Ni-MOF/g-C3N4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
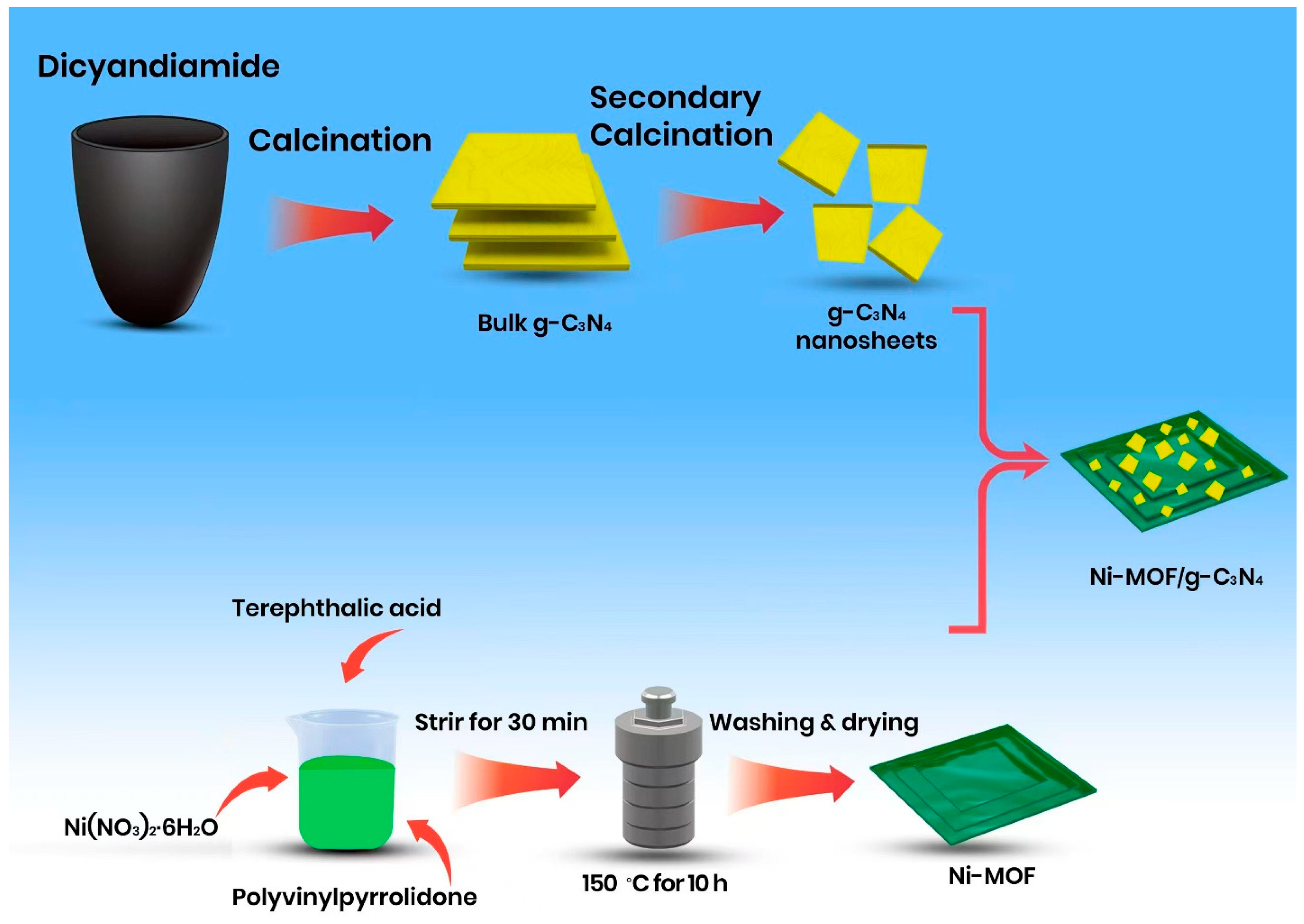
2.2. Synthesis of Photocatalyst
2.2.1. Synthesis of Ni-MOF
2.2.2. Synthesis of CN
2.2.3. Synthesis of Ni-MOF/CN
3. Results and Discussion
3.1. Phase Composition and FT-IR Analysis
3.2. Morphological Characterizations
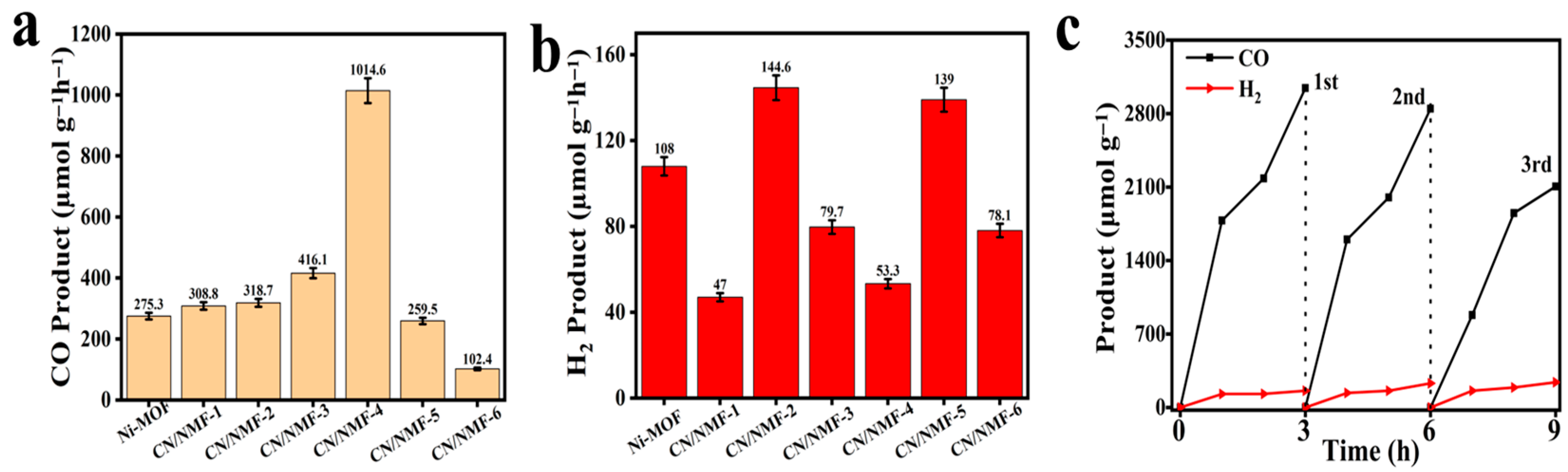
3.3. Photocatalytic CO2 Reduction Performance
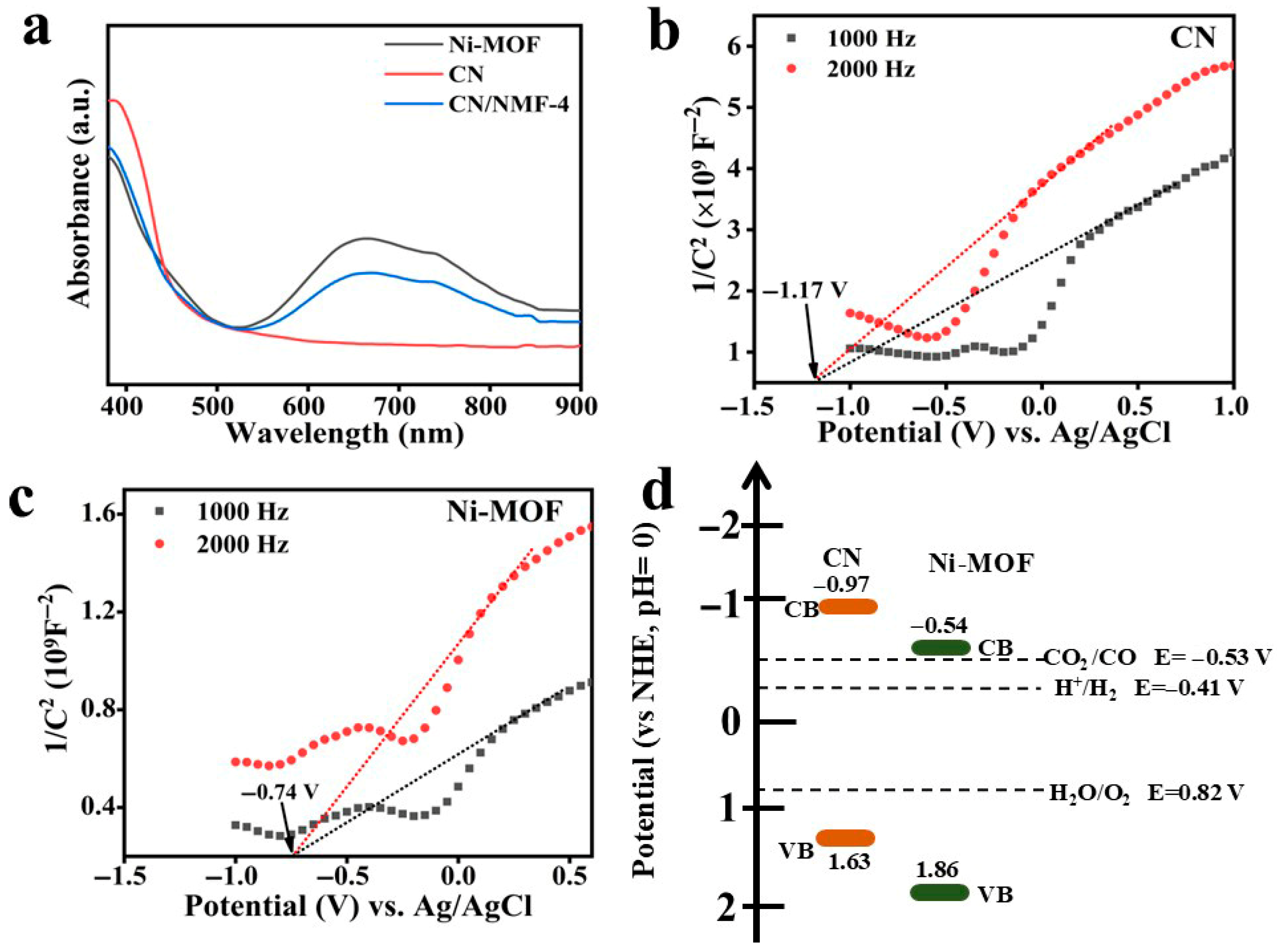
3.4. UV–Vis Diffuse Reflectance Absorption Analysis (DRS) and the Mott–Schottky Plots
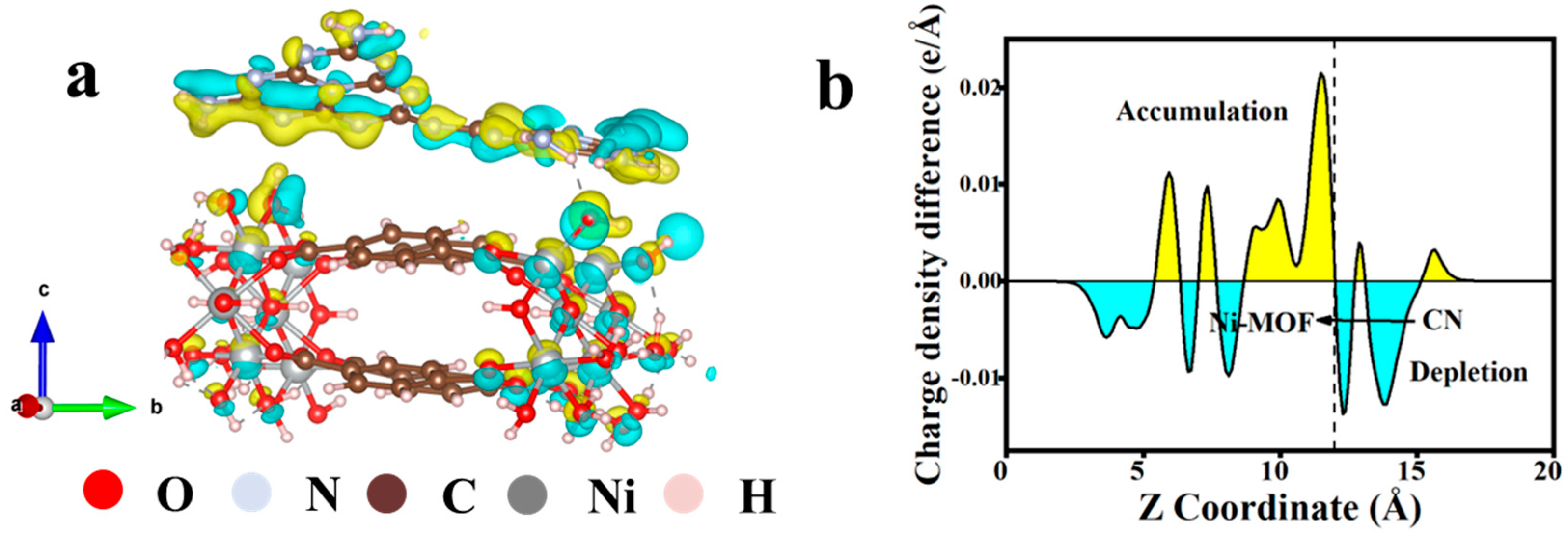
3.5. DFT Calculation
3.6. XPS and EPR Analysis
3.7. Photoluminescence and Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, M.; Yu, J.; Liu, G.; Jaroniec, M. Non-Noble Plasmonic Metal-Based Photocatalysts. Chem. Rev. 2022, 122, 10484–10537. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Materials 2024, 17, 3199. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.; Wang, X.; Zhu, X. Oxygen Vacancy Modification Mil-125 (Ti) Promotes CO2 Photoreduction to CO with near 100% Selectivity. Materials 2025, 18, 1343. [Google Scholar] [CrossRef]
- Sayed, M.; Xu, F.; Kuang, P.; Low, J.; Wang, S.; Zhang, L.; Yu, J. Sustained CO2-Photoreduction Activity and High Selectivity over Mn, C-Codoped ZnO Core-Triple Shell Hollow Spheres. Nat. Commun. 2021, 12, 4936. [Google Scholar] [CrossRef]
- Fang, Z.; Ge, H.; Lu, Y.; Liu, X.; Zhang, Z. Preparation, Stability, and Enhanced CO2 Absorption and Desorption of Nanofluids: Review and Perspectives. J. Environ. Chem. Eng. 2025, 13, 116056. [Google Scholar] [CrossRef]
- Dong, W.-W.; Jia, J.; Wang, Y.; An, J.-R.; Yang, O.-Y.; Gao, X.-J.; Liu, Y.-L.; Zhao, J.; Li, D.-S. Visible-Light-Driven Solvent-Free Photocatalytic CO2 Reduction to CO by Co-MOF/Cu2O Heterojunction with Superior Selectivity. Chem. Eng. J. 2022, 438, 135622. [Google Scholar] [CrossRef]
- Cho, J.; Medina, A.; Saih, I.; Il Choi, J.; Drexler, M.; Goddard, W.A., III; Alamgir, F.M.; Jang, S.S. 2d Metal/Graphene and 2d Metal/Graphene/Metal Systems for Electrocatalytic Conversion of CO2 to Formic Acid. Angew. Chem. 2024, 136, e202320268. [Google Scholar] [CrossRef]
