The Potential of Waste-Derived Sorbents for Absorbing Petroleum Substances in Firefighting Operations
Abstract
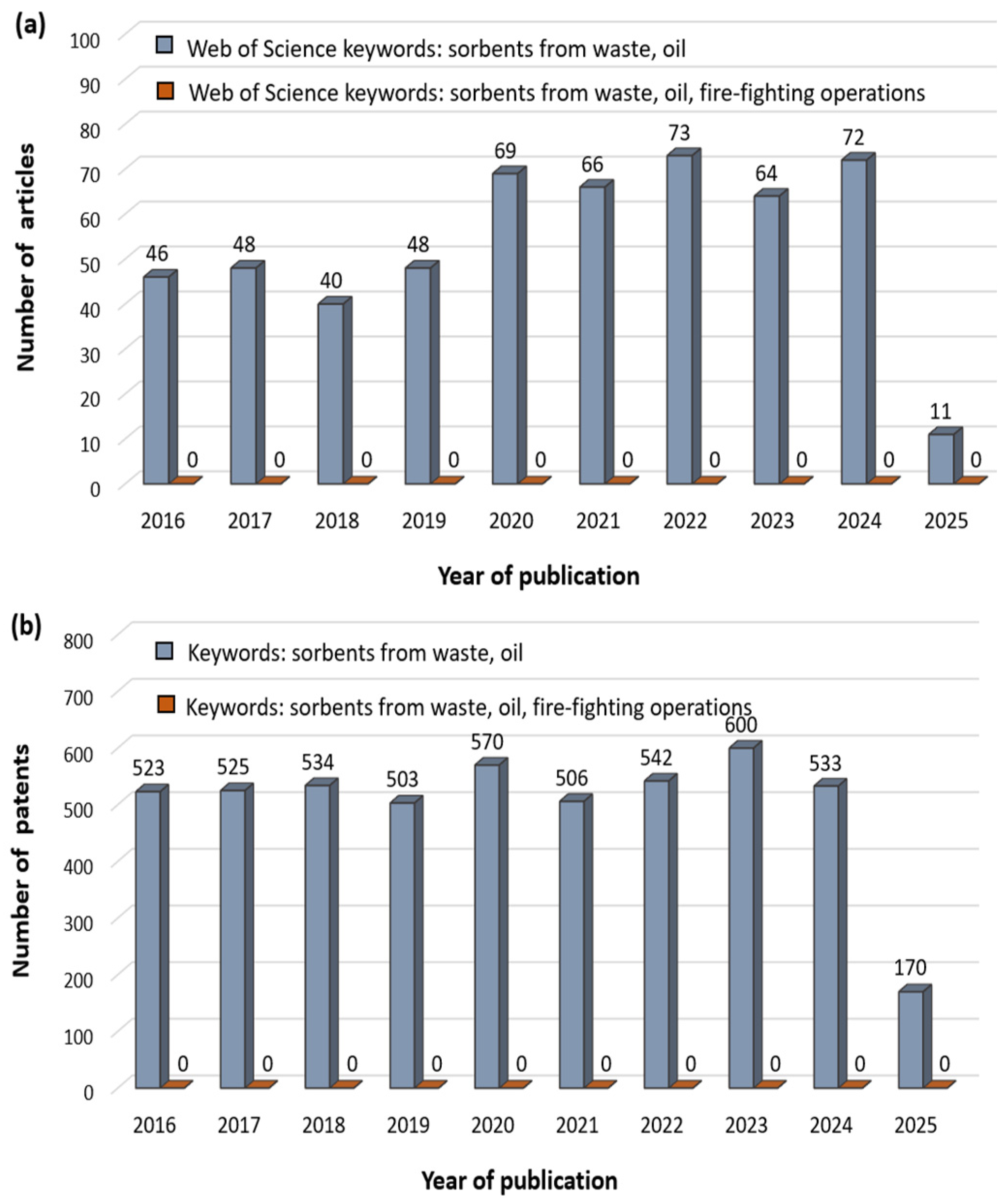
1. Introduction
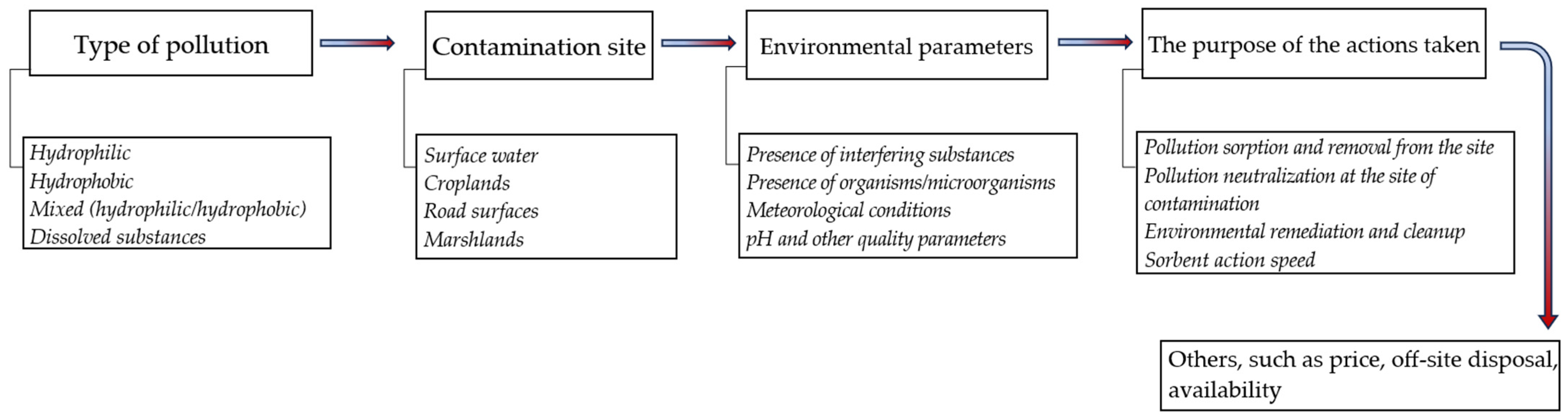
2. General Characteristics of Sorbents
3. Materials and Methods
3.1. Reagents and Materials
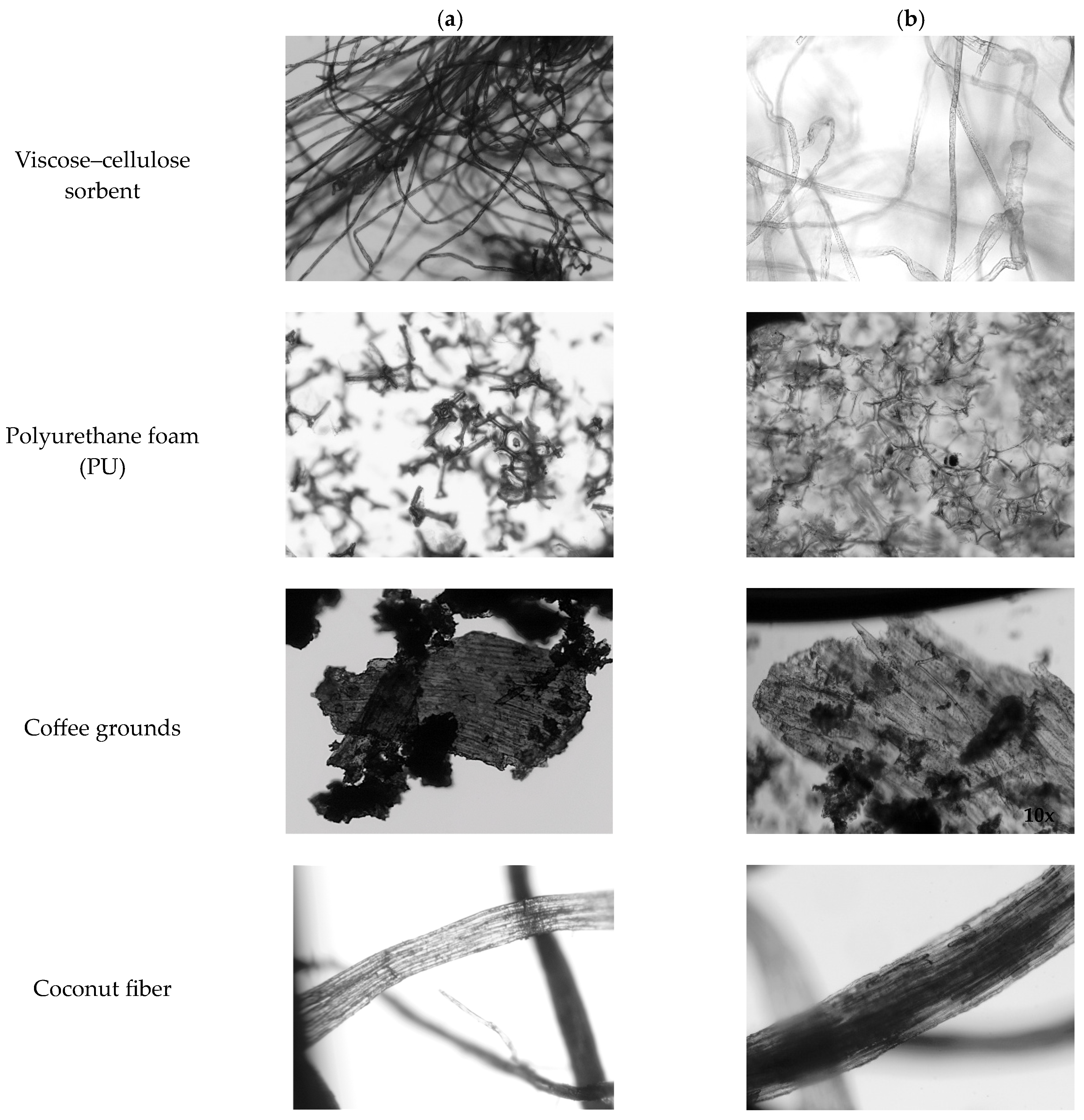
- Polyurethane foam (Figure 2a): hard, degassed polyurethane foam (PU) from recycling the thermal casing of refrigeration devices, with varying degrees of flammability; it was ground to obtain materials with varying degrees of fragmentation;
- Coffee grounds (Figure 2b): a mixture of Arabica and Robusta, MKCafe Select, obtained from a pressure espresso machine with grinding using a ceramic grinder; wet coffee ground disks were dried in a temperature chamber for 24 ± 0.5 h at 55 ± 0.5 °C and then poured onto a flat tray of 26 × 20 cm for another 24 ± 0.5 h at 20 ± 5 °C and dried in the open air;
- Coconut fiber (Figure 2c): ground coconut fiber mixed with ground coconut shell fragments;
- Viscose–cellulose sorbent (Figure 2d): viscose–cellulose nonwoven fabric with a composition of 50.3% viscose and 49.7% cotton, waste from the production of wet wipes and a material in fibrous form.
