Mechanism of Topotactic Reduction-Oxidation Between Mg-Doped SrMoO3 Perovskites and SrMoO4 Scheelites, Utilized as Anode Materials for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
Crystallographic Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steel, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.M.; Hossain, S.; Petra, P.M.; Ghasemi, M.; Azad, A.K. Achievements and trends of solid oxide fuel cells in clean energy field: A perspective review. Front. Energy 2020, 14, 359–382. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Ishihara, T. Perovskite Oxide for Solid Oxide Fuel Cells; Kyushu University: Fukuoka, Japan, 2009; p. 15. [Google Scholar]
- Ge, X.M.; Chan, S.H.; Liu, Q.L.; Sun, Q. Solid Oxide Fuel Cell Anode Materials for Direct Hydrocarbon Utilization. Adv. Energy Mater. 2012, 2, 1156–1181. [Google Scholar] [CrossRef]
- Lo Faro, M.; Zignani, S.C.; Arico, A.S. Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells. Materials 2020, 13, 3231. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Sunarso, J.; Hashim, S.S. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrogen Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Alonso, J.A.; Aguadero, A.; Fernandez-Díaz, M.T. Optimized energy conversion efficiency in solid oxide fuel cells implementing SrMo1-xFexO3-δ perovskites as anodes. J. Power Sources 2012, 208, 153–158. [Google Scholar] [CrossRef]
