Thermal Behavior and Smoke Suppression of Polyamide 6,6 Fabric Treated with ALD-ZnO and DOPO-Based Silane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZnO Coating by ALD
2.3. Sol–Gel Coating Protocol
2.4. Measurement and Characterization
3. Results
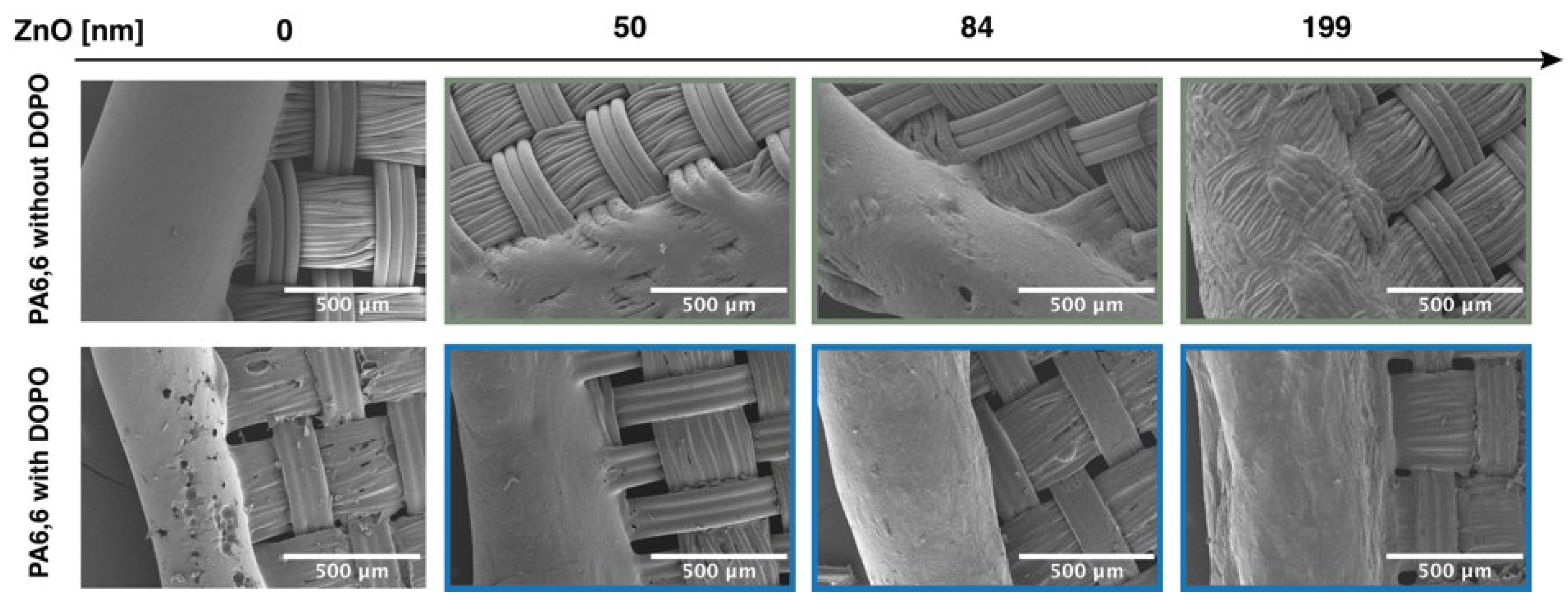
3.1. PA6,6 Finishing and Characterization
3.2. Thermal Decomposition and Combustion Behavior
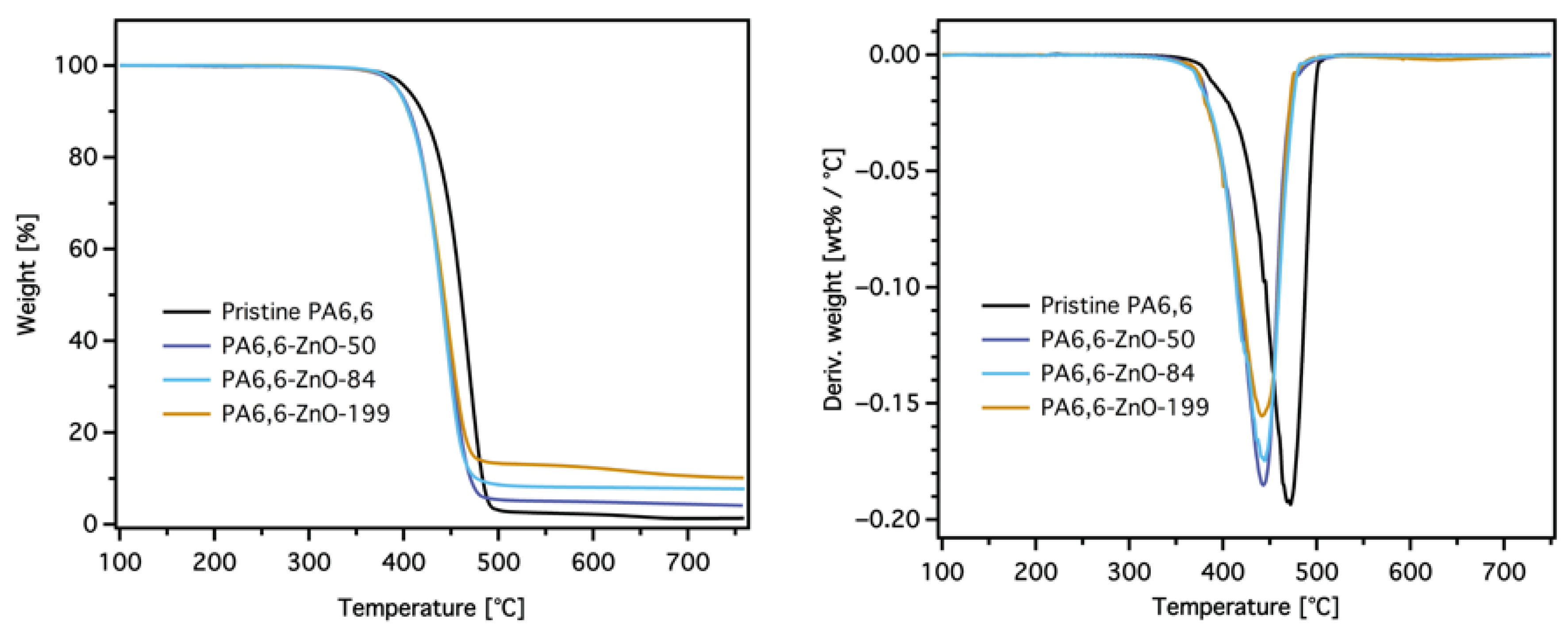
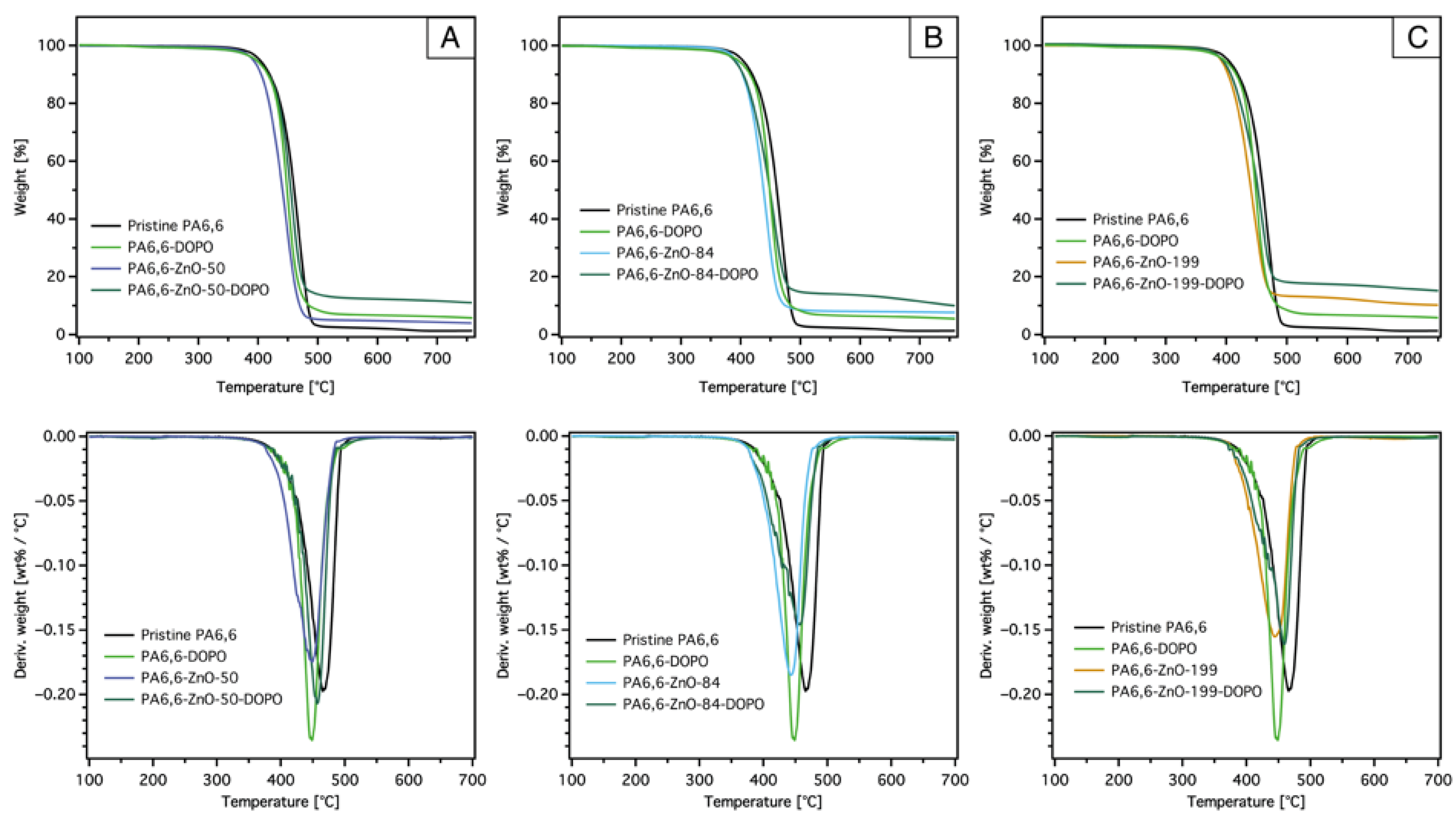
3.2.1. TG Test
3.2.2. MCC Test
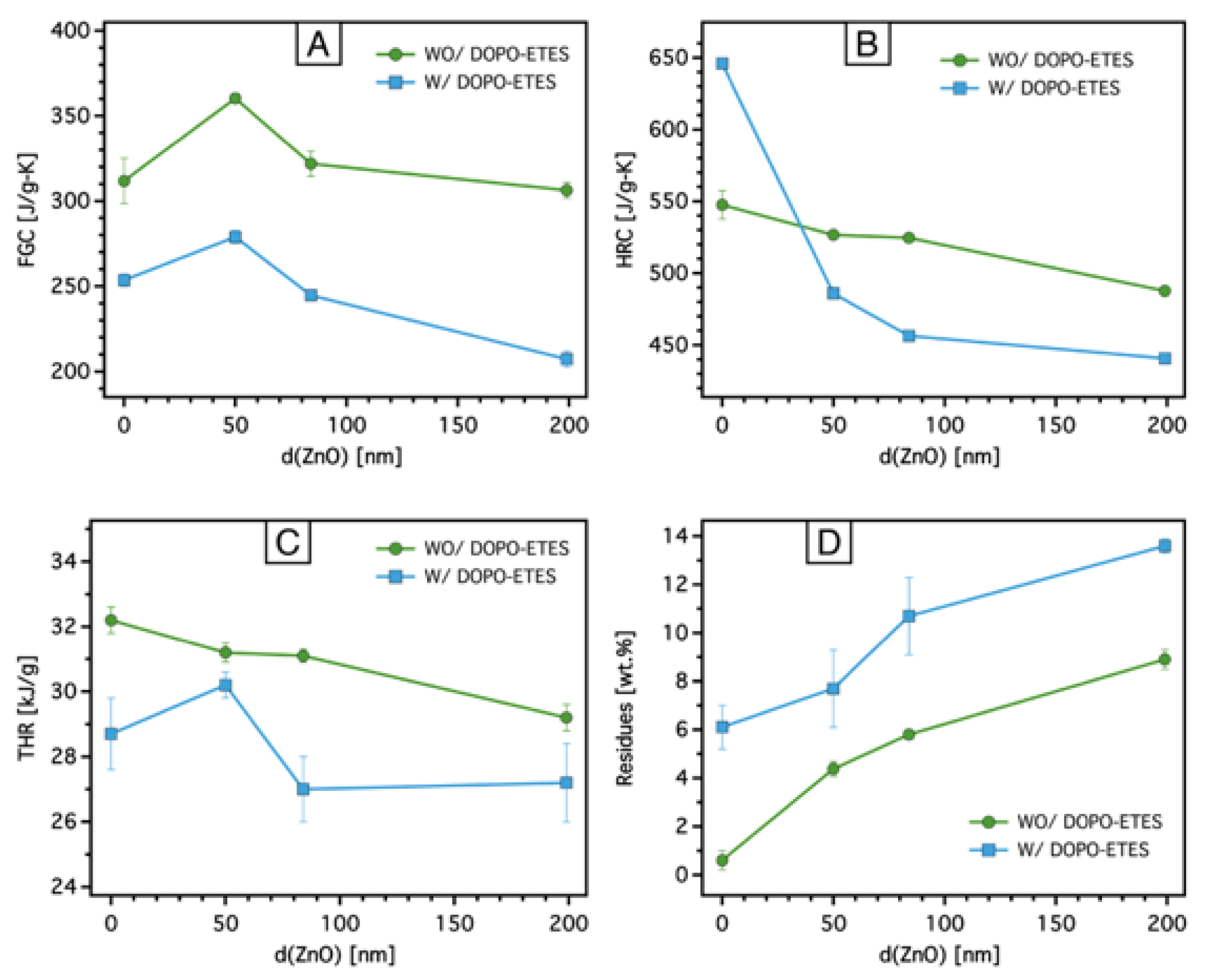
3.2.3. Cone Calorimeter Test
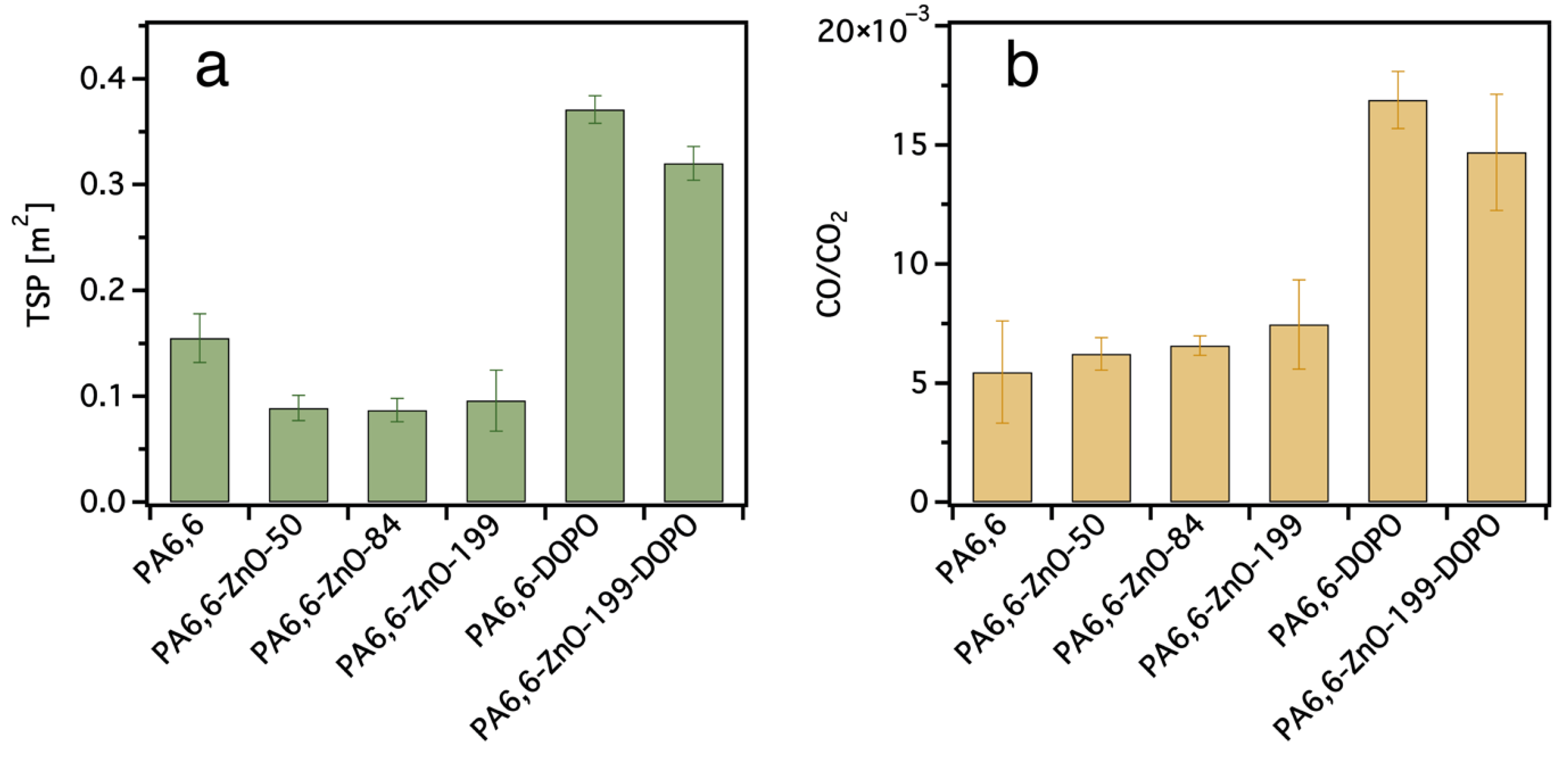
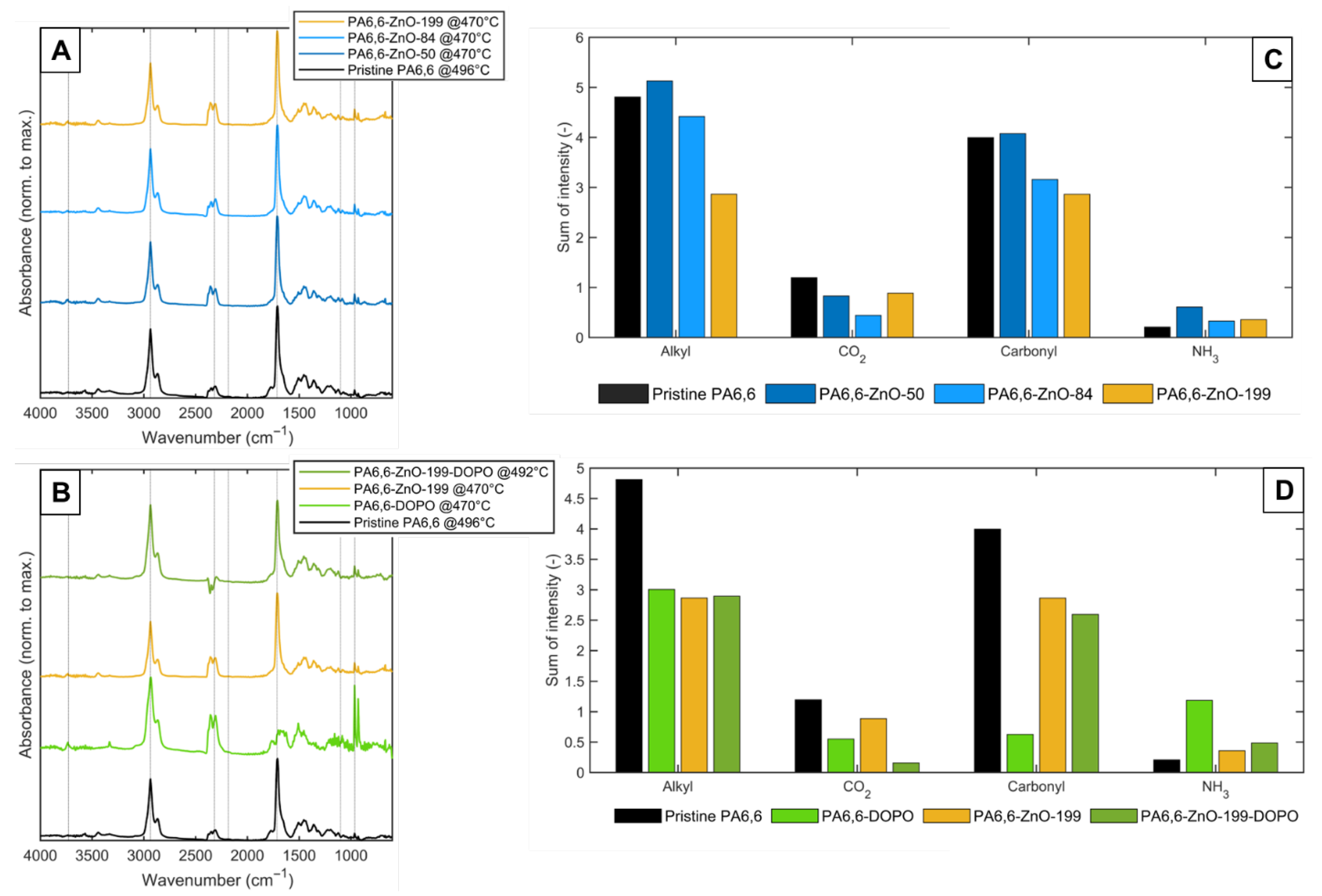
3.3. TG-IR Analysis
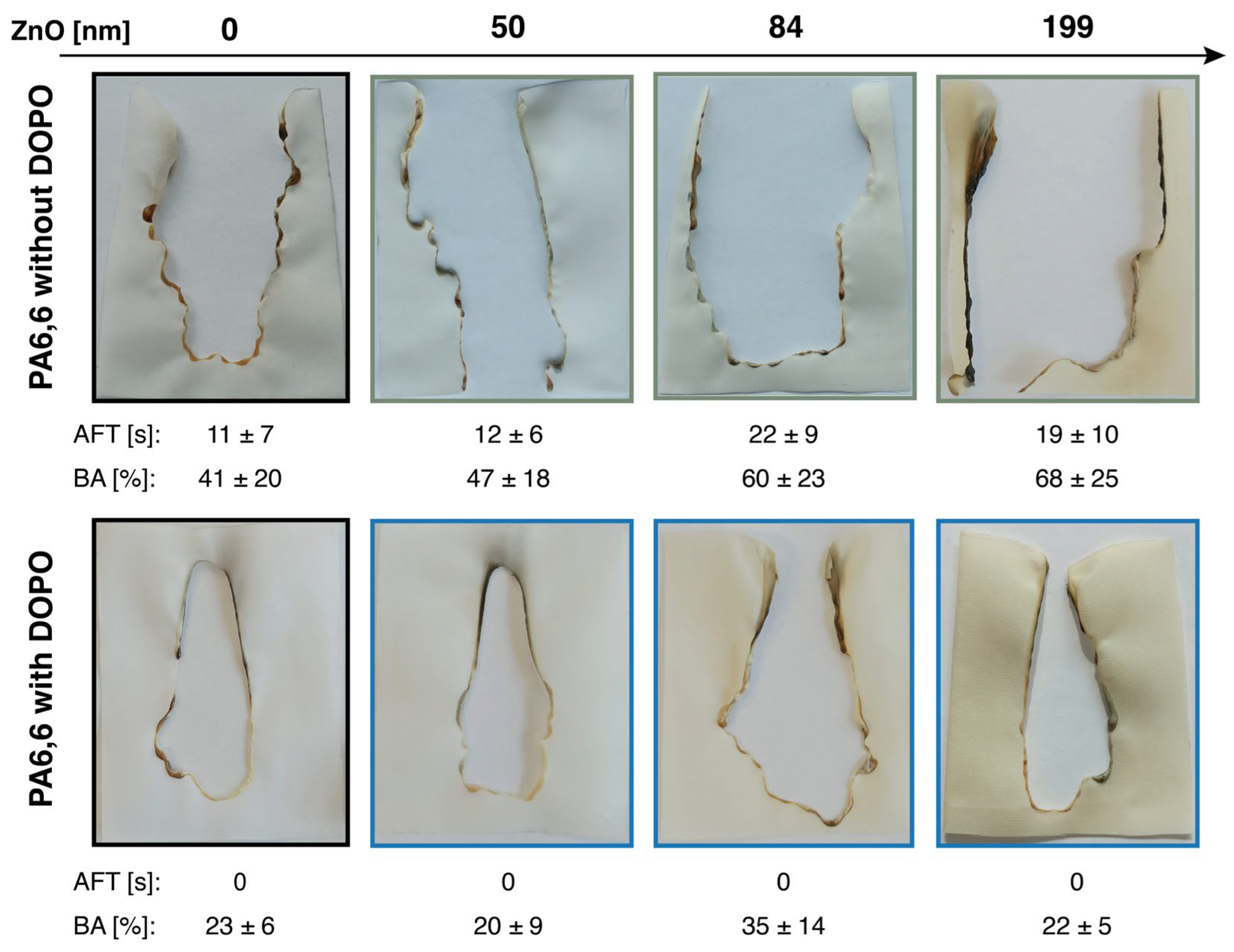
3.4. Flame Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horrocks, A.R.; Zhang, S. Char Formation in Polyamides (Nylons 6 and 6.6) and Wool Keratin Phosphorylated by Polyol Phosphoryl Chlorides. Text. Res. J. 2004, 74, 433–441. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Feng, H.; Li, D.; Xia, Z.; Yang, R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers 2023, 15, 2161. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, R.; Sitpalan, A.; Zhou, C.; Kandola, B.K. Flame Retardant Polyamide Fibres: The Challenge of Minimising Flame Retardant Additive Contents with Added Nanoclays. Polymers 2016, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, J.; Zuo, C.; Tan, W.; Ren, Y.; Liu, X. A green approach to endow PA66 with superhydrophobic, flame retardant and ultra UV resistance inspired by mussel chemistry. Appl. Surf. Sci. 2025, 681, 161589. [Google Scholar] [CrossRef]
- Jin, W.-J.; Cheng, X.-W.; Ma, S.-N.; Li, L.; Wu, R.-K.; Guan, J.-P. Core-shell DOPO/caramel nano-polymer with desirable UV shielding and flame retardancy for polyamide 6 fabric. Chem. Eng. J. 2024, 488, 151125. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Chen, F.; Cui, X.; Qi, P.