Exploring the Gas Permeability of Type IV Hydrogen Storage Cylinder Liners: Research and Applications
Abstract
1. Introduction
- (1)
- Regarding the gas permeability of plastic liners in Type IV hydrogen storage cylinders, this review provides a detailed introduction to the working conditions of plastic liners and compares their advantages with other forms of hydrogen storage. It clearly discusses and explores the key considerations in the selection of liner materials, focusing on compatibility with hydrogen, aging resistance, and mechanical properties.
- (2)
- Reveals the factors influencing the gas permeability of Type IV hydrogen storage cylinders, introducing the effects of material properties, temperature, pressure, and cylinder winding layers on gas permeability.
- (3)
- Clarifies the mechanism of hydrogen molecule dissolution and diffusion in polymer materials, compares and analyzes international standards for hydrogen permeation testing, and introduces the application of numerical simulation methods in hydrogen permeation through polymer materials.
- (4)
- Elucidates the safety of hydrogen storage systems, reviews the application of liner materials and manufacturing methods, and provides a comprehensive summary and discussion.
2. Permeability of Gas in Type IV Hydrogen Storage Cylinder Liners
2.1. Overview of Hydrogen Storage Cylinder Liners
2.2. Liner Material Selection and Characterization
2.2.1. The Compatibility of Materials with Hydrogen Gas
2.2.2. The Aging Resistance of Materials
2.2.3. The Mechanical Properties of Materials
2.3. The Influencing Factors of Gas Permeability
2.3.1. The Impact of Inner Liner Materials
2.3.2. The Influence of Temperature and Pressure
2.3.3. The Impact of Winding Layers and Coatings
2.4. Summary and Outlook of Gas Permeability in Liner Materials
3. Study Methods for Gas Permeability of Liners
3.1. Experimental Measurement Methods
3.2. Numerical Simulation Methods
3.3. Summary of Research Methods
4. The Application and Challenges of Inner Liner Gas Permeability
4.1. The Safety of Hydrogen Storage Systems
4.2. Manufacture and Application of Inner Liner
5. Limitations in Research on Inner Liner Gas Permeability
6. Future Perspectives
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lodewyckx, S.; Beasy, K.; Mattila, P. Pieces of a jigsaw: Opportunities and challenges in the nascent Australian hydrogen mobility market. Int. J. Hydrogen Energy 2023, 48, 19821–19833. [Google Scholar] [CrossRef]
- Smit, D.J.; Powell, J.B. Role of International Oil Companies in the Net-Zero Emission Energy Transition. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal carbonization of food waste for sustainable biofuel production: Advancements, challenges, and future prospects. Sci Total Environ. 2023, 897, 165327. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Sun, H.; Yin, H.; Xu, F.; Wang, Z. Evaluation of fuel properties and combustion behaviour of hydrochar derived from hydrothermal carbonisation of agricultural wastes. J. Energy Inst. 2023, 108, 101209. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Wu, S.; Hu, Z.; Bai, J. Critical upstream technologies for hydrogen energy industry: Research progress on ammonia decomposition catalysts. Sustain. Chem. Pharm. 2024, 38, 101492. [Google Scholar] [CrossRef]
- Martínez, L.; Fernández, D.; Mantz, R. Passivity-based control for an isolated DC microgrid with hydrogen energy storage system. Int. J. Hydrogen Energy 2024, 67, 1262–1269. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Jiang, A.; Chen, Y.; Duan, Y.; Ye, J.; Zhou, Y. Solar-Driven Hydrogen Evolution from Value-Added Waste Treatment. Advanced Energy Materials 2024, 14, 2470068. [Google Scholar] [CrossRef]
- Prajapati, M.; Shah, M. Geothermal-solar hybrid systems for hydrogen production: A systematic review. Int. J. Hydrogen Energy 2024, 67, 842–851. [Google Scholar] [CrossRef]
- Li, Y.; Hu, W.; Zhang, F.; Li, Y. Collaborative operational model for shared hydrogen energy storage and park cluster: A multiple values assessment. J. Energy Storage 2024, 82, 110507. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Tang, X.; He, Y. Solar-driven methanol steam reforming for low carbon and efficient hydrogen production: A review. J. Clean. Prod. 2024, 436, 140587. [Google Scholar] [CrossRef]
- Beiler, A.M.; Li, W.; Denisiuk, A.; Palomares, E.; Llobet, A. Solar hydrogen production from electrochemical ammonia splitting powered by a single perovskite solar cell. J. Energy Chem. 2024, 92, 292–295. [Google Scholar] [CrossRef]
- El-Hamalawy, A.F.; Farag, H.E.Z.; Asif, A. Optimal design of grid-connected green hydrogen plants considering electrolysis internal parameters and battery energy storage systems. Energy Convers. Manag. 2024, 302, 118127. [Google Scholar] [CrossRef]
- Hilali, İ.; Işıker, Y.; Ulker, N. The hydrogen perspective for Türkiye, which is on the Asia-Europe energy transition route. Can Türkiye become hydrogen hub? Int. J. Hydrogen Energy 2024, 75, 88–97. [Google Scholar] [CrossRef]
- Loewe, M.; Quittkat, C.; Knodt, M.; Ott, I. The Impact of the Russian War against Ukraine on the German Hydrogen Discourse. Sustainability 2024, 16, 773. [Google Scholar] [CrossRef]
- Hilali, I.; Dogan, E.; Altin, Y.; Servi, A.; Isiker, Y. The geostrategic and economic impact of Türkiye’s hydrogen energy vision on Europe. Int. J. Hydrogen Energy 2024, 57, 100–106. [Google Scholar] [CrossRef]
- Erivantseva, T.N.; Tuzova, S.Y.; Lyskov, N.B.; Salnikov, M.Y.; Tuzhilkina, E.A.; Palamarchuk, M.S.; Sedov, I.V.; Ryzhenko, P.I.; Skudro, M.I. Hydrogen energy vector in the sphere of intellectual property in Russian Federation. Int. J. Hydrogen Energy 2024, 60, 147–154. [Google Scholar] [CrossRef]
- Neumann, F.; Zeyen, E.; Victoria, M.; Brown, T. The potential role of a hydrogen network in Europe. Joule 2023, 7, 1793–1817. [Google Scholar] [CrossRef]
- Shi, M.; Bao, D.; Yan, J.; Zhong, H.; Zhang, X. Coordination and Architecture Regulation of Electrocatalysts for Sustainable Hydrogen Energy Conversion. Acc. Mater. Res. 2024, 5, 160–172. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, D.; Wang, W. Medium and long-term hydrogen production technology routes and hydrogen energy supply scenarios in Guangdong Province. Int. J. Hydrogen Energy 2024, 49, 1–15. [Google Scholar] [CrossRef]
- Boretti, A. Assessing the value of hydrogen thermal energy storage and electric thermal energy storage in NEOM city. Int. J. Hydrogen Energy 2024, 49, 1133–1147. [Google Scholar] [CrossRef]
- Gordon, J.A.; Balta-Ozkan, N.; Nabavi, S.A. Towards a unified theory of domestic hydrogen acceptance: An integrative, comparative review. Int. J. Hydrogen Energy 2024, 56, 498–524. [Google Scholar] [CrossRef]
- Ghirardi, E.; Brumana, G.; Franchini, G.; Aristolao, N.; Vedovati, G. The role of hydrogen storage and electric vehicles in grid-isolated hybrid energy system with high penetration of renewable. Energy Convers. Manag. 2024, 302, 118154. [Google Scholar] [CrossRef]
- Li, J.; Chai, X.; Gu, Y.; Zhang, P.; Yang, X.; Wen, Y.; Xu, Z.; Jiang, B.; Wang, J.; Jin, G.; et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. [Google Scholar] [CrossRef]
- Fu, H.; Lv, C.; Meng, J.; He, M.; Wu, J.; Gong, L. Strength and fatigue study of on-board type III cryo-compressed hydrogen storage cylinder. Int. J. Hydrogen Energy 2024, 57, 1081–1088. [Google Scholar] [CrossRef]
- Karayel, G.K.; Javani, N.; Dincer, I. A comprehensive assessment of energy storage options for green hydrogen. Energy Convers. Manag. 2023, 291, 117311. [Google Scholar] [CrossRef]
- Valant, M.; Luin, U. Chemistry of the iron-chlorine thermochemical cycle for hydrogen production utilizing industrial waste heat. J. Clean. Prod. 2024, 438, 140681. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, W.; Wei, Y.; Yan, Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Olutoki, J.O.; Ansari, M.N.M.; Abioye, K.J.; Rehman, Z.U.; Soleimani, H. Hydrogen-powered horizons: Transformative technologies in clean energy generation, distribution, and storage for sustainable innovation. Int. J. Hydrogen Energy 2024, 56, 1152–1182. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Y.; Li, J.; Bin, G.; Zhou, C.; Han, W.; Li, X. Hydrogen Permeability of Polyamide 6 Used as Liner Material for Type IV On-Board Hydrogen Storage Cylinders. Polymers 2023, 15, 3715. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Neogi, S. Performance-based design and manufacturing of filament wound Type-4 cylinders for compressed gas storage. Compos. Struct. 2023, 309, 116710. [Google Scholar] [CrossRef]
- Martinez-Boggio, S.; Di Blasio, D.; Fletcher, T.; Burke, R.; García, A.; Monsalve-Serrano, J. Optimization of the air loop system in a hydrogen fuel cell for vehicle application. Energy Convers. Manag. 2023, 283, 116911. [Google Scholar] [CrossRef]
- Andrade, T.S.; Thiringer, T. Low platinum fuel cell as enabler for the hydrogen fuel cell vehicle. J. Power Sources 2024, 598, 234140. [Google Scholar] [CrossRef]
- Sun, S.S.; Darzi, A.; Zargartalebi, M.; Guo, Y.; Sinton, D. Geothermal reforming crude glycerol to hydrogen. Energy Convers. Manag. 2024, 302, 118135. [Google Scholar] [CrossRef]
- Henry, A.; McStay, D.; Rooney, D.; Robertson, P.; Foley, A. Techno-economic analysis to identify the optimal conditions for green hydrogen production. Energy Convers. Manag. 2023, 291, 117230. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Sagade, A.A.; Mani, M.; Aroulmoji, V.; Rajan, V.F.; Manavalan, K. A concise review of recent biohydrogen production technologies. Sustain. Energy Technol. Assess. 2024, 62, 103606. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Z.; Liu, J.; Yan, H.; Feng, X.; Chen, D.; Yang, C. Towards low-carbon consumption and cleaner methanol production via hybrid hydrogen supply strategy: A techno-economic-environment assessment. Chem. Eng. Sci. 2024, 288, 119708. [Google Scholar] [CrossRef]
