Novel Methodology to Assess Salt Movement Between Mortar and Stones from Heritage in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Mortars
2.1.2. Stones
2.2. Sample Preparation
2.3. Methods
2.3.1. Capillarity Tests
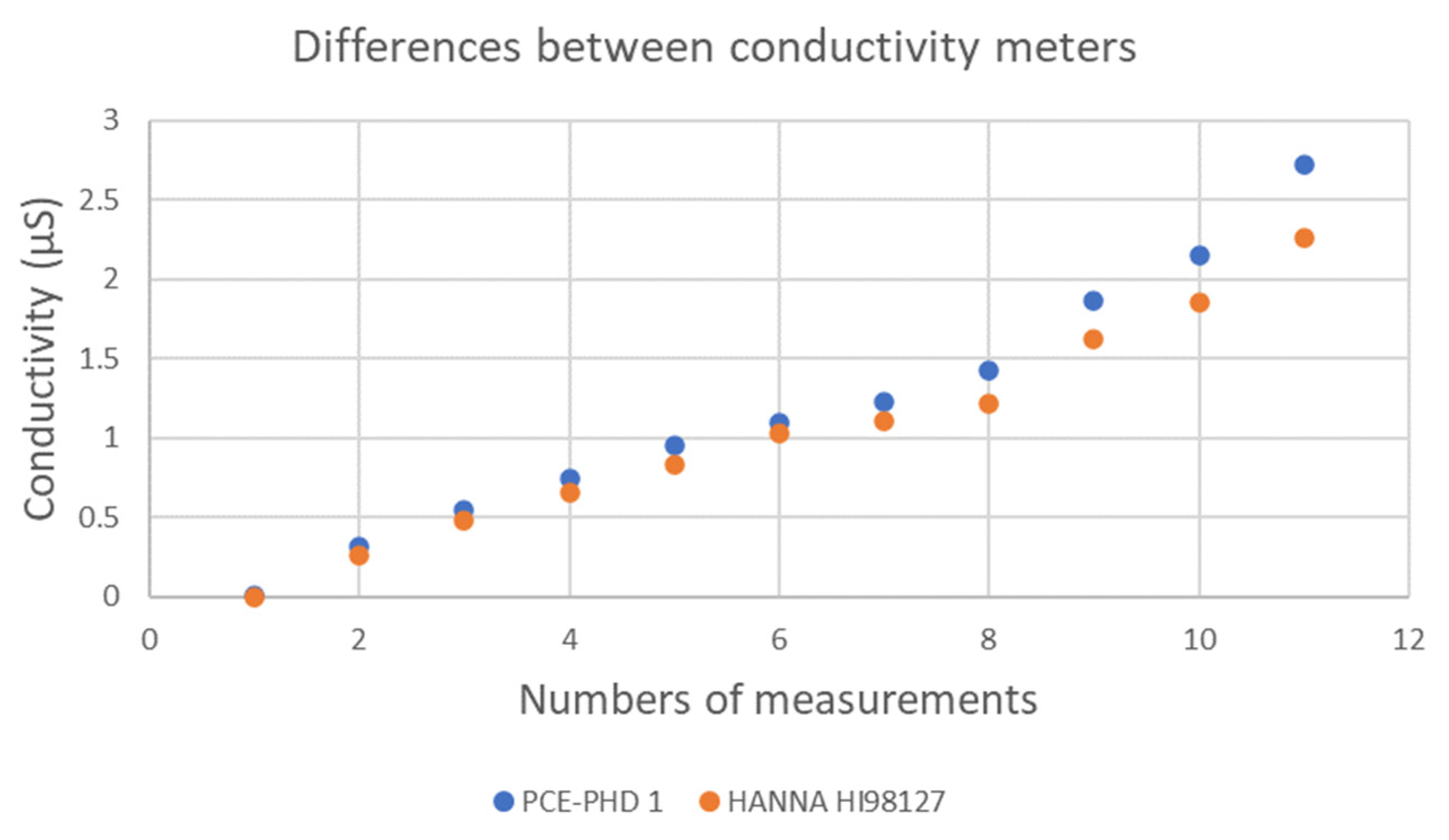
2.3.2. Conductivity Meter Modification and Calibration, and Conductivity Measurement on Solid Surfaces
2.3.3. Modified Conductivity Sensor Calibration
2.3.4. Natural Stone Surface Conductivity
2.3.5. Immersion Tests
3. Results and Discussion
3.1. Capillarity
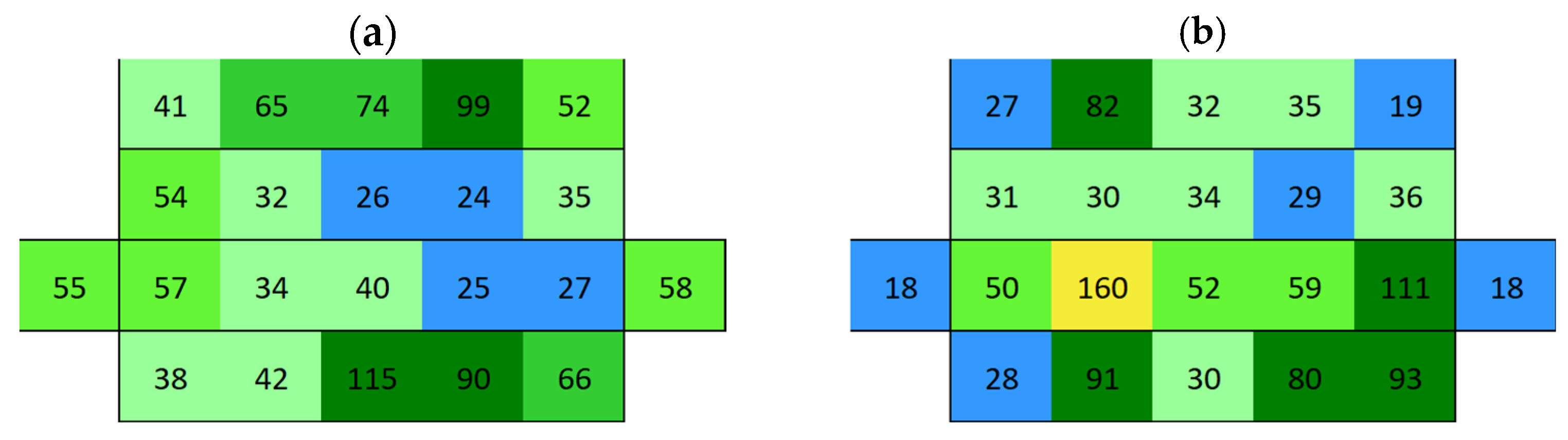
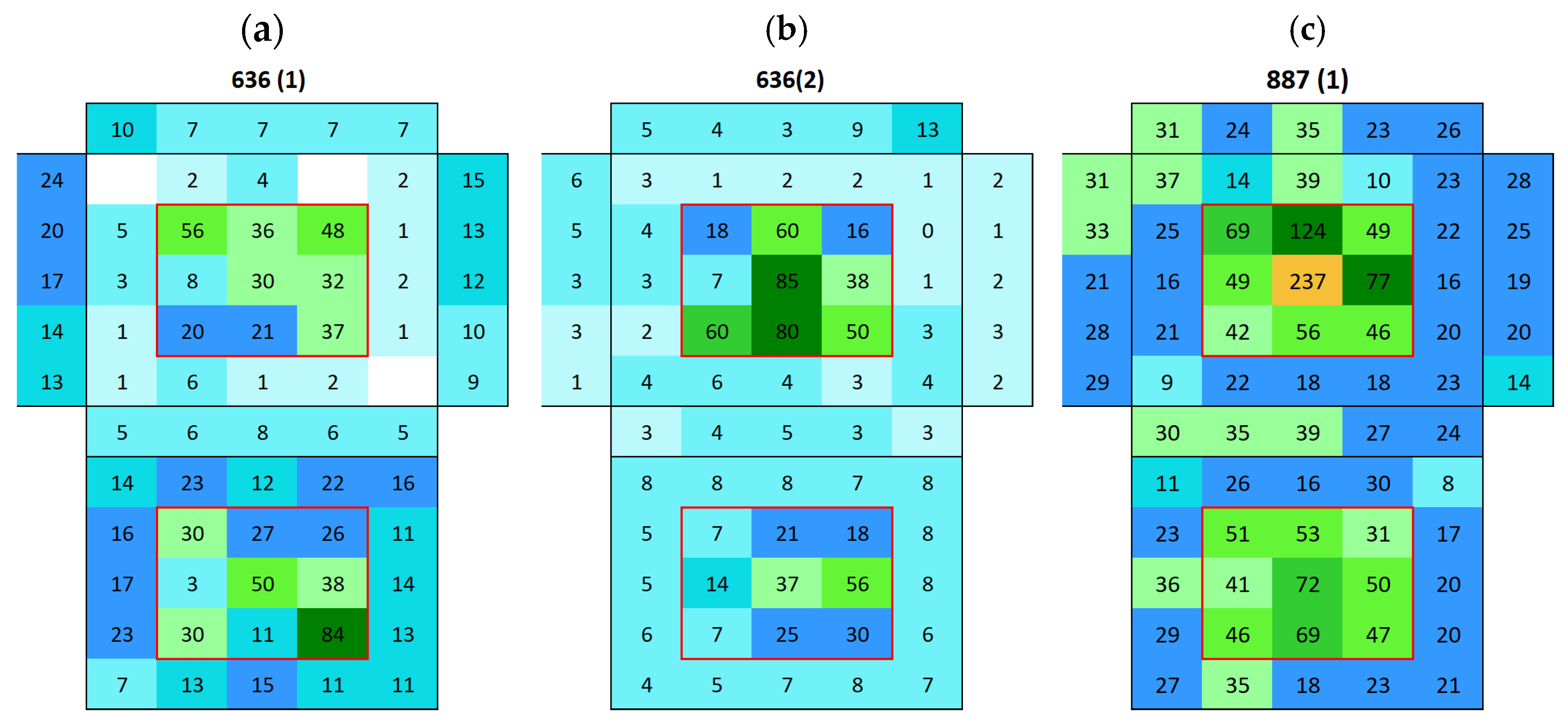
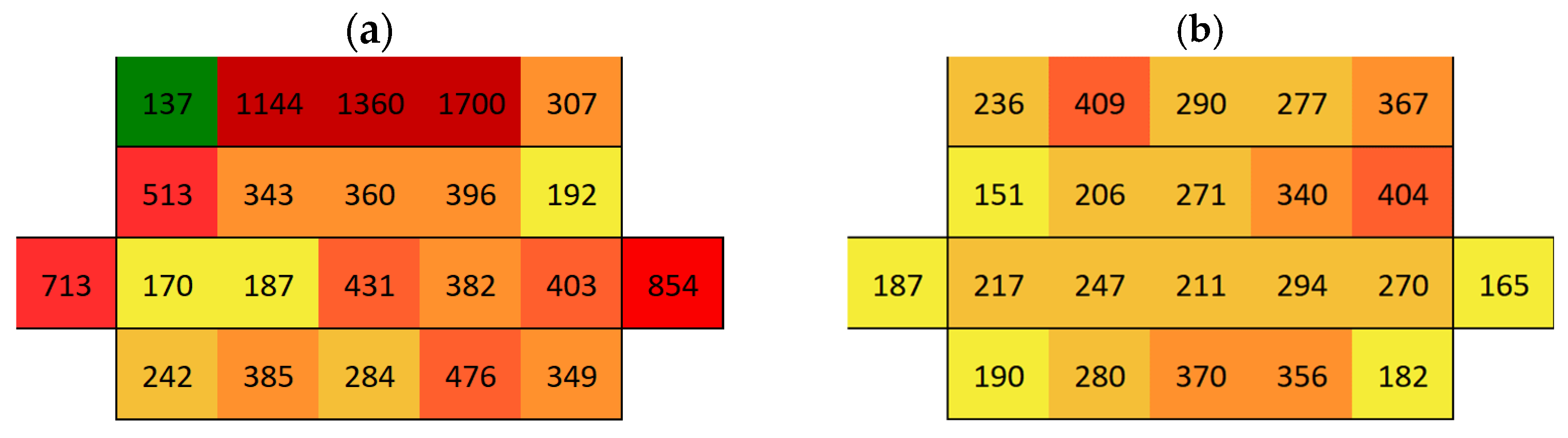
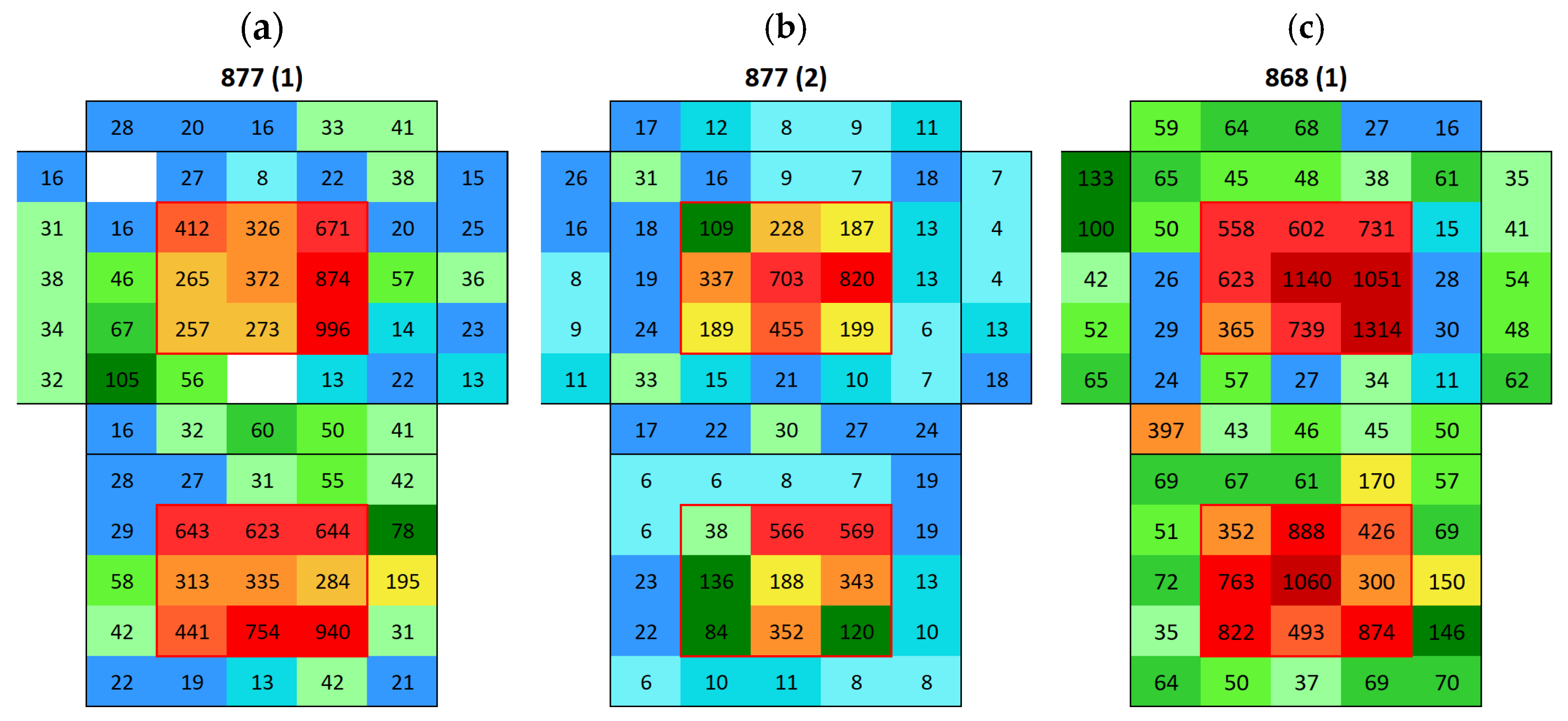
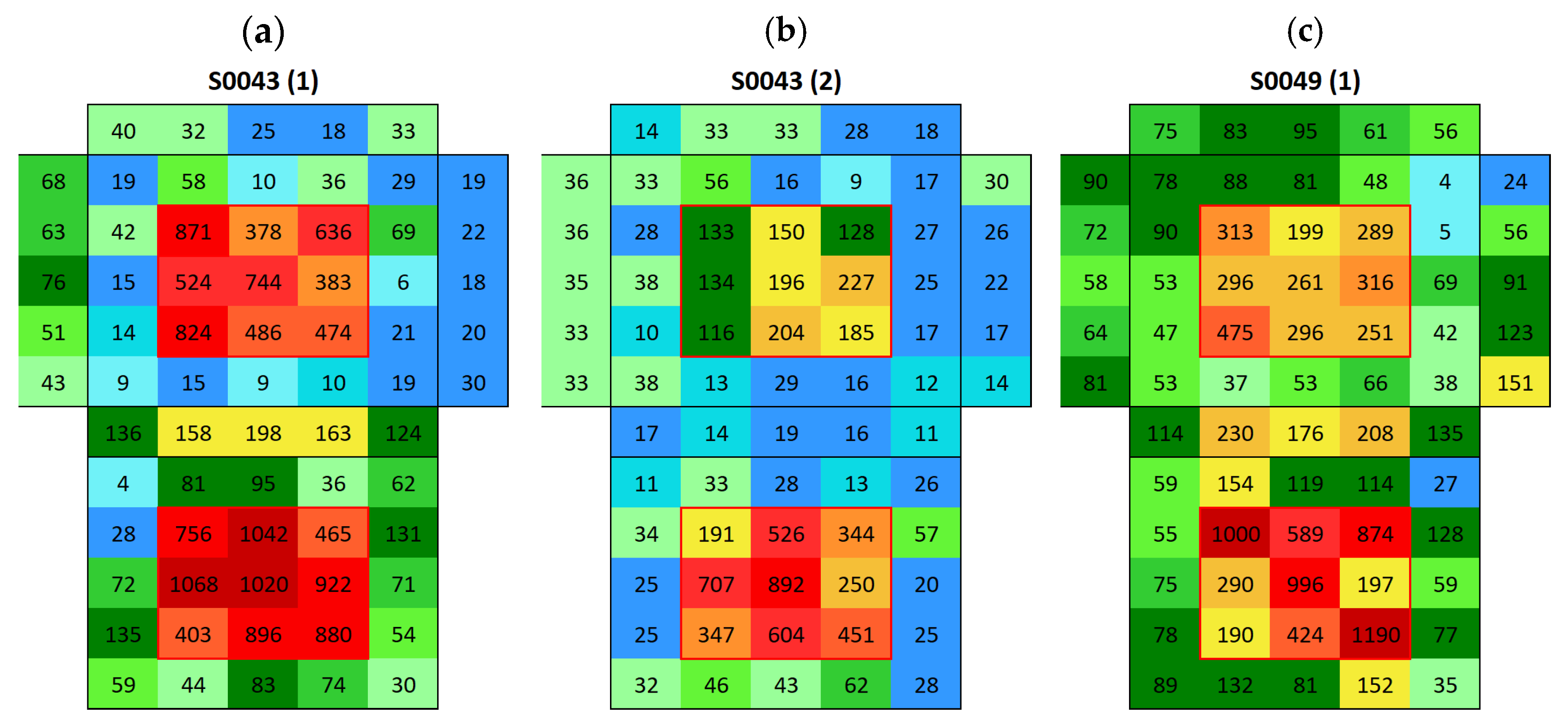
3.2. Surface Conductivity Before and After Immersion
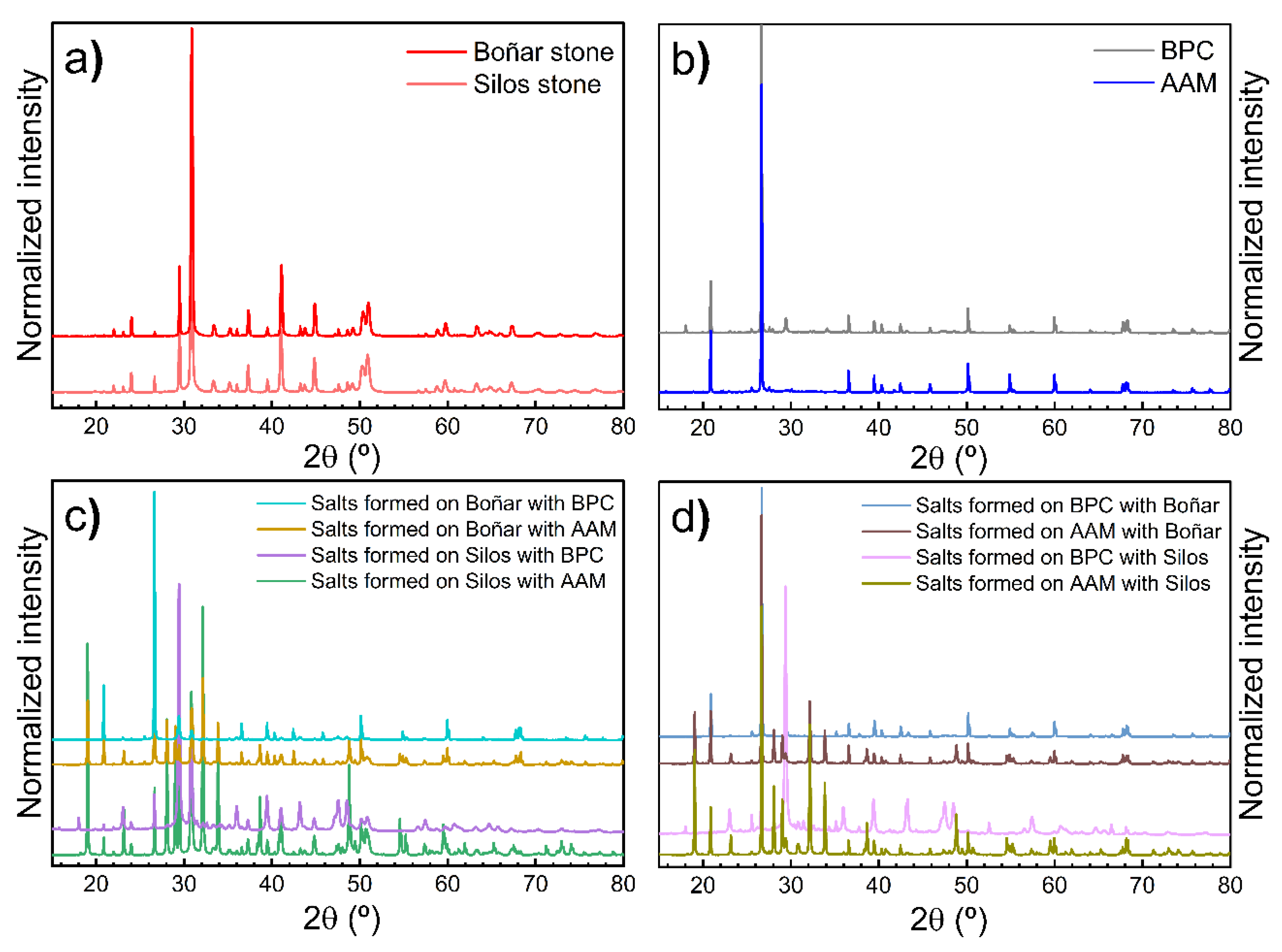
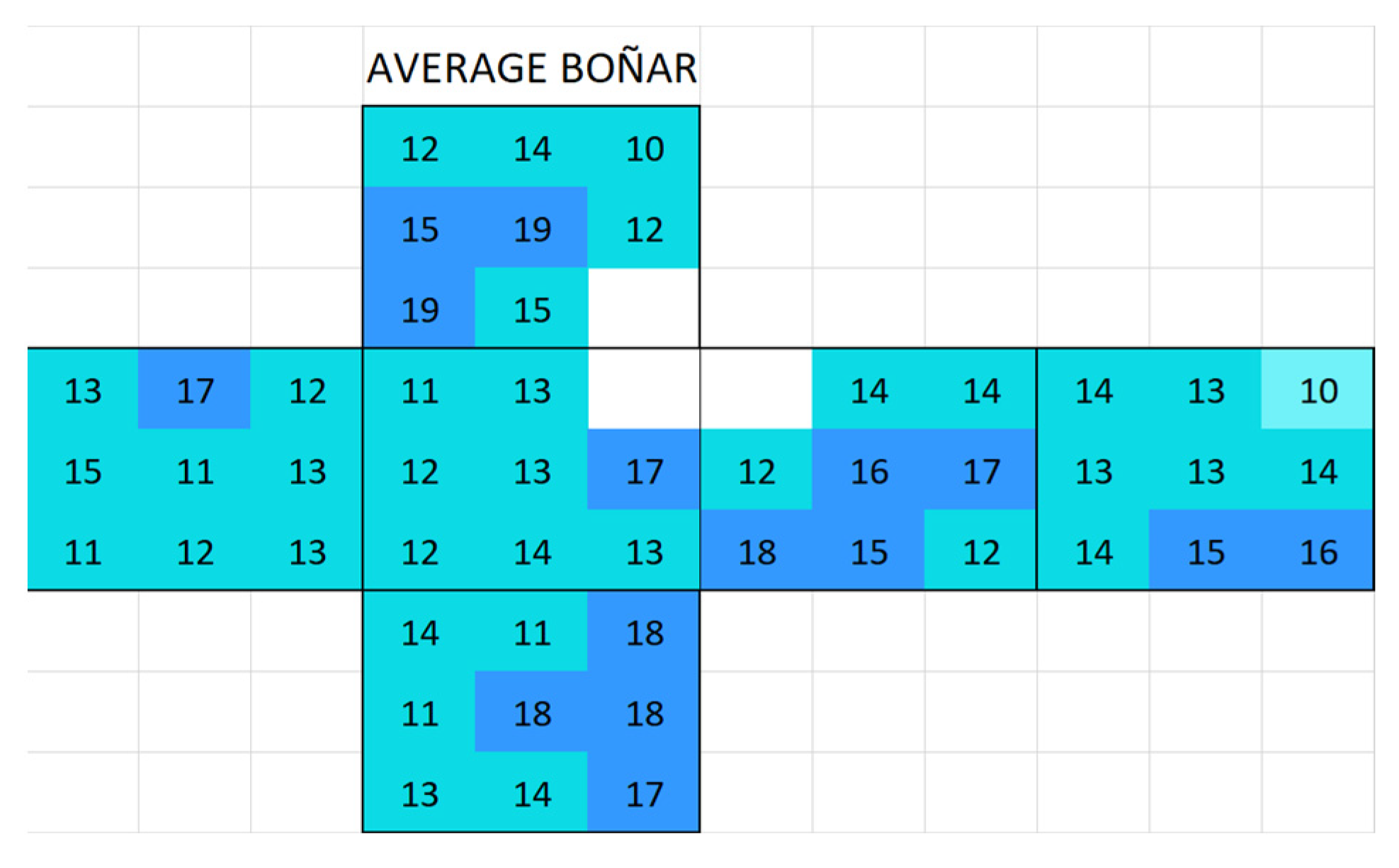
3.2.1. Boñar Stone Samples
