The Effect of Aging Treatment on the Properties of Cold-Rolled Cu-Ni-Si-Co Alloys with Different Mg Contents
Abstract
1. Introduction
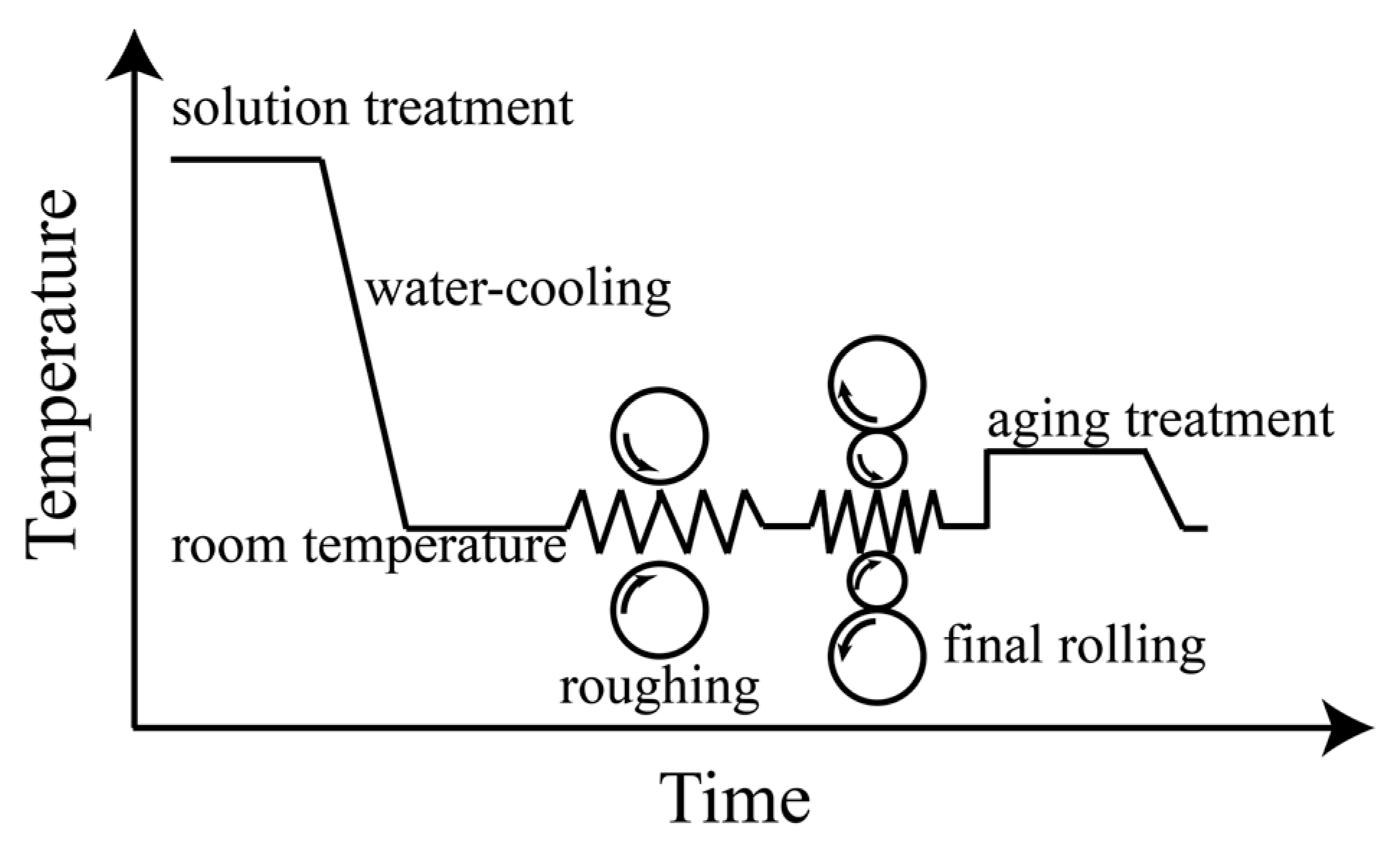
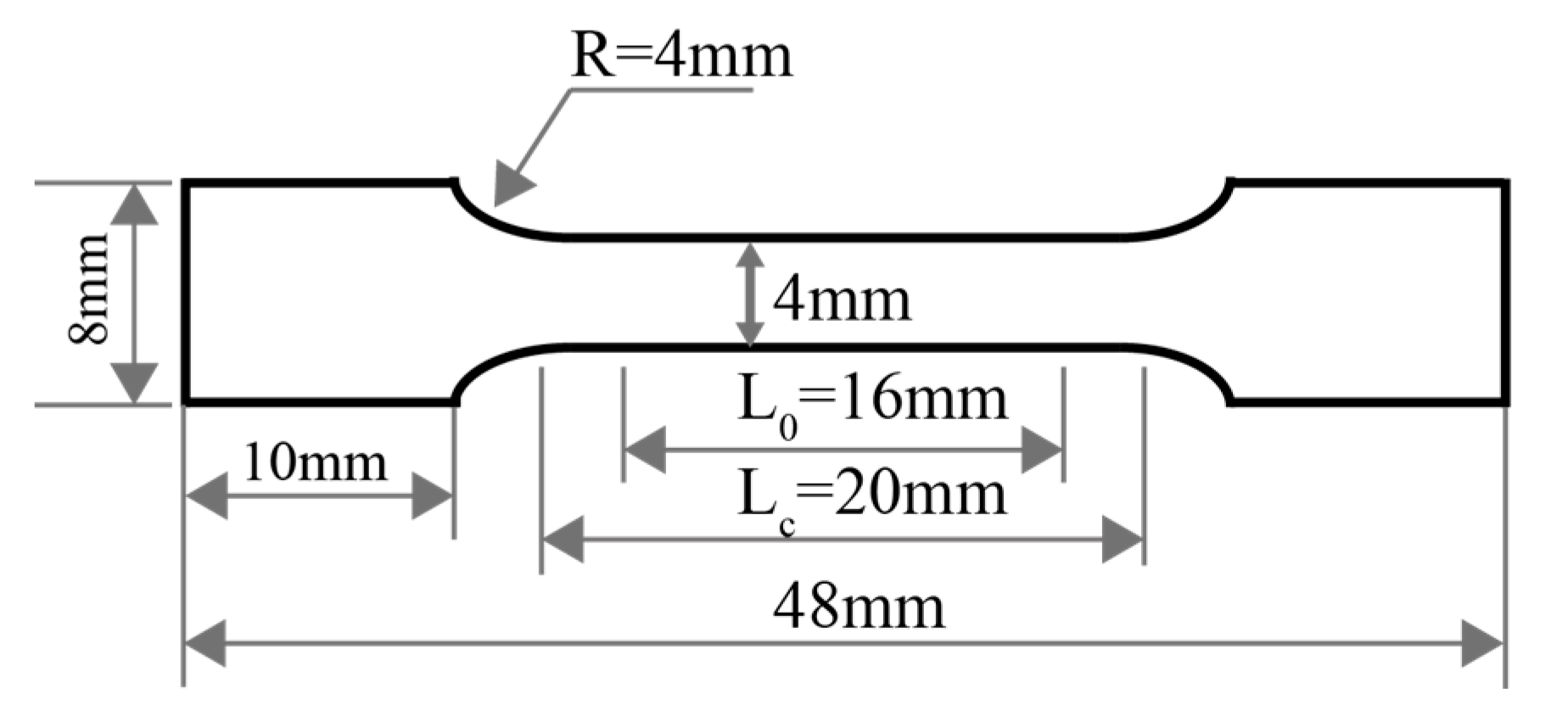
2. Materials and Methods