- Droessler, M.; Leach, A. Green with Envy? Hydrogen production in a carbon-constrained world. Energy Policy 2024, 186, 113982. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Liu, S. The impacts and instruments of energy transition regulations on environmental pollution. Environ. Impact Assess. Rev. 2024, 105, 107448. [Google Scholar] [CrossRef]
- Feng, W.; Ruiz, C. Risk management of energy communities with hydrogen production and storage technologies. Appl. Energy 2023, 348, 121494. [Google Scholar] [CrossRef]
- Chae, H.J.; Gye, H.R.; Lee, J.S.; Esmaeili, A.; Lee, G.Y.; Yoon, T.; Im, J.; Song, D.; Lee, C.J. Quantitative Risk Assessment of a Liquid Organic Hydrogen Carriers-Based Hydrogen Refueling Station. Korean J. Chem. Eng. 2024, 41, 1311–1327. [Google Scholar] [CrossRef]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on hydrogen safety issues: Incident statistics, hydrogen diffusion, and detonation process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- Mei, Y.; Shuai, J.; Li, Y. Analysis of the Consequences of Hydrogen-Blended Natural Gas Leakage Accidents. Combust. Sci. Technol. 2023, 196, 4768–4791. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, M.H.; Lee, M.C.; Kim, Y.J. Evaluating hydrogen risk management policy PR: Lessons learned from three hydrogen accidents in South Korea. Int. J. Hydrogen Energy 2023, 48, 24536–24547. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Hu, S.; Li, Y.; Yang, F.; Ouyang, M. Simulation and risk assessment of hydrogen leakage in hydrogen production container. Int. J. Hydrogen Energy 2023, 48, 20096–20111. [Google Scholar] [CrossRef]
- Liu, J.; Ma, H.; Zheng, S.; Zhang, Z.; Zheng, J.; Zhao, Y. Numerical investigation on temperature-rise of on-bus gaseous hydrogen storage cylinder with different thickness of liner and wrapping material. Int. J. Hydrogen Energy 2021, 46, 20607–20620. [Google Scholar] [CrossRef]
- Piras, M.; De Bellis, V.; Malfi, E.; Novella, R.; Lopez-Juarez, M. Hydrogen consumption and durability assessment of fuel cell vehicles in realistic driving. Appl. Energy 2024, 358, 122559. [Google Scholar] [CrossRef]
- Kandidayeni, M.; Trovão, J.P.; Soleymani, M.; Boulon, L. Towards health-aware energy management strategies in fuel cell hybrid electric vehicles: A review. Int. J. Hydrogen Energy 2022, 47, 10021–10043. [Google Scholar] [CrossRef]
- Sahwal, C.P.; Sengupta, S.; Dinh, T.Q. Advanced Equivalent Consumption Minimization Strategy for Fuel Cell Hybrid Electric Vehicles. J. Clean. Prod. 2024, 437, 140366. [Google Scholar] [CrossRef]
- Zheng, J.; He, Y.; Hou, Y.; Hu, J.; Li, X.; Su, Z. Interpretation of Phase II of the Global Technical Regulations for the Safety of Fuel Cell Electric Vehicles-Series III: Hydrogen Storage Systems. China Auto 2023, 12, 3–8. [Google Scholar]
- Zhao, Y.; Chen, G.; Xu, Q.; Lv, H.; Su, S.; Xia, L.; Zhang, G.; Yang, G.; Hu, K. Metering inaccuracy analysis and improvement measures of typical scenarios in hydrogen refueling station. Int. J. Hydrogen Energy 2024, 50, 815–828. [Google Scholar] [CrossRef]
- Melideo, D.; Baraldi, D.; Acosta-Iborra, B.; Cebolla, R.O.; Moretto, P. CFD simulations of filling and emptying of hydrogen tanks. Int. J. Hydrogen Energy 2017, 42, 7304–7313. [Google Scholar] [CrossRef]
- Li, J.Q.; Li, J.C.; Wang, X.Y.; Xu, H.; Kwon, J.T.; Leng, C.L. A numerical study on the thermal behavior of high pressure hydrogen in the on-board storage cylinder. AIP Adv. 2023, 13, 075222. [Google Scholar] [CrossRef]
- Bo, K.; Feng, H.; Jiang, Y.; Deng, G.; Wang, D.; Zhang, Y. Study of blister phenomena on polymer liner of type IV hydrogen storage cylinders. Int. J. Hydrogen Energy 2024, 54, 922–936. [Google Scholar] [CrossRef]
- Takahashi, T.; Ikeda, T.; Murata, K.; Hotaka, O.; Shigeki, H.; Tachikawa, Y.; Nishihara, M.; Matsuda, J.; Kitahara, T.; Lyth, S.M.; et al. Accelerated Durability Testing of Fuel Cell Stacks for Commercial Automotive Applications: A Case Study. J. Electrochem. Soc. 2022, 169, 044523. [Google Scholar] [CrossRef]
- Carignano, M.; Costa-Castelló, R. Toyota Mirai: Powertrain Model and Assessment of the Energy Management. IEEE Trans. Veh. Technol. 2023, 72, 7000–7010. [Google Scholar] [CrossRef]
- Toyota adds, hydrogen station at Motomachi. Fuel Cells Bull. 2018, 2018, 4. [CrossRef]
- Kojima, K.; Fukazawa, K. Current Status and Future Outlook of Fuel Cell Vehicle Development in Toyota. ECS Trans. 2025, 69, 213–219. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Q.; Shi, F.; Zhang, Z.; Liu, Y. Research progress on the inner liner material of carbon fiber fully-reinforced composite tank with plastic liner. New Chem. Mater. 2022, 50, 262–265. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, J.; Wang, X.; Tian, M.; Yang, W.; Xie, P. Preparation of high-density polyethylene/montmorillonite nanocomposites with high gas barrier by micro-nano torsional laminated extrusion. Polym. Compos. 2023, 44, 6747–6757. [Google Scholar] [CrossRef]
- Marais, S.; Lozay, Q.; Follain, N.; Soulestin, J.; Couvrat, N. Dargent, Multinanolayered PA6/Cloisite and PE/PA6/Cloisite composites: Structure, mechanical and barrier properties. Compos. Part B Eng. 2024, 271, 111167. [Google Scholar] [CrossRef]
- Somerday, B.P. Technical Reference on Hydrogen Compatibility of Materials; Sandia National Laboratories: Albuquerque, NM, USA, 2005; p. 1100. Available online: http://www.sandia.gov/matlsTechRef/ (accessed on 15 December 2018).
- Barth, R.R.; Simmons, K.L.; Marchi, C.W.S. Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems: Review of Literature and Analysis of Gaps; Sandia National Laboratories: Albuquerque, NM, USA, 2013. [Google Scholar] [CrossRef]
- Asl, F.H.; Saeb, M.R.; Jafari, S.H.; Khonakdar, H.A.; Goodarzi, V. Conceptualizing Physical and Chemical Interactions in the Compatibilized HDPE/PA6 and HDPE/EVOH Pairs: Theoretical and Experimental Analyses. Polym.-Plast. Technol. Eng. 2017, 56, 1986–1996. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, H.; Pan, Z.; Wang, X.; Zhao, Q.; Li, X. Hydrogen embrittlement of a precipitation-strengthened high-entropy alloy. Corros. Sci. 2024, 227, 111708. [Google Scholar] [CrossRef]
- Yin, S.; Cheng, G.; Chang, T.H.; Richter, G.; Zhu, Y.; Gao, H. Hydrogen embrittlement in metallic nanowires. Nat. Commun. 2019, 10, 2004. [Google Scholar] [CrossRef]
- Wang, H.; Tong, Z.; Zhou, G.; Zhang, C.; Zhou, H.; Wang, Y.; Zheng, W. Research and demonstration on hydrogen compatibility of pipelines: A review of current status and challenges. Int. J. Hydrogen Energy 2022, 47, 28585–28604. [Google Scholar] [CrossRef]
- Rahman, K.M.M.; Mohtadi-Bonab, M.A.; Ouellet, R.; Szpunar, J.; Zhu, N. Effect of electrochemical hydrogen charging on an API X70 pipeline steel with focus on characterization of inclusions. Int. J. Press. Vessel. Pip. 2019, 173, 147–155. [Google Scholar] [CrossRef]
- Park, C.; Kang, N.; Liu, S. Effect of grain size on the resistance to hydrogen embrittlement of API 2W Grade 60 steels using in situ slow-strain-rate testing. Corros. Sci. 2017, 128, 33–41. [Google Scholar] [CrossRef]
- Gijsman, P. Review on the thermo-oxidative degradation of polymers during processing and in service. e-Polymers 2008, 8, 065. [Google Scholar] [CrossRef]
- Lellinger, D.; Alig, I.; Oehler, H.; Rode, K.; Malz, F.; Herkenrath, L.M.; Youn, J.Y. Accelerated thermal aging of thermoplastic materials for the motor compartment: Characterization, degradation model and lifetime prediction. In Service Life Prediction of Polymers and Coatings; William Andrew Publishing: Norwich, NY, USA, 2020; pp. 117–161. [Google Scholar] [CrossRef]
- Li, R.; Hu, X. Study on discoloration mechanism of polyamide 6 during thermo-oxidative degradation. Polym. Degrad. Stab. 1998, 62, 523–528. [Google Scholar] [CrossRef]
- Alig, I.; Böhm, F.; Lellinger, D. Heat capacity spectroscopy by using stochastic temperature modulated calorimetry: Time-temperature superposition and fictive temperature at the glass transition of poly (vinyl acetate). Thermochim. Acta 2019, 677, 4–11. [Google Scholar] [CrossRef]
- Shu, Y.; Ye, L.; Yang, T. Study on the long-term thermal-oxidative aging behavior of polyamide 6. J. Appl. Polym. Sci. 2008, 110, 945–957. [Google Scholar] [CrossRef]
- Forsström, D.; Terselius, B. Thermo oxidative stability of polyamide 6 films I. Mechanical and chemical characterisation. Polym. Degrad. Stab. 2000, 67, 69–78. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Lv, H.; Liu, M.; Li, W.; Zhang, C. Review of Decompression Damage of the Polymer Liner of the Type IV Hydrogen Storage Tank. Polymers 2023, 15, 2258. [Google Scholar] [CrossRef]
- Alexis, F.; Castagnet, S.; Nadot-Martin, C.; Robert, G.; Havet, P. Effect of severe thermo-oxidative aging on the mechanical behavior and fatigue durability of short glass fiber reinforced PA6/6.6. Int. J. Fatigue 2023, 166, 107280. [Google Scholar] [CrossRef]
- Gijsman, P.; Hensen, G.; Mak, M. Thermal initiation of the oxidation of thermoplastic polymers (Polyamides, Polyesters and UHMwPE). Polym. Degrad. Stab. 2021, 183, 109452. [Google Scholar] [CrossRef]
- Schubert, K.; Kolb, J.; Wohlgemuth, F.; Lellinger, D.; Alig, I. Thermische Alterung und Eigenschaften von Polymermaterialien für das Selektive Lasersintern. In Additive Fertigung von Bauteilen und Strukturen; Springer: Berlin/Heidelberg, Germany, 2017; pp. 159–172. [Google Scholar] [CrossRef]
