Quantum Chemical Investigation on the Material Properties of Al-Based Hydrides XAl2H2 (X = Ca, Sr, Sc, and Y) for Hydrogen Storage Applications
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
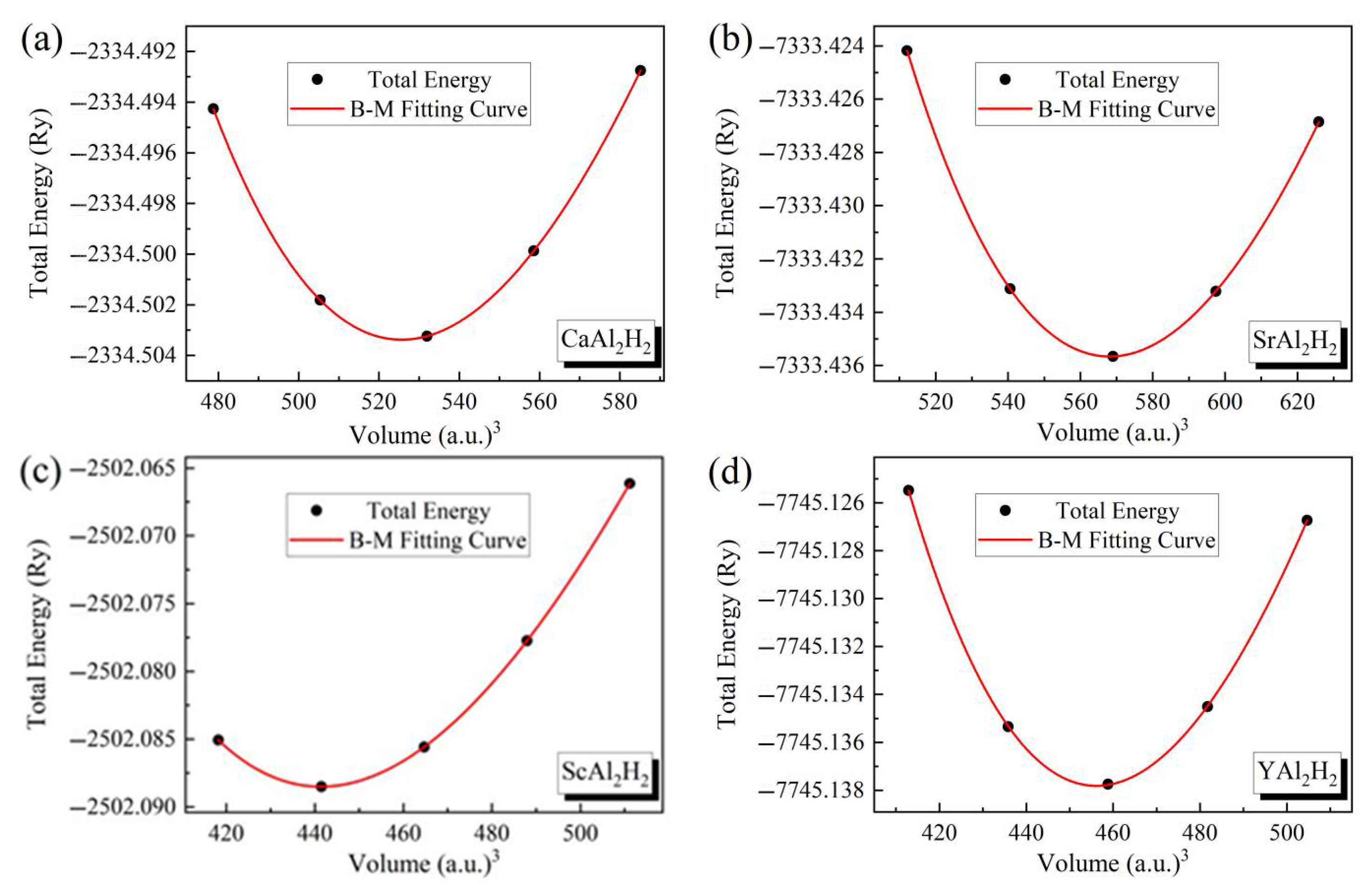
3.1. Structural and Hydrogen Storage Properties
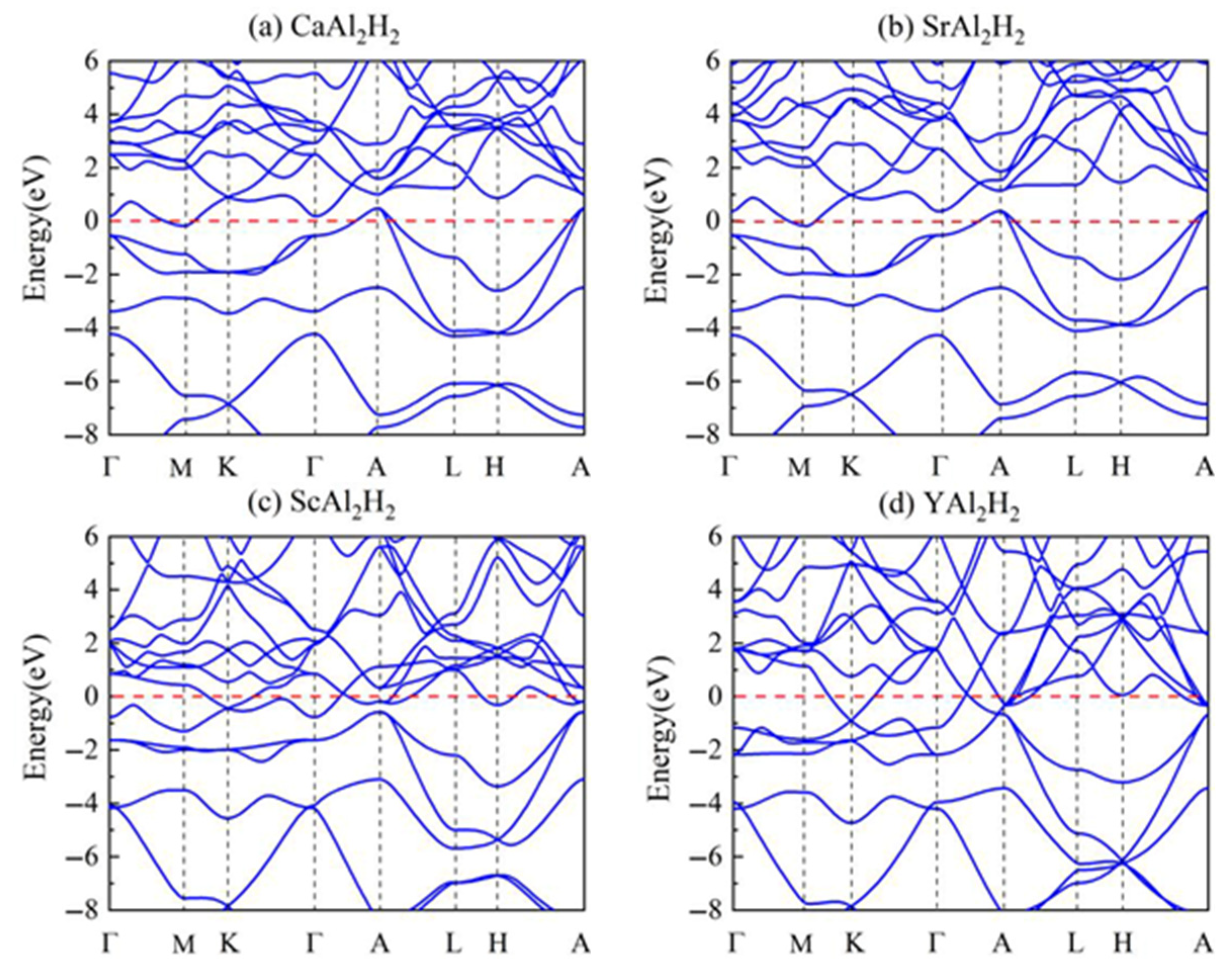
3.2. Electronic Properties
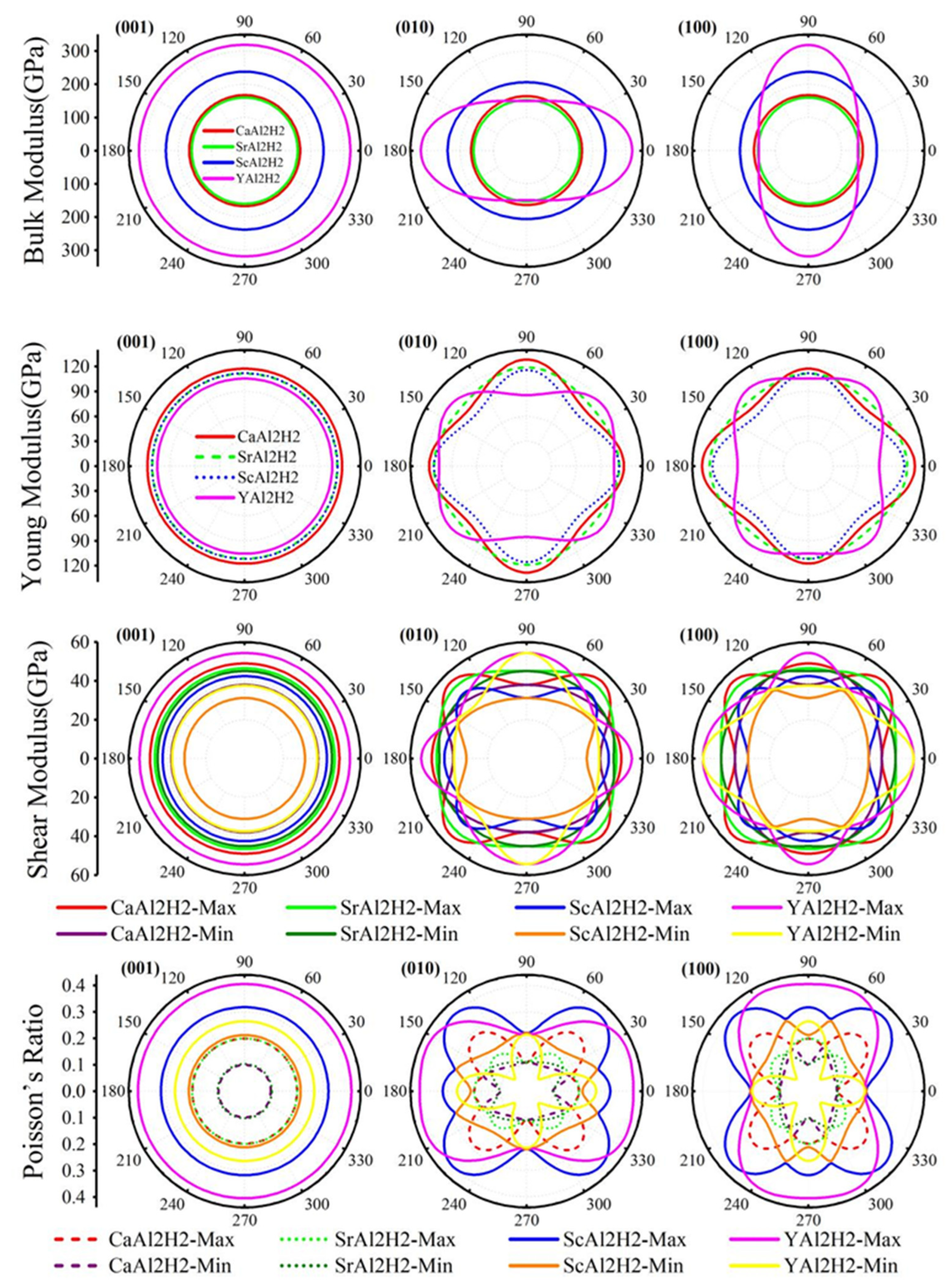
3.3. Mechanical Properties
3.4. Lattice Dynamical and Thermodynamic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, L.; Chen, K.; Jiang, J.; Yang, X.; Zhu, M. Hydrogen storage in light-metal based systems: A review. J. Alloys Compd. 2020, 829, 154597. [Google Scholar] [CrossRef]
- Murphy, R. What is undermining climate change mitigation? how fossil-fuelled practices challenge low-carbon transitions. Energy Res. Soc. Sci. 2024, 108, 103390. [Google Scholar] [CrossRef]
- Li, R.; Hu, F.; Xia, T.; Li, Y.; Zhao, X.; Zhu, J. Progress in the application of first principles to hydrogen storage materials. Int. J. Hydrogen Energy 2024, 56, 1079–1091. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Paul, A.R.; Mehla, S.; Bhargava, S. Intermetallic compounds for hydrogen storage: Current status and future perspectives. Small 2024, 20, 2408889. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, C.; Dong, S.; Liu, H.; Yang, W.; Li, Y.; Jiang, H.; Ding, Z.; Li, H.; et al. Unraveling the potential of solid-state hydrogen storage materials: Insights from first principle calculations. Fuel 2024, 373, 132340. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Hu, Q.; Si, N.; Zhang, C. Electrochemical Hydrogen Storage Materials: State-of-the-Art and Future Perspectives. Energy Fuels 2024, 38, 7579–7613. [Google Scholar] [CrossRef]