- Wu, H.L.; Li, X.B.; Tung, C.H.; Wu, L.Z. Semiconductor Quantum Dots: An Emerging Candidate for CO2 Photoreduction. Adv. Mater. 2019, 31, 1900709. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2d/2d/0d TiO2/C3N4/Ti3C2 Mxene Composite S-Scheme Photocatalyst with Enhanced CO2 Reduction Activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Sayed, M.; Qi, K.; Wu, X.; Zhang, L.; García, H.; Yu, J. Cu-Based S-Scheme Photocatalysts. Chem. Soc. Rev. 2025, 54, 4874–4921. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, X.; Fu, T.; Shen, C.; Ding, K.; Li, J.; Yang, Y.; Lin, H.; Liu, Z.; Hu, A.; et al. A Comprehensive Review of S-Scheme Heterojunction Photocatalysts for CO2 Reduction: Design Principles, Mechanisms, and Material Classification. J. CO2 Util. 2025, 95, 103087. [Google Scholar] [CrossRef]
- Wang, L.; Fei, X.; Zhang, L.; Yu, J.; Cheng, B.; Ma, Y. Solar Fuel Generation over Nature-Inspired Recyclable TiO2/g-C3N4 S-Scheme Hierarchical Thin-Film Photocatalyst. J. Mater. Sci. Technol. 2022, 112, 1–10. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cao, S.; Jiang, C.; Chen, T.; Xu, J.; Yu, J. Step-by-Step Mechanism Insights into the TiO2/Ce2S3 S-Scheme Photocatalyst for Enhanced Aniline Production with Water as a Proton Source. ACS Catal. 2021, 12, 164–172. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-Q.; Liao, J.; Zhang, X.; Feng, D.; Deng, H.; Ge, C. Infrared-to-Visible Energy Transfer Photocatalysis over Black Phosphorus Quantum Dots/Carbon Nitride. Chem. Eng. J. 2022, 431, 133453. [Google Scholar] [CrossRef]
- Liang, T.; Yu, Z.; Bin, Y.; Zhang, S.; Wei, J.; Liu, Y.; Zhu, T.; Fan, S.; Shen, Y.; Wang, S.; et al. Tungsten and Oxygen Dual Vacancies Regulation of the S-Scheme ZnSe/ZnWO4 Heterojunction with Local Polarization Electric Field for Efficient CO2 Photocatalytic Reduction. Chem. Eng. J. 2024, 479, 147942. [Google Scholar] [CrossRef]