- Sang, L.; Wang, C.; Wang, Y.; Wei, Z. Thermo-oxidative ageing effect on mechanical properties and morphology of short fibre reinforced polyamide composites–comparison of carbon and glass fibres. RSC Adv. 2017, 7, 43334–43344. [Google Scholar] [CrossRef]
- Shi, K.; Ye, L.; Li, G. Thermal oxidative aging behavior and stabilizing mechanism of highly oriented polyamide 6. J. Therm. Anal. Calorim. 2016, 126, 795–805. [Google Scholar] [CrossRef]
- Assink, R.A.; Celina, M.; Skutnik, J.M.; Harris, D.J. Use of a respirometer to measure oxidation rates of polymeric materials at ambient temperatures. Polymer 2005, 46, 11648–11654. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Molecular and macromolecular structure changes in polyamide 11 during thermal oxidation. Polym. Degrad. Stab. 2014, 108, 123–132. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Pery, F.; Fayolle, B. Quantification of hindered phenols in polyamide 11 during thermal aging. Polym. Test. 2016, 52, 63–70. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Fernagut, F.; Guilment, J.; Pery, F.; Fayolle, B.; Richaud, E. Thermal stabilization of polyamide 11 by phenolic antioxidants. Polym. Degrad. Stab. 2020, 179, 109206. [Google Scholar] [CrossRef]
- Richaud, E.; Diogo, O.O.; Fayolle, B.; Verdu, J.; Guilment, J.; Fernagut, F. Review: Auto-oxidation of aliphatic polyamides. Polym. Degrad. Stab. 2013, 98, 1929–1939. [Google Scholar] [CrossRef]
- Fayolle, B.; Richaud, E.; Colin, X.; Verdu, J. Review: Degradation-induced embrittlement in semi-crystalline polymers having their amorphous phase in rubbery state. J. Mater. Sci. 2008, 43, 6999–7012. [Google Scholar] [CrossRef]
- Fayolle, B.; Colin, X.; Audouin, L.; Verdu, J. Mechanism of degradation induced embrittlement in polyethylene. Polym. Degrad. Stab. 2007, 92, 231–238. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoît, G. Hydrogen influence on the tensile properties of mono and multi-layer polymers for gas distribution. Int. J. Hydrogen Energy 2010, 35, 7633–7640. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoît, G. Mechanical testing of polymers in pressurized hydrogen: Tension, creep and ductile fracture. Exp. Mech. 2012, 52, 229–239. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoît, G. Effect of long-term hydrogen exposure on the mechanical properties of polymers used for pipes and tested in pressurized hydrogen. Int. J. Press. Vessel. Pip. 2012, 89, 203–209. [Google Scholar] [CrossRef]
- Yuan, L.; Kyriakides, S. Liner wrinkling and collapse of bi-material pipe under axial compression. Int. J. Solids Struct. 2015, 60, 48–59. [Google Scholar] [CrossRef]
- Sapre, S.; Pareek, K.; Vyas, M. Investigation of structural stability of type IV compressed hydrogen storage tank during refueling of fuel cell vehicle. Energy Storage 2020, 2, e150. [Google Scholar] [CrossRef]
- Pepin, J.; Lainé, E.; Grandidier, J.C.; Castagnet, S.; Blanc-Vannet, P.; Papin, P.; Weber, M. Determination of key parameters responsible for polymeric liner collapse in hyperbaric type IV hydrogen storage vessels. Int. J. Hydrogen Energy 2018, 43, 16386–16399. [Google Scholar] [CrossRef]
- Pépin, J.; Lainé, E.; Grandidier, J.C.; Benoit, G.; Mellier, D.; Weber, M.; Langlois, C. Replication of liner collapse phenomenon observed in hyperbaric type IV hydrogen storage vessel by explosive decompression experiments. Int. J. Hydrogen Energy 2018, 43, 4671–4680. [Google Scholar] [CrossRef]
- Blanc-Vannet, P.; Papin, P.; Weber, M.; Renault, P.; Pepin, J.; Lainé, E.; Tantchou, G.; Castagnet, S.; Grandidier, J.-C. Sample scale testing method to prevent collapse of plastic liners in composite pressure vessels. Int. J. Hydrogen Energy 2019, 44, 8682–8691. [Google Scholar] [CrossRef]
- Cha, Y.; Wang, X.; Yang, W.; Xie, P. Numerical Simulation Study on the Mechanism of Collapse and Peeling of Plastic Liner of Type IV Hydrogen Storage Cylinders. In Proceedings of the 17th Annual CAE Engineering Analysis Technology Conference, Haikou, China, 12 November 2021; pp. 157–159, 173. [Google Scholar] [CrossRef]
- Nouri, M.; Ghasemi, F.A.; Sherbaf, G.R.; Kashyzadeh, K.R. Fatigue Analysis of a Type-IV CNG Composite Cylinder with Variable Wall-Thickness and Polyethylene Liner. Mech. Compos. Mater. 2023, 59, 927–944. [Google Scholar] [CrossRef]
- Santharam, P.; Marco, Y.; Le Saux, V.; Le Saux, M.; Robert, G.; Raoult, I.; Guévenoux, C.; Taveau, D.; Charrier, P. Fatigue criteria for short fiber-reinforced thermoplastic validated over various fiber orientations, load ratios and environmental conditions. Int. J. Fatigue 2020, 135, 105574. [Google Scholar] [CrossRef]
- Amiri, A.; Ulven, C.A.; Huo, S. Effect of Chemical Treatment of Flax Fiber and Resin Manipulation on Service Life of Their Composites Using Time-Temperature Superposition. Polymers 2015, 7, 1965–1978. [Google Scholar] [CrossRef]
- Amiri, A.; Hosseini, N.; Ulven, C.A. Long-Term Creep Behavior of Flax/Vinyl Ester Composites Using Time-Temperature Superposition Principle. J. Renew. Mater. 2015, 3, 224–233. [Google Scholar] [CrossRef]
- Huo, S.; Thapa, A.; Ulven, C.A. Effect of surface treatments on interfacial properties of flax fiber-reinforced composites. Adv. Compos. Mater. 2013, 22, 109–121. [Google Scholar] [CrossRef]
- Ota, W.N.; Amico, S.C.; Satyanarayana, K.G. Studies on the combined effect of injection temperature and fiber content on the properties of polypropylene-glass fiber composites. Compos. Sci. Technol. 2005, 65, 873–881. [Google Scholar] [CrossRef]
- Raphael, I.; Saintier, N.; Rolland, H.; Robert, G.; Laiarinandrasana, L. A mixed strain rate and energy based fatigue criterion for short fiber reinforced thermoplastics. Int. J. Fatigue 2019, 127, 131–143. [Google Scholar] [CrossRef]
- Duan, S.; Li, M.; Zhu, M.; Zeng, X.; Zhang, C.; Lu, X.; Shang, L. Reliability design of hydrogen storage cylinders based on nano-toughened composite materials. Int. J. Hydrogen Energy 2025, 126, 572–579. [Google Scholar] [CrossRef]
- Backens, S.; Siering, J.; Schmidt, S.; Glück, N.; Flügge, W. Comparative study of thermoplastic liner materials with regard to mechanical and permeation barrier properties before and after cyclic thermal aging. Mater. Test. 2021, 63, 311–316. [Google Scholar] [CrossRef]
- Parodi, E.; Peters, G.W.M.; Govaert, L.E. Prediction of plasticity-controlled failure in polyamide 6: Influence of temperature and relative humidity. J. Appl. Polym. Sci. 2018, 135, 45942. [Google Scholar] [CrossRef]
- Miri, V.; Persyn, O.; Lefebvre, J.M.; Seguela, R. Effect of water absorption on the plastic deformation behavior of nylon 6. Eur. Polym. J. 2009, 45, 757–762. [Google Scholar] [CrossRef]
- Kanjilal, A.; Duarte, M.J.; Scheu, C.; Dehm, G. Hydrogen Permeation Barrier Layers for the Hydrogen Economy. Annu. Rev. Mater. Res. 2025, 55. [Google Scholar] [CrossRef]
- Kim, J.; Yao, X.; Somjit, V.; Kim, S.Y.; Li, J.; Yildiz, B.; Tasan, C.C. Multilayer alumina/aluminum coatings for damage-resistant hydrogen permeation barrier. Int. J. Hydrogen Energy 2025, 106, 226–230. [Google Scholar] [CrossRef]
- Shi, M.; Yang, W.; Zhang, Y.; Tan, J.; Cheng, L.; Jiao, Z.; Zhen, X. Mechanical and dielectric properties and crystalline behavior of multilayer graphite-filled polyethylene composites. J. Appl. Polym. Sci. 2019, 136, 48131. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Yang, J.; Xie, P.; Yang, W. Research on torsional laminated extrusion for improving the permeability and mechanical properties of HDPE/PA6 composite for type IV storage tank liners. J. Appl. Polym. Sci. 2023, 140, e54567. [Google Scholar] [CrossRef]
- Moghri, M.; Garmabi, H.; Zanjanijam, A.R. Prediction of barrier properties of HDPE/PA-6/nanoclay composites by response surface approach: Effects of compatibilizer type and the contents of nanoclay, PA-6 and compatibilizer. Polym. Bull. 2017, 75, 2751–2767. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.Y.; Zhao, L.F.; He, H.F.; Shao, X.Y.; Fang, G.B.; Wan, Z.G.; Zeng, R.C. Research Progress of Graphene-Based Rubber Nanocomposites. Polym. Compos. 2016, 39, 1006–1022. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Rao, G.S.S.; Mathur, A.B.; Jasra, R. Polyolefin/graphene nanocomposites: A review. Rsc Adv. 2017, 7, 23615–23632. [Google Scholar] [CrossRef]
- Liu, G.; Yang, F.; Liu, W.; Bai, Y.; Han, C.; Jiao, W.; Wang, P.; Wang, R. Ultra-high gas barrier composites with aligned graphene flakes and polyethylene molecules for high-pressure gas storage tanks. J. Energy Storage 2021, 40, 102692. [Google Scholar] [CrossRef]
- Olivier, L.; Sabard, M.; Fulchiron, R.; Espuche, E.; David, L.; Guiu, A. Influence of α-ZrP fillers and process conditions on the morphology and the gas barrier properties of filled polyamide 6 films. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1734–1746. [Google Scholar] [CrossRef]
- Lozay, Q.; Beuguel, Q.; Follain, N.; Lebrun, L.; Guinault, A.; Miquelard-Garnier, G.; Tencé-Girault, S.; Sollogoub, C.; Dargent, E.; Marais, S. Structural and Barrier Properties of Compatibilized PE/PA6 Multinanolayer Films. Membranes 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- GB/T 42610-2023; State Administration for Market Supervision and Regulation. National Standardization Administration of China, Test Method for Compatibility of Plastic Liner and Hydrogen in High Pressure Hydrogen Cylinders. State Administration for Market Regulation. Standardization Administration of China: Beijing, China, 2023.