- Salman, M.; Rambhujun, N.; Pratthana, C.; Lai, Q.; Sapkota, P.; Aguey-Zinsou, K.F. Chapter 12-Solid-state hydrogen storage as a future renewable energy technology. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 263–287. [Google Scholar] [CrossRef]
- Ke, X.; Kuwabara, A.; Tanaka, I. Cubic and orthorhombic structures of aluminum hydride AlH3 predicted by a first-principles study. Phys. Rev. B 2005, 71, 184107. [Google Scholar] [CrossRef]
- Mei, Z.; Zhao, F.; Xu, S.; Ju, X. Theoretical investigations on the phase transition of pure and li-doped AlH3. RSC Adv. 2017, 7, 42024–42029. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effect of tixaly catalysts on hydrogen storage properties of LiAlH4 and LiAlH4. Int. J. Hydrogen Energy 2005, 30, 1417–1421. [Google Scholar] [CrossRef]
- Ianni, E.; Sofianos, M.V.; Rowles, M.R.; Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Synthesis of NaAlH4/Al composites and their applications in hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 17309–17317. [Google Scholar] [CrossRef]
- Weidenthaler, C. Crystal structure evolution of complex metal aluminum hydrides upon hydrogen release. J. Energy Chem. 2020, 42, 133–143. [Google Scholar] [CrossRef]
- Dragojlović, M.; Radaković, J.; Batalović, K. DFT study of crystal structure and electronic properties of metal-doped AlH3 polymorphs. Int. J. Hydrogen Energy 2022, 47, 6142–6153. [Google Scholar] [CrossRef]
- Xu, N.; Song, R.; Zhang, J.; Chen, Y.; Chen, S.; Li, S.; Jiang, Z.; Zhang, W. First-principles study on hydrogen storage properties of the new hydride perovskite XAlH3 (X=Na, K). Int. J. Hydrogen Energy 2024, 60, 434–440. [Google Scholar] [CrossRef]