- Cascos, V.; Chivite Lacaba, M.; Biskup, N.; Fernández-Díaz, M.T.; Alonso, J.A. SrMo0.9O3−δ Perovskite with Segregated Ru Nanoparticles Performing as Anode in Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2024, 16, 17474–17482. [Google Scholar] [CrossRef] [PubMed]
- Cascos, V.; Alonso, J.A.; Fernández-Díaz, M.T. Novel Mg-Doped SrMoO3 Perovskites Designed as Anode Materials for Solid Oxide Fuel Cells. Materials 2016, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Cascos, V.; Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T. Design of new Ga-doped SrMoO3 Perovskites performing as anode materials in SOFC. Renew. Energy 2017, 111, 476–483. [Google Scholar] [CrossRef]
- Hossain, K.M.; Hasan, M.Z.; Ali, M.L. Understanding the influences of Mg doping on the physical properties of SrMoO3 perovskite. Results Phys. 2020, 19, 103337. [Google Scholar] [CrossRef]
- Zhang, S.B.; Sun, Y.P.; Zhao, B.C.; Luo, X.; Hao, C.Y.; Zhu, X.B.; Song, W.H. Mn doping-induced semiconducting behavior in the perovskite molybdates SrMo1− xMnxO3 (≤x ≤ 0.20). J. Appl. Phys. 2007, 102, 103903. [Google Scholar] [CrossRef]
- Brixner, H. X-ray study and electrical properties of system BaxSr(1−x)MoO3. J. Inorg. Nucl. Chem. 1960, 14, 225–230. [Google Scholar] [CrossRef]
