Krístková, M.; Sojka, J.; Dagbert, C.; Hyspecká, L. Structural analysis and intergranular corrosion tests of AISI 316L steel. J. Microsc. 2006, 224, 62–64. [Google Scholar] [CrossRef]
- Liu, T.; Xia, S.; Bai, Q.; Zhou, B.; Lu, Y.; Shoji, T. Evaluation of Grain Boundary Network and Improvement of Intergranular Cracking Resistance in 316L Stainless Steel after Grain Boundary Engineering. Materials 2019, 12, 242. [Google Scholar] [CrossRef]
- Fujii, T.; Furumoto, T.; Tohgo, K.; Shimamura, Y. Crystallographic Evaluation of Susceptibility to Intergranular Corrosion in Austenitic Stainless Steel with Various Degrees of Sensitization. Materials 2020, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, Y.; Cheng, Y.; Li, J.; Jiang, Y. The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments. Materials 2019, 12, 1385. [Google Scholar] [CrossRef] [PubMed]

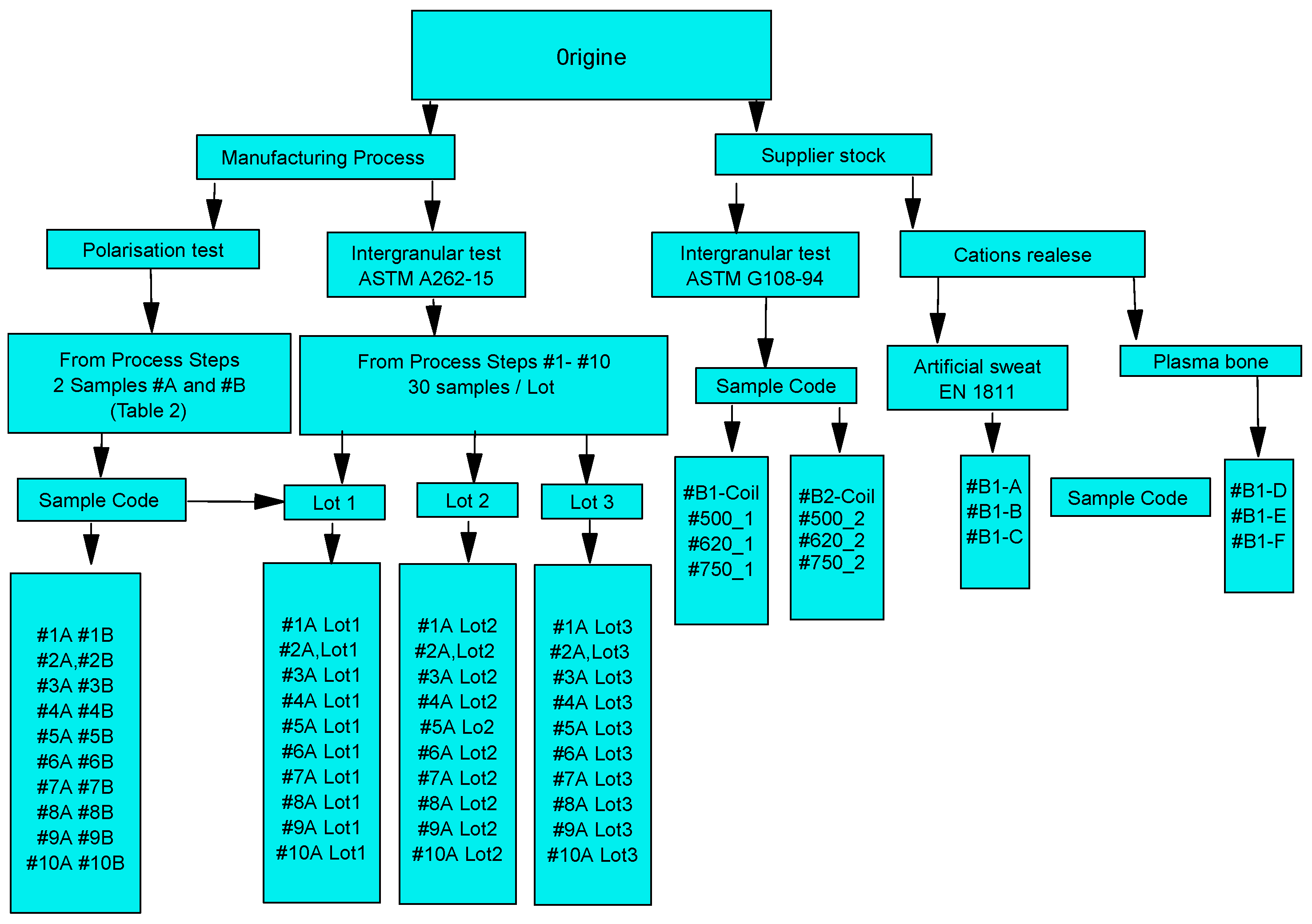
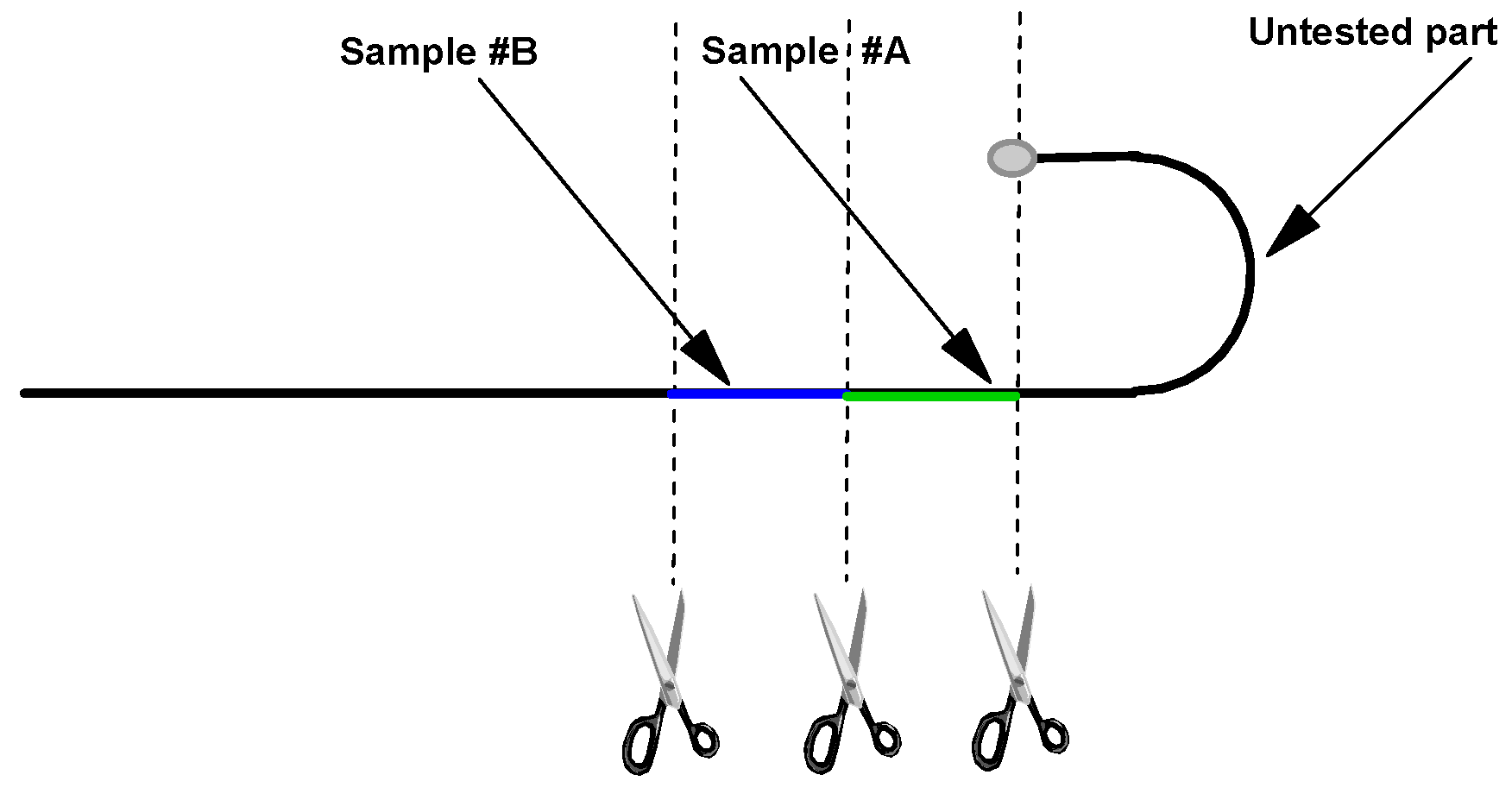
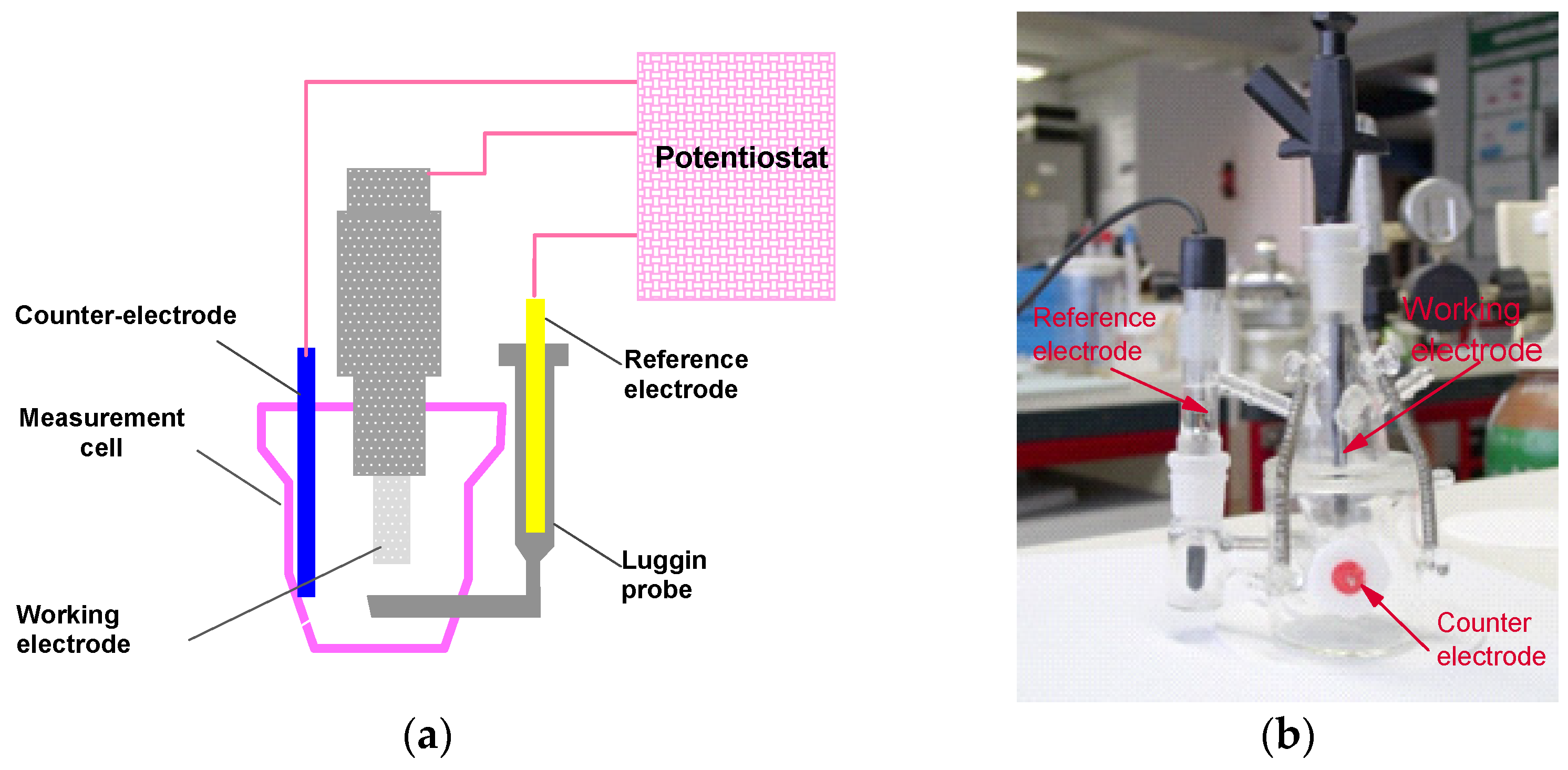






| Code | DIN | AISI | C | Si | Mn | P | S | Cr | Mo | Ni | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 1.4306 | 304L | <0.030 | <1.50 | <1.50 | <0.035 | <0.020 | 17.0–20.0 | -- | 8.0–12.0 | N 0.10-0-20 |
| Samples | Manufacturing Process Steps |
|---|---|
| #1A | Coil delivered 1 |
| #1B | |
| #2A | Mechanical straightening 2 |
| #2B | |
| #3A | Wire drawing 3 |
| #3B | |
| #4A | Cleaning 4 |
| #4B | |