- Umer, M.; Murtaza, G.; Ahmad, N.; Ayyaz, A.; Raza, H.H.; Usman, A.; Liaqat, A.; Manoharadas, S. First principles investigation of structural, mechanical, thermodynamic, and electronic properties of Al-based perovskites XAlH3 (X=K, Rb, Cs) for hydrogen storage. Int. J. Hydrogen Energy 2024, 61, 820–830. [Google Scholar] [CrossRef]
- Gingl, F.; Vogt, T.; Akiba, E. Trigonal SrAl2H2: The first Zintl phase hydride. J. Alloys Compd. 2000, 306, 127–132. [Google Scholar] [CrossRef]
- Ammi, H.; Charifi, Z.; Baaziz, H.; Ghellab, T.; Bouhdjer, L.; Adalla, S.; Ocak, H.Y.; Uğur, Ş.; Uğur, G. Investigation on the hydrogen storage properties, electronic, elastic, and thermodynamic of Zintl Phase Hydrides XGaSiH (X = sr, ca, ba). Int. J. Hydrogen Energy 2024, 87, 966–984. [Google Scholar] [CrossRef]
- Tang, F.; Chen, Y.; Yin, X.; Zhao, W.; Zhang, L.; Han, Z.; Zheng, R.; Zhang, X.; Fang, Y. Colossal magnetoresistance and Fermi surface topology in the layered Zintl-phase compound YbAl2Si2. Phys. Rev. B 2024, 110, 174408. [Google Scholar] [CrossRef]
- Björling, T.; Noréus, D.; Häussermann, U. Polyanionic hydrides from polar intermetallics AeE2 (Ae = Ca, Sr, Ba; E = Al, Ga, In). J. Am. Chem. Soc. 2006, 128, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Sankey, O.F.; Björling, T.; Moser, D.; Noréus, D.; Parker, S.T.; Häussermann, U. Vibrational properties of polyanionic hydrides SrAl2H2 and SrAlSiH: New insights into Al-H bonding interactions. Inorg. Chem. 2007, 46, 6987–6991. [Google Scholar] [CrossRef]
- Subedi, A.; Singh, D.J. Bonding in Zintl phase hydrides: Density functional calculations for SrAlSiH, SrAl2H2, SrGa2H2 and BaGa2H2. Phys. Rev. B 2008, 78, 045106. [Google Scholar] [CrossRef]