- Diesel oil compliant with EN 590 (ORLEN OIL Sp. z o.o., Gdańsk, Poland): oil intended for compression–ignition engines; density at 15 °C of 838.0 kg/m3, flash point of 65.0 °C, cetane index of 5.1, viscosity at 40 °C of 2,686 mm2/s, sulfur content of 6.1 mg/kg, and cetane number of 53.5;
- Jasol Machine Oil AN Z (46Z) machine oil (Flukar Ltd., Katowice, Poland): oil intended for lubrication of lightly or medium-loaded working elements of industrial machines and devices, piston and slide bearings, and open mechanical transmissions exposed to low temperatures; it is a mixture of mineral base oils and an additive improving low-temperature properties—heavy paraffinic distillates, hydrotreated (petroleum)—unspecified base oil, content 100% by weight; pour point < −24 °C; flash point > 200 °C; density at 15 °C of approx. 0.88 g/cm3; and kinematic viscosity at 40 °C of 28.8–74.8 mm2/s [15].
3.2. Research Equipment
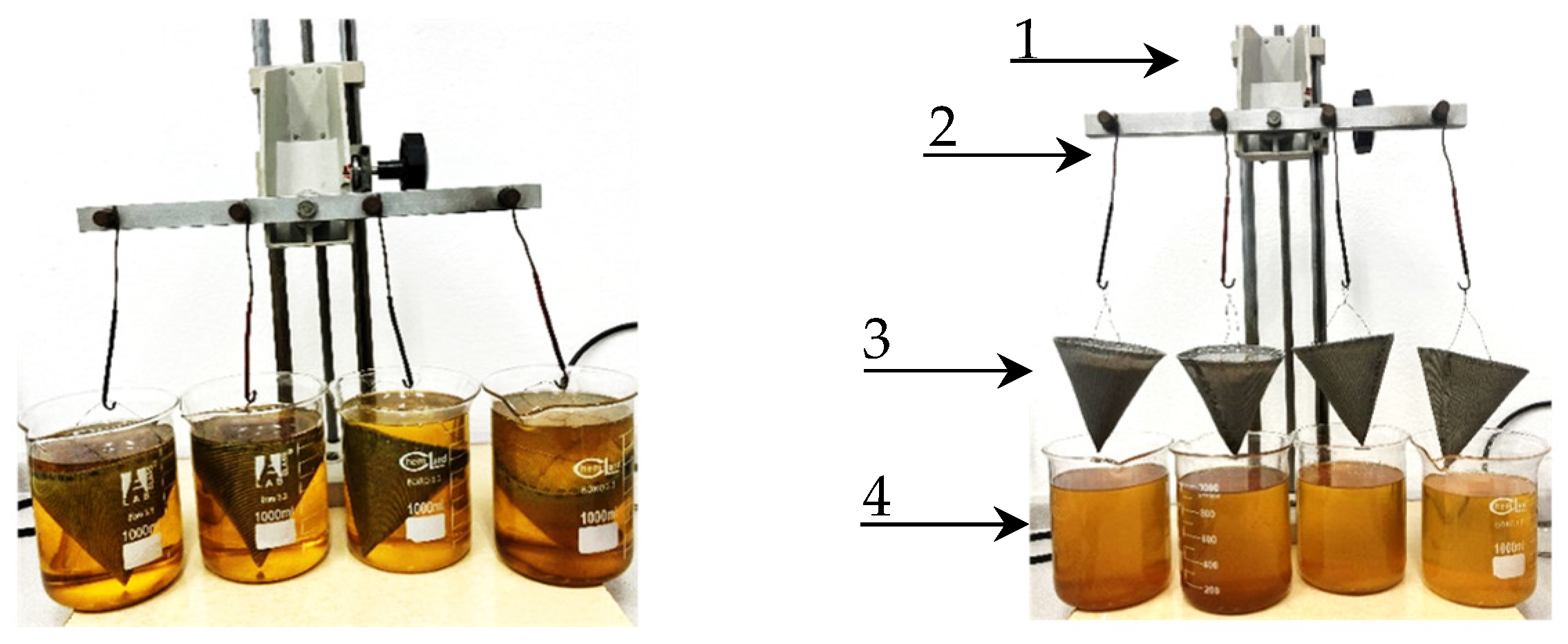
3.3. Methodology and Calculations
4. Results and Discussion
- The material’s absorption capacity for the selected pollutant (hydrophobic substances are better suited for absorbing oily pollutants);
- Bulk density, which determines its suitability for use in open environments (materials that are too light can be carried long distances by the wind);
- Costs resulting from the multiple processing stages, a complex production line, and modification/biomodification determine the price of the sorbent (the more complex the technological process, the higher the cost of producing the material);
- Post-use management, which influences further processes and increases the cost of using the material.
