Analysis of High-Speed Cutting Surface Layer Formation and Oxide Layer Thickness Prediction of Titanium Alloy (Ti6Al4V)
Abstract
1. Introduction
2. Experimental Material and Procedure
2.1. Cutting Surface Quality Test
2.2. Cutting Process Parameter Detection Experiment
3. Experimental Results and Analysis
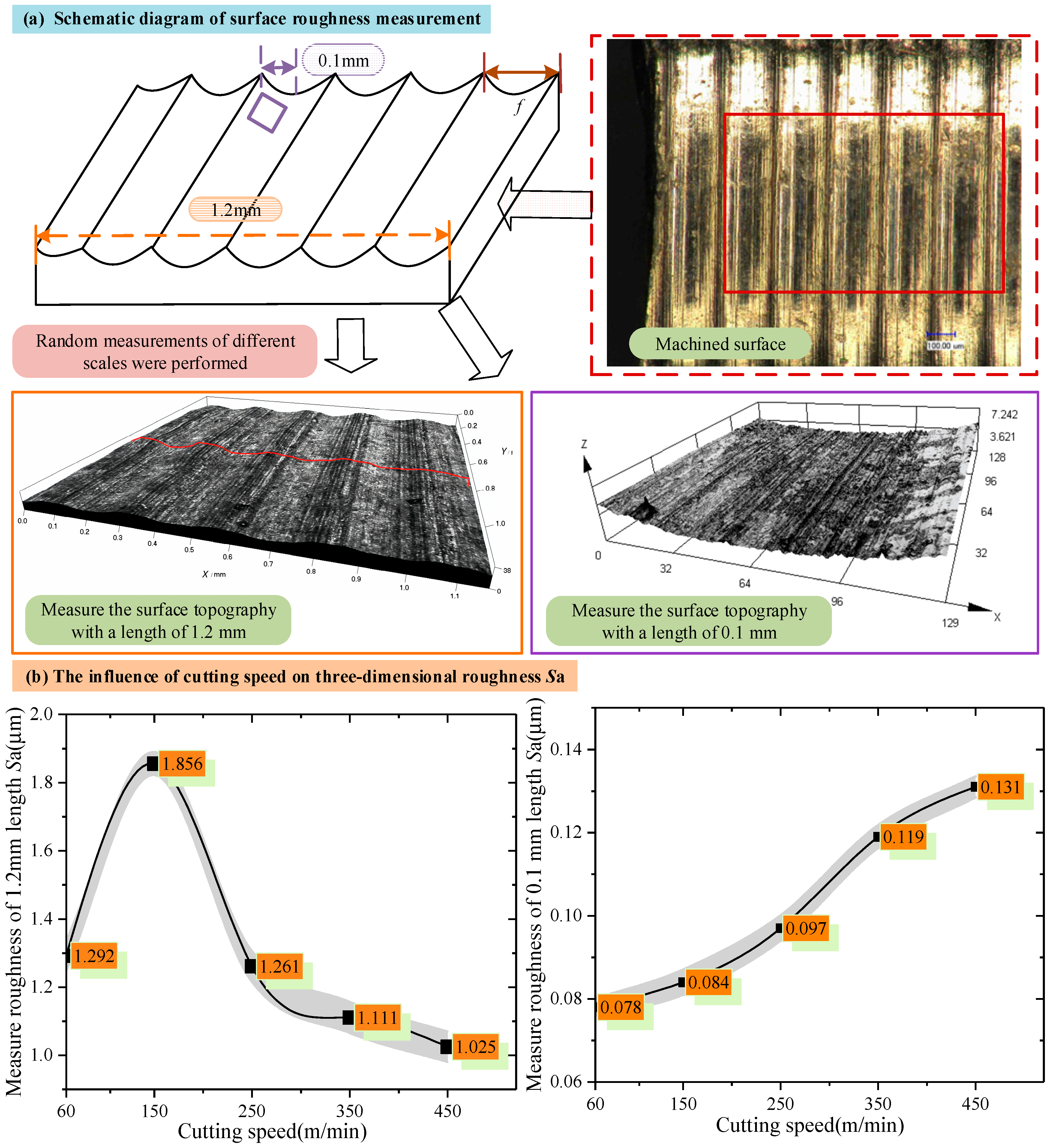
3.1. Effect of Cutting Speed on Cutting Surface
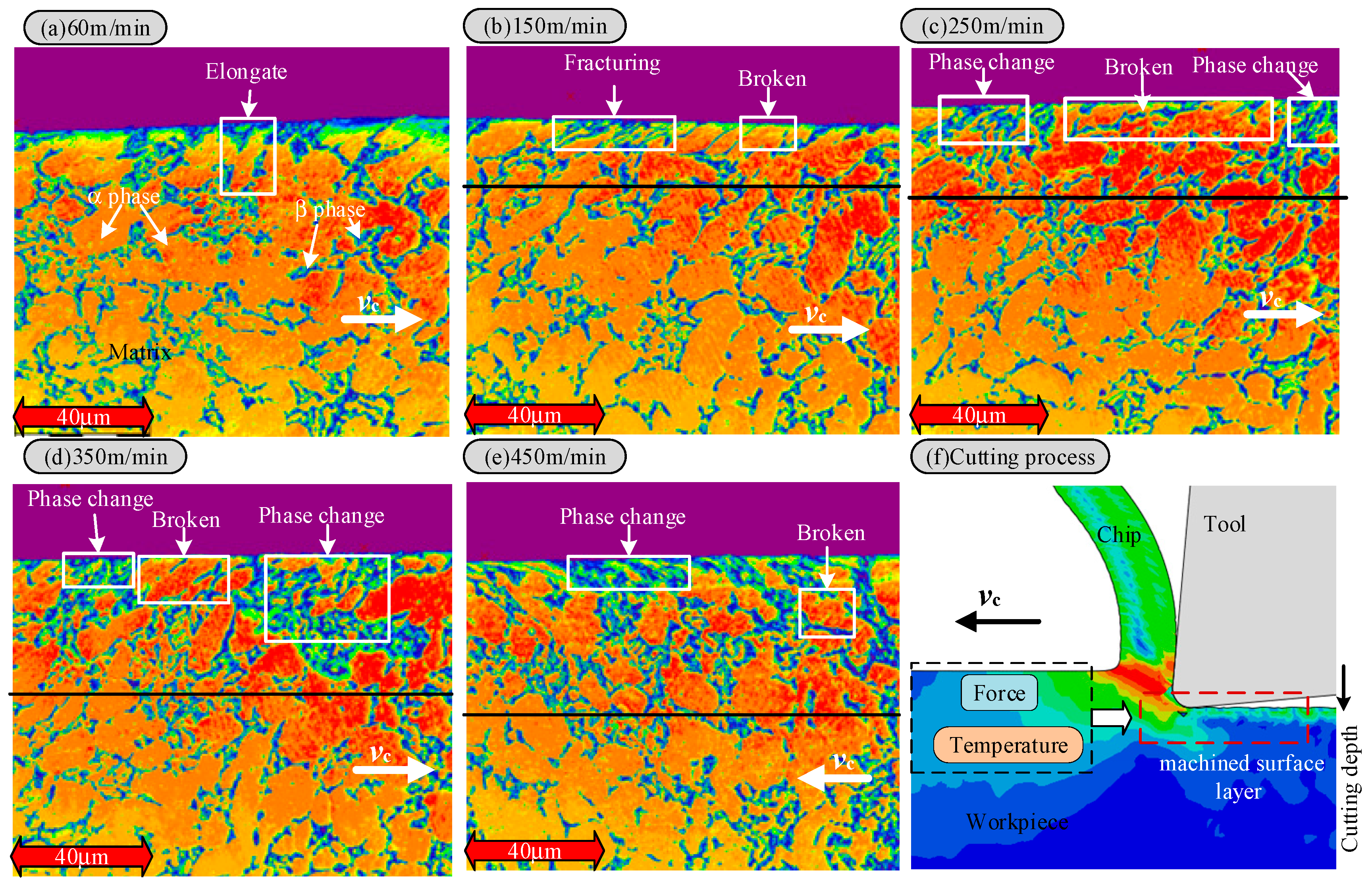
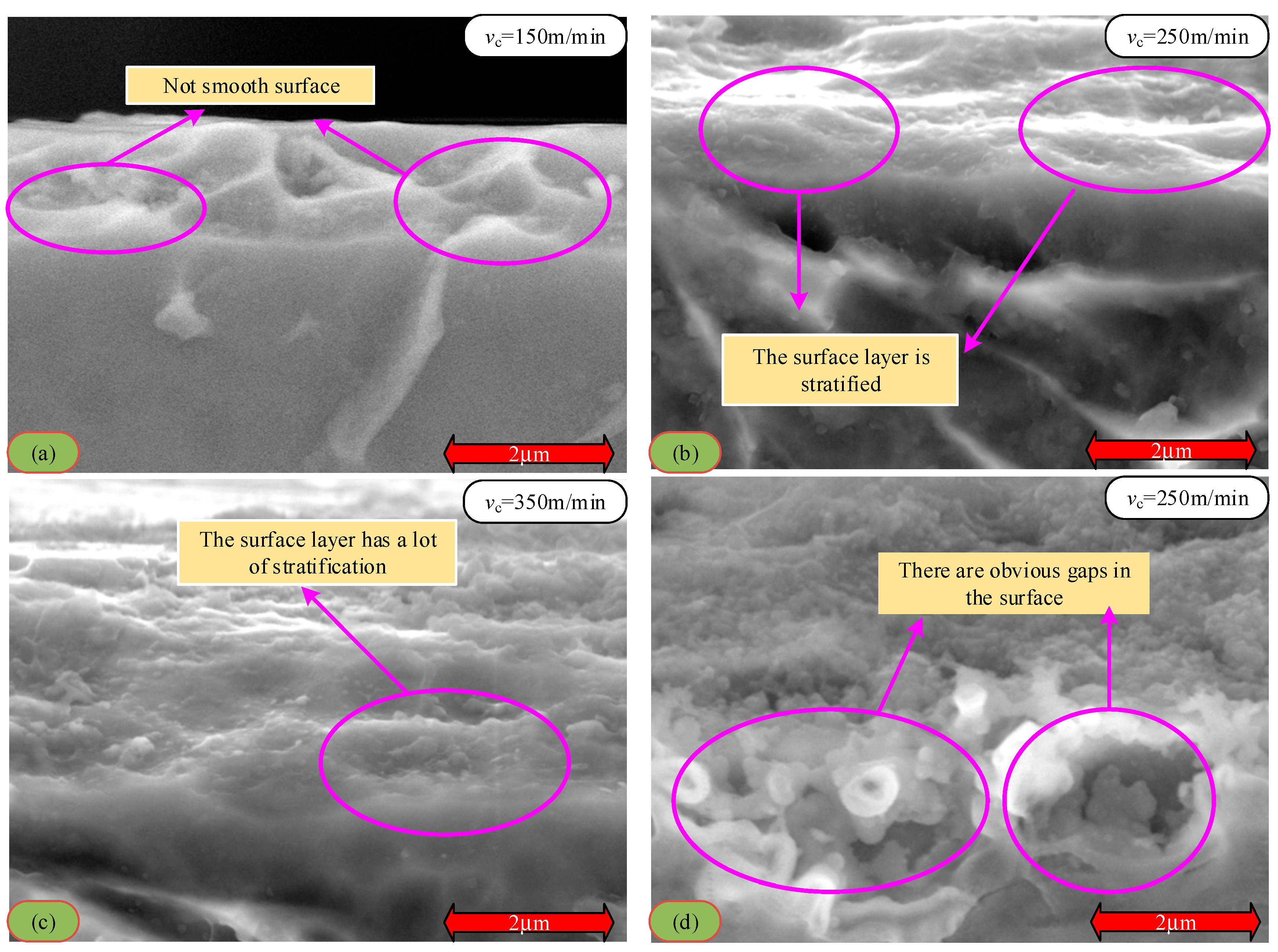
3.2. The Effect of Cutting Speed on the Cutting Surface Layer
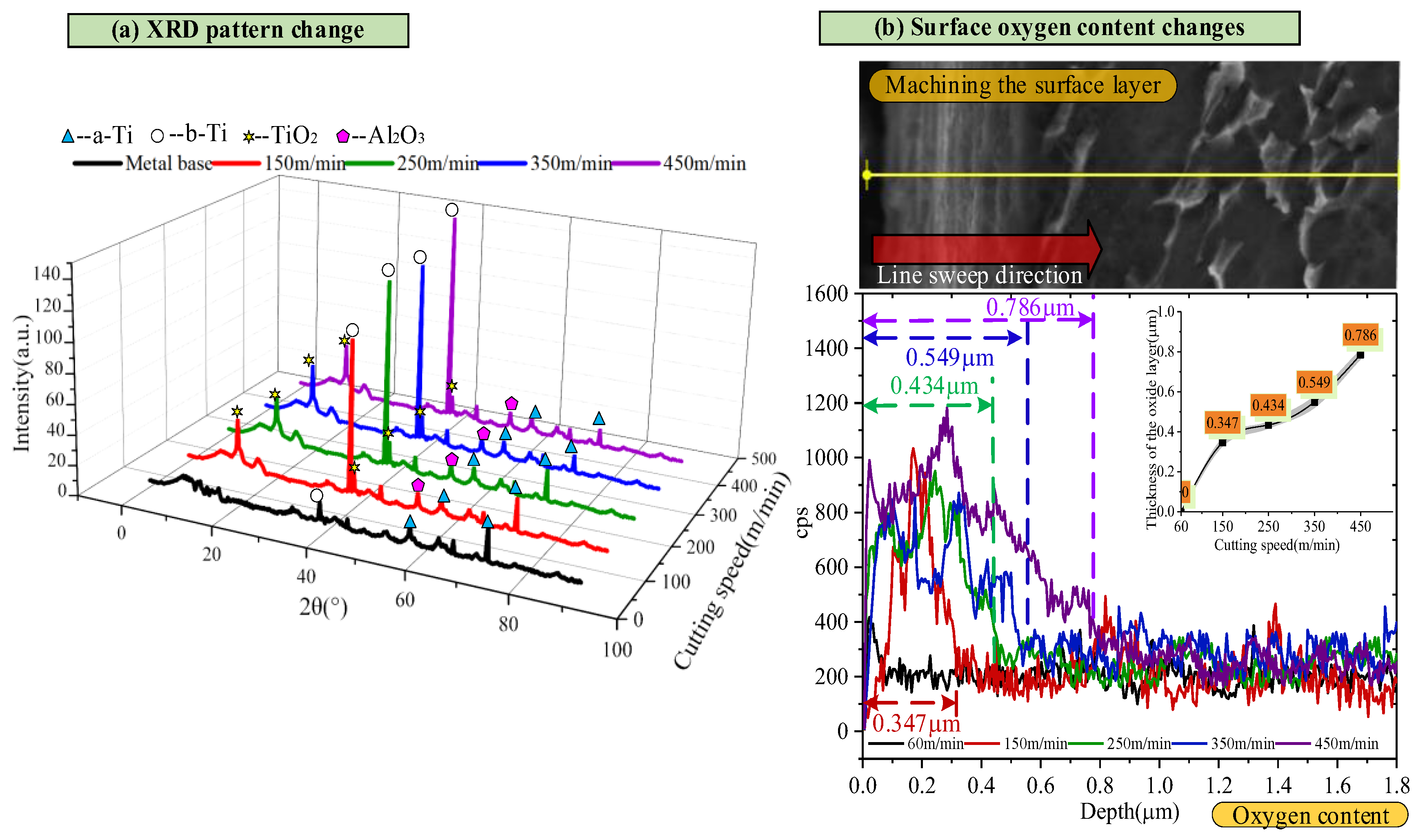