3. Results
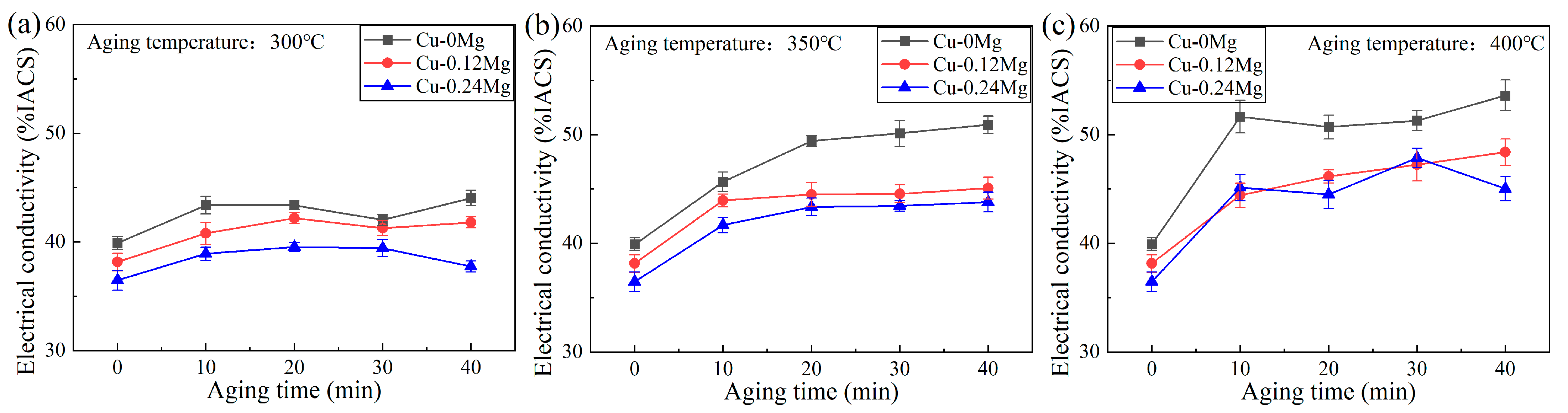
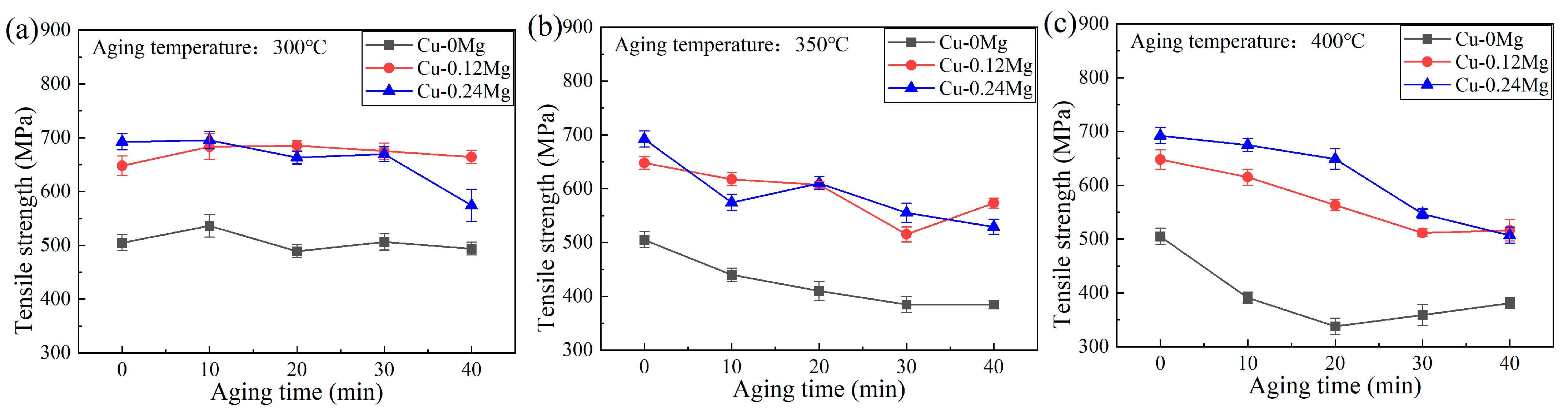
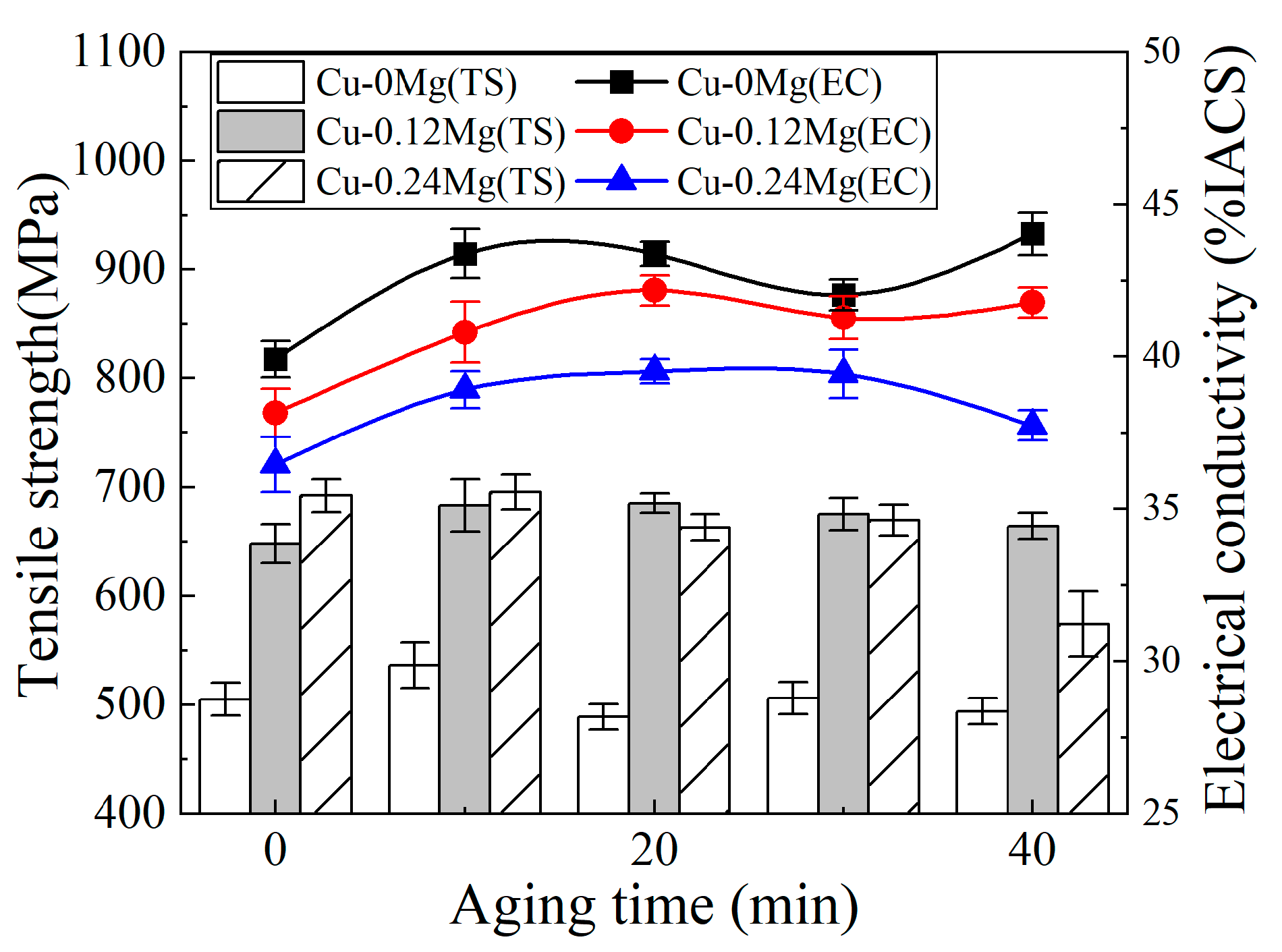
3.1. Conductivity and Strength
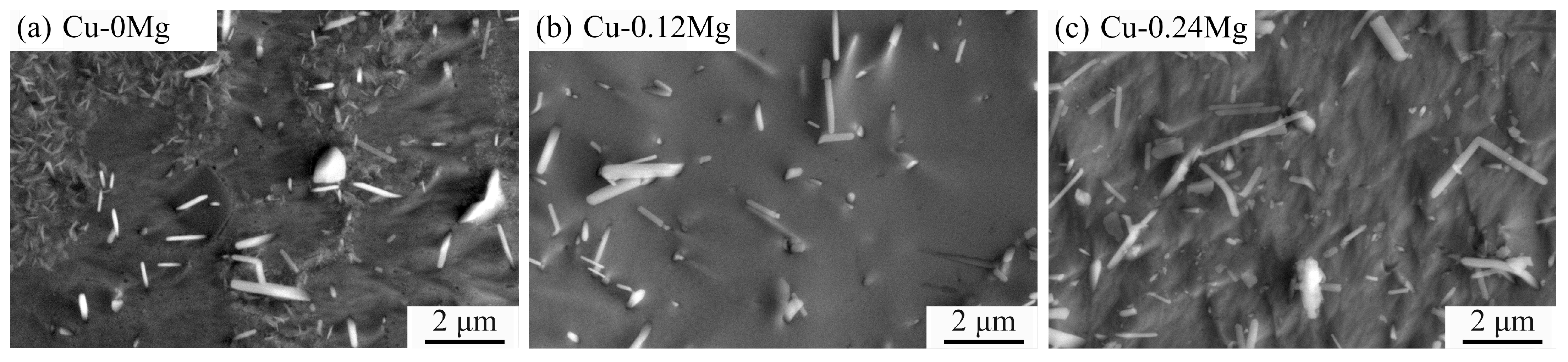
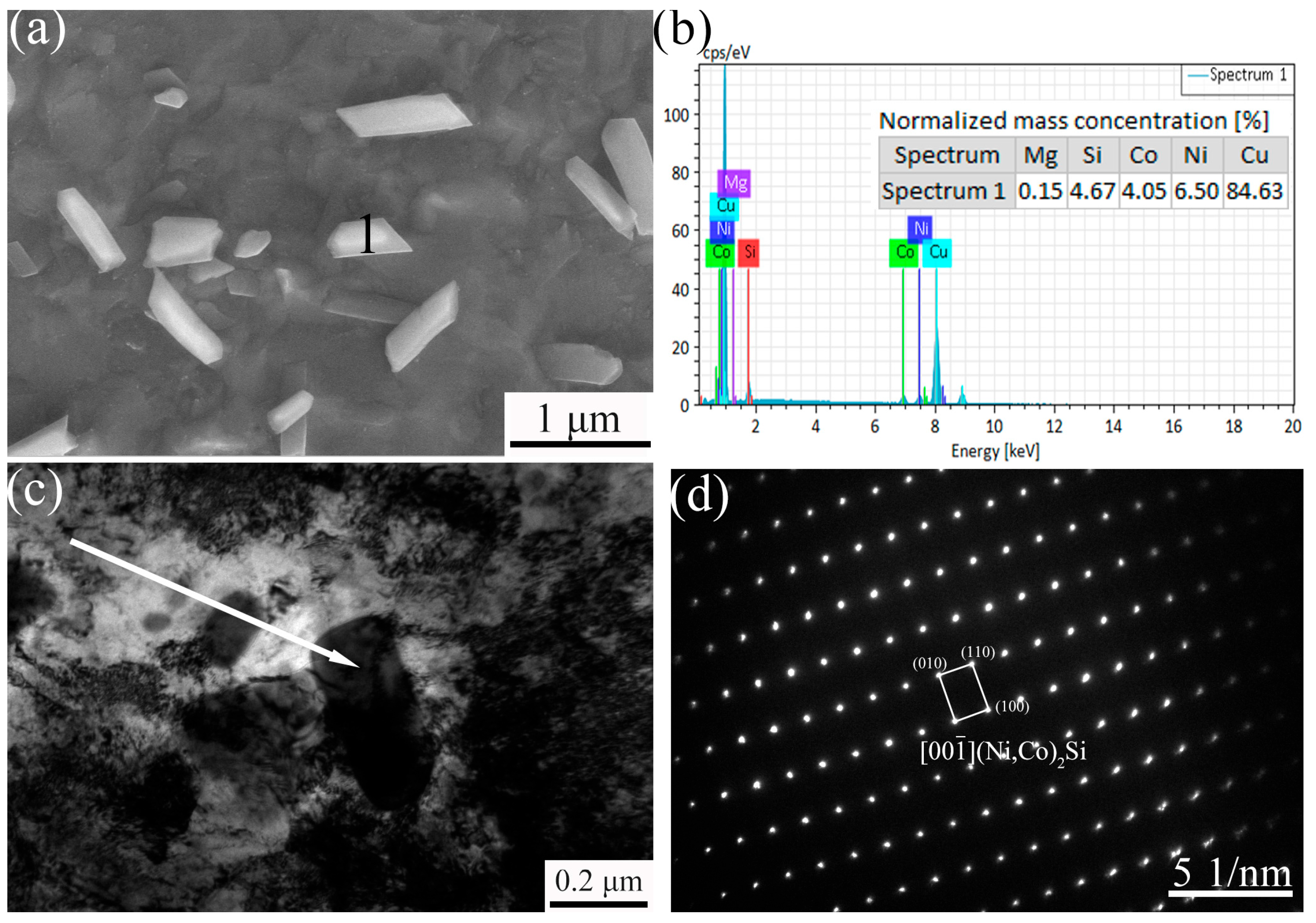