| #5A | Bevelled, rounded 5 |
| #5B | |
| #6A | Cleaning 6 |
| #6B | |
| #7A | Cold forming 7 |
| #7B | |
| #8A | Heat treatment 8 at 750 °C |
| #8B | |
| #9A | Heat treatment at 620 °C |
| #9B | |
| #10A | Heet treatment at 500 °C |
| #10B |
| Designation | Test | Temperature | Testing Time | Applicability | Evaluation Method |
|---|---|---|---|---|---|
| Practice E | 6% CuSO4 16% H2SO4 with Cu metallic | Boiling | 24 h | Chromium Carbide | Examination for fissures after bending |
| Practice F | CuSO4 50% H2SO4 with Cu metallic | Boiling 125 °C | 120 h | Chromium Carbide in 316 and 316L | Weight loss/ Corrosion rate |
| Code | Description |
|---|---|
| #B1 | Wire ϕ 5 mm diameter from supplier stock (reference) |
| #500_1 | Wire ϕ 5 mm supplier +500 °C heat treatment, 1 h |
| #620_1 | Wire ϕ 5 mm supplier stock +620 °C heat treatment, 1 h |
| #750_1 | Wire ϕ 5 mm supplier stock +750 °C heat treatment, 1 h |
| #B2 | Wier ϕ 5 mm from supplier stock (second reference) |
| #500_2 | Wire ϕ 5 mm supplier +500 °C heat treatment, 1 h |
| #620_2 | Wire ϕ 5 mm supplier stock +620 °C heat treatment, 1 h |
| #750_2 | Wire ϕ 5 mm supplier stock +750 °C heat treatment, 1 h |
| Code | E(i = 0) | Q E(i = 0) à 400 mV | Remarques |
|---|---|---|---|
| mV | µC/cm2 | Process Steps | |
| #1A | −2 | 66 | Coil delivered |
| #1B | −30 | 104 | |
| #2A | −10 | 64. | Mechanical straightening |
| #2B | −14 | 75 | |