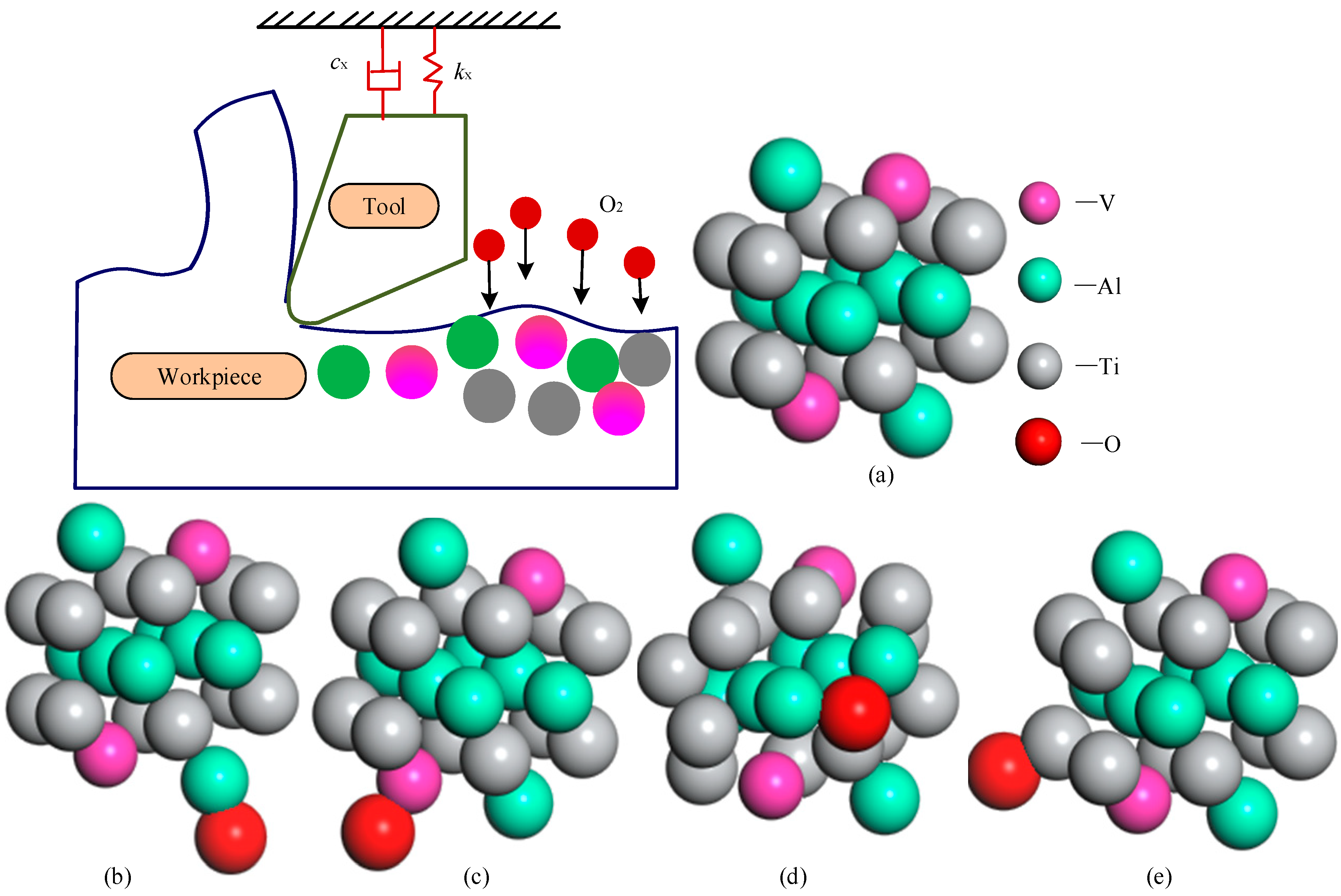
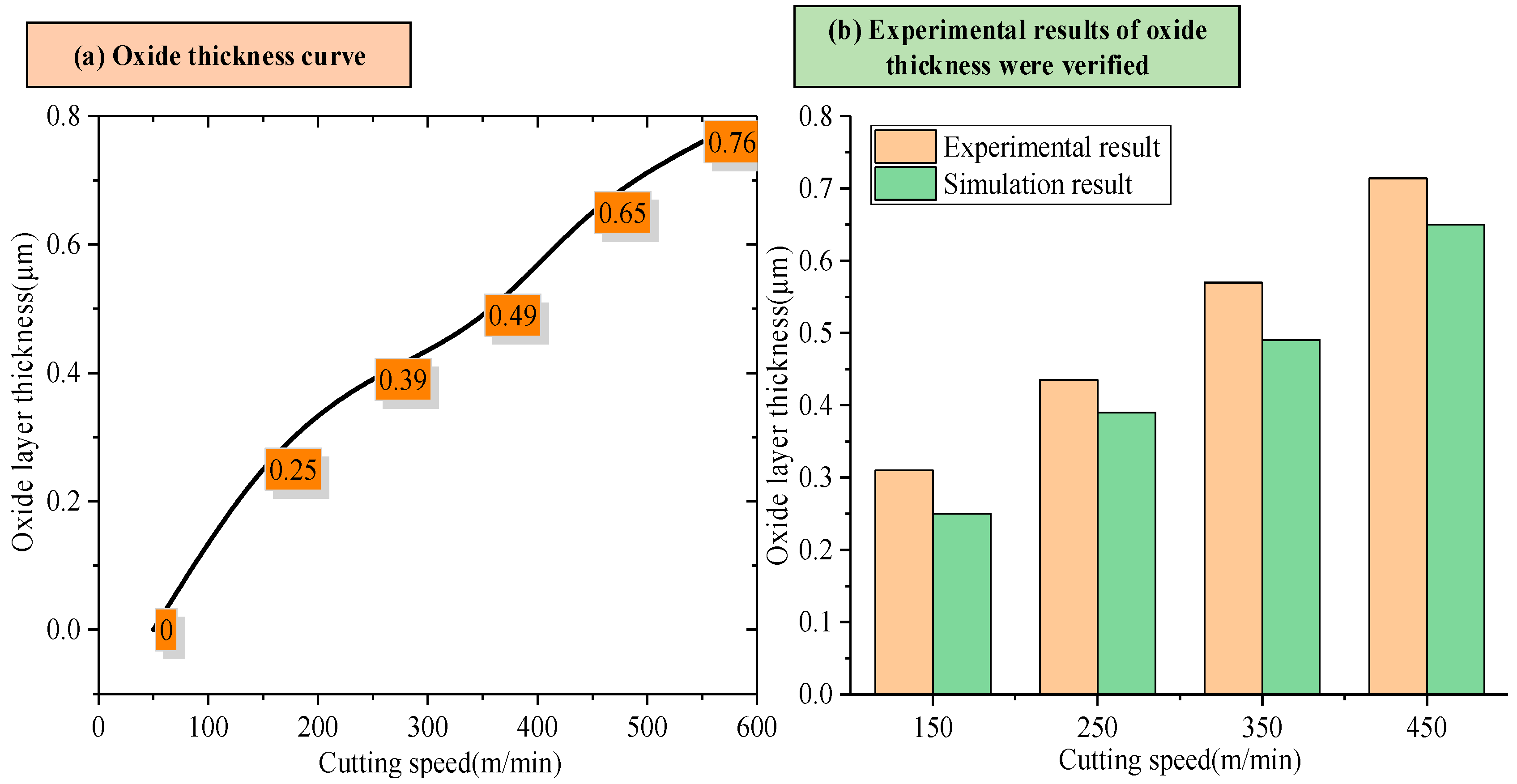
3.3. Prediction of Oxide Layer Thickness
4. Conclusions
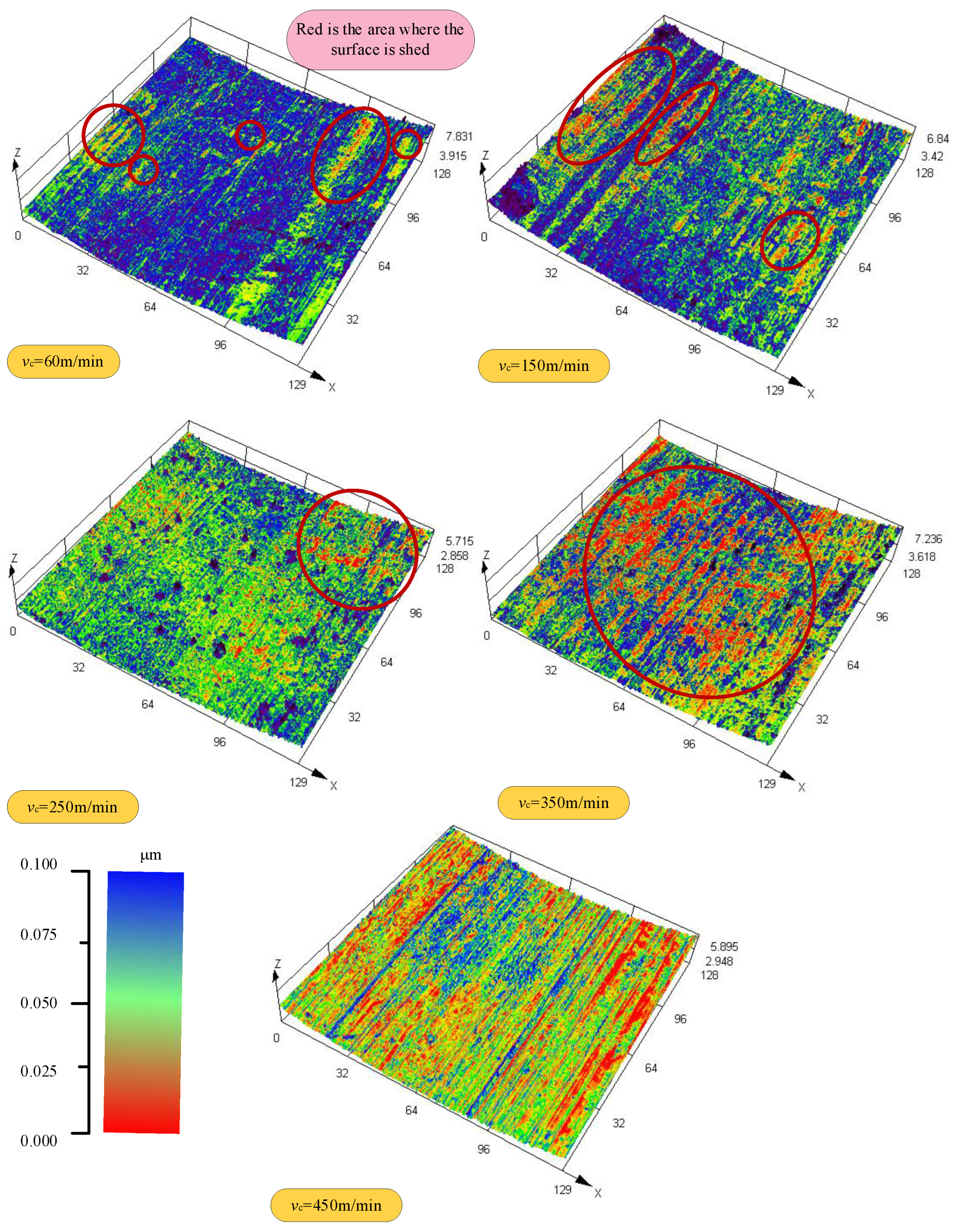
- With the increased cutting speed, the surface roughness increases first and then decreases under the detection scale more significantly than the feed amount (1.2 mm). It continues to increase under the detection scale, which is smaller than the feed amount (0.1 mm). This phenomenon reveals that the effect of cutting speed on the surface quality varies at different scales. With the increase in the cutting speed, the surface shedding area gradually increases, and the distribution of the red area (low roughness) changes from dispersion to concentration, reflecting the gradual deterioration of surface quality and the intensification of the shedding phenomenon.