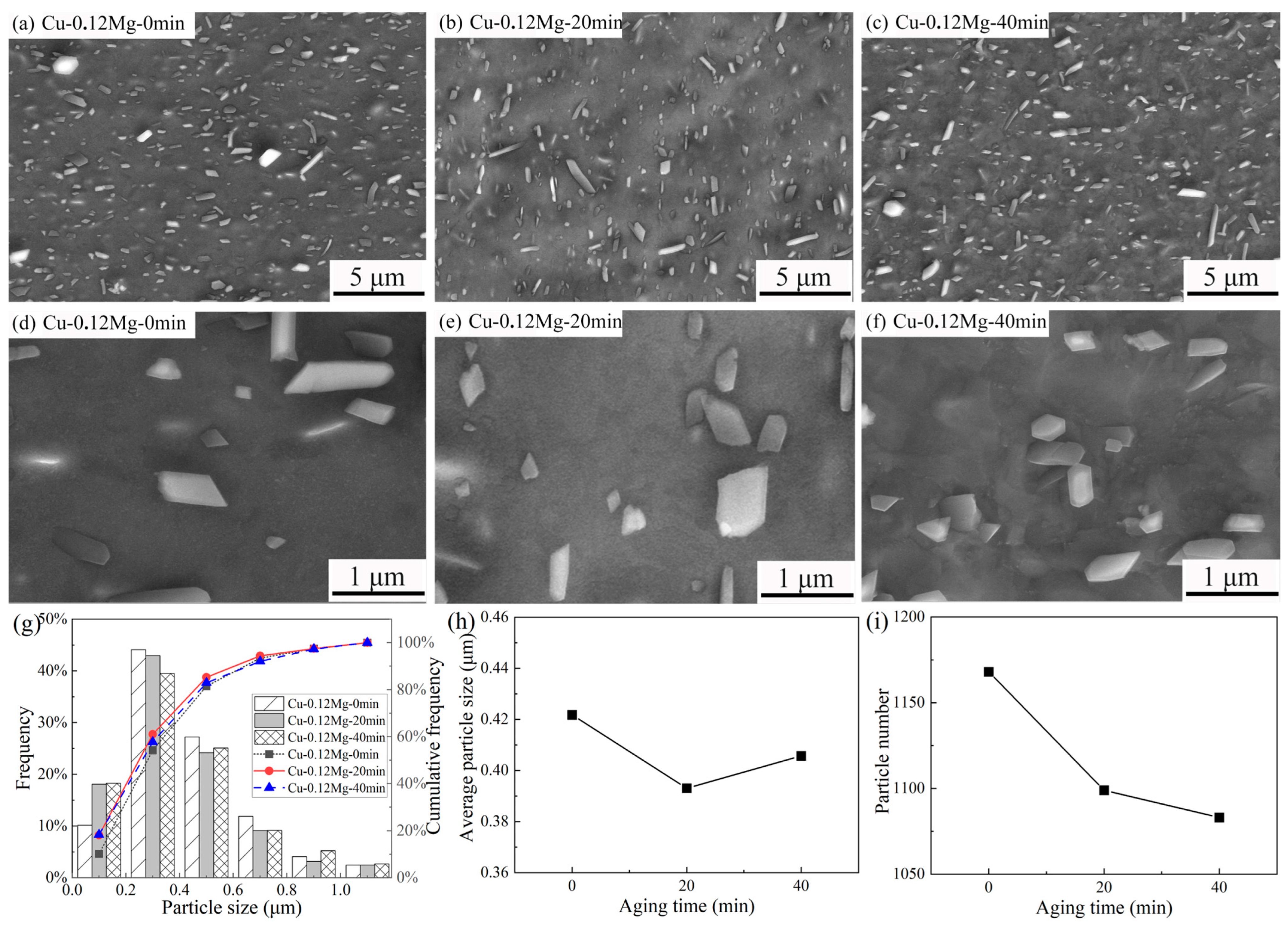
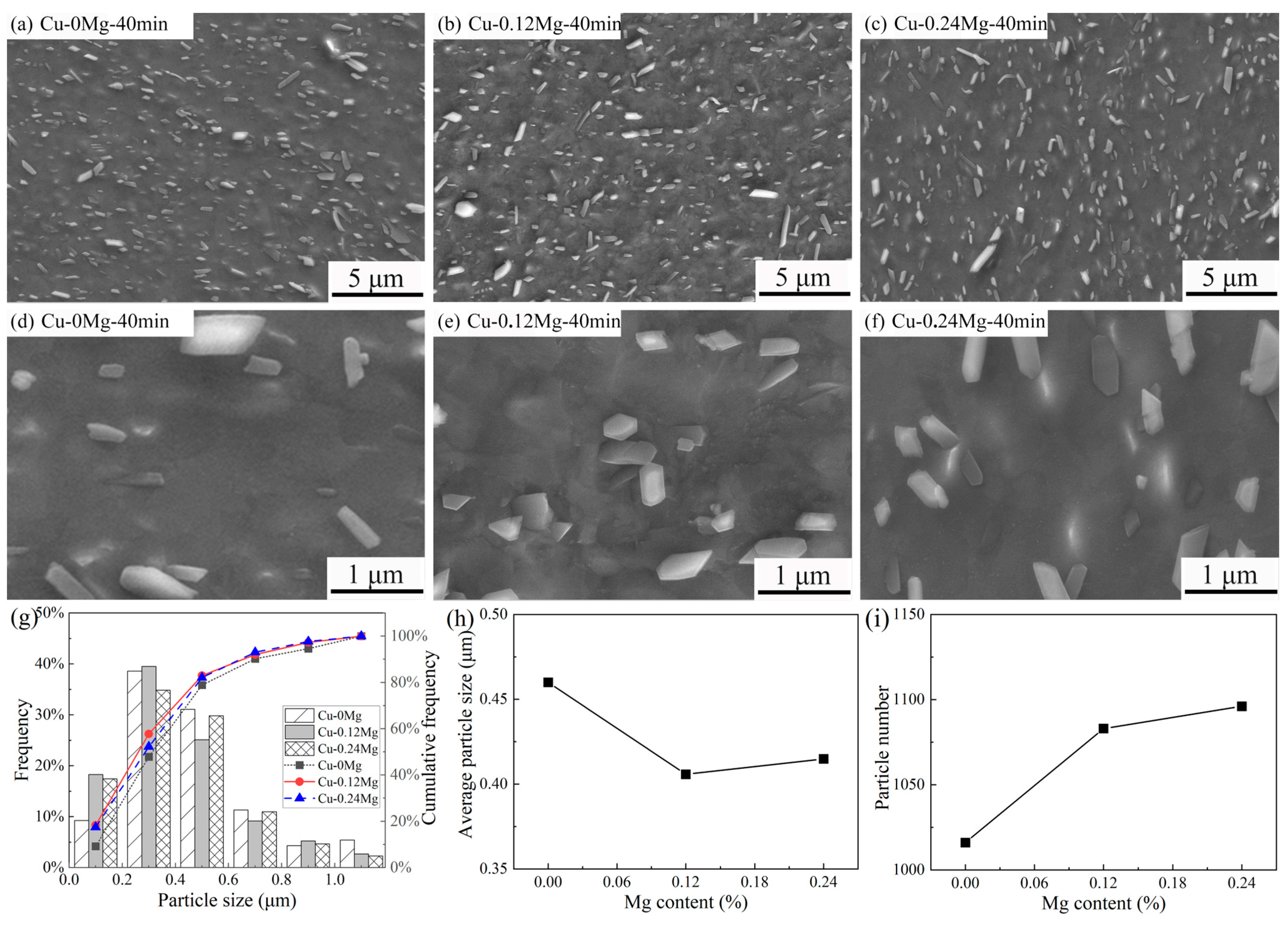
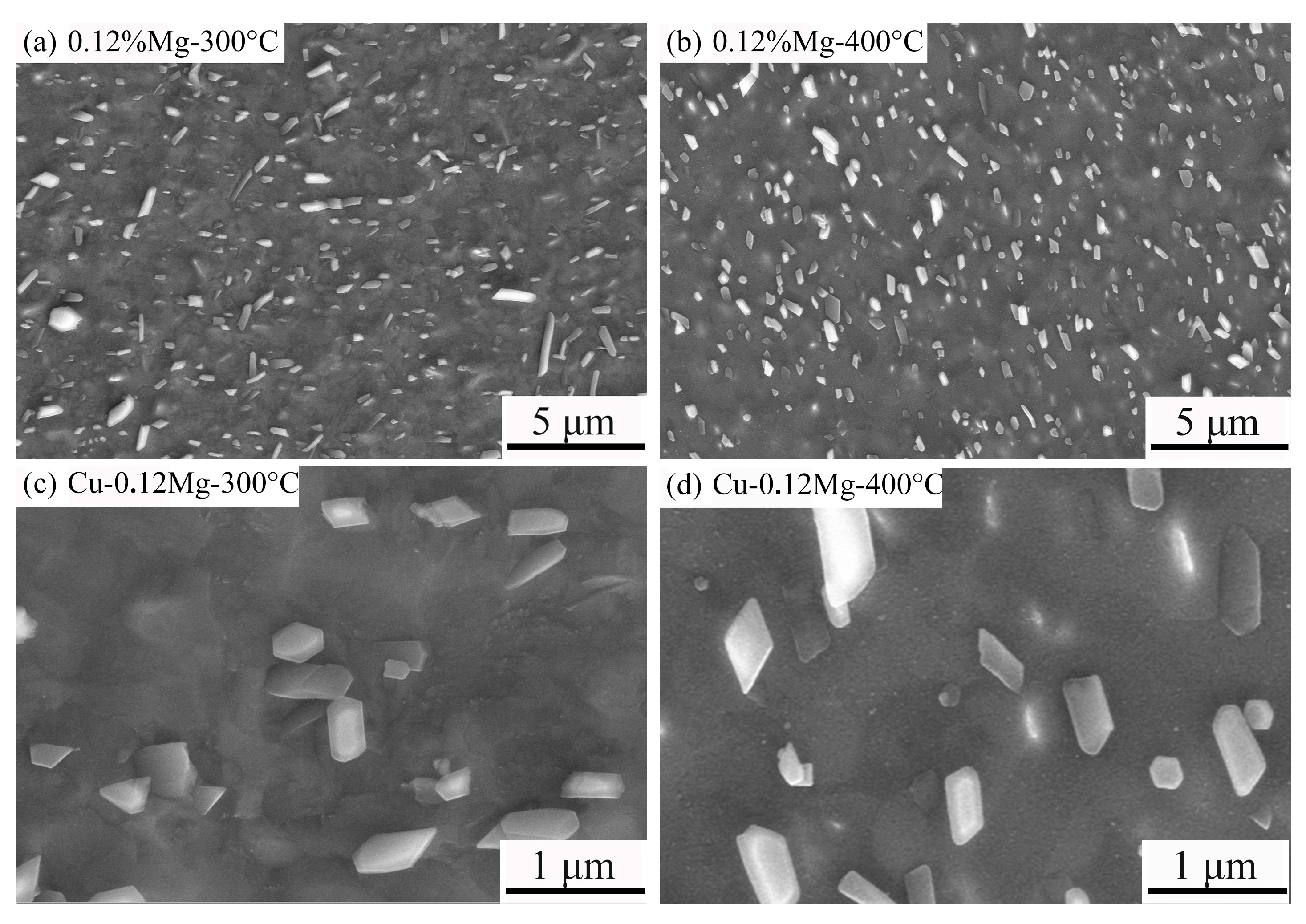
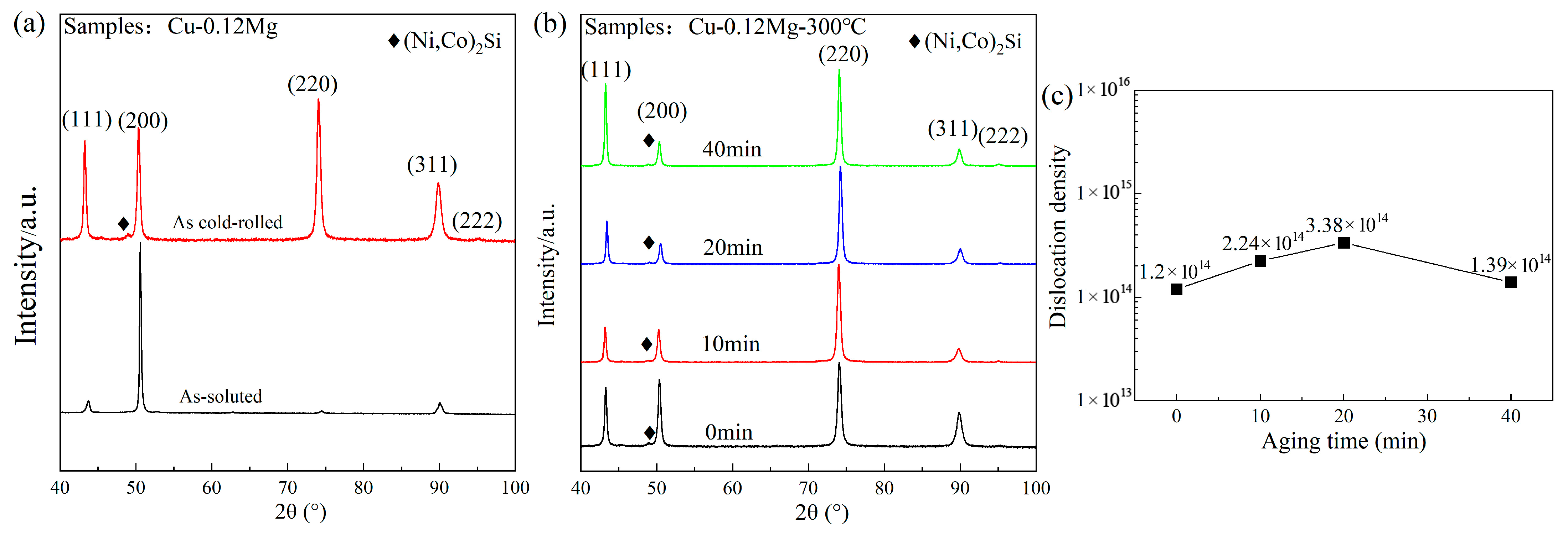
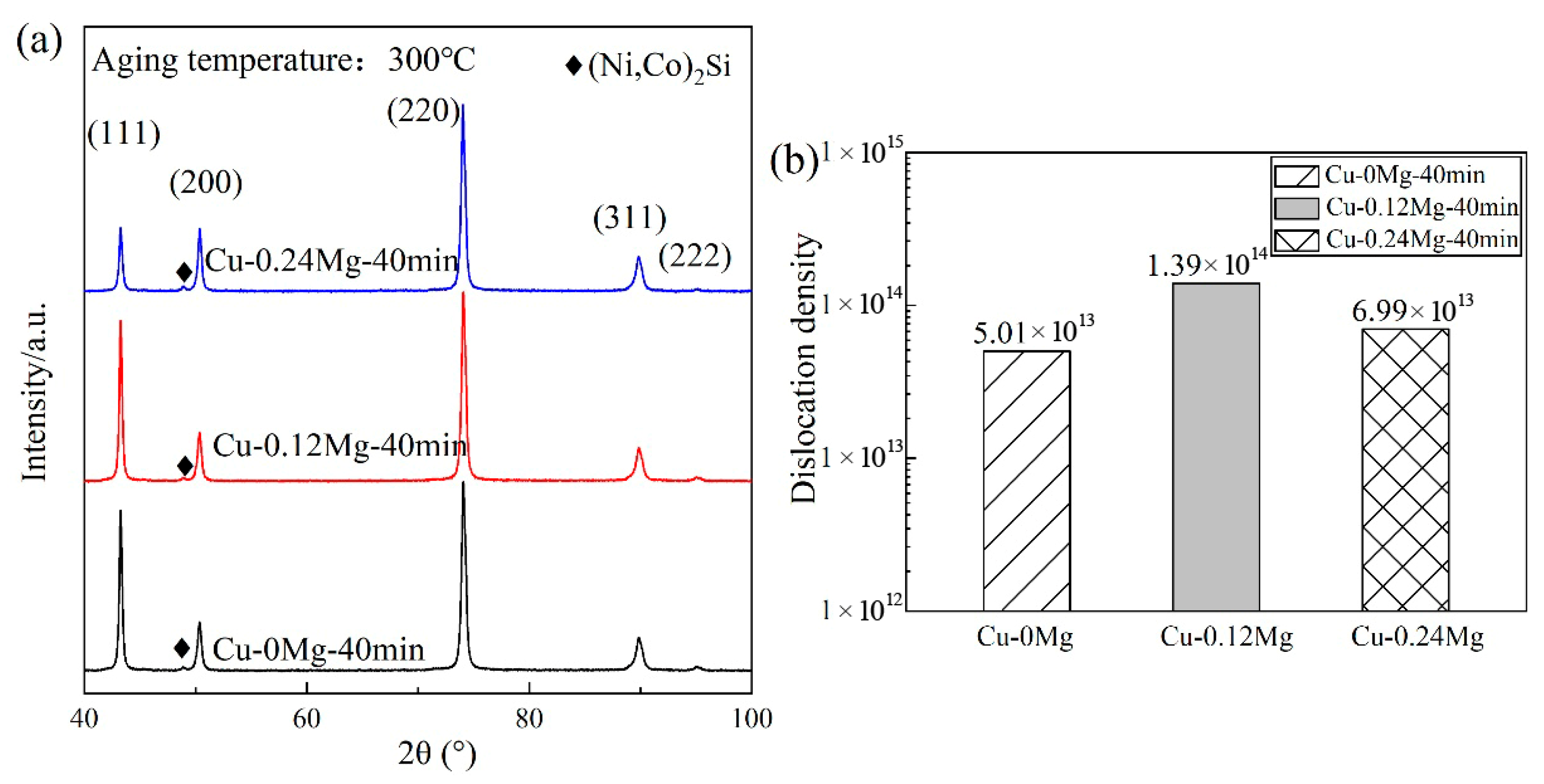
3.2. Precipitate Observation
4. Discussion