- Wang, H.H.; Cui, D.F.; Zhou, Y.L.; Chen, Z.H.; Chen, F.; Zhao, T.; Lu, H.B.; Yang, G.Z.; Xu, M.C.; Lan, Y.C.; et al. Growth and characterization of SrMoO3 thin films. J. Cryst. Growth 2001, 226, 261–266. [Google Scholar] [CrossRef]
- Rietveld, H.M. Profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radio and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
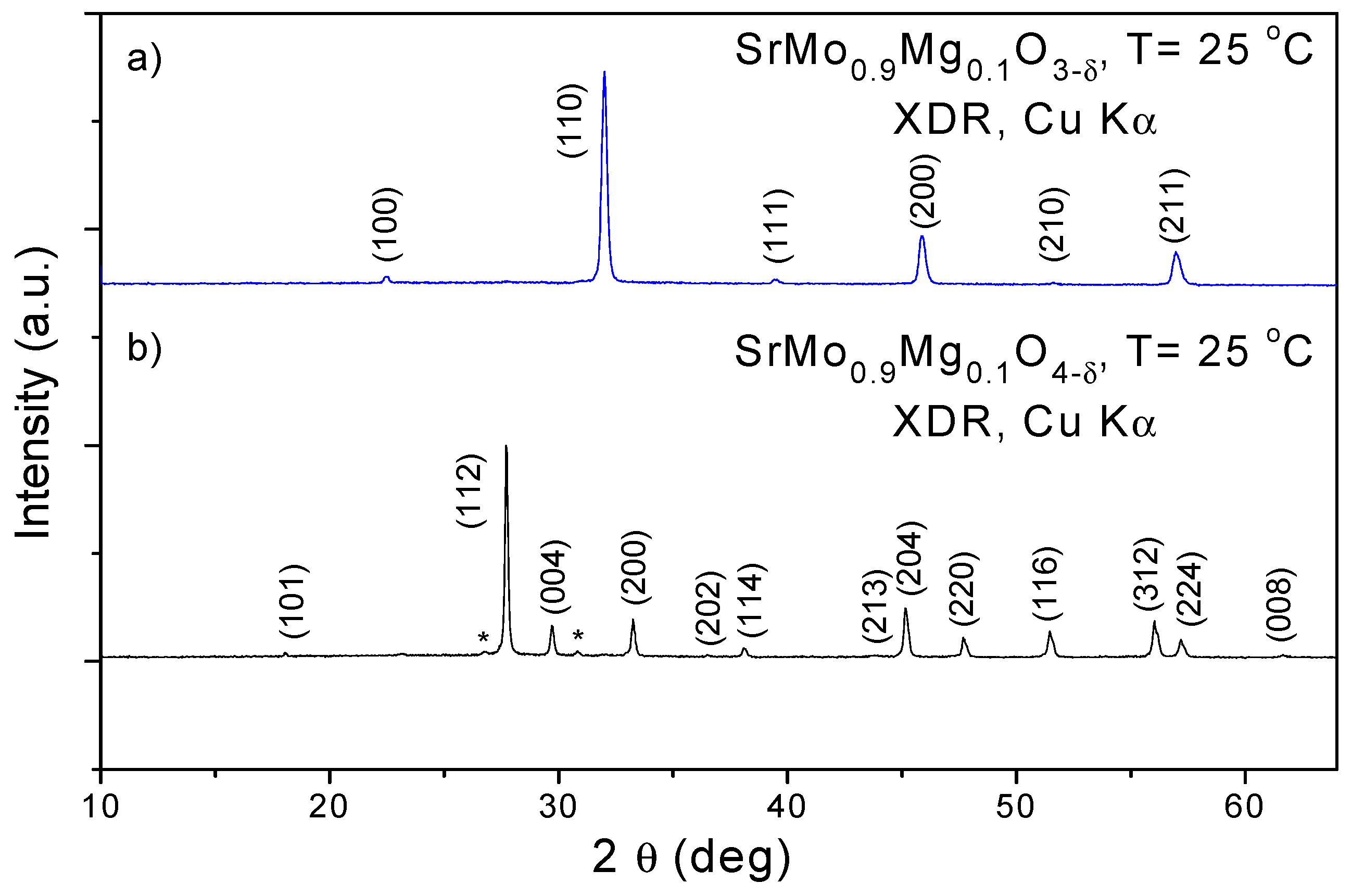
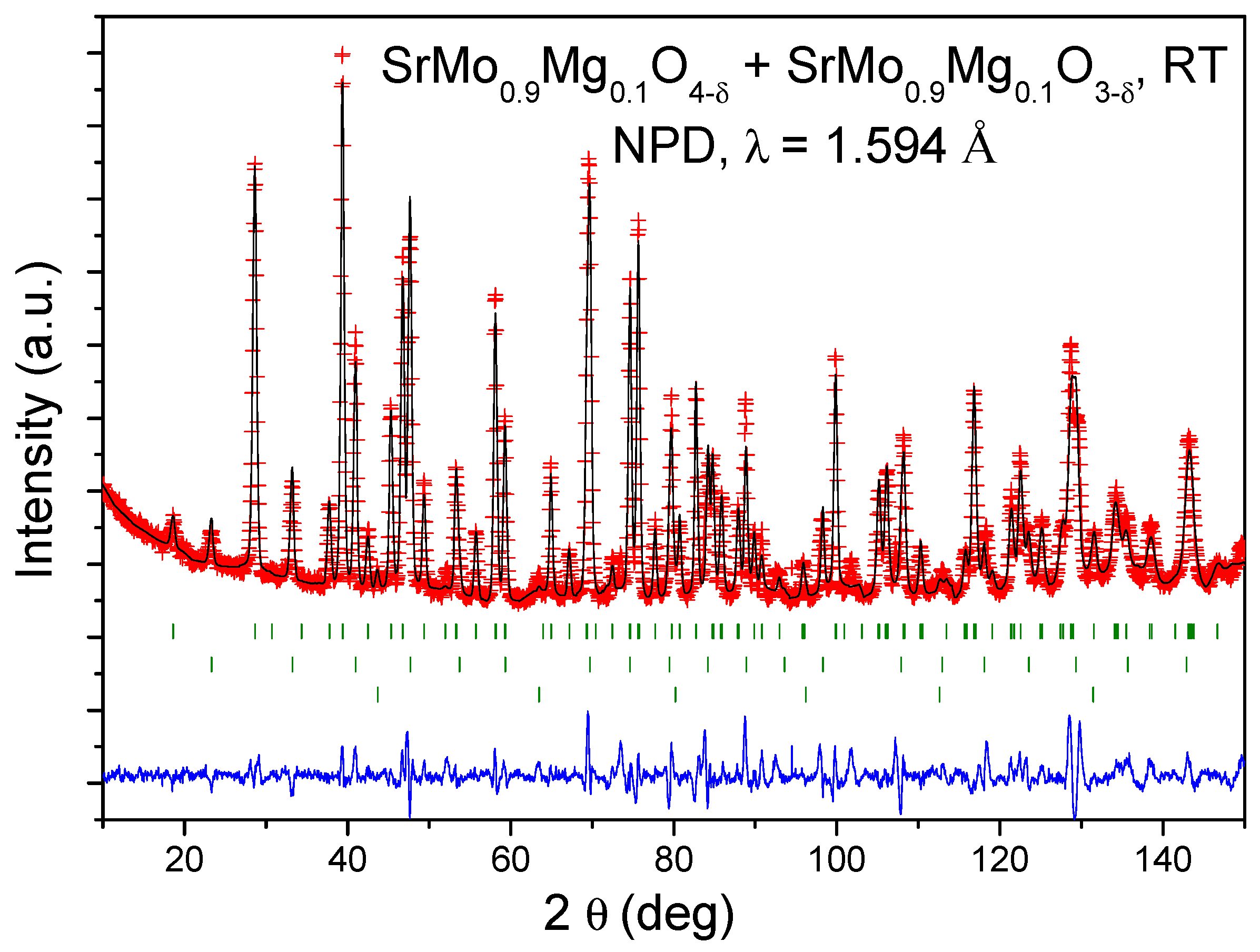
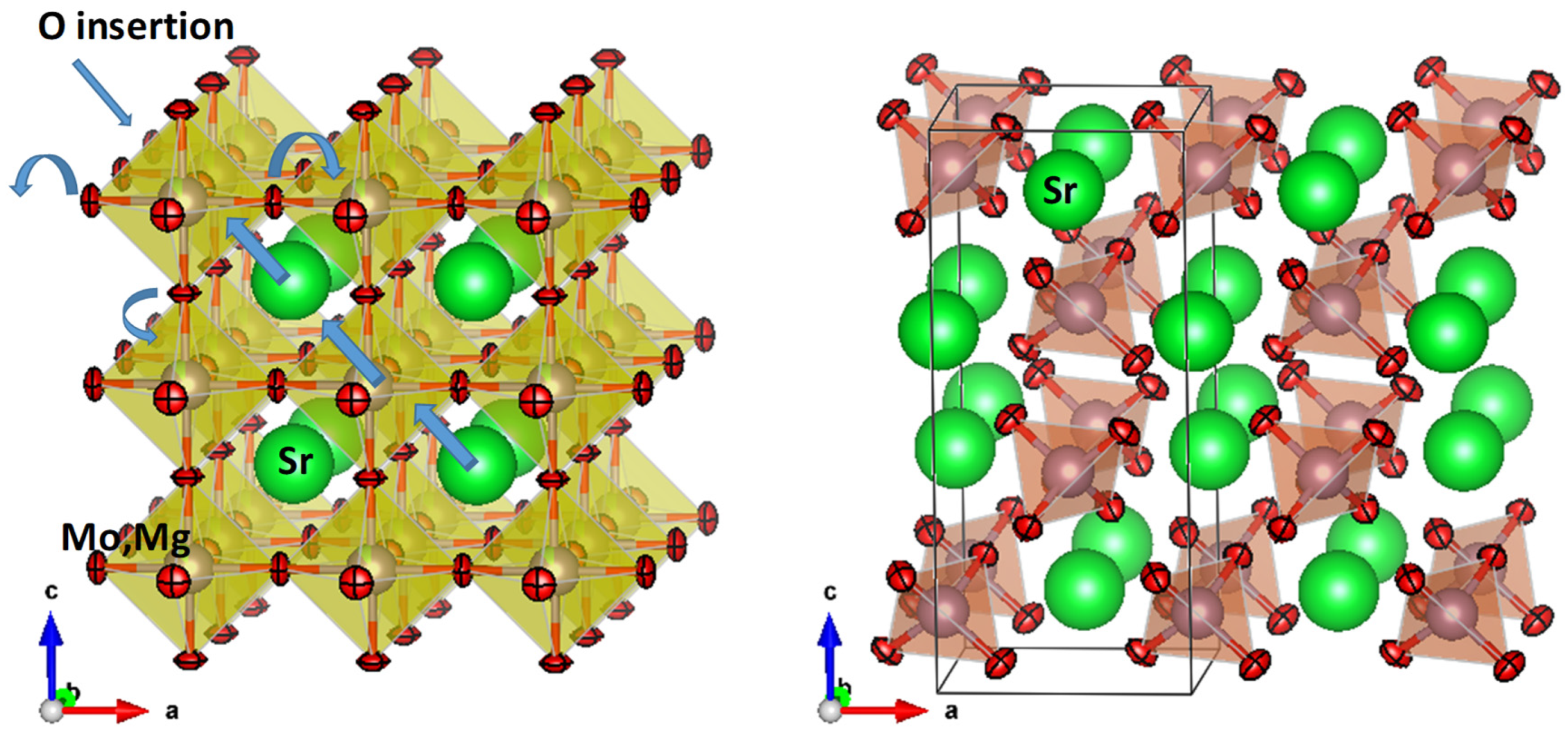
| RT | SrMo0.9Mg0.1O3-δ | SrMo0.9Mg0.1O4-δ |
|---|---|---|
| a (Å) | 3.9433 (2) | 5.3930 (2) |
| c (Å) | - | 12.0364 (7) |
| V (Å3) | 61.32 (1) | 350.06 (3) |
| Sr 1b (½,½,½)/4b (0, 1/4, 5/8) § | ||
| Biso (Å2) | 0.87 (1) | 0.69 (9) |
| focc | 1.00 | 1.00 |
| Mo, Mg 1a (0, 0, 0)/4a (0, 1/4, 1/8)§ | ||
| Biso (Å2) | 1.5 (1) | 0.011 (1) |
| Mo/Mg focc | 0.894 (1)/0.108(1) | 0.894 (1)/0.108 (1) |
| O1 3d (½, 0, 0)/16f (x, y, z) § | ||
| x | - | 0.2407 (6) |
| y | - | 0.1158 (4) |
| z | - | 0.0414 (3) |
| β11 * | - | 114 (10) |
| β22 * | - | −0.43 (7) |
| β33 * | - | 17 (2) |
| β12 * | - | 88 (8) |
| β13 * | - | 10 (3) |
| β23 * | - | −0.16 (4) |
| Biso/Beq (Å2) | 2.55 (9) | 0.61 (1) |
| focc | 0.97 (2) | 0.95 (2) |
| Reliability factors ** | - | - |