3.2.2. Silos Stone Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Surface Conductivity Maps Individual Boñar and Silos Stones
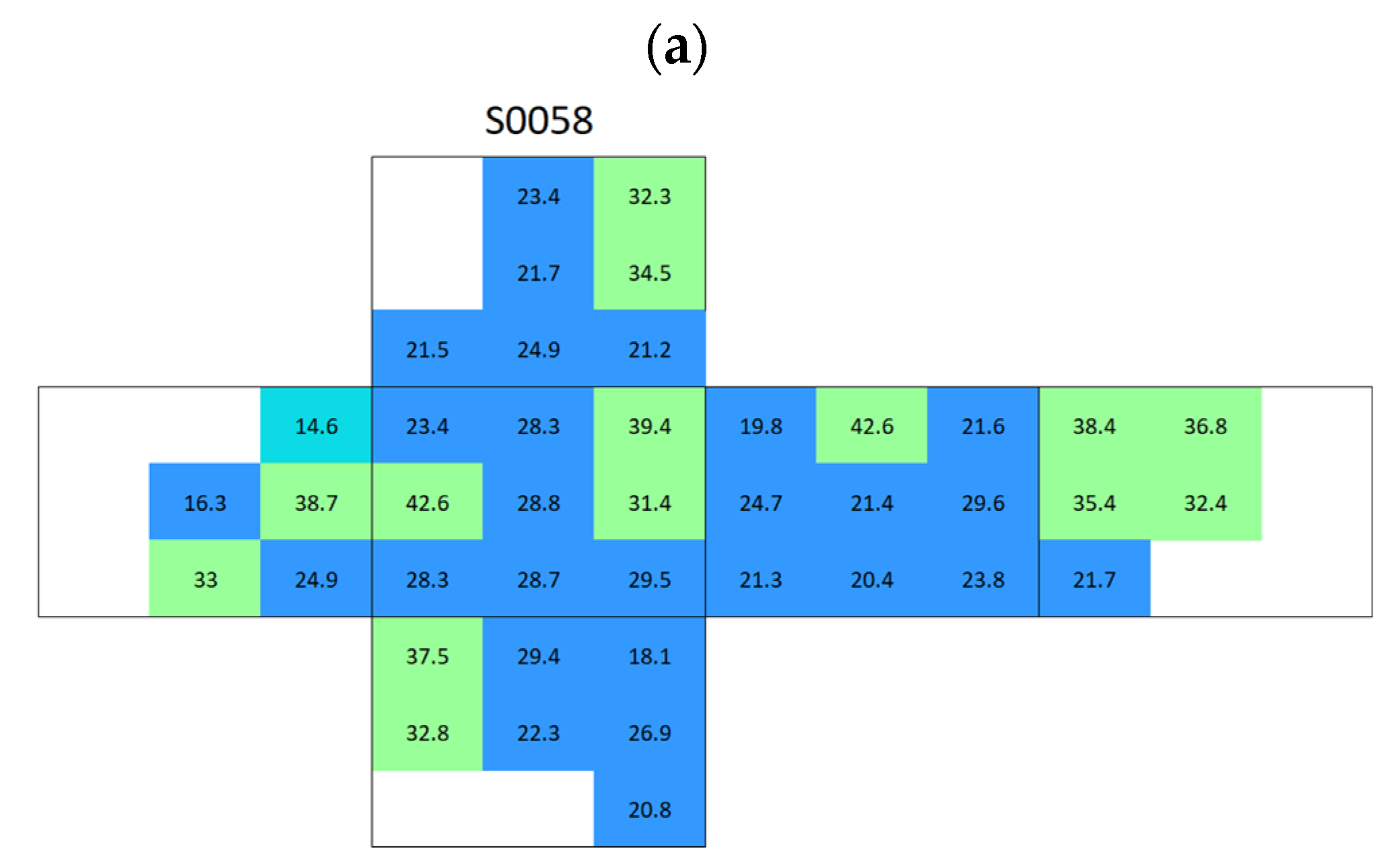
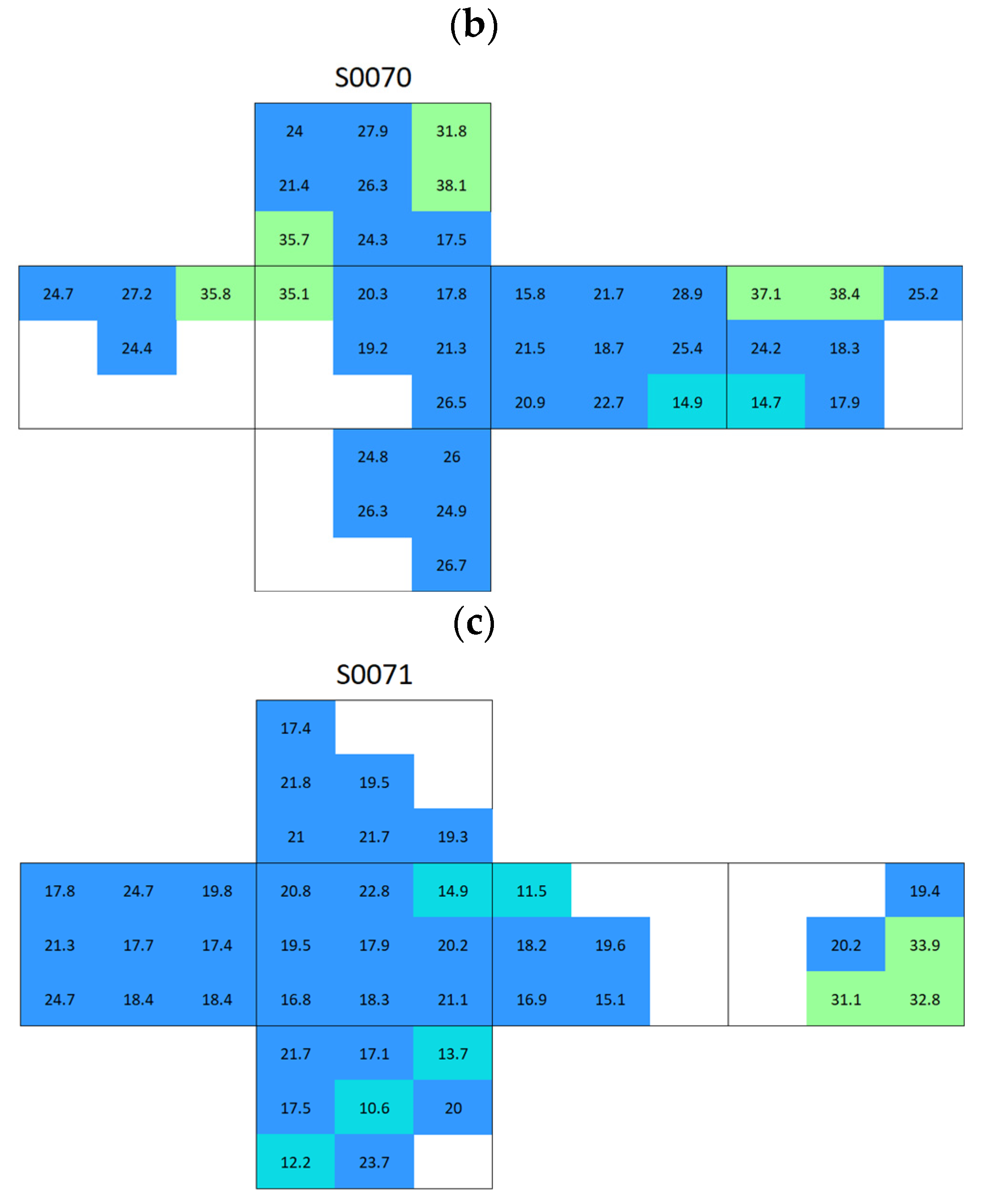
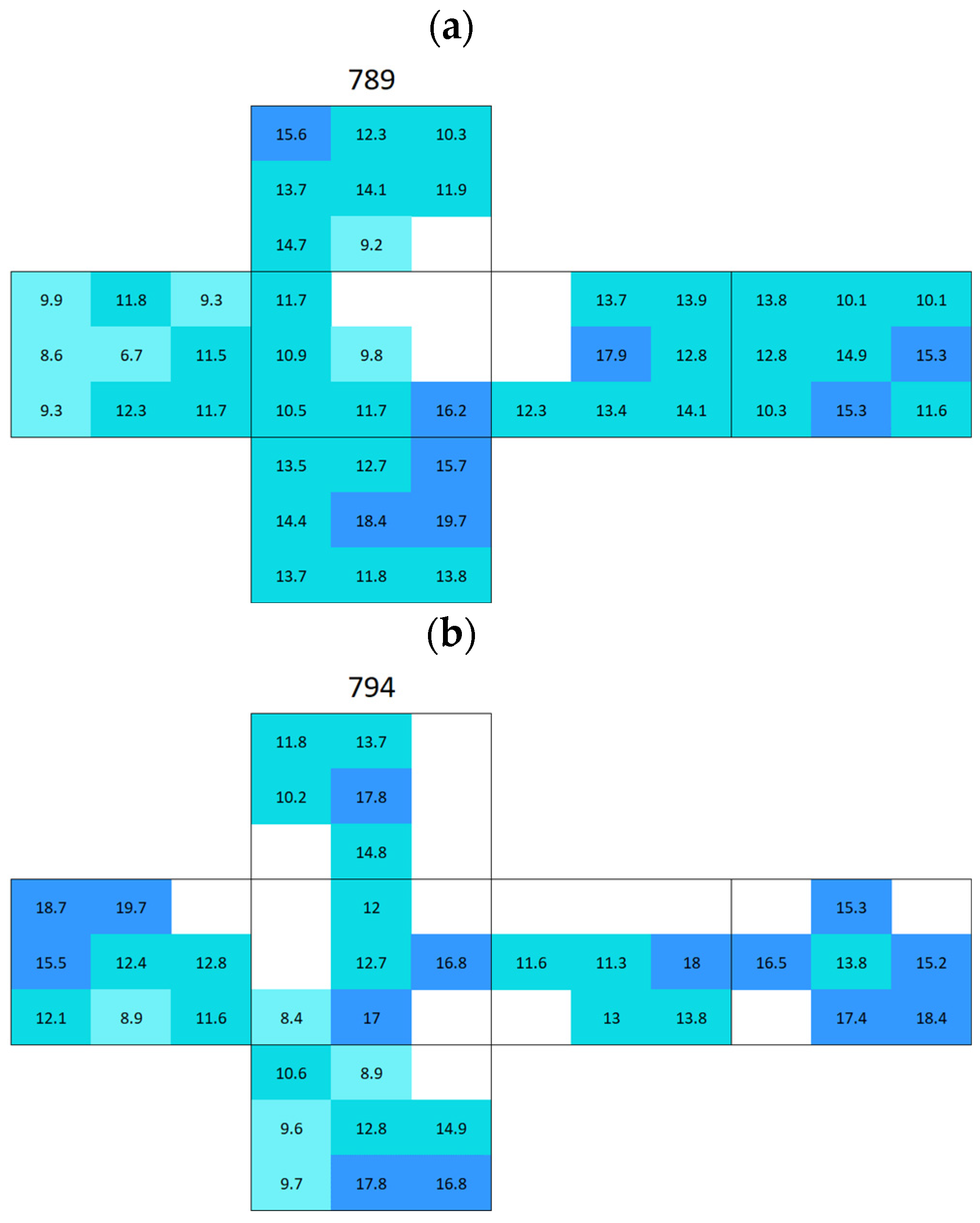
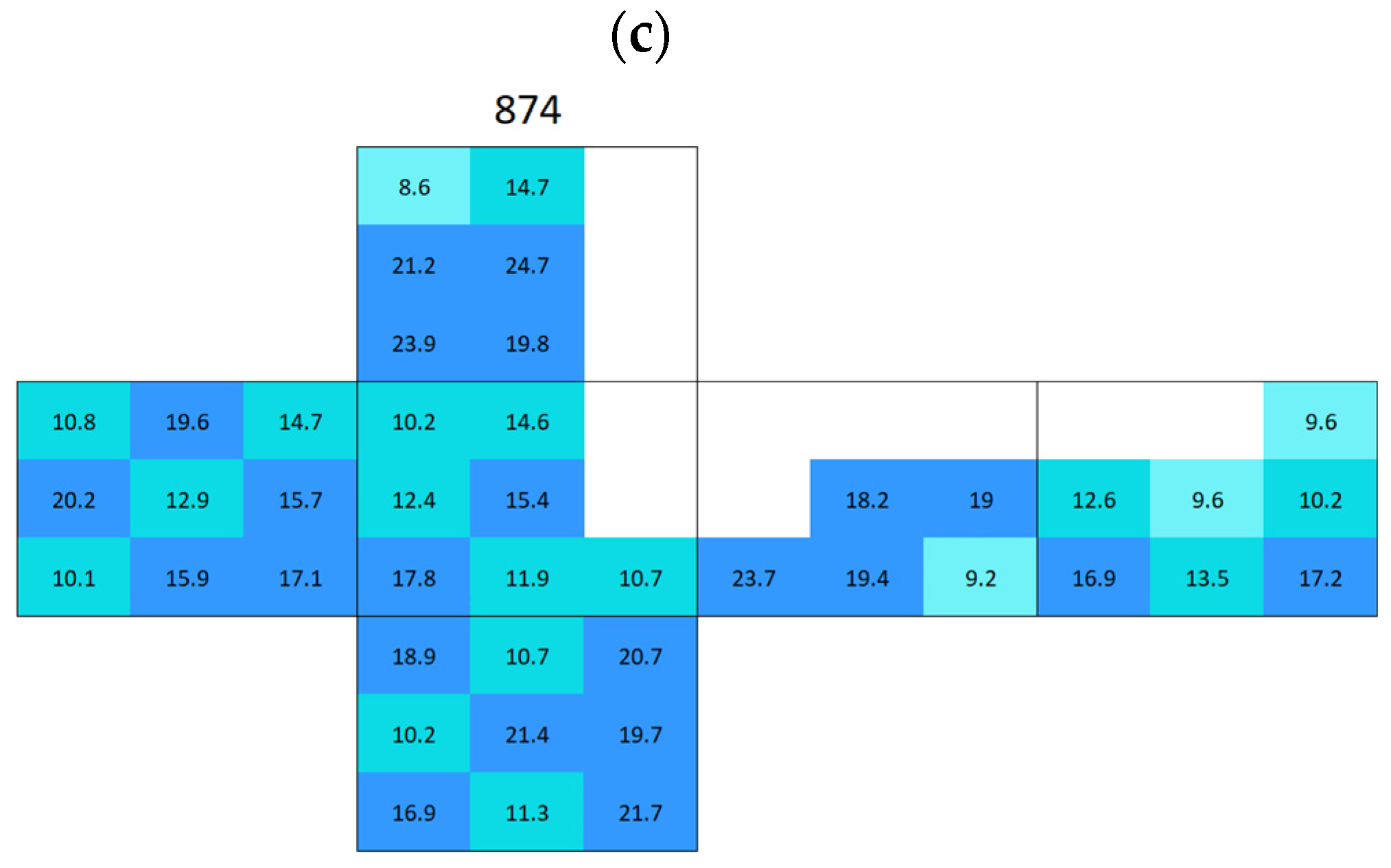
References
- Suchek, N.; Fernandes, C.I.; Kraus, S.; Filser, M.; Sjögrén, H. Innovation and the circular economy: A systematic literature review. Bus. Strategy Environ. 2021, 30, 3686–3702. [Google Scholar] [CrossRef]
- Ige, O.E.; Von Kallon, D.V.; Desai, D. Carbon emissions mitigation methods for cement industry using a systems dynamics model. Clean. Technol. Environ. Policy 2024, 26, 579–597. [Google Scholar] [CrossRef]
- Lanjewar, B.A.; Chippagiri, R.; Dakwale, V.A.; Ralegaonkar, R.V. Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies 2023, 16, 969. [Google Scholar] [CrossRef]