- Zhang, G.; Lee, P.C.; Jenkins, S.; Dooley, J.; Baer, E. The effect of confined spherulite morphology of high-density polyethylene and polypropylene on their gas barrier properties in multilayered film systems. Polymer 2014, 55, 4521–4530. [Google Scholar] [CrossRef]
- Lei, F.; Du, Q.; Li, T.; Li, J.; Guo, S. Effect of phase morphology and interfacial strength on barrier properties of high density polyethylene/polyamide 6 membranes. Polym. Eng. Sci. 2013, 53, 1996–2003. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Souza, C.; Feng, J.; Olah, A.; Wnek, G.; Baer, E. Thermoformable high oxygen barrier multilayer EVOH/LDPE film/foam. J. Appl. Polym. Sci. 2020, 137, 48903. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method -property of polymeric materials for high-pressure hydrogen devices (1)-. Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Rajasekar, R.; Kim, N.H.; Jung, D.; Kuila, T.; Lim, J.K.; Park, M.J.; Lee, J.H. Electrostatically assembled layer-by-layer composites containing graphene oxide for enhanced hydrogen gas barrier application. Compos. Sci. Technol. 2013, 89, 167–174. [Google Scholar] [CrossRef]
- Wang, X.; Tian, M.; Chen, X.; Xie, P.; Yang, J.; Chen, J.; Yang, W. Advances on materials design and manufacture technology of plastic liner of type IV hydrogen storage vessel. Int. J. Hydrogen Energy 2022, 47, 8382–8408. [Google Scholar] [CrossRef]
- Humpenöder, J. Gas permeation of fibre reinforced plastics. Cryogenics 1998, 38, 143–147. [Google Scholar] [CrossRef]
- Mokwena, K.K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef]
- Zhang, Z.; Britt, I.J.; Tung, M.A. Permeation of oxygen and water vapor through EVOH films as influenced by relative humidity. J. Appl. Polym. Sci. 2001, 82, 1866–1872. [Google Scholar] [CrossRef]
- Kim, S.W.; Cha, S.H. Thermal, mechanical, and gas barrier properties of ethylene–vinyl alcohol copolymer-based nanocomposites for food packaging films: Effects of nanoclay loading. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Simmons, K.L.; Kuang, W.; Burton, S.D.; Arey, B.W.; Shin, Y.; Menon, N.C.; Smith, D.B. H-Mat hydrogen compatibility of polymers and elastomers. Int. J. Hydrogen Energy 2021, 46, 12300–12310. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Roh, H.S.; Merkel, D.R.; Simmons, K.L. A predictive modeling tool for damage analysis and design of hydrogen storage composite pressure vessels. Int. J. Hydrogen Energy 2021, 46, 20573–20585. [Google Scholar] [CrossRef]
- Hu, J.; Chandrashekhara, K. Fracture analysis of hydrogen storage composite cylinders with liner crack accounting for autofrettage effect. Int. J. Hydrogen Energy 2009, 34, 3425–3435. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T.; Chiang, F.; Wang, L. An experimental investigation of the temperature effect on the mechanics of carbon fiber reinforced polymer composites. Compos. Sci. Technol. 2018, 154, 53–63. [Google Scholar] [CrossRef]
- Sapre, S.; Pareek, K.; Rohan, R.; Singh, P.K. H2 refueling assessment of composite storage tank for fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 23699–23707. [Google Scholar] [CrossRef]
- Monde, M.; Woodfield, P.; Takano, T.; Kosaka, M. Estimation of temperature change in practical hydrogen pressure tanks being filled at high pressures of 35 and 70 MPa. Int. J. Hydrogen Energy 2012, 37, 5723–5734. [Google Scholar] [CrossRef]
- Dong, W.; Gijsman, P. Influence of temperature on the thermo-oxidative degradation of polyamide 6 films. Polym. Degrad. Stab. 2010, 95, 1054–1062. [Google Scholar] [CrossRef]
- Jaravel, J.; Castagnet, S.; Grandidier, J.C.; Gueguen, M. Experimental real-time tracking and diffusion/mechanics numerical simulation of cavitation in gas-saturated elastomers. Int. J. Solids Struct. 2013, 50, 1314–1324. [Google Scholar] [CrossRef]
- Yersak, T.A.; Baker, D.R.; Yanagisawa, Y.; Slavik, S.; Immel, R.; Mack-Gardner, A.; Herrmann, M.; Cai, M. Predictive model for depressurization-induced blistering of type IV tank liners for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 28910–28917. [Google Scholar] [CrossRef]