| #3A | −4.9 | 70 | Wire drawing |
| #3B | −29 | 40 | |
| #4A | −41 | 163 | Cleaning |
| #4B | −26 | 180 | |
| #5A | −47 | 117 | Bevelled, Rounded |
| #5B | −49 | 104 | |
| #6A | −59 | 35 | Cleaning |
| #6B | +13 | 112 | |
| #7A | −52 | 69 | Cold forming |
| #7B | −48 | 56 | |
| #8A | −202 | 56,180 | Heat treatment at 750 °C |
| #8B | −166 | 196,600 | |
| #9A | −228 | 339,800 | Heat treatment at 620 °C |
| #9B | −280 | 154,600 | |
| #10A | −165 | 29,460 | Heat treatment at 500 °C |
| #10B | −151 | 37,320 |
| Stage | Initial | Final | Perte | Surface | Perte | |
|---|---|---|---|---|---|---|
| m1 [g] | m2 [g] | Δm [μg] | [cm2] | Δm [μg.cm−2] | ||
| #1A | Lot 1 | 0.0161 | 0.0161 | 0 | 0.46 | 0 |
| Lot 2 | 0.01509 | 0.01509 | 0 | 0.43 | 0 | |
| Lot 3 | 0.01608 | 0.01608 | 0 | 0.46 | 0 | |
| #2A | Lot 1 | 0.02399 | 0.02396 | 30 | 0.68 | 44 |
| Lot 2 | 0.02363 | 0.02363 | 0 | 0.67 | 0 | |
| Lot 3 | 0.02288 | 0.02287 | 10 | 0.65 | 15 | |
| #3A | Lot 1 | 0.01696 | 0.01686 | 100 | 0.48 | 208 |
| Lot 2 | 0.01865 | 0.01856 | 90 | 0.53 | 170 | |
| Lot 3 | 0.01465 | 0.01463 | 20 | 0.42 | 48 | |
| #5A | Lot 1 | 0.03132 | 0.03127 | 50 | 0.89 | 56 |
| Lot 2 | 0.03323 | 0.03322 | 10 | 0.94 | 11 | |
| Lot 3 | 0.03271 | 0.03266 | 50 | 0.93 | 54 | |
| #6A | Lot 1 | 0.02281 | 0.02272 | 90 | 0.65 | 139 |
| Lot 2 | 0.02363 | 0.02363 | 0 | 0.67 | 0 | |
| Lot 3 | 0.02389 | 0.02389 | 0 | 0.68 | 0 | |