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Enhancing fire safety, electromagnetic interference shielding, and photothermal conversion performances of wearable polyamide fabrics through eco-friendly coatings. Sustain. Mater. Technol. 2024, 40, e00949. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Zilke, O.; Ali, W.; Kamps, L.; Engels, T.; Schumacher, S.; Danielsiek, D.; Shabani, V.; Salma, A.; Plohl, D.; Wallmeier, R.; et al. Water-Soluble Cyclophosphazenes as Durable Flame-Retardant Finishes for Nylon/Cotton Blend Fabrics. ACS Appl. Polym. Mater. 2022, 4, 8833–8846. [Google Scholar] [CrossRef]
- Sun, X.; Miao, W.; Pan, Y.-T.; Song, P.; Gaan, S.; Ibarra, L.H.; Yang, R. Metal-Organic Frameworks: A Solution for Greener Polymeric Materials with Low Fire Hazards. Adv. Sustain. Syst. 2025, 9, 2400768. [Google Scholar] [CrossRef]
- Song, K.; Pan, Y.-T.; Zhang, J.; Song, P.; He, J.; Wang, D.-Y.; Yang, R. Metal–Organic Frameworks–Based Flame-Retardant System for Epoxy Resin: A Review and Prospect. Chem. Eng. J. 2023, 468, 143653. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, C.; Lin, J.; Wang, Y.; Ding, L.; Hu, J.; Gao, W.; Chi, Y.; Ma, M.; Huang, W. Construction of MOFs-based nanocomposites and their application in flame retardant polymers: A review. Polym. Degrad. Stab. 2024, 229, 110982. [Google Scholar] [CrossRef]
- Song, K.; Pan, Y.-T.; He, J.; Yang, R. Coordination bond cleavage of metal–organic frameworks and application to flame-retardant polymeric materials. Ind. Chem. Mater. 2024, 2, 556–570. [Google Scholar] [CrossRef]
- Gurbandurdyyev, G.; Mistry, K.; Delumeau, L.-V.; Loke, J.Y.; Teoh, C.H.; Cheon, J.; Ye, F.; Tam, K.C.; Musselman, K.P. Robust, Conformal ZnO Coatings on Fabrics via Atmospheric-Pressure Spatial Atomic Layer Deposition with In-Situ Thickness Control**. ChemNanoMat 2023, 9, e202200498. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Liu, H.; Sun, J.; Wu, J.; Liu, Q.; Zheng, Y.; Zheng, P. Recent advances in metal-family flame retardants: A review. RSC Adv. 2023, 13, 22639–22662. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106113. [Google Scholar] [CrossRef]