- Atiq, O.; Ricci, E.; Baschetti, M.G.; De Angelis, M.G. Molecular Simulations of Hydrogen Sorption in Semicrystalline High-Density Polyethylene: The Impact of the Surface Fraction of Tie-Chains. J. Phys. Chem. B 2024, 128, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure–Property Relationship of Cellulose Nanocrystal–Polyvinyl Alcohol Thin Films for High Barrier Coating Applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef]
- Layek, R.K.; Das, A.K.; Park, M.U.; Kim, N.H.; Lee, J.H. Layer-structured graphene oxide/polyvinyl alcohol nanocomposites: Dramatic enhancement of hydrogen gas barrier properties. J. Mater. Chem. A 2014, 2, 12158–12161. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Oxygen barrier of multilayer thin films comprised of polysaccharides and clay. Carbohydr. Polym. 2013, 95, 299–302. [Google Scholar] [CrossRef]
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A Review on Applicability, Limitations, and Improvements of Polymeric Materials in High-Pressure Hydrogen Gas Atmospheres. Polym. Rev. 2021, 62, 175–209. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Liu, Y.; Zhao, B.; Li, J. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials 2023, 16, 5366. [Google Scholar] [CrossRef] [PubMed]
- Zhevago, N.K.; Glebov, V.I. Hydrogen storage in capillary arrays. Energy Convers. Manag. 2007, 48, 1554–1559. [Google Scholar] [CrossRef]
- Ried, P.; Gaber, M.; Müller, R.; Deubener, J. Hydrogen permeability of a barium-aluminoborosilicate glass—A methodical approach. J. Non-Cryst. Solids 2014, 394–395, 43–49. [Google Scholar] [CrossRef]
- Holtappels, K.; Beckmann-Kluge, M.; Gebauer, M.; Eliezer, D. Pressure Resistance of Glass Capillaries for Hydrogen Storage. Mater. Test. 2011, 53, 14–18. [Google Scholar] [CrossRef]
- Prewitz, M.; Gaber, M.; Müller, R.; Marotztke, C.; Holtappels, K. Polymer coated glass capillaries and structures for high-pressure hydrogen storage: Permeability and hydrogen tightness. Int. J. Hydrogen Energy 2018, 43, 5637–5644. [Google Scholar] [CrossRef]
- Klopffer, M.; Flaconneche, B. Transport properties of gases in polymers: Bibliographic review. Oil Gas Sci. Technol. 2001, 56, 223–244. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C. Research on hydrogen permeability of polyamide 6 as the liner material for type IV hydrogen storage tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Macher, J.; Hausberger, A.; Macher, A.E.; Morak, M.; Schrittesser, B. Critical review of models for H2-permeation through polymers with focus on the differential pressure method. Int. J. Hydrogen Energy 2021, 46, 22574–22590. [Google Scholar] [CrossRef]
- Morsbach, S.; Giersch, D.; Zhang, K.A.I.; Schüßling, A.; Weberskirch, D.; Börger, A. Hydrogen Compatibility of Polymers for Fuel Cell Vehicles. Energy Technol. 2022, 10, 2200018. [Google Scholar] [CrossRef]
- Fang, Q.; Ji, D. Molecular simulation of hydrogen permeation behavior in liner polymer materials of Type IV hydrogen storage vessels. Mater. Today Commun. 2023, 35, 106302. [Google Scholar] [CrossRef]
- ISO-11114-5; ISO Copyright Office, Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents—Part 5:Test Methods for Evaluating Plastic Liners. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/77441.html#:~:text=This%20document (accessed on 22 January 2022).
- Fujiwara, H.; Ono, H.; Ohyama, K.; Kasai, M.; Kaneko, F.; Nishimura, S. Hydrogen permeation under high pressure conditions and the destruction of exposed polyethylene-property of polymeric materials for high-pressure hydrogen devices (2)-. Int. J. Hydrogen Energy 2021, 46, 11832–11848. [Google Scholar] [CrossRef]
- CSA/ANSI CHMC 2:19; Test Methods for Evaluating Material Compatibility in a Compressed Hydrogen Applications-Polymers. CSA Group. Canadian Standards Association: Toronto, ON, Canada, 2019. Available online: https://img.antpedia.com/standard/files/pdfs_ora/20230615/CSA/CHMC/CSA%20ANSI%20CHMC%202-19.pdf#:~:text=Test%20methods%20for (accessed on 23 May 2019).
- T/CATSI 02 007-2020; Fully-Wrapped Carbon Fiber Reinforced Cylinder with a Plastic Liner for On-Board Storage of Compressed Hydrogen for Land Vehicles. China Association for Technical Supervision Information: Beijing, China, 2020.