| #8A | Lot 1 | 0.01486 | 0.01482 | 40 | 0.42 | 95 |
| Lot 2 | 0.02520 | 0.02516 | 40 | 0.72 | 56 | |
| Lot 3 | 0.03154 | 0.03149 | 50 | 0.90 | 56 | |
| #9A | Lot 1 | 0.01482 | 0.01474 | 82 | 0.64 | 128 |
| Lot 2 | 0.02307 | 0.02299 | 75 | 0.65 | 115 | |
| Lot 3 | 0.03264 | 0.03256 | 79 | 0.65 | 122 | |
| #10A | Lot 1 | 0.01383 | 0.01382 | 10 | 0.39 | 25 |
| Lot 2 | 0.02394 | 0.02392 | 20 | 0.68 | 29 | |
| Lot 3 | 0.03095 | 0.0309 | 50 | 0.88 | 57 |
| Code | Eoc [mV] | Ir [mA/cm2] | Q [C/cm2] | Pa [C/cm2] |
|---|---|---|---|---|
| #B1-Coil | −387 | 11.84 | 0.28 | 1.15 |
| #B2-Coil | −410 | 8.21 | 0.33 | 1.37 |
| #500_1 | −388 | 59.22 | 1.94 | 8.10 |
| #500_2 | −410 | 30.70 | 1.33 | 5.54 |
| #620_1 | −404 | 173.30 | 8.17 | 34.04 |
| #620_2 | −395 | 162.20 | 9.94 | 41.42 |
| #750_1 | −407 | 71.86 | 4.49 | 18.69 |
| #750_2 | −407 | 59.22 | 4.01 | 16.70 |
| Milieu | Code | Ba | Cr | Fe | Ni | Ti | |||
|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/cm2 Week | µg/L | µg/cm2 Week | µg/L | µg/cm2 Week | µg/L | ||
| Blank | <0.2 | <1 | 3.8 | <1 | 1.8 | ||||
| Sweat | #B1-A | 1.3 | 33.6 | 0.034 | 1325 | 1.35 | 45.3 | 0. 05 | 2.0 |
| #B1-B | 1.6 | 36.0 | 0.037 | 2262 | 2.31 | 115.3 | 0.12 | 2.2 | |
| ##B1-C | 1.5 | 45.2 | 0.046 | 1475 | 1.51 | 101.8 | 0.10 | 2.5 | |
| Plasma bone | Blank | 0.3 | <1 | 1.5 | <1 | <0.5 | |||
| #B1-D | 0.8 | <2 | 58.8 | 0.06 | 22.5 | 0.02 | <0.5 | ||