- Moser, D.; Häussermann, U.; Utsumi, T.; Björling, T.; Noréus, D. A series of BaAl2−xSixH2−x (0.4<x<1.6) hydrides with compositions and structures in between BaSi2 and BaAl2H2. J. Alloys Compd. 2010, 505, 1–5. [Google Scholar] [CrossRef]
- Iwaszczuk, J.; Baj, A.; Walejko, P. Sialic acids-structure and properties. Prospect. Pharm. Sci. 2024, 22, 31–38. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.; Marks, L. WIEN2k: An APW+lo program for calculating the properties of solids. J. Chem. Phys. 2020, 152, 074101. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Gay, D.M. Algorithm 611: Subroutines for unconstrained minimization using a model/trust-region approach. ACM Trans. Math. Softw. 1983, 9, 503–524. [Google Scholar] [CrossRef]
- Jamal, M.; Bilal, M.; Ahmad, I.; Jalali-Asadabadi, S. IRelast package. J. Alloys Compd. 2018, 753, 569–579. [Google Scholar] [CrossRef]
- Yalameha, S.; Nourbakhsh, Z.; Vashaee, D. ElATools: A tool for analyzing anisotropic elastic properties of the 2D and 3D materials. Comput. Phys. Commun. 2022, 271, 108195. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-principles determination of the soft mode in cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Song, R.; Chen, Y.; Chen, S.; Xu, N.; Zhang, W. First-principles to explore the hydrogen storage properties of XPtH3 (X=Li, Na, K, Rb) perovskite type hydrides. Int. J. Hydrogen Energy 2024, 57, 949–957. [Google Scholar] [CrossRef]
- Surucu, G.; Gencer, A.; Candan, A.; Gullu, H.H.; Isik, M. CaXH3 (X= Mn, Fe, Co) perovskite-type hydrides for hydrogen storage applications. Int. J. Energy Res. 2019, 44, 2345. [Google Scholar] [CrossRef]
