The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstocks and Additional Materials
2.2. Physico-Chemical Analysis of Substrates and Carriers
2.2.1. Feedstock, Inoculum, and Batch Analysis
2.2.2. Analysis of Carrier Materials
2.3. Experimental Setup for Biogas Generation
2.3.1. Sample Preparation Procedure
2.3.2. Anaerobic Digestion Procedure
2.3.3. Physico-Chemical Analysis of Samples
2.3.4. Microbiological Analysis of Samples
3. Results and Discussion
3.1. Physico-Chemical Properties of Carrier Materials
3.1.1. Morphological and Microstructural Properties
3.1.2. BET Surface Area and Pore Structures
3.1.3. Elemental Analysis and FTIR Spectra
3.1.4. DSC
3.2. Bacterial Community Abundance and Composition
3.3. Physico-Chemical Analysis of Sludge Samples and Biogas Performance
3.4. Literature Review and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABET | Brunauer–Emmett–Teller (BET) surface area |
| AD | Anaerobic digestion |
| ASVs | Amplicon sequence variants |
| BJH | Barrett–Joyner–Halenda method for calculating pore size distributions |
| CE | Cheese |
| DSC | Differential scanning calorimetry |
| EDS | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier–transform infrared spectroscopy |
| MU | Measurement uncertainty |
| NGS | Next-generation sequencing |
| PCR | Polymerase chain reaction |
| PdI | Polydispersity index |
| RDP | Ribosomal Database Project |
| SEM | Scanning electron microscope |
| Sp | Pore diameter |
| SSA | Specific surface area |
| TA | Total alkalinity |
| TCD | Thermal conductivity detector |
| TD | Dehydration temperature |
| TG/DTA | Thermogravimetric/differential thermal analysis |
| TS | Total solids |
| VFA | Volatile fatty acids |
| Vp | Pore volume |
| VS | Volatile solids |
| WF–control | Vample of waste wafers |
| WF–Ch/P | Sample of waste wafers with carrier Ch/P |
| WF–Ch/P | Sample of waste wafers and waste cheese |
| WFC–Ch/P | Sample of waste wafers and waste cheese with carrier Ch/P |
| WF–Control 1, WF–Ch/P 1, WFC–Control 1, WFC–Ch/P 1 | Samples collected from the first stage (1) of AD |
| WF–Control 2, WF–Ch/P 2, WFC–Control 2, WFC–Ch/P 2 | Samples collected from the last stage (2) of AD |
References
- Wang, T.; Wang, J.; Jiazi Niu, J.; Guo, P.; Peng, C.; He, R.; Hui, Z.; Gao, W.; Zhang, Q. Synchronous improvement of methane production and digestate dewaterability in sludge anaerobic digestion by nanobubble. Bioresour. Technol. 2024, 402, 130791. [Google Scholar] [CrossRef]
- Assis, T.I.; Gonçalves, R.F. Valorization of food waste by anaerobic digestion: A bibliometric and systematic review focusing on optimization. J. Environ. Manag. 2022, 320, 115763. [Google Scholar] [CrossRef]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Reprint of Organic waste conversion through anaerobic digestion: A critical insight into the metabolic pathways and microbial interactions. Metab. Eng. 2022, 17, 62–76. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, H.; Zheng, D.; Pu, X.; Liu, Y.; Wang, L.; Zhang, Y.; Deng, L. Methanogenic activity and microbial communities characteristics in dry and wet anaerobic digestion sludges from swine manure. Biochem. Eng. J. 2019, 15, 107390. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Jia, H.; Yong, X.; Zhang, L.; Zhou, J.; Cao, Z.; Kruse, A.; Wei, P. Effects of different biofilm carriers on biogas production during anaerobic digestion of corn straw. Bioresour. Technol. 2017, 244, 445–451. [Google Scholar] [CrossRef]
- Jin, H.J.; Yao, X.Y.; Tang, C.C.; Zhou, A.J.; Liu, W.; Ren, Y.X.; Li, Z.; Wang, A.; He, Z.W. Magnetite modified zeolite as an alternative additive to promote methane production from anaerobic digestion of waste activated sludge. Renew. Energy 2024, 224, 120181. [Google Scholar] [CrossRef]
- Langxian, S.; Lintong, Z.; Xin, Y.; Maoyou, Y.; Jialin, L.; Minchun, H.; Xidan, F.; Lianhua, L. Dual roles in interspecies electron transfer of carbon-based materials for accelerating anaerobic digestion of food waste. Biochem. Eng. J. 2024, 203, 109182. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Adamski, M.; Zaborowicz, M.; Dorota Cais-Sokolińska, D.; Wolna-Maruwka, A.; Niewiadomska, A. Eco-friendly and effective diatomaceous earth/peat (DEP) microbial carriers in the anaerobic biodegradation of food waste products. Energies 2022, 15, 3442. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Jarosław Grządziel, J.; Gałązka, A.; Paluch, E.; Borowiak, K.; Pilarski, K. Quantitative and qualitative changes in the genetic diversity of bacterial communities in anaerobic bioreactors with the diatomaceous earth/peat cell carrier. Cells 2022, 11, 2571. [Google Scholar] [CrossRef]
- Fenice, M.; Gorrasi, S. Advances in chitin and chitosan science. Molecules 2021, 26, 1805. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with natural additives as a potential food packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Baselga, J.; Florian, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Li, B.; Shi, Y.; Shan, C.; Zhou, Q.; Ibrahim, M.; Wang, Y.; Wu, G.; Li, H.; Xie, G.; Sun, G. Effect of chitosan solution on the inhibition of Acidovoraxcitrulli causing bacterial fruit blotch of watermelon. J. Sci. Food Agric. 2013, 93, 1010–1015. [Google Scholar] [CrossRef]
- Bonde, S.; Chandarana, C.; Prajapati, P.; Vashi, V. A comprehensive review on recent progress in chitosan composite gels for biomedical uses. Int. J. Biol. Macromol. 2024, 272, 132723. [Google Scholar] [CrossRef]
- Olajire, A.A.; Bamigbade, L.A. Green synthesis of chitosan-based iron@ silver nanocomposite as adsorbent for wastewater treatment. Water Res. Ind. 2021, 26, 100158. [Google Scholar] [CrossRef]
- Yin, M.; Chen, H. Unveiling the dual faces of chitosan in anaerobic digestion of waste activated sludge. Bioresour. Technol. 2022, 344, 126182. [Google Scholar] [CrossRef]
- Nie, W.; Lin, Y.; Wu, X.; Wu, S.; Li, X.; Cheng, J.J.; Yang, C. Chitosan-Fe3O4 composites enhance anaerobic digestion of liquor wastewater under acidic stress. Bioresour. Technol. 2023, 377, 128927. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Biogas production from wastewater: Comparing biostimulation impact of magnetised-chitosan and-titania chitosan. Mater. Today Proc. 2022, 62, S85–S90. [Google Scholar] [CrossRef]
- Yilmazer, S.; Ozdeniz, M.B. The effect of moisture content on sound absorption of expanded perlite plates. Build. Environ. 2005, 40, 311–318. [Google Scholar] [CrossRef]
- Ates, A.; Altintig, E.; Demirel, H.; Yilmaz, M. Comparative study on adsorptive removal of Cu, Pb, Zn heavy metals by modified perlite composites. Desalin. Water Treat. 2017, 98, 244–253. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Masoumi, H.; Ghaemi, A. On the performance of perlite as a mineral adsorbent for heavy metals ions and dye removal from industrial wastewater: A review of the state of the art. Case Stud. Chem. Environ. Eng. 2023, 8, 100385. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, G.; Zhang, Y.; Gao, Y.; Li, Z. The influence of expanded perlite as a bio-carrier on the freeze-thaw properties of self-healing concreto. Constr. Build. Mater. 2023, 409, 133891. [Google Scholar] [CrossRef]
- Ivankovic, T.; Kontek, M.; Mihalic, V.; Ressler, A.; Jurisic, V. Perlite as a biocarrier for augmentation of biogas-producing reactors from olive (Olea europaea) waste. Appl. Sci. 2022, 12, 8808. [Google Scholar] [CrossRef]
- Vijaya, Y.; Subbaiah, M.V.; Reddy, A.S.; Krishnaiah, A. Equilibrium and kinetic studies of fluoride adsorption by chitosan coated perlite. Desalin. Water Treat. 2010, 20, 272–280. [Google Scholar] [CrossRef]
- Demirçivi, P. Synthesis and characterization of Zr(IV) doped immobilized cross-linked chitosan/perlite composite for acid orange II adsorption. Int. J. Biol. Macromol. 2018, 118, 340–346. [Google Scholar] [CrossRef]
- Farrokhi, Z.; Sadjadi, S.; Raouf, F.; Bahri-Laleh, N. Novel bio-based Pd/chitosan-perlite composite bead as an efficient catalyst for rapid decolorization of azo dye. Inorg. Chem. Comm. 2022, 143, 109734. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef]
- Cabuk, M. Electrorheological properties of biodegradable chitosan/expanded perlite composites. JOTCSA 2016, 3, 119–132. [Google Scholar] [CrossRef]
- Norm VDI 4630; Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Engineers Club: Düsseldorf, Germany, 2006.