- Lawrynowicz, A.; Palo, E.; Nizamov, R.; Miettunen, K. Self-cleaning and UV-blocking cotton—Fabricating effective ZnO structures for photocatalysis. J. Photochem. Photobiol. A Chem. 2024, 450, 115420. [Google Scholar] [CrossRef]
- Mohammadipour-Nodoushan, R.; Shekarriz, S.; Shariatinia, Z.; Heydari, A.; Montazer, M. Improved cotton fabrics properties using zinc oxide-based nanomaterials: A review. Int. J. Biol. Macromol. 2023, 242, 124916. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Etemad-Parishanzadeh, O.; Leven, Y.; Kamps, L.; Engels, T.; Gutmann, J.S.; Mayer-Gall, T.; Textor, T. Combinatorial coating based on light sensitive photocatalyst-biostatic, self-cleaning and UV protective textiles. Melliand Int. 2022, 2022, 34–36. [Google Scholar]
- Xu, H.; Ye, X.; Shi, X.; Zhong, H.; He, D.; Jin, B.; Jin, F. ZnO as a simple and facile catalyst for acid-base coordination transformation of biomass-based monosaccharides into lactic acid. Mol. Catal. 2022, 522, 112241. [Google Scholar] [CrossRef]
- Wang, W.; Meng, D.; Wang, J.; Li, H.; Sun, J.; Zhang, S.; Gu, X. Oriented growth of LDH for constructing multifunctional cotton fabric with flame retardancy, smoke suppression, and filtering capability. Appl. Clay Sci. 2022, 226, 106588. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic effects of organically modified montmorillonite on the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, D.; Yan, L.; Xie, X. Synergistic effect of sepiolite and polyphosphate ester on the fire protection and smoke suppression properties of an amino transparent fire-retardant coating. Prog. Org. Coat. 2020, 141, 105572. [Google Scholar] [CrossRef]
- Kroenke, W.J. Low-melting sulphate glasses and glass-ceramics, and their utility as fire and smoke retarder additives for poly(vinyl chloride). J. Mater. Sci. 1986, 21, 1123–1133. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, S.; Shan, H.; Qian, L.; Wang, J.; Xin, F. Effect of two boron compounds on smoke-suppression and flame-retardant properties for rigid polyurethane foams. Polym. Int. 2022, 71, 1210–1219. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Li, N.; Tang, T.; Xie, X.; Zhang, C.; Zuo, Y. The Flame Retardancy and Smoke Suppression Performance of Polyvinyl Chloride Composites with an Efficient Flame Retardant System. Coatings 2023, 13, 1814. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame retardant effect of boron compounds in polymeric materials. Compos. Part B Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- Goller, S.M.; Krüger, S.; Schartel, B. No business as usual: The effect of smoke suppressants commonly used in the flame retardant PA6.6 on smoke and fire properties. Polym. Degrad. Stab. 2023, 209, 110276. [Google Scholar] [CrossRef]
- Goller, S.M.; Schartel, B.; Krüger, S. Block it and rock it: Smoke suppressants that form a protective layer in PA 6.6. J. Fire Sci. 2024, 42, 117–141. [Google Scholar] [CrossRef]
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 010801. [Google Scholar] [CrossRef]
- Hyde, G.K.; Scarel, G.; Spagnola, J.C.; Peng, Q.; Lee, K.; Gong, B.; Roberts, K.G.; Roth, K.M.; Hanson, C.A.; Devine, C.K.; et al. Atomic layer deposition and abrupt wetting transitions on nonwoven polypropylene and woven cotton fabrics. Langmuir 2010, 26, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, A.J.; Sarnes, L.; Townsend, R.; Mikkonen, J.; Karppinen, M. Flexible Thermoelectric ZnO–Organic Superlattices on Cotton Textile Substrates by ALD/MLD. Adv. Electron. Mater. 2017, 3, 1600459. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, T.; Huang, Z.; He, A.; Luo, Y.; Hong, Y.; Wang, M.; Shi, Z.; Tong, A.; Qiao, S.; et al. An innovative strategy towards highly efficient flame-retardant silk. Chem. Eng. J. 2024, 489, 151356. [Google Scholar] [CrossRef]