- Kumar, S.S.; Kanagaraj, G. Investigation on Mechanical and Tribological Behaviors of PA6 and Graphite-Reinforced PA6 Polymer Composites. Arab. J. Sci. Eng. 2016, 41, 4347–4357. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.; Hu, S.; Dong, S.; Wei, P. Investigations of filling mass with the dependence of heat transfer during fast filling of hydrogen cylinders. Int. J. Hydrogen Energy 2014, 39, 4380–4388. [Google Scholar] [CrossRef]
- Jung, J.K. Review of Developed Methods for Measuring Gas Uptake and Diffusivity in Polymers Enriched by Pure Gas under High Pressure. Polymers 2024, 16, 723. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Li, Y.; Huang, R.; Xu, J.; Yang, L. Effects of molecular structures of poly α-olefin mixture on nano-scale thin film lubrication. Mater. Today Commun. 2020, 25, 101500. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, R.; Lu, D. Investigation of Polymer Aging Mechanisms Using Molecular Simulations: A Review. Polymers 2023, 15, 1928. [Google Scholar] [CrossRef]
- Karlsson, G.E.; Gedde, U.W.; Hedenqvist, M.S. Molecular dynamics simulation of oxygen diffusion in dry and water-containing poly(vinyl alcohol). Polymer 2004, 45, 3893–3900. [Google Scholar] [CrossRef]
- Pavel, D.; Shanks, R. Molecular dynamics simulation of diffusion of O2 and CO2 in blends of amorphous poly(ethylene terephthalate) and related polyesters. Polymer 2005, 46, 6135–6147. [Google Scholar] [CrossRef]
- Cha, J.; Lee, W.; Baek, J. Penetration of Hydrogen into Polymer Electrolyte Membrane for Fuel Cells by Quantum and Molecular Dynamics Simulations. Polymers 2021, 13, 947. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, L.; Li, H.; Qi, G.; Gao, X.; Yang, W. Molecular Simulation Study on the Hydrogen Permeation Behavior and Mechanism of Common Polymers. Polymers 2024, 16, 953. [Google Scholar] [CrossRef] [PubMed]
- Voyiatzis, E.; Stroeks, A. Atomistic Modeling of Hydrogen and Oxygen Solubility in Semicrystalline PA-6 and HDPE Materials. J. Phys. Chem. B 2022, 126, 6102–6111. [Google Scholar] [CrossRef]
- Zheng, D.; Li, J.; Liu, B.; Yu, B.; Yang, Y.; Han, D.; Li, J.; Huang, Z. Molecular dynamics investigations into the hydrogen permeation mechanism of polyethylene pipeline material. J. Mol. Liq. 2022, 368, 120773. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, J.; Yang, Z.; Zhao, J. Molecular Dynamics Simulation of Helium Barrier Performance of Modified Polyamide 6 Lining of IV Hydrogen Storage Tank with Montmorillonite. Molecules 2023, 28, 3333. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Pan, Z.-B.; Liu, J.; Ma, L.-H.; Zhou, J.-Y.; Su, Y.-F. Review on optimization design, failure analysis and non-destructive testing of composite hydrogen storage vessel. Int. J. Hydrogen Energy 2022, 47, 38862–38883. [Google Scholar] [CrossRef]
- Kwan, T.H.; Zhang, Z.; Huang, J.; Yao, Q. Fire safety parametric analysis of vehicle mounted hydrogen tanks based on a coupled heat transfer and thermomechanics model. Int. J. Hydrogen Energy 2024, 50, 792–803. [Google Scholar] [CrossRef]
- Zhang, T.; Uratani, J.; Huang, Y.; Xu, L.; Griffiths, S.; Ding, Y. Hydrogen liquefaction and storage: Recent progress and perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113204. [Google Scholar] [CrossRef]
- Huang, H.; Wang, D.; Xu, H.; Yan, Y.; WEI, Z.; Liu, D. Characterization of 70 MPa Hydrogen Storage Cylinder Safety Release for Hydrogen Vehicles. Valves 2023, 5, 570–577. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shi, X.; Fan, R. Safety analysis of hydrogen leakage accident with a mobile hydrogen refueling station. Process Saf. Environ. Prot. 2023, 171, 619–629. [Google Scholar] [CrossRef]
- Yamashita, A.; Kondo, M.; Goto, S.; Ogami, N. Development of High-Pressure Hydrogen Storage System for the Toyota “Mirai”; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- Xu, K.; Wen, Y.; Guo, D.; Yan, X. Effect of lamellae thickness on bubble dissolution in rotomoulding. Polym. Eng. Sci. 2022, 62, 3144–3153. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Tian, M.; Zhang, L.; Yang, W.; Xie, P. Effect of process parameters on the performance of PA6 products fabricated by rotational molding. Polym. Eng. Sci. 2023, 63, 3762–3770. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Tian, M.; Zha, Y.; Duan, Y.; Jin, Y.; Yang, W.; Xie, P. Mechanical and gas barrier properties of polyamides 6/organically modified montmorillonite/maleic anhydride grafted polyolefin elastomer composites produced by rotational molding. Polym. Compos. 2024, 45, 8721–8731. [Google Scholar] [CrossRef]