- ISO. Guide to the Expression of Uncertainty in Measurement (GUM); ISO: Geneva, Switzerland, 1993. [Google Scholar]
- DIN Guideline 38 414-S8; Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Institute for Standardization: Berlin, Germany, 1985.
- Pilarska, A.A.; Marzec-Grządziel, A.; Paluch, E.; Pilarski, K.; Wolna-Maruwka, A.; Kubiak, A.; Kałuża, T.; Kulupa, T. Biofilm formation and genetic diversity of microbial communities in anaerobic batch reactor with polylactide (PLA) addition. Int. J. Mol. Sci. 2023, 24, 10042. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, Q.; Cole, J.R. Updated RDP taxonomy and RDP Classifier for more accurate taxonomic classification. Microbiol. Resour. Announc. 2024, 13, e0106323-23. [Google Scholar] [CrossRef]
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 2018, 6, 140. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. cPhyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Sweah, Z.J.; Malk, F.H.; Hussain, W.A. Determination of the Optical Parameter from Chitosan Doping with Nicotine. AIP Conf. Proc. 2020, 2213, 020065. [Google Scholar] [CrossRef]
- Hasan, S.; Ghosh, T.K.; Boddu, V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008, 152, 826–837. [Google Scholar] [CrossRef]
- Swayampakula, K.; Boddu, V.M.; Abburi, K. Competitive adsorption of Cu (II), Co (II) and Ni (II) from their binary and tertiary aqueous solutions using chitosan–coated perlite beads as biosorbent. J. Hazard. Mater. 2009, 170, 680–689. [Google Scholar] [CrossRef]