4.1. Physicochemical Parameters of Sorbents Obtained from Waste
- Chemical inertness, which allows for sorption to be based on physical processes;
- Buoyancy, which allows us to determine whether the material is suitable for use on water or paved surfaces;
- Bulk density, which allows us to determine whether the material is lightweight and will be carried by the wind during use or whether it is heavier and poses no such risk.
4.2. Oil Absorption Capacity
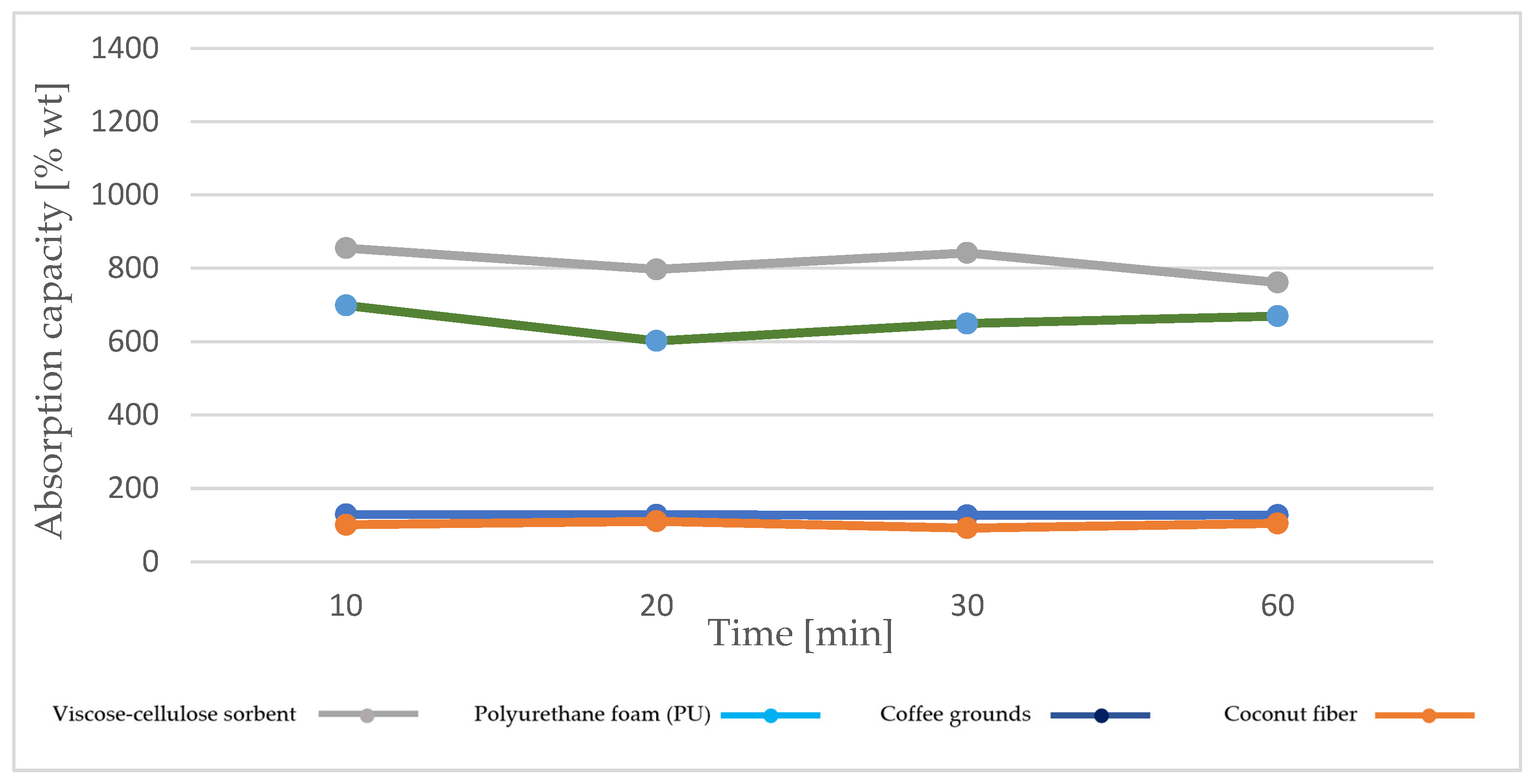
4.2.1. Diesel Fuel Absorption Capacity
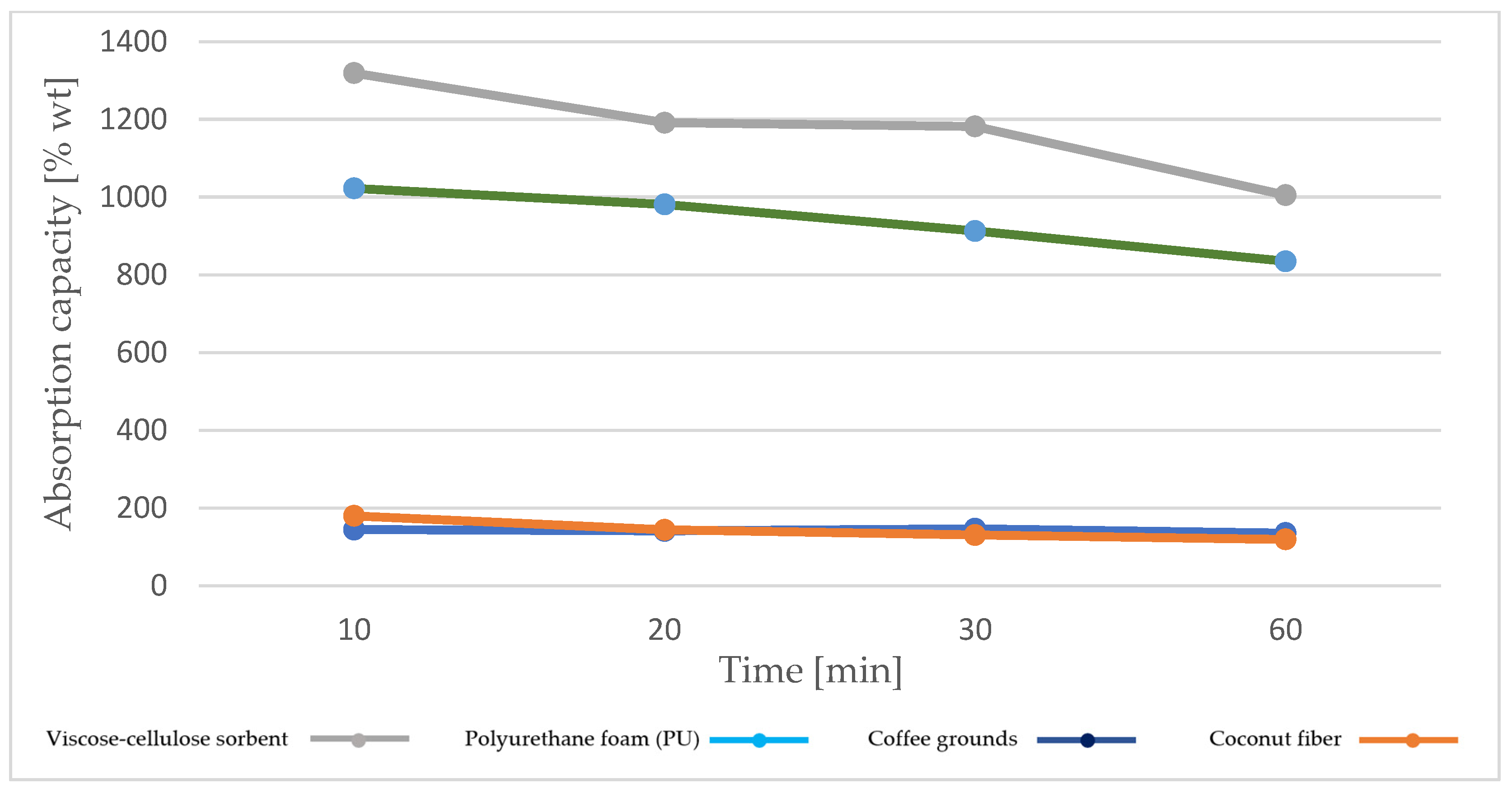
4.2.2. Machine Oil Absorption Capacity AN Z (46Z)
4.2.3. Possibility of Using Waste-Based Sorbents
4.3. Disposal of Sorbents
5. Conclusions
- The ease and speed of preparing sorbents (simple processes such as crushing, grinding, and drying)—each additional process generates costs and the potential for further waste generation;
- The possibility of easy and quick use, without negative impacts on humans and the environment;
- Methods for disposing of used sorbents that are simple, energy-efficient, and environmentally safe, preferably based on biodegradation.
- The best operating parameters are obtained for the viscose–cellulose sorbent for both oils, with higher absorption capacity values obtained for machine oil;