- Ramkumar, P.L.; Gupta, N. A systematic approach for the selection of additives for rotational molding process. Sādhanā 2024, 49, 47. [Google Scholar] [CrossRef]
- Luciano, G.; Vignolo, M.; Brunengo, E.; Utzeri, R.; Stagnaro, P. Study of Microwave-Active Composite Materials to Improve the Polyethylene Rotomolding Process. Polymers 2023, 15, 1061. [Google Scholar] [CrossRef]
- Benrabah, Z.; Ilinca, F.; Giraldeau, F.; Bournival, S.; Bardetti, A. Contribution to modeling hydrogen permeation and thickness optimization in blow molded plastic liners for on-board compressed hydrogen tanks. Int. J. Hydrogen Energy 2024, 51, 688–694. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Luo, Y.M.; Chevalier, L.; Baron, A.; Lesueur, F.; Utheza, F. Numerical Simulation of Infrared Heating and Ventilation before Stretch Blow Molding of PET Bottles. Int. J. Mater. Form. 2023, 16, 37. [Google Scholar] [CrossRef]
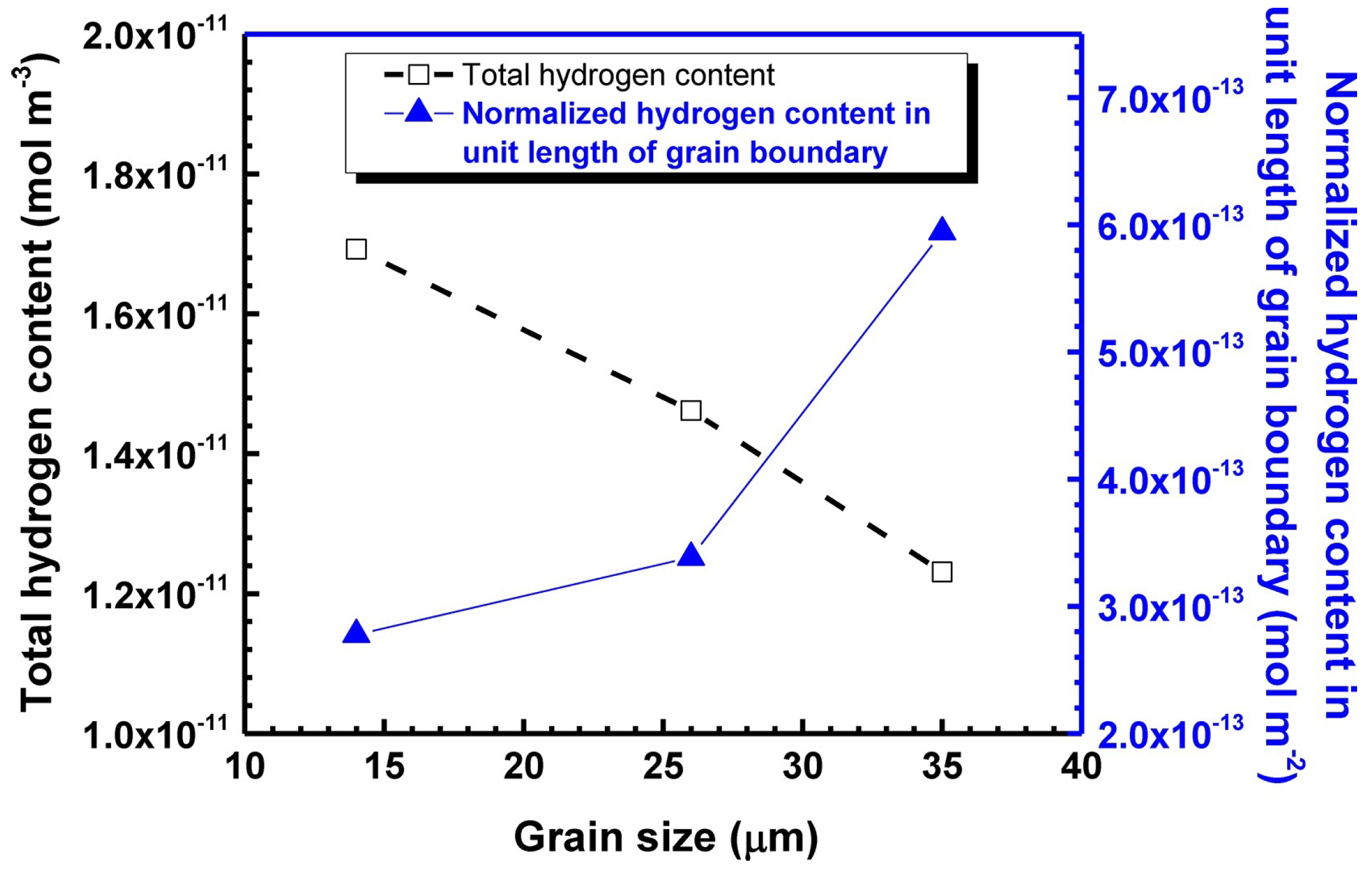
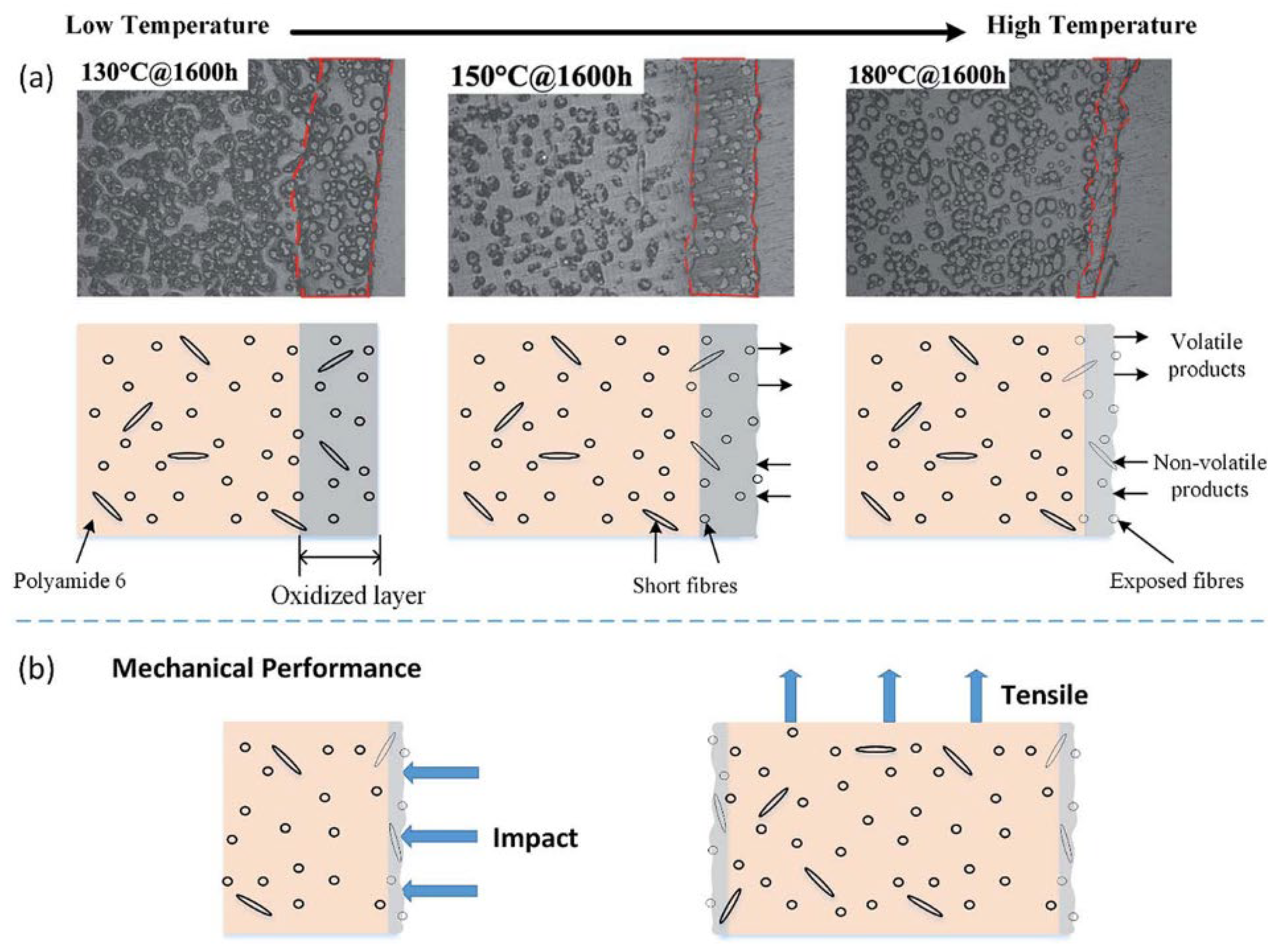
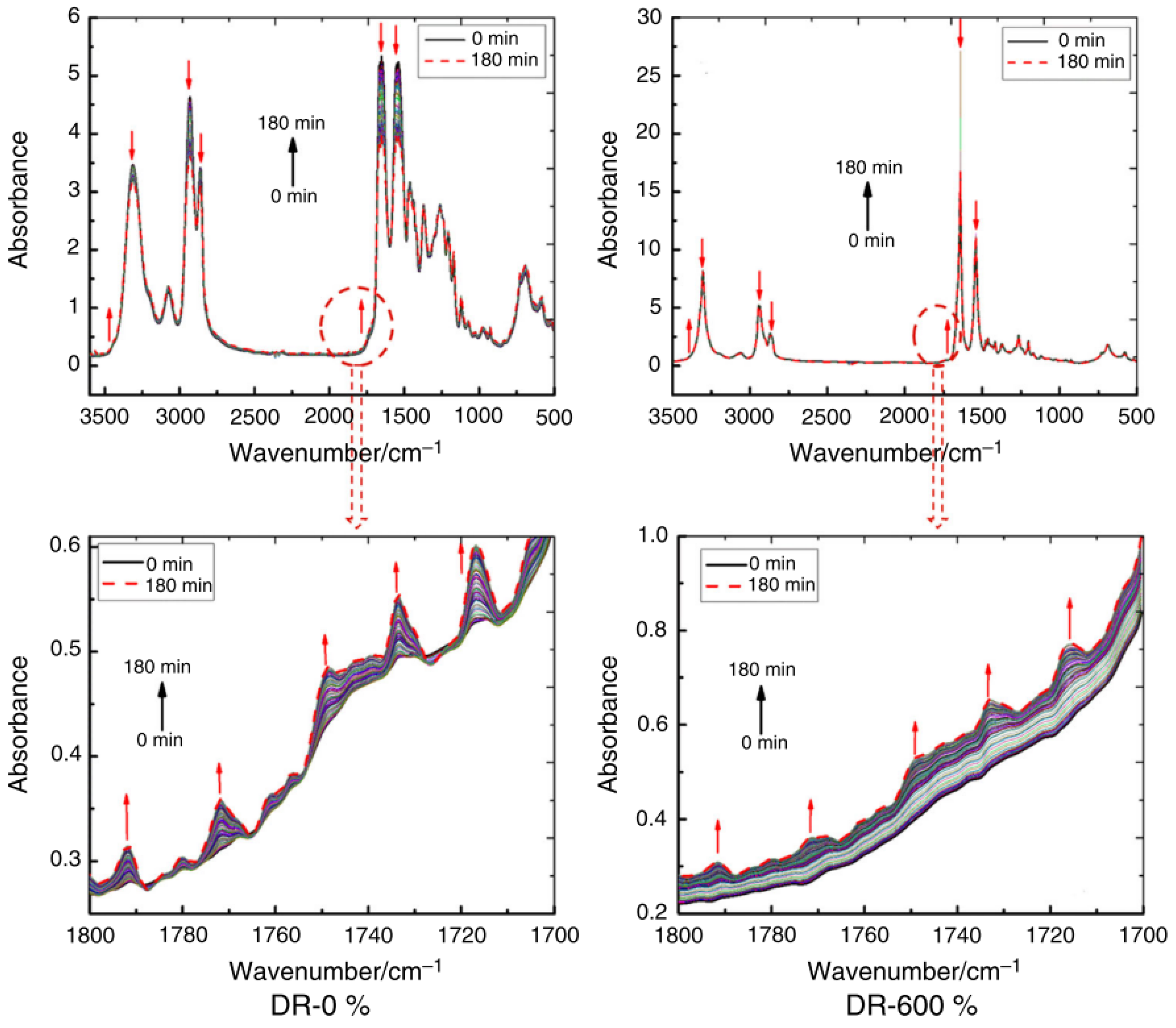
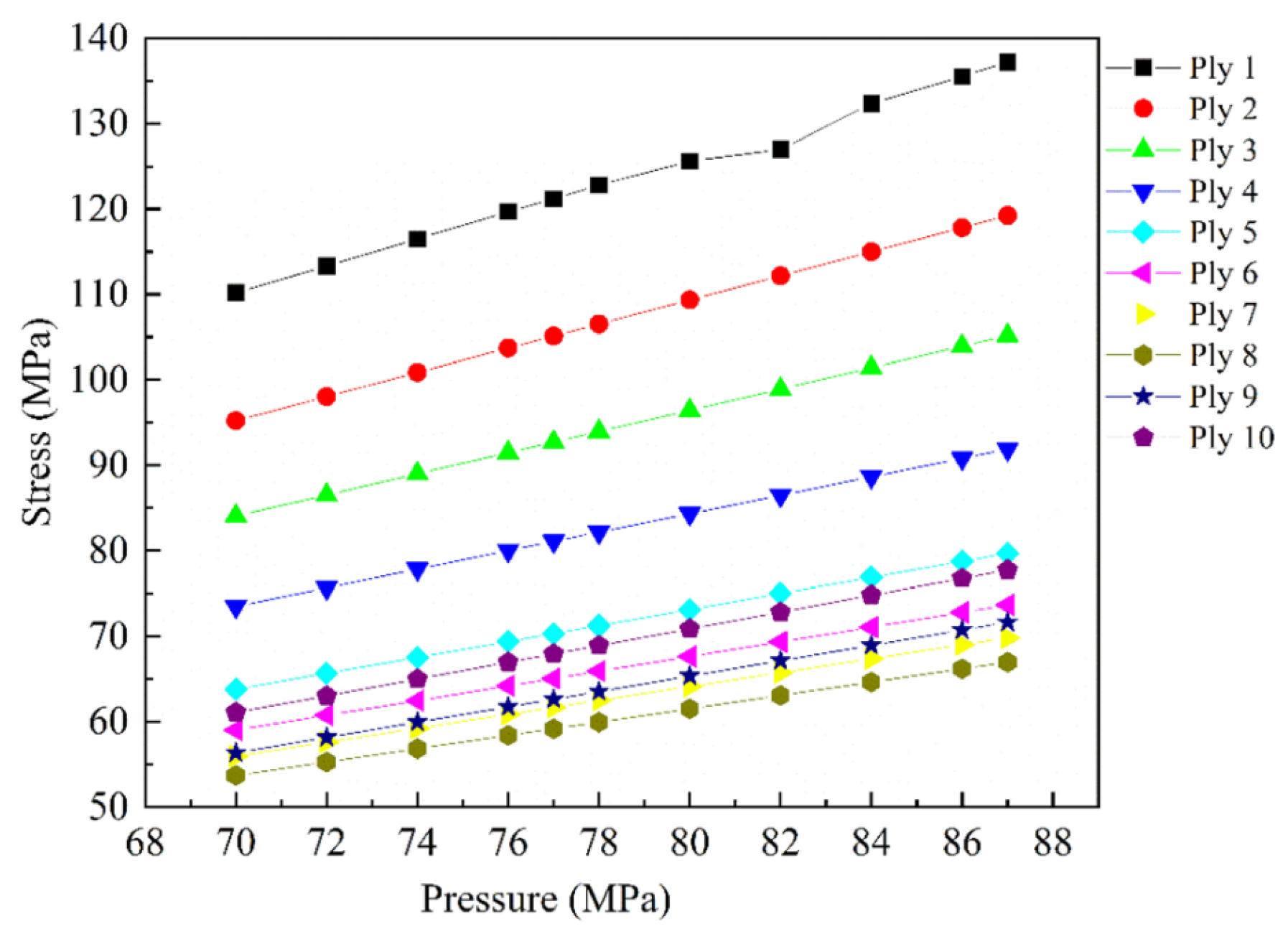
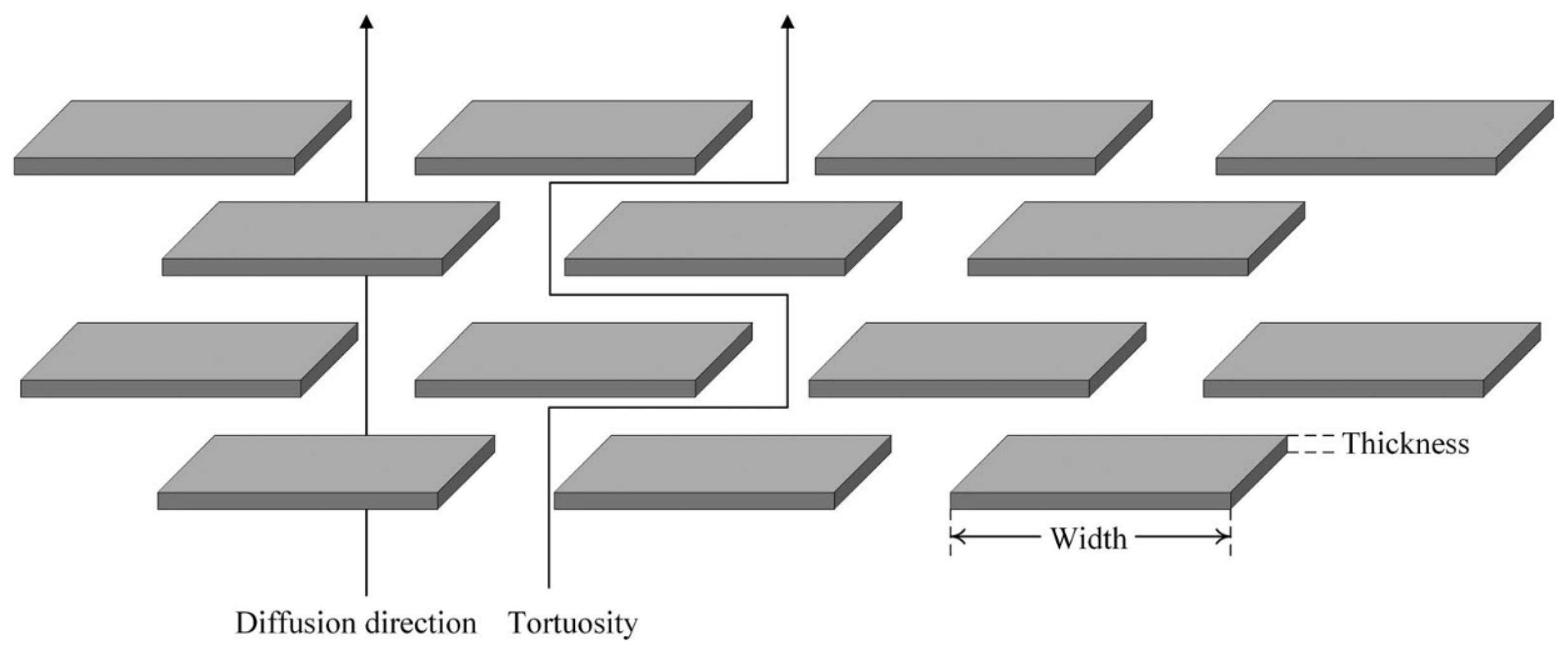
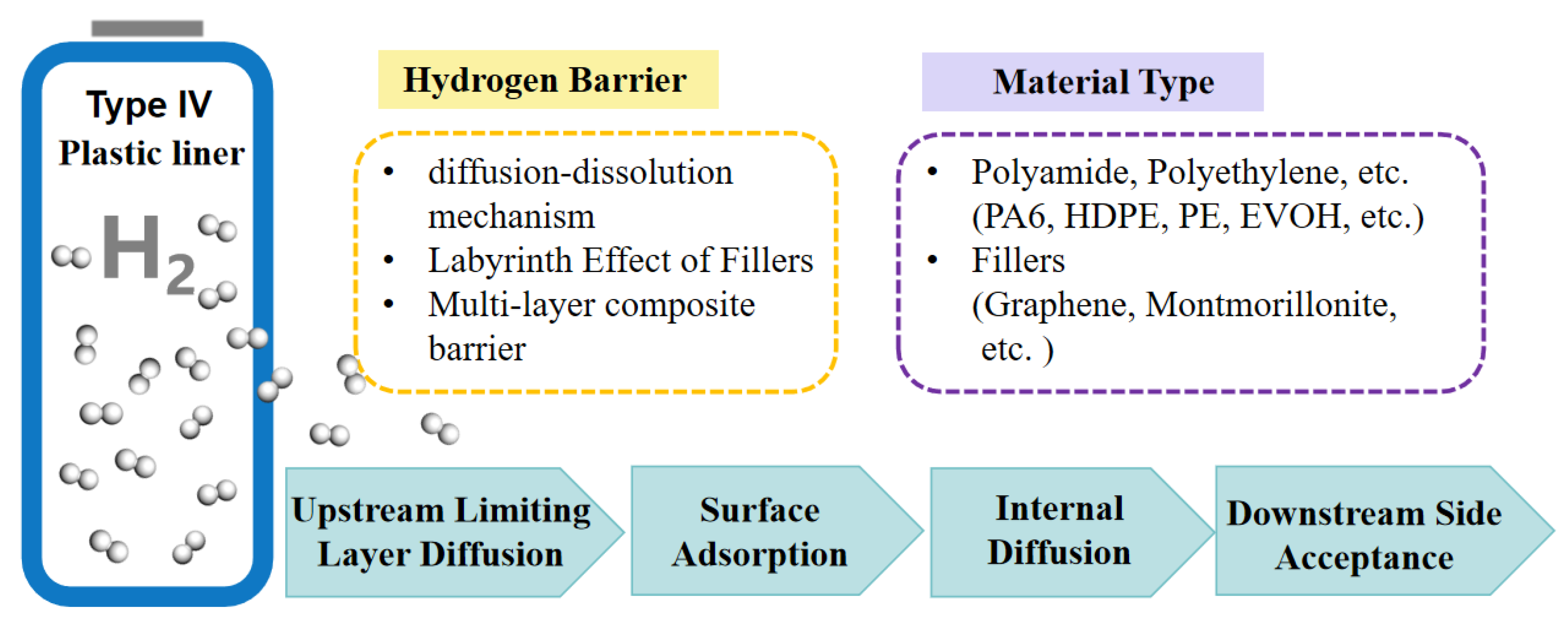
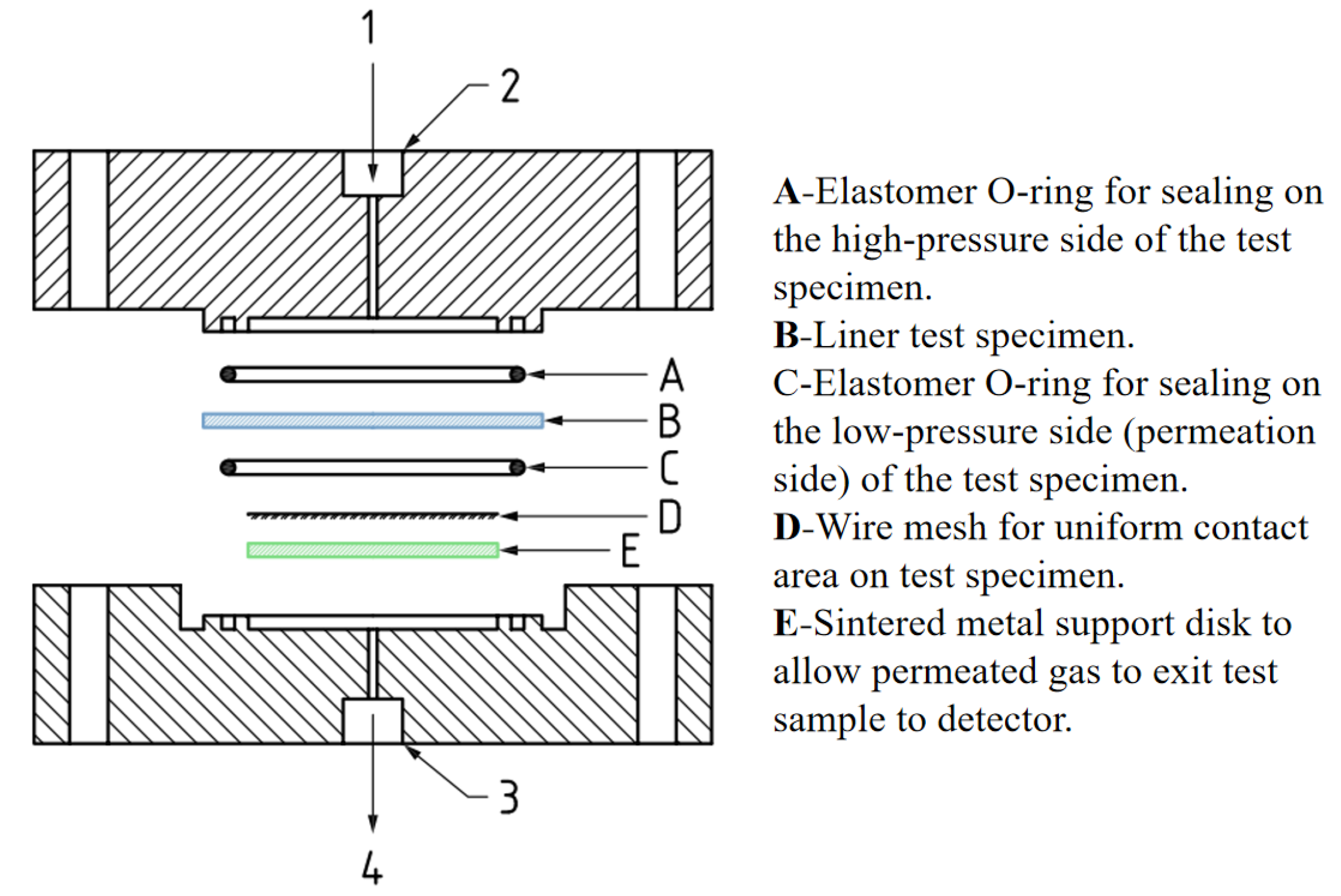

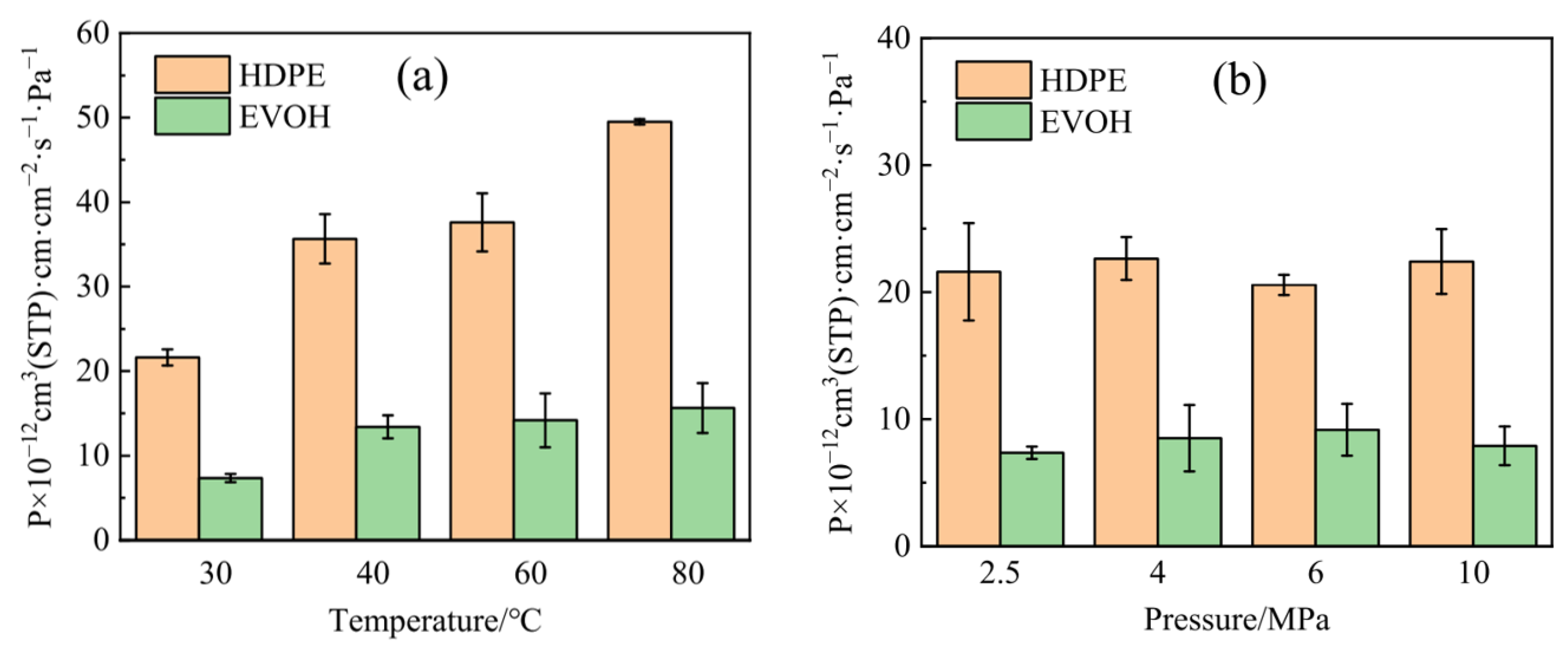

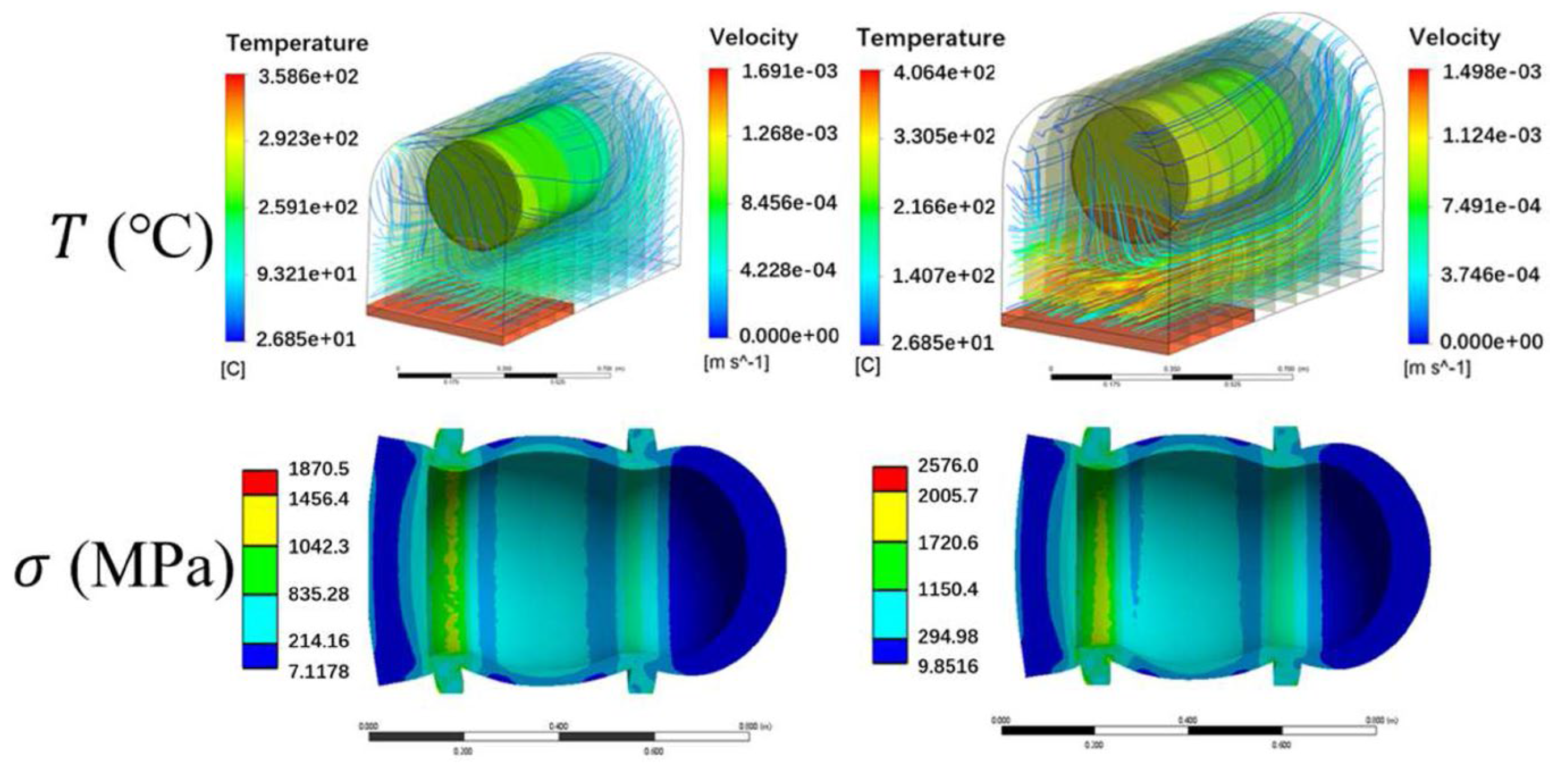
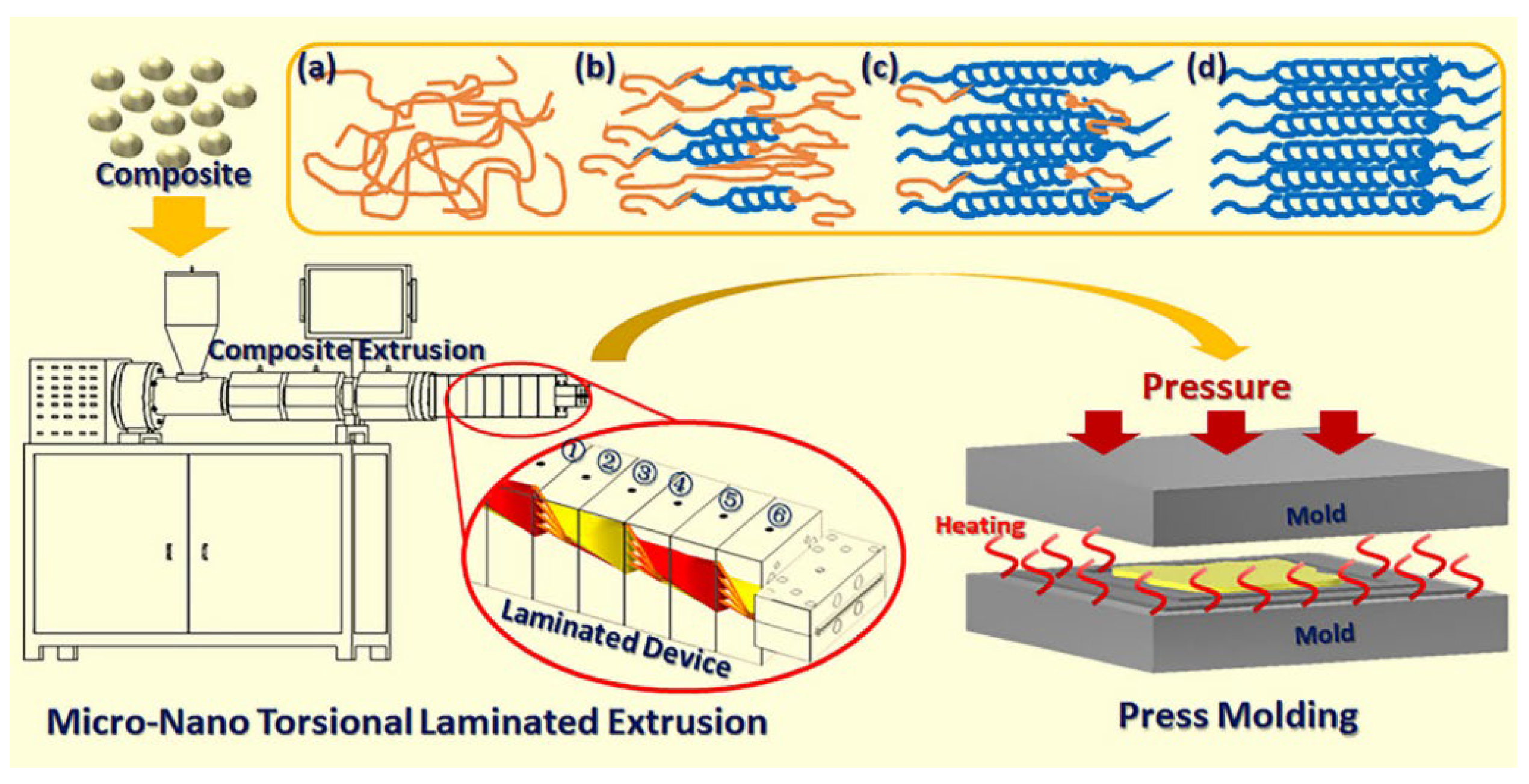
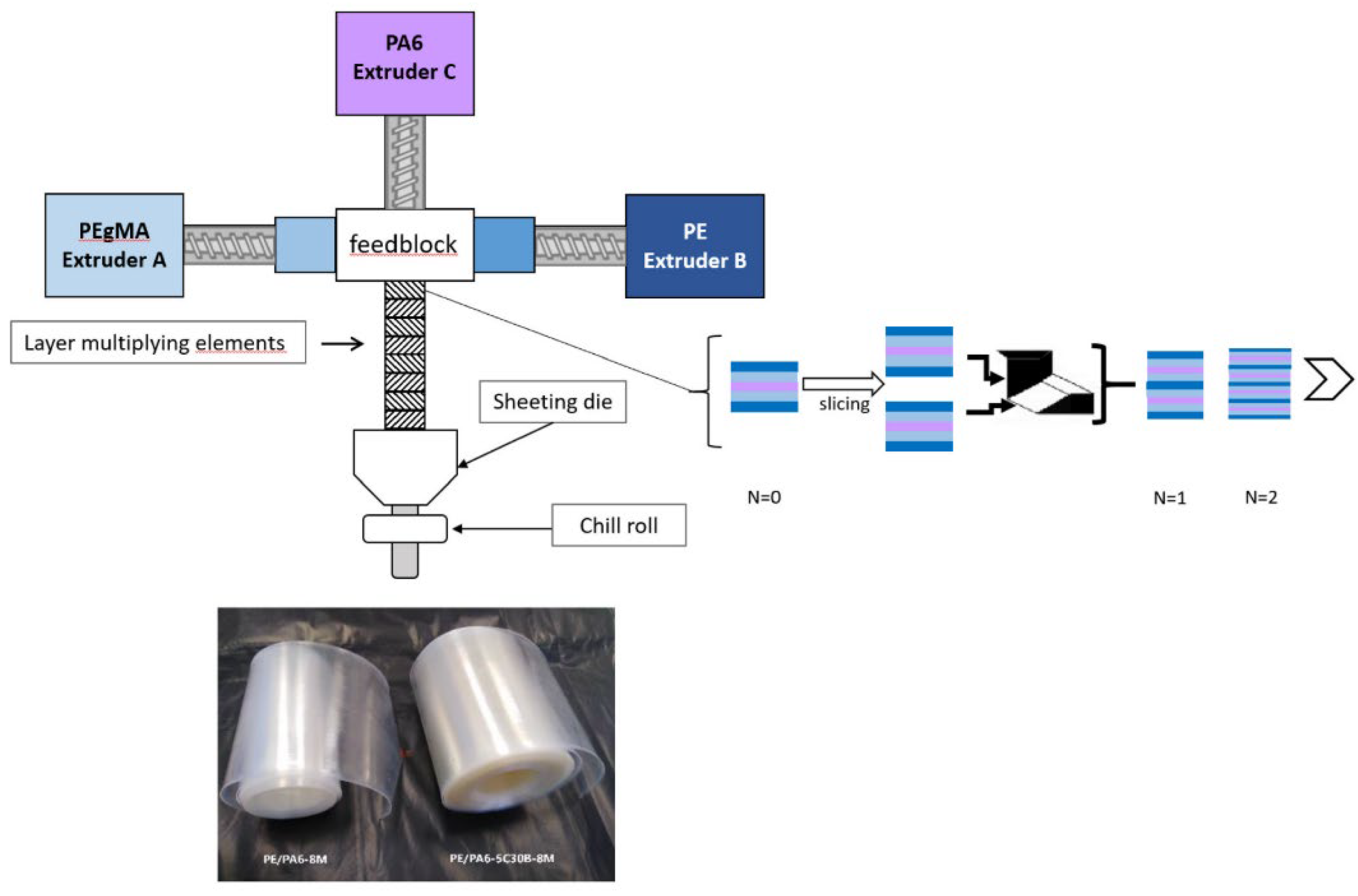
| Materials | Technical Means | Barrier Property | Mechanical Property | Ref. |
|---|---|---|---|---|
| Graphite/PE | Lamination | Tensile strength increased by 68.39% compared to pure PE. | [114] | |
| PE/GF | Boundary constraint-free hot-pressing method | Helium gas permeability 3.92 × 10−9 Pa·m3/s. | Tensile strength 23.24 MPa, Young’s modulus 1.78 GPa. | [119] |
| PA6/α-ZrP | Injection or blow-molded | Helium permeability coefficient 0.8 × 10−10 cmSTP cm cm−2 s−1 cmHg−1. | [120] | |
| PE/PA6 | A coextrusion process with layer multiplier elements (LME) | Combination of moisture resistance of PE and barrier properties of PA6. | Young’s modulus of 956 MPa, tensile strength of 37 MPa, elongation at break of 609%. | [121] |
| PA6/Cloisite | Coextrusion with layer multiplier elements (LMEs) | Permeability coefficient P: PN2 < PO2 < PCO2. | Fracture stress 80–85 MPa, Young’s modulus 0.9–1.0 GPa. | [63] |
| PE/PA6/Cloisite | A coextrusion process with layer multiplier elements (LME) | Improved oxygen barrier effect 58%. | Packing has an effect on Young’s modulus and elongation at break, but a much smaller effect on stress at break. | [63] |
| PA6 | Injection molding according to GB/T 42610-2023 standards [122] | Hydrogen permeability coefficient 1.72 × 10−14 cm3·cm/cm2·s Pa. | [32] | |
| PA11 | Injection molding according to GB/T 42610-2023 standards [122] | Hydrogen permeability coefficient 1.87 × 10−14 cm3·cm/cm2·s Pa. | [32] | |
| HDPE | Injection molding according to GB/T 42610-2023 standards [122] | Hydrogen permeability coefficient 5.88 × 10−14 cm3·cm/cm2·s Pa. | [32] | |
| HDPE/MMT | MNTLE | Oxygen permeability coefficient 2.485 × 10−14 cm3·cm/cm2·s Pa. | Tensile Strength 35.42 MPa, Elongation at break 848.31%. | [62] |
| HDPE/HP030 | Multi-layer extrusion technology | Oxygen barrier increased by 2 times, water vapor barrier increased by 5 times. | [123] | |
| HDPE/PA6 | Micro-layer coextrusion technology | Nitrogen permeability coefficient 2.89 × 10−12 cm3·cm/cm2·s Pa. | [124] | |
| EVOH32 | Oxygen permeability 6 cm3 mm/(m2 day atm). | [125] | ||
| EVOH/LDPE | Continuous multilayer coextrusion | OTR 0.60 ± 0.06 cm3/(m2 day). | Tensile strength 7.3 ± 0.3 MPa, elongation at break 202 ± 60%, Young’s modulus 181 ± 9 MPa. | [126] |
| HDPE | Hot-press method | Pressure 90 MPa Hydrogen permeability coefficient 4.95 × 1016 mol·m/(m2·s·Pa) | [127] | |
| PDDA/SPVDF-GO | Layer-by-layer assembly (LBL) | Hydrogen transmission rate 11.7 cc/m2 d atm. | Tensile strength 366.2 MPa, Young’s modulus approx. 7 GPa, elongation at break approx. 220%. | [128] |