- Patil, S.B.; Sawant, K.K. Chitosan microspheres as a delivery system for nasal insufflation. Colloids Surf. B Biointerfaces 2011, 84, 384–389. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2017, 43, 3527–3537. [Google Scholar] [CrossRef]
- Vaezifar, S.; Razavi, S.; Golozar, M.A.; Karbasi, S.; Morshed, M.; Kamali, M. Effects of some parameters on particle size distribution of chitosan nanoparticles prepared by ionic gelation method. J. Clust. Sci. 2013, 24, 891–903. [Google Scholar] [CrossRef]
- Dhawade, P.P.; Jagtap, R.N. Characterization of the glass transition temperature of chitosan and its oligomers by temperature modulated differential scanning calorimetry. Adv. Appl. Sci. Res. 2012, 3, 1372–1382. [Google Scholar]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Conv. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Major, N.; Sørensen, S.J.S.J.; Ban, D.; Nesme, J.; Grosch, R.; Ban, S.G.; Schikora, A.; Cerne, M.; Schierstaedt, J. Influence of sewage sludge stabilization method on microbial community and the abundance of antibiotic resistance genes. Waste Manag. 2022, 154, 126–135. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Microbiota of anaerobic digesters in a full-scale wastewater treatment plant. Arch. Environ. Prot. 2017, 43, 53–60. [Google Scholar]
- Deng, P.; Wang, L.; Li, X.; Zhang, J.; Jiang, H. Geobacter grbiciae—A new electron donor in the formation of co-cultures via direct interspecies electron transfer. Microbiol. Res. 2023, 14, 1774–1787. [Google Scholar] [CrossRef]