- The metamorphic layer’s thickness gradually increases as the cutting speed increases. When the cutting speed is 60 m/min, the surface layer of the newly formed material is not observed. When the velocity increases to 150 m/min, the thickness of the metamorphic layer is about 0.31 μm. When the velocity is further increased to 450 m/min, the thickness of the metamorphic layer increases to 0.714 μm. The increase in cutting speed leads to an increase in the cutting temperature, accelerates the oxidation reaction, and increases the thickness of the oxide layer. The binding force between the oxide layer and the matrix is weakened, and it is easy for this to fall off under the action of the cutting force, which leads to defects on the machined surface and affects the machining accuracy.
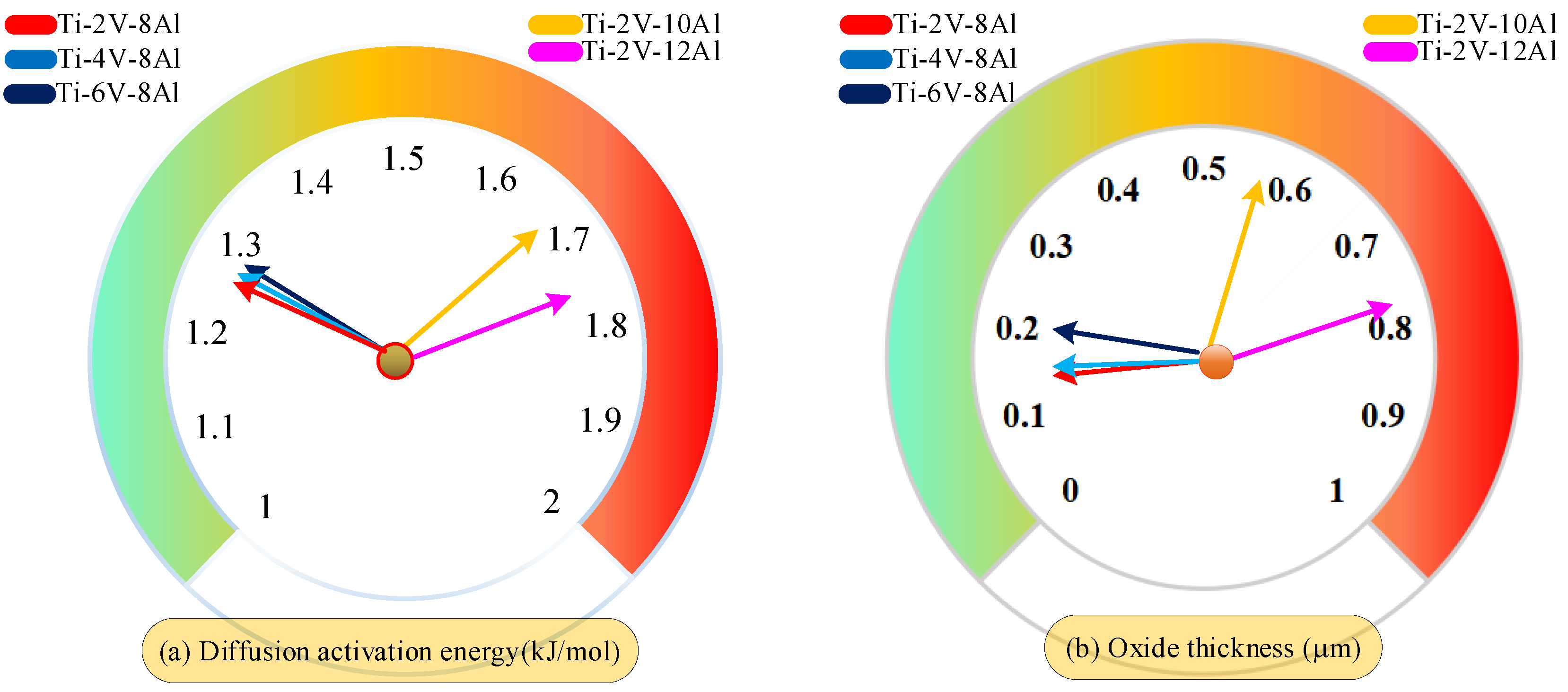
- In this study, the first-principles calculation method established a prediction model of oxide layer thickness based on the diffusion activation energy of oxygen atoms in the Ti6Al4V alloy system. Compared with experimental data, it is verified that the prediction error of this model is less than 15%. Further calculation and analysis revealed that in the study investigating the influence of different element proportions on the thickness of the oxide layer, the increase of aluminum content had more significant effects on the diffusion activation energy of oxygen atoms and the thickness of the oxide layer than that of the vanadium content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Chu, S.; Zhang, X.; Wang, Y.; Zhao, H.; Zhou, B.; Wang, H.; Wu, G.; Mao, B. Laser shock processing of titanium alloys: A critical review on the microstructure evolution and enhanced engineering performance. J. Mater. Sci. Technol. 2025, 209, 262–291. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, Y.; Hu, J.; Sun, X.; Deng, C.; Sun, Y.; Zhang, X.; Tang, S. Effects of heating time on corrosion behavior and surface conductivity of Ti-Al coated Ti6Al4V. Surf. Coat. Technol. 2024, 485, 130928. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; He, D.; Du, X.; Wang, R. Theoretical Analysis and Experimental Research into the Mechanical Properties of Al-Ti-Al Symmetrical Laminated Plate. Adv. Mater. Sci. Eng. 2020, 2020, 8942585. [Google Scholar] [CrossRef]
- Huo, Y.; Niu, Y.; Sun, Z.; Li, Y.; Niu, J. Surface/subsurface damage mechanisms and inhibition strategies in machining of hard and brittle materials: A systematic review. Surf. Interfaces 2024, 54, 105088. [Google Scholar] [CrossRef]
- Obikawa, T.; Matsumoto, W.; Hayashi, M.; Morigo, C. Boiling of Coolant Near the Cutting Edge in High Speed Machining of Difficult-to-Cut Materials: Special Issue on Advanced Metal Cutting Technologies. Int. J. Autom. Technol. 2024, 18, 400–405. [Google Scholar] [CrossRef]
- Alshibi, A.; Nasreldin, A.; Pervaiz, S. Chip morphology and tool wear investigation in high-speed machining of AZ61 magnesium alloys using vegetable-oil based cutting fluid. Adv. Mater. Process. Technol. 2024, 10, 3430–3450. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Ding, W.; Song, J.; Li, H. Wear mechanisms of coated carbide tools during high-speed face milling of Ti2AlNb intermetallic alloys. Int. J. Adv. Manuf. Technol. 2023, 131, 2881–2892. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Yao, H.; Kou, F. Dynamic simulation and experimental study of cutting force by rake angle of multi-axis high-speed ball-end milling tool. Int. J. Adv. Manuf. Technol. 2022, 122, 377–390. [Google Scholar] [CrossRef]
- Xinjian, L.; Guigen, Y.; Zhimin, W.; Shifeng, X.; Yi, Z. Optimization on the Johnson-Cook parameters of Ti6Al4V used for high speed cutting simulation. J. Phys. Conf. Ser. 2020, 1653, 012034. [Google Scholar] [CrossRef]
- Gurevich, L.M.; Novikov, R.; Bannikov, A. Simulation of High-Speed Cutting Using Deform 3D Software. Solid State Phenom. 2020, 299, 340–344. [Google Scholar] [CrossRef]