- The absorption capacity values for diesel oil are lower than for machine oil in the case of all sorption materials analyzed;
- The viscose–cellulose sorbent and polyurethane foam are characterized by higher absorption capacity for both oils compared to sorbents based on coffee grounds and coconut fiber;
- Sorbents based on coffee grounds and coconut fiber show stability over time in terms of absorption capacity for both types of oil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kainth, S.; Sharma, P.; Pandey, O.P. Green sorbents from agricultural wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Valentukeviciene, M.; Zurauskiene, R. Investigating the Effectiveness of Recycled Agricultural and Cement Manufacturing Waste Materials Used in Oil Sorption. Materials 2022, 15, 218. [Google Scholar] [CrossRef]
- El-Din, G.A.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; McKay, G. Up-cycling plastic waste into swellable super-sorbents. J. Hazard. Mater. 2023, 453, 131356. [Google Scholar] [CrossRef] [PubMed]
- Łudzik, M. Neutralization Systems of Wastewater Post-decontamination After Decontamination of Vehicles. Saf. Fire Technol. 2013, 31, 69–80. [Google Scholar] [CrossRef]
- EPA. United States Environmental Protection Agency, Emergency Management. Sorbents. Available online: https://archive.epa.gov/emergencies/content/learning/web/html/sorbents.html (accessed on 14 April 2025).