- Liu, S.; Liang, D.; Wang, Y.; He, W.; Feng, Y. Impact of carrier capacitance on Geobacter enrichment and direct interspecies electron transfer under anaerobic conditions. Bioresour. Technol. 2025, 419, 132079. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Tian, L.; Yan, X.; Du, L.; Leininger, A.; Zhang, M.; Li, N.; Ren, Z.J.; Wang, X. DIET-like mutualism of Geobacter and methanogens at specific electrode potential boosts production of both methane and hydrogen from propionate. Water Res. 2023, 235. Available online: https://www.sciencedirect.com/journal/water-research/vol/235/suppl/C119911 (accessed on 10 August 2024). [CrossRef]
- Hu, P.; Xiao, M.; Wang, N.; Zhang, S.; Shi, J.; Shi, J.; Tang, T.; Liu, L. Metagenome reveals the possible mechanism that microbial strains promote methanogenesis during anaerobic digestion of food waste. Environ. Res. 2024, 251, 118723. [Google Scholar] [CrossRef]
- Matheri, A.N.; Eloko, N.S.; Ntuli, F.; Ngila, J.C. Influence of pyrolyzed sludge use as an adsorbent in removal of selected trace metals from wastewater treatment. Case Stud. Chem. Environ. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Hantoko, D.; Kanchanatip, A.E.; Yan, M.; Weng, Z.; Gao, Z.; Zhong, Y. Assessment of sewage sludge gasification in supercritical water for H2-rich syngas production. Process Saf. Environ. Prot. 2019, 131, 63–72. [Google Scholar] [CrossRef]
- Mathaba, M.; Daramola, M.O. Effect of chitosan’s degree of deacetylation on the performance of PES membrane infused with chitosan during AMD treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Sikorski, D.; Gzyra-Jagieła, K.; Draczyński, Z. The kinetics of chitosan degradation in organic acid solutions. Mar. Drugs 2021, 19, 236. [Google Scholar] [CrossRef]