- Prasad, K.K.; Tamang, S.K.; Chandrasekaran, M. Comparative Study on Cutting Force Simulation Using DEFORM 3D Software during High Speed Machining of Ti6Al4V. Key Eng. Mater. 2020, 856, 50–56. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Xia, Z. A thermo-mechanical fully coupled model for high-speed machining of Ti6Al4V. Ind. Lubr. Tribol. 2024, 76, 906–914. [Google Scholar] [CrossRef]
- Han, G.; Ye, Z.; Xu, J.; Ma, Y.; Xu, C.; Zhao, X.; Yu, L.; Feng, P.; Feng, F. Investigation on cutting forces and tool wear in high-speed milling of Ti6Al4V assisted by longitudinal torsional ultrasonic vibrations. Int. J. Adv. Manuf. Technol. 2023, 129, 783–799. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, R.L.; Zhou, J.; M’sAoubi, R.; Gustafsson, D.; Moverare, J. Effect of machining parameters on cutting force and surface integrity when high-speed turning AD 730™ with PCBN tools. Int. J. Adv. Manuf. Technol. 2019, 100, 2601–2615. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, D.; Huang, K.; Zhang, J.; Wang, H.; Chen, X.; Xiao, J.; Xu, J. Modeling and analysis of surface integrity transition in cutting of Sip/Al composites based on coordination deformation effect of particle-matrix. Tribol. Int. 2024, 199, 110024. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Chen, H.; Zou, J.; Ma, L.; Yin, G. Study on surface quality and subsurface damage mechanism of nickel-based single-crystal superalloy in precision turning. J. Manuf. Process. 2023, 99, 230–242. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Li, W.; Wang, Q.; Li, Y. A study on microstructure evolution and corrosion resistance of cutting layer metal of 7055 aluminum alloy based on extreme environment. Mater. Corros. 2018, 69, 1389–1397. [Google Scholar] [CrossRef]
- Potekaev, A.I.; Gromov, V.E.; Yuriev, A.B.; Ivanov, Y.F.; Konovalov, S.V.; Minenko, S.S.; Semin, A.P.; Chapaikin, A.S.; Litovchenko, I.Y. Transition Zone Structure in the Fast-Cutting Surfaced Layer—Substrate System. Russ. Phys. J. 2024, 67, 1107–1113. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Zhou, B.; Chen, Y.; Feng, R.; Cao, H.; Lei, C. Effect of structure tool on nano-cutting surface integrity of single crystal γ-TiAl alloy via atomic simulation. Mater. Today Commun. 2024, 40, 110034. [Google Scholar] [CrossRef]
- Chen, G.; Caudill, J.; Chen, S.; Jawahir, I.S. Machining-induced surface integrity in titanium alloy Ti6Al4V: An investigation of cutting edge radius and cooling/lubricating strategies. J. Manuf. Process. 2022, 74, 353–364. [Google Scholar] [CrossRef]
- Li, G.; Qu, S.; Guan, S.; Wang, F. Study on the tensile and fatigue properties of the heat-treated HIP Ti6Al4V alloy after ultrasonic surface rolling treatment. Surf. Coat. Technol. 2019, 379, 124971. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Lai, X.; Zhao, G.; Yang, Y. Effect of Cutting Surface Integrity on Fatigue Properties of TC17 Titanium Alloy. Materials 2023, 16, 5658. [Google Scholar] [CrossRef]
- Matuszak, J.; Zaleski, K.; Zyśko, A. Investigation of the Impact of High-Speed Machining in the Milling Process of Titanium Alloy on Tool Wear, Surface Layer Properties, and Fatigue Life of the Machined Object. Materials 2023, 16, 5361. [Google Scholar] [CrossRef]
- Zhao, X.; Li, R.; Liu, E.; Lan, C. Effect of cryogenic cutting surface integrity on fatigue life of titanium alloy Ti-5553. Ferroelectrics 2022, 596, 115–125. [Google Scholar] [CrossRef]
- Wu, S.; He, Y.; Zhu, T.; Zhang, W.; Chen, W.; Wang, C. Microstructure and mechanical properties of superficial surface and subsurface layers in the cutting of hardened steel under cryogenic cooling. J. Mater. Process. Technol. 2023, 322, 118165. [Google Scholar] [CrossRef]