- O’Brien, S.; Cosgrave, L.; Lodge, V.; Povey, I.M. Atomic Layer Deposition on Fabrics for Flame Resistance. ECS Trans. 2015, 66, 31–35. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Jerman, I.; Simončič, B.; Demšar, A.; Samaki, Y.; Šobak, M.; Šest, E.; Golja, B.; Leskovšek, M.; et al. In situ prepared polyamide 6/DOPO-derivative nanocomposite for melt-spinning of flame retardant textile filaments. Polym. Degrad. Stab. 2019, 166, 50–59. [Google Scholar] [CrossRef]
- Kundu, C.K.; Yu, B.; Gangireddy, C.S.R.; Mu, X.; Wang, B.; Wang, X.; Song, L.; Hu, Y. UV Grafting of a DOPO-Based Phosphoramidate Monomer onto Polyamide 66 Fabrics for Flame Retardant Treatment. Ind. Eng. Chem. Res. 2017, 56, 1376–1384. [Google Scholar] [CrossRef]
- Kundu, C.K.; Song, L.; Hu, Y. Sol-gel coatings from DOPO-alkoxysilanes: Efficacy in fire protection of polyamide 66 textiles. Eur. Polym. J. 2020, 125, 109483. [Google Scholar] [CrossRef]
- Sahyoun, J.; Bounor-Legaré, V.; Ferry, L.; Sonnier, R.; Da Cruz-Boisson, F.; Melis, F.; Bonhommé, A.; Cassagnau, P. Synthesis of a new organophosphorous alkoxysilane precursor and its effect on the thermal and fire behavior of a PA66/PA6 copolymer. Eur. Polym. J. 2015, 66, 352–366. [Google Scholar] [CrossRef]
- Šehić, A.; Tomšič, B.; Jerman, I.; Vasiljević, J.; Medved, J.; Simončič, B. Synergistic inhibitory action of P- and Si-containing precursors in sol–gel coatings on the thermal degradation of polyamide 6. Polym. Degrad. Stab. 2016, 128, 245–252. [Google Scholar] [CrossRef]
- Chernyy, S.; Ulah, S.; Sørensen, G.; Tordrup, S.W.; Pedersen, P.B.; Almdal, K. DOPO-VTS-based coatings in the realm of fire retardants for cotton textile. J. Appl. Polym. Sci. 2015, 132, 41955. [Google Scholar] [CrossRef]
- Ali, W.; Zilke, O.; Danielsiek, D.; Salma, A.; Assfour, B.; Shabani, V.; Caglar, S.; Phan, H.M.; Kamps, L.; Wallmeier, R.; et al. Flame-retardant finishing of cotton fabrics using DOPO functionalized alkoxy- and amido alkoxysilane. Cellulose 2023, 30, 2627–2652. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- EN ISO 15025:2016; Protective Clothing—Protection Against Flame—Method of Test for Limited Flame Spread. ISO: Geneva, Switzerland, 2016.