- Masood, M.K.; Elaggoune, W.; Chaoui, K.; Bibi, S.; Khan, M.I.; Usman, M.; Alothman, A.A.; Rehman, J. The investigation of the physical properties of nickel-based hydrides and the prediction of their suitability for hydrogen storage application. Mat. Sci. Semicon. Proc. 2024, 186, 109094. [Google Scholar] [CrossRef]
- Murtaza, H.; Ain, Q.; Alshgari, R.A.; Akhter, T.; Munir, J. Exploring hydrogen storage attributes of alkali metal XNH6 (X=Li, Na, K) perovskite hydrides using DFT calculations. J. Power Sources 2025, 641, 236788. [Google Scholar] [CrossRef]
- Zhou, R.; Mo, X.; Huang, Y.; Hu, C.; Zuo, X.; Ma, Y.; Wei, Q.; Jiang, W. Dehydrogenation of Alkali Metal Aluminum Hydrides MAlH4 (M = Li, Na, K, and Cs): Insight from First-Principles Calculations. Batteries 2023, 9, 179. [Google Scholar] [CrossRef]
- Ammi, H.; Charifi, Z.; Baaziz, H.; Ghellab, T.; Bouhdjer, L.; Adalla, S. Electronic, elastic, and thermodynamic properties of complex hydrides XAlSiH (X = Sr, Ca, and Ba) intended for hydrogen storage: An ab-initio study. Phys. Scr. 2024, 99, 0659a2. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, T.; Li, S. The structural, electronic, mechanical, lattice dynamics and thermodynamic properties of Rh5B4: First-principles calculations. Mod. Phys. Lett. B 2017, 31, 1750245. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Hill, R. The Elastic behavior of a crystalline aggregate. Proc. Phys. Soc. 1952, 65, 349. [Google Scholar] [CrossRef]