| #B1-E | 0.9 | 2.6 | 0.03 | 17.4 | 0.07 | 26.8 | 0.03 | <0.5 | |
| #B1-F | 0.9 | 2.0 | 0.02 | 46.8 | 0.05 | 33.4 | 0.04 | <0.5 |
| Parameters | Quantity of Ni Release | |
|---|---|---|
| Raw Materials | Variable, In Function of the Lot | Strong Dispersion |
| Heat Treatment | 100% N2 100% H2 | Strong Decrease Light Decrease |
| Surface | Rough Polished Satiny | Slight Influence |
| Work Hardening | Strain >10% | Increase |
| Structure | Inclusions and Second Phases | Increase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reclaru, L.; Grecu, A.F.; Grecu, D.F.; Lungulescu, C.V.; Grecu, D.C. Corrosion and Ion Release in 304L Stainless Steel Biomedical Stylets. Materials 2025, 18, 3769. https://doi.org/10.3390/ma18163769
Reclaru L, Grecu AF, Grecu DF, Lungulescu CV, Grecu DC. Corrosion and Ion Release in 304L Stainless Steel Biomedical Stylets. Materials. 2025; 18(16):3769. https://doi.org/10.3390/ma18163769
Chicago/Turabian StyleReclaru, Lucien, Alexandru Florian Grecu, Daniela Florentina Grecu, Cristian Virgil Lungulescu, and Dan Cristian Grecu. 2025. "Corrosion and Ion Release in 304L Stainless Steel Biomedical Stylets" Materials 18, no. 16: 3769. https://doi.org/10.3390/ma18163769
APA StyleReclaru, L., Grecu, A. F., Grecu, D. F., Lungulescu, C. V., & Grecu, D. C. (2025). Corrosion and Ion Release in 304L Stainless Steel Biomedical Stylets. Materials, 18(16), 3769. https://doi.org/10.3390/ma18163769