- Mohammadnezhad, J.; Khodabakhshi-Soreshjani, F.; Bakhshi, H. Preparation and evaluation of chitosan-coated eggshell particles as copper(II) biosorbent. Desalin. Water Treat. 2016, 57, 1693–1704. [Google Scholar] [CrossRef]
- Dandil, S.; Sahbaz, D.A.; Acikgoz, C. Adsorption of Cu(II) ions onto crosslinked chitosan/Waste Active Sludge Char (WASC) beads: Kinetic, equilibrium, and thermodynamic study. Int. J. Biol. Macromol. 2019, 136, 668–675. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, G.; Jianga, C.; Li, Z. Sugar-coated expanded perlite as a bacterial carrier for crack-healing concrete applications. Constr. Build. Mater. 2020, 232, 117222. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, W.; Li, Z.; Jia, G.; Zhang, Y.; Ma, G.; Gao, Y. Mechanical properties and frost resistance of self-healing concrete based on expanded perlite with different particle sizes as microbial carrier. Constr. Build. Mater. 2024, 422, 135450. [Google Scholar] [CrossRef]
- Sahbaza, D.A.; Acikgoz, C. Adsorption of a textile dye Ostazin Black NH from aqueous solution onto chitosan-coated perlite beads. Desalin. Water Treat. 2017, 67, 332–338. [Google Scholar] [CrossRef]
- Zamrisham, N.A.F.; Idrus, S.; Harun, M.R.; Ab Razak, M.S.; Jaman, K. Biogas production by integrating lava rock, red clay & ceramic bio ring as support carrier in treatment of landfill leachate with liquidised food waste. Biochem. Eng. J. 2024, 204, 109221. [Google Scholar] [CrossRef]


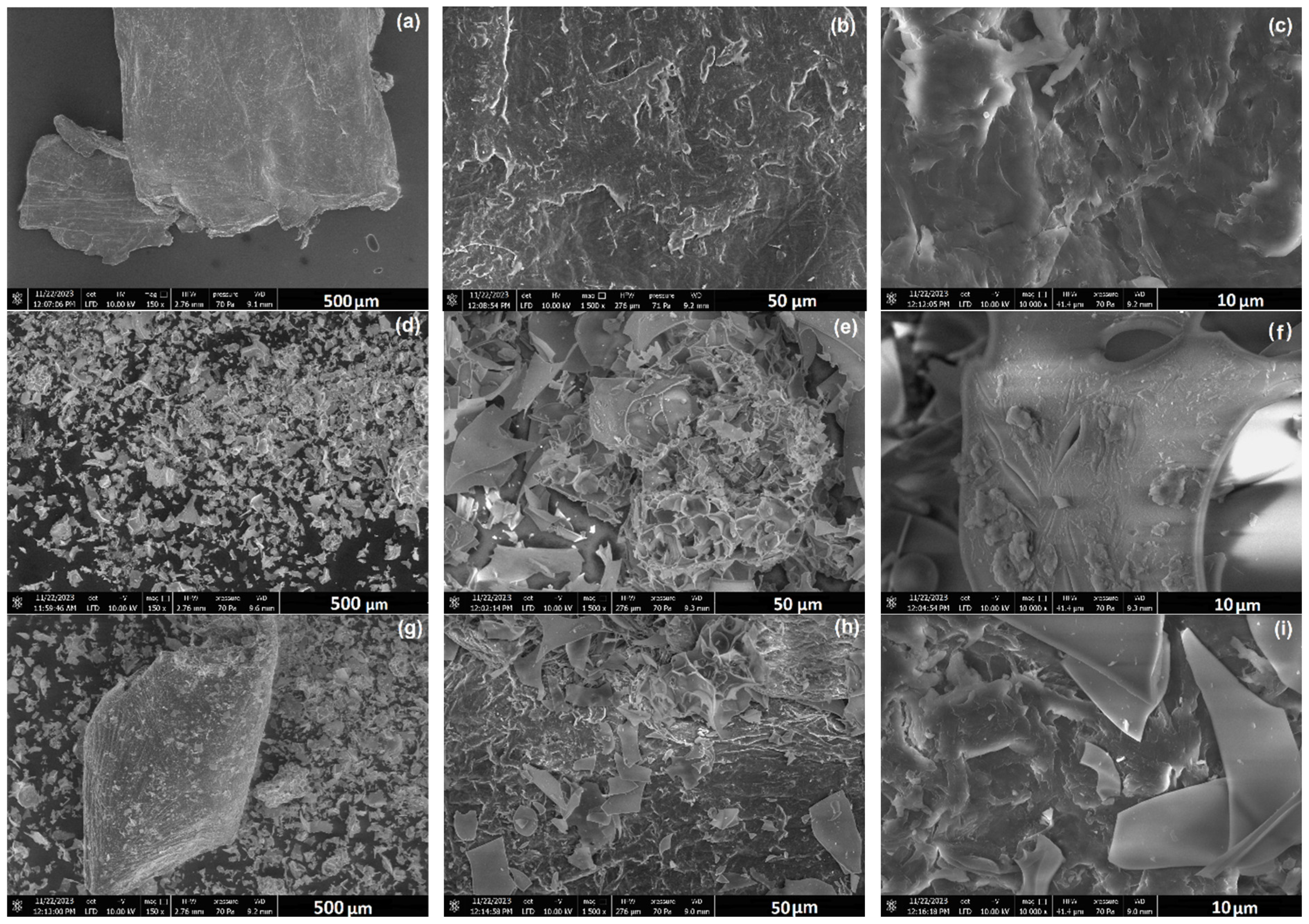

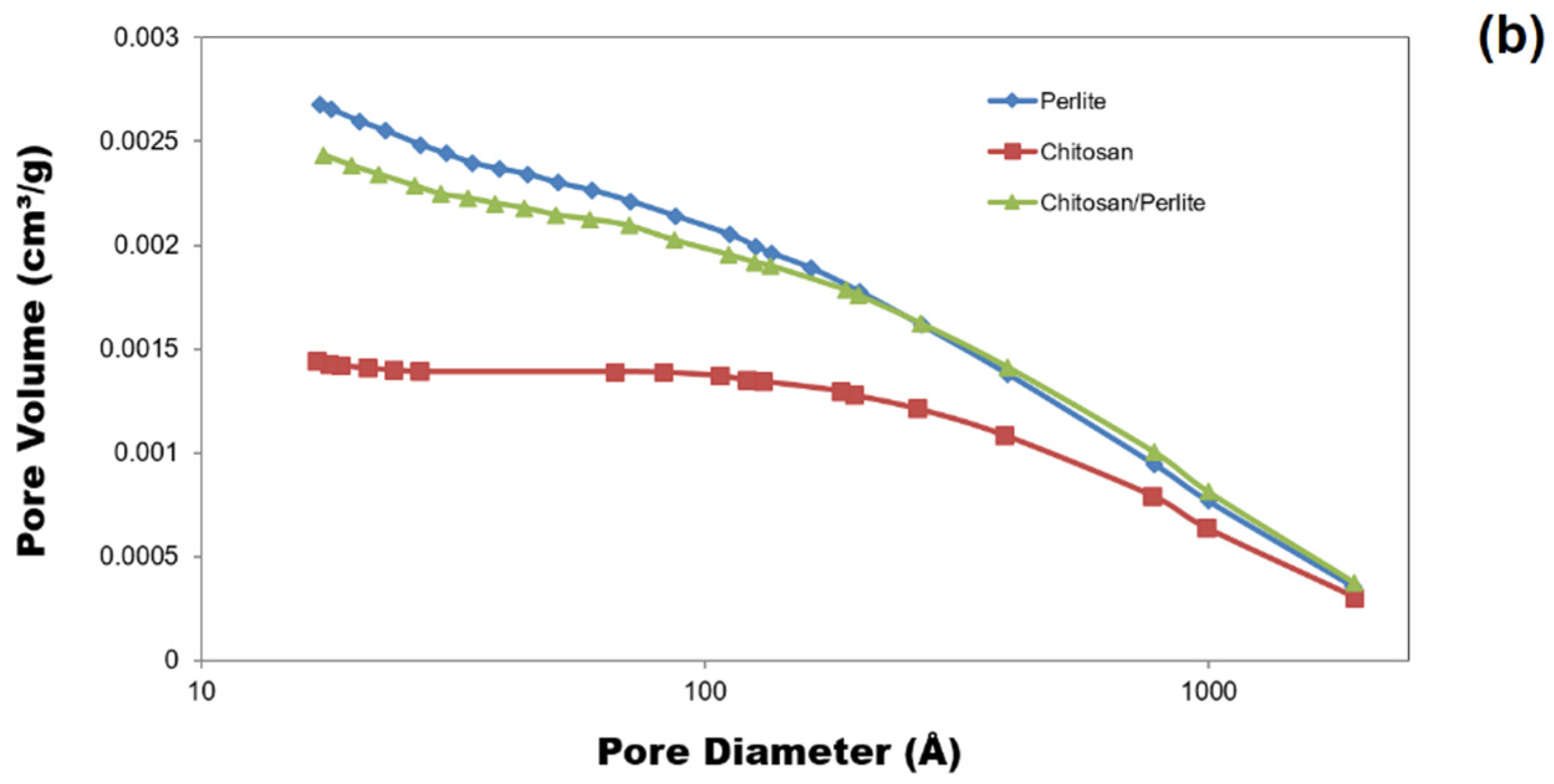

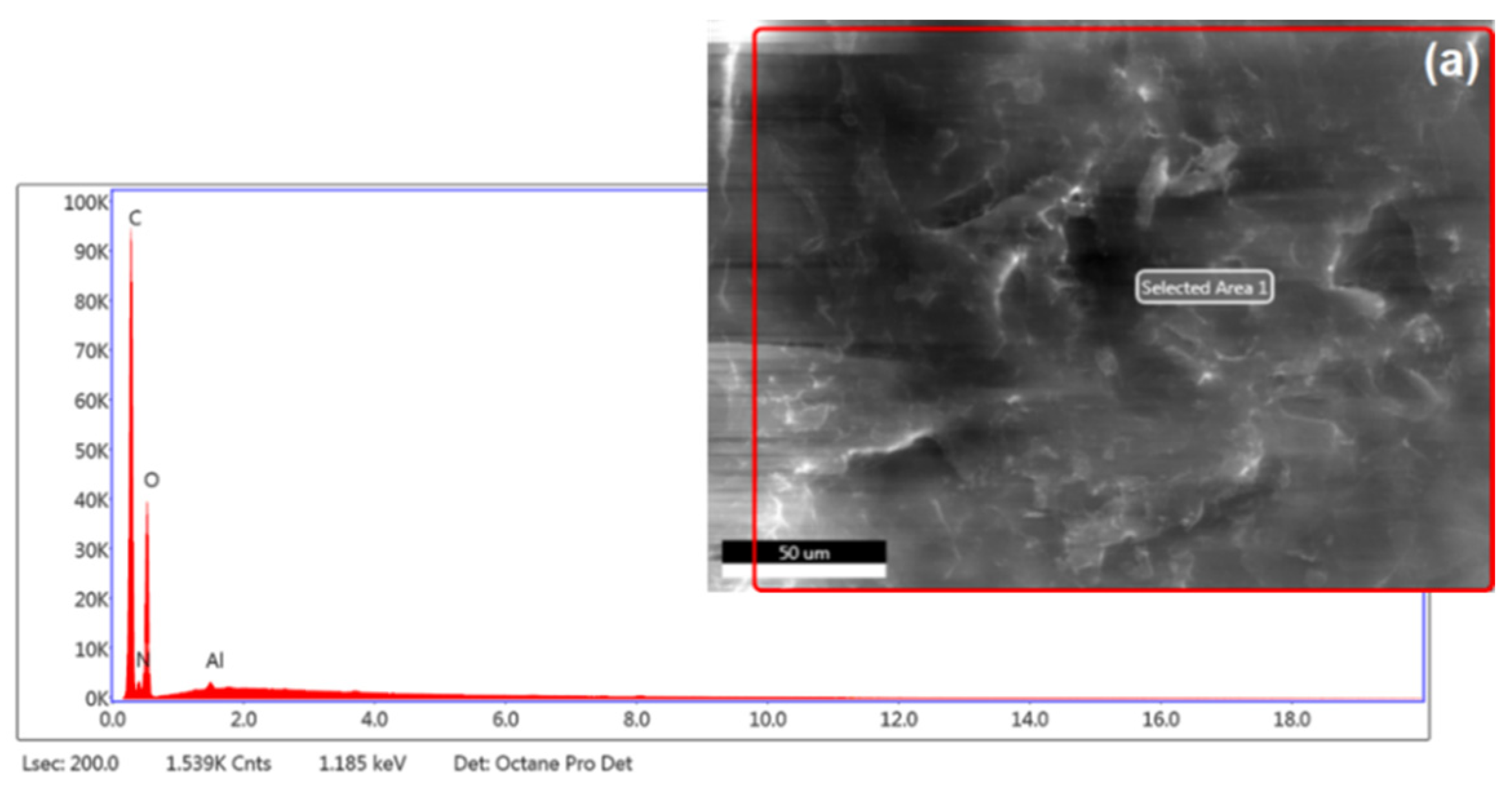