- ISO 11611:2024; Protective Clothing for Use in Welding and Allied Processes. ISO: Geneva, Switzerland, 2024.
- ISO 5660; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate. Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement); ISO: Geneva, Switzerland, 2015.
- Marques, F.; Tǎbǎcaru, A.; Bușilǎ, M.; Pinheiro, T.; Matos, A.P.A. Modification of ZnO nanoparticles with silanes enables their application as anticancer agents. Ann. Med. 2021, 53, S43. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol-Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Zhai, J.; Tao, X.; Pu, Y.; Zeng, X.-F.; Chen, J.-F. Core/shell structured ZnO/SiO2 nanoparticles: Preparation, characterization and photocatalytic property. Appl. Surf. Sci. 2010, 257, 393–397. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Grigorie, A.C.; Muntean, C.; Vlase, T.; Locovei, C.; Stefanescu, M. ZnO-SiO2 based nanocomposites prepared by a modified sol-gel method. Mater. Chem. Phys. 2017, 186, 399–406. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Cogen, J.M.; Lin, T.S.; Lyon, R.E. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame retardant polyolefin compounds. Fire Mater. 2009, 33, 33–50. [Google Scholar] [CrossRef]
- Mensah, R.A.; Xu, Q.; Asante-Okyere, S.; Jin, C.; Bentum-Micah, G. Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments. J. Therm. Anal. Calorim. 2019, 136, 589–599. [Google Scholar] [CrossRef]
- Walters, N.R. Development of Instrumental and Computational Tools for Investigation of Polymer Flammability. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2013. [Google Scholar]
- DIN 4102-1; Fire Behaviour of Building Materials and Elements—Classification of Building Materials—Requirements and Testing. European Standards: Plzen, Czech Republic, 1998.
- DIN 7500; Trilobular Thread-Forming System. Bossard: Zug, Switzerland, 2017.
- Rahman, M.Z.; Wang, X.; Song, L.; Hu, Y. A novel green phosphorus-containing flame retardant finishing on polysaccharide-modified polyamide 66 fabric for improving hydrophilicity and durability. Int. J. Biol. Macromol. 2023, 239, 124252. [Google Scholar] [CrossRef] [PubMed]
- Ziaur Rahman, M.; Kundu, C.K.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Hybrid coatings for durable flame retardant and hydrophilic treatment of Polyamide 6.6 fabrics. Prog. Org. Coat. 2020, 144, 105640. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Chitosan-based flame retardant coatings for polyamide 66 textiles: One-pot deposition versus layer-by-layer assembly. Int. J. Biol. Macromol. 2020, 143, 1–10. [Google Scholar] [CrossRef]
- Kumar Kundu, C.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef]
- ISO 6940-2004; Textile Fabrics—Burning Behaviour—Determination of Ease of Ignition of Vertically Oriented Specimens. ISO: Geneva, Switzerland, 2004.
- Meng, D.; Guo, J.; Wang, A.; Gu, X.; Wang, Z.; Jiang, S.; Zhang, S. The fire performance of polyamide66 fabric coated with soybean protein isolation. Prog. Org. Coat. 2020, 148, 105835. [Google Scholar] [CrossRef]