- Bazargan, A.; Tan, J.; Mckay, G. Standardization of Oil Sorbent Perfomance Testing. J. Test. Eval. 2015, 43, 1271–1278. [Google Scholar] [CrossRef]
- ASTM F726-12; Standard Test Method for Sorbent Performance of Adsorbents. ASTM International: West Conshohocken, PA, USA, 2012.
- NF T90-362; Water and Soil Pollution Control Products—Classification of Sorbents. Association Francaise de Normalisation: Paris, France, 2022.
- Merlin, F.X.; Le Guerroue, P. Use of Sorbents for Spill Response, Operational Guide. 2009. Available online: https://wwz.cedre.fr/en/content/download/1776/140008/file/extract-sorbents.pdf (accessed on 22 May 2025).
- Regulation of the Minister of Internal Affairs and Administration of 31 October 2022 on the List of Products Used to Ensure Public Safety or Protect Health, Life and Property, as Well as the Principles of Issuing Approvals for Use of These Products (Journal of Laws 2022, Item 2282). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20220002282/O/D20222282.pdf (accessed on 22 May 2025). (In Polish)
- Tefora, J.L.L.; Tomon, T.R.B.; Ungang, J.I.D.S.; Malaluan, R.M.; Lubguban, A.A.; Bacosa, H.P. The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications. J. Mar. Sci. Eng. 2025, 13, 1176. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Betus, M.; Koncek, M.; Sofranko, M.; Rosova, A.; Szucs, M.; Cvoliga, M. Effectiveness of Sorbents in the Equipment of Firefighting Units in Practice. Fire 2024, 7, 449. [Google Scholar] [CrossRef]
- SAFETY DATA SHEET. JASOL Machine Oil AN. Available online: https://flukar.eu/wp-content/uploads/2017/03/MSDS_Jasol_Machine_Oil_AN_Z.pdf (accessed on 22 May 2025). (In Polish).
- Accreditation Certificate No. AB 060 Confirms That the Performed Activity Is Based on Regulations Pursuant to PN-EN ISO/IEC 17025:2018-02 Standard “General Requirements for the Competence of Testing and Calibration Laboratories”. Available online: https://cnbop.pl/en/our-offer/laboratory-tests/laboratory-of-fire-extinguishing-agents-and-equipment-bu/akredytacja-zakres-badan-en/ (accessed on 22 May 2025).
- Suni, S.; Kosunen, A.-L.; Hautala, M.; Pasila, A.; Romantschuk, M. Use of a by-product of peat excavation, cotton grass fibre, as a sorbent for oil-spills. Mar. Pollut. Bull. 2004, 49, 916–921. [Google Scholar] [CrossRef]
- Sobolewski, M.; Gancarczyk, D.; Książek, P. Efficiency of sorbents used to restore the grip of the surface of the oily road. MATEC Web Conf. 2018, 247, 00024. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Nasr, S.M.; Thabet, W. Palm fibers and modified palm fibres adsorbents for different oils. Alex. Enf. J. 2017, 56, 749–755. [Google Scholar] [CrossRef]
- Hussain, F.A.; Zamora, J.; Ferrer, I.M.; Kinyua, M.; Velázquez, J.M. Adsorption of crude oil from crude oil–water emulsion by mesoporous hafnium oxide ceramics. Environ. Sci. Water Res. Technol. 2020, 6, 2035–2042. [Google Scholar] [CrossRef]
- Dziubak, T. Operating fluids contaminantions and their effect on the wear of elements of motor vehicle’s combustion engine. Arch. Automot. Eng. 2016, 72, 43–72. [Google Scholar] [CrossRef]
- Stauffer, E. Chapter 11—Analysis of vehicle fluids. In Forensic Investigation of Stolen-Recovered and Other Crime-Related Vehicles; Academic Press: Cambridge, MA, USA, 2006; pp. 283–299. [Google Scholar] [CrossRef]
- Salamonowicz, Z.; Grudziecki, A.; Lipiński, D.; Dmochowska, A. Comparative analysis of the absorbency of sorbents determined by various methods for diesel oil and white spirit. Sci. Rep. Fire Univ. 2023, 1, 297–310. [Google Scholar] [CrossRef]