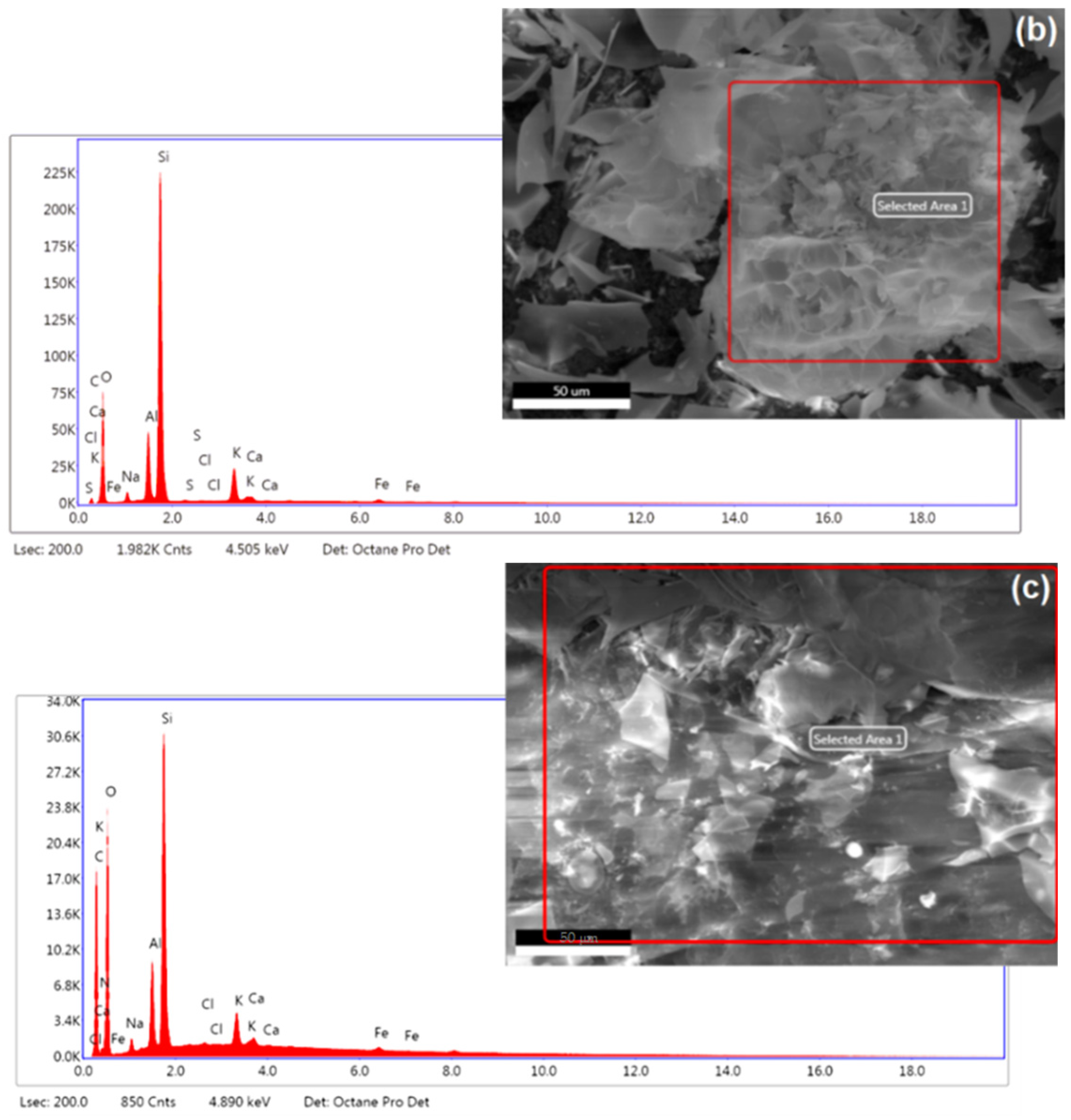

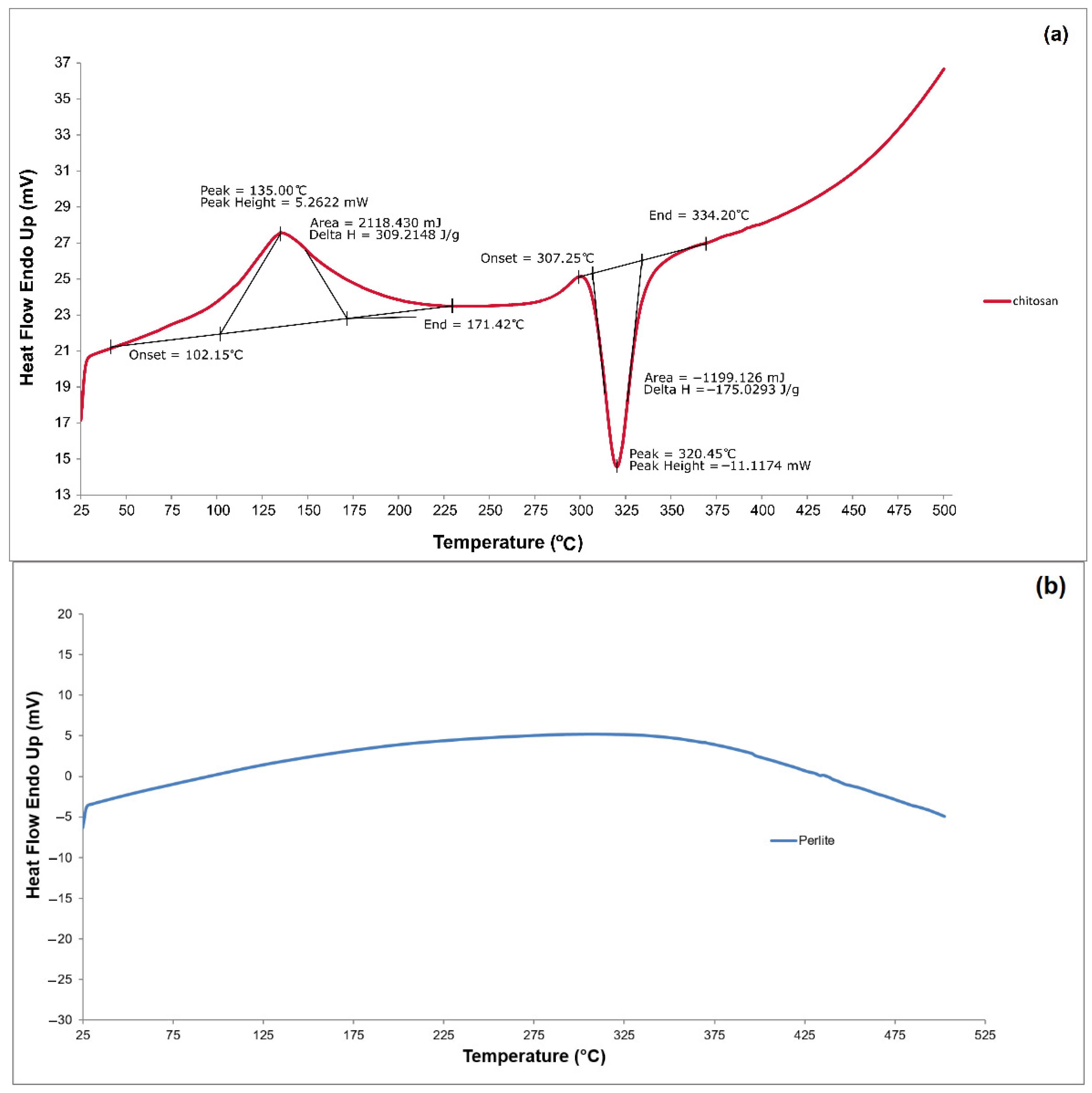
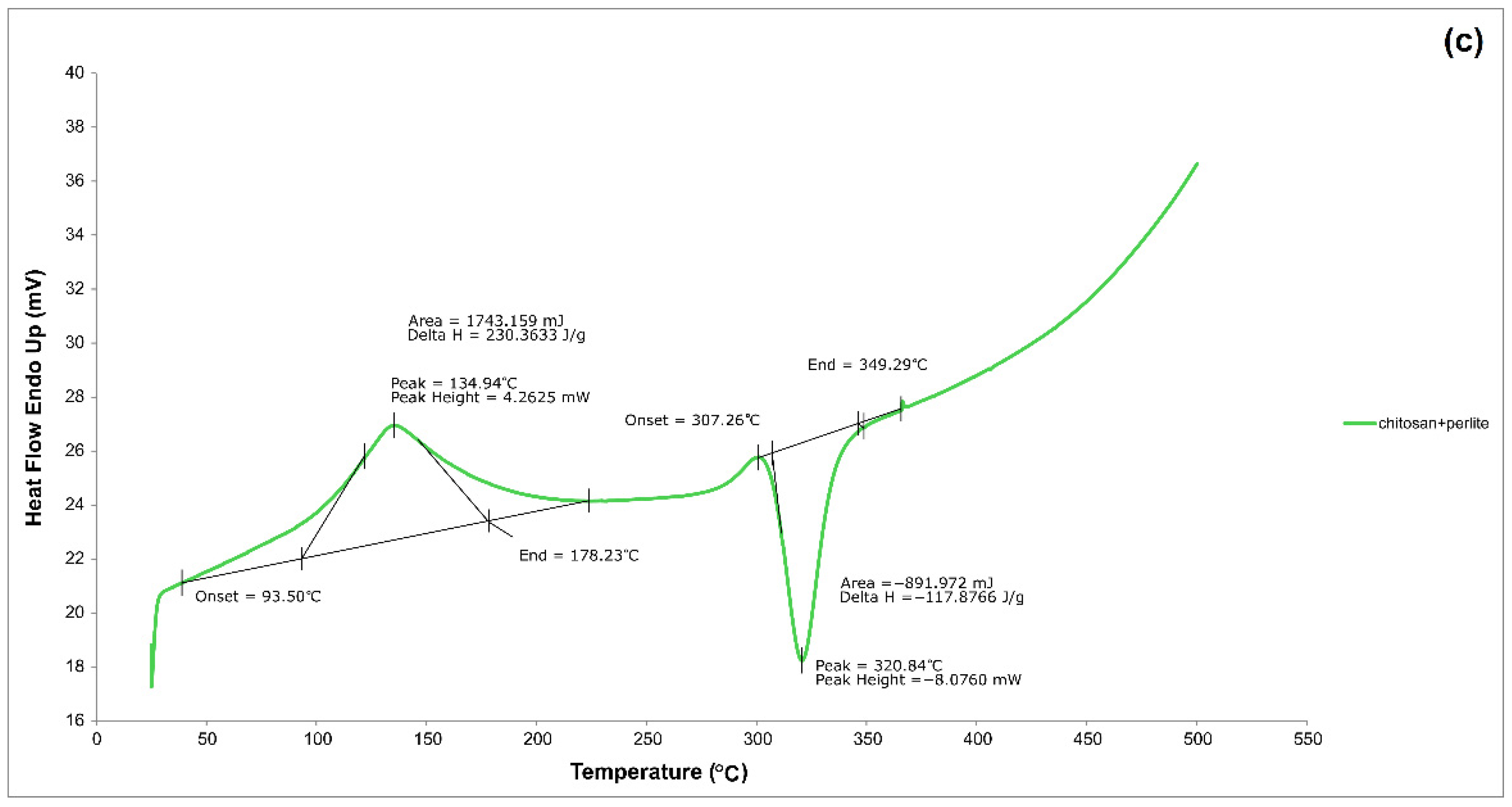
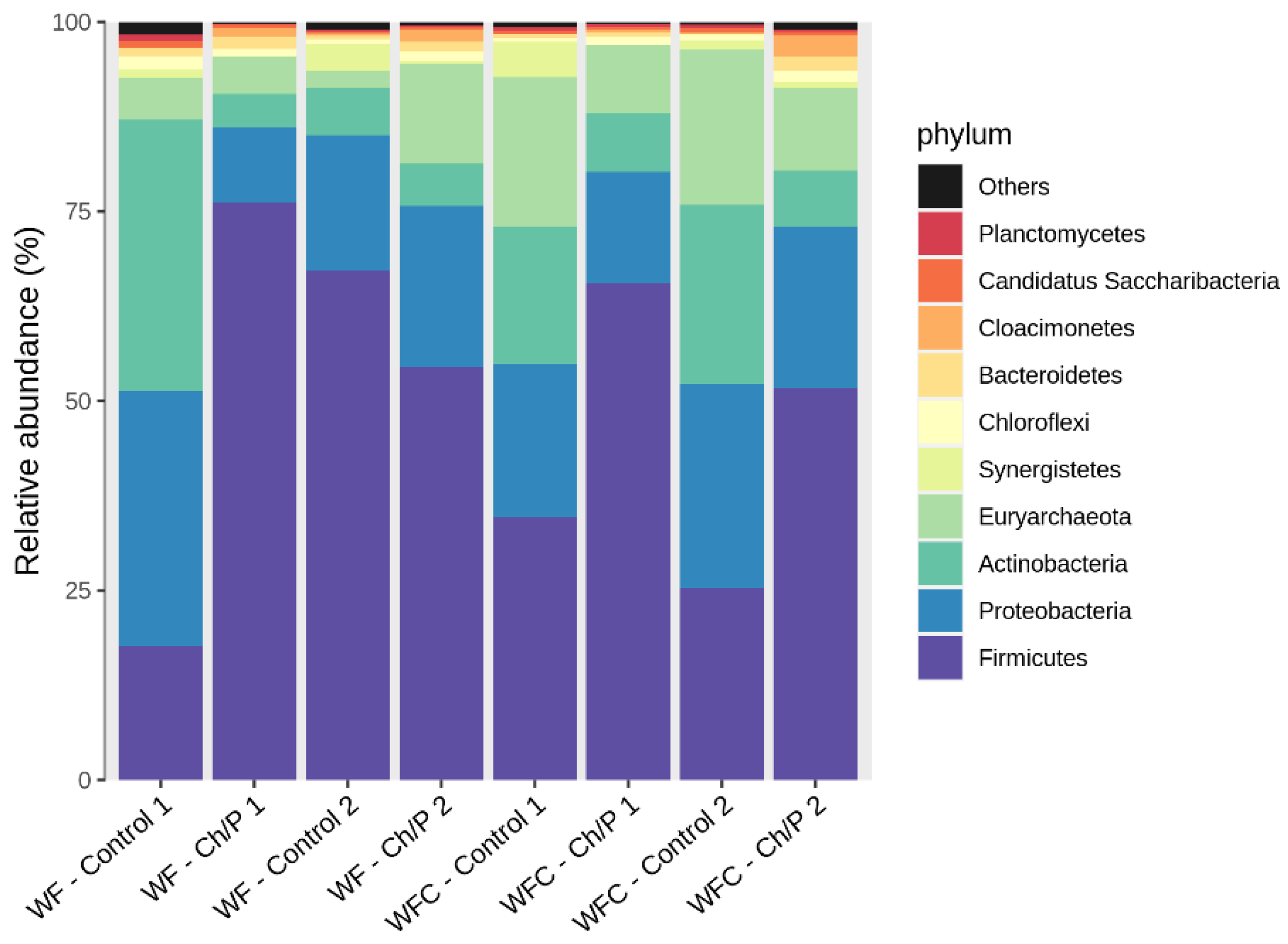
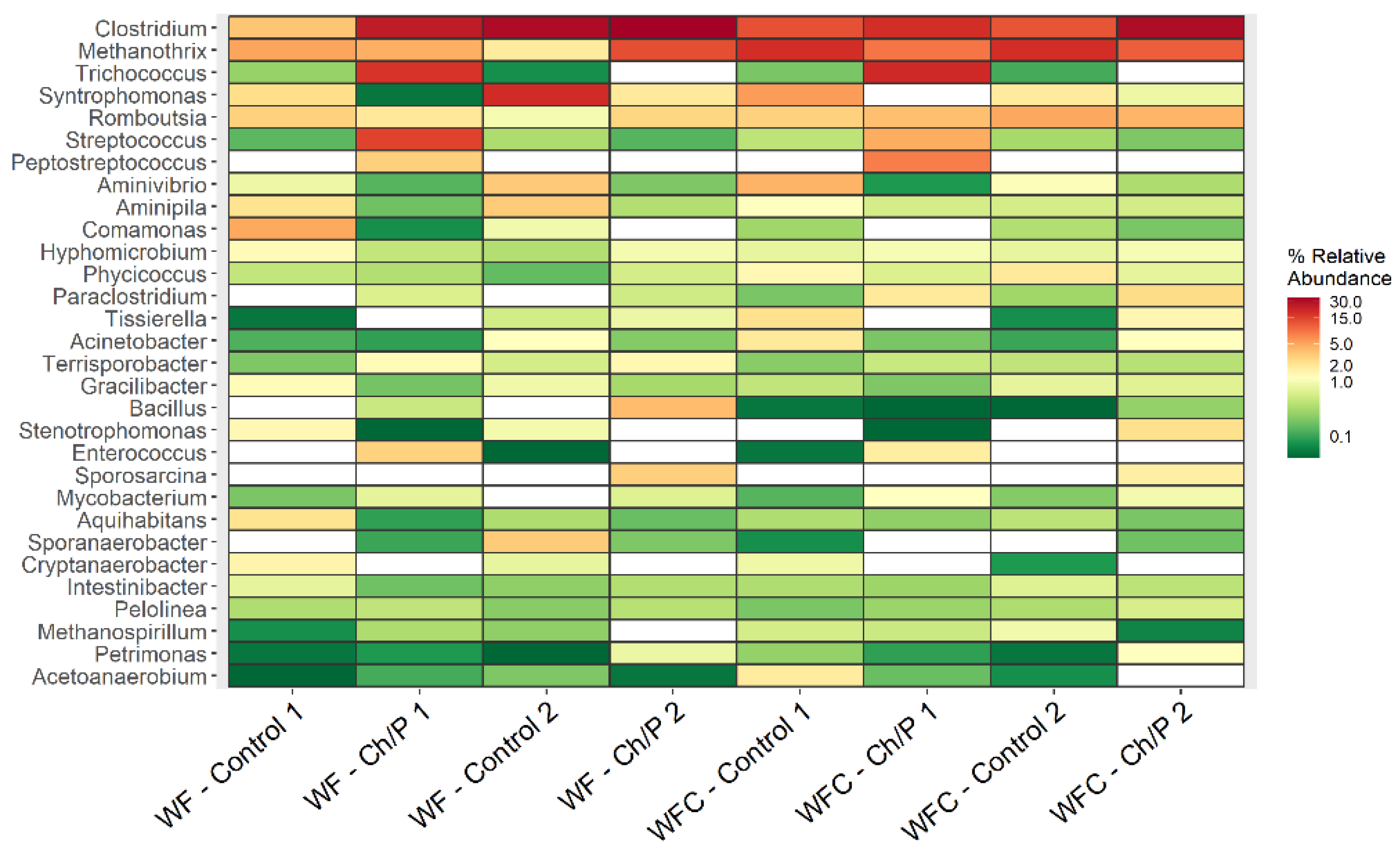
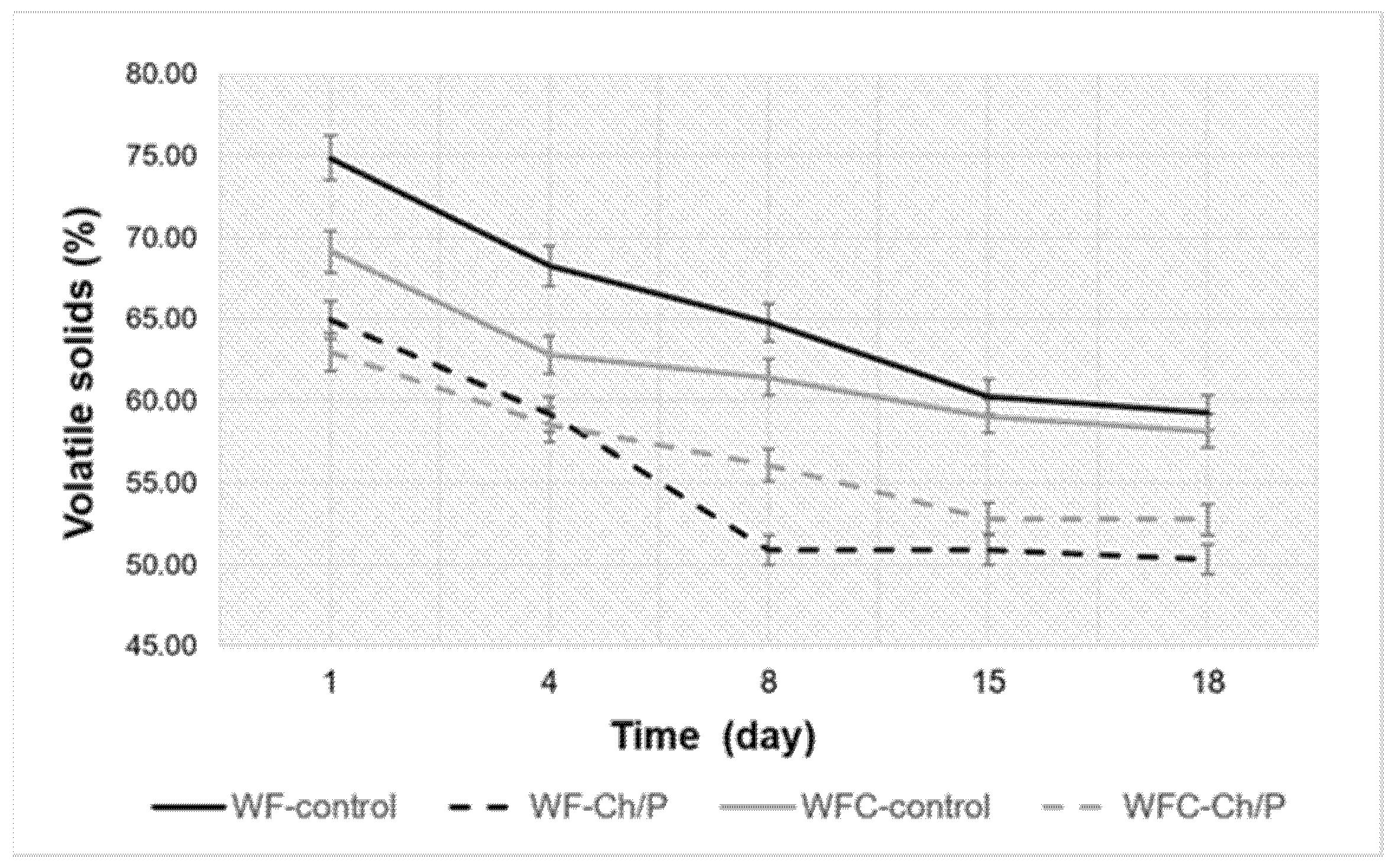
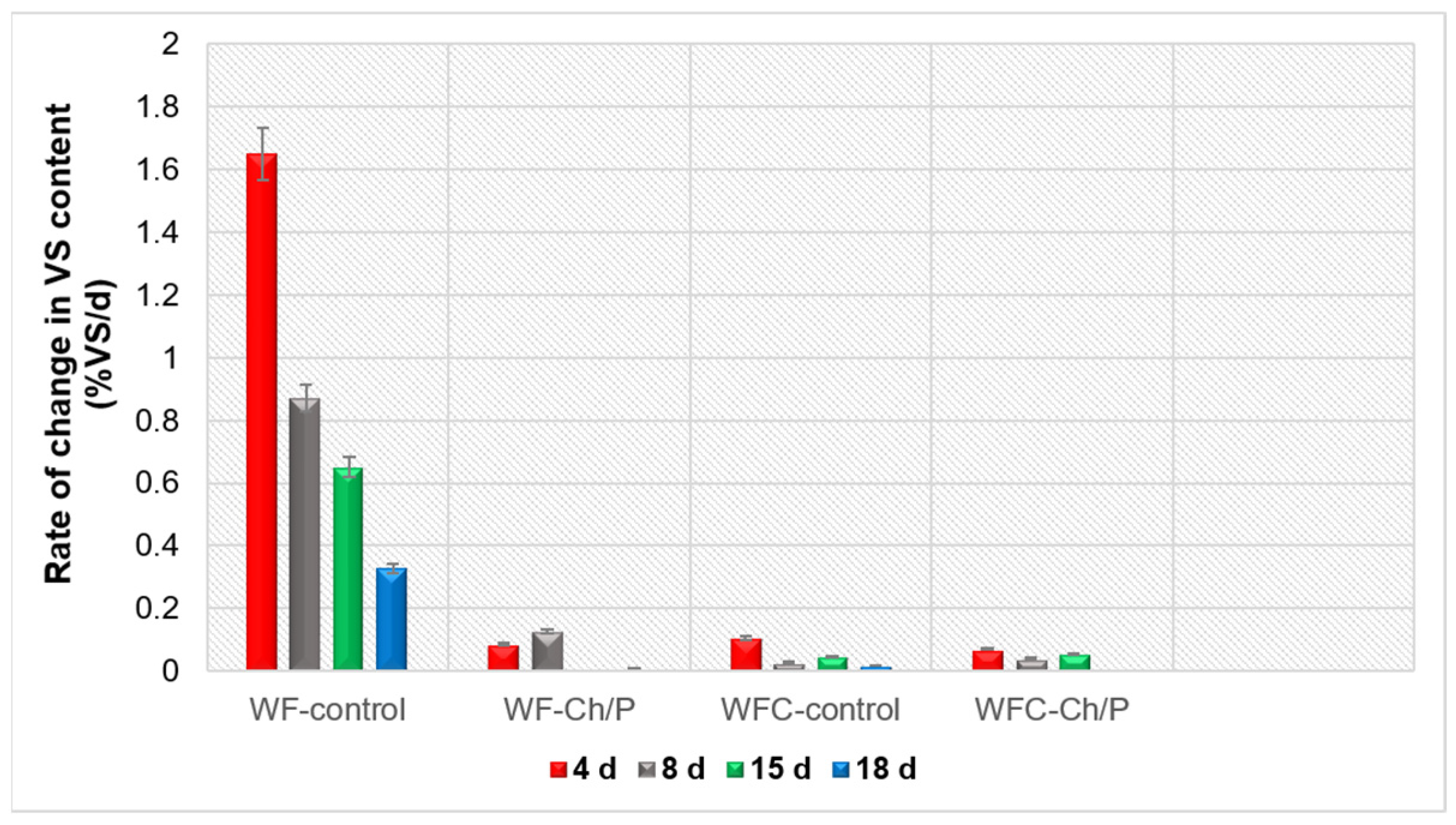
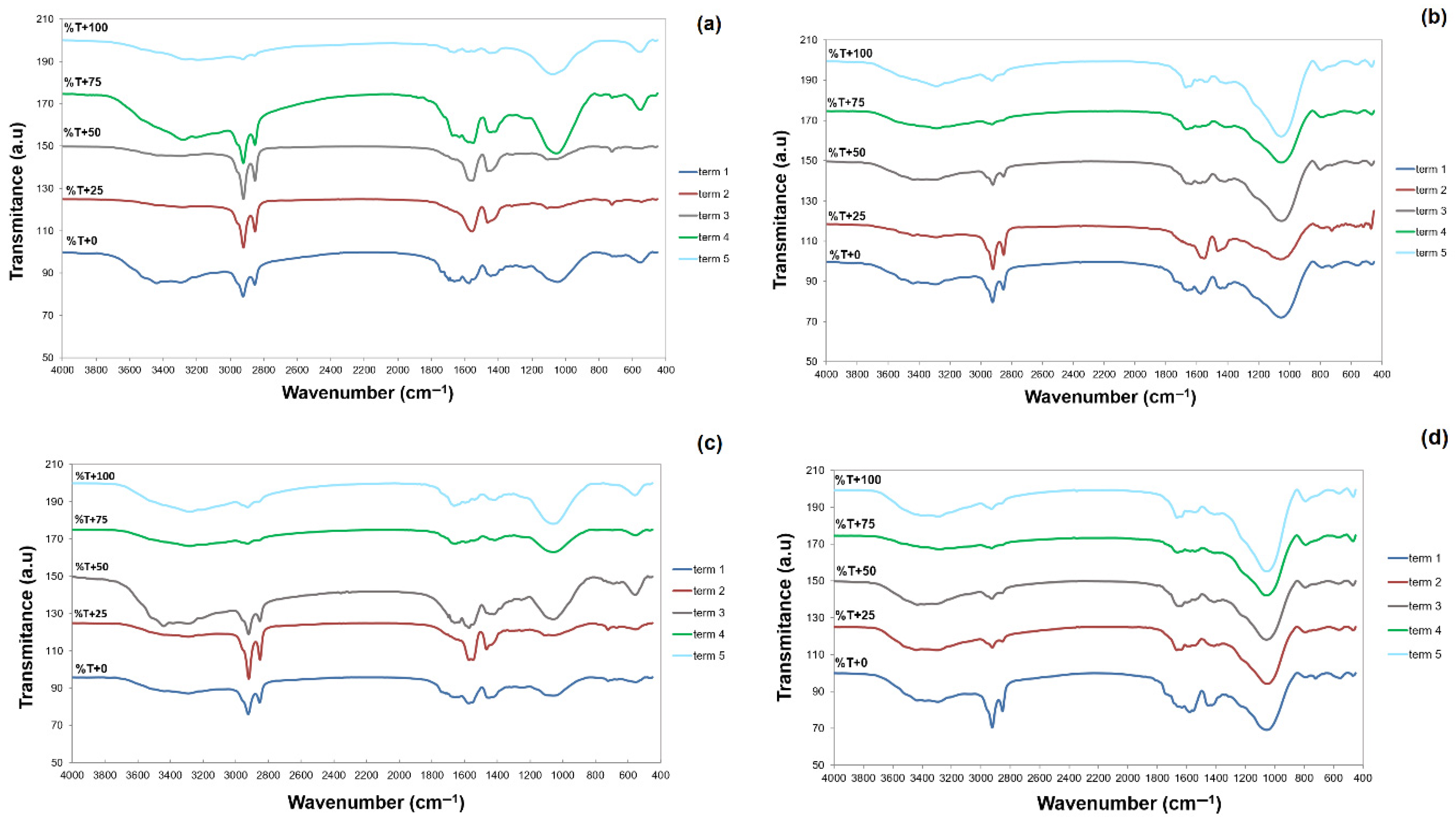
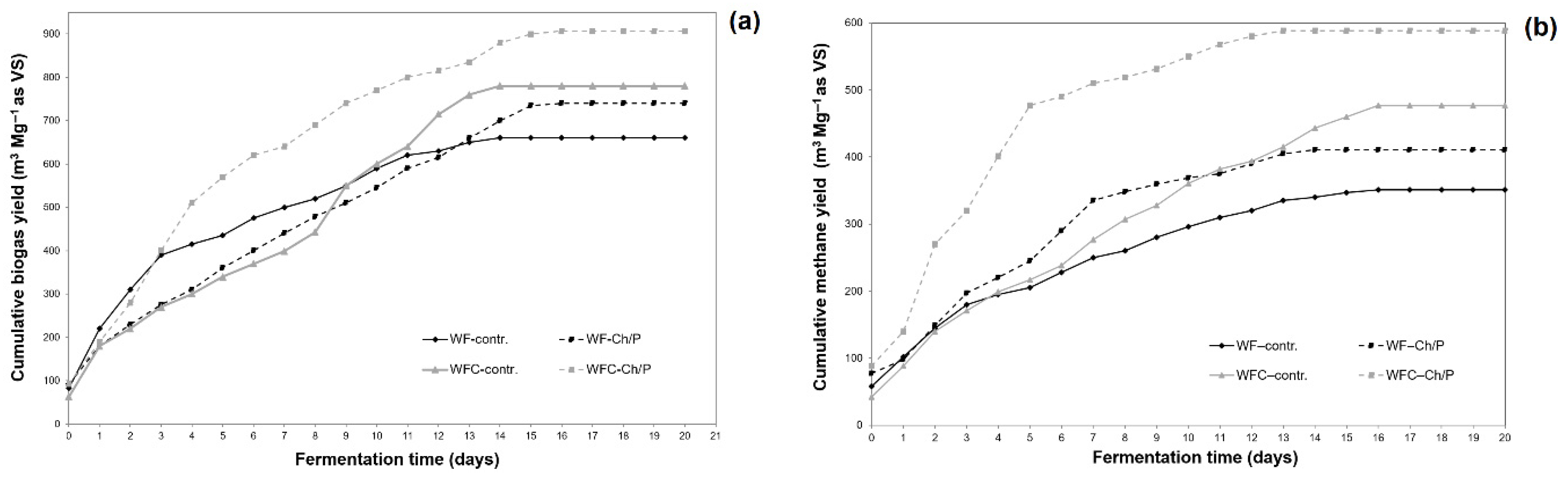
| Mat. | pH | MU | Cond. | MU | TS | MU | VS | MU | C/N Ratio | MU | C | MU | N | MU | N–NH4+ | MU | Ptotal | MU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – | (±) | (mS cm−1) | (±) | (wt %) | (±) | (wt %TS) | (±) | – | (±) | (wt %TS) | (±) | (wt %TS) | (±) | (wt %TS) | (±) | (wt %TS) | (±) | |
| Wafers | 7.02 | 0.39 | 1.62 | 0.08 | 73.24 | 4.49 | 96.81 | 6.12 | 37.50 | 2.07 | 42.37 | 2.27 | 1.13 | 0.06 | 0.36 | 0.02 | 0.15 | 0.01 |
| Cheese | 4.32 | 0.24 | 72.96 | 3.74 | 34.25 | 2.10 | 92.56 | 5.85 | 3.60 | 0.20 | 45.87 | 2.46 | 12.73 | 0.69 | 0.53 | 0.03 | 1.62 | 0.10 |
| Inoc. | 7.93 | 0.45 | 26.50 | 1.36 | 3.47 | 0.21 | 71.43 | 4.52 | 11.21 | 0.62 | 33.06 | 1.77 | 2.95 | 0.16 | 2.48 | 0.14 | 2.57 | 0.15 |
| Samples | WF | MU | CE | MU | Ch/P | MU | Inoc. | MU | pH | MU | Cond. | MU | TS | MU | VS | MU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (±) | (g) | (±) | (g) | (±) | (g) | (±) | – | (±) | (mS cm−1) | (±) | (%) | (±) | (%) | (±) | |
| WF–control | 9.5 | 0.10 | – | – | – | – | 835.5 | 8.36 | 7.16 | 0.40 | 62.95 | 3.23 | 4.08 | 0.25 | 73.46 | 4.6 |
| WF–Ch/P | 9.5 | 0.10 | – | – | 20.0 | 0.20 | 835.5 | 8.36 | 7.03 | 0.40 | 69.25 | 3.55 | 3.97 | 0.24 | 72.62 | 4.6 |