- ASTMD 6413-08; Standard Test Method for Flame Resistance of Textiles (Vertical Test). ASTM: Pennsylvania, PA, USA, 2008.
- Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z.; Hu, Y. Graphene oxide functionalized biomolecules for improved flame retardancy of Polyamide 66 fabrics with intact physical properties. Int. J. Biol. Macromol. 2020, 156, 362–371. [Google Scholar] [CrossRef]
- GB/T 5455-1997; Textiles-Burning Behaviour-Vertical Method. National Standards of the People’s Republic of China: Beijing, China, 1997.
- Li, L.; Chen, G.; Liu, W.; Li, J.; Zhang, S. The anti-dripping intumescent flame retardant finishing for nylon-6,6 fabric. Polym. Degrad. Stab. 2009, 94, 996–1000. [Google Scholar] [CrossRef]
- Maydannik, P.S.; Kääriäinen, T.O.; Lahtinen, K.; Cameron, D.C.; Söderlund, M.; Soininen, P.; Johansson, P.; Kuusipalo, J.; Moro, L.; Zeng, X. Roll-to-roll atomic layer deposition process for flexible electronics encapsulation applications. J. Vac. Sci. Technol. A Vac. Surf. Film. 2014, 32, 051603. [Google Scholar] [CrossRef]
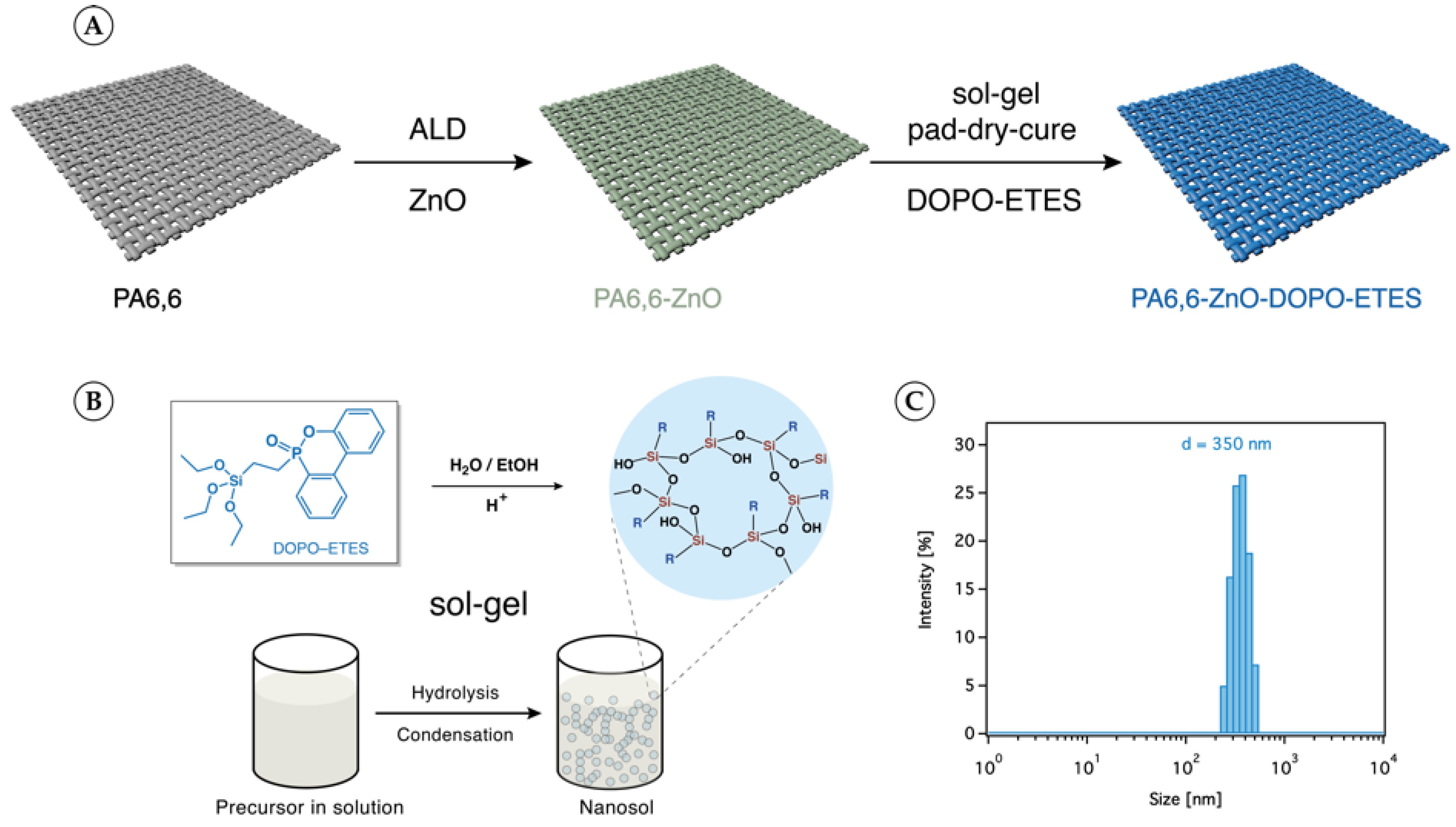
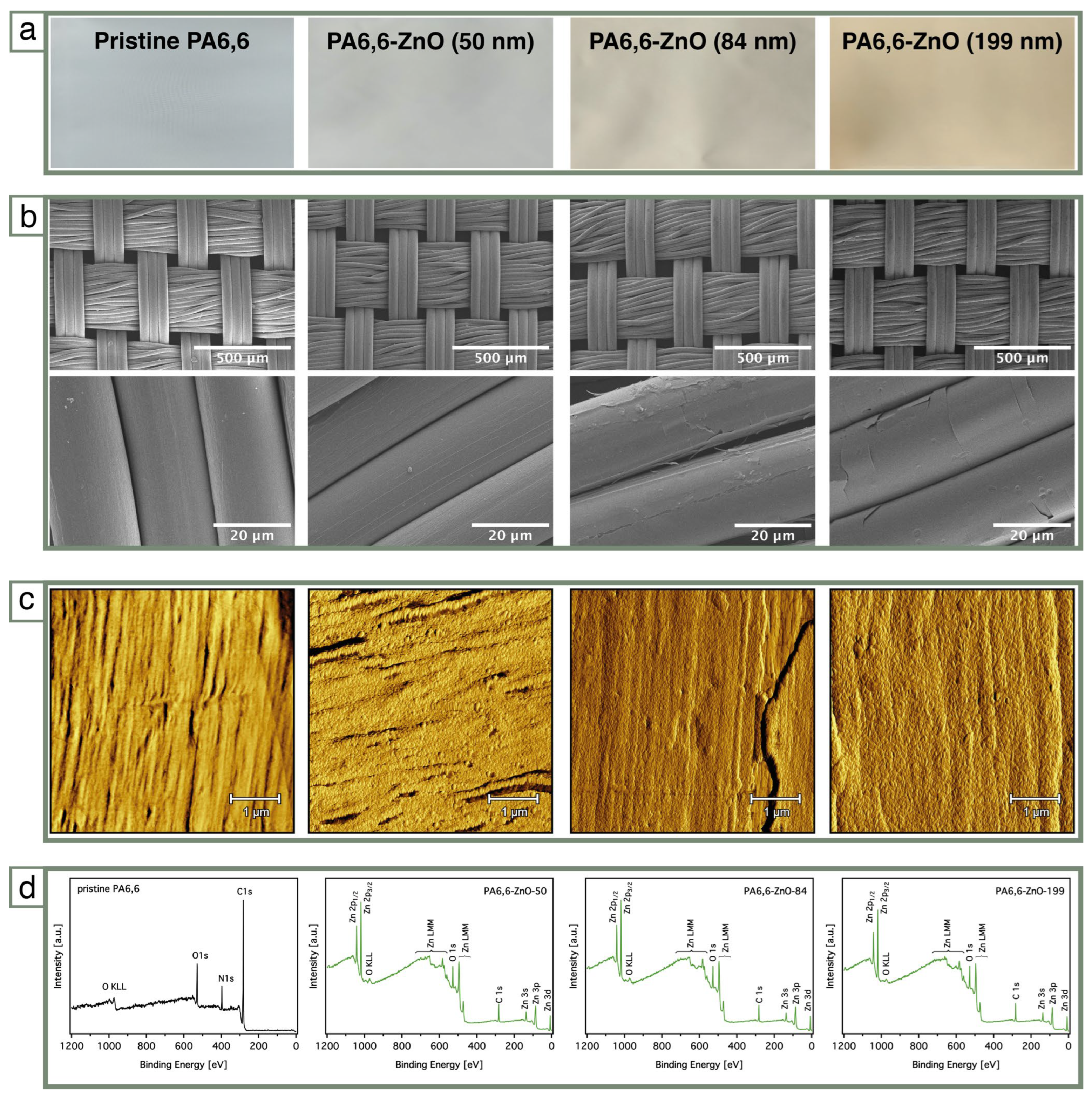






| Sample Code | ZnO | ||
|---|---|---|---|
| Cycles [-] | Layer Thickness [nm] | Add-on [wt%] | |
| PA6,6-ZnO-50 | 300 | 50 ± 0.5 | 3.2 ± 0.5 |
| PA6,6-ZnO-84 | 600 | 84 ± 0.8 | 4.3 ± 0.6 |
| PA6,6-ZnO-199 | 1200 | 199 ± 1.9 | 8.5 ± 0.7 |
| Sample Code | DOPO-ETES [wt%] | PTheor. [%] | PXPS [%] | SiTheor. [%] | SiXPS [%] |
|---|---|---|---|---|---|
| PA6,6-DOPO | 15 ± 1.4 | 1.57 ± 0.15 | 2.3 | 1.43 ± 0.13 | 3.1 |
| PA6,6-ZnO-50-DOPO | 14.6 ± 1.2 | 1.53 ± 0.13 | 1.9 | 1.39 ± 0.11 | 1.5 |
| PA6,6-ZnO-84-DOPO | 16.1 ± 0.9 | 1.69 ± 0.09 | 2.1 | 1.53 ± 0.09 | 2.6 |
| PA6,6-ZnO-199-DOPO | 14.8 ± 1.1 | 1.55 ± 0.12 | 1.6 | 1.41 ± 0.10 | 2.8 |
| Sample | T5% [°C] | Tmax [°C] | Degradation Rate @ Tmax [wt%/°C] × 10−2 | Res.700 [%] | Res.Theor [%] (PA + ZnO) a | Res.Theor [%] (PA + ZnO + SiO2) b |
|---|---|---|---|---|---|---|
| PA6,6 | 401 | 471 | 19.3 | 1.0 | – | – |
| PA6,6-DOPO | 391 | 449 | 23.5 | 5.4 | – | 4.0 |
| PA6,6-ZnO-50 | 390 | 443 | 18.4 | 3.8 | 4.2 | – |
| PA6,6-ZnO-50-DOPO | 393 | 457 | 20.6 | 9.8 | 4.2 | 7.2 |
| PA6,6-ZnO-84 | 387 | 449 | 17.4 | 6.6 | 5.3 | – |
| PA6,6-ZnO-84-DOPO | 386 | 457 | 14.5 | 11.1 | 5.3 | 8.6 |
| PA6,6-ZnO-199 | 391 | 445 | 15.5 | 10.4 | 9.5 | – |
| PA6,6-ZnO-199-DOPO | 391 | 459 | 16.1 | 15.3 | 9.5 | 12.5 |
| Sample | pHRR [W/g] | TpHRR [°C] | FGC [J/g-K] | THR [kJ/g] | Residues [%] |
|---|---|---|---|---|---|
| PA6,6 | 547.5 ± 9.9 | 475.2 ± 1.3 | 311.7 ± 13.3 | 33.2 ± 0.4 | 0.6 ± 0.3 |
| PA6,6-DOPO | 646.1 ± 2.9 | 453.7 ± 0.5 | 253.6 ± 3.6 | 28.7 ± 1.1 | 6.1 ± 0.9 |
| PA6,6-ZnO-50 | 526.7 ± 2.5 | 459.4 ± 0.5 | 360.4 ± 2.7 | 31.2 ± 0.3 | 4.38 ± 1.2 |
| PA6,6-ZnO-50-DOPO | 485.9 ± 1.2 | 458.0 ± 0.1 | 278.9 ± 3.7 | 30.2 ± 0.4 | 7.7 ± 1.6 |
| PA6,6-ZnO-84 | 524.6 ± 2.9 | 453.9 ± 0 | 321.9 ± 7.4 | 31.1 ± 0.2 | 5.8 ± 0.2 |
| PA6,6-ZnO-84-DOPO | 456.5 ± 3.3 | 461.8 ± 0.4 | 244.7 ± 0.8 | 27.0 ± 1.0 | 10.7 ± 1.6 |
| PA6,6-ZnO-199 | 487.7 ± 0.7 | 455.1 ± 0.8 | 306.3 ± 4.7 | 29.2 ± 0.4 | 8.9 ± 0.6 |