- Cheng, S.; Sun, Z.; Lim, K.H.; Wibowo, A.A.; Zhang, T.; Du, T.; Liu, L.; Nguyen, H.T.; Li, G.K.; Yin, Z.; et al. Dual-Defective Two-Dimensional/Two-Dimensional Z-Scheme Heterojunctions for CO2 Reduction. ACS Catal. 2023, 13, 7221–7229. [Google Scholar] [CrossRef]
- Hu, C.; Cao, J.; Jia, X.; Sun, H.; Lin, H.; Chen, S. Difunctional Ni2P Decorated Novel Z-Scheme BiVO4/g-C3N4 Heterojunction for Achieving Highly Efficient CO2 Reduction and Tetracycline Oxidation. Appl. Catal. B Environ. 2023, 337, 122957. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, B.; Xu, J.; Yu, J.; Cao, S. Organic–Inorganic S-Scheme Heterojunction Photocatalysts: Design, Synthesis, Applications, and Challenges. eScience 2024, 5, 100354. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Tong, L.; Wang, Y.; Zhang, P.; Zhu, M.; Dong, H. Recent Progress and Challenges of Photocatalytic CO2 Conversion into Value-Added Multi-Carbon Products. Coord. Chem. Rev. 2024, 502, 215623. [Google Scholar] [CrossRef]
- Kumagai, H.; Tamaki, Y.; Ishitani, O. Photocatalytic Systems for CO2 Reduction: Metal-Complex Photocatalysts and Their Hybrids with Photofunctional Solid Materials. Acc. Chem. Res. 2022, 55, 978–990. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Ji, X. Deep Eutectic Solvent-Based Slurry for CO2 Capture: Enhanced Efficiency and Kinetics. J. CO2 Util. 2025, 95, 103065. [Google Scholar] [CrossRef]
- Singh, S.; Verma, R.; Kaul, N.; Sa, J.; Punjal, A.; Prabhu, S.; Polshettiwar, V. Surface Plasmon-Enhanced Photo-Driven CO2 Hydrogenation by Hydroxy-Terminated Nickel Nitride Nanosheets. Nat. Commun. 2023, 14, 2551. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, Z.; Guo, H.; Lei, Z.; Liu, Z.; Wang, L. Solvent Engineering of Oxygen-Enriched Carbon Dots for Efficient Electrochemical Hydrogen Peroxide Production. Small 2023, 19, 2303156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Loh, H.; Low, B.Q.L.; Zhu, H.; Low, J.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.; Loh, X.J.; Ye, E.; et al. Role of Oxygen Vacancy in Metal Oxides for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Shen, Q.; Lu, Z.; Bi, F.; Fang, Y.; Song, L.; Yang, Y.; Wu, M.; Zhang, X. Effect of Actual Working Conditions on Catalyst Structure and Activity for Oxidation of Volatile Organic Compounds: A Review. Fuel 2023, 343, 128012. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 Reduction over Metal-Organic Framework-Based Materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Bi, F.; Ma, S.; Gao, B.; Liu, B.; Huang, Y.; Qiao, R.; Zhang, X. Boosting Toluene Deep Oxidation by Tuning Metal-Support Interaction in Mof-Derived Pd@ ZrO2 Catalysts: The Role of Interfacial Interaction between Pd and ZrO2. Fuel 2024, 357, 129833. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Yang, W.; Mao, G.; Zheng, L.; Jiang, H.-L. Modulating Coordination Environment of Single-Atom Catalysts and Their Proximity to Photosensitive Units for Boosting Mof Photocatalysis. J. Am. Chem. Soc. 2021, 143, 12220–12229. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, C.; Gao, Y.; Tao, X.; Ding, C.; Fan, F.; Jiang, H.L. Charge Separation by Creating Band Bending in Metal–Organic Frameworks for Improved Photocatalytic Hydrogen Evolution. Angew. Chem. 2022, 134, e202204108. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Lv, Z.; Qi, Z.; Xu, Y.; Miao, T.; Fu, X.; Li, L. Coupled Visible-Light Driven Photocatalytic Reactions over Porphyrin-Based Mof Materials. Chem. Eng. J. 2022, 442, 136186. [Google Scholar] [CrossRef]
- Yue, X.; Cheng, L.; Li, F.; Fan, J.; Xiang, Q. Highly Strained Bi-Mof on Bismuth Oxyhalide Support with Tailored Intermediate Adsorption/Desorption Capability for Robust CO2 Photoreduction. Angew. Chem. Int. Ed. 2022, 61, e202208414. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-L.; Liu, H.-R.; Wang, S.-M.; Guan, G.-W.; Yang, Q.-Y. Immobilizing Isatin-Schiff Base Complexes in NH2-UiO-66 for Highly Photocatalytic CO2 Reduction. ACS Catal. 2023, 13, 2547–2554. [Google Scholar] [CrossRef]