| WFC–control | 6.5 | 0.07 | 3.0 | 0.03 | – | – | 833.0 | 8.33 | 6.91 | 0.39 | 77.54 | 3.96 | 4.15 | 0.25 | 69.38 | 4.4 |
| WFC–Ch/P | 6.5 | 0.07 | 3.0 | 0.03 | 20.0 | 0.20 | 833.0 | 8.33 | 6.83 | 0.38 | 78.86 | 4.04 | 4.23 | 0.26 | 68.55 | 4.3 |
| Materials | ABET (m2/g) | Vp (cm3/g) | Sp (nm) |
|---|---|---|---|
| Chitosan | 0.5061 | 0.001441 | 27.366 |
| Perlite | 1.3730 | 0.002678 | 11.842 |
| Chitosan/perlite | 1.0746 | 0.002432 | 13.714 |
| Elemental Content, Weight (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | C K | N K | O K | Na K | Al K | Si K | S K | Cl K | K K | Ca K | Fe K |
| Chitosan | 41.10 | 5.22 | 53.55 | – | 0.13 | – | – | – | – | – | – |
| Perlite | 2.14 | – | 48.09 | 2.00 | 7.38 | 34.42 | 0.13 | 0.06 | 4.64 | 0.64 | 0.49 |
| Chitosan/Perlite | 24.43 | 3.56 | 60.66 | 0.79 | 2.27 | 7.20 | – | 0.05 | 0.72 | 0.20 | 0.13 |
| Chao1 | Shannon | Simpson | |
|---|---|---|---|
| WF–Control 1 | 140 | 3.773 | 0.956 |
| WF–Ch/P 1 | 117 | 2.994 | 0.88 |
| WF–Control 2 | 111 | 2.923 | 0.855 |
| WF–Ch/P 2 | 96 | 2.932 | 0.849 |
| WFC–Control 1 | 120 | 3.407 | 0.929 |
| WFC–Ch/P 1 | 111 | 3.154 | 0.903 |
| WFC–Control 2 | 124 | 3.36 | 0.926 |
| WFC–Ch/P 2 | 105 | 3.24 | 0.882 |
| Bray–Curtis Index | |
|---|---|
| WF–Control 1 vs. WF–Ch/P 1 | 0.749 |
| WF–Control 2 vs. WF–Ch/P 2 | 0.466 |
| WFC–Control 1 vs. WFC–Ch/P 1 | 0.532 |
| WFC–Control 2 vs. WFC–Ch/P 2 | 0.468 |
| WF–Ch/P 1 vs. WFC–Ch/P 1 | 0.264 |
| WF–Ch/P 2 vs. WFC–Ch/P 2 | 0.228 |
| WF–Ch/P 1 vs. WF–Ch/P 2 | 0.509 |
| WFC–Ch/P 1 vs. WFC–Ch/P 2 | 0.451 |