| PA6,6-ZnO-199-DOPO | 440.8 ± 1.3 | 447.1 ± 4.1 | 207.3 ± 4.5 | 27.2 ± 1.2 | 13.6 ± 0.3 |
| No. | FR Type | Finishing Method | FR Content [wt%] | LOI [%] | Reduction in pHRR [%] | Flame Test | Dripping | Smoke Behavior [%] | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PCS + PAS | LbL + UV/thermal | 6.5 | 23 ± 0.5 | 25 (CC) | UL 94 (V-1) AFT = 50 ± 8 | no | SPR decreased @ FR content of 2.1% | [60] |
| 2 | CS/PA/OSA | LbL | 9.3 | 20.7 | 23 (CC) | UL 94 (V-1) AFT = 18 s | no | N/A | [61] |
| 3 | AA/CS/ME/ UREA/PA + CS/GO | UV-grafting + LbL + pad–dry–cure | 15.7 | 25 | 44.3 (MCC) | UL 94 (V-1) AFT = 33 s | no | N/A | [59] |
| 4 | CS/PAA | UV-grafting + pad–dry–cure | 30.1 | 24 | 52.3 (MCC) | UL 94 (V-1) AFT = 24 s | no | N/A | [58] |
| 5 | soybean protein + thiourea | pad–dry–cure | 9.4 | 25 ± 0.4 | 12.8 (CC) | vertical test ISO 6940-2004 [62] AFT = 0 s | no | N/A | [63] |
| 6 | GO-lignin/CS/PA | pad–dry–cure | 6.0 ± 0.30 | 25.5 ± 1 | 25 (CC) | vertical test ASTMD 6413-08 [64] AFT = 66 ± 13 s | yes | TSR increased by 17.6% | [65] |
| 7 | intumescent FR (APP, ME, PER) | pad–dry–cure | 40.6 | 27.9 | N/A | vertical test GB/T 5455-1997 [66] AFT = 5.3 s | no | N/A | [67] |
| 8 | DOPO-APTES | sol–gel (pad–dry–cure) | 14.6 | 21.5 | 36 (CC) | UL-94 (V-1) AFT = 38 s | no | SPR increased by 38% | [37] |
| 9 | DOPO-DAAM | UV-grafting | 20.3 | 23.0 | 22 (CC) | UL-94 (V-0) AFT = 10 s | no | N/A | [36] |
| 10 | ZnO | ALD | 8.5 ± 0.7 | N/A | 10.9 ± 1.2 (MCC) | EN ISO 15025 (surface ignition) AFT = 19 ± 10 s | no | TSP decreased by 44 ± 8 (vs. pristine PA6,6) | This work |
| ZnO + DOPO-ETES | ALD + sol–gel (pad–dry–cure) | 23.3 ± 1.8 | N/A | 19.5 ± 1.6 (MCC) | EN ISO 15025 (surface ignition) AFT = 0 s | no | TSP decreased by 14 ± 1 (vs. PA6,6-DOPO) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, W.; Otto, R.; Lema Jimenez, A.R.; Lehmann, S.; Shin, E.-Y.; Feng, Y.; Jovic, M.; Gaan, S.; Gutmann, J.S.; Nielsch, K.; et al. Thermal Behavior and Smoke Suppression of Polyamide 6,6 Fabric Treated with ALD-ZnO and DOPO-Based Silane. Materials 2025, 18, 3195. https://doi.org/10.3390/ma18133195
Ali W, Otto R, Lema Jimenez AR, Lehmann S, Shin E-Y, Feng Y, Jovic M, Gaan S, Gutmann JS, Nielsch K, et al. Thermal Behavior and Smoke Suppression of Polyamide 6,6 Fabric Treated with ALD-ZnO and DOPO-Based Silane. Materials. 2025; 18(13):3195. https://doi.org/10.3390/ma18133195
Chicago/Turabian StyleAli, Wael, Raphael Otto, Ana Raquel Lema Jimenez, Sebastian Lehmann, Eui-Young Shin, Ying Feng, Milijana Jovic, Sabyasachi Gaan, Jochen S. Gutmann, Kornelius Nielsch, and et al. 2025. "Thermal Behavior and Smoke Suppression of Polyamide 6,6 Fabric Treated with ALD-ZnO and DOPO-Based Silane" Materials 18, no. 13: 3195. https://doi.org/10.3390/ma18133195
APA StyleAli, W., Otto, R., Lema Jimenez, A. R., Lehmann, S., Shin, E.-Y., Feng, Y., Jovic, M., Gaan, S., Gutmann, J. S., Nielsch, K., Bahrami, A., & Mayer-Gall, T. (2025). Thermal Behavior and Smoke Suppression of Polyamide 6,6 Fabric Treated with ALD-ZnO and DOPO-Based Silane. Materials, 18(13), 3195. https://doi.org/10.3390/ma18133195