- Murtaza, H.; Ain, Q.; Jbara, A.S.; Munir, J.; Aldwayyan, A.S.; Ghaithan, H.M.; Ahmed, A.A.A.; Qaid, S.M. The prediction of hydrogen storage capacity and solar water splitting applications of Rb2AlXH6 (X= In, Tl) perovskite halides: A DFT study. J. Phys. Chem. Solids 2025, 198, 112427. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Connétable, D.; Thomsa, O. First-principles study of the structural, electronic, vibrational, and elastic properties of orthorhombic NiSi. Phys. Rev. B 2009, 79, 094101. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, F.; Lu, Y.; Liu, Z.; Liu, W.; Liu, Q. First-principles calculations of the structural, mechanical, electronic, and optical properties of BaX2 (X=O, S, Se and Te) compounds. Mat. Sci. Semicon. Proc. 2022, 147, 106755. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef]
- Hahn, T. Space Group Symmetry; Springer: Berlin, Germany, 2005; Volume A. [Google Scholar]
- Vajeeston, P.; Fjellvg, H. Revised electronic structure, Raman and IR studies of AB2H2 and ABCH (A = Sr, Ba; B = Al, Ga; C = Si, Ge) phases. RSC Adv. 2014, 4, 22–31. [Google Scholar] [CrossRef]
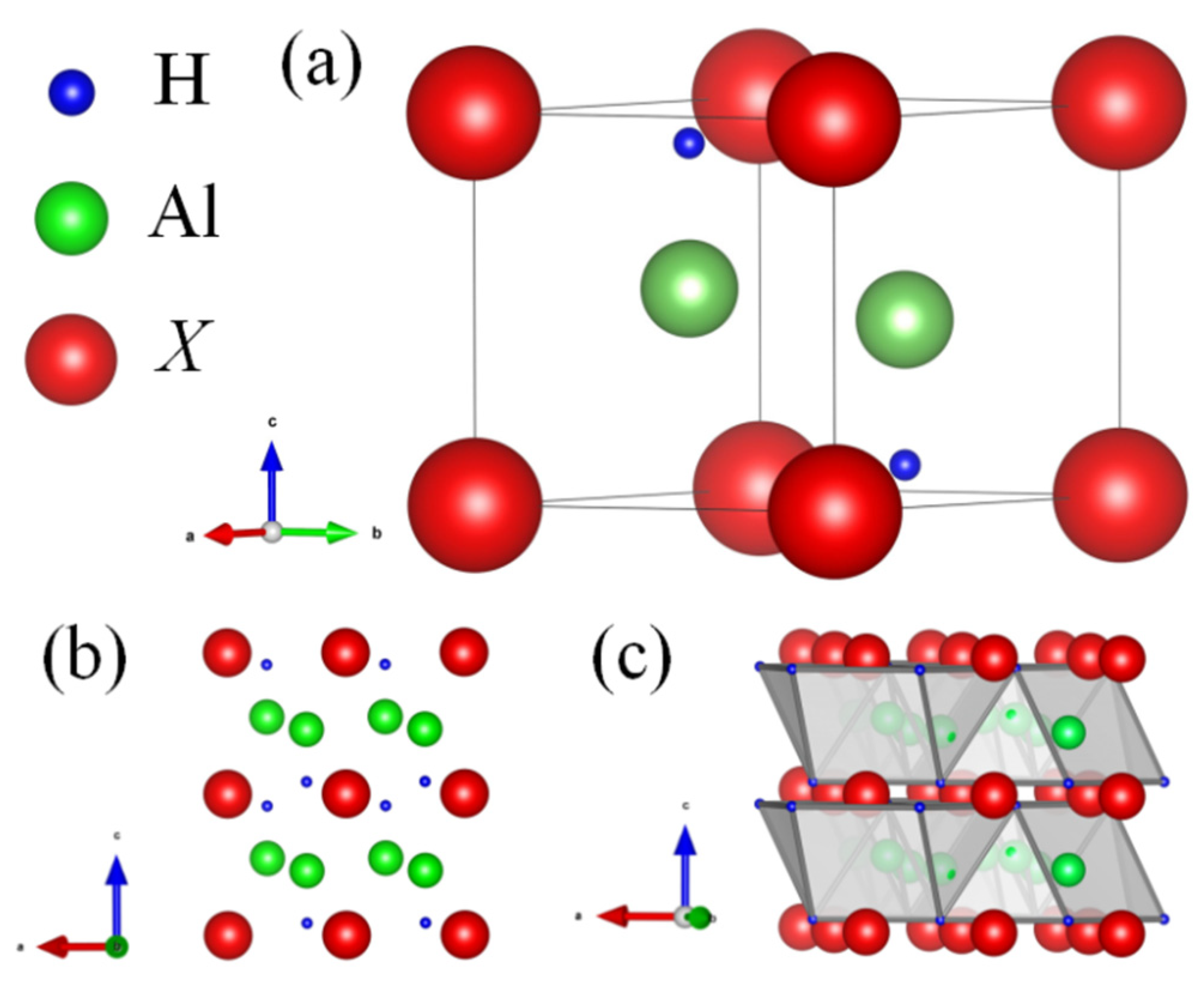




| Compounds | Al | H | dAl-H (Å) | Refs. |
|---|---|---|---|---|
| CaAl2H2 | (1/3, 2/3, 0.456027) | (1/3, 2/3, 0.085312) | 1.697 | Present |
| SrAl2H2 | (1/3, 2/3, 0.460439) | (1/3, 2/3, 0.097497) | 1.716 | Present |
| SrAl2H2 | (1/3, 2/3, 0.4589) | (1/3, 2/3, 0.0976) | 1.71 | Exp. [17] |
| SrAl2H2 | (1/3, 2/3, 0.4608) | (1/3, 2/3, 0.0964) | 1.721 | Theo. [22] |
| ScAl2H2 | (1/3, 2/3, 0.441807) | (1/3, 2/3, 0.033534) | 1.720 | Present |
| YAl2H2 | (1/3, 2/3, 0.500094) | (1/3, 2/3, 0.000166) | 2.067 | Present |
| Compounds | a | c | c/a | V0 | B0 | B’ | ΔH | Cwt% | Tdes | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| (Å) | (Å) | (Å3) | (GPa) | (eV/f.u.) | (K) | |||||