| Samples | Biogas Efficiency | Methane Efficiency | CH4 Content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (m3 Mg−1 as TS) | MU (±) | (m3 Mg−1 as VS) | MU (±) | (m3 Mg−1 as TS) | MU (±) | (m3 Mg−1 as VS) | MU (±) | (%) | MU (±) | |
| WF–contr. | 468.19 | 16.68 | 660.13 | 26.15 | 249.08 | 8.80 | 351.19 | 14.03 | 53.2 | 2.2 |
| WF–Ch/P | 524.61 | 18.69 | 740.08 | 29.30 | 291.16 | 10.28 | 410.74 | 16.40 | 55.5 | 2.3 |
| WFC–contr. | 545.21 | 19.42 | 780.42 | 30.86 | 333.12 | 11.76 | 476.84 | 19.02 | 61.1 | 2.5 |
| WFC–Ch/P | 633.53 | 22.57 | 906.85 | 35.88 | 411.16 | 14.50 | 588.55 | 23.49 | 64.9 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Marzec-Grządziel, A.; Makowska, M.; Kolasa-Więcek, A.; Jambulingam, R.; Kałuża, T.; Pilarski, K. The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste. Materials 2025, 18, 3504. https://doi.org/10.3390/ma18153504
Pilarska AA, Marzec-Grządziel A, Makowska M, Kolasa-Więcek A, Jambulingam R, Kałuża T, Pilarski K. The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste. Materials. 2025; 18(15):3504. https://doi.org/10.3390/ma18153504
Chicago/Turabian StylePilarska, Agnieszka A., Anna Marzec-Grządziel, Małgorzata Makowska, Alicja Kolasa-Więcek, Ranjitha Jambulingam, Tomasz Kałuża, and Krzysztof Pilarski. 2025. "The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste" Materials 18, no. 15: 3504. https://doi.org/10.3390/ma18153504
APA StylePilarska, A. A., Marzec-Grządziel, A., Makowska, M., Kolasa-Więcek, A., Jambulingam, R., Kałuża, T., & Pilarski, K. (2025). The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste. Materials, 18(15), 3504. https://doi.org/10.3390/ma18153504