- Sun, D.; Kim, D.-P. Hydrophobic Mofs@ Metal Nanoparticles@ Cofs for Interfacially Confined Photocatalysis with High Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 20589–20595. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Zhang, L.; Li, S.; Song, H.; Fan, Y.; Su, C.-Y. Engineering Single-Atom Sites into Pore-Confined Nanospaces of Porphyrinic Metal–Organic Frameworks for the Highly Efficient Photocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2022, 144, 22747–22758. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Fan, H.; Yang, X.; Dong, W.; Wang, W. Synergism between Chemisorption and Unique Electron Transfer Pathway in S-Scheme AgI/g-C3N4 Heterojunction for Improving the Photocatalytic H2 Evolution. J. Colloid Interface Sci. 2023, 631, 269–280. [Google Scholar] [CrossRef]
- Anus, A.; Park, S. The Synthesis and Key Features of 3d Carbon Nitrides (C3N4) Used for CO2 Photoreduction. Chem. Eng. J. 2024, 486, 150213. [Google Scholar] [CrossRef]
- Hu, D.-D.; Guo, R.-T.; Li, C.-F.; Yan, J.-S.; Pan, W.-G. Construction of Indirect Dual S-Scheme Heterojunction CN/AP/AW Mediated by Ag Nanoparticles Enables Efficient CO2-to-CH4 Photoreduction. Sep. Purif. Technol. 2025, 353, 128473. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Jin, Z. Phosphorus Modified Ni-Mof–74/Bivo4 S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. Energy 2022, 307, 121166. [Google Scholar] [CrossRef]
- Sayed, M.; Zhu, B.; Kuang, P.; Liu, X.; Cheng, B.; Ghamdi, A.A.A.; Wageh, S.; Zhang, L.; Yu, J. Epr Investigation on Electron Transfer of 2d/3d g-C3N4/ZnO S-Scheme Heterojunction for Enhanced CO2 Photoreduction. Adv. Sustain. Syst. 2022, 6, 2100264. [Google Scholar] [CrossRef]
- Javad Kalbasi, R.; Parishani, P.; Mazaheri, O. Encapsulation of Nickel Nanoparticles and Homopoly (Vinylsulfonic Acid) in Mesoporous Carbon Cmk-3 as an Acid–Metal Bifunctional Catalyst for Tandem Reductive Amination. J. Clust. Sci. 2018, 29, 561–575. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1, 3, 5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.; Sayed, M.; Zeng, Z.; Cheng, B.; Wang, W.; Wang, C.; Xu, J.; Cao, S. Enhancing CO2 Photoreduction by Construction of g-C3N4/Co-Mofs S-Scheme Heterojunction. Appl. Surf. Sci. 2025, 693, 162752. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–Organic Frameworks: A New Promising Class of Materials for a High Performance Supercapacitor Electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Maruthapandian, V.; Kumaraguru, S.; Mohan, S.; Saraswathy, V.; Muralidharan, S. An Insight on the Electrocatalytic Mechanistic Study of Pristine Ni Mof (Btc) in Alkaline Medium for Enhanced Oer and Uor. ChemElectroChem 2018, 5, 2795–2807. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled Adsorption and Photocatalysis of g-C3N4 Based Composites: Material Synthesis, Mechanism, and Environmental Applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Gallo, E.; Gorelov, E.; Guda, A.A.; Bugaev, A.L.; Bonino, F.; Borfecchia, E.; Ricchiardi, G.; Gianolio, D.; Chavan, S.; Lamberti, C. Effect of Molecular Guest Binding on the D–D Transitions of Ni2+ of CPO-27-Ni: A Combined UV–Vis, Resonant-Valence-to-Core X-Ray Emission Spectroscopy, and Theoretical Study. Inorg. Chem. 2017, 56, 14408–14425. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Huo, P. 3d Fe-Mof Embedded into 2d Thin Layer Carbon Nitride to Construct 3d/2d S-Scheme Heterojunction for Enhanced Photoreduction of CO2. Chin. J. Catal. 2022, 43, 2625–2636. [Google Scholar] [CrossRef]
- Li, G.; Sun, Y.; Zhang, Q.; Gao, Z.; Sun, W.; Zhou, X. Ag Quantum Dots Modified Hierarchically Porous and Defective TiO2 Nanoparticles for Improved Photocatalytic CO2 Reduction. Chem. Eng. J. 2021, 410, 128397. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron Doping Induced Charge Transfer Switching of a C3N4/ZnO Photocatalyst from Z-Scheme to Type II to Enhance Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2021, 282, 119538. [Google Scholar] [CrossRef]
- Dong, Y.L.; Jiang, Y.; Ni, S.; Guan, G.W.; Zheng, S.T.; Guan, Q.; Pei, L.M.; Yang, Q.Y. Ligand Defect-Induced Active Sites in Ni-Mof-74 for Efficient Photocatalytic CO2 Reduction to CO. Small 2024, 20, 2308005. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0d/2d S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, J.; Zhu, B.; Liang, G.; Zhang, L.; Yu, J. Verifying the Charge-Transfer Mechanism in S-Scheme Heterojunctions Using Femtosecond Transient Absorption Spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202218688. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhu, B.; Qin, X.; Ho, W.; Yu, J. Zinc Porphyrin/g-C3N4 S-Scheme Photocatalyst for Efficient H2O2 Production. Chem. Eng. J. 2023, 467, 143528. [Google Scholar] [CrossRef]
- Pan, J.; Wang, D.; Zhang, B.; Zhao, C.; Liu, D.; Liu, S.; Zeng, Z.; Chen, T.; Liu, G.; Jiao, S.; et al. Atomic-Level Charge Separation Boosting the Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2024, 487, 150536. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Ma, S.; Zhang, Y.; Wang, L.; Zhu, G.; Huang, W.; Wang, S. Induced Dipole Moments in Amorphous ZnCdS Catalysts Facilitate Photocatalytic H2 Evolution. Nat. Commun. 2024, 15, 2600. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Chen, S. Use of NiFe Layered Double Hydroxide as Electrocatalyst in Oxygen Evolution Reaction: Catalytic Mechanisms, Electrode Design, and Durability. Acta Phys. Chim. Sin. 2024, 40, 2303059. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+ U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Song, K.; Liang, S.; Zhong, X.; Wang, M.; Mo, X.; Lei, X.; Lin, Z. Tailoring the crystal forms of the Ni-MOF catalysts for enhanced photocatalytic CO2-to-CO performance. Appl. Catal. B Environ. 2022, 309, 121232. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.J.; Lin, Z. Nickel metal–organic framework monolayers for photoreduction of diluted CO2: Metal-node-dependent activity and selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Xu, M.; Sun, C.; Zhao, X.; Jiang, H.; Wang, H.; Huo, P. Fabricated hierarchical CdS/Ni-MOF heterostructure for promoting photocatalytic reduction of CO2. Appl. Surf. Sci. 2022, 576, 151792. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Li, Y.-R.; Zhang, F.-J.; Wang, Y.-R. Novel honeycomb-like Ni-MOF enhanced hierarchical Bi2MoO6 microspheres for high efficient photocatalytic CO2 reduction. Inorg. Chem. Commun. 2023, 156, 111271. [Google Scholar] [CrossRef]