| Category | Content Summary |
|---|---|
| Research Theme | Comprehensive review of gas permeability in Type IV hydrogen cylinder liners, including materials, oxidation, pressure–temperature effects, and coating strategies. |
| Main Materials | PA6, PA11, PA12 (good mechanical/gas barrier); HDPE (easily processable but low barrier); and nanocomposites (e.g., PE + MMT, PE + GF, PE/PA multilayers) improved both strength and permeability. |
| Key Influencing Factors | Material selection, crystallinity, functional groups, multilayer structures, temperature–pressure conditions, thermal oxidation, and overwrap–liner interface. |
| Research Highlights | Nanofillers (e.g., GF, ZrP, MMT) significantly enhanced gas barrier properties, reducing hydrogen permeability by up to 98.6%; multilayer co-extrusion improved both tensile strength and gas resistance; mechanical performance was retained under low-pressure H2 conditions. |
| Limitations | Lack of studies simulating real service environments; insufficient understanding of multilayer interface failure (e.g., buckling, foaming); limited engineering-scale testing and standardization. |
| Application Areas | Primarily in fuel cell vehicles; potential for aerospace, deep-sea, and other extreme-environment hydrogen storage. |
| Future Outlook | Development of high-barrier polymer systems, functionalized nanocomposites, interface engineering, and multiphysics simulation; focus on scalable processing and material standardization. |
| Method Type | Representative Techniques | Advantages | Limitations | Application Scenarios | Ref. |
|---|---|---|---|---|---|
| Experimental Methods | High-Pressure Hydrogen Permeation (HPHP) | High accuracy; aligned with real service conditions; standardized | Time-consuming; requires specialized equipment | Standard qualification testing of liner materials | [32,122,148,159] |
| Thermal Desorption Analysis (TDA) | Rapid hydrogen dissolution analysis; suitable for screening | Less accurate under high pressure; prone to distortion during decompression | Preliminary assessment of dissolved hydrogen content | [127] | |
| Pressure Difference Method | Suitable for low-permeability membranes; simple setup | Limited to low or moderate pressure; slower response under dynamic conditions | Membrane-level gas permeation testing | [62,124,125] | |
| Simulation Methods | Molecular Dynamics (MD) | Microscopic insight; predicts free volume, diffusion behavior, and polymer–gas interactions | High computational cost; requires accurate force fields | Mechanism study, temperature/pressure effect evaluation | [168,169,170,171,173] |
| Grand Canonical Monte Carlo (GCMC) + MD | Quantifies solubility and diffusion; effective under varying thermodynamic conditions | Less effective for non-equilibrium phenomena | Simulation of sorption and transport processes | [170,172] | |
| Materials Studio (MS) molecular modeling | Visual modeling of complex systems; useful for filler and nanocomposite studies | Requires expertise in simulation software and validation | Design of nanocomposite structures; prediction of barrier performance | [157,173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Q.; Wu, S.; Wu, D.; Wu, C.; Cui, D.; Bai, J. Exploring the Gas Permeability of Type IV Hydrogen Storage Cylinder Liners: Research and Applications. Materials 2025, 18, 3127. https://doi.org/10.3390/ma18133127
Li X, Wang Q, Wu S, Wu D, Wu C, Cui D, Bai J. Exploring the Gas Permeability of Type IV Hydrogen Storage Cylinder Liners: Research and Applications. Materials. 2025; 18(13):3127. https://doi.org/10.3390/ma18133127
Chicago/Turabian StyleLi, Xinshu, Qing Wang, Shuang Wu, Dongyang Wu, Chunlei Wu, Da Cui, and Jingru Bai. 2025. "Exploring the Gas Permeability of Type IV Hydrogen Storage Cylinder Liners: Research and Applications" Materials 18, no. 13: 3127. https://doi.org/10.3390/ma18133127
APA StyleLi, X., Wang, Q., Wu, S., Wu, D., Wu, C., Cui, D., & Bai, J. (2025). Exploring the Gas Permeability of Type IV Hydrogen Storage Cylinder Liners: Research and Applications. Materials, 18(13), 3127. https://doi.org/10.3390/ma18133127