- Hussein, M.; Amer, A.A.; Hoda, F.; Zahran, H.F.; Ali, S.M.; Elgohary, M.; Nasr, M. Agricultural waste as a biosorbent for oil spills. Int. J. Dev. 2013, 2, 127–135. [Google Scholar]
- Hussein, M.; Amer, A.A.; El-Maghraby, A.; Taha, N.A. Availability of Barley Straw Application on Oil SPill Clean Up. J. Environ. Sci. Technol. 2008, 6, 123–130. [Google Scholar] [CrossRef]
- Sayed, S.A.; Zayed, A.M. Investigation of the effectiveness of some adsorbent materials in oil spill clean-ups. Desalination 2006, 194, 90–100. [Google Scholar] [CrossRef]
- Tan, J.Y.; Low, S.Y.; Ban, Z.H.; Siwayanan, P. A review on oil spill clean-up using bio-sorbent materials with special emphasis on utilization of kenaf core fibers. BioResources 2021, 16, 8394–8416. [Google Scholar] [CrossRef]
- Rengasamy, R.S.; Das, D.; Karan, C.P. Study of oil sorption behavior of filled and structured fiber assemblies made from polypropylene, kapok and milkweed fibers. J. Hazard. Mater. 2011, 186, 526–532. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Coated kapok fiber for removal of spilled oil. Mar. Pollut. Bull. 2013, 69, 91–96. [Google Scholar] [CrossRef]
- Rajakovic, V.; Aleksic, G.; Radetic, M.; Rajakovic, L. Efficiency of oil removal from real wastewater with different sorbent materials. J. Hazard. Mater. 2007, 143, 494–499. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Wang, F. Oil spill cleanup by structured natural sorbents made from cattail fibers. Ind. Crops Prod. 2015, 76, 25–33. [Google Scholar] [CrossRef]
- Statistical Data of the National Headquarters of the State Fire Service of Poland. Available online: https://www.gov.pl/web/kgpsp/interwencje-psp (accessed on 28 April 2025). (In Polish)
- Minnesota Department of Transportation. Regulated Materials. Used Oil Sorbent Management. Available online: https://www.dot.state.mn.us/environment/regulatedmaterials/guidance/used-oil-sorbent.html (accessed on 25 June 2025).
- ITU AbsorbTech. The Ultimate Guide: How to Dispose of Used Rags and Oil Absorbents. Available online: https://ituabsorbtech.com/manage-used-rags-and-absorbents-federal-state-guide/ (accessed on 25 June 2025).
- Dereli, B.; Gürel, B.; Dolgun, G.K.; Keçebaş, A. Comprehensive study on incineration-based disposal of hazardous gas and liquid wastes from used lubricating oil refineries. Process Saf. Environ. Prot. 2024, 184, 79–95. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Daystar, J.S.; Venditti, R.A.; Pawlak, J.J.; Zambrano, M.C.; Barlaz, M.; Ankeny, M.; Pires, S. Biodegradability of Cellulose Fibers, Films, and Particles: A Review. BioResources 2025, 20, 2391–2458. [Google Scholar] [CrossRef]
- Royer, S.J.; Wiggin, K.; Kogler, M.; Deheyn, D.D. Degradation of synthetic and wood-based cellulose fabrics in the marine environment: Comparative assessment of field, aquarium, and bioreactor experiments. Sci. Total Environ. 2021, 791, 148060. [Google Scholar] [CrossRef]
- Jishnu, V.P.; Sankar, N.; Chandrakaran, S. Strength behavior of cohesionless soil reinforced with coconut leaf let as a natural material. Mater. Today Proc. 2020, 31 (Suppl. S2), S340–S347. [Google Scholar] [CrossRef]
- Mir, I.A.; Bawa, E.R.A. Utilization of waste coconut coir fiber in soil reinforcement. Int. J. Civ. Eng. Technol. (IJCIET) 2018, 9, 774–781. Available online: http://iaeme.com/Home/issue/IJCIET?Volume=9&Issue=9 (accessed on 25 June 2025).