| χ2 | 7.11 | 7.11 |
| Rp (%) | 4.08 | 4.08 |
| Rwp (%) | 5.61 | 5.61 |
| Rexp (%) | 2.12 | 2.12 |
| RBragg (%) | 6.34 | 4.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos, V.; Fernández-Díaz, M.T.; Alonso, J.A. Mechanism of Topotactic Reduction-Oxidation Between Mg-Doped SrMoO3 Perovskites and SrMoO4 Scheelites, Utilized as Anode Materials for Solid Oxide Fuel Cells. Materials 2025, 18, 3424. https://doi.org/10.3390/ma18153424
Cascos V, Fernández-Díaz MT, Alonso JA. Mechanism of Topotactic Reduction-Oxidation Between Mg-Doped SrMoO3 Perovskites and SrMoO4 Scheelites, Utilized as Anode Materials for Solid Oxide Fuel Cells. Materials. 2025; 18(15):3424. https://doi.org/10.3390/ma18153424
Chicago/Turabian StyleCascos, Vanessa, M. T. Fernández-Díaz, and José Antonio Alonso. 2025. "Mechanism of Topotactic Reduction-Oxidation Between Mg-Doped SrMoO3 Perovskites and SrMoO4 Scheelites, Utilized as Anode Materials for Solid Oxide Fuel Cells" Materials 18, no. 15: 3424. https://doi.org/10.3390/ma18153424
APA StyleCascos, V., Fernández-Díaz, M. T., & Alonso, J. A. (2025). Mechanism of Topotactic Reduction-Oxidation Between Mg-Doped SrMoO3 Perovskites and SrMoO4 Scheelites, Utilized as Anode Materials for Solid Oxide Fuel Cells. Materials, 18(15), 3424. https://doi.org/10.3390/ma18153424