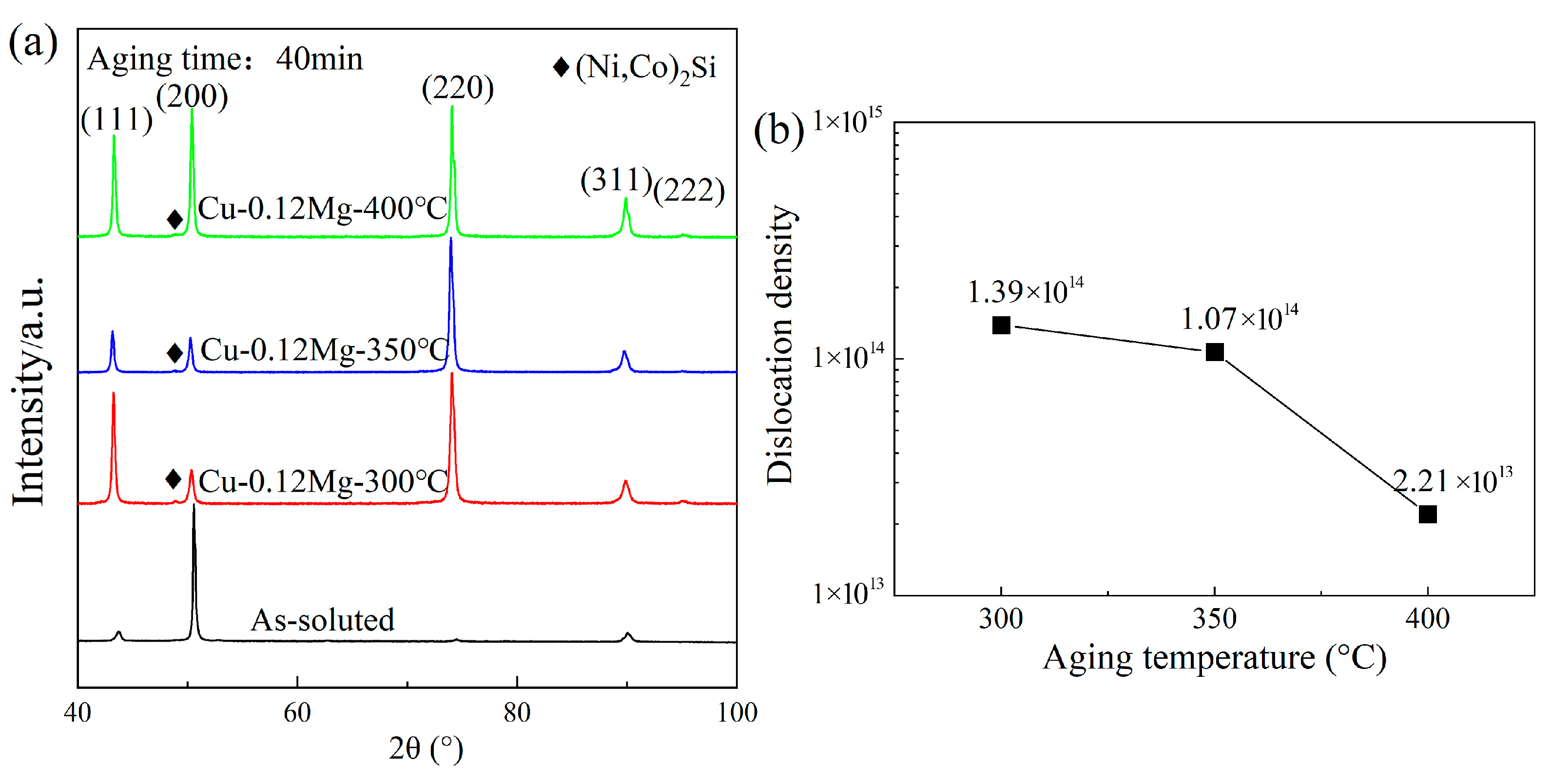
4.1. Dislocation Analysis
4.2. Effects of Mg Addition on the Precipitates and Mechanical Properties
5. Conclusions
- (1)
- The precipitate was (Ni, Co)2Si and exhibited a strip shape. For the Cu-0.12Mg-300 °C samples, a substantial quantity of small (Ni, Co)2Si precipitates were formed in the early stage of aging. However, as the aging time increased, the coarsening of the precipitates became increasingly dominant. The addition of 0.12%Mg promoted the precipitation of small precipitates. The number of precipitates increased by 6.6%, while the average particle size decreased by 11.8%. However, the number of precipitates increased by just 1.2% as the Mg content increased from 0.12% to 0.24%.