- Li, A.; Pang, J.; Zhao, J. Crystallographic texture evolution and tribological behavior of machined surface layer in orthogonal cutting of Ti6Al4V alloy. J. Mater. Res. Technol. 2019, 8, 4598–4611. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Xie, H.; Zhao, W.; Xu, C.; Liu, J. Effect of cutting parameters on the depth of subsurface deformed layers of single γ-TiAl alloy during nano-cutting process. Appl. Phys. A 2022, 128, 189. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, R.; Dong, Z.; Bao, Y.; Guo, D.; Bai, Q. Effect of dislocation density distribution in work-hardened layer on cutting characteristics in the multi-cutting of 49Fe49Co2V alloy. J. Manuf. Process. 2023, 85, 1187–1196. [Google Scholar] [CrossRef]
- Yi, X.; Wang, H.; Sun, B.; Sun, K.; Huang, C.; Gao, Z.; Meng, X.; Cai, W.; Zhao, L. The microstructural characteristics and high temperature mechanical properties of quaternary Ti–V–Al–Co shape memory alloys. J. Alloys Compd. 2020, 835, 155416. [Google Scholar] [CrossRef]
- Ratochka, I.V.; Naydenkin, E.V.; Lykova, O.N.; Mishin, I.P. Development of Superplastic Deformation in Ultrafine-Grained α–β-Titanium Alloys with a Large Content of β-Phase. Russ. Phys. J. 2022, 64, 2193–2201. [Google Scholar] [CrossRef]
- Chen, X.; Huang, L.; Song, J.; Zhou, S.; Wang, S.; Zhang, R.; Geng, L. Towards strength-ductility synergy of titanium matrix composites through configuration design: Mesoscale deformation and damage behaviors. Compos. Part B 2024, 283, 111651. [Google Scholar] [CrossRef]
- Safari, H.; Sharif, S.; Izman, S.; Jafari, H. Surface integrity characterization in high-speed dry end milling of Ti-6Al-4V titanium alloy. Int. J. Adv. Manuf. Technol. 2015, 78, 651–657. [Google Scholar] [CrossRef]
- Ji, H.; Peng, Z.; Huang, X.; Wang, B.; Xiao, W.; Wang, S. Characterization of the Microstructures and Dynamic Recrystallization Behavior of Ti-6Al-4V Titanium Alloy through Experiments and Simulations. J. Mater. Eng. Perform. 2021, 30, 8257–8275. [Google Scholar] [CrossRef]
- Lou, Y.; Chen, L.; Wu, H.; To, S. Influence of cutting velocity on surface roughness during the ultra-precision cutting of titanium alloys based on a comparison between simulation and experiment. PLoS ONE 2023, 18, e0288502. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, B.; Liu, E.; Chai, Y.; Wang, X.; Yang, L.; Li, G.; Wang, J. Surface integrity investigation into longitudinal-torsional ultrasonic vibration side milling for a TC18 titanium alloy—Part I: The effects of cutting speed on cutting force and surface integrity. Int. J. Adv. Manuf. Technol. 2022, 120, 2701–2713. [Google Scholar] [CrossRef]






| Test Number | vc (m/min) | f (mm/r) | ap (mm) |
|---|---|---|---|
| 1 | 60 | 0.2 | 0.2 |
| 2 | 150 | 0.2 | 0.2 |
| 3 | 250 | 0.2 | 0.2 |
| 4 | 350 | 0.2 | 0.2 |
| 5 | 450 | 0.2 | 0.2 |
| Ti (wt.%) | Al (wt.%) | V (wt.%) | Fe (wt.%) | C (wt.%) | N (wt.%) | H (wt.%) | O (wt.%) |
|---|---|---|---|---|---|---|---|
| Allowance | 5.5~6.8 | 3.5~4.5 | 0.3 | 0.08 | 0.05 | 0.015 | 0.20 |
| Yield Strength (τ) | Thermal Conductivity (λ) | Specific Heat (ϲ) | Density (ρ) | Thermal Softening Coefficient (α) |
|---|---|---|---|---|
| MPa | W(m·K)−1 | J(kg·K)−1 | kg/m3 | 1/K |
| 825 | 6.8 | 520 | 4.44 × 103 | 6.13 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, C.; Miao, H.; Tan, Z.; Sun, W. Analysis of High-Speed Cutting Surface Layer Formation and Oxide Layer Thickness Prediction of Titanium Alloy (Ti6Al4V). Materials 2025, 18, 3160. https://doi.org/10.3390/ma18133160
Wang C, Li C, Miao H, Tan Z, Sun W. Analysis of High-Speed Cutting Surface Layer Formation and Oxide Layer Thickness Prediction of Titanium Alloy (Ti6Al4V). Materials. 2025; 18(13):3160. https://doi.org/10.3390/ma18133160
Chicago/Turabian StyleWang, Chenyu, Changyou Li, Huihui Miao, Zhi Tan, and Wei Sun. 2025. "Analysis of High-Speed Cutting Surface Layer Formation and Oxide Layer Thickness Prediction of Titanium Alloy (Ti6Al4V)" Materials 18, no. 13: 3160. https://doi.org/10.3390/ma18133160
APA StyleWang, C., Li, C., Miao, H., Tan, Z., & Sun, W. (2025). Analysis of High-Speed Cutting Surface Layer Formation and Oxide Layer Thickness Prediction of Titanium Alloy (Ti6Al4V). Materials, 18(13), 3160. https://doi.org/10.3390/ma18133160