- Bang, J.; Choi, J.; Hong, W.T.; Jung, J.; Kim, G.M.; Yang, B. Influences of binder composition and carbonation curing condition on the compressive strength of alkali-activated cementitious materials: A review. J. CO2 Util. 2023, 74, 102551. [Google Scholar] [CrossRef]
- Xue, L.; Ni, Z.; Zhou, Z.; Zhang, Z.; Xiong, H.; Wang, H.; Zhuge, X.; Liu, H. Effects of desulfurized gypsum on shrinkage behavior of alkali-activated slag (AAS) and hybrid alkali-activated cement (HAC). Case Stud. Constr. Mater. 2025, 22, E04320. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- Lamaa, G.; Duarte, A.P.C.; Silva, R.V.; de Brito, J. Carbonation of Alkali-Activated Materials: A Review. Materials 2023, 16, 3086. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, A.; Jebter, A.A.; Hejazi, F. Investigation on durability of concrete exposed to chloride, sulfate attack and steel corrosion. IOP Conf. Ser. Earth Environ. Sci. 2019, 357, 012011. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Geng, J.; Zhang, Y.; Hu, J. Pore-structure and damage behaviors of cement stone subjected to dry-wet cycles. J. Rock Mech. Geotech. Eng. 2024, 16, 4944–4954. [Google Scholar] [CrossRef]
- Kramar, S.; Mladenović, A.; Pristacz, H.; Mirtič, B. DETERIORATION OF THE BLACK DRENOV GRIČ LIMESTONE ON HISTORICAL MONUMENTS (LJUBLJANA, SLOVENIA). Acta Carsologica 2011, 40, 483–495. [Google Scholar] [CrossRef]
- Kramar, S.; Urosevic, M.; Pristacz, H.; Mirtič, B. Assessment of limestone deterioration due to salt formation by micro-Ramanspectroscopy: Application to architectural heritage. J. Raman Spectrosc. 2010, 41, 1441–1448. [Google Scholar] [CrossRef]
- Schiro, M.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Damage mechanisms of porous materials due to in-pore salt crystallization. Phys. Rev. Lett. 2012, 109, 265503. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, L.; Wang, J.; Yue, L.; Shi, E.; Wang, Z.; Yang, T. Impact of Acid, Salt, and Wetting-Drying Cycles on Weathering Deterioration of the Red Sandstone Used for Leshan Giant Buddha China. Int. J. Archit. Herit. 2024, 19, 457–484. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.-W.; Ouyang, K.-G.; Ji, T.-T.; Gao, Y.-F.; Geng, X.-H.; Niu, R.; Huang, J.-Z.; Yan, H.-B.; Wan, L. The mechanisms of salt weathering responsible for sandstone deterioration in the Yungang Grottoes, China. Eng. Geol. 2025, 350, 107989. [Google Scholar] [CrossRef]
- Angeli, M.; Bigas, J.-P.; Benavente, D.; Menéndez, B.; Hébert, R.; David, C. Salt crystallization in pores: Quantification and estimation of damage. Environ. Geol. 2006, 52, 205–213. [Google Scholar] [CrossRef]
- Aguilar, J.G. El papel de la reproducción experimental de agentes/procesos en los estudios sobre el deterioro de la piedra de nuestro patrimonio histórico. In La Piedra en el Patrimonio Monumental; En Azofra Agustín, E., García-Talegón, J., M. Gutiérrez-Hernández, A., Eds.; Ediciones Univesidad de Salamanca: Salamanca, Spain, 2023; pp. 113–135. [Google Scholar] [CrossRef]
- Patil, S.M.; Kasthurba, A.K.; Patil, M.V. Characterization and assessment of stone deterioration on Heritage Buildings. Case Stud. Constr. Mater. 2021, 15, e00696. [Google Scholar] [CrossRef]
- Begonha, A. Mineralogical study of the deterioration of granite stones of two Portuguese churches and characterization of the salt solutions in the porous network by the presence of diatoms. Mater. Charact. 2009, 60, 621–635. [Google Scholar] [CrossRef]
- Buj, O.; Gisbert, J.; Franco, B.; Mateos, N.; Bauluz, B. Decay of the campanile limestone used as building material in tudela cathedral (Navarra, Spain). Geol. Soc. Spec. Publ. 2010, 331, 195–202. [Google Scholar] [CrossRef]
- Balboni, E.; Espinosa-Marzal, R.M.; Doehne, E.; Scherer, G.W. Can drying and re-wetting of magnesium sulfate salts lead to damage of stone? Environ. Earth Sci. 2011, 63, 1463–1473. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.; Zhao, G.; Zhou, J. Electrical resistivity analysis for the internal capillary water migration mechanism of porous stone. Acta Geophys. 2024, 72, 213–231. [Google Scholar] [CrossRef]
- Wang, J.; Yan, H.; Ren, W. Driving mechanisms and hydro-chemical variation for assessing salt-induced weathering of sandstone in grotto relics. Earth Surf. Process. Landf. 2024, 49, 2855–2869. [Google Scholar] [CrossRef]
- Monaco, M.; Aurilio, M.; Tafuro, A.; Guadagnuolo, M. Sustainable Mortars for Application in the Cultural Heritage Field. Materials 2021, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- EN 197-1; Cement-Part 1: Composition, Specifications and Conformity Criteria For Common Cements. European Committee for Standarization: Brussels, Belgium, 2011.