- (2)
- For the Cu-0.12Mg-300 °C samples, the dislocation density showed an increasing and then decreasing trend with the aging time. The value increased from 1.2 × 1014/m2 (at 0 min) to 3.38 × 1014/m2 (at 20 min) and then decreased to 1.39 × 1014/m2 (at 40 min). The aging temperature had a significant effect on the dislocation density, which decreased from 1.39 × 1014/m2 to 2.21 × 1013/m2 as the aging temperature increased from 300 °C to 400 °C. The addition of 0.12%Mg resulted in a corresponding increase in the dislocation density.
- (3)
- The addition of 0.12%Mg increased the tensile strength by around 150–200 MPa, but caused a slight reduction in the electrical conductivity. However, as the Mg content increased from 0.12% to 0.24%, there was little change in tensile strength, but the electrical conductivity decreased significantly. The electrical conductivities of the Cu-0Mg-300 °C-20 min, Cu-0.12Mg-300 °C-20 min and Cu-0.24Mg-300 °C-20 min samples were 43.4%IACS, 42.2%IACS and 39.5%IACS, respectively. Meanwhile, their respective tensile strengths were 489 MPa, 685 MPa and 663 MPa. The Cu-0.12Mg-300 °C-20 min sample had the best performance in terms of overall electrical conductivity and tensile strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Wang, Y.; Guo, M.; Wang, H.; Mo, Y.; Dong, X.; Lou, H. Recent development of advanced precipitation-strengthened Cu alloys with high strength and conductivity: A review. Prog. Mater. Sci. 2023, 138, 101141. [Google Scholar] [CrossRef]
- Sun, X.; Jie, J.; Wang, T.; Li, T. Effect of two-step cryorolling and aging on mechanical and electrical properties of a Cu–Cr–Ni–Si alloy for lead frames applications. Mater. Sci. Eng. A 2021, 809, 140521. [Google Scholar] [CrossRef]
- Jiang, Y.; Lou, H.; Xie, H.; Li, T.; Song, K.; Liu, X.; Yun, X.; Wang, H.; Xiao, Z.; Li, Z. Development status and prospects of advanced copper alloy. Chin. J. Eng. Sci. 2020, 22, 84–92. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Z.; Lei, C.; Meng, L.; Fang, Y.; Liu, J.; Wang, H. High strength and high conductivity Cu alloys: A review. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Song, H.; Zhang, S. Enhanced mechanical properties and strengthening mechanism of Cu-Ni-Si alloy with trace chromium addition. Mater. Today Commun. 2024, 38, 107729. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Aliyu, A.; Bishop, D.P.; Nasiri, A. Additive manufacturing of copper-based alloys for high-temperature aerospace applications: A review. Mater. Today Commun. 2024, 38, 108395. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Zhu, Q.; Wang, H.; Chen, Q.; Feng, J.; Zhou, Y.; Liu, H.; Li, Q.; Song, K. Microstructure and residual stress gradient evolution mechanism of electronic copper strip for lead frame. J. Alloy. Compd. 2024, 1009, 176902. [Google Scholar] [CrossRef]
- Monzen, R.; Watanabe, C. Microstructure and mechanical properties of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2008, 483, 117–119. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Zeng, W.; Liu, Y.; Lu, L.; Sun, B. Study on high-strength and high-conductivity Cu–Fe–P alloys. Mater. Sci. Eng. A 2006, 421, 254–259. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, Y.; Song, K.; Zhang, Y.; Yang, R.; Yang, S.; Lu, L.; Yu, Y.; Liu, L.; Chen, J.; et al. The influence of microalloying and preparation process on phase transformation, mechanical properties, and friction/corrosion behavior of Cu–Ni–Sn alloys: A review. J. Mater. Res. Technol. 2024, 28, 951–966. [Google Scholar] [CrossRef]
- Qi, F.; Fu, H.; Yun, X. Achieving high strength and high electrical conductivity Cu–Cr–Zr alloys by continuous extrusion and thermomechanical treatment. Mater. Sci. Eng. A 2024, 915, 147242. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu–Ni–Si alloy. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Lei, Q.; Xiao, Z.; Hu, W.; Derby, B.; Li, Z. Phase transformation behaviors and properties of a high strength Cu-Ni-Si alloy. Mater. Sci. Eng. A 2017, 697, 37–47. [Google Scholar] [CrossRef]
- Han, S.Z.; Choi, E.; Lim, S.H.; Kim, S.; Lee, J. Alloy design strategies to increase strength and its trade-offs together. Prog. Mater. Sci. 2021, 117, 100720. [Google Scholar] [CrossRef]
- Han, S.Z.; Lee, J.; Lim, S.H.; Ahn, J.H.; Kim, K.; Kim, S. Optimization of conductivity and strength in Cu-Ni-Si alloys by suppressing discontinuous precipitation. Met. Mater. Int. 2016, 22, 1049–1054. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Effect of Ni/Si Mass Ratio and Thermomechanical Treatment on the Microstructure and Properties of Cu-Ni-Si Alloys. Materials 2019, 12, 2076. [Google Scholar] [CrossRef] [PubMed]
- Akkaş, M.; Hakan, A.; Polat, Ş. Effect of Ni:Si ratio on microstructure and properties of powder metallurgical Corson alloy. Sci. Sinter. 2023, 55, 425–436. [Google Scholar] [CrossRef]
- Ahn, J.H.; Han, S.Z.; Choi, E.; Lee, G.Y.; Kim, K.; Lee, J.; Han, H.N. Simple optimization for strength and conductivity of Cu-Ni-Si alloy with discontinuous precipitation. Mater. Charact. 2022, 184, 111605. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, W.; Zhang, W.; Xie, H.; Yang, Z.; Li, Z.; Peng, L.; Zhu, Y. Accelerating Discontinuous Precipitation to Increase Strength by Pre-Deformation in Cu-Ni-Si Alloys. Materials 2024, 17, 5658. [Google Scholar] [CrossRef]
- Ozawa, A.; Watanabe, C.; Monzen, R. Influence of Co on Strength of Cu-Ni-Co-Si Alloy. Mater. Sci. Forum 2014, 783–786, 2468–2473. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Fan, Z.; Zhang, Y.; Hui, S.; Peng, L.; Huang, G.; Xie, H.; Mi, X. Effects of Co Addition on the Microstructure and Properties of Elastic Cu-Ni-Si-Based Alloys for Electrical Connectors. Materials 2021, 14, 1996. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Zhou, Y.; Zhao, S.; Shan, Y.; Yang, Y.; Song, K. The influences of Cr and Mn on the strength, electrical conductivity, and stress relaxation properties of Cu-Ni-Si-Co-Mg alloys. Mater. Today Commun. 2025, 42, 111328. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.M.; Yang, W.W.; Chen, D.L. Effect of aluminum on microstructure and property of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Lee, E.; Han, S.; Euh, K.; Lim, S.; Kim, S. Effect of Ti addition on tensile properties of Cu-Ni-Si alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, B.; Volinsky, A.A.; Sun, H.; Chai, Z.; Liu, P.; Chen, X.; Liu, Y. Microstructure and Precipitate’s Characterization of the Cu-Ni-Si-P Alloy. J. Mater. Eng. Perform. 2016, 25, 1336–1341. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Wang, Z.; Yang, T.; Zhang, R. Microstructure and texture evolution in Cu–Ni–Si–Mg alloy processed by secondary cold rolling. Mater. Res. Express 2017, 4, 116522. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Wang, M.P.; Zhang, L.; Xiao, Z.; Jia, Y.L. The evolution of microstructure in Cu–8.0Ni–1.8Si–0.15Mg alloy during aging. Mater. Sci. Eng. A 2010, 527, 6728–6733. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, F.; Huang, F. Effects of Co Addition on the Properties and Microstructure of Cu-Ni-Si-P-Mg Alloys. Materials 2021, 14, 368. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. Standards Press of China: Beijing, China, 2021.