| Standard | Division of Sorbents | Ref. |
|---|---|---|
| ASTM F726-12: Standard Test Method for Sorbent Performance of Adsorbents, Annual Book of ASTM Standards, ASTM International, West Conshohocken, PA, 2012 |
| [7,8] |
| NF T90-362: Water and soil pollution control products—Classification of sorbents | Division based on selectivity:
| [9,10] |
| Regulation of the Minister of Internal Affairs and Administration of 31 October 2022 on the list of products used to ensure public safety or protect health, life and property, as well as the principles of issuing approvals for use of these products (Journal of Laws 2022, item 2282) | Division according to sorption capacity:
| [11] |
| Parameter | Requirement |
|---|---|
| Oil absorption capacity | We required ≥50% by weight of the sorbent for diesel fuel meeting the requirements of EN 590. Measurement error of the method used: +/−3.74% |
| Sieve analysis | According to ISO 2591-1:2000, we used the dry sieving method, with the following range of sieves: 40 µm, 63 µm, 125 µm, 4 mm. The content of fractions with a grain size above 4 mm and fractions with a grain size smaller than 0.125 mm cannot differ by more than ±10% of the value declared by the manufacturer. Measurement error of the method used: +/−0.22% |
| Chemical inactivity | The chemical passivity test was performed during the oil absorption capacity test by observing four aspects, i.e., (i) gas release (organoleptic observation of smoke or change in smell), (ii) change in the color of the absorbed liquid, (iii) an increase in the temperature of the absorbed medium, (iv) change in the form of the sorbent. The sorbent cannot enter into chemical reactions with the absorbed substances. Organoleptic examination. Temperature measurement error: +/−0.2 °C |
| Buoyancy | This is assessed according to the method developed by the Laboratory of Fire Extinguishing Devices and Agents BU, which is within the scope of PCA accreditation (AB060) [16]—a sample previously tested for oil absorption capacity is used for testing. After the required time has elapsed, the amount of submerged sorbent should be assessed. This amount should not exceed 5% of the sorbent subjected to testing. In total, 95% of the sorbent used on water surfaces, both ready for use and used, fully saturated with a reference hydrocarbon according to EN 590, remains on the surface of standing water for 24 h. The requirement applies only to sorbents for collecting contaminants from water surfaces. Organoleptic examination |
| Bulk density | According to PN-C-04532:1980 method B, the bulk density value expressed in g/L cannot differ by more than ±10% of the manufacturer’s declared value. The requirement applies only to bulk sorbent. Measurement error of the method used: +/−0.95% |
| Sorption Material | Chemical Inertness | Buoyancy | Bulk Density [g/L] | Grain Composition |
|---|---|---|---|---|
| Polyurethane foam (PU) | Passive | Floating | 52.66 | <0.125 mm: 4.95% wt.; >4.0 mm: 0.05% wt. |
| Coffee grounds | Passive | Non-floating | 341.49 | <0.125 mm: 4.88% wt.; >4.0 mm: 0% wt. |
| Viscose–cellulose sorbent | Passive | Non-floating | NA * | NA * |
| Coconut fiber | Passive | Non-floating | NA * | NA * |
| Sorptio Material | Sorption Capacity [% wt] | Notes | Ref. |
|---|---|---|---|
| Commercial sorbents | |||
| Compakt | 91.0 | t = 10 min soaking and draining; Westinghouse method | [23] |
| ECOBARK | 104.0 | ||
| Quartz sand | 21.0 | ||
| Waste sorbents | |||
| Viscose–cellulose sorbent | 796.8 *–1191.2 ** | t = 20 min soaking and draining; ambient temperature 20.5 ± 1 °C | Measured in this study |
| Polyurethane foam | 601.9 *–981.1 ** | ||
| Coconut fibers | 110.5 *–144.1 ** | ||
| Coffee grounds | 129.7 *–140.9 ** | ||
| Corn with cotton | 3790 | t = 15 min; test: 0.5 L of artificial seawater with a concentration of 3.5% NaCl; layer of diesel oil/crude oil with different thicknesses of 1, 3, and 5 mL; ambient temperature 25 ± 1 °C | [24] |
| Barley straw | 780–1220 | t = 15 min soaking, 5 min draining; particle size 250 μm; ambient temperature 25 ± 1 °C; test: oil + seawater, film thickness 5 mm | [25] |
| Banana peels | 531–663 | t = 15 min soaking and draining; particle size 0.3625 mm; ambient temperature 25 °C | [3] |
| Onion peels | 45.5 | t = 90, 30, and 30 s contact; test: 0.6 g crude oil in physiological saline solution; 750 mL 0.5 M NaCl; ambient temperature 30 °C | [26] |
| Garlic peels | 38.5 | ||
| Product | Purpose |
|---|---|
| Polyurethane foam (PU) | A product designed to eliminate oil and petroleum spills in industrial halls, garages, and underground car parks and in open areas |
| Coffee grounds | A product designed to eliminate oil and petroleum spills in industrial halls, garages, and underground car parks and in open areas |
| Viscose–cellulose sorbent | A product designed to collect water, oil, and petroleum contaminants from solid surfaces |
| Coconut fiber | Designed for collecting oil and petroleum contamination from solid surfaces. The product is used in the absorption of oils, gasoline, kerosene, cutting fluids, cooling fluids, emulsions, rinsing fluids and paints |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gniazdowska, J.; Rabajczyk, A.; Wilczyński, T.; Małozięć, D. The Potential of Waste-Derived Sorbents for Absorbing Petroleum Substances in Firefighting Operations. Materials 2025, 18, 3752. https://doi.org/10.3390/ma18163752
Gniazdowska J, Rabajczyk A, Wilczyński T, Małozięć D. The Potential of Waste-Derived Sorbents for Absorbing Petroleum Substances in Firefighting Operations. Materials. 2025; 18(16):3752. https://doi.org/10.3390/ma18163752
Chicago/Turabian StyleGniazdowska, Justyna, Anna Rabajczyk, Tomasz Wilczyński, and Daniel Małozięć. 2025. "The Potential of Waste-Derived Sorbents for Absorbing Petroleum Substances in Firefighting Operations" Materials 18, no. 16: 3752. https://doi.org/10.3390/ma18163752
APA StyleGniazdowska, J., Rabajczyk, A., Wilczyński, T., & Małozięć, D. (2025). The Potential of Waste-Derived Sorbents for Absorbing Petroleum Substances in Firefighting Operations. Materials, 18(16), 3752. https://doi.org/10.3390/ma18163752