- Ali, R.N.; Qureshi, W.A.; Naz, H.; Jiang, H.; Yaseen, M.; Yu, X.; Liu, Q. Synthesis of a highly active core–shell Ni-MOF@ CdS S-scheme heterojunction for enhanced photoreduction of CO2 to CO. New J. Chem. 2023, 47, 15534–15542. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Yu, S.; Qin, W.; Xie, Y. Organic-inorganic hybridization strategy for promoting BiOBr CO2 photoreduction via enhanced CO2 adsorption and photogenerated carrier migration. J. Catal. 2024, 429, 115295. [Google Scholar] [CrossRef]
- He, B.; Wang, Y.-J.; Bai, X.; Bian, H.; Xie, Y.; Li, R.; Li, J.-R. Rational construction of MOF-on-MOF heterojunction with an array of flexible two-dimensional microsheets for efficient CO2 photoreduction. Chem. Eng. J. 2024, 482, 149000. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Xu, H.; Wang, G.; Qu, Y.; Zhu, P.; Wang, D. Co-MOF as an electron donor for promoting visible-light photoactivities of g-C3N4 nanosheets for CO2 reduction. Chin. J. Catal. 2020, 41, 514–523. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabir, M.; Sayed, M.; Riaz, I.; Qiu, G.; Tahir, M.; Alibrahim, K.A.; Wang, W. Ni-MOF/g-C3N4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction. Materials 2025, 18, 3419. https://doi.org/10.3390/ma18143419
Sabir M, Sayed M, Riaz I, Qiu G, Tahir M, Alibrahim KA, Wang W. Ni-MOF/g-C3N4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction. Materials. 2025; 18(14):3419. https://doi.org/10.3390/ma18143419
Chicago/Turabian StyleSabir, Muhammad, Mahmoud Sayed, Iram Riaz, Guogen Qiu, Muhammad Tahir, Khuloud A. Alibrahim, and Wang Wang. 2025. "Ni-MOF/g-C3N4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction" Materials 18, no. 14: 3419. https://doi.org/10.3390/ma18143419
APA StyleSabir, M., Sayed, M., Riaz, I., Qiu, G., Tahir, M., Alibrahim, K. A., & Wang, W. (2025). Ni-MOF/g-C3N4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction. Materials, 18(14), 3419. https://doi.org/10.3390/ma18143419