- Goto, M.; Yamamoto, T.; Choi, E.; Kim, S.; Hirano, R.; Lim, S.H.; Ahn, J.; Lee, J.; Han, S.Z. Physical background of significant increase in mechanical properties and fatigue strength of groove-rolled Cu-Ni-Si alloy with discontinuous precipitates. J. Alloys Compd. 2023, 947, 169569. [Google Scholar] [CrossRef]
- Wang, C.; Fu, H.; Zhang, H.; He, X.; Xie, J. Simultaneous enhancement of mechanical and electrical properties of Cu–Ni–Si alloys via thermo-mechanical process. Mater. Sci. Eng. A 2022, 838, 142815. [Google Scholar] [CrossRef]
- Lockyer, S.A.; Noble, F.W. Precipitate structure in a Cu-Ni-Si alloy. J. Mater. Sci. 1994, 29, 218–226. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Y.; Li, Z.; Shan, Q.; Yu, W.; Tang, D. Microstructure evolution and dislocation strengthening mechanism of Cu–Ni–Co–Si alloy. Mater. Sci. Eng. A 2021, 826, 142023. [Google Scholar] [CrossRef]
- Révész, Á.; Ungár, T.; Borbély, A.; Lendvai, J. Dislocations and grain size in ball-milled iron powder. Nanostruct. Mater. 1996, 7, 779–788. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E. III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Ungár, T.; Dragomir, I.; Révész, Á.; Borbély, A. The contrast factors of dislocations in cubic crystals: The dislocation model of strain anisotropy in practice. J. Appl. Cryst. 1999, 32, 992–1002. [Google Scholar] [CrossRef]
- Ungár, T.; Borbély, A. The effect of dislocation contrast on x-ray line broadening: A new approach to line profile analysis. Appl. Phys. Lett. 1996, 69, 3173–3175. [Google Scholar] [CrossRef]
- Wang, W.; Guo, E.; Chen, Z.; Kang, H.; Chen, Z.; Zou, C.; Li, R.; Yin, G.; Wang, T. Correlation between microstructures and mechanical properties of cryorolled CuNiSi alloys with Cr and Zr alloying. Mater. Charact. 2018, 144, 532–546. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kang, M.; Sills, R.B.; Ramesh, K.T.; Cai, W. Direct comparison between experiments and dislocation dynamics simulations of high rate deformation of single crystal copper. Acta Mater. 2023, 250, 118851. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Lu, J.; Tan, S.; Jiang, F.; Sun, J. Correlations between microstructures and properties of Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2018, 731, 403–412. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wang, S.; Song, H.; Zhang, S. Coarsening mechanism of over-aged δ-Ni2Si nanoscale precipitates in Cu-Ni-Si-Cr-Mg alloy. Mater. Charact. 2024, 209, 113693. [Google Scholar] [CrossRef]
- Ma, M.; Li, Z.; Xiao, Z.; Jia, Y.; Meng, X.; Jiang, Y.; Hu, Y. Microstructure and properties of Cu–Ni–Co–Si–Cr–Mg alloys with different Si contents after multi-step thermo-mechanical treatment. Mater. Sci. Eng. A 2022, 850, 143532. [Google Scholar] [CrossRef]
- Qi, F.; Fu, H.; Song, Y.; Yun, X. Regulation of dislocation density and precipitates to maximize the mechanical strength and electrical conductivity in continuously extruded Cu–Ni–Si alloys. J. Mater. Res. Technol. 2024, 30, 8519–8528. [Google Scholar] [CrossRef]
| No. | Alloy | Ni | Co | Si | Mg | Cu |
|---|---|---|---|---|---|---|
| Cu-0Mg | Cu-1.86Ni-1.1Co-0.6Si-0Mg | 1.82 | 1.05 | 0.6 | 0 | Bal. |
| Cu-0.12Mg | Cu-1.86Ni-1.1Co-0.6Si-0.12Mg | 1.82 | 1.06 | 0.59 | 0.12 | Bal. |
| Cu-0.24Mg | Cu-1.86Ni-1.1Co-0.6Si-0.24Mg | 1.90 | 1.08 | 0.61 | 0.23 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Hu, J.; Hu, Q.; Wu, L.; Guan, B.; Zeng, S.; Xing, Z.; Wang, J.; Xu, J.; Huang, G.; et al. The Effect of Aging Treatment on the Properties of Cold-Rolled Cu-Ni-Si-Co Alloys with Different Mg Contents. Materials 2025, 18, 3263. https://doi.org/10.3390/ma18143263
Wu D, Hu J, Hu Q, Wu L, Guan B, Zeng S, Xing Z, Wang J, Xu J, Huang G, et al. The Effect of Aging Treatment on the Properties of Cold-Rolled Cu-Ni-Si-Co Alloys with Different Mg Contents. Materials. 2025; 18(14):3263. https://doi.org/10.3390/ma18143263
Chicago/Turabian StyleWu, Dan, Jinming Hu, Qiang Hu, Lingkang Wu, Bo Guan, Siqi Zeng, Zhen Xing, Jiahao Wang, Jing Xu, Guojie Huang, and et al. 2025. "The Effect of Aging Treatment on the Properties of Cold-Rolled Cu-Ni-Si-Co Alloys with Different Mg Contents" Materials 18, no. 14: 3263. https://doi.org/10.3390/ma18143263
APA StyleWu, D., Hu, J., Hu, Q., Wu, L., Guan, B., Zeng, S., Xing, Z., Wang, J., Xu, J., Huang, G., & Liu, J. (2025). The Effect of Aging Treatment on the Properties of Cold-Rolled Cu-Ni-Si-Co Alloys with Different Mg Contents. Materials, 18(14), 3263. https://doi.org/10.3390/ma18143263