| CaAl2H2 | 4.4328 | 4.5773 | 1.03 | 77.90 | 55.16 | 3.80 | −1.46 | 1.41 | 1076 | Present |
| SrAl2H2 | 4.5323 | 4.7395 | 1.05 | 84.18 | 51.64 | 3.93 | −1.80 | 0.94 | 1326 | Present |
| SrAl2H2 | 4.5283 | 4.7215 | 1.04 | Exp. [17] | ||||||
| SrAl2H2 | 4.528 | 4.722 | 1.04 | Theo. [22] | ||||||
| ScAl2H2 | 4.2343 | 4.2123 | 0.99 | 65.41 | 76.01 | 3.94 | −0.47 | 1.34 | 349 | Present |
| YAl2H2 | 4.3440 | 4.1364 | 0.95 | 67.60 | 74.82 | 4.01 | −1.33 | 0.93 | 983 | Present |
| Compounds | C11 | C12 | C13 | C33 | C44 | C66 | Refs. |
|---|---|---|---|---|---|---|---|
| CaAl2H2 | 124.21 | 26.30 | 16.31 | 131.92 | 37.94 | 48.96 | Present |
| SrAl2H2 | 118.20 | 25.15 | 15.71 | 122.46 | 45.09 | 46.52 | Present |
| ScAl2H2 | 137.37 | 52.43 | 41.60 | 133.95 | 31.16 | 42.47 | Present |
| YAl2H2 | 146.95 | 71.84 | 46.83 | 105.56 | 54.40 | 37.54 | Present |
| CaAlSiH | 175.6237 | 39.1212 | 23.7807 | 94.061 | 43.1177 | 68.25125 | Theo. [38] |
| SrAlSiH | 163.2624 | 32.5895 | 27.2531 | 97.4601 | 46.2550 | 65.3364 | Theo. [38] |
| Present Work | Other Al-Base Hydrides | |||||||
| Compounds | CaAl2H2 | SrAl2H2 | ScAl2H2 | YAl2H2 | CaAlSiH [38] | SrAlSiH [38] | Rb2AlTlH6 [42] | NaAlH3 [15] |
| B (GPa) | 55.35 | 52.44 | 75.47 | 79.15 | 65.350 | 64.468 | 27.4 | 43.669 |
| G (GPa) | 45.77 | 47.38 | 38.53 | 43.97 | 53.091 | 52.919 | 18.2 | 8.702 |
| E (GPa) | 107.64 | 109.23 | 98.78 | 111.30 | 125.332 | 124.650 | 44.6 | 24.479 |
| B/G | 1.21 | 1.11 | 1.96 | 1.80 | 1.2309 | 1.2182 | 1.50 | 5.018 |
| G/B | 0.83 | 0.90 | 0.51 | 0.56 | ||||
| ν | 0.176 | 0.152 | 0.281 | 0.265 | 0.180 | 0.177 | 0.23 | 0.407 |
| A | 0.775 | 0.969 | 0.734 | 1.450 | 0.72 | |||
| ρ | 2.05 | 2.83 | 2.56 | 3.56 | ||||