- EN 15167-1; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout-Part 1: Definitions, Specifications and Conformity Criteria. Bureau voor Normalisatie (NEN): Brussels, Belgium, 2006.
- EN 196-1; Methods of Testing Cement-Part 1: Determination of Strength. European Committee for Standarization: Brussels, Belgium, 2018.
- İnce, İ.; Bozdağ, A.; Barstuğan, M.; Fener, M. Evaluation of the relationship between the physical properties and capillary water absorption values of building stones by regression analysis and artificial neural networks. J. Build. Eng. 2021, 42, 103055. [Google Scholar] [CrossRef]
- İnce, İ. Relationship Between Capillary Water Absorption Value, Capillary Water Absorption Speed, and Capillary Rise Height in Pyroclastic Rocks. Min. Metall. Explor. 2021, 38, 841–853. [Google Scholar] [CrossRef]
- Sariisik, A.; Sariisik, G.; Senturk, A. Characterization of physical and mechanical properties of natural stones affected by ground water under different ambient conditions. Ekoloji 2010, 19, 88–96. [Google Scholar] [CrossRef]






| Na | Mg | Al | Si | P | S | Ca | Sr | |
|---|---|---|---|---|---|---|---|---|
| PG | 4.6 | - | 0.8 | - | 0.21 | 18 | 21 | 1.6 |
| GGBFS | 1.2 | 7.8 | 9 | 25 | - | 1.3 | 38 | 0.1 |
| Boñar Stone | Silos Stone | |||
|---|---|---|---|---|
| BPC | AAM | BPC | AAM | |
| Capillarity | 631 (with) | 784 (with) | S0052 (without) | S0044 (with) |
| Immersion | 636 (with) | 868 (with) | S0055 (with) | S0043 (without) |
| 887 (without) | 877 (without) | S0056 (without) | S0049 (without) | |
| Boñar Stone | Silos Stone | |||
|---|---|---|---|---|
| BPC | AAM | BPC | AAM | |
| 631 (with sand) | 784 (with sand) | S0052 (without sand) | S0044 (with sand) | |
| 1st experiment | 0.176 | 0.232 | 0.459 | 0.369 |
| 2nd experiment | 0.181 | 0.291 | 0.153 | 0.256 |
| 3rd experiment | 0.275 | 0.281 | 0.790 | 0.346 |
| Mean | 0.211 | 0.268 | 0.467 | 0.324 |
| StDev | 0.056 | 0.032 | 0.319 | 0.060 |
| Dolomite MgCa(CO3)2 | Calcite CaCO3 | Quartz SiO2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Boñar stone | 93.122 | 6.471 | 0.408 | |||||
| Silos stone | 87.899 | 11.668 | 0.433 | |||||
| Dolomite MgCa(CO3)2 | Quartz SiO2 | Orthoclase KAlSi3O8 | Oligoclase (Ca, Na) (Al, Si)4O8 | Alite Ca3SiO5 | Muscovite KAl2(AlSi3O10) (F,OH)2 | Ettringite Ca6Al2(SO4)3 (OH)12·26H2O | Lime Ca(OH)2 | |
| BPC | 16.744 | 65.535 | 5.866 | 1.101 | 5.449 | 5.303 | ||
| AAM | 68.863 | 11.206 | 1.865 | 4.098 | 2.393 | 11.574 | ||
| Dolomite MgCa(CO3)2 | Calcite CaCO3 | Quartz SiO2 | Larnite Ca2SiO4 | Corundum Al2O3 | Thenardite Na2SO4 | Anhydrite CaSO4 | Lime Ca(OH)2 | |
| Salts formed on Boñar with BPC | 16.542 | 28.034 | 52.806 | 2.347 | 0.271 | |||
| Salts formed on Boñar with AAM | 26.35 | 7.598 | 21.328 | 44.723 | ||||
| Salts formed on Silos with BPC | 32.761 | 59.028 | 1.57 | 4.861 | 1.78 | |||
| Salts formed on Silos with AAM | 35.824 | 6.682 | 3.193 | 54.301 | ||||
| Salts formed on BPC with Boñar | 23.646 | 61.394 | 14.96 | |||||
| Salts formed on AAM with Boñar | 9.64 | 25.77 | 60.125 | 4.465 | ||||
| Salts formed on BPC with Silos | 2.287 | 81.389 | 1.358 | 3.663 | 9.34 | 1.964 | ||
| Salts formed on AAM with Silos | 6.665 | 6.758 | 27.15 | 2.347 | 57.02 | 2.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollet, L.; Antolín-Rodríguez, A.; Gisbert-Aguilar, J.; Búrdalo-Salcedo, G.; Juan-Valdés, A.; García-Álvarez, C.; Raga-Martín, A.; Schroeyers, W.; Calvo, V.; Fernández-Raga, M. Novel Methodology to Assess Salt Movement Between Mortar and Stones from Heritage in Spain. Materials 2025, 18, 3340. https://doi.org/10.3390/ma18143340
Pollet L, Antolín-Rodríguez A, Gisbert-Aguilar J, Búrdalo-Salcedo G, Juan-Valdés A, García-Álvarez C, Raga-Martín A, Schroeyers W, Calvo V, Fernández-Raga M. Novel Methodology to Assess Salt Movement Between Mortar and Stones from Heritage in Spain. Materials. 2025; 18(14):3340. https://doi.org/10.3390/ma18143340
Chicago/Turabian StylePollet, Linde, Andrea Antolín-Rodríguez, Josep Gisbert-Aguilar, Gabriel Búrdalo-Salcedo, Andrés Juan-Valdés, César García-Álvarez, Angel Raga-Martín, Wouter Schroeyers, Víctor Calvo, and María Fernández-Raga. 2025. "Novel Methodology to Assess Salt Movement Between Mortar and Stones from Heritage in Spain" Materials 18, no. 14: 3340. https://doi.org/10.3390/ma18143340
APA StylePollet, L., Antolín-Rodríguez, A., Gisbert-Aguilar, J., Búrdalo-Salcedo, G., Juan-Valdés, A., García-Álvarez, C., Raga-Martín, A., Schroeyers, W., Calvo, V., & Fernández-Raga, M. (2025). Novel Methodology to Assess Salt Movement Between Mortar and Stones from Heritage in Spain. Materials, 18(14), 3340. https://doi.org/10.3390/ma18143340