| θD (K) | 620.6 | 521.9 | 546.0 | 488.6 | 606.615 | 502.165 | 208.18 | |
| vt (m/s) | 4727.66 | 4089.59 | 3877.55 | 3514.88 | 4756.12 | 4048.46 | 1870.3 | |
| vl (m/s) | 7538.60 | 6388.36 | 7035.45 | 6221.7 | 7616.09 | 6466.87 | 3160.11 | |
| vm (m/s) | 5206.06 | 4492.98 | 4321.33 | 3909.41 | 5239.81 | 4458.98 | 2071.09 | |
| The Point Group: O3d (−3m) | |||
| Mode | CaAl2H2 | SrAl2H2 | SrAl2H2 [48] |
| A2u (IR) | 132, 1397 | 134, 1332 | 132, 1333 |
| Eu (IR) | 121, 414 | 135, 541 | 143, 593 |
| A1g (R) | 247, 1466 | 254, 1406 | 267, 1412 |
| Eg (R) | 376, 670 | 348, 735 | 377, 765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Guo, R.; Wan, L.; Zhang, Y. Quantum Chemical Investigation on the Material Properties of Al-Based Hydrides XAl2H2 (X = Ca, Sr, Sc, and Y) for Hydrogen Storage Applications. Materials 2025, 18, 3521. https://doi.org/10.3390/ma18153521
Guo Y, Guo R, Wan L, Zhang Y. Quantum Chemical Investigation on the Material Properties of Al-Based Hydrides XAl2H2 (X = Ca, Sr, Sc, and Y) for Hydrogen Storage Applications. Materials. 2025; 18(15):3521. https://doi.org/10.3390/ma18153521
Chicago/Turabian StyleGuo, Yong, Rui Guo, Lei Wan, and Youyu Zhang. 2025. "Quantum Chemical Investigation on the Material Properties of Al-Based Hydrides XAl2H2 (X = Ca, Sr, Sc, and Y) for Hydrogen Storage Applications" Materials 18, no. 15: 3521. https://doi.org/10.3390/ma18153521
APA StyleGuo, Y., Guo, R., Wan, L., & Zhang, Y. (2025). Quantum Chemical Investigation on the Material Properties of Al-Based Hydrides XAl2H2 (X = Ca, Sr, Sc, and Y) for Hydrogen Storage Applications. Materials, 18(15), 3521. https://doi.org/10.3390/ma18153521






